Abstract
1,2-Propanediol (1,2-PD) added exogenously to cultures or produced endogenously from l-rhamnose is metabolized to n-propanol and propionate in Listeria innocua Lin11. The pduD gene, which encodes a diol dehydratase ß subunit homolog, is required for 1,2-PD catabolism. pduD and 16 other genes within the pduA-to-pduF region of a large gene cluster are induced in medium containing 1,2-PD.
1,2-Propanediol (1,2-PD) is a three-carbon glycol that is produced during the catabolism of rhamnose and fucose, which are abundant sugars in plant matter and mammalian glycoconjugates (1, 3). 1,2-PD metabolism has been investigated extensively in Salmonella species that can use 1,2-PD as a carbon source and in which its metabolism appears to be important for virulence (3). More recently, operons consisting of genes for 1,2-PD utilization (pdu) have been identified in Listeria monocytogenes EGDe and L. innocua CLIP11262 by bioinformatic analyses of genome sequences (7). It has been proposed that, as in Salmonella, these genes may be important for virulence in L. monocytogenes (7). So far, only a limited number of studies indicate that pdu genes may be functional in Listeria. For example, it has been shown for L. monocytogenes that the transcription of some pdu genes increases when glucose is depleted from culture media (22), and several pdu genes are switched on during intracellular growth in mammalian cells (17). Experiments showing that pdu genes actually serve in 1,2-PD catabolism in Listeria have not yet been performed.
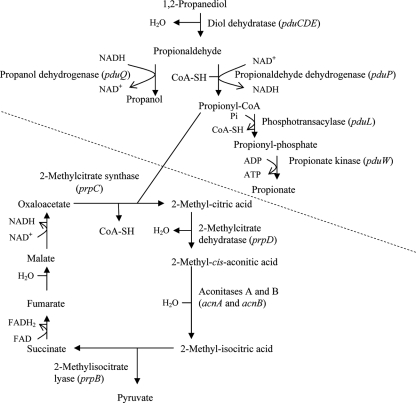
In Salmonella, 1,2-PD catabolism begins with the conversion of 1,2-PD to propionaldehyde by a hexameric vitamin B12-dependent diol dehydratase encoded by the pduCDE genes (11) (Fig. 1). Under fermentation conditions, two molecules of propionaldehyde are subsequently converted to n-propanol and propionate (3, 15, 20). These steps produce ATP and regenerate NAD+ but do not provide a source of carbon for biosynthesis. Nonetheless, it has been shown that 1,2-PD catabolism augments anaerobic growth in the presence of poor-quality carbon sources (25). Under aerobic conditions, and anaerobic respiration with tetrathionate as the electron acceptor, Salmonella can use 1,2-PD as both a carbon and an energy source (25). The propionyl coenzyme A (CoA) produced in the 1,2-PD pathway is converted into the biosynthesis precursor pyruvate by the 2-methylcitric acid cycle (16, 23) (Fig. 1). ATP is produced by respiration, using reducing equivalents derived from 2-methylcitric acid cycle reactions. Salmonella assembles polyhedral bodies wherein 1,2-PD is formed and converted into propionyl CoA (15, 28). Polyhedral bodies are proteinaceous shells that are formed from proteins encoded by the pduA, -B, -J, -K, -N, -T, and -U genes. These structures serve as microcompartments wherein propionaldehyde is formed from 1,2-PD and is sequestered to protect the cell from its toxic effects (28).
FIG. 1.
Pathways of 1,2-PD catabolism in Salmonella and the putative pathway of 1,2-PD catabolism in L. innocua Lin11. The pdu pathway steps in the upper part of the diagram (above the dashed line) appear to occur in both species. The 2-methylcitric acid cycle reactions shown in the lower part of the diagram do not appear to take place in Listeria. The figure has been adapted from work published in references 3, 4, and 23.
In Salmonella, all 23 pdu genes are clustered together and located adjacent to a gene cluster (cbi/cob) carrying genes needed for vitamin B12 biosynthesis (7, 12, 33). Both the pdu and the cbi/cob genes are coordinately regulated by the pocR gene, which is located between the two gene clusters (2, 26). The PocR transcription factor is activated by 1,2-PD and stimulates transcription by binding to control sites upstream of these operons (10, 27). Possibly due to the sensitivity of cob gene products to O2 inactivation, vitamin B12 can be synthesized de novo only under anaerobic conditions, and it or the precursor cobinamide must be imported into cells for growth on 1,2-PD under aerobic conditions (26).
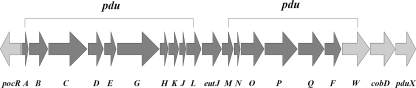
In Listeria, pdu genes reside within a large cluster that is interspersed with cbi, cob, and ethanolamine utilization (eut) genes (3, 7, 18). The genes appear to be organized into several operons, and a pocR ortholog also is located within the cluster (Fig. 2). As an initial step in the analysis of the pdu gene function in Listeria, we studied the requirement for diol dehydratase in 1,2-PD catabolism and the regulation of the diol dehydratase-encoding portion of the pdu genes by 1,2-PD under aerobic growth conditions in L. innocua.
FIG. 2.
