Abstract
Broiler flocks often become infected with Campylobacter and Salmonella, and the exact contamination routes are still not fully understood. Insects like darkling beetles and their larvae may play a role in transfer of the pathogens between consecutive cycles. In this study, several groups of beetles and their larvae were artificially contaminated with a mixture of Salmonella enterica serovar Paratyphi B Variant Java and three C. jejuni strains and kept for different time intervals before they were fed to individually housed chicks. Most inoculated insects were positive for Salmonella and Campylobacter just before they were fed to the chicks. However, Campylobacter could not be isolated from insects that were kept for 1 week before they were used to mimic an empty week between rearing cycles. All broilers fed insects that were inoculated with pathogens on the day of feeding showed colonization with Campylobacter and Salmonella at levels of 50 to 100%. Transfer of both pathogens by groups of insects that were kept for 1 week before feeding to the chicks was also observed, but at lower levels. Naturally contaminated insects that were collected at a commercial broiler farm colonized broilers at low levels as well. In conclusion, the fact that Salmonella and Campylobacter can be transmitted via beetles and their larvae to flocks in successive rearing cycles indicates that there should be intensive control programs for exclusion of these insects from broiler houses.
Salmonella and Campylobacter are responsible for many cases of human food-borne disease. Many of these cases can be related to the handling or consumption of contaminated chicken meat, and these pathogens occur frequently in broiler husbandry. Due to the introduction of pathogen control programs in The Netherlands, the prevalence of Salmonella-positive broiler flocks (cecal carriage) has been reduced from 22% in 1997 to approximately 5% in 2006. At the retail level, this resulted in a contamination rate of about 8% for poultry meat in 2006. For Campylobacter, however, the prevalence at the cecal level remained quite stable at 30% of the broiler flocks positive, leading to a prevalence of 16% for poultry meat products in 2006 (38).
So far, how broilers become infected with these microorganisms is not fully understood, but it is assumed that there are horizontal transmission routes with multiple sources of infection (40). Insects like flies, beetles, and larvae are some of the potential sources, as these animals are reported to be frequent carriers of pathogens such as Escherichia coli, Shigella, Salmonella, and Campylobacter around the world, especially near animal-rearing facilities (3, 13, 14, 25, 26, 27, 29, 36, 42). The insects that frequently occur in poultry houses include Alphitobius diaperinus, the darkling beetle, and its larvae, the lesser mealworm (19, 30, 34). These insects are persistent in poultry houses and can be carriers of zoonotic bacteria, such as Salmonella and Campylobacter, and viruses (10, 27, 41), and they are considered to be a risk factor for Campylobacter contamination in broilers (28). Beetles and their larvae can survive in empty broiler houses between rearing cycles and may cause damage to the building's infrastructure by eating their way into insulation material in ceilings and walls, or they may hide under floors and in cracks and joints. When litter is taken out of the houses, the beetles may spread into the neighborhood (20).
It is not clear whether darkling beetles can be a vector for transmission of Salmonella and Campylobacter in consecutive broiler rearing cycles. The time spans between cycles are important for pathogen survival in these insects. These time spans may be different in different countries; e.g., in The Netherlands new rotations start after 1 week, but the time span is up to 6 weeks in Scandinavian countries (6, 32). Workers have described contradictory results regarding the survival of pathogens in insects, and the survival time ranges from 1 to 20 days (2, 16, 35, 37). In a study of broiler houses (32), Salmonella survived in beetles in the period (up to 2 weeks) between rearing cycles in broiler houses; however, during the same period no Campylobacter was found in beetles even after a first rotation with positive chickens. On the other hand, this could be explained by the fact that stressed Campylobacter cells may not grow on selective media. In the case of a less selective growth environment, such as the intestines of young chickens, sublethally damaged bacteria may still grow and colonize the chickens (9).
Therefore, in this study, different groups of darkling beetles (A. diaperinus) and their larvae that were either artificially contaminated with Salmonella and Campylobacter or caught in commercial broiler houses that were regularly positive for both pathogens were fed to young chickens to examine the possibility that the insects were a vector for pathogen transmission. Cecal droppings of the chickens were examined for the presence of the pathogens regularly, and isolated strains were characterized by antibiotic resistance testing (Salmonella) or amplified fragment length polymorphism (AFLP) typing (Campylobacter).
