Abstract
The effectiveness of two major UV technologies against a highly prevalent species of Mycobacterium avium complex was investigated. Our study indicates that M. avium is much more resistant to UV irradiation than most waterborne pathogens and that it is one of the rare microorganisms that are highly resistant to both chemical and UV disinfection in water.
There is a growing concern about human exposure to Mycobacterium avium complex (MAC) through drinking water (16) due to its high resistance to most chemical disinfectants in water treatment processes (15) and its high prevalence in biofilms in water distribution systems (6). On the other hand, many water utilities in the United States are interested in UV disinfection as an alternative to conventional chemical disinfection in compliance with a recent U.S. federal regulation (the Long Term 2 Enhanced Surface Water Treatment Rule), due to its remarkable effectiveness against highly chlorine-resistant protozoan parasites, such as Cryptosporidium parvum and Giardia lamblia (9, 12).
Despite the important public health implications of MAC and the anticipated widespread use of UV disinfection in drinking water treatment, little is known about the effectiveness of UV irradiation against MAC in water disinfection. Early studies of UV disinfection against MAC (5, 10) were performed with different purposes (control of airborne mycobacterial infection) and different experimental procedures (for air/surface disinfection), so it is difficult to determine the effectiveness of UV irradiation against the MAC in water disinfection. For example, there was no correction for UV absorption in suspending liquid in the early studies, which may result in overestimation of the UV doses delivered to the microorganisms. Also, there was no correction for distribution of UV irradiance across the irradiated surface and no use of collimated-beam UV apparatus (with irradiation in only one direction) in those studies, which makes it difficult to determine the true UV doses delivered to the microorganisms. Most importantly, the early studies were done with low-pressure (LP) UV irradiation alone and therefore provide no information on the effectiveness of another promising UV technology, medium-pressure (MP) UV irradiation. Therefore, we investigated the effectiveness of two major UV technologies (LP and MP UV) against one of the most prevalent species of MAC in water disinfection by using a standard UV apparatus and dosimetry in this study.
A clinical isolate of M. avium subsp. hominissuis, HMC02 (2), was chosen for this study. Two morphotypic clones, white transparent (WT) and white opaque (WO), were analyzed. These morphotypes are frequently isolated from patient samples (11) and from natural biofilm samples (7). The WT morphotype is the most virulent and multidrug-resistant form of MAC (3, 11). M. avium was grown and assayed with Middlebrook 7H10 agar containing oleic acid-albumin-dextrose-catalase enrichment and 0.5% glycerol as previously described (3). For UV disinfection, a small amount (∼109 CFU) of M. avium cells in early stationary phase was scraped from agar slants and suspended in 10 ml of phosphate-buffered saline (PBS; pH 7.4) in 15-ml conical tubes (BD Biosciences, Franklin Lakes, NJ). In order to disperse cells, cells were vortexed with glass beads (710 to 1,180 μm; Sigma Chemical Co., St. Louis, MO) for 1 min before UV irradiation.
The bench scale collimated-beam apparatus consisted of two 15-watt germicidal lamps (41 cm in length and 2.5 cm in width; model XX-15G; Spectroline, Westbury, NY) and a 400-watt MP UV lamp (11.5 cm in length and 2.5 cm in width; model 7825 immersion lamp; Hanovia Ltd., Slough, United Kingdom) for LP and MP UV, respectively. The distances from the UV lamps to the surfaces of the test suspensions were 37 and 104 cm for the LP and MP UV disinfection apparatus, respectively, and the lamps were mounted on top of the UV irradiation apparatus and provided with incident radiation perpendicular to the surface of the test suspension in 60-mm cell culture petri dishes. UV irradiance was measured with a calibrated International Light IL1700 radiometer (International Light Inc., Newburyport, MA). The delivered UV dose, accounting for the UV absorbance in the liquid and the depth of the suspension (0.255 cm), was calculated based on the measurement of the irradiance incident on the petri dishes, a series of correction factors (petri factor, reflection factor, water factor, divergence factor, sensor factor [for MP UV irradiation only], and germicidal factor [for MP UV irradiation only]) as described by Bolton and Linden (1), and the exposure time in seconds.
UV disinfection experiments were performed as previously described (12). Briefly, Escherichia coli type B and M. avium cells were mixed and diluted in PBS to give final concentrations of ∼106 CFU/ml. Aliquots of 5 ml each in 60-mm cell culture petri dishes were irradiated with the aforementioned collimated-beam UV sources while the samples were stirred slowly on a magnetic stir plate. After predetermined exposure times (23 to 100 s for M. avium, depending on the UV dose applied), the samples were removed from the UV irradiation systems, diluted serially, and immediately plated for corresponding infectivity assays. All the experiments were conducted in dimmed light.