Genetic organization of the pdu gene cluster in L. innocua (7). Genes induced by 1,2-PD are shown in dark gray. The putative function of each gene is as follows (2, 3, 6, 7, 13, 15, 20, 28; http://genolist.pasteur.fr/ListiList). pocR (lin1114), a transcriptional activator of the pdu operon; pduA (lin1115), polyhedral body component; pduB (lin1116), polyhedral body component; pduC (lin1117), diol dehydratase large (α) subunit; pduD (lin1118), diol dehydratase medium (β) subunit; pduE (lin1119), diol dehydratase small (γ) subunit; pduG (lin1120), diol dehydratase-reactivating factor large subunit; pduH (lin1121), diol dehydratase-reactivating factor small subunit; pduK (lin1122), polyhedral body component; pduJ (lin1123), polyhedral body component; pduL (lin1124), phosphotransacylase; eutJ (lin1125), unknown; pduM (lin1126), unknown; pduN (lin1127), polyhedral body component; pduO (lin1128), adenosyltransferase; pduP (lin1129), propionaldehyde dehydrogenase; pduQ (lin1130), propanol dehydrogenase; pduF (glpF, lin1131), propanediol diffusion facilitator; pduW (lin1132), propionate kinase; cobD (lin1133), l-threonine-O-3-phosphate decarboxylase; pduX (lin1134), l-threonine kinase. Gene names and numbers used at the ListiList server are provided in parentheses to facilitate searches at that website.
Growth of L. innocua Lin11 in 1,2-PD, l-rhamnose, and l- and d-fucose media.
To test for growth on 1,2-PD and other carbon sources, cultures of the wild-type L. innocua Lin11 strain (36) were grown aerobically at 30°C in synthetic liquid minimal medium (HTM) (32) supplemented with 20 nM vitamin B12. Growth was observed in HTM when 0.2% l-rhamnose or 0.2% d-glucose was added as the carbon source but did not occur in medium using 0.2% l-fucose or 52 mM 1,2-PD as the sole carbon source. Additional experiments performed with an API 50 CHB/E carbohydrate fermentation test system (bioMérieux, France) confirmed that the strain can grow on l-rhamnose but not on l- or d-fucose medium.
As discussed above, Salmonella metabolizes propionyl CoA produced from 1,2-PD catabolism via the 2-methylcitric acid cycle to obtain energy and a source of carbon for growth under aerobic conditions (Fig. 1). While BLAST-p searches conducted at the ListiList website identified L. innocua CLIP11262 and L. monocytogenes EGDe homologs of Salmonella genes encoding the 2-methylcitric acid cycle enzymes PrpC (CitZ), AcnA (CitB), AcnB (LeuC), and PrpB (Lmo0075/Lin0067), a PrpD homolog could not be identified (Fig. 1). Searches also revealed that prp homologs are scattered throughout the Listeria genomes, whereas they reside in one operon (prpBCDE) in Salmonella (16). We also searched for homologs of AcnD (2-methylcitrate dehydratase) and its PrpF accessory factor that, together, have been shown to substitute for PrpD in Shewanella oneidensis and Vibrio cholerae (14). Although L. innocua appears to contain homologs of AcnD (CitB and LeuC), no homolog of PrpF was found. Because AcnD activity alone cannot convert 2-methylcitric acid to 2-methyl-cis-aconitic acid, it is proposed that L. innocua Lin11 may not be able to utilize 1,2-PD for aerobic growth due to the lack of a complete 2-methylcitric acid cycle pathway.
The pduD gene encodes a diol dehydratase that participates in 1,2-PD catabolism in L. innocua Lin11.
Two approaches were used to determine if L. innocua Lin11 can nonetheless degrade 1,2-PD in a pdu-dependent manner. These involved (i) quantitation of n-propanol and propionate production in growth medium containing 1,2-PD, and (ii) measurement of diol dehydratase activities in cell extracts. Analyses were conducted with the wild-type L. innocua Lin11 strain, a mutant with an in-frame deletion in pduD, which encodes a homolog of the ß subunit of diol dehydratase (Fig. 1), and the deletion strain complemented with the pduD gene cloned in plasmid pAM401 (35). Note that the deletion strains retain intact copies of the pduC and pduE genes, which encode homologs of the α and γ subunits of diol dehydratase.
The deletion mutant was constructed by replacing the wild-type pduD gene (lin1118) that encodes the 219-amino-acid PduD polypeptide with a truncated pduD gene that encodes only the N-terminal 16 amino acids and the C-terminal 13 amino acids of PduD. The deletion gene was constructed by splice-by-overlap extension PCR in plasmid pKSV7 and introduced into Lin11 by homologous recombination (9, 37). The plasmid used in complementation experiments (pJX1118) was constructed by separately amplifying the promoter sequence of the tetL gene present in plasmid pLTV3 (8) and the complete L. innocua Lin11 pduD coding sequence and fusing them together by splice-by-overlap extension PCR. Subsequently, the tetL-pduD hybrid gene was cloned into the shuttle vector pAM401 and transformed into the deletion mutant (24). The tetL promoter sequence was fused to pduD because this gene resides at an internal location in the pdu cluster and appears to lack its own promoter. The PCR primers used to construct the deletion mutant and pJX1118 are listed in Table 1. The sequences of all constructs were verified by DNA sequencing.
TABLE 1.
