Abstract
Two strains of Bifidobacterium animalis subsp. lactis were indistinguishable by several nucleic acid-based techniques; however, the type strain DSMZ 10140 was glucose utilization positive, while RB 4825, an industrially employed strain, was unable to grow rapidly on glucose as the principal carbon source. This difference was attributed to the presence of a low-affinity facilitated-diffusion glucose transporter identified in DSMZ 10140 but lacking in RB 4825. Uptake of d-[U-14C]glucose in DSMZ 10140 was stimulated by monovalent cations (ammonium, sodium, potassium, and lithium) and inhibited by divalent cations (calcium and magnesium). When competitor carbohydrates were included in the uptake assays, stereospecific inhibition was exhibited, with greater competition by methyl-β-glucoside than methyl-α-glucoside. Significant inhibition (>30%) was observed with phloretin, an inhibitor of facilitated diffusion of glucose, whereas there was no inhibition by sodium fluoride, iodoacetate, sodium arsenate, sodium azide, 2,4-dinitrophenol, monensin, or valinomycin, which typically reduce energy-driven transport. Based on kinetic analyses, the mean values for Kt and Vmax were 14.8 ± 3.4 mM d-glucose and 0.13 ± 0.03 μmol glucose/min/mg cell protein, respectively. Glucose uptake by several glucose-utilizing commercial strains of B. animalis subsp. lactis was also inhibited by phloretin, indicating the presence of facilitated diffusion glucose transporters in those strains. Since DSMZ 10140 has been previously reported to lack a functional glucose phosphoenolpyruvate phosphotransferase system, the glucose transporter identified here is responsible for much of the organism's glucose uptake.
Probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit on the host (11). With increasing scientific evidence to support their value for human health, interest has grown in the addition of probiotic organisms to dairy products and to pharmaceutical preparations. Bifidobacterium animalis subsp. lactis strains have been isolated from a majority of surveyed probiotic dairy products originating in the United States, Canada, and Europe (15, 16, 18, 27). This subspecies of bifidobacteria possesses some unique technological attributes (e.g., acid and oxygen tolerance) which enable it to better withstand the adverse conditions of starter culture and product manufacture and to maintain viability and stability during product shelf life (28). Strains of B. animalis subsp. lactis have demonstrated better survival and viability than other bifidobacterial species in food products (yogurt and fermented or acidified milk products) (8, 16, 18).
Colonization and survival in the large intestine are dependent on utilization of fermentable carbohydrates not absorbed or metabolized by the host. Several nondigestible carbohydrates (e.g., raffinose, galactooligosaccharides, and inulin) have been identified as prebiotic compounds (i.e., they stimulate growth or activity of bifidobacteria in the large intestine) (13). Monosaccharides generally occur in the large intestine, either in small amounts which have escaped digestion or as the result of extracellular hydrolytic activity by microorganisms in the gastrointestinal tract. While glucose may not be a major nutrient for bifidobacteria in the human intestinal tract, it is often included in bifidobacterial media in industry and laboratory settings, and many strains of bifidobacteria are able to metabolize exogenously supplied glucose.
Even though carbohydrate utilization is critical to understanding bifidobacterial activity in the gut, sugar transport in this genus has only recently been examined. The genome sequence of Bifidobacterium longum NCC 2705 revealed as many as 10% of its genes are involved in carbohydrate transport and metabolism (41). Direct experimental as well as sequence-based evidence has been used to identify a few carbohydrate transporters in bifidobacteria. Activity of a glucose phosphoenolpyruvate phosphotransferase system (PTS) was demonstrated biochemically in Bifidobacterium breve NCFB 2257 (9), while in Bifidobacterium bifidum DSM 20082, activities of a lactose/proton symport, a glucose/potassium symport, and an unsaturable galactose permease were identified (23, 24). In B. longum NCC 2705, glucose-PTS activity and an inducible glucose/proton symport subject to lactose repression were detected, suggesting multiple glucose transport systems occur in this species (30, 31). However, neither B. animalis subsp. lactis DSMZ 10140 nor B. bifidum MB245 exhibited glucose-PTS activity in assays performed by Parche et al.; only a fructose-specific PTS was detected (30).
Although most strains of bifidobacteria are able to utilize glucose, glucose-nonfermenting strains of B. pseudolongum (33), B. gallinarum (44), B. animalis subsp. lactis (27, 40), and an uncharacterized Bifidobacterium sp. (4, 34) have been reported in the literature. The objective of the present research was to develop an understanding of what is responsible for the difference in glucose utilization between strains of B. animalis subsp. lactis that can and cannot grow on glucose. The present study compares two closely related strains of B. animalis subsp. lactis, namely DSMZ 10140 (the type strain) which is glucose fermenting, and RB 4825 (from a commercial starter culture manufacturer), which was identified as a glucose-negative strain by the supplier. The conclusion of this work is that glucose utilization by B. animalis subsp. lactis requires the activity of a low-affinity, facilitated diffusion-based transporter, the properties of which are described here.
MATERIALS AND METHODS
Bacterial strains.
Bifidobacterium animalis subsp. lactis DSMZ 10140 was obtained from the DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany), and B. animalis subsp. lactis RB 4825 was received from a commercial supplier. Additional strains of B. animalis subsp. lactis used in several studies were obtained from either culture collections (ATCC 27536) or from commercial starter culture companies (RB 0171, RB 1280, and RB 1573). Commercial strains (with the RB designation) are available from the corresponding author for noncommercial purposes.
Bifidobacteria were maintained in liver lactose (LL) broth (25). All medium components were obtained from BD Diagnostic Systems (Sparks, MD). Broth media were incubated at 37°C for 24 h in an anaerobic incubator (10% carbon dioxide, 5% hydrogen, 85% nitrogen [VWR, West Chester, PA]).
Growth of bifidobacteria in LL and LG media.
Strains of B. animalis subsp. lactis were grown in LL broth, harvested in mid- to late-log phase by centrifugation (21,000 × g for 10 min), and resuspended in sodium phosphate buffer with cysteine (0.05 M phosphate buffer [pH 6.5], 500 mg/liter cysteine). Aliquots were transferred (1% [vol/vol]) to warm LL or liver glucose (LG) broth (same composition as LL broth but with 10 g/liter glucose replacing lactose) and incubated anaerobically at 37°C. The optical density at 600 nm (OD600) was monitored using a Spectronic 21D spectrophotometer (Milton Roy, Rochester, NY). All growth curves were performed at least in triplicate.