MATERIALS AND METHODS
Bacterial strains.
Stock cultures of Campylobacter jejuni strains C224 (isolated from darkling beetles), C356 (isolated from broilers), and C81116 (often used in colonization experiments) and Salmonella enterica serovar Paratyphi B variant Java (nalidixic acid-resistant strain) were kept in cryovials with beads in heart infusion broth supplemented with 20% (vol/vol) glycerol at −80°C.
Insects.
A. diaperinus beetles and their larvae were purchased from a commercial supplier (Kreca, Ermelo, The Netherlands) or were collected from a commercial broiler farm in The Netherlands known to commonly have Salmonella- and Campylobacter-positive broiler flocks. The insects were kept in glass containers and were fed rat feed and water.
Inoculation of insects with bacteria.
Equal amounts of freshly prepared cultures of the Salmonella strain and the three Campylobacter strains in heart infusion broth were mixed, and the resulting bacterial suspension (containing about 107 CFU/ml of Salmonella and 108 CFU/ml of Campylobacter) was fed as droplets to the beetles and their larvae. The amount of the cocktail consumed was at least 1 μl, resulting in infection levels of 104 to 105 CFU of each of the pathogens per insect. Five groups of beetles and their larvae were used (Table 1): (i) insects inoculated with pathogens on the day of feeding to the chicks, simulating incidental contamination (groups A-b and A-l); (ii) insects inoculated daily for 4 weeks but not 1 week prior to feeding to the chicks, simulating the Dutch situation in broiler houses between rearing cycles (groups B-b and B-l); (iii) insects inoculated daily for 5 weeks until they were fed to the chicks, simulating a contaminated broiler house during rearing (groups C-b and C-l); (iv) insects inoculated daily for 5 to 6 weeks until they were fed to the chicks daily for 7 days, simulating repeated exposure of the chicks to contaminated insects during rearing (groups D-b and D-l); and (v) insects collected at a commercial broiler farm 2 days before feeding to the chicks to study possibly naturally contaminated insects (groups E-b and E-l).
TABLE 1.
Isolation of Campylobacter and Salmonella from beetles and their larvae at the start of the chicken feeding experiment and total numbers of chickens positive for Campylobacter and Salmonella at any time
| Groupsa | Treatment | Isolation of pathogens from beetles and larvae
|
No. of positive chickens/total no. tested
|
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Campylobacter
|
Salmonella
|
Campylobacter
|
Salmonella
|
||||||
| Beetles | Larvae | Beetles | Larvae | Beetles | Larvae | Beetles | Larvae | ||
| A-b and A-l | Beetles and larvae, respectively, inoculated on the day of feeding to chicks | + | + | + | + | 11/12 | 10/12 | 12/12 | 10/12 |
| B-b and B-l | Beetles and larvae, respectively, inoculated daily for 4 wk but not 1 wk prior to feeding to chicks | − | − | + | + | 1/15 | 2/15 | 3/15 | 4/15 |
| C-b and C-l | Beetles and larvae, respectively, daily inoculated for 5 wk prior to feeding to chicks | +b | +b | + | + | 13/15 | 8/14 | 15/15 | 7/14 |
| D-b and D-l | Beetles and larvae, respectively, inoculated daily for 5 to 6 wk until feeding to chicks and fed daily to chicks for 7 days | +b | +b | + | + | 13/15 | 15/15 | 14/15 | 14/15 |
| E-b and E-l | Beetles and larvae, respectively, collected at a commercial broiler farm 2 days before feeding to chicks | − | +b | + | + | 1/15 | 1/16 | 3/15 | 1/16 |
b, beetles; l, larvae.
Positive only after enrichment.
Salmonella and Campylobacter isolation.
The presence of pathogens in the insects was checked by crushing the insects in a mortar and subsequent specific enrichment and/or plating using Preston broth (Oxoid CM067, SR0117, and 5% lysed horse blood) or cefoperazone charcoal desoxycholate broth and agar (Oxoid CM0963 and CM0739, each with SR0155) for Campylobacter and using buffered peptone water (Oxoid CM0509), brilliant green agar (Oxoid CM0329 with 100 mg/liter nalidixic acid), or modified semisolid Rappaport-Vassiliadis agar (Oxoid CM0910 with SR0161 incubated for 24 h at 41.5°C) for Salmonella. Campylobacter preparations were incubated microaerobically at 42°C for 2 days, and Salmonella preparations were incubated for 1 day at 37°C unless indicated otherwise. The identity of Campylobacter was confirmed microscopically and serologically (Microscreen Campylobacter; Microgen Bioproducts Ltd., Camberley, United Kingdom). The identity of Salmonella was confirmed by using the standard biochemical test and serological agglutination (Pro-Lab Diagnostics, BioTrading, Mijdrecht, The Netherlands).
