Abstract
Several strategies currently exist for control of Salmonella enterica serovar Typhimurium colonization in the chicken intestine, among which the use of probiotics is of note. Little is known about the underlying mechanisms of probiotic-mediated reduction of Salmonella colonization. In this study, we asked whether the effect of probiotics is mediated by antimicrobial peptides, including avian beta-defensins (also called gallinacins) and cathelicidins. Four treatment groups were included in this study: a negative-control group, a probiotic-treated group, a Salmonella-infected group, and a probiotic-treated and Salmonella-infected group. On days 1, 3, and 5 postinfection (p.i.), the cecal tonsils were removed, and RNA was extracted and used for measurement of avian beta-defensin 1 (AvBD1), AvBD2, AvBD4, AvBD6, and cathelicidin gene expression by real-time PCR. The expressions of all avian beta-defensins and cathelicidin were detectable in all groups, irrespective of treatment and time point. Probiotic treatment and Salmonella infection did not affect the expression of any of the investigated genes on day 1 p.i. Furthermore, probiotic treatment had no significant effect on the expression of the genes at either 3 or 5 days p.i. However, the expression levels of all five genes were significantly increased (P < 0.05) in response to Salmonella infection at 3 and 5 days p.i. However, administration of probiotics eliminated the effect of Salmonella infection on the expression of antimicrobial genes. These findings indicate that the expression of antimicrobial peptides may be repressed by probiotics in combination with Salmonella infection or, alternatively, point to the possibility that, due to a reduction in Salmonella load in the intestine, these genes may not be induced.
Chickens may harbor food-borne pathogens, including Salmonella enterica serovar Typhimurium, in their intestines, and administration of live bacterial cultures in the form of probiotics can reduce intestinal colonization with these bacteria (9, 18). Probiotics have also been shown to exert other functions, such as maintenance of health and promotion of growth, in chickens (11).
In spite of considerable published data regarding the efficacy of probiotics in reducing intestinal colonization by enteric pathogens, the mechanisms of action of probiotics are not fully understood. Several mechanisms have been proposed for probiotic functions, among which modulation of the immune system has recently received attention. Probiotic bacteria can exert immunomodulatory activities through their interactions with the host immune system. These interactions may lead to enhancement of natural and antigen-specific antibodies (6, 7), activation or suppression of T cells (3, 19), and changes in cytokine expression profiles (5, 12, 29). Moreover, probiotics are able to induce the expression of antimicrobial peptides by host cells (23, 30). Collectively, the above-mentioned mechanisms contribute to the immunomodulatory activities of probiotics.
Antimicrobial peptides are a part of innate defense mechanisms (25) and are divided into two main families, defensins and cathelicidins (4). According to the positions of their disulfide bonds, defensins are subdivided into two major classes: alpha-defensins and beta-defensins (16). In addition, a class of defensins known as teta-defensins, with a circular structure and a different disulfide motif, has been identified in rhesus monkeys but not in any other species (4, 26). Defensins have a wide range of antimicrobial activity against different groups of microorganisms, including gram-negative and gram-positive bacteria, as well as fungi and certain enveloped viruses (4). In addition to their direct antimicrobial activities, defensins might also participate in regulation of acquired immune response against pathogens by facilitating maturation of dendritic cells and recruitment of effector T cells to infected tissues (34).
Avian species express only beta-defensins (15), and avian heterophils or epithelial cells have been reported to express these molecules (25). Members of the beta-defensin family in chickens are also called gallinacins. Recently, a new nomenclature was proposed for avian beta-defensins, which will be used hereafter. To date, 14 avian beta-defensins (avian beta-defensin 1 [AvBD1] to AvBD14) have been discovered (15). Among these molecules, AvBD1 and AvBD2 are expressed in different sections of the chicken intestine, lungs, and bone marrow (1, 36). AvBD6 is expressed in different parts of the digestive system, especially in the proximal part of the intestine (27). AvBD4 was shown to have an expression pattern similar to those of AvBD1 and AvBD2 (14).
Beta-defensin genes are inducible, and inflammation or bacterial infection in host tissues can induce the expression of these genes (16, 31). Induction of beta-defensins following infection with bacteria or their components has also been shown to occur in chickens. Yoshimura et al. (35) reported that inoculation of cell cultures of the hen oviduct with Salmonella enterica serovar Enteritidis and lipopolysaccharide (LPS) increased the expression levels of AvBD1, AvBD2, and AvBD3. Upregulation of AvBD1, AvBD7, and AvBD12 in the theca layer of ovarian follicles in LPS-injected laying hens has also been demonstrated (24). AvBD4 was expressed significantly in the liver of chickens in response to Salmonella serovar Enteritidis and serovar Typhimurium (17).
