Abstract
φSG-JL2 is a newly discovered lytic bacteriophage infecting Salmonella enterica serovar Gallinarum biovar Gallinarum but is nonlytic to a rough vaccine strain of serovar Gallinarum biovar Gallinarum (SG-9R), S. enterica serovar Enteritidis, S. enterica serovar Typhimurium, and S. enterica serovar Gallinarum biovar Pullorum. The φSG-JL2 genome is 38,815 bp in length (GC content, 50.9%; 230-bp-long direct terminal repeats), and 55 putative genes may be transcribed from the same strand. Functions were assigned to 30 genes based on high amino acid similarity to known proteins. Most of the expected proteins except tail fiber (31.9%) and the overall organization of the genomes were similar to those of yersiniophage φYeO3-12. φSG-JL2 could be classified as a new T7-like virus and represents the first serovar Gallinarum biovar Gallinarum phage genome to be sequenced. On the basis of intraspecific ratios of nonsynonymous to synonymous nucleotide changes (Pi[a]/Pi[s]), gene 2 encoding the host RNA polymerase inhibitor displayed Darwinian positive selection. Pretreatment of chickens with φSG-JL2 before intratracheal challenge with wild-type serovar Gallinarum biovar Gallinarum protected most birds from fowl typhoid. Therefore, φSG-JL2 may be useful for the differentiation of serovar Gallinarum biovar Gallinarum from other Salmonella serotypes, prophylactic application in fowl typhoid control, and understanding of the vertical evolution of T7-like viruses.
T7-like viruses have short noncontractile tails and are members of the family Podoviridae. To date, eight strains have been assigned as Enterobacteria phage T7 and three strains (T3, T7, and φYeO3-12) have been characterized genomically (http://www.ncbi.nlm.nih.gov/ICTVdb/Ictv/index.htm) (19, 50, 51). Genetic recombination between T7-like viruses infecting different bacterial genera or different species has been demonstrated, and T3 may have evolved from an ancient phage generated by recombination between yersiniophages φA1122 and φYeO3-12 (20, 51). Horizontal genetic transfer results in genomic mosaicism of phages, which hinders their hierarchical classification (22, 37). However, common genetic components and layouts observed among T7-like viruses may support the idea that they crossed a “Darwinian threshold” and have been undergoing vertical evolution (26, 79). Therefore, they may be useful in understanding genetic variations of closely related T7-like phages during host adaptation. However, current genomic data are insufficient to permit such detailed analysis. Additional genome sequences of closely related T7-like viruses are required to gain insight into their vertical evolution.
Fowl typhoid is an acute septicemic disease occurring in adult chickens. The disease is characterized by anemia, leukocytosis, and hemorrhage and is an economically disastrous disease in the poultry industry (53). The causative agent, Salmonella enterica serovar Gallinarum biovar Gallinarum, is classified in serogroup D and is both nonmotile and host adapted (3, 53). Differentiation of serovar Gallinarum biovar Gallinarum from frequent avian serogroup D Salmonella strains, such as S. enterica serovar Gallinarum biovar Pullorum and S. enterica serovar Enteritidis, has been partially successful (33, 34), and differentiation of field strains of serovar Gallinarum biovar Gallinarum from the rough vaccine strain SG-9R has become important because of nationwide vaccination in some countries. The appearance of multidrug-resistant serovar Gallinarum biovar Gallinarum strains in the field has prompted increasing concerns about phage therapy, similar to other bacterial diseases (5, 30, 35, 64, 68), but candidate phages that are lytic to broad ranges of serovar Gallinarum biovar Gallinarum strains have never been reported. Fowl typhoid has been reported to spread via the fecal-oral route, but recently, fowl typhoid was reproduced by intratracheal challenge with serovar Gallinarum biovar Gallinarum (4).
In this study, we report the basic biological properties and complete genomic sequence of a new Salmonella T7-like virus, φSG-JL2. It is lytic to serovar Gallinarum biovar Gallinarum and has a double-stranded DNA of 38,815 bp with 55 putative genes. Comparative genomic analyses have demonstrated the close relationships of φSG-JL2 with φYeO3-12 from Yersinia enterocolitica O3 and with T3 from Escherichia coli and have provided molecular clues to understand host adaptations of related phages. The obligate specificity and broad lytic activity of φSG-JL2 may be useful for differentiation of serovar Gallinarum biovar Gallinarum from S. enterica serovar Enteritidis and serovar Gallinarum biovar Pullorum, and the prophylactic efficacy of φSG-JL2 against fowl typhoid was tested with a respiratory model of fowl typhoid.
MATERIALS AND METHODS
Bacteria, phage, and media.
Serovar Gallinarum biovar Pullorum (four strains) and some serovar Gallinarum biovar Gallinarum strains used in the present study were identified and reported previously (33, 52). Other serovar Gallinarum biovar Gallinarum strains were isolated from commercial chickens consigned to diagnosis from 2000 to 2005 and were identified as described previously (52). The SG-9R rough vaccine strain was cultured from a commercial live-vaccine product (Intervet, Boxmeer, The Netherlands), and reference strains of S. enterica serovar Typhimurium (KCTC12400) and E. coli (ATCC 43896) were purchased from the Korea Culture Collection of Microorganisms (Seoul, Korea). S. enterica serovar Enteritidis strains (20 strains) were isolated from poultry farms in Korea and identified as described previously (33, 34, 52). All Salmonella strains were cultured with MacConkey agar and tryptic soy broth (TSB) (Difco, Detroit, MI). A lytic serovar Gallinarum biovar Gallinarum-specific bacteriophage isolated from a sample of final processed sewage water collected in Seoul as described below was designated φSG-JL2. Tryptic soy agar (Difco) and TSB were used for plaque tests and phage propagation as described below.
Phage isolation, cloning, and propagation.
A portion of the final outflow from a sewage-processing plant in Seoul was collected and centrifuged at 15,000 × g for 30 min to precipitate debris. The supernatant was filtered through a membrane filter with a 0.45-μm pore size. A 26-ml portion of the filtered sewage water was transferred to a 50-ml conical tube. Three milliliters of 10× TSB and 107 CFU/ml of serovar Gallinarum biovar Gallinarum strain 002 (SG002) were added, mixed, and incubated at 37°C for 5 h. The incubated culture was centrifuged (15,000 × g; 30 min), and the supernatant was diluted 10-fold from 10−1 to 10−8. Five hundred microliters of each dilution was mixed with 500 μl of serovar Gallinarum biovar Gallinarum (109 CFU/ml) and plated on a 90-mm-diameter tryptic soy agar plate. A typically large and well-isolated plaque was retrieved with a sterilized yellow tip and suspended in TSB following preparation of 10−1 to 10−5 dilutions. This process was repeated five times for cloning. The isolated phage was propagated in TSB with the host and filtered through a 0.2-μm-pore-size membrane filter after centrifugation as detailed above. The PFU count of the filtered phage was determined as described above. Phage preparations were stored at −70°C until they were required.
Host range determination.
A 5-μl volume of each serial dilution (10−5 to 10−9) of cloned and filtered phage (1010 PFU/ml) was dispensed on lawns of serovar Gallinarum biovar Gallinarum field strains (106 strains, including SG002 and SG101) and SG-9R, serovar Gallinarum biovar Pullorum (4 strains), S. enterica serovar Enteritidis (20 strains), S. enterica serovar Typhimurium (KCTC12400), and E. coli (ATCC 43896). The PFU count was determined after overnight incubation at 37°C.
Electron microscopy.
Purified phage were applied to carbon-shadowed Parlodion-coated grids and stained with 1% uranyl acetate. Electron micrographs of the phage were taken with a Zeiss EM902 transmission electron microscope operating at 80 kV.
Heat and pH susceptibility tests.
The heat susceptibility of φSG-JL2 was measured at 55°C for 30 and 60 min, together with that of the host strain, SG002. The pH susceptibility of φSG-JL2 was tested at final pH 3.0, pH 4.0, and pH 6.0 by mixing equal volumes of φSG-JL2 and acidic phosphate-buffered saline solution (pH 2.0, pH 3.0, and pH 5.0, adjusted with 1 M HCl) for 10, 30, and 60 min.
One-step growth curve.
At mid-logarithmic growth phase (determined in preliminary experiments to be an optical density of 0.5 at 600 nm), SG101 was harvested by centrifugation (15,000 × g; 15 min) and resuspended in 0.5 volume of the original culture (108 CFU/ml). The phage was added at a multiplicity of infection (MOI) of 0.001 and was allowed to adsorb for 5 min. The adsorbed phage and bacteria were centrifuged (15,000 × g; 15 min) and resuspended in 10 ml of TSB. During the incubation of the resuspension at 37°C, samples were taken at 5-min intervals for 25 min. The samples were immediately diluted and plated for phage titration.
DNA extraction, cloning, PCR, and sequencing.
TSB containing the phage was centrifuged at 15,000 × g for 30 min and filtered through a 0.22-μm-pore-size membrane filter. Proteinase K (100 μg/ml) was added and incubated at 65°C for 1 h. Then, an equal volume of phenol-chloroform/isoamyl alcohol was mixed with the broth and centrifuged as described above. The aqueous phase was collected, and the same volume of isopropanol was added. Precipitated phage DNA was collected at 15,000 × g for 30 min. After the DNA was washed by resuspension in 70% ethanol and centrifugation under the same conditions, the phage DNA was resuspended in sterilized deionized distilled water. For the shotgun cloning to obtain partial nucleotide sequences of φSG-JL2, the phage genomic DNA and pBluescript II SK(+) were digested with HpaII and ClaI, respectively; ligated with T4 DNA ligase; and used to transform competent E. coli (Invitrogen, Carlsbad, CA). Inserted DNA was directly amplified by colony PCR with M13 forward and reverse primers as previously described (32). The nucleotide sequences of amplicons were determined using an automatic DNA sequencer and a Dye Terminator kit (Perkin Elmer, Foster City, CA).