Primers used for construction of the pduD deletion and complementation mutant strainsa
| Primer | Site | Sequence |
|---|---|---|
| Deletion mutants | ||
| lin1118-A-EcoRI | F | CGGAATTCTGCCAGAACGTAACATGG |
| lin1118-B | R | GCGTTTTTACCTTGAACAACTACTTCTGAGATAATTCCG |
| lin1118-C | F | GTTGTTCAAGGTAAAAACGC |
| lin1118-D-BamHI | R | CGGGATCCCTAAAATACGCTCATCTGG |
| Complementation mutants | ||
| tetLP-F-XbaI | F | GCTCTAGAACGGGCCATATTGTTGTATAAG |
| tetLP-R | R | ATTTCACCCTCCAATAATGAGG |
| lin1118-F2 | F | CCTCATTATTGGAGGGTGAAATATGGTTGAAATTAACGAAAAAGTG |
| lin1118-R2-SalI | R | ACGCGTCGACTTAATTTACTTGTAGTTCGACTGC |
F, forward primer; R, reverse primer. The restriction sites incorporated into primers for cloning purposes are underlined. The overhangs complementary to the lin1118-C primer and to the tetLP-R primer (both used for splice-by-overlap extension PCR) are underlined in the primers lin1118-B and lin1118-F2, respectively. Primers were designed using the L. innocua CLIP11262 genome sequence available at ListiList (http://genolist.pasteur.fr/ListiList).
Cell cultures used in the analysis of n-propanol and propionate production were grown aerobically for 48 h at 30°C in HTM liquid medium supplemented with 0.2% yeast extract, 20 nM vitamin B12, and 52 mM 1,2-PD or 0.2% l-rhamnose. A medium lacking glucose was selected because glucose represses pdu transcription in Salmonella (2) and possibly in L. monocytogenes (22 and see discussion below). A poor carbon source (0.2% [wt/vol] yeast extract) was added so that the strains would grow in 1,2-PD medium and to see if growth is improved in the presence of 1,2-PD (25).
1,2-PD, n-propanol, and propionate concentrations in culture media were determined using a gas-liquid chromatography method that offers the advantage of simultaneously determining the concentrations of the three compounds (21, 34). Prior to analysis, media samples were centrifuged and filtered to remove cells, and proteins were precipitated using metaphosphoric acid at a final concentration of 25% (wt/vol). Subsequently, 1-μl samples of processed culture media were analyzed using an HP 5890-II model gas chromatograph (Agilent Technologies, Santa Clara, CA) equipped with an Equity-1 capillary column (30-m by 0.53-mm inside diameter by 1.50-μm film thickness; Supelco, Bellefonte, PA) and flame ionization detector. Injector and detector temperatures were set at 225°C and 275°C, respectively. Oven temperature was initiated at 50°C and then after 5 min was increased to 225°C at a rate of 8°C/min. Ultra-high-purity hydrogen was the carrier gas. The retention times of 1,2-PD, n-propanol, and propionate were determined using commercially available standards (Sigma, St. Louis, MO). The identities of 1,2-PD, n-propanol, and propionate peaks in medium chromatograms were further confirmed by spiking samples with each standard and verifying that endogenous and exogenous compounds coeluted as single peaks. Peak areas were integrated using an HP 3396-II integrator and quantified using 1 mg of acetate or 1 mg of 2-ethylbutyrate as the internal standard (5). Data represent the averages of three to six separate experiments and were analyzed by analysis of variance using GLM software of SAS (SAS Institute, Cary, NC).
All three strains grew poorly in 1,2-PD medium, reaching a final optical density at 600 nm (OD600) range of only 0.2 to 0.3. In addition, 1,2-PD supplementation did not improve the growth of any of the strains compared to the control medium lacking 1,2-PD. Nonetheless, 1,2-PD was depleted, and n-propanol and propionate were produced in the medium used to grow the wild-type Lin11 strain (Fig. 3). In marked contrast, the level of 1,2-PD was not reduced compared to that in the unincubated medium, and n-propanol and propionate were not produced in the medium used to grow the deletion strain Lin11Δ1118. Finally, the expression of pduD from pJX1118 in the complementation strain (Lin11Δ1118/pJX1118) restored 1,2-PD degradation and synthesis of n-propanol and propionate. The results observed for the complementation strain eliminate the possibility that 1,2-PD is not catabolized in the deletion mutant due to a polar effect on the transcription of genes located downstream of the pduD deletion site.
FIG. 3.