Statistical analysis of inactivation kinetics was performed by using SAS (SAS Institute Inc., Cary, NC) and EXCEL (Microsoft, Redmond, WA). Linear regression analysis was used to calculate the values for IT (UV intensity times exposure time) with EXCEL, and analysis of covariance (ANCOVA) was applied to compare the inactivation kinetics (slope difference) by use of the SAS General Linear Model procedure.
Figure 1 shows the kinetics of inactivation of E. coli B by both LP and MP UV irradiation in PBS at room temperature based on four replicate experiments. The inactivations of E. coli B (the reference bacterium) by both LP and MP UV irradiation were very rapid (approximately first-order), and a 1-log10 inactivation was achieved with a UV dose of ∼1.5 mJ/cm2. The inactivation kinetics of E. coli B in this study were similar to those in previous studies (4, 14, 17).
FIG. 1.
Inactivation of E. coli B by LP and MP UV irradiation in PBS at room temperature. Each data point is an average of results for four replicate experiments, and the error bars represent 1 standard deviation.
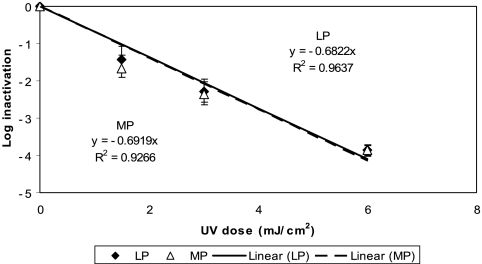
Figure 2 shows the kinetics of inactivation of the two M. avium morphotypes by LP UV irradiation in PBS at room temperature based on three replicate experiments. The inactivations of both M. avium morphotypes were much slower than those of E. coli B, and a 1-log10 reduction was achieved with a UV dose of ∼6 mJ/cm2. In order to achieve a significant inactivation (e.g., 4 log10), a UV dose of more than 20 mJ/cm2 (24 and 23 mJ/cm2 for WT and WO morphotypes, respectively) was required. There was no statistically significant difference between the WT and WO morphotypes in their responses to LP UV irradiation (ANCOVA; P = 0.97).
FIG. 2.
Inactivation of M. avium by LP UV irradiation in PBS at room temperature. Each data point is an average of results for three replicate experiments, and the error bars represent 1 standard deviation.
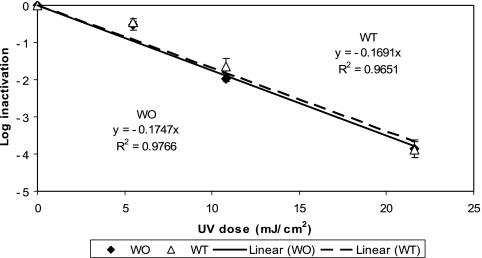
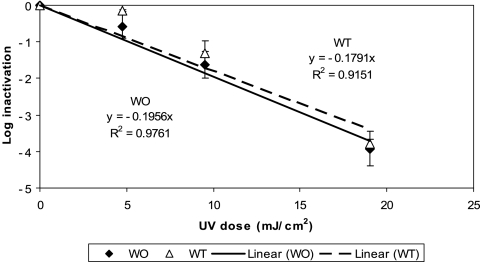
The kinetics of inactivation of the two M. avium morphotypes by MP UV irradiation (Fig. 3) was similar to that by LP UV irradiation. That is, the inactivations of both M. avium morphotypes by MP UV irradiation were also slow (1-log10 reduction with a UV dose of ∼6 mJ/cm2), and a UV dose of more than 20 mJ/cm2 (22 and 20 mJ/cm2 for WT and WO morphotypes, respectively) was required to achieve a 4-log10 inactivation. Again, there was no statistically significant difference between the WT and WO morphotypes in their responses to MP UV irradiation (ANCOVA; P = 0.93), and there was no statistically significant difference between the two different UV technologies in terms of their effectiveness against the two morphotypes (ANCOVA; P = 0.17 and 0.10 for WT and WO morphotypes, respectively).
FIG. 3.
Inactivation of M. avium by MP UV irradiation in PBS at room temperature. Each data point is an average of results for three replicate experiments, and the error bars represent 1 standard deviation.
It is difficult to directly compare the results of the current study to those of the early studies (5, 10) because the early studies were performed with different purposes and different experimental procedures. Nonetheless, the UV dose needed to achieve a 1-log10 inactivation of M. avium HMC02 in the current study (∼6 mJ/cm2) was higher than that needed for M. avium DM9 (3.5 mJ/cm2) (10) but lower than that needed for M. avium-intracellulare T-931-72 (7 mJ/cm2) (5). Among waterborne pathogens, the UV dose needed to achieve a 4-log10 inactivation of M. avium HMC02 was much higher than those needed for all the other pathogenic bacteria studied so far (8, 13). In fact, M. avium HMC02 is much more resistant than most protozoan parasites and pathogenic bacteria and as resistant as many pathogenic viruses but not rotaviruses or adenoviruses (8, 13).