The whole genomic nucleotide sequence was determined by aligning the genomic nucleotide sequences of φYeO3-12 (AJ251805) and φT3 (AJ318471) and designing primer sets from the conserved regions. Based on the amplicon nucleotide sequences, additional primer sets were designed to amplify and determine the nucleotide sequences. Terminal-repeat sequences were determined by sequencing of an amplicon that contained right (RTR) and left (LTR) terminal repeats and which might originate from the genomic concatemers of φSG-JL2 (24). For PCR amplification, 20 μl containing 1 mM MgCl2, 1 mM deoxynucleotide triphosphates, 10 μM of each forward and reverse primer, and 1 unit of Taq polymerase (iNtRON Biotechnology, Sungnam, Korea) were mixed together, and PCR was conducted on the mixture at 94°C for 3 min; 35 cycles of 94°C for 20 s, 52°C for 20 s, and 72°C for 90 s; and 72°C for 7 min. The amplicons were purified with a PCR purification kit (iNtRON Biotechnology) according to the manufacturer's protocol, and the nucleotide sequences were determined as described above.
Sequence analysis.
The nucleotide sequences were compared with those of other genes in GenBank by the BLASTN program (http://www.ncbi.nlm.nih.gov/BLAST/). The open reading frames (ORFs) were identified with the ORF Finder at the National Center for Bioinformatics site (http://www.ncbi.nlm.nih.gov/gorf.html) and GenMark.hmm prokaryotic (version 2.5a) (http://opal.biology.gatech.edu/GeneMark/). Confirmation was provided by the presence of an appropriately located potential Shine-Dalgarno sequence upstream of the start codon and comparison of corresponding ORFs with those of φYeO3-12 and φT3. The molecular weight and isoelectric point were calculated (6) with the Compute pI/Mw program (http://www.expasy.ch/tools/pi_tool.html). The analogous promoters of host and phage RNA polymerases (RNAPs), rho-independent terminators, and RNase III recognition sites were manually compared with those of φYeO3-12 and φT3, and the secondary structures and free energies were calculated with RNAfold (http://rna.tbi.univie.ac.at/cgi-bin/RNAfold.cgi). The genomic nucleotide sequence of φSG-JL2 was compared with those of φYeO3-12, φT3, and other Salmonella phages (SP6 [NC_004831], P22 [NC_002371], ES18 [NC_006949], Gifsy-1 [NC_010392], ST64B [NC_004313], ST64T [NC_004348], Gifsy-2 [NC_010393], Fels-1 [NC_010391], and Fels-2 [NC_010463]) with the BLAST 2 sequence tool (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi), and the synteny plots were generated by the Nucmer program in the Mummer software package (17). The nucleotide and deduced amino acid sequences of phages and host genes were aligned by the Clustal method in the MEGA program (32), and the Pi[a]/Pi[s] and Ka/Ks ratios (ratios of the average number of asynonymous nucleotide changes to the average number of synonymous nucleotide changes) were measured with the DnaSP program (version 4.20) (60).
Prophylactic efficacy of φSG-JL2 against fowl typhoid in chickens.
To test the prophylactic efficacy of φSG-JL2, 106 CFU/ml of SG101 was treated with φSG-JL2 at MOIs of 0.1, 1, and 10 in tryptic soy broth at room temperature for 4 h, and 80 13-day-old commercial male brown layer chicks were assigned to one control (SG101 only) and three treated (SG101 plus φSG-JL2 at an MOI of 0.1, 1, or 10) groups. Respiratory reproduction of fowl typhoid was performed as described previously (4). Briefly, 5 μl from each tube was inoculated into each chick via the intratracheal route, and they were observed for mortality for 15 days after inoculation. After 15 days, the surviving chicks were sacrificed to observe lesions on the livers (hepatic necrotic foci). The dead chicks were not included in the counting of lesion-positive chicks. The surviving lesion-negative chicks were used for the calculation of the protection rate.
Statistical analysis.
The Kaplan-Meier survival curves were drawn and the log rank test for comparison between survival curves was performed using SAS (version 9.1.3). Also, the protection rate of each group was evaluated via chi-square and Fisher's exact tests (95% confidence interval).
Nucleotide sequence accession number.
The genomic nucleotide sequence of φSG-JL2 was deposited in GenBank under accession number EU547803.
RESULTS AND DISCUSSION
Host range of φSG-JL2.
φSG-JL2 plated at an efficiency of <0.5 × 10−6 on serovar Gallinarum biovar Pullorum strain SP4 but at an efficiency of <6.5 × 10−9 on S. enterica serovar Enteritidis, S. enterica serovar Typhimurium, SG-9R (a rough vaccine strain of serovar Gallinarum biovar Gallinarum), and E. coli. Determination of the host range of φSG-JL2 using 106 strains of serovar Gallinarum biovar Gallinarum isolated in Korea between 1994 and 2006 demonstrated that φSG-JL2 was lytic to 98.1% of the isolates, indicative of its utility in the identification of serovar Gallinarum biovar Gallinarum and for prophylactic application against fowl typhoid.
The receptors of T7-like viruses have been reported to be lipopolysaccharide (LPS), but different phages bind different moieties of LPS (45, 54). Neither the T3 nor the T7 type of T7-like viruses forms plaques on smooth E. coli strains, and binding is to glucose residues in the outer core (T3) and more inner moieties (T7) of LPS (45, 54). Salmonella phage SP6 grows on both rough and smooth strains, but φYeO3-12 is specific to the O3 antigen of Y. enterocolitica (1, 45). No plaque formation of φSG-JL2 occurs on SG-9R, which lacks LPS O side chains (67), consistent with the participation of the O antigen as the receptor.
Morphology of φSG-JL2.
Electron microscopy of negatively stained preparations of φSG-JL2 virions revealed hexagonal heads with a diameter of about 54 nm (data not shown), similar to those of other T7-like viruses (49).
One-step growth curve of φSG-JL2.
A very short latent period (<10 min) was evident, and burst-out of phage particles occurred between 10 and 15 min (data not shown). The overall one-step growth cycle was slightly shorter than that of φYeO3-12, but the burst size (about 100 PFU per infected cell) was similar to that of φYeO3-12 (49).
Heat and pH susceptibilities of φSG-JL2.
The PFU count of φSG-JL2 was slightly decreased from 2 × 109 to 1.5 × 109 and 1 × 109 at 55°C for 30 and 60 min, respectively, but the CFU count of the host bacteria, SG002, decreased from 2 × 108 to 0. The relative heat resistance of φSG-JL2 may be useful to inactivate residual pathogenic serovar Gallinarum biovar Gallinarum during production of φSG-JL2 for phage therapy in terms of reduction of chloroform use. According to the pH susceptibility test, φSG-JL2 was completely inactivated just after being mixed with pH 3.0 and pH 2.0 solutions and incubation for 10 min, and it was highly susceptible to low-pH conditions.
Determination of the φSG-JL2 genome sequence.
The φSG-JL2 genome was found to contain 38,815 bp of nucleotides and to possess an overall GC content of 50.9%. The latter is slightly higher than that of T7 (48.4%) but similar to those of φYeO3-12 (50.6%) and T3 (50.0%) (19, 50, 51). Fifty-five putative genes were identified in the same strand, and functions were assigned to 30 genes based on high amino acid similarity to known proteins (Table 1). φSG-JL2 showed no significant similarity to other Salmonella phages compared.
TABLE 1.