Chicken experiments.
A previous feeding trial showed that, once accustomed to the insects, chicks ate the beetles and their larvae eagerly. One-day-old chicks obtained from a commercial Salmonella-free breeder flock were placed on a litter floor for 5 days. On day 5, broilers were individually transferred to battery cages that were separated from each other by one empty cage and then had ad libitum access to feed and water in a strictly controlled broiler facility. Upon arrival, chicks were screened for the presence of Salmonella and Campylobacter by testing the paper liners of the chicken transport crates. Furthermore, eight pooled samples were taken from fecal droppings on day 7 just before insect feeding started, and all samples were negative. At the age of 7 days, each broiler was fed three beetles or three larvae after feed deprivation for 2 h. The number of chicks in groups A-b and A-l was 12, the number of chicks in group C-l was 14, the number of chicks in group E-l was 16, and all other groups contained 15 chicks. The broilers that received insects belonging to the different groups were distributed randomly in the cages.
Sampling of the chickens.
Trays covered with clean paper sheets were placed below the cages in order to sample the cecal (or, if not present, fecal) droppings since the cecum is reported to be the primary site of colonization (1, 5). After each sampling, trays were cleaned and new paper was applied. Swab samples were taken from the droppings of all chickens on days 1, 2, 5, 7, 9, and 12 after the first insects were fed to the chickens. Chickens were sacrificed after 14 days, ceca were removed, and swab samples of the contents were taken. All swabs were streaked on cefoperazone charcoal desoxycholate agar and subsequently mixed with buffered peptone water, incubated for 16 to 20 h at 37°C, and streaked onto brilliant green agar. The incubation and confirmation procedures used were the procedures described above. Isolated strains were collected and kept at −80°C until they were characterized further.
Antibiotic resistance.
A random selection of the isolated Salmonella strains was tested for antibiotic resistance as described previously (18). In brief, Salmonella (108 CFU) was plated on Mueller-Hinton agar plates, and four antibiotic-containing filter disks (Neo-sensitabs; Rosco Diagnostics A/S, Taastrup, Denmark) were applied to each plate. The plates were incubated for 24 h at 37°C, and inhibition zones were measured. The antibiotics used were ampicillin (33 μg per disk), nalidixic acid (130 μg), amoxicillin (30 μg), gentamicin (40 μg), ciprofloxacin (5 μg), doxycycline (80 μg), trimethoprim (5.2 μg), spectinomycin (200 μg), norfloxacin (40 μg), chloramphenicol (60 μg), enrofloxacin (10 μg), flumequine (30 μg), streptomycin (100 μg), and tetracycline (80 μg).
AFLP typing and data processing.
A range of the Campylobacter strains isolated from beetles, larvae, and chicken ceca and the three strains used for inoculation were typed using AFLP genotyping, essentially as described previously (12). In short, chromosomal DNA was digested with HindIII and HhaI and ligated to restriction site-specific adapters. A preselective PCR and then a selective PCR were performed using a D4-labeled HindIII selective primer (Biolegio, Malden, The Netherlands). The final products were mixed with an internal standard (Size Standard-600; Beckman) and separated using a CEQ 8000 capillary sequencer (Beckman). Data in SCF 3.00 format were imported into BioNumerics 3.5 (Applied Maths, Sint-Martens-Latem, Belgium). An AFLP analysis was performed for fragments ranging from 60 to 500 nucleotides long using Bionumerics 3.5. The similarity of patterns was calculated using the Pearson product-moment correlation coefficient. The unweighted pair group method using arithmetic averages and 1% optimization for position tolerance was used for cluster analysis.