For high-performance liquid chromatography analyses to monitor carbohydrate utilization (21), samples of broth cultures were centrifuged for 10 min at 5,000 × g to pellet the cells. A 300-μl portion of the supernatant was diluted with 1.2 ml of 0.005 M sulfuric acid and centrifuged at 21,000 × g for 10 min. The supernatant was filtered (0.2-μm filter) and stored at 4°C. The chromatographic system consisted of a Waters 510 solvent delivery system (Waters Corporation, Milford, MA), a Rheodyne 7125 sample injector (20-μl loop; Rheodyne, Inc., Cotati, CA), a Bio-Rad IG cation H guard column (Bio-Rad, Hercules, CA), an Aminex HPX-87H column (300 by 7.8 mm; Bio-Rad), and a Waters 410 differential refractometer. The column and detector were maintained at 35°C, while the solvent, 0.005 M sulfuric acid, was delivered at a flow rate of 0.6 ml/min. Data were acquired and processed using Empower software (Waters). Standard curves were prepared to quantify lactose, glucose, galactose, lactic acid, and acetic acid and were linear over the range of 0.1 to 10 g/liter.
Nucleic acid-based characterization of bifidobacteria.
Strains were confirmed as B. animalis subsp. lactis using the 16S rRNA-targeted species-specific PCR primers described by Ventura and Zink (42). A rapid pulsed-field gel electrophoresis (PFGE) method (6) was then used to further characterize strains of B. animalis subsp. lactis, comparing restriction patterns of chromosomal DNA digested with XbaI or SpeI.
For strain comparison by randomly amplified polymorphic DNA (RAPD)-PCR analyses, DNA was extracted as described by Vincent et al. (43) and RAPD-PCR was performed using seven different primers. The sequences for primers #103 (5′-GTGACGCCGC-3′), #127 (5′-ATCTGGCAGC-3′), and #173 (5′-CAGGCGGCGT-3′) are from Sakata et al. (36). Primer sequences AB-1 (5′-GGTGCGGGAA-3′) and AB-5 (5′-AACGCGCAAC-3′) were from the Amersham Biosciences Ready-to-Go RAPD analysis beads technical insert. Primer sequences OPV-07 (5′-GAAGCCAGCC-3′) and OPR-13 (5′-GGACGACAAG-3′) were from Mayer et al. (29).
Preparation of cells for glucose uptake assays.
Prior to all glucose uptake assays, strains were subcultured in either LL or LG broth for at least five consecutive days. Strains of B. animalis subsp. lactis were grown anaerobically in the appropriate medium until mid-log phase (OD600 of ∼0.5). Cells were harvested by centrifugation (8,000 × g for 10 min at 4°C), washed twice with an equal volume of buffer (4°C), and then resuspended to a uniform cell density of 1.0 OD unit (Microscan turbidity meter; Dade Behring, Inc., Deerfield, IL). Unless otherwise specified, 0.05 M potassium phosphate buffer (pH 7.5) containing 5 mM NaCl was used to prepare cells and reagents. Cell suspensions (750-μl portions) were transferred to sterile microcentrifuge tubes and held on ice until analysis. Holding samples up to 4 h did not affect glucose uptake.
Glucose uptake assays.
Glucose uptake was determined by incubating cells with d-[U-14C]glucose (GE Healthcare, Buckinghamshire, United Kingdom). Aliquots of resuspended cells were sequentially removed from ice and held in a water bath at 37°C for 10 min. A 500-μl volume of the cell suspension was added to 50 μl of a 37°C glucose solution, and then the sample was immediately vortexed and returned to the 37°C bath for an incubation time of 5 min unless specified otherwise. For standard assays, the final concentrations of glucose were 1 mM and 0.2 μCi/ml. After incubation, a 500-μl portion of the cell-glucose mixture was removed and quickly filtered through a nitrocellulose membrane filter (Whatman, Florham Park, NJ [0.45-μm pore, 25-mm diameter]) using a vacuum flask and aspirator. Filters were rapidly washed twice with 1-ml aliquots of 37°C 0.05 M phosphate buffer (pH 7.5) containing 5 mM NaCl. Generally, sample filtration and washing occurred in less than 15 s. Filters were transferred to 20-ml scintillation vials and dissolved in 2 ml of 2-methoxyethanol, and then 12 ml EcoScint A scintillation fluid (National Diagnostics, Atlanta, Georgia) was added. Radioactivity was determined with an LS 6500 scintillation system (Beckman Instruments, Fullerton, CA). Assays were performed at least in duplicate. Kinetic parameters of glucose uptake were estimated at evenly spaced reciprocal substrate concentrations while holding the radioactivity in each assay constant (0.2 μCi/ml); velocity data were analyzed by double-reciprocal plots and linear regression. Although this assay is actually a determination of glucose-carbon incorporation into cells rather than a precise measure of glucose transport or uptake, similar methods have been widely used to study bacterial transporters even though efflux or other routes for loss of carbon are likely occur to some extent.
Carbohydrate competitor experiments were performed in the same manner as described above, except with the addition of nonradiolabeled carbohydrates to the glucose solution. The glucose concentration in the assay was held at 1 mM (0.2 μCi/ml), and competitors were evaluated at 30 mM unless otherwise noted.
To evaluate the effect of cations on glucose uptake, cells were harvested in mid-log phase, washed twice, and resuspended to 1.0 OD unit with 0.05 M imidazole-HCl buffer (pH 7.5). Portions (700 μl) of cell suspension were mixed with 35 μl of cation solution and preincubated for 10 min at 37°C. A sample of the cell-cation mixture (525 μl) was then combined with 25 μl of labeled glucose solution prepared in imidazole buffer (pH 7.5) to give 1 mM glucose and 0.2 μCi/ml. After incubation, 500-μl samples were removed, filtered, and prepared for liquid scintillation counting as described above. Sodium chloride was evaluated at final concentrations from 1 to 50 mM, and all other cations were evaluated at a final concentration of 5 mM. Imidazole buffer (no added cations) was used as the control for these experiments.