Gene and protein identities of φSG-JL2 with φYeO3-12, φT3, and other bacteriophages
| Gene | Range | Length (aa)a | Mass (kDa) | pI | Ribosome binding site and initiation codonb | Identity (%)
|
Function | |
|---|---|---|---|---|---|---|---|---|
| φYeO3-12 | φT3 | |||||||
| 0.3 | 1050-1505 | 152 | 17.01 | 6.82 | GAGGTaacaccaaAUG | 99.3 | 98.0 | S-Adenosyl-l-methionine hydrolase |
| 0.3B | 1131-1505 | 125 | 13.90 | 8.30 | GAGGTGaacAUG | 98.4 | 97.6 | |
| 0.45 | 1730-1927 | 66 | 7.45 | 6.80 | AGGActaacaccAUG | 98.5 | ||
| 0.6A | 2105-2341 | 79 | 9.30 | 10.63 | GGTGaaacacgcAUG | 64.6 | 74.7 | |
| 0.6B | 2105-2498 | 131 | 15.31 | 10.99 | GGTGaaacacgcAUG | 77.9 | ||
| 0.7 | 2516-3622 | 369 | 42.45 | 8.06 | AGGAcactgaacgAUG | 87.3 | 91.9 | Protein kinase |
| 1 | 3696-6347 | 884 | 98.80 | 7.32 | GAGGTaagcaAUG | 99.2 | 99.0 | RNAP |
| 1.05 | 6449-6955 | 169 | 19.5 | 9.26 | GAGgtttactttAUG | 17.8 | 18.3 | Gene 1.05 protein |
| 1.1 | 7051-7188 | 46 | 5.90 | 10.93 | GAGGtaagatactAUG | 100 | 97.8 | |
| 1.2 | 7191-7466 | 92 | 10.60 | 7.94 | GGAGtggaactaAUG | 98.9 | 94.6 | dGTP triphosphohydrolase inhibitor |
| 1.3 | 7564-8577 | 338 | 38.37 | 4.98 | GAGGaacaaccgtAUG | 90.5 | 93.2 | DNA ligase |
| 1.5 | 8658-8732 | 25 | 2.82 | 3.32 | AGGAGacacaccAUG | 92.0 | 96.0 | |
| 1.6 | 8748-9002 | 85 | 9.83 | 11.18 | TAAGGAGacaacatcAUG | 98.8 | 97.6 | |
| 1.7 | 9005-9493 | 163 | 18.53 | 9.04 | TAAGGAGGTtctgtaAUG | 77.9 | 91.4 | Gene 1.7 protein |
| 1.8 | 9483-9626 | 48 | 5.51 | 4.77 | AGGggctgtgctAUG | 89.6 | 89.6 | |
| 2 | 9616-9777 | 54 | 6.26 | 4.67 | TAAGGAGGctcaaaGTG | 65.4 | 94.4 | Host RNAP inhibitor |
| 2.5 | 9833-10528 | 232 | 25.95 | 4.83 | AAGGAGaaacattAUG | 99.1 | 98.7 | Single-stranded DNA-binding protein |
| 3 | 10531-10989 | 153 | 17.64 | 9.48 | GAGGacttctaAUG | 100 | 92.8 | Endonuclease |
| 3.5 | 10985-11437 | 151 | 16.93 | 8.79 | AAGGAGtaaagaaaaAUG | 98.0 | 96.0 | Amidase (lysozyme) |
| 3.7 | 11445-11549 | 35 | 4.16 | 8.47 | GAGGgtgataccAUG | 100 | 97.1 | |
| 4A | 11619-13316 | 566 | 62.90 | 5.13 | AAGGAatgtacaAUG | 95.2 | 99.3 | DNA primase/helicase |
| 4B | 11805-13316 | 504 | 55.89 | 5.11 | AGGAGGcagcaagcctAUG | 98.8 | 99.2 | DNA helicase |
| 4.15 | 11885-11989 | 35 | 3.78 | 9.49 | AGGAGacAUG | 94.3 | ||
| 4.3 | 13416-13625 | 70 | 7.73 | 10.00 | AGGAGacacatcAUG | 100 | 97.1 | |
| 4.5 | 13641-13922 | 94 | 10.75 | 9.89 | TAAGGAGcgcacactAUG | 100 | 96.8 | |
| 5 | 13993-16104 | 704 | 79.80 | 6.42 | AAGGAGGgcattAUG | 97.4 | 87.4 | DNA polymerase |
| 5.5 | 16124-16426 | 101 | 11.22 | 6.83 | TAGGAGaaacattAUG | 100 | 52.5 | Growth on lambda lysogen? |
| 5.5-5.7 | 16124-16632 | 170 | 18.46 | 9.25 | GGAGaaacattAUG | 100 | 35.3 | H-NS inhibitor? |
| 5.7 | 16426-16632 | 69 | 7.26 | 9.81 | GAGGTGttcaaAUG | 100 | 88.4 | |
| 5.9 | 16632-16811 | 60 | 6.77 | 4.04 | GGAGGTtgcgtAUG | 98.3 | 21.7 | RecBCD nuclease inhibitor |
| 6 | 16811-17719 | 303 | 34.73 | 4.88 | GGAGGatgacgaAUG | 98.0 | 78.2 | Exonuclease |
| 6.1 | 16848-17075 | 76 | 9.16 | 11.71 | GGAGatgcGUG | 97.4 | ||
| 6.3 | 17704-17814 | 37 | 4.15 | 9.69 | AAGGAGatttacttAUG | 100 | 97.3 | |
| 6.5 | 17910-18152 | 81 | 9.34 | 5.88 | GAGGTGAatttAUG | 98.8 | 100 | |
| 6.7 | 18160-18408 | 83 | 8.85 | 9.13 | AGGAGtaacgatAUG | 98.8 | 100 | Excreted head protein |
| 7.3 | 18439-18756 | 106 | 10.96 | 9.78 | GGAGaaacatcAUG | 97.2 | 94.3 | Tail protein (host specificity) |
| 8 | 18770-20374 | 535 | 58.67 | 4.54 | AGGAGGactgaAUG | 99.6 | 98.7 | Head-to-tail joining protein |
| 9 | 20479-21408 | 310 | 33.71 | 4.29 | AGGAGatttaacaAUG | 99.0 | 95.2 | Capsid assembly protein |
| 10A | 21568-22611 | 348 | 36.90 | 6.46 | TAAGGAGattcaacAUG | 98.3 | 97.7 | Major capsid protein 10A |
| 10B | 21568-22745 | 393 | 41.78 | 6.47 | TAAGGAGattcaacAUG | 82.7 | 82.2 | Minor capsid protein 10B |
| 11 | 22830-23417 | 196 | 22.22 | 4.48 | AGGAGGTaacatcAUG | 99.5 | 99.5 | Tail tubular protein A |
| 12 | 23436-25838 | 801 | 89.99 | 6.20 | AAGGAGGctctAUG | 98.4 | 97.6 | Tail tubular protein B |
| 13 | 25914-26321 | 136 | 15.82 | 5.58 | GGTtaaagcattAUG | 97.1 | 96.3 | Internal virion protein A |
| 14 | 26327-26917 | 197 | 21.31 | 9.21 | AGGAGGtaactAUG | 99.5 | 98.0 | Internal virion protein B |
| 15 | 26923-29163 | 747 | 85.12 | 5.47 | GGAGGTaataAUG | 98.9 | 69.5 | Internal virion protein C |
| 16 | 29185-33144 | 1320 | 143.71 | 8.26 | TAAGGAGGctccAUG | 98.7 | 66.7 | Internal virion protein D |
| 17 | 33219-35192 | 658 | 70.23 | 6.03 | AAGGAGGTcacAUG | 31.9 | 33.8 | Tail fiber protein |
| 17.5 | 35206-35406 | 67 | 7.28 | 6.16 | AGGAGGacataAUG | 91.0 | 86.6 | Lysis protein (holin) |
| 18 | 35413-35676 | 88 | 9.89 | 4.70 | TAAGGAGtaacctAUG | 98.9 | 72.7 | DNA-packaging protein A |
| 18.5 | 35769-36218 | 150 | 16.95 | 9.41 | GGAGGTGttAUG | 98.7 | 52.7 | Endopeptidase; lambda Rz homolog |
| 18.7 | 35884-36135 | 84 | 9.37 | 9.78 | AAGGAGGTaatccaaaAUG | 98.8 | 47.6 | Lambda Rz1 homolog |
| 19 | 36196-37959 | 587 | 66.73 | 5.32 | TAAGGAGatgcagaAUG | 99.7 | 97.6 | DNA-packaging protein B |
| 19.2 | 36845-37075 | 77 | 8.32 | 10.88 | AAGGAactcgaagataaccGUG | 100 | 81.8 | |
| 19.3 | 37382-37507 | 42 | 4.75 | 11.88 | GGTtccgcgAUG | 100 | 95.2 | |
| 19.5 | 38203-38349 | 49 | 5.44 | 7.87 | AAGGAGGTGgctcaAUG | 98.0 | 95.9 | |
aa, amino acids.
Lowercase letters indicate spacer nucleotides.
Regulatory elements of φSG-JL2.
Three major early promoters (A1, positions 460 to 489; A2, 589 to 618; and A3, 700 to 728) and a minor leftward promoter, A0 (142 to 116), for host RNAP were identified in the noncoding region near the left end of the φSG-JL2 genome. The nucleotide sequences of host promoters were exactly the same as those of φYeO3-12 and T3 (A1) or identical only to φYeO3-12 (A0) or T3 (A2 and A3).
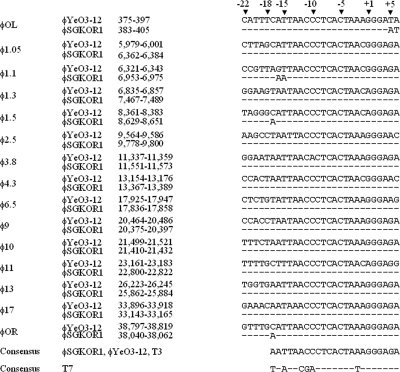
Altogether, 15 putative φSG-JL2 promoters were identified in the phage genome (Fig. 1 and 2), and most of them were similar in position and sequence to those of φYeO3-12 with slight nucleotide changes (φOL, φ1.1, φ1.5, and φOR). The consensus promoter sequence of φSG-JL2 is exactly the same as those of φYeO3-12 and T3 but is apparently different from that of T7. The T7 promoter (−17 to +6) has three distinct elements: RNAP binding (−17 to −6), promoter opening (−4 to −1), and initiation and elongation sites (+1 to +6) (2, 7-10, 19, 23, 25, 38, 56, 59, 77). Mutations in the RNAP binding site decrease the affinity of RNAP and bases in the region interacting with amino acid residues of RNAP (11, 12). The residues 93 to 101 and 739 to 770 contacted the −17 to −13 and −11 to −7 regions of the T7 RNA promoter, respectively. Comparison of amino acid residues of φSG-JL2 in the regions revealed 100% (739 to 770) or high (93 to 101) similarity to those of φYeO3-12 and T3 but apparent differences from those of T7. The transcription efficiency of T7 RNAP can apparently be decreased by mutations (A to C at −10 or C to A at −12) and even abrogated by a G-to-C mutation at −11 in the T7 promoter (25). Therefore, high nucleotide variations between φSG-JL2/φYeO3-12/T3 and T7 in the −17 to −7 region of the consensus sequences may be the result of coevolution of the RNAP and promoter, which results in phage-specific promoter recognition (Fig. 1). The consensus sequence of the promoter opening site of φSG-JL2/φYeO3-12/T3 is similar to that of T7 (TAAA versus TATA). The selection of the transcription start site in the T7 promoter is determined by H784 of T7 RNAP (7), and the presence of H785 and similar amino acid residues around it in RNAPs of φSG-JL2, φYeO3-12, and T3 may be related to identical consensus sequences of the initiation and elongation sites between φSG-JL2/φYeO3-12/T3 and T7. The stronger activities of the class III T7 promoters are linked to an A+T-rich region without interruption of G or C nucleotides between −22 and −18. It increases the affinity of the T7 RNAP (74), but there were no such evident differences between class II and class III promoters of φSG-JL2, φYeO3-12, and T3 (Fig. 1). The A+T-rich recognition loop of T7 RNAP consists of amino acid residues from 93 to 101, and K93 and K95 are suspected to interact with the A+T-rich region (74). The RNAPs of φSG-JL2, φYe-O3-12, and T3 have the same (K95) and different (A93) residues; therefore, they may recognize class II and class III promoters differently than does T7 RNAP.