RESULTS
Insects (n = 50) from all groups were examined for the presence of the pathogens just before they were fed to the chickens on day 0, and all of them were positive for Salmonella. Most groups were positive for Campylobacter as well; however, this pathogen could not be isolated from group B-b, B-l, and E-b insects (Table 1). For groups C, D, and E-l, Campylobacter was found only after enrichment and not by direct plating.
All chicken groups fed insects that were inoculated with the pathogens on the day of feeding (groups A, C, and D) showed colonization with Campylobacter and Salmonella at levels of 50 to 100% (Table 1). Insects from groups B and E, however, colonized chickens at levels of 10 and 25% for Campylobacter and Salmonella, respectively.
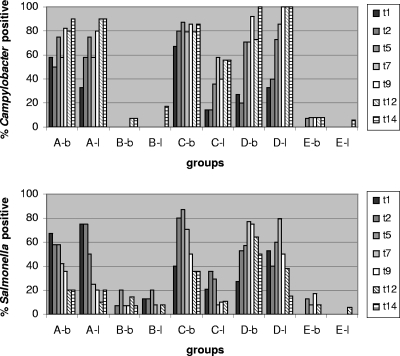
In general, with groups A, C, and D the number of Campylobacter-carrying chicks increased during the first 5 to 9 days after feeding and remained stable at a high value until the end of the experiment (Fig. 1). In contrast, the colonization percentages for Salmonella were initially high and decreased gradually toward the end of the experiment most clearly for groups A, C, and D. Salmonella-positive birds fed group B insects were found from the beginning of the experiment, but Campylobacter colonization for this group was found only after 12 to 14 days. With group E (insects collected from a commercial broiler farm), low numbers of chicks were colonized with Campylobacter and Salmonella from day 5 onward.
FIG. 1.
Percentages of Campylobacter-positive (upper panel) and Salmonella-positive (lower panel) chickens, arranged by feeding group. The different bars for each group indicate the results for samples taken 1, 2, 5, 7, 9, 12, and 14 days after the insects were fed to the chickens. For a description of the groups see Table 1.
The AFLP types of Campylobacter strains C81116, C224, and C356 used for inoculation were designated types R, S, and T, respectively. Seven of the strains isolated from the insects directly were identical to the type R strain, and the type of one strain was designated type T2 (similar to type T with minor differences). A total of 31 strains isolated from chickens were typed; type R was the predominant type found, and some type T-related types were observed as well (Table 2). Furthermore, a new AFLP type was identified, which was designated type P. No type S strains were recovered from insects or chickens.
TABLE 2.
AFLP typing results for Campylobacter strains used for inoculation of the insects and for isolates from beetles, larvae, and chickens
| Strain or source | Group | No. of strains for AFLP type:
|
||||||
|---|---|---|---|---|---|---|---|---|
| R | S | T | T1 | T2 | T3 | P | ||
| C81116 | 1 | |||||||
| C224 | 1 | |||||||
| C356 | 1 | |||||||
| Beetles | 4 | 1 | ||||||
| Larvae | 3 | |||||||
| Chicken | A-b | 2 | ||||||
| Chicken | A-l | 5 | ||||||
| Chicken | B-b | 1 | ||||||
| Chicken | B-l | 1 | 1 | |||||
| Chicken | C-b | 5 | 1 | 1 | ||||
| Chicken | C-l | 1 | 1 | |||||
| Chicken | D-b | 7 | ||||||
| Chicken | D-l | 1 | 2 | |||||
| Chicken | E-b | 1 | ||||||
| Chicken | E-l | 1 | ||||||
A total of 19 Salmonella isolates from insects and chickens were characterized further, and all strains exhibited antibiotic resistance patterns similar to those of the Salmonella variant Java strain used for inoculation (data not shown).
DISCUSSION
The easy transfer of Salmonella and Campylobacter from beetles and their larvae to chickens in groups A, C, and D confirmed that these insects may have a role as vectors in one rearing cycle. There were no major differences between incidental contact (groups A and C) and repeated exposure to the inoculated insects (group D), except for group C larvae, which showed an inexplicably lower level of transfer of both pathogens. Apparently, just a single exposure of chicks to contaminated insects may be sufficient for colonization of the intestines, which was also observed for Campylobacter in a previous study (35). In general, no differences between beetles and their larvae were observed, indicating that the two types of insects are equally important in transmission of the pathogens.