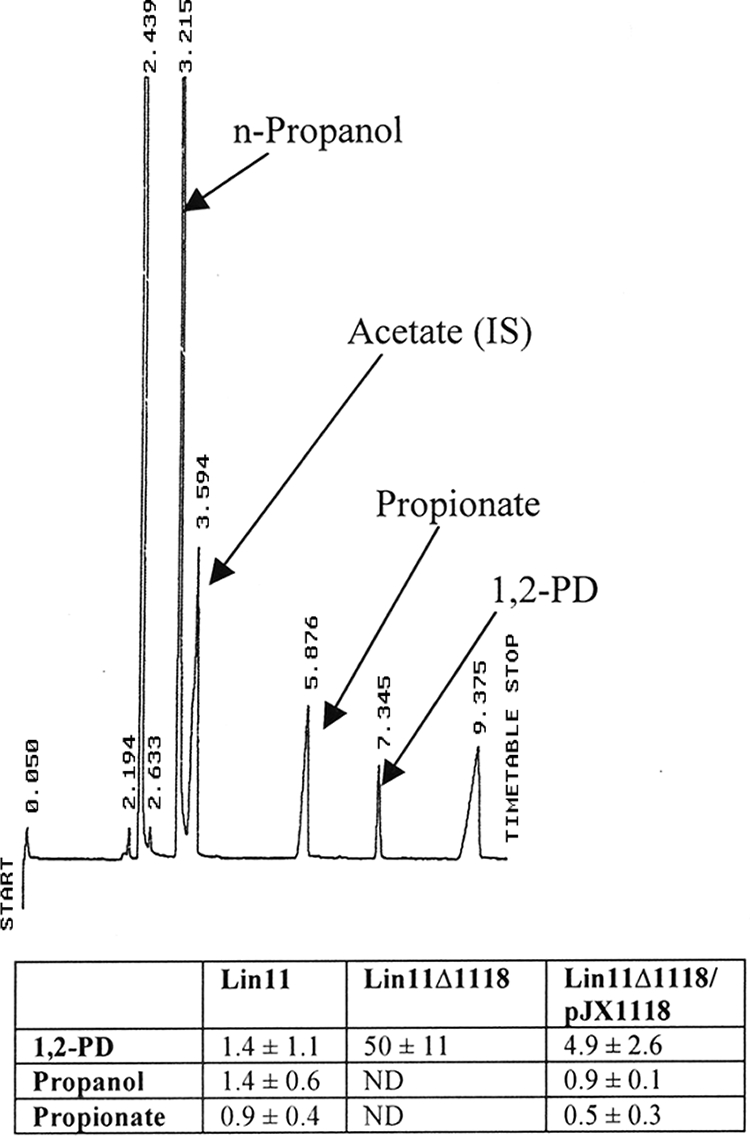
Gas-liquid chromatography analysis of 1,2-PD metabolites in culture media. A representative chromatogram of medium composition obtained from the wild-type Lin11 strain grown for 48 h in HTM, 0.2% yeast extract, 20 nM vitamin B12, 52 mM 1,2-PD medium is shown. Peaks were separated using an Equity-1 capillary column, identified by using commercially available standards, and quantified by using 1 mg of acetate as the internal standard (IS). Numbers above each peak are on-column retention times in minutes. Note that while peak areas are proportional to metabolite concentrations, the detector response factors differ for each compound, and thus, the relative peak heights do not mirror the relative concentrations listed in the table. Values for metabolite concentrations in culture media are expressed as mM for the three strains and are reported as means ± standard deviations (n = 3) in the table. In all cases, metabolite concentration values for the deletion and complementation strains differed significantly from those in Lin11 (P < 0.01). The peak eluting at 2.439 min is ethanol, which is derived from the solvent used for the addition of lipoic acid to HTM medium. ND, not detected, indicating that concentrations are at least 50-fold lower than that observed for the Lin11 strain.
The growth of all three strains in l-rhamnose medium was better, with strains reaching final OD600 nm values of 0.4 to 0.6. While the strains all produced 1,2-PD from l-rhamnose metabolism, only the wild-type and complementation strains converted 1,2-PD to n-propanol and propionate (Table 2). Note that the relative levels of n-propanol and propionate differed in the culture media obtained from the wild-type and complementation strain when strains are grown in l-rhamnose medium (Table 2), but not in 1,2-PD medium (Fig. 3). This suggests that the fluxes through the two branches of the 1,2-PD pathway (upper part of Fig. 1) may possibly be regulated differently in l-rhamnose and 1,2-PD media.
TABLE 2.
Concentrations of 1,2-PD metabolites produced by L. innocua strains grown in l-rhamnose mediuma
| Carbon source | Avg concn (mM) ± SD of metabolite producedb
|
P valuec | ||
|---|---|---|---|---|
| Lin11 (significant difference value) | Lin11Δ1118 (significant difference value) | Lin11Δ1118/pJX1118 (significant difference value) | ||
| 1,2-PD | 0.04 ± 0.02 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.67 |
| n-Propanol | 0.06 ± 0.02 (2) | ND (3) | 0.28 ± 0.03 (1) | 0.001 |
| Propionate | 0.25 ± 0.07 (1) | ND (3) | 0.09 ± 0.02 (2) | <0.001 |
Values are expressed as average mM concentrations ± standard deviations (SD) (n = 6). ND, not detected, i.e., concentrations are at least 50-fold lower than that observed for the Lin11 strain.
Metabolite concentration values that are significantly different between strains are indicated by numbers in parentheses (1, 2, or 3), with 1 assigned to the highest concentration and 3 assigned to the lowest concentration.
P values were calculated to determine if the concentration of each metabolite differed for the three strains.
We also examined the ability of L. innocua to convert 1,2-PD to propionaldehyde in a pduD-dependent manner by measuring diol dehydratase activities in cell-free protein extracts obtained from the wild type, the deletion mutant, and the complementation strain. The cultures used in these assays were grown aerobically at 30°C in Luria-Bertani (LB) medium with or without 20 nM vitamin B12 and 52 mM 1,2-PD, until early stationary phase. Cells were broken by glass bead disruption using a FastPrep-24 apparatus (MP Biomedicals, Solon, OH), and cell-free protein extracts were prepared following an established procedure (29). Diol dehydratase activity was measured by the 3-methyl-2-benzothiazolinone hydrazone method (31) and was verified to be dependent on the vitamin B12-derived cofactor adenosylcobalamin, in vitro.
The results of the assay (Table 3) are consistent with the data obtained by measurement of the levels of n-propanol and propionate in culture media. Diol dehydratase activity was relatively high in the wild-type strain, very low in the deletion mutant, and partially restored in the complementation strain. Although clearly present in the wild-type strain, activity was considerably lower than that reported for Salmonella (15). Importantly, the activity levels observed for the wild-type and complementation strains were induced by the addition of vitamin B12 and 1,2-PD to the growth medium. We speculate that the residual activity in the deletion mutant may arise from a second dehydratase, such as vitamin B12-dependent glycerol dehydratase, which can use 1,2-PD as substrate, because no activity was observed when adenosylcobalamin was deleted from the assay buffer used to analyze the deletion strain extract (data not shown). Glycerol dehydratases are present along with diol dehydratases in species such as Klebsiella pneumoniae and other members of the family Enterobacteriaceae (11, 30). However, a BLAST-p search of the L. innocua CLIP11262 genome, using the K. pneumoniae DhaBCE glycerol dehydratase sequences, matched only PduCDE. Thus, the source of the residual activity in the deletion strain remains unknown.