FIG. 1.
Comparison of φSG-JL2 and φYe-O3-12 promoters. The 15 putative promoter sequences of φSG-JL2 are aligned with those of φYeO3-12. The positions of the first nucleotides of the promoter sequences in the phage genome are given. Homologous nucleotides are represented by dashes.
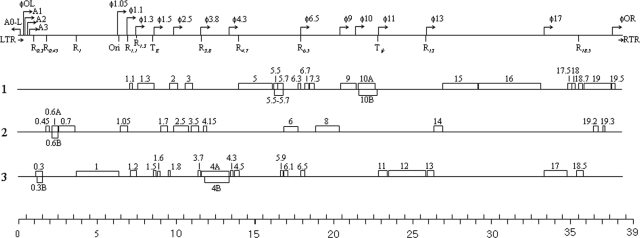
FIG. 2.
Putative genome organization of φSG-JL2. The locations of putative regulatory elements, host (A0 to A3) and phage (φL to φR) promoters, RNase III recognition sites (R0.3 to R18.5), terminators (TE and Tφ), and replication origin (Ori) are represented at the top, and the predicted ORFs are numbered and arranged according to reading frame (1, 2, and 3). A point on the scale represents 0.5 kb.
The CJ (concatemer junction) terminator (5′-ATCTGTT-3′) was located just after the LTR (231 to 237) and was conserved among T7-like viruses. A putative rho-independent early transcriptional terminator, TE, for the host RNAP has been identified at positions 8,591 to 8,612, and the stem-loop structure (ΔG = −14.9 kcal/mol) and following U tract (UUUCUU) are identical to those of T3 (50, 51). The TE of φSG-JL2 is located immediately downstream of gene 1.3, as are those of φYeO3-12, T3, and T7 (19, 50, 51). A putative major terminator, Tφ, has been identified just downstream of gene 10 at positions 22772 to 22792, and the stem-loop structure (ΔG = −6.4 kcal/mol) and following U tract (UUUUUU) are similar to those of φYeO3-12 (50, 51).
T3 and T7 RNAs are cleaved by the host enzyme RNase III at specific sites that form a stem-loop structure. Overall, 10 putative RNase III sites analogous in their positions and sequences to those of φYeO3-12 and T3 phages have been identified in φSG-JL2 (50, 51); the sequences and the free energies are summarized in Table 2. R0.3, R3.8, and R4.7 are identical to those of φYeO3-12 and T3, and R13 is identical only to that of φYeO3-12. The nucleotide sequences of R0.45, R1.3, and R18.5 are relatively variable among the compared phages.
TABLE 2.
Comparison of predicted RNase III sites of bacteriophages φYeO3-12 and φSG-JL2
| Name of putative RNase III site | Range | ΔG (kcal/mol) | Sequence of predicted stem-loopa |
|---|---|---|---|
| φYeO3-12 R0.3 | 956-1009 | −17.6 | UAAGCGAAUAACUCAAGGUCGCACUGAAAGCGUGGCCUUUAU/GAUAUUCACUUA |
| T3 R0.3 | 822-875 | −17.6 | —————————————————————————————————————/——————————— |
| φSG-JL2 R0.3 | 969-1022 | −17.6 | —————————————————————————————————————/—————————— |
| φYeO3-12 R0.45 | 1490-1545 | −21.5 | GUAAGUGUUAAACUCAAGGUCGCUCCAUGCGAGUGGCCUUUAU/GAUUAUCACUUAU |
| T3 R0.5 | 1359-1408 | −20.8 | ...————————————————AU——A—G————..————————/—————A——.——.. |
| φSG-JL2 R0.45 | 1662-1717 | −26.5 | ——————A——G———————————A—UG—AU—U—————————/————————————— |
| φYeO3-12 R1 | 3245-3293 | −20.7 | GAGUCUUUUCUUACAGGUCAUCAUGUGGUGGCCUGAAU/AGGAACGAUUU |
| T3 R1 | 2908-2956 | −20.5 | —————————————————————————A———————/——————————— |
| φSG-JL2 R1 | 3628-3676 | −20.9 | ——————A—————————————————AUC——————/————U—————— |
| φYeO3-12 R1.1 | 6340-6391 | −20.4 | GAGAGUUAAACUUAAGGUCAUCACCGACGGUGGCCUUUGU/GAUUAACUUUC |
| T3 R1.1 | 6003-6053 | −20.4 | ——————————————————————A————————————/—————————— |
| φSG-JL2 R1.1 | 6972-7022 | −20.2 | ———————GC——C———————————————————————/—————————— |
| φYeO3-12 R1.3 | 6856-6896 | −18.2 | GAAUCCU/UAAGGUCACUU AACAUGAGUGGCCUUUGU/GAUUC |
| T3 R1.3 | 6519-6558 | −16.9 | ——————/——————————U————..——————C——/————— |
| φSG-JL2 R1.3 | 7488-7527 | −16.3 | —————/————————UC——U———UG————————-/——-——— |
| φYeO3-12 R3.8 | 11350-11377 | −14.9 | UAAAGGGAGACUUAACGGUUUCCCUUUG |
| T3 R3.8 | 10616-10642 | −13.7 | ———————————————.————————— |
| φSG-JL2 R3.8 | 11564-11591 | −14.9 | ————————————————————————— |
| φYeO3-12 R4.7 | 13706-13754 | −21.7 | AAGUGAUAAACUCAAGGUCGCCCAAGGGUGGCCUUUAU/GAUUAUCAUUU |
| T3 R4.7 | 12970-13018 | −21.7 | ————————————————————————————————-—/———————--- |
| φSG-JL2 R4.7 | 13920-13968 | −21.7 | —————————————————————————————-———-/———------- |
| φYeO3-12 R6.5 | 17901-17971 | −25.9 | AAGUGAUAAACUCAAGGCUCUCUGUA UUAACCCUCACUAAAGGGAAGAGGGAGCCUUUAU/GAUUAUUACUU |
| T3 R6.5 | 17135-17206 | −26.3 | —————————————————————AC—A——————————————————————————————/———-—————— |
| φSG-JL2 R6.5 | 17812-17882 | −24.8 | ————————C———————————————————————————-————————————————/———-—————— |
| φYeO3-12 R13 | 26211-26248 | −21.0 | GUCUCCCUGUGGUGAAUUAACCCUCACUAAAGGGAGAC |
| T3 R13 | 25443-25480 | −19.9 | ————————C————————————————————————— |
| φSG-JL2 R13 | 25848-25885 | −21.0 | —————————————————————————————————— |
| φYeO3-12 R18.5 | 36435-36488 | −23.4 | UAAGUGACAUACUCAAGGUUCUCCACUCGGGGGAGCCUUUAU/GGAUGUUAUUUG |
| T3 R18.5 | 35035-35085 | −23.6 | AC————U——G———————.————.UA—.——UG——————/———————C——GU |
| φSG-JL2 R18.5 | 35677-35729 | −23.5 | —————————————————————————————.——————/———————————— |
Slashes indicate cleavage sites; periods indicate deletions; homologous nucleotides are represented by dashes.
Origins of DNA replication.
The T7 primary replication origin is located between the noncoding regions of gene 1 and gene 1.1 and is characterized by two phage promoters (φ1.1A and φ1.1B), a highly A+T-rich region, and a primase site (T7 type; 5′-GACCC-3′) that can initiate rightward leading-strand synthesis (61). The primary replication origins of φYeO3-12 and T3 have been mapped downstream of gene 1 overlapping the 5′ end of gene 1.05 (50, 62), and they include a phage promoter (φ1.05), a putative stem-loop sequence (5′-GGGAGACtacttaagGTCTCCC-3′; lowercase letters indicate loop-forming nucleotides), and an A+T-rich region containing a primase site (T3 type; 5′-GACAC-3′) near the stem-loop sequence (50, 62). φSG-JL2 had a 12-nucleotide deletion just after the stem-loop sequence that resulted in the loss of the primase site in the A+T-rich region (78.3%; 6401 to 6460). The first T7-type primase site appeared downstream (6610 to 6614) of the A+T-rich region. The primase-helicase of T7 binds randomly to single-stranded DNA and then translocates in a 5′-to-3′ direction until it reaches the priming signal (61). Thus, the putative primary replication origin of φSG-JL2 DNA (R) (Fig. 2) could be tentatively placed at positions 6363 to 6614 between genes 1 and 1.05, slightly different from those of φYeO3-12, T3, and T7. The T7 φOL and φOR promoters are proposed to be secondary origins of replication (19). Counterparts to both promoters were found in the φSG-JL2 genome; they contained A+T-rich regions (334 to 338) and primase sites (38111 to 38115).