Other compounds tested for possible inhibition of glucose transport were analyzed in the same manner as described for the cation assays, but with imidazole buffer containing 50 mM NaCl. For the control, 35 μl of the solvent used to prepare the inhibitors (e.g., ethanol) was added to the cell suspension and was incubated for 10 min at 37°C as described above.
Galactose was obtained from VWR, and sodium azide was obtained from Fisher Scientific (Pittsburgh, PA). All other carbohydrates, salts, and inhibitors were obtained from Sigma (St. Louis, MO).
Statistical analysis of effect of various treatments on glucose uptake.
General linear model tests were performed using Minitab statistical software (Minitab, Inc., State College, PA) to identify significant differences among mean values of glucose uptake. Pairwise differences of the mean responses were evaluated using Tukey's procedure (P = 0.05).
RESULTS
Similarity of DSMZ 10140 and RB 4825 revealed using nucleic acid-based techniques.
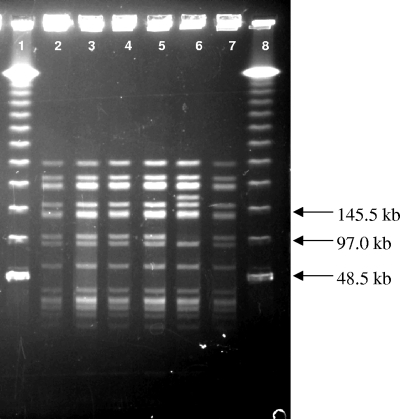
Both DSMZ 10140 and RB 4825 were confirmed as B. animalis subsp. lactis strains by PCR. RAPD-PCR performed with seven different primers was unable to distinguish between the two strains, although B. animalis subsp. animalis ATCC 25527 and ATCC 27672, as well as a number of less closely related species of Bifidobacterium, did differ from DSMZ 10140 and RB 4825 (data not shown). PFGE analysis employing two different restriction enzymes was conducted on DSMZ 10140, RB 4825, and several other B. lactis strains obtained from commercial sources. As shown in Fig. 1, all strains exhibited an identical SpeI digest pattern that differed from that of B. animalis subsp. lactis ATCC 27536. However, all strains of B. animalis subsp. lactis exhibited an identical XbaI digest pattern (data not shown). The inability of sensitive nucleic acid-based methods to differentiate between DSMZ 10140 and RB 4825 (as well as the other strains used here) indicates a very high degree of relatedness, a feature common among commercial isolates of B. animalis subsp. lactis (4, 8, 15, 42).
FIG. 1.
PFGE of commercial and reference B. animalis subsp. lactis strains restricted using SpeI. Lanes 1 and 8, Lambda molecular weight marker; lanes 2 to 5, commercial strains of B. animalis subsp. lactis (lane 2, RB 0171; lane 3, RB 1280; lane 4, RB 1573; lane 5, RB 4825); lane 6, B. animalis subsp. lactis ATCC 27536; lane 7, B. animalis subsp. lactis DSMZ 10140.
Growth of DSMZ 10140 and RB 4825 in LL and LG media.
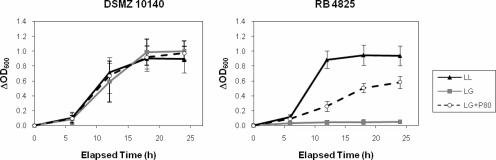
Representative growth curves for B. animalis subsp. lactis strains in LL and LG media are illustrated in Fig. 2. Growth rates in LL and LG media were similar for DSMZ 10140, as well as for strains RB 1280 and RB 1573. Strain RB 4825 grew well in LL broth, but only slightly in LG broth, with similar behavior exhibited by RB 0171. However, RB 4825 and RB 0171 grew in LG medium if 0.1% polysorbate 80 was added. One possible explanation is polysorbate 80 increased permeability of the cell membrane (7), resulting in increased glucose uptake and subsequent cell growth.
FIG. 2.
Growth of B. animalis subsp. lactis strains DSMZ 10140 and RB 4825 in LL and LG media. Strains DSMZ 10140 and RB 4825 were grown in LL medium to mid- to late-log phase, harvested, washed with phosphate buffer, and then inoculated into LL broth, LG broth, or LG broth with 0.1% polysorbate 80 (LG+P80) and incubated at 37°C anaerobically. Growth was monitored as OD600. Values represent the means of triplicate experiments; error bars represent standard deviations.
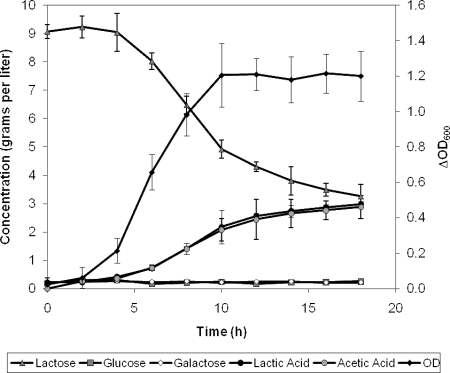
Lactose utilization, product formation, and growth of DSMZ 10140 in LL medium appear in Fig. 3, and similar behavior was observed with RB 4825 (data not shown). Because both strains utilize lactose and can metabolize glucose derived from it, it was concluded the difference in glucose phenotype was related to the ability of the strains to transport glucose. Recognizing previous work had shown DSMZ 10140 lacked the activity of a glucose-specific PTS (30), attention was focused on analyzing the activity of glucose transporters that function independent of phosphoenolpyruvate hydrolysis.
FIG. 3.
Utilization of lactose, product formation, and growth of B. animalis subsp. lactis DSMZ 10140 in LL medium. Strain DSMZ 10140 was grown in LL broth to mid- to late-log phase, harvested, washed with phosphate buffer, and then inoculated into LL broth and incubated at 37°C anaerobically. Growth was monitored as OD600. Glucose, galactose, lactic acid, and acetic acid were monitored by HPLC. Data represent the mean of replicates; error bars represent standard deviations.
Comparative glucose uptake rates.
Glucose uptake rates were typically measured over 5 min of incubation, which was within the linear range for glucose uptake. Glucose uptake rates by DSMZ 10140 and RB 4825, when harvested from LL broth, were approximately 5 and 1 nmol/min/mg protein, respectively, when measured at a supplied glucose concentration of 1 mM (Table 1). Under these conditions, the glucose-positive DSMZ 10140 strain transported glucose at a rate five times higher than the glucose-negative RB 4825 strain, suggesting the lower level of glucose taken up by RB 4825 is insufficient for normal growth in LG medium. Glucose-utilizing strains RB 1280 and RB 1573 exhibited even higher uptake rates than DSMZ 10140 when grown on LL medium. While both RB 4825 and RB 0171 are glucose-nonfermenting strains unable to grow on LG broth, RB 0171 exhibited a glucose transport rate (when grown on LL broth) lower than RB 4825.
TABLE 1.
Comparative glucose uptake rates of B. animalis subsp. lactis strains
| Strain | Glucose uptake rate (nmol/min/mg cell protein)a
|
|
|---|---|---|
| LL medium | LG medium | |
| DSMZ 10140 | 4.9 ± 0.3 | 9.6 ± 0.4 |
| RB 1280 | 8.3 ± 0.2 | 7.4 ± 0.2 |
| RB 1573 | 9.0 ± 0.1 | 8.0 ± 0.3 |
| RB 4825 | 1.4 ± 0.1 | ND |
| RB 0171 | 0.8 ± 0.1 | ND |
The glucose uptake rate was determined with strains of B. animalis subsp. lactis grown to mid-log phase in either LL or LG broth. Glucose uptake assays were performed in 0.05 M potassium phosphate buffer (pH 7.5) with 5 mM NaCl and 1 mM glucose. Values represent the means of duplicate assays ± standard deviations. ND, not determined.
It should be noted from Table 1 that uptake of glucose by those strains capable of utilizing glucose (DSMZ 10140, RB 1280, and RB 1573) was not consistently elevated when grown on glucose compared to lactose, with only DSMZ 10140 exhibiting evidence of possible lactose repression of glucose transport. Moreover, analysis of culture media during growth of DSMZ 10140 on a mixture of glucose and lactose revealed coutilization of both carbohydrates (data not shown). The combined results argue that glucose uptake in these strains of B. animalis subsp. lactis is not subject to strong repression by lactose, in contrast to what has been concluded for B. longum NCC 2705 (31).
Determination of kinetic parameters for glucose uptake by DSMZ 10140.
A more detailed examination of uptake rate as a function of glucose concentration by DSMZ 10140 revealed values for Kt (the apparent affinity constant for a transport protein) and Vmax of 14.8 ± 3.4 mM d-glucose and 130 ± 30 nmol glucose/min/mg cell protein, respectively, from three independent experiments using glucose concentrations up to 30 mM. Low-affinity glucose transporters are sometimes paired with high-affinity glucose transporters and can be identified by the presence of biphasic kinetics when uptake is evaluated over a wide range of concentrations (12, 14, 20, 32). To search for additional transporters with higher affinities, uptake assays were performed with glucose concentrations as low as 30 μM and biphasic kinetics were not observed. Thus, in DSMZ 10140, either a high-affinity glucose transporter does not exist or its activity is so low as to be overshadowed by that of the low-affinity transporter.
The low level of glucose uptake observed in strain RB 4825 when grown on LL broth did not allow a determination of Kt or Vmax values because saturation kinetics were not observed. This suggests the uptake measured in this strain was due to simple diffusion, although the possibility of a weak transporter having a very high Kt value, unmeasurable under the experimental conditions, cannot be completely excluded. Further studies are needed to distinguish between these situations.
Characterization of glucose transport in DSMZ 10140.
Additional characterization of glucose uptake by strain DSMZ 10140 included evaluation of the competitive effect of other carbohydrates in order to gain information on the recognition specificity of the transporter for other sugars. When tested at a 30-fold molar excess over glucose, compounds showing competition for uptake included 2-deoxyglucose, galactose, maltose, melibiose, N-acetylglucosamine, methyl-α-glucoside, and methyl-β-glucoside (Table 2). Inhibition by 2-deoxyglucose and N-acetylglucosamine indicates the absence of a C2 hydroxyl group, as observed with these glucose analogues, did not prevent their recognition by the transporter. Similarly, galactose, an epimer of d-glucose at the C4 position, was inhibitory. The α- and β-methyl-glucosides were markedly different in reducing glucose uptake, the β-glucoside being a better competitor. This difference suggests stereospecificity for glucose uptake, a key characteristic of carrier-mediated transporters. Compounds not inhibitory under the conditions employed include d-xylose, l-arabinose, fructose, cellobiose, amygdalin, lactose (assayed at 15 mM), raffinose, and inulin.
TABLE 2.
Competition by other carbohydrates for glucose uptake in B. animalis subsp. lactis DSMZ 10140
| Competitor (assayed at 30 mM)a | % Change in glucose uptakeb |
|---|---|
| None | 0 ± 2c |
| d-Ribose | −2 ± 2 |
| d-Melibiose | −7 ± 2* |
| Methyl-α-glucoside | −11 ± 3* |
| Maltose | −13 ± 5* |
| 2-Deoxy-d-glucose | −20 ± 3* |
| d-Galactose | −23 ± 3* |
| N-Acetylglucosamine | −25 ± 8* |
| Methyl-β-glucoside | −35 ± 1* |
Glucose uptake was determined by growing DSMZ 10140 in LG broth to mid-log phase. Glucose uptake assays were performed in 0.05 M potassium phosphate buffer containing 5 mM NaCl, 1 mM glucose, and 30 mM competitor.
Values represent means ± standard deviations from duplicate assays and at least two replications. Values with an asterisk are statistically different from the control without added competitor (α = 0.05).
Mean value for control without added competitor, 7.0 nmol/min/mg cell protein.