In The Netherlands, an empty 1-week period between rearing cycles is a common practice to clean out broiler houses. Removal of the litter results in removal of many of the insects, but some insects remain and hide in insulation material or in crevices in the building material or beneath the floor. Transmission to the next rearing cycle occurs if the microorganisms grow in the insects or just survive, which is most likely the case for Campylobacter due to its high minimal growth temperature (30°C). The empty week between rearing cycles was mimicked with group B, where inoculated beetles and their larvae were isolated for 1 week before they were fed to the chicks. Just before feeding, all insects were positive for Salmonella, but Campylobacter could not be isolated from group B insects. The rapid decrease in the number of campylobacters in insects may be explained by normal die-off kinetics due to temperature or humidity levels, which are season dependent, and also by the presence of antimicrobials in insects, which has been described for flies and unicorn beetles (17, 21). However, although the levels of colonization were low, with group B transfer of both pathogens to the chicks was observed. Stressed Campylobacter cells may not grow on selective media under these conditions (9), but in a less selective growth environment, such as the intestines of young chickens, sublethally damaged bacteria may still grow and colonize the birds. Slow recovery of damaged campylobacters could explain the fact that these pathogens were isolated from the chickens only at the end of the trial. The possibility of cross-contamination between different birds cannot be completely excluded, but cross-contamination was unlikely since empty cages were placed between the separate chickens and no direct contact was possible. Even though the numbers of positive chicks fed groups B and E insects were low, one positive chicken in a broiler house soon results in most chickens becoming colonized quite quickly and stably until slaughter (19). Furthermore, once a chick was found to be Campylobacter positive, it was also found to be positive postmortem, which also confirms that there was stable colonization.
On the other hand, shedding of Salmonella by the birds was intermittent and decreased toward the end of the experiment, similar to results described previously (23). Salmonella was isolated in all broiler groups throughout the trial, indicating that A. diaperinus can indeed be a vector for this organism in broiler houses, even after an empty week between rearing cycles. This is in accordance with the results of a previous study (32), in which similar genotypes of S. enterica serovar Indiana were found in broilers in two successive cycles and in beetles in a 2-week empty period between flocks. Salmonella variant Java was chosen for this study since this pathogen is known to be a cause of gastroenteritis (8) and it is an increasingly common organism found in broiler houses in The Netherlands (39), which was confirmed by the fact that it was found in insects from the commercial broiler farm (group E).
Some chickens carried one of the pathogens, but concomitant infection was also observed frequently for all groups (data not shown), which is consistent with previous data showing no differences in single-colonization and cocolonization levels in chicks for both Salmonella and Campylobacter (33).
AFLP typing of the Campylobacter strains showed that mainly type R was isolated from beetles, larvae, and chickens, which indicates that strain C81116 either survives best in insects or colonizes chickens best or both. C81116 showed enhanced colonization of chickens after a single passage in vivo, which may be an explanation for the rapid spread of Campylobacter in broiler houses once a chicken is infected (7). Furthermore, some type T-related strains were also isolated, indicating colonization with strain C356. Compared to this original strain, there were minor differences in the genotypes, which is not uncommon since it is known that Campylobacter is genetically unstable and intragenomic alterations may occur, especially after chicken passage (11). Type S was not recovered, which is remarkable since strain C224 was originally isolated from beetles, which suggests that there would be some level of survival in the insects. However, considering the fact that not all isolates were typed and the fact that only one colony per plate was isolated, some types might have been missed, especially if low numbers were present. A new AFLP type (type P) was also found in the cecum of a group C-b chicken, which could be explained by the genetic rearrangement within or between the inoculated strains mentioned above. A great variety of Campylobacter genotypes in broiler flocks and less variation in beetles were reported previously, which was explained by preferential survival of certain clones in beetles or by different levels of recovery via enrichment (beetles) and direct plating (chickens) (4).
Type R was also isolated from group E-b and E-l chickens, which was unexpected, since these groups were fed insects that were not artificially contaminated but were caught at a commercial broiler farm. Cross-contamination with the insects from the other groups was not likely, since even at day 0 group E-l larvae were shown to carry a strain whose type was similar to type R, indicating that there was coincidental resemblance to C81116. This is plausible since this type was previously isolated from the majority of the samples in a human outbreak and chicken isolates from geographically distant sources were type R (24).