Genome ends of φSG-JL2.
The left-end noncoding region of the φSG-JL2 genome contains the LTR; the CJ (231 to 237) terminator that is sensitive to lysozyme-mediated RNAP instability; repeats of short sequences (16 repeats of CCTAAAG and single-nucleotide variants); an A+T-rich region (361 to 390; 68.5%) that contains the φL replication origin; the A1, A2, and A3 promoters for host RNAP; the R0.3 RNase III cleavage site; and the start of the coding sequence of gene 0.3 (19, 50). The right end of φSG-JL2 DNA contains the RTR, repeats of short sequences similar to those found near the left end (12 repeats of CCTAAAG and single-nucleotide variants); the coding sequence of gene 19.5; an A+T-rich region (38008 to 38167; 65%) that contains the φR replication origin; and the end of the coding sequence of gene 19. The 230-bp terminal repeats (LTR and RTR) are 94.4% and 91.4% identical, respectively, to those of φYeO3-12 and T3, and the length is similar to those of T3 (231 bp) and φYe-O3-12 (232 bp) (50, 51).
Other features of the nucleotide sequence.
Restriction and modification (R-M) of foreign DNA by bacteria is a basic defense mechanism, and phages have evolved to evade the host R-M system. Genome analysis of φYeO3-12 has revealed the markedly less frequent methylation of GATC and CC(A/T)GG by DNA cytosine methyltransferase (Dcm) and DNA adenine methyltransferase (Dam), respectively (42, 50). Furthermore, the φYeO3-12 and T3 genomes are not methylated because S-adenosyl-l-methionine hydrolase (SAMase) degrades the methyl group donor in the host and because almost all recognition sites are present downstream of gene 0.3, encoding SAMase (Table 3) (19, 70). Just like φYeO3-12, Dam and Dcm recognition sites were found to be infrequent in the φSG-JL2 genome (Table 3), but one Dam site was located upstream of the 0.3 gene. Considering the high processing activity of Dam, the recognition site can be methylated before SAMase translation, but the low copy number and localized presence of Dam in the replication site of bacterial genomic DNA may explain the normal replication of φSG-JL2 in serovar Gallinarum biovar Gallinarum (78).
TABLE 3.
Frequencies of restriction enzyme and methylase recognition sites in the genomes of φSG-JL2, φYeO3-12. and T3
| R-M system/family | Enzyme | Recognition sequence | No. of recognition sites (location of first recognition site)
|
|||
|---|---|---|---|---|---|---|
| φSG-JL2 (1050-1505)a | φYeO3-12 (1035-1490) | T3 (901-1359) | T7 (925-1276) | |||
| Methylase | Dcm | CC(A/T)GG | 0 | 0 | 2 (19053) | 2 (2366) |
| Methylase | Dam | GATC | 5 (921) | 3 (7382) | 10 (2380) | 6 (8312) |
| Type I/A | EcoKI | AAC(N)6GTGC | 7 (3383) | 5 (3110) | 4 (5490) | 4 (15161) |
| Type I/A | StySBI | GAG(N)6RTAYG | 1 (23718) | 2 (7562) | 2 (3295) | 7 (1491) |
| Type I/A | StySPI | AAC(N)6GTRC | 8 (3383) | 9 (3000) | 7 (2663) | 9 (6356) |
| Type I/B | EcoAI | GAG(N)7GTCA | 6 (9106) | 5 (8232) | 5 (8162) | 0 |
| Type I/B | StySKI | CGAT(N)7GTTA | 2 (3953) | 2 (3570) | 1 (3233) | 0 |
| Type I/D | StySBLI | CGA(N)6TACC | 3 (10375) | 6 (5091) | 7 (4754) | 13 (2107) |
| Type III | EcoP15I | CAGCAG/CTGCTG | 4 (9724)/40 | 4 (8543)/38 | 5 (1938)/47 | 0/36 |
| Type III | StyLTI | CAGAG/CTCTG | 11 (14056)/78 | 13 (12520)/72 | 14 (4795)/63 | 15 (2287)/72 |
Location of gp0.3 (SAMase).
The T3 and T7 genomes were also resistant to the type I restriction enzyme EcoKI (28, 29, 72, 73) because of SAMases and the downstream locations of the first recognition sequences, 5490 to 5502 and 15161 to 15173, respectively, from gene 3 (Table 3). Four Salmonella type 1 restriction enzymes (StySBI, StySPI, StySKI, and StySBLI) have been identified in S. enterica serovar Typhimurium, S. enterica serovar Potsdam, S. enterica serovar Kaduna, and S. enterica serovar Blegdam, respectively (46, 75, 76). The StySBI and StySBLI sites in the φSG-JL2 genome occur only once and three times, respectively, and are distant from gene 0.3, at positions 23718 and 10375, respectively. Only the frequencies and locations of StySBI and StySBLI recognition sites in φYeO3-12, T3, and T7 apparently contrasted with those of φSG-JL2 (Table 3).
A type III R-M enzyme, EcoP15, methylates the second adenine of the CAGCAG sequence but recognizes two CAGCAG sequences in the inverse orientation for restriction (44). The resistance of T7 and susceptibility of T3 to EcoP15 restriction can be explained by the absence and multiple presence of the inverted sequences, respectively (Table 3) (63). StyLTI is a type III R-M enzyme and is encoded by chromosomal genes of S. enterica serovar Typhimurium LT7 (16). The enzyme recognizes the sequence CAGAG and methylates the second adenine in one strand, but whether it requires two inverse recognition sites is unclear. The frequencies of CAGAG and CTCTG sequences were different (11 versus 79, respectively) in the φSG-JL2 genome, and CAGAG appeared first far downstream (position 14056) from the 0.3 gene (Table 3). Therefore, the strand bias of CAGAG may support the hypothesis that StyLTI recognizes two inverted recognition sites, just as EcoP15 does, but further study is required to understand the biological meaning of the location bias of CAGAG in the φSG-JL2 genome.
The genomic nucleotide sequence of φSG-JL2 was compared with those of φYeO3-12 and T3. Synteny plots revealed that, similar to φYeO3-12, the genome sequence of φSG-JL2 was dissimilar to that of T3 in two distinct regions, genes 5 to 6.1 and genes 15 to 18.7 (data not shown).
Translational features of φSG-JL2.
Just like those of other T7-like viruses, the φSG-JL2 genome is highly packaged and the coding region covers 90% of the genome, which is slightly lower than T3 (91%) and φYeO3-12 (92%) (47). The gene content of φSG-JL2 was found to be similar to those of φYeO3-12 and T3, and the identities of the putative φSG-JL2 proteins ranged from 17.8% to 100% compared to those of φYeO3-12 and T3 (Table 1).
The initiation codon for gp2, gp6.1, and gp19.2 was GUG, but all other genes started with AUG. The preferred stop codons were UAA (69.1%) and UGA (29.1%). It has been shown that all predicted genes are preceded by a potential Shine-Dalgarno sequence of 3 to 10 nucleotides capable of uninterrupted pairing with nucleotides near the 3′ end of the 16S rRNA (3′-AUUCCUCCACUAG) (62, 66). The use of GCU (alanine) as the second codon in highly expressed genes of T7 and φYeO3-12 is also observed in comparable genes of φSG-JL2 (50).
The ribosomal +1 and −1 frameshifts during the translation of genes 0.6A, 5.5, and 10A in T7 generate gp0.6B, gp5.5 to -5.7, and gp10B (13, 18). The nucleotide sequences of the 0.6A and 5.5 frameshifting regions of φSG-JL2 were observed to be exactly the same as those of φYeO3-12 but were different from those of T7. Although the experimental data on frameshifts in 0.6A and 5.5 of φYeO3-12 are unavailable, the putative gp0.6B and gp5.5 to -5.7 of φSG-JL2 are listed in Table 1. In T7 and T3, overlapping valine-phenylalanine and proline-lysine codons by −1 frame, respectively, near the stop codon of 10A render base pairing of corresponding tRNAs with −1 frame codons, and the hypothetical pseudoknots may enhance the ribosomal frameshifting (13-15). As observed with T3 and φYeO3-12, φSG-JL2 shared the same 10A motif for frameshifting.
Homing nuclease is contained in a group I intron and functions in site-specific gene conversion of the group I intron by catalyzing double-strand breaks in the recipient target site (18). Relative to φSG-JL2, φYeO3-12 acquired genes 1.45, 4.2, 5B, 5.3, and 13.5, and among them, genes 1.45, 5.3, and 13.5 represent putative group I introns or homing endonucleases grouped into the ββα-Me family (31, 50, 51). The homing endonucleases are common in other T7-like viruses, such as T3, T7, φYeO3-12, φA1122, φgh-1, φVpV262, and φKMV, but the copy numbers vary from one to four (20, 21, 26, 36, 50, 51). The origins of homing endonucleases are unclear, but the lack of known homing endonuclease homologs in the φSG-JL2 genome reflects the relatively low rate of genetic exchanges with genetic pools containing homing endonucleases during its evolution.