Overall, the results of this study indicate that M. avium is quite resistant to UV (both LP and MP UV) disinfection in water. M. avium appears to be one of the rare microorganisms that are highly resistant to both chemical and UV disinfection in water treatment processes. It is plausible that water disinfection may select MAC over less resistant competitor microorganisms and thereby facilitate the colonization of MAC in water distribution systems, which could increase human exposure to MAC through drinking water. Further studies of different species of MAC and more effort to develop effective control measures against MAC in water treatment processes are needed before the anticipated widespread implementation of UV disinfection.
Acknowledgments
This research was supported by Cooperative Agreement no. 83303001-0 and grant R833011 from the U.S. Environmental Protection Agency.
Footnotes
Published ahead of print on 26 September 2008.
REFERENCES
- 1.Bolton, J. R., and K. G. Linden. 2003. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J. Environ. Eng. 129:209-215. [Google Scholar]
- 2.Cangelosi, G. A., C. O. Palermo, J.-P. Laurent, A. M. Hamlin, and W. H. Brabant. 1999. Colony morphotypes on Congo red agar segregate along species and drug susceptibility lines in the Mycobacterium avium-intracellulare complex. Microbiology 145:1317-1324. [DOI] [PubMed] [Google Scholar]
- 3.Cangelosi, G. A., C. O. Palermo, and L. E. Bermudez. 2001. Phenotypic consequences of red-white colony type variation in Mycobacterium avium. Microbiology 147:527-533. [DOI] [PubMed] [Google Scholar]
- 4.Chang, J. C. H., S. F. Osoff, D. C. Lobe, M. H. Dorfman, C. M. Dumais, R. G. Qualls, and J. D. Johnson. 1985. UV inactivation of pathogenic and indicator microorganisms. Appl. Environ. Microbiol. 49:1361-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.David, H. L. 1973. Response of mycobacteria to ultraviolet light radiation. Am. Rev. Respir. Dis. 108:1175-1185. [DOI] [PubMed] [Google Scholar]
- 6.Falkinham, J. O., III, C. D. Norton, and M. W. LeChevallier. 2001. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford, T. E., P. Prommasith, and G. A. Cangelosi. 2003. Public health implications of mycobacterial survival in municipal water biofilms, p. 21. ASM Conf. Biofilms 2003, Victoria, BC, Canada.
- 8.Hijnen, W. A. M., and G. J. Medema. 2005. Inactivation of viruses, bacteria, spores and protozoa by ultraviolet irradiation in drinking water practice: a review. Water Sci. Technol. Water Supply 5(5):93-99. [Google Scholar]
- 9.Linden, K. G., G. Shin, G. Faubert, W. Carns, and M. D. Sobsey. 2002. Inactivation of Giardia lamblia cysts by low pressure UV radiation. Environ. Sci. Technol. 36:2519-2522. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy, C. M., and J. O. Schaeffer. 1974. Response of Mycobacterium avium to ultraviolet irradiation. Appl. Microbiol. 28:151-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee, S., M. Petrofsky, K. Yaraei, L. E. Bermudez, and G. A. Cangelosi. 2001. The white morphotype of Mycobacterium avium-intracellulare is common in infected humans and virulent in infection models. J. Infect. Dis. 184:1480-1484. [DOI] [PubMed] [Google Scholar]
- 12.Shin, G., K. G. Linden, M. J. Arrowood, and M. D. Sobsey. 2001. Low-pressure UV inactivation and DNA repair potential of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 67:3029-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobsey, M. D. 1989. Inactivation of health-related microorganisms in water by disinfection processes. Water Sci. Technol. 21(3):179-195. [Google Scholar]
- 14.Sommer, R., G. Weber, A. Cabaj, J. Wekerle, G. Keck, and G. Schauberger. 1989. UV inactivation of microorganisms in water. Zentralbl. Hyg. Umweltmed. 189:214-224. [PubMed] [Google Scholar]
- 15.Taylor, R. H., J. O. Falkinham III, C. D. Norton, and M. W. LeChevallier. 2000. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol. 66:1702-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Reyn, C. F., J. N. Maslow, T. W. Barber, J. O. Falkinham III, and R. D. Arbeit. 1994. Persistent colonization of portable water as a source of Mycobacterium avium infection in AIDS. Lancet 343:1137-1141. [DOI] [PubMed] [Google Scholar]
- 17.Wilson, B. R., P. F. Rossler, E. Van Dellen, M. Abbaszadegan, and C. P. Gerba. 1992. Coliphage MS-2 as a UV water disinfection efficacy test surrogate for bacterial and viral pathogens, p. 219-235. In Proceedings of the Water Quality Technology Conference, Toronto, ON, Canada.