In conclusion, the finding that Salmonella and, to a lesser extent, Campylobacter may be transmitted via beetles or their larvae to successive rearing cycles indicates that there should be intensive control programs for elimination of insects from broiler houses. Depending on the type of house, controlling insect populations in broiler houses with physical barriers (15) and insecticides is possible (31), but only when carefully planned strategies are used (22). Another option might be to increase the time between rearing cycles, but, at least in countries where this type of husbandry is used, this is not economically feasible, especially in the case of Salmonella, which might survive for several weeks. Such measures should contribute to reducing the levels of Campylobacter and Salmonella in poultry meat products and thereby improve public health.
Acknowledgments
We thank Joost Fiddelaers, Frans Putirulan, and Albert de Boer for excellent technical assistance and Fimme-Jan van der Wal for critical reading of the manuscript. The skillful assistance of the staff of the animal facilities is also acknowledged.
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Achen, M., T. Y. Morishita, and E. C. Ley. 1998. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter. Avian Dis. 42:732-737. [PubMed] [Google Scholar]
- 2.Bang, B. D., J. Blom, F. Scheutz, M. Lund, J. B. Jespersen, K. Pedersen, and M. Madsen. 2001. Experimental infection of the lesser mealworm beetle (Alphitobius diaperinus) with Campylobacter jejuni. Int. J. Med. Microbiol. 291(Suppl. 31):130. [Google Scholar]
- 3.Barro, N., S. Aly, O. C. A. Tidiane, and T. A. Sababenedjo. 2006. Carriage of bacteria by proboscises, legs, and feces of two species of flies in street food vending sites in Ouagadougou, Burkina Fasso. J. Food Prot. 69:2007-2010. [DOI] [PubMed] [Google Scholar]
- 4.Bates, C., K. L. Hiett, and N. J. Stern. 2004. Relationship of Campylobacter isolated from poultry and from darkling beetles in New Zealand. Avian Dis. 48:138-147. [DOI] [PubMed] [Google Scholar]
- 5.Beery, J. T., M. B. Hugdahl, and M. P. Doyle. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berndtson, E., U. Emanuelson, A. Engvall, and M.-L. Danielsson-Tham. 1996. A 1-year epidemiological study of campylobacters in 18 Swedish chicken farms. Prev. Vet. Med. 26:167-185. [Google Scholar]
- 7.Cawthraw, S. A., T. M. Wassenaar, R. Ayling, and D. G. Newell. 1996. Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implication on the rate of transmission within flocks. Epidemiol. Infect. 117:213-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chart, H. 2003. The pathogenicity of strains of Salmonella paratyphi B and Salmonella java. J. Appl. Microbiol. 94:340-348. [DOI] [PubMed] [Google Scholar]
- 9.Corry, J. E. L., H. I. Atabay, S. J. Forsythe, and L. P. Mansfield. 2003. Culture media for the isolation of campylobacters, heliobacters and arcobacters, p. 271-316. In J. E. L. Corry, G. D. W. Curtis, and R. M. Baird (ed.), Handbook of culture media for food microbiology, 2nd ed. Elsevier, Amsterdam, The Netherlands.
- 10.Davies, R. H., and M. Breslin. 2003. Persistence of Salmonella Enteritidis phage type 4 in the environment and arthropod vectors on an empty free-range chicken farm. Environ. Microbiol. 5:79-84. [DOI] [PubMed] [Google Scholar]
- 11.de Boer, P., J. A. Wagenaar, R. P. Achterberg, J. P. M. van Putten, L. M. Schouls, and B. Duim. 2002. Generation of Campylobacter jejuni genetic diversity in vivo. Mol. Microbiol. 44:351-359. [DOI] [PubMed] [Google Scholar]
- 12.Duim, B., P. A. R. Vandamme, A. Rigter, S. Laevens, J. R. Dijkstra, and J. A. Wagenaar. 2001. Differentiation of Campylobacter species by AFLP fingerprinting. Microbiology 147:2729-2737. [DOI] [PubMed] [Google Scholar]
- 13.Förster, M., S. Klimpel, H. Mehlhorn, K. Sievert, S. Messler, and K. Pfeffer. 2007. Pilot study on synanthropic flies (e.g. Musca, Sarcophaga, Calliphora, Fannia, Lucilia, Stomoxys) as vectors of pathogenic microorganisms. Parasitol. Res. 101:243-246. [DOI] [PubMed] [Google Scholar]
- 14.Hald, B., H. Skovgard, D. D. Bang, K. Pedersen, J. Dybdahl, J. B. Jespersen, and M. Madsen. 2004. Flies and Campylobacter infection of broiler flocks. Emerg. Infect. Dis. 10:1490-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansson, I., I. Vagsholm, L. Svensson, and E. Olsson Engvall. 2007. Correlations between Campylobacter spp. prevalence in the environment and broiler flocks. J. Appl. Microbiol. 103:640-649. [DOI] [PubMed] [Google Scholar]
- 16.Hazeleger, W. C., G. J. Coenen, and R. R. Beumer. 2001. Survival of Campylobacter jejuni in darkling beetles (Alphitobius diaperinus). Int. J. Med. Microbiol. 291(Suppl. 31):37. [Google Scholar]
- 17.Hou, L., Y. Shi, P. Zhai, and G. Le. 2007. Inhibition of foodborne pathogens by Hf-1, a novel antibacterial peptide from the larvae of the housefly (Musca domestica) in medium and orange juice. Food Control 18:1350-1357. [Google Scholar]
- 18.Jacobs-Reitsma, W. F., P. M. F. J. Koenraad, N. M. Bolder, and R. W. A. W. Mulder. 1994. In vitro susceptibility of Campylobacter and Salmonella isolates from broilers to quinolones, ampicillin, tetracycline, and erythromycin. Vet. Q. 16:206-208. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs-Reitsma, W. F., A. W. van de Giessen, N. M. Bolder, and R. W. A. W. Mulder. 1995. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol. Infect. 114:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman, P. E., M. Burgess, and D. A. Rutz. 2002. Population dynamics of manure inhabiting arthropods under an integrated pest management (IPM) program in New York poultry facilities—3 case studies. J. Appl. Poult. Res. 11:90-103. [Google Scholar]
- 21.Koyama, Y., M. Motobu, K. Hikosaka, M. Yamada, K. Nakamura, H. Saaka, A. Asaoka, M. Yamakawa, K. Sekikawa, H. Kitani, K. Shimura, Y. Nakai, and Y. Hirota. 2006. Protective effects of antimicrobial peptides derived from the beetle Allomyrina dichotoma defensin on endotoxic shock in mice. Int. Immunopharmacol. 6:234-240. [DOI] [PubMed] [Google Scholar]
- 22.Lambkin, T. A., and S. J. Rice. 2007. Baseline responses of Alphitobius diaperinus (Coleoptera: Tenebrionidae) to spinosad, and susceptibility of broiler populations in Eastern and Southern Australia. J. Econ. Entomol. 100:1423-1427. [DOI] [PubMed] [Google Scholar]
- 23.Linton, A. H., Z. A. M. Al-Chalaby, and M. H. Hinton. 1985. Natural subclinical Salmonella infection in chicken: a potential model for testing the effects of various procedures on Salmonella shedding. Vet. Rec. 116:361-364. [DOI] [PubMed] [Google Scholar]
- 24.Manning, G., B. Duim, T. Wassenaar, J. A. Wagenaar, A. Ridley, and D. G. Newell. 2001. Evidence for a genetically stable strain of Campylobacter jejuni. Appl. Environ. Microbiol. 76:1185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAllister, J. C., C. D. Steelman, J. K. Skeeles, L. A. Newberry, and E. E. Gbur. 1996. Reservoir competence of Alphitobius diaperinus (Coleoptera: Tenebrionidae) for Escherichia coli (Eubacteriales: Enterobacteriaceae). J. Med. Entomol. 33:983-987. [DOI] [PubMed] [Google Scholar]
- 26.Mian, L. S., H. Maag, and J. V. Tacal. 2002. Isolation of Salmonella from muscoid flies at commercial animal establishments in San Bernardino County, California. J. Vector Ecol. 27:82-85. [PubMed] [Google Scholar]
- 27.Olsen, A. R., and T. S. Hammack. 2000. Isolation of Salmonella spp. from the housefly, Musca domestica L., and the dump fly, Hydrotaea aenescens (Wiedemann) (Diptera: Muscidae), at caged-layer houses. J. Food Prot. 63:958-960. [DOI] [PubMed] [Google Scholar]
- 28.Refrégier-Petton, J., N. Rose, M. Denis, and G. Salvat. 2001. Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 29.Rosef, O., and G. Kapperud. 1983. House flies (Musca domestica) as possible vectors of Campylobacter fetus subsp. jejuni. Appl. Environ. Microbiol. 45:381-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salin, C., Y. R. Delettre, M. Cannavacciuolo, and P. Vernon. 2000. Spatial distribution of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) in the soil of a poultry house along a breeding cycle. Eur. J. Soil Biol. 36:107-115. [Google Scholar]
- 31.Salin, C., Y. R. Delettre, and P. Vernon. 2003. Controlling the mealworm Alphitobius diaperinus (Coleoptera: Tenebrionidae) in broiler and turkey houses: field trials with a combined insecticide treatment: insect growth regulator and pyrethroid. J. Econ. Entomol. 96:126-130. [DOI] [PubMed] [Google Scholar]
- 32.Skov, M. N., A. G. Spencer, B. Hald, L. Petersen, B. Nauery, B. Carstensen, and M. Madsen. 2004. The role of litter beetles as potential reservoir for Salmonella enterica and thermophilic Campylobacter spp. between broiler flocks. Avian Dis. 48:9-18. [DOI] [PubMed] [Google Scholar]
- 33.Stern, N. J., J. S. Bailey, R. J. Meinersmann, N. A. Cox, and L. C. Blankenship. 1991. Simultaneous colonization of Campylobacter jejuni and Salmonella typhimurium in day-old chicks. Poult. Sci. 70:790-795. [DOI] [PubMed] [Google Scholar]
- 34.Strother, K. O., and C. D. Steelman. 2001. Spatial analysis of Alphitobius diaperinus (Coleoptera: Tenebrionidae) in broiler production facilities. Environ. Entomol. 30:556-561. [Google Scholar]
- 35.Strother, K. O., C. Dayton Steelman, and E. E. Gbur. 2005. Reservoir competence of lesser mealworm (Coleoptera: Tenebrionidae) for Campylobacter jejuni (Campylobacterales: Campylobacteriaceae). J. Med. Entomol. 42:42-47. [DOI] [PubMed] [Google Scholar]
- 36.Szalanski, A. L., C. B. Owens, T. McKay, and C. D. Steelman. 2004. Detection of Campylobacter and Escherichia coli 157:H7 from filth flies by polymerase chain reaction. Med. Vet. Entomol. 18:241-246. [DOI] [PubMed] [Google Scholar]
- 37.Templeton, J. M., A. J. de Jong, P. J. Blackall, and J. K. Miflin. 2006. Survival of Campylobacter spp. in darkling beetles (Alphitobius diaperinus) and their larvae in Australia. Appl. Environ. Microbiol. 72:7909-7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valkenburgh, S., R. van Oosteron, O. Stenvers, M. Aalten, M. Braks, B. Schimmer, A. van de Giessen, W. van Pelt, and M. Langelaar (ed.). 2007. Zoonoses and zoonotic agents in humans, food, animals and feed in the Netherlands. Report no. 330152001. National Institute of Public Health and the Environment, Bilthoven, The Netherlands.
- 39.van de Giessen, A. W., M. Bouwknegt, W. D. C. Dam-Deisz, W. van Pelt, W. J. B. Wannet, and G. Visser. 2006. Surveillance of Salmonella spp. and Campylobacter spp. in poultry production flocks in The Netherlands. Epidemiol. Infect. 134:1266-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagenaar, J. A., W. Jacobs-Reitsma, M. Hofshagen, and D. Newell. 2008. Poultry colonization with Campylobacter and its control at the primary production level, p. 667-678. In I. Nachamkin, C. M. Szymanski, and M. J. Blaser (ed.), Campylobacter, 3rd ed. ASM Press, Washington, DC.
- 41.Watson, D. W., J. S. Guy, and S. M. Stringham. 2000. Limited transmission of turkey coronavirus in young turkeys by adult Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Med. Entomol. 37:480-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright, E. P. 1983. The isolation of Campylobacter jejuni from flies. J. Hyg. 91:223-226. [DOI] [PMC free article] [PubMed] [Google Scholar]