Holins are grouped into two classes on the basis of the number of transmembrane domains. Class I holins have three transmembrane domains, and class II holins have two transmembrane domains (80). The holins of φYeO3-12, T3, and T7 are predicted to have two transmembrane domains and so represent class II holins (49, 80), but our present analysis using version 2.0 of the TMHMM program (27) revealed only one transmembrane domain in the holins of φSG-JL2, φYeO3-12, T3, and T7 and charged N termini and C termini in the periplasm and cytosol, respectively. In view of the transmembrane domains of other class I (λS and Hol500) and class II (λ21S and HolTW) holins (39, 40, 80) accurately predicted by the program and the presence of dozens of holins containing a single transmembrane domain in GenBank (accession no. NP_795652, YP_238508, AAM83087, YP_001333670, CAC17008, BAD51461, NP_813783, AAD04658, NP_043494, NP_536830, AAQ75055, CAK25980, YP_001522836, YP_655476, CAA81341, YP_001468955, NP_839939, YP_399007, NP_853599, NP_700424, YP_003932, NP_803401, ABF72775, NP_795484, NP_795705, NP_268941, ABF31779, ABF33660, YP_001430016, CAB52539, YP_025044, AAP42307, YP_001671761, CAC48115, NP_835573, YP_908848, YP_001469228, NP_061647, YP_803187, NP_891825, and AAX11974), assignment of a new class to the holins of T7-like phages should be considered.
Identification of proteins involved in host adaptation of φSG-JL2.
Among the proteins with known functions, nonstructural proteins (gp0.3, gp0.7, gp1, gp1.2, gp1.3, gp2, gp2.5, gp3.5, gp4A, gp4B, gp5, and gp6) and the host specificity-related proteins gp17 (tail fiber) and gp7.3 (tail protein) were targeted for polymorphism analyses among φSG-JL2, φYeO3-12, and T3. We computed the Pi[a]/Pi[s] ratios of the target genes with the DnaSP program (window length, 50; sliding size, 10). The Pi[a]/Pi[s] ratios of genes 0.3, 1, 2.5, 3.5, 4B, and 7.3 ranged from 0.033 to 0.059, but those of genes 0.7, 1.2, 1.3, 2, 4A, 5, and 6 ranged from 0.094 to 1.264. The Pi[a]/Pi[s] ratio of gene 2 exceeded 1, indicative of positive Darwinian selection (Table 4). gp2 of T7 is reported to inhibit host RNAP by interaction with a dispensable region of the β′ subunit, and mutants carrying an E1158K or E1188K mutation in rpoC are resistant to T7 (47). T3 productively infected a mutant carrying an E1188K mutation; therefore, gp2 of T3 may interact with a different site of host RNAP from gp2 of T7 (8, 47). The Ka/Ks ratios of rpoA, rpoB, and rpoC of E. coli, Yersinia spp. (Yersinia pestis, Y. enterocolitica, and Yersinia pseudotuberculosis), and Salmonella serotypes (S. enterica serovar Typhimurium, S. enterica serovar Paratyphi A, S. enterica serovar Paratyphi B, and S. enterica serovar Typhi) ranged from 0.007 to 0.015, 0.028 to 0.040, and 0.035 to 0.072, respectively, but only rpoC contained one to three variable regions whose Ka/Ks ratios exceeded 1 (Salmonella serotypes versus E. coli, 1,870 to 1,884, 2,140 to 2,157, and 3,472 to 3,492 nucleotides; Salmonella serotypes versus Yersinia spp., 1,651 to 1,700 and 1,661 to 1,710 nucleotides; Yersinia spp. versus E. coli, 1,684 to 1,704 and 1,945 to 1,965 nucleotides). Glutamic acids at positions 1158 and 1188 were conserved among E. coli, the Salmonella serotypes, and the Yersinia spp. compared. Therefore, the gp2 proteins of φSG-JL2, φYeO3-12, and T3 are likely to interact with different regions of the β′ subunit of host RNAP.
TABLE 4.
Pi[a]/Pi[s] ratios of φSG-JL2, φYeO3-12, and T3 genes
| Gene | Protein | Mean Pi[a]/Pi[s] ratioa | Variable region (Pi[a]/Pi[s] ratio)b |
|---|---|---|---|
| 0.7 | Protein kinase | 0.155 | 757-777 (1.007) |
| 2 | Host RNA polymerase inhibitor | 1.264 | 1-93 (1.724), 81-101 (1.685), 90-110 (4.126) |
| 6 | Exonuclease | 0.241 | 337-357 (1.738), 364-384 (1.000), 373-393 (2.828) |
Window length, 50; sliding size, 10.
Region whose Pi[a]/Pi[s] ratio is more than 1.000 (window length, 21; sliding size, 9).
The genes 0.7 and 6 possessed local polymorphic regions whose Pi[a]/Pi[s] ratios exceeded 1 (Table 4). The reasons for and functions of local polymorphisms of the proteins are unclear, but they can be explained in part by evolution for optimal interaction with host proteins. gp0.7 is a serine/threonine protein kinase and phosphorylates translational components (IF1, IF2, IF3, elongation factor G, and ribosomal proteins S1 and S6), host RNAP β′ subunit, and enzymes related to mRNA metabolism (RNase III and RNase E), resulting in exclusive phage gene expression (41, 43, 48, 55, 57, 58, 65, 81). gp6 is an exonuclease and contributes to the packaging of concatemerized phage DNA by suppressing the packaging of host DNA (69). To date, interaction of gp6 with host proteins has been unknown; therefore, the reasons why gp6 possesses polymorphic regions need to be resolved.
gp17 is a tail fiber protein that attaches to a host receptor and determines host specificity. The conserved N terminus of T7 gp17 interacts with a head-tail connector protein, and the hypervariable C terminus interacts with host receptor (71). The amino acid similarities of gp17 proteins among the compared phages are only 30.1% to 33.8%.
During the early phase of the evolution of an organism, horizontal genetic transfer may play a key role, but when it crosses the “Darwinian threshold,” vertical genetic changes become more important (26, 79). T7 group phages have been proposed to be descendants of an ancient species that crossed the “Darwinian threshold” because of severely limited horizontal genetic exchange and conservation of essential genes and their layout (26). The comparison of closely related phages, φSG-JL2, φYeO3-12, and T3, in the present study also revealed conservation of essential genes and their layout but the presence of species-specific genes, especially gene 2, that may play key roles during host adaptation (47). Therefore, φSG-JL2 and variable genes identified in the present study may be useful for understanding vertical evolution of a phage during its adaptation to a specific host.
Prophylactic efficacy of φSG-JL2 against fowl typhoid in chickens.
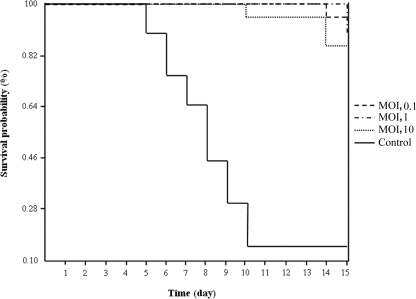
Serovar Gallinarum biovar Gallinarum is an intracellular pathogen, and the therapeutic application of φSG-JL2 against fowl typhoid may be limited. However, the clear lysis of a broad range of serovar Gallinarum biovar Gallinarum strains and obligate lytic infection of φSG-JL2 may still be valuable properties for prophylactic application to fowl typhoid control. Fowl typhoid can be reproduced more easily and consistently by intratracheal challenge with serovar Gallinarum biovar Gallinarum than by oral challenge (4). Therefore, we applied the respiratory model system to test the prophylactic efficacy of φSG-JL2 against fowl typhoid. The untreated control group showed 85% (17/20) mortality, but the groups that were treated with φSG-JL2 at different MOIs (0.1, 1, and 10) showed 5%, 10%, and 15% mortality, respectively (Fig. 3). The survival curves were significantly different between untreated and treated groups (P < 0.05). The protection rates of the untreated group and the groups treated at MOIs of 0.1, 1, and 10 were 10%, 70%, 80%, and 65%, respectively, and the differences between untreated and treated groups were significant (P < 0.05). The protection rates of the treated groups were not significantly different from each other (P > 0.05).
FIG. 3.
Survival curves of φSG-JL2-treated and untreated groups. Eighty 13-day-old commercial male brown layer chicks were challenged with a field strain of serovar Gallinarum biovar Gallinarum (SG101) directly or after it was mixed with φSG-JL2 (MOIs, 0.1, 1, and 10) for 4 h at room temperature, and mortality was observed for 15 days.
To date, prophylactic or therapeutic phage therapies against S. enterica serovar Typhimurium, E. coli, and Bacillus anthracis have been reported (5, 30, 64, 68), but phage therapy against serovar Gallinarum biovar Gallinarum has been rare. The high susceptibility of φSG-JL2 to low pH can be a drawback for oral treatment because of gastric acid, but mixing it with acid-neutralizing reagents or directly spraying a phage solution onto chickens, floors, and the environment may improve the prophylactic efficacy of φSG-JL2. To control fowl typhoid, “test and slaughter” of a positive flock has been the best policy, but in countries where fowl typhoid is enzootic, prophylactic application of bacteriophage can be one measure to reduce horizontal transmission of multidrug-resistant serovar Gallinarum biovar Gallinarum between chickens, flocks, or farms. Therefore, further studies to verify the preventive efficacy of φSG-JL2 under various conditions that simulate field conditions may be valuable to minimize economic losses caused by fowl typhoid and antibiotic use.
Acknowledgments
This study was partially supported by Korea Research Foundation grants (KRF-2006-005-J02901 and KRF-2006-005-J02903).
Footnotes
Published ahead of print on 26 September 2008.
REFERENCES
- 1.Al-Hendy, A., P. Toivanen, and M. Skurnik. 1991. Expression cloning of the Yersinia enterocolitica O:3 rfb gene cluster in Escherichia coli K12. Microb. Pathog. 10:47-59. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, J. N., J. F. Klement, and W. T. McAllister. 1983. Relationship between promoter structure and template specificities exhibited by the bacteriophage T3 and T7 RNA polymerases. Proc. Natl. Acad. Sci. USA 80:2814-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow, P. A., M. B. Huggins, and M. A. Lovell. 1994. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect. Immun. 62:4602-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basnet, H. B., H. J. Kwon, S. H. Cho, S. J. Kim, H. S. Yoo, Y. H. Park, S. I. Yoon, N. S. Shin, and H. J. Youn. 2008. Reproduction of fowl typhoid by respiratory challenge with Salmonella Gallinarum. Avian Dis. 52:156-159. [DOI] [PubMed] [Google Scholar]
- 5.Berchieri, A. Jr., M. A. Lovell, and P. A. Barrow. 1991. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res. Microbiol. 142:541-549. [DOI] [PubMed] [Google Scholar]
- 6.Bjellqvist, B., G. J. Hughes, C. H. Pasquali, N. Paquet, F. Ravier, J. C. Sanchez, S. Frutiger, and D. F. Hochstrasser. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14:1023-1031. [DOI] [PubMed] [Google Scholar]
- 7.Brieba, L. G., R. Padilla, and R. Sousa. 2002. Role of T7 RNA polymerase His784 in start site selection and initial transcription. Biochemistry 41:5144-5149. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlin, M. 1974. Isolation and characterization of prototrophic mutants of Escherichia coli unable to support the intracellular growth of T7. J. Virol. 14:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman, K. A., and R. R. Burgess. 1987. Construction of bacteriophage T7 late promoters with point mutations and characterization by in vitro transcription properties. Nucleic Acids Res. 15:5413-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman, K. A., S. I. Gunderson, M. Anello, R. D. Wells, and R. R. Burgess. 1988. Bacteriophage T7 late promoters with point mutations: quantitative footprinting and in vivo expression. Nucleic Acids Res. 16:4511-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheetham, G., D. Jeruzalmi, and T. A. Steitz. 1999. Structural basis for initiation of transcription from an RNA polymerase-promoter complex. Nature 399:80-83. [DOI] [PubMed] [Google Scholar]
- 12.Cheetham, G., and T. A. Steitz. 1999. Structure of a transcribing T7 RNA polymerase initiation complex. Science 286:2305-2309. [DOI] [PubMed] [Google Scholar]
- 13.Condreay, J. P., S. E. Wright, and I. J. Molineux. 1989. Nucleotide sequence and complementation studies of the gene 10 region of bacteriophage T3. J. Mol. Biol. 207:555-561. [DOI] [PubMed] [Google Scholar]
- 14.Condron, B. G., R. F. Gesteland, and J. F. Atkins. 1991. An analysis of sequences stimulating frameshifting in the decoding of gene 10 of bacteriophage T7. Nucleic Acids Res. 19:5607-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayhuff, T. J., J. F. Atkins, and R. F. Gesteland. 1986. Characterization of ribosomal frameshift events by protein sequence analysis. J. Biol. Chem. 261:7491-7500. [PubMed] [Google Scholar]
- 16.De Backer, O., and C. Colson. 1991. Identification of the recognition sequence for the M.StyLTI methyltransferase of Salmonella typhimurium LT7: an asymmetric site typical of type-III enzymes. Gene 97:103-107. [DOI] [PubMed] [Google Scholar]
- 17.Delcher, A. L., A. Phillippy, J. Carlton, and S. L. Salzberg. 2002. Fast algorithms for large-scale genome alignment and comparision. Nucleic Acids Res. 30:2478-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dujon, B. 1989. Group I introns as mobile genetic elements: facts and mechanistic speculations—a review. Gene 82:91-114. [DOI] [PubMed] [Google Scholar]
- 19.Dunn, J. J., and F. W. Studier. 1983. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J. Mol. Biol. 166:477-535. [DOI] [PubMed] [Google Scholar]
- 20.Garcia, E., J. M. Elliott, E. Ramanculov, P. S. G. Chain, M. C. Chu, and I. J. Mollineux. 2003. The genome sequence of Yersinia pestis bacteriophage φA1122 reveals an intimate history with the coliphage T3 and T7 genomes. J. Bacteriol. 185:5248-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardies, S. C., A. M. Comeau, P. Serwer, and C. A. Suttle. 2003. The complete sequence of marine bacteriophage VpV262 infecting Vibrio parahaemolyticus indicates that an ancestral component of a T7 viral supergroup is widespread in the marine environment. Virology 310:359-371. [DOI] [PubMed] [Google Scholar]
- 22.Hendrix, R. W., M. C. M. Smith, R. N. Burns, M. E. Ford, and G. F. Hatfull. 1999. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc. Natl. Acad. Sci. USA 96:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imbrugio, D., M. Rong, K. Ma, and W. T. McAllister. 2000. Studies of promoter recognition and start site selection by T7 RNA polymerase using a comprehensive collection of promoter variants. Biochemistry 39:10419-10430. [DOI] [PubMed] [Google Scholar]
- 24.Kelly, T. J., Jr., and C. A. Thomas, Jr. 1969. An intermediate in the replication of bacteriophage T7 DNA molecules. J. Mol. Biol. 44:459-475. [DOI] [PubMed] [Google Scholar]
- 25.Klement, J. F., M. B. Moorefield, E. Jorgensen, J. E. Brown, S. Risman, and W. T. McAllister. 1990. Discrimination between bacteriophage T3 and T7 promoters by the T3 and T7 RNA polymerases depends primarily upon a three base-pair region located 10 to 12 base-pairs upstream from the start site. J. Mol. Biol. 215:21-29. [DOI] [PubMed] [Google Scholar]
- 26.Kovalyova, I. V., and A. M. Kropinski. 2003. The complete genomic sequence of lytic bacteriophage gh-1 infecting Pseudomonas putida—evidence for close relationship to the T7 group. Virology 311:305-315. [DOI] [PubMed] [Google Scholar]
- 27.Krogh, A., B. Larsson, G. von Heijne, and E. L. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genome. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 28.Krüger, D. H., C. Schroeder, S. Hansen, and H. A. Rosenthal. 1977. Active protection by bacteriophages T3 and T7 against E. coli B- and K-specific restriction of their DNA. Mol. Gen. Genet. 153:99-106. [DOI] [PubMed] [Google Scholar]
- 29.Krüger, D. H., L. S. Chernin, S. Hansen, H. A. Rosenthal, and D. M. Goldfarb. 1978. Protection of foreign DNA against host-controlled restriction in bacterial cells. I. Protection of F′ plasmid DNA by preinfecting with bacteriophages T3 or T7. Mol. Gen. Genet. 159:107-110. [DOI] [PubMed] [Google Scholar]
- 30.Kudva, I. T., S. Jelacic, P. I. Tarr, P. Youderian, and C. J. Hovde. 1999. Biocontrol of Escherichia coli O157 with O157-specific bacteriophages. Appl. Environ. Microbiol. 65:3767-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kühlmann, U. C., G. R. Moore, R. James, C. Kleanthous, and A. M. Hemmings. 1999. Structural parsimony in endonuclease active sites: should the number of homing endonuclease families be redefined? FEBS Lett. 463:1-2. [DOI] [PubMed] [Google Scholar]
- 32.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 33.Kwon, H. J., K. Y. Park, H. S. Yoo, J. Y. Park, Y. H. Park, and S. J. Kim. 2000. Differentiation of Salmonella serotype Gallinarum biotype Pullorum from biotype Gallinarum by analysis of flagellin C gene (fliC). J. Microbiol. Methods 40:33-38. [DOI] [PubMed] [Google Scholar]
- 34.Kwon, H. J., K. Y. Park, S. J. Kim, and H. S. Yoo. 2001. Application of nucleotide sequence of RNA polymerase β-subunit gene (rpoB) to molecular differentiation of serovars of Salmonella enterica subsp. enterica. Vet. Microbiol. 82:121-129. [DOI] [PubMed] [Google Scholar]
- 35.Kwon, H. J., T. E. Kim, S. H. Cho, J. G. Seol, B. J. Kim, J. W. Hyun, K. Y. Park, S. J. Kim, and H. S. Yoo. 2002. Distribution and characterization of class 1 integrons in Salmonella enterica serotype Gallinarum biotype Gallinarum. Vet. Microbiol. 89:303-309. [DOI] [PubMed] [Google Scholar]
- 36.Lavigne, R., M. V. Burkal'tseva, J. Robben, N. N. Sykilinda, L. P. Kurochkina, B. Grymonprez, B. Jonckx, V. N. Krylov, V. V. Mesyanzhinov, and G. Volckaert. 2003. The genome of bacteriophage φKMV, a T7-like infecting Pseudomonas aeruginosa. Virology 312:49-59. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence, J. G., G. F. Hatfull, and R. W. Hendrix. 2002. Imbroglios of viral taxonomy: genetic exchange and failings of phonetic approaches. J. Bacteriol. 184:4891-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, T., H. H. Ho, M. Maslak, C. Schick, and C. T. Martin. 1996. Major groove recognition elements in the middle of the T7 RNA polymerase promoter. Biochemistry 35:3722-3727. [DOI] [PubMed] [Google Scholar]
- 39.Loessner, M. J., G. Wendlinger, and S. Scherer. 1995. Heterogeneous endolysin in Listeria monocytogenes bacteriophages: a new class of enzymes and evidence for conserved holin genes within the siphoviral lysis cassettes. Mol. Microbiol. 16:1231-1241. [DOI] [PubMed] [Google Scholar]
- 40.Loessner, M. J., S. Gaeng, G. Wendlinger, K. S. Maier, and S. Scherer. 1998. The two component lysis system of Staphylococcus aureus bacteriophage Twort: a large TTG-start and an associated amidase endolysin. FEMS Microbiol. Lett. 162:265-274. [DOI] [PubMed] [Google Scholar]
- 41.Marchand, I., A. W. Nicholson, and M. Dreyfus. 2001. Bacteriophage T7 protein kinase phosphorylates RNase E and stabilizes mRNAs synthesized by T7 RNA polymerase. Mol. Microbiol. 42:767-776. [DOI] [PubMed] [Google Scholar]
- 42.Marinus, M. G. 1996. Methylation of DNA, p. 782-791. In F. C. Neidhart, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, vol. I. ASM Press, Washington, DC. [Google Scholar]
- 43.Mayer, J. E., and M. Schweiger. 1983. RNase III is positively regulated by T7 protein kinase. J. Biol. Chem. 258:5340-5343. [PubMed] [Google Scholar]
- 44.Meisel, A., T. A. Bickle, D. H. Krüger, and C. Schroeder. 1992. Type III restriction enzymes need two inversely oriented recognition sites for DNA cleavage. Nature 355:467-469. [DOI] [PubMed] [Google Scholar]
- 45.Mollineux, I. J. 2006. The T7 group, p. 277-301. In R. Calendar (ed.), The bacteriophages. Oxford University Press, New York, NY.
- 46.Nagaraja, V., J. C. W. Shepherd, T. Pripfl, and T. A. Bickle. 1985. Two type I restriction enzymes from Salmonella species: purification and DNA recognition sequences. J. Mol. Biol. 182:579-587. [DOI] [PubMed] [Google Scholar]
- 47.Nechaev, S., and K. Severinov. 1999. Inhibition of E. coli RNA polymerase by bacteriophage T7 gene 2 protein. J. Mol. Biol. 289:815-826. [DOI] [PubMed] [Google Scholar]
- 48.Pai, S. H., H. J. Rahmsdorf, H. Ponta, M. Hirsch-Kauffmann, P. Herrlich, and M. Schweiger. 1975. Protein kinase of bacteriophage T7. 2. Properties, enzyme synthesis in vitro and regulation of enzyme synthesis and activity in vivo. Eur. J. Biochem. 55:305-314. [DOI] [PubMed] [Google Scholar]
- 49.Pajunen, M., S. Kiljunen, and M. Skurnik. 2000. Bacteriophage φYeO3-12, specific for Yersinia enterocolitica serotype O:3, is related to coliphage T3 and T7. J. Bacteriol. 182:5114-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pajunen, M. I., S. J. Kiljunen, M. E. L. Söderholm, and M. Skurnik. 2001. Complete genomic sequence of the lytic bacteriophage φYe-O3-12 of Yersinia enterocolitica serotype O:3. J. Bacteriology. 183:1928-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pajunen, M. I., M. R. Elizondo, M. Skurnik, J. Kieleczawa, and I. J. Molineux. 2002. Complete nucleotide sequence and likely recombinatorial origin of bacteriophage T3. J. Mol. Biol. 319:1115-1132. [DOI] [PubMed] [Google Scholar]
- 52.Park, K. Y., S. U. Lee, H. S. Yoo, and J. K. Yeh. 1996. Epidemiological studies of Salmonella gallinarum infection in Korea: infection routes, biochemical characteristics, antimicrobial drug susceptibility pattern and plasmid profile. Korean J. Infect. Dis. 28:413-421. [Google Scholar]
- 53.Pomeroy, B. S. 1991. Fowl typhoid: diseases of poultry, 9th ed. Iowa State University Press, Ames.
- 54.Prehm, P., B. Jann, K. Jann, G. Schmidt, and S. Stirm. 1976. On a bacteriophage T3 and T4 receptor region within the cell wall lipopolysaccharide of Escherichia coli. J. Mol. Biol. 101:277-281. [DOI] [PubMed] [Google Scholar]
- 55.Rahmsdorf, H. J., S. H. Pai, H. Ponta, P. Herrlich, R. Roskoski, Jr., M. Schweiger, and F. W. Studier. 1974. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc. Natl. Acad. Sci. USA 71:586-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raskin, C. A., G. A. Diaz, K. Joho, and W. T. McAllister. 1993. Hierarchy of base-pair preference in the binding domain of the bacteriophage T7 promoter. J. Mol. Biol. 229:805-811. [DOI] [PubMed] [Google Scholar]
- 57.Robertson, E. S., and A. W. Nicholson. 1990. Protein kinase of bacteriophage T7 induces the phosphorylation of only a small number of proteins in the infected cell. Virology 175:525-534. [DOI] [PubMed] [Google Scholar]
- 58.Robertson, E. S., L. A. Aggison, and A. W. Nicholson. 1994. Phosphorylation of elongation factor G and ribosomal protein S6 in bacteriophage T7-infected Escherichia coli. Mol. Microbiol. 11:1045-1057. [DOI] [PubMed] [Google Scholar]
- 59.Rong, M., B. He, and W. T. McAllister. 1998. Promoter specificity determinants of T7 RNA polymerase. Proc. Natl. Acad. Sci. 95:515-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rozas, J., and R. Rozas. 1999. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15:174-175. [DOI] [PubMed] [Google Scholar]
- 61.Saito, H., S. Tabor, F. Tamanoi, and C. C. Richardson. 1980. Nucleotide sequence of the primary origin of bacteriophage T7 DNA replication: relationship to adjacent genes and regulatory elements. Prog. Natl. Acad. Sci. 77:3917-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmitt, M. P., P. J. Beck, C. A. Kearney, J. L. Spence, D. DiGiovanni, J. P. Condreay, and I. J. Mollineux. 1987. Sequence of a conditionally essential region of bacteriophage T3, including the primary origin of DNA replication. J. Mol. Biol. 193:479-495. [DOI] [PubMed] [Google Scholar]
- 63.Schroeder, C., H. Jurkschat, A. Meisel, J. G. Reich, and D. H. Krüger. 1986. Unusual occurrence of EcoP1 and EcoP15 recognition sites and counterselection of type II methylation and restriction sequences in bacteriophage T7 DNA. Gene 45:77-86. [DOI] [PubMed] [Google Scholar]
- 64.Schuch, R., D. Nelson, and V. A. Fischetti. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884-889. [DOI] [PubMed] [Google Scholar]
- 65.Severinova, E., and K. Severinov. 2006. Localization of the Escherichia coli RNA polymerase β′ subunit residue phosphorylated by bacteriophage T7 kinase Gp0.7. J. Bacteriol. 188:3470-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shine, J., and L. Dalgarno. 1974. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc. Natl. Acad. Sci. USA 71:1342-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith, H. W. 1956. The use of live vaccines in experimental Salmonella gallinarum infection in chickens with observations on their interference effect. J. Hyg. 54:419-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith, H. W., M. B. Huggins, and K. M. Shaw. 1987. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J. Gen. Microbiol. 133:1111-1126. [DOI] [PubMed] [Google Scholar]
- 69.Son, M., and P. Serwer. 1992. Role of exonuclease in the specificity of bacteriophage T7 DNA packaging. Virology 190:824-833. [DOI] [PubMed] [Google Scholar]
- 70.Spoerel, N., P. Herrlich, and T. A. Bickle. 1979. A novel bacteriophage defence mechanism: the anti-restriction protein. Nature 278:30-34. [DOI] [PubMed] [Google Scholar]
- 71.Steven, A. C., B. L. Trus, J. V. Maizel, M. Unser, D. A. D. Parry, J. S. Wall, J. F. Hainfeld, and F. W. Studier. 1988. Molecular substructure of a viral receptor-recognition protein. The gp17 tail-fiber of bacteriophage T7. J. Mol. Biol. 200:351-365. [DOI] [PubMed] [Google Scholar]
- 72.Studier, F. W. 1975. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J. Mol. Biol. 94:283-295. [DOI] [PubMed] [Google Scholar]
- 73.Studier, F. W., and N. R. Movva. 1976. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J. Virol. 19:136-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang, G. Q., R. P. Bandwar, and S. S. Patel. 2005. Extended upstream A-T sequence increases T7 promoter strength. J. Biol. Chem. 280:40707-40713. [DOI] [PubMed] [Google Scholar]
- 75.Thorpe, P. H., D. Ternent, and N. E. Murray. 1997. The specificity of StySKI, a type I restriction enzyme, implies a structure with rotational symmetry. Nucleic Acids Res. 25:1694-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Titheradge, A. J., D. Ternent, and N. E. Murray. 1996. A third family of allelic hsd genes in Salmonella enterica: sequence comparisons with related proteins identify conserved regions implicated in restriction of DNA. Mol. Microbiol. 22:437-447. [PubMed] [Google Scholar]
- 77.Ujvari, A., and C. T. Martin. 1997. Identification of a minimal binding element within the T7 RNA polymerase promoter. J. Mol. Biol. 273:775-781. [DOI] [PubMed] [Google Scholar]
- 78.Urig, S., H. Gowher, A. Hermann, C. Beck, M. Fatemi, A. Humeny, and A. Jeltsch. 2002. The Escherichia coli Dam DNA methyltransferase modifies DNA in a highly processive reaction. J. Mol. Biol. 319:1085-1096. [DOI] [PubMed] [Google Scholar]
- 79.Woese, C. R. 2002. On the evolution of cells. Proc. Natl. Acad. Sci. USA 99:8742-8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young, R., and U. Bläsi. 1995. Holins: form and function in bacteriophage lysis. FEMS Microbiol. Rev. 17:191-205. [DOI] [PubMed] [Google Scholar]
- 81.Zillig, W., H. Fujiki, W. Blum, D. Janekovic, M. Schweiger, H. J. Rahmsdorf, H. Ponta, and M. Hirsch-Kauffmann. 1975. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage T7-induced protein kinase. Proc. Natl. Acad. Sci. USA 7:2506-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]