Antimicrobial properties of avian beta-defensins in chickens have been demonstrated in several studies. Higgs et al. (10) reported that AvBD13 can kill Salmonella serovar Typhimurium and Listeria monocytogenes at a concentration of 500 μg/ml. By use of an in vitro assay, it was demonstrated that AvBD6, AvBD4, and AvBD5 possess bactericidal activity against Salmonella species (17). In another study, the expression of AvBD6 in the cecal tonsils of 6-week-old broiler chickens and its antimicrobial activity against enteric pathogens were shown (27). Finally, antimicrobial activity of chicken cathelicidins has also been reported (2, 32). Also of interest are the observations that probiotic bacterial strains, including some strains of lactobacilli as well as a cell wall preparation of Enterococcus faecalis (EC-12), can induce the expression of defensins, such as AvBD2, in various tissues, including the tongue and the bursa of Fabricius (22, 23).
In our previous studies, we showed the immunomodulatory effects of a probiotic product in newly hatched chicks (5, 6, 7). Particularly, in a recent study, we examined cytokine gene expression in the cecal tonsils of chickens treated with probiotics and infected with Salmonella (5). The aim of the present study was to extend our previous studies and to investigate the effects of this probiotic in combination with Salmonella infection on innate host defenses by measuring antimicrobial peptide gene expression in the cecal tonsils of broiler chicks during their first week of life.
MATERIALS AND METHODS
Chicks and experimental design.
Seventy-two 1-day-old female broiler chicks were purchased from a local hatchery (Maple Leaf Food Inc., New Hamburg, ON, Canada). After arrival, the chicks were divided into four groups and randomly assigned to four separate rooms in an isolation unit (Ontario Veterinary College, University of Guelph, ON, Canada). All groups were provided with a broiler starter diet during the experimental period. All chicks had free access to feed and water during the experiment. The animal experimentations were approved by the Animal Care Committee, University of Guelph. The treatment groups were as follows: (i) the negative control (no probiotic treatment and no Salmonella infection), (ii) the probiotic-treated group, (iii) the Salmonella-infected group, and (iv) the probiotic-treated and Salmonella-infected group.
Probiotic treatment and Salmonella infection.
The probiotic used in this study was a commercial preparation consisting of three species of beneficial bacteria: Lactobacillus acidophilus, Bifidobacterium bifidum, and Enterococcus faecalis (Intervet, Whitby, ON, Canada). Immediately after arrival, the chicks and their boxes were sampled for the presence of Salmonella. On the first day of age, all chicks in groups ii and iv (the probiotic-treated group and the probiotic-treated and Salmonella-infected group, respectively) received 0.5 ml phosphate-buffered saline (PBS) containing 1 × 106 CFU of the probiotic via oral gavage. On the following day (the second day of age), the chicks in groups iii and iv (the Salmonella-infected group and the probiotic-treated and Salmonella-infected group, respectively) were orally infected with 1 × 104 CFU of nalidixic acid-resistant Salmonella enterica serovar Typhimurium of phage type 193 in 0.5 ml PBS. PBS was used as a placebo in untreated and/or uninfected groups on either the first or the second day of age.
Tissue collection and storage.
On days 1, 3, and 5 postinfection (p.i.) (3, 5, and 7 days of age, respectively), six chicks from each group were randomly selected and euthanized by cervical dislocation. The cecal tonsils were removed and kept in RNAlater (Qiagen Inc., Mississauga, ON, Canada) at −20°C until processing for RNA extraction.
Total RNA extraction and reverse transcription.
Total RNA from cecal tonsil samples was extracted using Trizol reagent (Invitrogen Canada Inc., Burlington, ON, Canada) according to the manufacturer's instructions. First-strand cDNA synthesis was performed using 2 μg of extracted total RNA, oligo(dT)12-18 primers, and a reverse transcription kit (Superscript first-strand synthesis system; Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. The obtained cDNA was then stored at −20°C until use.
Construction of standard DNA plasmids.