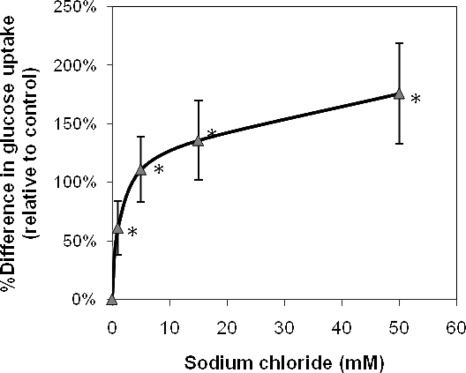
Because dependence of uptake on a specific cation is often used to reveal the type of transporter involved, several cations were tested for their effect on glucose incorporation by DSMZ 10140 (Table 3). Monovalent cations NH4+, Na+, K+, and Li+, but not Cs+, significantly stimulated glucose uptake, while the divalent cations Mg2+ and Ca2+ were inhibitory; all were evaluated as their chloride salts. Moreover, sodium and potassium phosphate stimulated glucose uptake similar to sodium and potassium chloride when tested at identical cation concentrations (data not shown). Sodium chloride concentrations ≥1 mM promoted glucose uptake, with the effect continuing to at least 50 mM, as illustrated in Fig. 4. The stimulation of uptake by multiple monovalent cations in B. animalis subsp. lactis was unexpected since glucose uptake in B. bifidum DSM 20082 specifically required potassium ions and was inhibited by the ionophore lasalocid, indicating a potassium symport (24). Lactose uptake in the same strain was by a proton symport system that could be inhibited by proton ionophores and was unaffected by sodium, potassium, or lithium ions (23).
TABLE 3.
Effect of cations on glucose uptake by B. animalis subsp. lactis DSMZ 10140
| Cation (assayed at 5 mM)a | % Change in glucose uptakeb |
|---|---|
| None added | 0 ± 4c |
| Potassium chloride | 150 ± 38* |
| Ammonium chloride | 143 ± 41* |
| Sodium chloride | 112 ± 23* |
| Lithium chloride | 87 ± 19* |
| Cesium chloride | 22 ± 11 |
| Magnesium chloride | −17 ± 7* |
| Calcium chloride | −82 ± 2* |
Glucose uptake was determined with strain DSMZ 10140 grown in LG broth to mid-log phase. Uptake assays were performed in 0.05 M imidazole-HCl buffer (pH 7.5) with 1 mM glucose and 5 mM cation.
Values represent the means ± standard deviations from duplicate assays with at least two experimental replications. Values with an asterisk are statistically different from the control without added cation (α = 0.05).
Mean uptake value for control without added cation, 2.5 nmol/min/mg cell protein.
FIG. 4.
Effect of sodium chloride concentration on glucose uptake by B. animalis subsp. lactis DSMZ 10140. Glucose uptake was determined by growing DSMZ 10140 in LG broth to mid-log phase. Glucose uptake assays were performed in 0.05 M imidazole buffer with 1 mM glucose. Change in glucose uptake is expressed relative to the control, without added sodium chloride (mean value of 2.6 nmol/min/mg cell protein). Values represent the means of at least two replicate experiments; error bars represent standard deviations. Values with an asterisk are statistically different from the control without added sodium chloride (α = 0.05).
Evidence for facilitated diffusion of glucose in DSMZ 10140 and other B. animalis subsp. lactis strains.
A number of inhibitors known to affect active transport systems were evaluated, and all failed to have a measurable effect on glucose uptake in DSMZ 10140 under the conditions employed. The inhibitors examined included 15 mM sodium fluoride, 0.5 mM iodoacetate, 50 mM sodium arsenate, 10 mM sodium azide, 1 mM 2,4-dinitrophenol, 0.01 mM monensin (a sodium ionophore), and 0.015 mM valinomycin (a potassium ionophore). Assays with the ionophores were performed in imidazole buffer containing either 50 mM sodium chloride, 50 mM potassium chloride, or no added salt. Thus, although glucose uptake in DSMZ 10140 was stimulated by addition of monovalent cations, the transporter was not a typical proton- or alkali metal-dependent symport in that ionophores (2,4-dinitrophenol, monensin, and valinomycin) did not affect glucose uptake. Furthermore, energy poisons such as fluoride, iodoacetate, arsenate, and azide also had no effect on uptake, indicating the uptake process was likely not energy linked through ATP hydrolysis.
Additional information on the nature of the glucose transporter in DSMZ 10140 was sought by examining the effect of compounds typically used to disrupt facilitated diffusion sugar transporters on glucose uptake. Phloretin and phloridzin, tested at 1 mM, decreased glucose uptake by 32% ± 3% and 6% ± 2%, respectively, relative to a control without added inhibitor. Phloretin has been identified as an inhibitor of facilitated diffusion of glucose, whereas phloridzin, the 2′-β-glucoside of phloretin, is not (19, 26). In red blood cells, phloretin interferes with glucose binding on the inside and outside of the cell membrane and competitively inhibits glucose uptake (2, 26), while in Escherichia coli, phloretin noncompetitively inhibits facilitated diffusional transport of β-galactosides (3).
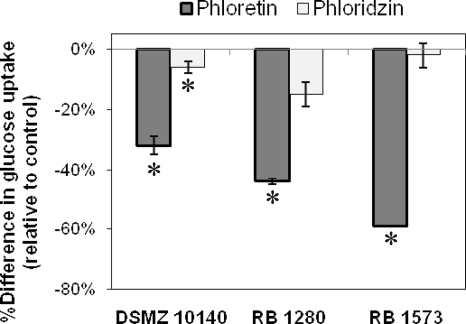
Several commercially obtained strains of B. animalis subsp. lactis which were able to grow rapidly on LG medium and demonstrated glucose uptake were also studied for the effects of phloretin and phloridzin on their glucose uptake (Fig. 5). Phloretin significantly inhibited glucose uptake for all of the strains evaluated, indicating facilitated diffusion-based transporters were present and involved in the uptake of glucose for these strains. Phloridzin minimally affected their glucose uptake.
FIG. 5.
Effect of phloretin and phloridzin on glucose uptake by selected strains of B. animalis subsp. lactis. Glucose uptake was determined after growth in LG broth to mid-log phase. Glucose uptake assays were performed in 0.05 M imidazole buffer with 50 mM NaCl containing 1 mM glucose. Phloretin and phloridzin were prepared in ethanol and assayed with a 1 mM final concentration. An equivalent volume of ethanol was added to each control (1.25%). Change in glucose uptake is expressed relative to the control, without added inhibitor. Mean values of controls were 6.3 (DSMZ 10140), 6.4 (RB 1280), and 4.1 (RB 1573) nmol/min/mg cell protein from duplicate experiments; error bars represent standard deviations. Values with an asterisk are statistically different from the control without added inhibitor (α = 0.05).
DISCUSSION
In this work, two highly related strains of B. animalis subsp. lactis, DSMZ 10140 and RB 4825, could not be differentiated by the PFGE or RAPD-PCR techniques employed but were easily distinguished by their difference in utilization of glucose as a carbon source. Bifidobacterium strain differentiation will be improved once more genome sequences are available to permit comparisons over a broad range of genes. A total genomic sequence has been reported for B. longum (38), and a full genome sequence analysis for DSMZ 10140 has just been completed in our lab, but at this time, the only detectable difference within the group of B. animalis subsp. lactis strains examined here concerns their variable ability to utilize glucose.
Growth of strains of B. animalis subsp. lactis in a glucose-based medium correlated with the ability of these strains to exhibit glucose uptake. Further characterization of the glucose-positive strain DSMZ 10140 demonstrated glucose uptake occurs via a low-affinity, facilitated-diffusion, monovalent cation-enhanced transporter. Evidence for a carrier-mediated transport system in DSMZ 10140 included (i) saturable kinetics with a Kt of 14.8 mM; (ii) stereospecificity, with greater competition by methyl-β-glucoside than methyl-α-glucoside; and (iii) decreased glucose uptake of more than 30% by phloretin, an inhibitor of facilitated diffusion of glucose. A range of monovalent cations stimulated uptake but appear not to be absolutely required, thereby suggesting that uptake is not energetically driven by a cation-dependent symport process but only enhanced by certain cations.
The low-affinity transport kinetics for glucose observed in this study with B. animalis subsp. lactis DSMZ 10140 (Kt of 15 mM and Vmax of 0.13 μmol/min/mg cell protein) are similar to those seen for the glucose transporter in B. bifidum DSM 20082, which exhibited a Kt of 9.2 mM and Vmax of 0.027 μmol/min/mg cell protein (24). In B. longum NCC 2705, an inducible glucose-proton symporter of the MFS family was identified, and kinetic analyses gave a Kt of 70 μM when performed in a heterologous system: i.e., the glcP gene was cloned and expressed in a glucose transport-deficient strain of E. coli (31). While heterologous complementation offers the advantage of characterizing a single transporter, the kinetics may be affected by altered conformations and quantities of the transport protein in membranes with different compositions. For example, Kt values for a glucose transporter were 0.42 mM in Vibrio parahaemolyticus and 0.03 mM when the gene was cloned and expressed in transformed E. coli (37).
Likewise, similar selectivity toward analogues to that seen in DSMZ 10140 was noted for the glucose transporter in B. bifidum DSM 20082, where 2-deoxyglucose, N-acetylglucosamine, and galactose were also strong competitors for glucose uptake, with decreases in uptake of 66%, 54%, and 34%, respectively, when added at a 20-fold excess over glucose (24). In B. longum NCC 2705, galactose inhibited glucose uptake by nearly 70% (concentrations 50 times in excess of glucose) (31). However, these studies did not examine the effects of methylglucosides on uptake.
Unlike the potassium ion-specific glucose transporter in B. bifidum DSM 20082, glucose uptake by DSMZ 10140 was stimulated by any of several cations, thus suggesting the cations may function indirectly rather than as a specific ion symport. Moreover, there was no discernible effect of potassium or sodium ionophores on glucose uptake, arguing that transport was not being driven by an ion gradient. While no detailed explanation can be offered at this time, it is likely that interactions between cations and the glucose transporter in DSMZ 10140 result in a change to the protein conformation producing a favorable effect on its subsequent activity.
Inhibition of glucose transport by divalent cations, particularly as was noted with calcium ions in DSMZ 10140, is seldom studied, so the prevalence of this behavior is uncertain. In red blood cells, calcium inhibited uptake of 3-O-methyl-α-glucoside, an effect which was reduced by ATP (17). In mutants of E. coli K-12, glucose uptake was inhibited by zinc chloride, but not by calcium chloride (1). In contrast, the ability to utilize maltose was restored when a mutant strain of E. coli lacking a maltose-binding protein was treated with calcium chloride (5). Calcium disrupted the outer membrane, resulting in an increase in permeability and restoration of maltose transport. No observation similar to that observed with DSMZ 10140 (i.e., glucose uptake stimulation by monovalent cations and inhibition by divalent cations) appears to have been reported. It is plausible that calcium plays a regulatory role in glucose uptake by Bifidobacterium since milk contains 30 mM total calcium, of which approximately 2 mM free ionic calcium exists, although this varies with processing conditions, temperature, and pH (39). Bifidobacteria are among the major genera of bacteria isolated from infants fed breast milk and have been used in the manufacture of cultured dairy products. Calcium ions derived from milk may suppress glucose uptake in bifidobacteria, focusing the cell's resources on utilization of the primary carbohydrates present (i.e., lactose and complex oligosaccharides), but conversely, inhibition by calcium ions could also reduce the undesired loss of intracellular glucose under conditions in which the external glucose concentration is low relative to its internal level.
Glucose transport in B. longum NCC 2705 is repressed by lactose (31). When grown on a mixture of lactose and glucose, NCC 2705 sequentially utilized lactose before glucose. Additionally, when NCC 2705 was grown on a lactose-containing medium, glucose uptake was 30% of that seen when grown on a glucose-containing medium. By comparison, growth on lactose had less effect on glucose uptake in strains of B. animalis subsp. lactis. Although DSMZ 10140 grown in LL broth exhibited glucose uptake only 50% of that observed when grown in LG broth, other strains of B. animalis subsp. lactis (RB 1280 and RB 1573) demonstrated no effect attributable to lactose repression (Table 1). Moreover, analysis of supernatants of DSMZ 10140 grown on a mixture of lactose and glucose revealed co-utilization of both carbohydrates (data not shown). These results suggest glucose uptake is regulated differently in B. animalis subsp. lactis than in B. longum, in agreement with evidence that indicates different glucose transport systems are operative in the two species.
Glucose transport through facilitated diffusion, while perhaps uncommon overall, is certainly well recognized in a variety of organisms; yeast and red blood cells have served as model systems for this process. Glucose transport by facilitated diffusion was reported for the rumen bacterium, Streptococcus bovis (35), and a low-affinity facilitated diffusion glucose transporter (Kt of 5 to 15 mM) was identified in Zymomonas mobilis (10). More recently, the presence of facilitated diffusion transporters in E. coli has been examined by Kornberg and associates (22). Situations exist in which an isoform of the membrane-bound enzyme II for a specific sugar is produced, usually through mutation, but the modified enzyme II species does not interact with other PTS components to permit phosphoenolpyruvate-dependent uptake and concurrent phosphorylation. Instead, the enzyme II isoform is able to bind and participate in the uptake of a sugar via facilitated diffusion. In view of the fact that B. lactis does not have a functional glucose-PTS, whereas B. longum does, the possibility that facilitated diffusion of glucose in B. animalis subsp. lactis could be accomplished by an altered form of the enzyme IIGlc is worthy of further consideration.
Facilitated diffusion glucose transporters have not been frequently recognized in bacteria, likely because such systems may be overlooked if there are active transporters that can be more easily identified and characterized. Unlike active transport systems, no energy requirement exists, and thus facilitated diffusion transporters cannot concentrate a substrate against a gradient. Although free glucose concentrations in the large intestine are rather low, being formed primarily by the action of bacterial sugar hydrolases on poorly digested polysaccharides, bifidobacteria could take up glucose as long as its internal glucose concentration is even lower and divalent cations like calcium are limiting. Nevertheless, it appears that by specializing in the utilization of large oligosaccharides, Bifidobacterium lactis strains have adapted to their environment by competing for whatever carbohydrate substrates are available, without the need for high-affinity, energy-driven glucose transporters (38).
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Anraku, Y. 1968. Transport of sugars and amino acids in bacteria. III. Studies on the restoration of active transport. J. Biol. Chem. 243:3128-3135. [PubMed] [Google Scholar]
- 2.Basketter, D. A., and W. F. Widdas. 1978. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J. Physiol. 278:389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batt, E. R., and D. Schachter. 1971. Effects of phloretin and synthetic estrogens on β-galactoside transport in Escherichia coli. Biochim. Biophys. Acta 233:189-200. [DOI] [PubMed] [Google Scholar]
- 4.Bonaparte, C., and G. Reuter. 1997. Bifidobacteria in commercial dairy products: which species are used? Microecol. Ther. 28:181-197. [Google Scholar]
- 5.Brass, J. M., W. Boos, and R. Hengge. 1981. Reconstitution of maltose transport in malB mutants of Escherichia coli through calcium-induced disruptions of the outer membrane. J. Bacteriol. 146:10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briczinski, E. P., and R. F. Roberts. 2006. Technical note: a rapid pulsed-field gel electrophoresis method for analysis of bifidobacteria. J. Dairy Sci. 89:2424-2427. [DOI] [PubMed] [Google Scholar]
- 7.Brown, M. R. W., E. M. Geaton, and P. Gilbert. 1979. Additivity of action between polysorbate 80 and polymyxin B towards spheroplasts of Pseudomonas aeruginosa NCTC 6750. J. Pharm. Pharmacol. 31:168-170. [DOI] [PubMed] [Google Scholar]
- 8.Crittenden, R. G., L. F. Morris, M. L. Harvey, L. T. Tran, H. L. Mitchell, and M. J. Playne. 2001. Selection of a Bifidobacterium strain to complement resistant starch in a synbiotic yoghurt. J. Appl. Microbiol. 90:268-278. [DOI] [PubMed] [Google Scholar]
- 9.Degnan, B. A., and G. T. Macfarlane. 1993. Transport and metabolism of glucose and arabinose in Bifidobacterium breve. Arch. Microbiol. 160:144-151. [DOI] [PubMed] [Google Scholar]
- 10.DiMarco, A. A., and A. H. Romano. 1985. d-Glucose transport system of Zymomonas mobilis. Appl. Environ. Microbiol. 49:151-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FAO/WHO. 2002. Guidelines for the evaluation of probiotics in foods: Joint FAO/WHO Working Group Meeting, London, Ontario, Canada, 30 April to 1 May 2002. World Health Organization, Geneva, Switzerland.
- 12.Garcia-Dominguez, M., J. F. Martin, and P. Liras. 1989. Characterization of sugar uptake in wild-type Streptomyces clavuligerus, which is impaired in glucose uptake, and in a glucose-utilizing mutant. J. Bacteriol. 171:6808-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 14.Gourdon, P., M. Raherimandimby, H. Dominguez, M. Cocaign-Bousquet, and N. D. Lindley. 2003. Osmotic stress, glucose transport capacity and consequences for glutamate overproduction in Corynebacterium glutamicum. J. Biotechnol. 104:77-85. [DOI] [PubMed] [Google Scholar]
- 15.Grand, M., M. Küffer, and A. Baumgartner. 2003. Quantitative analysis and molecular identification of bifidobacteria strains in probiotic milk products. Eur. Food Res. Technol. 217:90-92. [Google Scholar]
- 16.Gueimonde, M., S. Delgado, B. Mayo, P. Ruas-Madiedo, A. Margolles, and C. G. de los Reyes-Gavilán. 2004. Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res. Int. 37:839-850. [Google Scholar]
- 17.Helgerson, A. L., D. N. Hebert, S. Naderi, and A. Carruthers. 1989. Characterization of two independent modes of action of ATP on human erythrocyte sugar transport. Biochemistry 28:6410-6417. [DOI] [PubMed] [Google Scholar]
- 18.Jayamanne, V. S., and M. R. Adams. 2006. Determination of survival, identity and stress resistance of probiotic bifidobacteria in bio-yoghurts. Lett. Appl. Microbiol. 42:189-194. [DOI] [PubMed] [Google Scholar]
- 19.Jennings, M. L., and A. K. Solomon. 1976. Interaction between phloretin and the red blood cell membrane. J. Gen. Physiol. 67:381-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kearns, D. B., and J. B. Russell. 1996. Catabolite regulation in a diauxic strain and a nondiauxic strain of Streptococcus bovis. Curr. Microbiol. 33:216-219. [DOI] [PubMed] [Google Scholar]
- 21.Kimmel, S. A., R. F. Roberts, and G. R. Ziegler. 1998. Optimization of exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus RR grown in a semidefined medium. Appl. Environ. Microbiol. 64:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornberg, H. L., L. T. M. Lambourne, and A. A. Sproul. 2000. Facilitated diffusion of fructose via the phosphoenolpyruvate/glucose phosphotransferase system of Escherichia coli. Proc. Natl. Acad. Sci. USA 97:1808-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krzewinski, F., C. Brassart, F. Gavini, and S. Bouquelet. 1996. Characterization of the lactose transport system in the strain Bifidobacterium bifidum DSM 20082. Curr. Microbiol. 32:301-307. [DOI] [PubMed] [Google Scholar]
- 24.Krzewinski, F., C. Brassart, F. Gavini, and S. Bouquelet. 1997. Glucose and galactose transport in Bifidobacterium bifidum DSM 20082. Curr. Microbiol. 35:175-179. [DOI] [PubMed] [Google Scholar]
- 25.Lapierre, L., P. Undeland, and L. J. Cox. 1992. Lithium chloride-sodium propionate agar for the enumeration of bifidobacteria in fermented dairy products. J. Dairy Sci. 75:1192-1196. [DOI] [PubMed] [Google Scholar]
- 26.LeFevre, P. G., and K. R. Marshall. 1959. The attachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. J. Biol. Chem. 234:3022-3026. [PubMed] [Google Scholar]
- 27.Masco, L., G. Huys, E. De Brandt, R. Temmerman, and J. Swings. 2005. Culture-dependent and culture-independent qualitative analysis of probiotic products claimed to contain bifidobacteria. Int. J. Food Microbiol. 102:221-230. [DOI] [PubMed] [Google Scholar]
- 28.Mättö, J., H.-L. Alakomi, A. Vaari, I. Virkajärvi, and M. Saarela. 2006. Influence of processing conditions on Bifidobacterium animalis ssp. lactis functionality with a special focus on acid tolerance and factors affecting it. Int. Dairy J. 16:1029-1037. [Google Scholar]
- 29.Mayer, H. K., E. Amtmann, E. Philippi, G. Steinegger, S. Mayrhofer, and W. Kneifel. 2007. Molecular discrimination of new isolates of Bifidobacterium animalis subsp. lactis from reference strains and commercial probiotic strains. Int. Dairy J. 17:565-573. [Google Scholar]
- 30.Parche, S., J. Amon, I. Jankovic, E. Rezzonico, M. Beleut, H. Barutçu, I. Schendel, M. P. Eddy, A. Burkovski, F. Arigoni, and F. Titgemeyer. 2007. Sugar transport systems of Bifidobacterium longum NCC 2705. J. Mol. Microbiol. Biotechnol. 12:9-19. [DOI] [PubMed] [Google Scholar]
- 31.Parche, S., M. Beleut, E. Rezzonico, D. Jacobs, F. Arigoni, F. Titgemeyer, and I. Jankovic. 2006. Lactose-over-glucose preference in Bifidobacterium longum NCC2705: glcP, encoding a glucose transporter, is subject to lactose repression. J. Bacteriol. 188:1260-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker, C., and R. Hutkins. 1997. Listeria monocytogenes Scott A transports glucose by high-affinity and low-affinity glucose transport systems. Appl. Environ. Microbiol. 63:543-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rada, V., J. Bartonová, and E. Vlková. 2002. Specific growth rate of bifidobacteria cultured on different sugars. Folia Microbiol. 47:477-480. [DOI] [PubMed] [Google Scholar]
- 34.Rada, V., and J. Petr. 2000. A new selective medium for the isolation of glucose non-fermenting bifidobacteria from hen caeca. J. Microbiol. Methods 43:127-132. [DOI] [PubMed] [Google Scholar]
- 35.Russell, J. B., H. J. Strobel, and S. A. Martin. 1990. Strategies of nutrient transport by ruminal bacteria. J. Dairy Sci. 73:2996-3012. [DOI] [PubMed] [Google Scholar]
- 36.Sakata, S., M. Kitahara, M. Sakamoto, H. Hayashi, M. Fukuyama, and Y. Benno. 2002. Unification of Bifidobacterium infantis and Bifidobacterium suis as Bifidobacterium longum. Int. J. Syst. Evol. Microbiol. 52:1945-1951. [DOI] [PubMed] [Google Scholar]
- 37.Sarker, R. I., W. Ogawa, T. Shimamoto, T. Shimamoto, and T. Tsuchiya. 1997. Primary structure and properties of the Na+/glucose symporter (Sg1S) of Vibrio parahaemolyticus. J. Bacteriol. 179:1805-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M.-C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swaisgood, H. E. 1996. Characteristics of milk, p. 841-878. In O. R. Fennema (ed.), Food chemistry, 3rd ed. Marcel Dekker, Inc., New York, NY.
- 40.Van der Meulen, R., L. Avonts, and L. De Vuyst. 2004. Short fractions of oligofructose are preferentially metabolized by Bifidobacterium animalis DN-173 010. Appl. Environ. Microbiol. 70:1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ventura, M., C. Canchaya, G. F. Fitzgerald, R. S. Gupta, and D. Van Sinderen. 2007. Genomics as a means to understand bacterial phylogeny and ecological adaptation: the case of bifidobacteria. Antonie van Leeuwenhoek 91:351-372. [DOI] [PubMed] [Google Scholar]
- 42.Ventura, M., and R. Zink. 2002. Rapid identification, differentiation, and proposed new taxonomic classification of Bifidobacterium lactis. Appl. Environ. Microbiol. 68:6429-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent, D., D. Roy, F. Mondou, and C. Déry. 1998. Characterization of bifidobacteria by random DNA amplification. Int. J. Food Microbiol. 43:185-193. [DOI] [PubMed] [Google Scholar]
- 44.Watabe, J., Y. Benno, and T. Mitsuoka. 1983. Bifidobacterium gallinarum sp. nov.: a new species isolated from the ceca of chickens. Int. J. Syst. Bacteriol. 33:127-132. [Google Scholar]