TABLE 3.
Diol dehydratase activities of cell-free protein extracts of strains grown in media with and without 1,2-PDa
| Strain | Diol dehydratase activity (avg units/mg total cell protein ± SD)c
|
P valueb | |
|---|---|---|---|
| +1,2-PD | −1,2-PD | ||
| Lin11 | 6.7 × 10−2 ± 2.1 × 10−2 | 4.0 × 10−3 ± 0 | <0.01 |
| Lin11Δ1118 | 3.0 × 10−3 ± 0 | 4.0 × 10−3 ± 1.0 × 10−3 | >0.05 |
| Lin11Δ1118/pJX1118 | 1.3 × 10−2 ± 3.0 × 10−3 | 7.0 × 10−3 ± 3.0 × 10−3 | <0.05 |
Values are expressed as average units of diol dehydratase activity per mg of total cellular protein ± standard deviations (SD) in medium with (+) and without (−) 1,2-PD (n = 3; n = 4 for Lin11 in medium +1,2-PD). One unit of diol dehydratase activity is equivalent to the amount of enzyme that converts 1 μmol/min of 1,2-PD to propionaldehyde.
P values were calculated to determine if enzyme activities differed significantly due to the addition of 1,2-PD to the medium.
P values were calculated to determine if enzyme activities differed significantly between strains; with 1,2-PD, the P value in a comparison of strains Lin11, Lin11Δ1118, and Lin11Δ1118/pJX1118 was <0.01, and without 1,2-PD, the P value among these strains was >0.05.
Taken together, the data show that the pduD gene product is a functional diol dehydratase that participates in the catabolism of 1,2-PD to n-propanol and propionate in L. innocua Lin11. Interestingly, the amounts of catabolites detected in culture medium from the wild-type L. innocua Lin11 strain grown in 1,2-PD are smaller than would be expected for Salmonella (23, 28). This implies that Listeria deals more efficiently with propionaldehyde and further metabolizes propionate, although probably not via the 2-methylcitric acid cycle, as discussed above. We speculate that propionate is converted to another end product, which may serve to decrease its toxicity to Listeria (19). Last, the less efficient conversion of 1,2-PD to n-propanol and propionate observed for the complementation strain appears to be due to the relatively low level of diol dehydratase activity produced in this strain (Table 3).
Stimulation of pduA-pduF transcription in L. innocua Lin11 by 1,2-PD.
To determine whether transcription of pduD and other genes is positively regulated by 1,2-PD, RNA was isolated from wild-type L. innocua Lin11 grown in medium with and without 1,2-PD and analyzed by real-time reverse transcription-PCR (RT-PCR) using primers complementary to pdu genes. Cells were grown aerobically at 30°C in LB medium with or without 20 nM vitamin B12 and 52 mM 1,2-PD for ∼4 h from a starting OD600 of 0.03 to 0.5 for RNA isolation. No additional carbon sources were added. The mRNA quantitation method has been described previously (36), and primers complementary to pdu genes and a 16S rRNA endogenous control gene are listed in Table 4. REST statistics software was used to compare mRNA levels (36).
TABLE 4.
Primers used for real-time RT-PCR analysis of pdu gene transcript levelsa
| Primer | Sequence |
|---|---|
| pocR (lin1114) | |
| F | AAATGTCGCTCGCAAAAGAT |
| R | TAGCTCGTTTGGGTGAATCC |
| pduA (lin1115) | |
| F | GAGGGGATGTAGGTGCAGTT |
| R | ATGCGGACGAGGAATAACAT |
| pduB (lin1116) | |
| F | GGCCTAGTGATTGCGAATGT |
| R | CACCAGTACGGGCTCCTAAA |
| pduC (lin1117) | |
| F | ACAATATGTTTGCGGGTTCC |
| R | ACTGGCGTTAAACCACCATC |
| pduD (lin1118) | |
| F | CTTACCGGGCAATTGGTAAA |
| R | AATTTTGGACGAGCCATTTG |
| pduE (lin1119) | |
| F | AAAATGCAAGCGCAAGTAGC |
| R | TACGCTCATCTGGAACAACG |
| pduG (lin1120) | |
| F | AGCGCGTTTTTGTAACGAAT |
| R | GGCTGATCCTCCGACAATAA |
| pduH (lin1121) | |
| F | TTTGGTTGGAATTACGCTCA |
| R | TGCGCAAAAACGATATTCTG |
| pduK (lin1122) | |
| F | ACAAACGGCAATAGCCTCAG |
| R | GGCACGATTTCTTCTTTTGG |
| pduJ (lin1123) | |
| F | TGATGTTGGCGCAGTAAAAG |
| R | CATTGTGTGGACGTGGGATA |
| pduL (lin1124) | |
| F | TAAAGCAACCTGGCGAATTT |
| R | AAAATTTTCGAAGCGGACCT |
| eutJ (lin1125) | |
| F | TACCGCGAGTCCGTAGAAGT |
| R | CCAGGTGGAATAGCTCCAGA |
| pduM (lin1126) | |
| F | ATTCGTGCCGCAAATAAAAC |
| R | CGAACTGTCCCACTTTCCAT |
| pduN (lin1127) | |
| F | GAACATGTGCGCTCAGAAGA |
| R | CTGCAAATACGCTCCTAGCC |
| pduO (lin1128) | |
| F | TCAGGTGCAGGTTGTCTCAG |
| R | AGCGTGTTCCATTCGGTAAT |
| pduP (lin1129) | |
| F | CGACAAGAGCAGTTGCAGAG |
| R | ATCTTCTACCCCTGGCGTTT |
| pduQ (lin1130) | |
| F | CAAATGCCACGAACATTGAC |
| R | CAAGCAAGCGTCTTTCAGTG |
| pduF (lin1131) | |
| F | TTGGCGGAATTTATTGGAAC |
| R | CCCAGTTTGCTCCTTGTGAT |
| pduW (lin1132) | |
| F | CGGATGTTTACTGGCCAAAT |
| R | CACCAACTCCAGCCGTAAAT |
| cobD (lin1133) | |
| F | CGTATTGCCTCGTTTCATCA |
| R | TTTGCGCCTTGGTTACTTTT |
| pdu X (lin1134) | |
| F | AAAAGGGTCCGTGATTGAAA |
| R | TTCTTGCCGATATGTTGCAG |
| 16S rRNA | |
| F | AAGCAACGCGAAGAACCTTA |
| R | TGCACCACCTGTCACTTTGT |
F, forward primer; R, reverse primer. Primers were designed using the L. innocua CLIP11262 genome sequence available at the ListiList server (http://genolist.pasteur.fr/ListiList).
As shown in Table 5, transcripts for all genes in the pduA-to-pduF region were significantly induced (range, 84- to 3.2-fold; P < 0.01) by 1,2-PD. The mRNA level for the pduD gene increased by 12-fold due to the addition of 1,2-PD to the medium (P < 0.01). Transcript levels for pduW, cobD, and pduX, which are downstream of pduF, were unchanged, indicating that these genes are not induced by 1,2-PD, at least under the conditions studied. In addition, the mRNA of the divergently transcribed pocR gene was induced 3.1-fold (P < 0.05) by 1,2-PD. Taken together, the data indicate that the gene encoding the putative 1,2-PD transporter (pduF) and all but one of the pdu genes (pduW) that may serve in the putative pathway by which 1,2-PD is converted to n-propanol and propionate are induced by 1,2-PD (Fig. 1). The finding that L. innocua pduW is not induced by 1,2-PD varies from observations with Salmonella, where pduW appears to be part of a longer pdu operon (13, 28). Perhaps in L. innocua, the basal activity of PduW (propionate kinase) is sufficient to keep pace with flux from propionaldehyde to propionate so that pduW induction is not required for 1,2-PD metabolism.
TABLE 5.
Relative levels of pdu mRNAs in L. innocua Lin11a
| Gene | Relative fold induction of mRNA (range [lowest-highest])
|
|
|---|---|---|
| Lin11 in LB medium − 1,2-PD | Lin11 in LB medium + 1,2-PDb | |
| pocR (lin1114) | 1.0 (0.6-1.6) | 3.1 (2.1-4.5)* |
| pduA (lin1115) | 1.0 (0.8-1.2) | 84 (67-110)* |
| pduB (lin1116) | 1.0 (0.7-1.4) | 75 (51-100)* |
| pduC (lin1117) | 1.0 (0.8-1.2) | 43 (35-54)* |
| pduD (lin1118) | 1.0 (0.6-1.8) | 12 (9.2-17)* |
| pduE (lin1119) | 1.0 (0.9-1.1) | 13 (11-15)* |
| pduG (lin1120) | 1.0 (0.8-1.3) | 15 (13-19)* |
| pduH (lin1121) | 1.0 (0.7-1.3) | 12 (9.4-16)* |
| pduK (lin1122) | 1.0 (0.8-1.2) | 11 (8.8-13)* |
| pduJ (lin1123) | 1.0 (0.9-1.1) | 7.6 (6.6-8.9)* |
| pduL (lin1124) | 1.0 (0.8-1.2) | 7.6 (6.0-9.8)* |
| eutJ (lin1125) | 1.0 (0.8-1.3) | 7.8 (6.4-9.6)* |
| pduM (lin1126) | 1.0 (0.8-1.2) | 5.2 (4.5-6.0)* |
| pduN (lin1127) | 1.0 (0.8-1.2) | 5.5 (4.7-6.6)* |
| pduO (lin1128) | 1.0 (0.9-1.2) | 3.2 (2.8-3.8)* |
| pduP (lin1129) | 1.0 (0.5-1.8) | 5.0 (3.7-6.7)* |
| pduQ (lin1130) | 1.0 (0.9-1.2) | 4.2 (3.5-5.1)* |
| pduF (lin1131) | 1.0 (0.6-1.7) | 3.2 (1.4-7.0)* |
| pduW (lin1132) | 1.0 (0.8-1.3) | 1.1 (0.7-1.9) |
| cobD (lin1133) | 1.0 (0.6-1.5) | 0.9 (0.7-1.1) |
| pduX (lin1134) | 1.0 (0.7-1.5) | 0.9 (0.7-1.2) |
The values shown are averages of relative levels of pdu mRNAs in L. innocua Lin11 grown aerobically in LB/vitamin B12 medium with (+) or without (−) 52 mM 1,2-PD, obtained using three independent RNA preparations. Transcript levels were measured in triplicate for each RNA preparation. The cycle threshold values for PCR products were compared to those measured in strain lin11 grown in LB medium without 1,2-PD to determine differences. Values in parentheses signify the lowest and highest values, respectively, measured for each mRNA.
Asterisks indicate values that are significantly different between the two media (P < 0.01).
Interestingly, we observed that transcript levels for genes in the pdu cluster decreased across the pduA-to-pduF series of genes (Table 5). We speculate that the reduction in mRNA levels could be caused by stability differences for the coding regions within a long pduA-to-pduF polycistronic transcript. Alternatively, the data could be explained by the presence of internal promoter or terminator sequences within the gene cluster. However, we did not find evidence by sequence analysis for strong consensus σ70 promoter sequences or ρ-independent terminator sequences, even immediately after pduF, within this gene cluster.
Last, we determined, by real-time RT-PCR analysis, that the pduA-to-pduF genes are not induced by 1,2-PD in brain heart infusion medium, which contains 0.2% glucose (data not shown). This is consistent with findings in Salmonella, where it has been demonstrated that glucose inhibits the pdu operon expression via repression of pocR transcription (2).
Conclusions.
It has been demonstrated for the first time that an L. innocua strain can degrade 1,2-PD to n-propanol and propionate via a pduD-dependent pathway and that, based on comparative studies with Salmonella, degradation may proceed as shown in Fig. 1. We also determined that pduD and all but one other gene (pduW) needed for 1,2-PD transport and conversion to n-propanol and propionate by the conventional pathway are coordinately upregulated by 1,2-PD, possibly via a PocR-dependent mechanism. Because pdu genes are highly conserved between L. innocua and L. monocytogenes, it seems possible that the pathogen also may be able to catabolize 1,2-PD to n-propanol and propionate. Although L. innocua Lin11 did not grow aerobically on 1,2-PD medium, we propose that 1,2-PD catabolism nonetheless could be important for growth in poor-quality medium containing l-rhamnose. In this regard, dihydroxyacetone phosphate and 1,2-PD are both formed during l-rhamnose catabolism (25, 33). While metabolism of dihydroxyacetone phosphate probably supplies most of the energy needed for growth, the breakdown of 1,2-PD to n-propanol and propionate could contribute to energy production.
Acknowledgments
We thank Thomas A. Bobik for helpful discussions.
We thank The University of Wyoming for financial support.
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Badía, J., J. Ros, and J. Aguilar. 1985. Fermentation mechanism of fucose and rhamnose in Salmonella typhimurium and Klebsiella pneumoniae. J. Bacteriol. 161:435-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobik, T. A., M. Ailion, and J. R. Roth. 1992. A single regulatory gene integrates control of vitamin B12 synthesis and propanediol degradation. J. Bacteriol. 174:2253-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobik, T. A., G. D. Havemann, R. J. Busch, D. S. Williams, and H. C. Aldrich. 1999. The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B12-dependent 1,2-propanediol degradation. J. Bacteriol. 181:5967-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramer, C. O., and A. Steinbuchel. 2001. The methylcitric acid pathway in Ralstonia eutropha: new genes identified involved in propionate metabolism. Microbiology 147:2203-2214. [DOI] [PubMed] [Google Scholar]
- 5.Brokaw, L., B. W. Hess, and D. C. Rule. 2001. Supplemental soybean oil or corn for beef heifers grazing summer pasture: effects on forage intake, ruminal fermentation, and site and extent of digestion. J. Anim. Sci. 79:2704-2712. [DOI] [PubMed] [Google Scholar]
- 6.Brushaber, K. R., G. A. O'Toole, and J. C. Escalante-Semerena. 1998. CobD, a novel enzyme with L-threonine-O-3-phosphate decarboxylase activity, is responsible for the synthesis of (R)-1-amino-2-propanol O-2-phosphate, a proposed new intermediate in cobalamin biosynthesis in Salmonella typhimurium LT2. J. Biol. Chem. 273:2684-2691. [DOI] [PubMed] [Google Scholar]
- 7.Buchrieser, C., C. Rusniok, F. Kunst, P. Cossart, P. Glaser, and the Listeria Consortium. 2003. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: clues for evolution and pathogenicity. FEMS Immunol. Med. Microbiol. 35:207-213. [DOI] [PubMed] [Google Scholar]
- 8.Camilli, A., D. A. Portnoy, and P. Youngman. 1990. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J. Bacteriol. 172:3738-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, P., M. Ailion, T. Bobik, G. Stormo, and J. Roth. 1995. Five promoters integrate control of the cob/pdu regulon in Salmonella typhimurium. J. Bacteriol. 177:5401-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel, R., T. A. Bobik, and G. Gottschalk. 1999. Biochemistry of coenzyme B12-dependent glycerol and diol dehydratases and organization of the encoding genes. FEMS Microbiol. Rev. 22:553-566. [DOI] [PubMed] [Google Scholar]
- 12.Escalante-Semerena, J. C. 2007. Conversion of cobinamide into adenosylcobamide in bacteria and archaea. J. Bacteriol. 189:4555-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan, C., and T. A. Bobik. 2008. The PduX enzyme of Salmonella enterica is an L-threonine kinase used for coenzyme B12 synthesis. J. Biol. Chem. 283:11322-11329. [DOI] [PubMed] [Google Scholar]
- 14.Grimek, T. L., and J. C. Escalante-Semerena. 2004. The acnD genes of Shewanella oneidensis and Vibrio cholerae encode a new Fe/S-dependent 2-methylcitrate dehydratase enzyme that requires prpF function in vivo. J. Bacteriol. 186:454-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havemann, G. D., and T. A. Bobik. 2003. Protein content of polyhedral organelles involved in coenzyme B12-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 185:5086-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horswill, A. R., and J. C. Escalante-Semerena. 1999. Salmonella typhimurium LT2 catabolizes propionate via the 2-methylcitric acid cycle. J. Bacteriol. 181:5615-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph, B., K. Przybilla, C. Stuhler, K. Schauer, J. Slaghuis, T. M. Fuchs, and W. Goebel. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J. Bacteriol. 188:556-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kofoid, E., C. Rappleye, I. Stojiljkovic, and J. Roth. 1999. The 17-gene ethanolamine (eut) operon of Salmonella typhimurium encodes five homologues of carboxysome shell proteins. J. Bacteriol. 181:5317-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouassi, Y., and L. A. Shelef. 1996. Metabolic activities of Listeria monocytogenes in the presence of sodium propionate, acetate, lactate and citrate. J. Appl. Bacteriol. 81:147-153. [DOI] [PubMed] [Google Scholar]
- 20.Liu, Y., N. A. Leal, E. M. Sampson, C. L. Johnson, G. D. Havemann, and T. A. Bobik. 2007. PduL is an evolutionarily distinct phosphotransacylase involved in B12-dependent 1,2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 189:1589-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livesey, J. F., S. L. Perkins, N. E. Tokessy, and M. J. Maddock. 1995. Simultaneous determination of alcohols and ethylene glycol in serum by packed- or capillary-column gas chromatography. Clin. Chem. 41:300-305. [PubMed] [Google Scholar]
- 22.Nilsson, L., T. B. Hansen, P. Garrido, C. Buchrieser, P. Glaser, S. Knochel, L. Gram, and A. Gravesen. 2005. Growth inhibition of Listeria monocytogenes by a nonbacteriocinogenic Carnobacterium piscicola. J. Appl. Microbiol. 98:172-183. [DOI] [PubMed] [Google Scholar]
- 23.Palacios, S., V. J. Starai, and J. C. Escalante-Semerena. 2003. Propionyl coenzyme A is a common intermediate in the 1,2-propanediol and propionate catabolic pathways needed for expression of the prpBCDE operon during growth of Salmonella enterica on 1,2-propanediol. J. Bacteriol. 185:2802-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, S. F., and G. S. A. B. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 25.Price-Carter, M., J. Tingey, T. A. Bobik, and J. R. Roth. 2001. The alternative electron acceptor tetrathionate supports B12-dependent anaerobic growth of Salmonella enterica serovar Typhimurium on ethanolamine or 1,2-propanediol. J. Bacteriol. 183:2463-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rondon, M. R., and J. C. Escalante-Semerena. 1992. The poc locus is required for 1,2-propanediol-dependent transcription of the cobalamin biosynthetic (cob) and propanediol utilization (pdu) genes of Salmonella typhimurium. J. Bacteriol. 174:2267-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rondon, M. R., and J. C. Escalante-Semerena. 1996. In vitro analysis of the interactions between the PocR regulatory protein and the promoter region of the cobalamin biosynthetic (cob) operon of Salmonella typhimurium LT2. J. Bacteriol. 178:2196-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson, E. M., and T. A. Bobik. 2008. Microcompartments for B12-dependent 1,2-propanediol degradation provide protection from DNA and cellular damage by a reactive metabolic intermediate. J. Bacteriol. 190:2966-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauvageot, N., V. Pichereau, L. Louarme, A. Hartke, Y. Auffray, and J.-M. Laplace. 2002. Purification, characterization and subunits identification of the diol dehydratase of Lactobacillus collinoides. Eur. J. Biochem. 269:5731-5737. [DOI] [PubMed] [Google Scholar]
- 30.Toraya, T., and S. Fukui. 1977. Immunochemical evidence for the difference between coenzyme-B12-dependent diol dehydratase and glycerol dehydratase. Eur. J. Biochem. 76:285-289. [DOI] [PubMed] [Google Scholar]
- 31.Toraya, T., K. Ushio, S. Fukui, and H. P. C. Hogenkamp. 1977. Studies on the mechanism of the adenosylcobalamin-dependent diol dehydrase reaction by the use of analogs of the coenzyme. J. Biol. Chem. 252:963-970. [PubMed] [Google Scholar]
- 32.Tsai, H. N., and D. A. Hodgson. 2003. Development of a synthetic minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 69:6943-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter, D., M. Ailion, and J. Roth. 1997. Genetic characterization of the pdu operon: use of 1,2-propanediol in Salmonella typhimurium. J. Bacteriol. 179:1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams, R. H., S. M. Shah, J. A. Maggiore, and T. B. Erickson. 2000. Simultaneous detection and quantitation of diethylene glycol, ethylene glycol, and the toxic alcohols in serum using capillary column gas chromatography. J. Anal. Toxicol. 24:621-626. [DOI] [PubMed] [Google Scholar]
- 35.Wirth, R., F. Y. An, and D. B. Clewell. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J. Bacteriol. 165:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue, J., I. Hunter, T. Steinmetz, A. Peters, B. Ray, and K. W. Miller. 2005. Novel activator of mannose-specific phosphotransferase system permease expression in Listeria innocua, identified by screening for pediocin AcH resistance. Appl. Environ. Microbiol. 71:1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue, J., and K. W. Miller. 2007. Regulation of the mpt operon in Listeria innocua by the ManR protein. Appl. Environ. Microbiol. 73:5648-5652. [DOI] [PMC free article] [PubMed] [Google Scholar]