The sequences of the AvBD1, AvBD2, AvBD4, and AvBD6 beta-defensin genes were obtained from GenBank. These genes were selected because their expression in cecal tonsils or in the chicken intestine had previously been shown (1, 14, 27, 36). We also attempted to amplify AvBD3 and AvBD13 but were not successful; therefore, we did not pursue study of the expression of these genes further. In addition to beta-defensins, we also examined cathelicidin gene expression. The sequence used for amplification of the cathelicidin gene has 100% identity with that for fowlicidin1 (a chicken cathelicidin), identified by Xiao et al. (32). In order to construct standard plasmid DNA required for relative quantification of target and reference (β-actin) genes, cDNA was amplified by PCR using primer pairs specific to each gene (Table 1). Amplification of target fragment was done with a reaction mixture (25-μl total volume) containing 1 μl of cDNA, 1× PCR buffer, 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 0.4 μM of each gene-specific primer, and 1 unit Taq DNA polymerase. The cycling parameters were as follows: initial denaturation at 94°C for 4 min, followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Amplification was terminated by a final extension at 72°C for 10 min. PCR products were then purified using a commercial kit (PCR purification kit; Qiagen Inc., Mississauga, ON, Canada) and subsequently cloned into the pDrive cloning vector (Qiagen PCR cloning kit; Qiagen Inc., Mississauga, ON, Canada). Clones were screened by restriction enzyme digestion, and positive clones were sequenced. Plasmid concentration was determined by measurement of absorbance at 260 nm. To convert the plasmid concentrations to copy numbers, the following equation was used: number of copies per μl of target plasmid DNA = DNA concentration in grams per μl × 6 × 1023 copies per mol/molecular weight of the plasmid DNA in grams per mol. In order to generate standard curves, 10-fold serial dilutions (10−1 to 10−9) of standard plasmids were made and amplified in duplicate by real-time PCR.
TABLE 1.
Sequences of primer pairs used for amplification of target and reference genes
| Primer target | Orientationa | Sequence | Product size (bp) | Temp (°C)b |
|---|---|---|---|---|
| AvBD1 | F | GGTTCTTACTGCCTTGCTGT | 158 | 58 |
| R | TGACTTCCTTCCTAGAGCCT | |||
| AvBD2 | F | GGACTGCCTGCCACATACAT | 239 | 58 |
| R | TTGCAGCAGGAACGGAAC | |||
| AvBD4 | F | TACCTGCTGCTGTCTGTCCT | 158 | 58 |
| R | AGTCCACTGCCACATGATCC | |||
| AvBD6 | F | TTGTGGCAGTTCATGGAG | 188 | 58 |
| R | ACTTCTGGAGATCCTGTGC | |||
| Cathelicidin | F | CTGCACAACCTCAACTTCAC | 231 | 65 |
| R | GTATCCTGCAATCACAGTCC | |||
| β-Actin | F | CAACACAGTGCTGTCTGGTGG | 205 | 64 |
| R | ATCGTACTCCTGCTTGCTGAT |
F, forward; R, reverse.
The annealing duration for each primer was 5 seconds.
Real-time PCR.
Quantifications of target and reference genes in cDNA samples were carried out by fluorometric real-time PCR using a LightCycler 480 instrument (Roche Diagnostic GmbH, Manheim, Germany) and a real-time PCR kit (LightCycler 480 DNA Sybr green Ι master; Roche Diagnostic GmbH, Manheim, Germany). The assay was performed using a microplate (LightCycler 480 multiwell plate 384; Roche Diagnostic GmbH, Manheim, Germany) with final volume of 20 μl/well, consisting of 0.25 μM of each gene-specific primer (Table 1), 10 μl of LightCycler 480 DNA Sybr green Ι master mix (containing fast-start Taq DNA polymerase, Sybr green Ι dye, and MgCl2), 5 μl of the cDNA template, and PCR-grade water.
The thermal cycling protocol consisted of an initial denaturation at 95°C for 10 min, followed by amplification for 40 cycles at 95°C for 1 s, 58°C for 5 s, and 72°C for 9 to 11 s. The specificity of amplification for each product was determined by melting curve analysis at 95°C for 1 s and 65°C for 15 s, followed by progressive rising of the temperature to 95°C, with continued measurement of fluorescence, and finally cooling of the plate at 40°C for 30 s. Alongside each real-time PCR assay, a 10−4 dilution of a related standard DNA plasmid and a blank control were run to serve as a calibrator and a negative control, respectively.
Data analysis.
The efficiency of the real-time PCR assays was calculated by the LightCycler 480 software program. Relative expression was calculated as a ratio between expression of target genes (the AvBD1, AvBD2, AvBD4, AvBD6, and cathelicidin genes) and expression of the β-actin gene (as a reference gene) in the same cDNA sample. To achieve this, the following equation, which considers differences in the efficiencies obtained for the target and reference genes, was used (20): 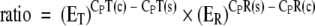 . In this equation, ET and ER represent the efficiencies of real-time PCR from the target and reference genes, CPT(c) and CPT(s) represent the crossing points of the target gene for the calibrator and the samples, and CPR(s) and CPR(c) represent the crossing points of the reference gene for the samples and the calibrator, respectively.
. In this equation, ET and ER represent the efficiencies of real-time PCR from the target and reference genes, CPT(c) and CPT(s) represent the crossing points of the target gene for the calibrator and the samples, and CPR(s) and CPR(c) represent the crossing points of the reference gene for the samples and the calibrator, respectively.
All data were analyzed according to a completely randomized design, consisting of four treatments and six replicates, using the General Linear Models procedure in SAS. Treatment means were compared by the new Duncan multiple-range test at an α value of 0.05 (SAS).
RESULTS
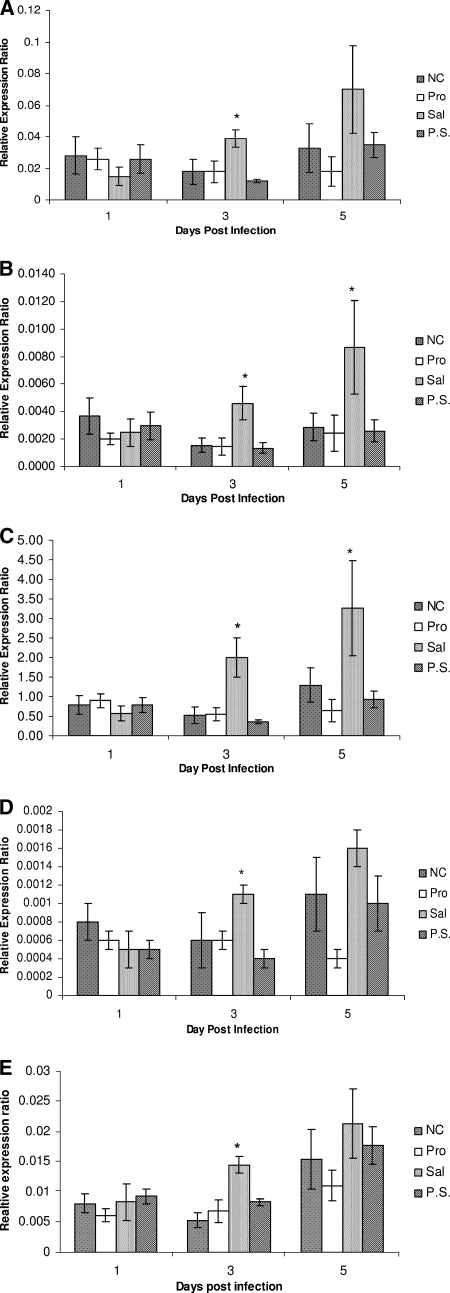
The results of Salmonella infection and probiotic treatment effects on the expressions of AvBD1, AvBD2, AvBD4, AvBD6, and cathelicidin are illustrated in Fig. 1A to E. Irrespective of treatment group and time point, the expressions of all antimicrobial peptide genes investigated in this study (AvBD1, AvBD2, AvBD4, AvBD6, and cathelicidin) were detectable in the cecal tonsils of chicks.
FIG. 1.
Relative expression levels of AvBD1 (A), AvBD2 (B), AvBD6 (C), AvBD4 (D), and cathelicidin (E) in the cecal tonsils of chicks. The treatment groups were as follows: the negative control (NC; no probiotic treatment, no Salmonella infection), the probiotic-treated group (Pro), the Salmonella-infected group (Sal), and the probiotic-treated and Salmonella-infected group (P.S.). Target gene expression is presented relative to β-actin expression and normalized to a calibrator. Error bars represent standard errors of the means. The differences in gene expression among groups at each time point were tested by Duncan's new multiple range test and were considered significant at P values of ≤0.05 (*).
The expressions of the AvBD1, AvBD2, AvBD4, AvBD6, and cathelicidin genes in the cecal tonsils of chicks were not affected by probiotic treatment or Salmonella infection on day 1 p.i. Furthermore, probiotic treatment had no significant effect on the expressions of these genes either at 3 or at 5 days p.i., compared to what was found for chickens that were not treated with probiotics or infected with Salmonella. However, the expression levels of AvBD1, AvBD2, AvBD4, AvBD6 and cathelicidin were significantly increased (P < 0.05) in response to Salmonella infection at 3 days p.i. (2.7-, 2.87-, 3.7-, 1.83-, and 2.72-fold increases for AvBD1, AvBD2, AvBD4, AvBD6, and cathelicidin, respectively, compared to control levels). However, this upregulation of genes due to Salmonella infection was not observed in chicks which had been treated with probiotics before being infected with Salmonella (the probiotic-treated and Salmonella-infected group). The elevated expression levels of the genes in response to Salmonella infection continued until 5 days p.i. (2.12-, 3.0-, 2.50-, 1.45-, and 1.39-fold increases for AvBD1, AvBD2, AvBD4, AvBD6, and cathelicidin, respectively, compared to negative-control levels). However, at this time point, the increased expression levels were significant (P < 0.05) only for AvBD2 and AvBD6. Similar to what was observed at 3 days p.i., the expressions of these genes were unaffected in the probiotic-treated and Salmonella-infected group at 5 days p.i.
DISCUSSION
In this study, the possible role of antimicrobial peptides in Salmonella infection and probiotic-mediated protection against Salmonella in chickens was investigated. This was evaluated by measuring the expressions of AvBD1, AvBD2, AvBD4, AvBD6, and cathelicidin in the cecal tonsils of chickens infected with Salmonella serovar Typhimurium or treated with probiotics prior to Salmonella infection. Expressions of the avian beta-defensin and cathelicidin genes were detectable in all groups during the first week of age. This observation confirmed and extended the previous observations that AvBD1 and AvBD2 are expressed in the small and large intestines of healthy chicks during the first week of age (1). In addition, we showed that while Salmonella infection led to elevated expression of all the antimicrobial genes studied here, probiotic treatment prior to infection dampened the expression of these genes.
We have previously shown that administration of the probiotic product used in the present study could reduce cecal Salmonella counts by 1.3 to 3 logs (5; J. R. Chambers, H. R. Haghighi, and S. Sharif, unpublished data). In the present study, increases were observed in the expression levels of AvBD1, AvBD2, AvBD4, AvBD6, and cathelicidin genes in the cecal tonsils of the chicks in response to Salmonella infection on day 3 p.i. and, in the cases of some genes, on day 5 p.i. as well. However, the probiotic bacteria used in this study did not evoke any significant increase or decrease in the expression of any of the defensin genes. The increases in the expression levels of these antimicrobial genes in response to Salmonella infection may be an indication that all of these genes are involved in host responses to infection. In agreement with our results, Sadeyen et al. (21) also reported increases in the expression levels of AvBD1 and AvBD2 in the cecal tonsils of laying hens in response to infection with Salmonella serovar Enteritidis. Furthermore, upregulation of AvBD1, AvBD7, and AvBD12 in the theca layer of ovarian follicles in LPS-injected laying hens has been demonstrated (24).
Probiotics may also induce the expression of beta-defensins. Wehkamp et al. (30) have reported the induction of human beta-defensin 2 in Caco-2 intestinal epithelial cells through a number of probiotic bacterial strains, such as Escherichia coli Nissle 1917 and some lactobacilli strains. The upregulation of the human beta-defensin 2 gene in Caco-2 cells by several probiotic Lactobacillus strains also has been demonstrated by Schlee et al. (23). However, there is no report of effects of live probiotic bacteria on the expression of avian beta-defensin genes in chickens. Nevertheless, it has been shown that oral administration of a cell wall preparation of Enterococcus faecalis strain EC-12 in newly hatched broiler chicks increased expression of Gal-2 in the tongue and the bursa of Fabricius (22).
Our results indicate that although Salmonella infection is able to induce the expression of avian beta-defensin and cathelicidin genes in the cecal tonsils of chickens, early intestinal colonization by probiotic bacteria does not result in enhancements in the expression levels of these genes. Moreover, when Salmonella inoculation was done after probiotic treatment (in the probiotic-treated and Salmonella-infected group), the expression levels of these genes were similar to those observed in uninfected chickens. Therefore, it may be concluded that when probiotic bacteria are combined with Salmonella, they may have a direct antagonistic effects against Salmonella-induced expression of defensin genes. Alternatively, the downregulation of these genes in the probiotic-treated and Salmonella-infected group may be the result of inhibitory effects of probiotic bacteria on Salmonella colonization. Consequently, a reduction in intestinal colonization by Salmonella may have eliminated the inflammatory conditions needed for the induction of these genes. We have also previously observed that the same probiotic used in the present study was able to reduce intestinal colonization by Salmonella serovar Typhimurium as well as expression of proinflammatory cytokines, such as interleukin 12 and gamma interferon, in cecal tonsils.
Despite some reports that the expression of beta-defensins is enhanced by mixed cultures or individual probiotic bacteria (23, 30), we did not observe a significant change in the expressions of avian beta-defensins and cathelicidin genes in the group that was treated with probiotics only. Enhanced expression of defensin genes is usually associated with pathogenic infections and inflammations caused by these infections (16). Hence, probiotic bacteria, which are usually regarded as members of the commensal microbiota, may be well adapted to their host, and as a result, their colonization will not result in inflammation or ensuing enhancement in expression of antimicrobial peptides.
In conclusion, we have demonstrated that infection of young chicks with Salmonella serovar Typhimurium significantly increases the expression of several of the antimicrobial peptide genes in cecal tonsils. Furthermore, when chickens were treated with probiotics prior to Salmonella infection, the expressions of avian beta-defensin and cathelicidin genes were reduced to levels comparable to those seen in the negative-control group. Further studies are needed to reveal the source of antimicrobial peptides in the cecal tonsils of Salmonella-infected chicks as well as the mechanisms of action of probiotics in downregulating antimicrobial peptide genes in infected chickens.
Acknowledgments
This study was funded by the NSERC-CRD program, the Ontario Ministry of Agriculture and Food, the Saskatchewan Chicken Industry Development Fund, and the Poultry Industry Council. M. R. Akbari was a recipient of a fellowship from Ferdowsi University of Mashhad, Iran.
Footnotes
Published ahead of print on 30 September 2008.
REFERENCES
- 1.Bar-Shira, E., and A. Friedman. 2006. Development and adaptations of innate immunity in the gastrointestinal tract of the newly hatched chick. Dev. Comp. Immunol. 30:930-941. [DOI] [PubMed] [Google Scholar]
- 2.Bommineni, Y. R., H. Dai, Y. X. Gong, J. L. Soulages, S. C. Fernando, U. Desilva, O. Prakash, and G. Zhang. 2007. Fowlicidin-3 is an alpha-helical cationic host defense peptide with potent antibacterial and lipopolysaccharide-neutralizing activities. FEBS J. 274:418-428. [DOI] [PubMed] [Google Scholar]
- 3.Castellazzi, A. M., C. Valsecchi, L. Montagna, P. Malfa, G. Ciprandi, M. A. Avanzini, and G. L. Marseglia. 2007. In vitro activation of mononuclear cells by two probiotics: Lactobacillus paracasei I 1688, Lactobacillus salivarius I 1794, and their mixture (PSMIX). Immunol. Investig. 36:413-421. [DOI] [PubMed] [Google Scholar]
- 4.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 5.Haghighi, H. R., M. F. Abdul-Careem, R. A. Dara, J. R. Chambers, and S. Sharif. 2008. Cytokine gene expression in chicken cecal tonsils following treatment with probiotics and Salmonella infection. Vet. Microbiol. 126:225-233. [DOI] [PubMed] [Google Scholar]
- 6.Haghighi, H. R., J. Gong, C. L. Gyles, M. A. Hayes, B. Sanei, P. Parvizi, H. Gisavi, J. R. Chambers, and S. Sharif. 2005. Modulation of antibody-mediated immune response by probiotics in chickens. Clin. Diagn. Lab. Immunol. 12:1387-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haghighi, H. R., J. Gong, C. L. Gyles, M. A. Hayes, H. Zhou, B. Sanei, J. R. Chambers, and S. Sharif. 2006. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 13:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Higgins, J. P., S. E. Higgins, J. L. Vicente, A. D. Wolfenden, G. Tellez, and B. M. Hargis. 2007. Temporal effects of lactic acid bacteria probiotic culture on Salmonella in neonatal broilers. Poult. Sci. 86:1662-1666. [DOI] [PubMed] [Google Scholar]
- 10.Higgs, R., D. J. Lynn, S. Gaines, J. McMahon, J. Tierney, T. James, A. T. Lloyd, G. Mulcahy, and C. O'Farrelly. 2005. The synthetic form of a novel chicken beta-defensin identified in silico is predominantly active against intestinal pathogens. Immunogenetics 57:90-98. [DOI] [PubMed] [Google Scholar]
- 11.Khan, M., D. Raoult, H. Richet, H. Lepidi, and B. La Scola. 2007. Growth-promoting effects of single-dose intragastrically administered probiotics in chickens. Br. Poult. Sci. 48:732-735. [DOI] [PubMed] [Google Scholar]
- 12.Kim, Y. G., T. Ohta, T. Takahashi, A. Kushiro, K. Nomoto, T. Yokokura, N. Okada, and H. Danbara. 2006. Probiotic Lactobacillus casei activates innate immunity via NF-kappaB and p38 MAP kinase signaling pathways. Microbes Infect. 8:994-1005. [DOI] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Lynn, D. J., R. Higgs, S. Gaines, J. Tierney, T. James, A. T. Lloyd, M. A. Fares, G. Mulcahy, and C. O'Farrelly. 2004. Bioinformatic discovery and initial characterisation of nine novel antimicrobial peptide genes in the chicken. Immunogenetics 56:170-177. [DOI] [PubMed] [Google Scholar]
- 15.Lynn, D. J., R. Higgs, A. T. Lloyd, C. O'Farrelly, V. Herve-Grepinet, Y. Nys, F. S. Brinkman, P. L. Yu, A. Soulier, P. Kaiser, G. Zhang, and R. I. Lehrer. 2007. Avian beta-defensin nomenclature: a community proposed update. Immunol. Lett. 110:86-89. [DOI] [PubMed] [Google Scholar]
- 16.Menendez, A., and B. Brett Finlay. 2007. Defensins in the immunology of bacterial infections. Curr. Opin. Immunol. 19:385-391. [DOI] [PubMed] [Google Scholar]
- 17.Milona, P., C. L. Townes, R. M. Bevan, and J. Hall. 2007. The chicken host peptides, gallinacins 4, 7, and 9 have antimicrobial activity against Salmonella serovars. Biochem. Biophys. Res. Commun. 356:169-174. [DOI] [PubMed] [Google Scholar]
- 18.Pascual, M., M. Hugas, J. I. Badiola, J. M. Monfort, and M. Garriga. 1999. Lactobacillus salivarius CTC2197 prevents Salmonella enteritidis colonization in chickens. Appl. Environ. Microbiol. 65:4981-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pessi, T., E. Isolauri, Y. Sutas, H. Kankaanranta, E. Moilanen, and M. Hurme. 2001. Suppression of T-cell activation by Lactobacillus rhamnosus GG-degraded bovine casein. Int. Immunopharmacol. 1:211-218. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadeyen, J. R., J. Trotereau, J. Protais, C. Beaumont, N. Sellier, G. Salvat, P. Velge, and A. C. Lalmanach. 2006. Salmonella carrier-state in hens: study of host resistance by a gene expression approach. Microbes Infect. 8:1308-1314. [DOI] [PubMed] [Google Scholar]
- 22.Sakai, Y., T. Tsukahara, W. Bukawa, N. Matsubara, and K. Ushida. 2006. Cell preparation of Enterococcus faecalis strain EC-12 prevents vancomycin-resistant enterococci colonization in the cecum of newly hatched chicks. Poult. Sci. 85:273-277. [DOI] [PubMed] [Google Scholar]
- 23.Schlee, M., J. Harder, B. Koten, E. F. Stange, J. Wehkamp, and K. Fellermann. 2008. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin. Exp. Immunol. 151:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subedi, K., N. Isobe, M. Nishibori, and Y. Yoshimura. 2007. Changes in the expression of gallinacins, antimicrobial peptides, in ovarian follicles during follicular growth and in response to lipopolysaccharide in laying hens (Gallus domesticus). Reproduction 133:127-133. [DOI] [PubMed] [Google Scholar]
- 25.Sugiarto, H., and P. L. Yu. 2004. Avian antimicrobial peptides: the defense role of beta-defensins. Biochem. Biophys. Res. Commun. 323:721-727. [DOI] [PubMed] [Google Scholar]
- 26.Tang, Y. Q., G. Osapay, K. Osapay, D. