Abstract
To determine cell-mediated immune mechanisms involved in the resolution of chlamydial genital infection of mice, we utilized an established murine model in which it has been demonstrated that resolution of infection occurs independently of the antibody response. Splenic T lymphocytes were obtained from mice that had previously been immunized with viable elementary bodies of the mouse pneumonitis agent (MoPn), a Chlamydia trachomatis biovar. Antigen-reactive T lymphocytes were maintained and expanded in vitro by frequent restimulation with UV light-inactivated MoPn in the presence of antigen-presenting cells and recombinant interleukin-2 (rIL-2). Flow cytometry indicated that this cell line was at least 92% positive for the pan-specific T-cell marker Thy1.2. Stimulation of the cells in the presence of syngeneic antigen-presenting cells plus MoPn antigen and in the absence of exogenous IL-2 induced the cells to produce IL-2 activity in culture supernatants. Following adoptive transfer, this T-lymphocyte line was effective in inducing resolution of an ongoing MoPn genital infection in congenitally athymic nude mice which otherwise maintain chronic unresolved infections. The line was less efficient in resolving the infection after longer periods in culture. An additional T-lymphocyte line was derived from the spleens of athymic mice that had received the first line and had resolved the infection. These T cells were also capable of inducing resolution of the infection. Lastly, this cell line was treated with specific antibody and complement to delete either CD4+ or CD8+ T lymphocytes in an attempt to enrich for T-cell subpopulations prior to transfer into infected athymic mice. The anti-CD4-treated line was essentially depleted of CD4 cells, while the anti-CD8-treated line was only partially enriched for CD4 cells, with a large proportion of CD8 cells still present. Nude mice that received either of the treated T-cell lines or the parental cell line were capable of resolving the infection, although the line with increased numbers of CD4 cells was more efficient than either the parental line or the CD8 line.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron A. L., Rank R. G., Moses E. B. Immune response in mice infected in the genital tract with mouse pneumonitis agent (Chlamydia trachomatis biovar). Infect Immun. 1984 Apr;44(1):82–85. doi: 10.1128/iai.44.1.82-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A. L., White H. J., Rank R. G., Soloff B. L., Moses E. B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981 Jan;143(1):63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Kuo C. C., Cles L., Holmes K. K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983 Mar;39(3):1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Grubbs B., Dickey T. J., Schachter J., Williams D. M. Interferon in recovery from pneumonia due to Chlamydia trachomatis in the mouse. J Infect Dis. 1987 Dec;156(6):993–996. doi: 10.1093/infdis/156.6.993. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Krueger D. A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983 Dec;42(3):1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley R. B. The regulation of T cell growth: requirements for the activation and replication of antigen-specific interleukin 2 producing T cells. Immunobiology. 1982 Oct;163(1):63–76. doi: 10.1016/s0171-2985(82)80107-1. [DOI] [PubMed] [Google Scholar]
- Dailey M. O., Fathman C. G., Butcher E. C., Pillemer E., Weissman I. Abnormal migration of T lymphocyte clones. J Immunol. 1982 May;128(5):2134–2136. [PubMed] [Google Scholar]
- Evans R. T., Taylor-Robinson D. Comparison of various McCoy cell treatment procedures used for detection of Chlamydia trachomatis. J Clin Microbiol. 1979 Aug;10(2):198–201. doi: 10.1128/jcm.10.2.198-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W. R., Meade R., Pettinicchi A., Ptak W., Askenase P. W. Nude mice produce a T cell-derived antigen-binding factor that mediates the early component of delayed-type hypersensitivity. J Immunol. 1989 Mar 15;142(6):1803–1812. [PubMed] [Google Scholar]
- Hough A. J., Jr, Rank R. G. Induction of arthritis in C57B1/6 mice by chlamydial antigen. Effect of prior immunization or infection. Am J Pathol. 1988 Jan;130(1):163–172. [PMC free article] [PubMed] [Google Scholar]
- Klein J. R., Raulet D. H., Pasternack M. S., Bevan M. J. Cytotoxic T lymphocytes produce immune interferon in response to antigen or mitogen. J Exp Med. 1982 Apr 1;155(4):1198–1203. doi: 10.1084/jem.155.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung J. T., Thomas C. A., 3rd Athymic nude CD4+8- T cells produce IL-2 but fail to proliferate in response to mitogenic stimuli. J Immunol. 1988 Dec 1;141(11):3691–3696. [PubMed] [Google Scholar]
- Lammert J. K., Wyrick P. B. Modulation of the host immune response as a result of Chlamydia psittaci infection. Infect Immun. 1982 Feb;35(2):537–545. doi: 10.1128/iai.35.2.537-545.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979 Jul 19;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Blanc C., Lees R. K., Sordat B. Abnormal distribution of T cell subsets in athymic mice. J Immunol. 1986 Jun 15;136(12):4337–4339. [PubMed] [Google Scholar]
- Pasternack M. S., Bevan M. J., Klein J. R. Release of discrete interferons by cytotoxic T lymphocytes in response to immune and nonimmune stimuli. J Immunol. 1984 Jul;133(1):277–280. [PubMed] [Google Scholar]
- Pavia C. S., Schachter J. Failure to detect cell-mediated cytotoxicity against Chlamydia trachomatis-infected cells. Infect Immun. 1983 Mar;39(3):1271–1274. doi: 10.1128/iai.39.3.1271-1274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvigstad E., Hirschberg H. Lack of cell-mediated cytotoxicity towards Chlamydia trachomatis infected target cells in humans. Acta Pathol Microbiol Immunol Scand C. 1984 Jun;92(3):153–159. doi: 10.1111/j.1699-0463.1984.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Qvigstad E., Onsrud M., Degré M., Skaug K. Cell-mediated and humoral immune responses to chlamydial and herpesvirus antigens in patients with cervical carcinoma. Gynecol Oncol. 1985 Feb;20(2):184–189. doi: 10.1016/0090-8258(85)90140-4. [DOI] [PubMed] [Google Scholar]
- Qvigstad E., Onsrud M., Skaug K. T cell proliferative responses to Chlamydia trachomatis antigen in vitro in patients with a history of gynaecological chlamydia infection. Br J Vener Dis. 1984 Apr;60(2):132–132. doi: 10.1136/sti.60.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. H., Soderberg L. S., Rank R. G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988 May;56(5):1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Effect of antithymocyte serum on the course of chlamydial genital infection in female guinea pigs. Infect Immun. 1983 Aug;41(2):876–879. doi: 10.1128/iai.41.2.876-879.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect Immun. 1983 Jan;39(1):463–465. doi: 10.1128/iai.39.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Soderberg L. S., Barron A. L. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect Immun. 1985 Jun;48(3):847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
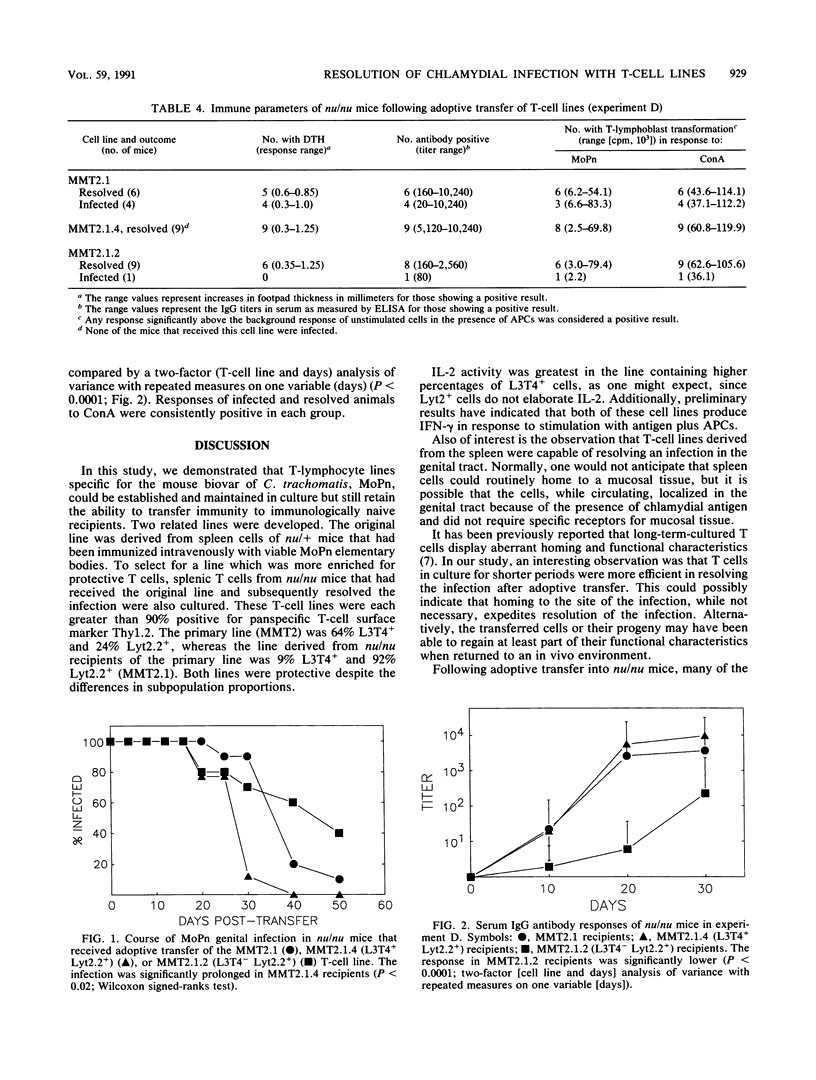
- Rank R. G., Soderberg L. S., Sanders M. M., Batteiger B. E. Role of cell-mediated immunity in the resolution of secondary chlamydial genital infection in guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1989 Mar;57(3):706–710. doi: 10.1128/iai.57.3.706-710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., White H. J., Barron A. L. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1979 Nov;26(2):573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Byrne G. I., Havell E. A. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells). Infect Immun. 1983 Jan;39(1):362–370. doi: 10.1128/iai.39.1.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Byrne G. I., Grubbs B., Marshal T. J., Schachter J. Role in vivo for gamma interferon in control of pneumonia caused by Chlamydia trachomatis in mice. Infect Immun. 1988 Nov;56(11):3004–3006. doi: 10.1128/iai.56.11.3004-3006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



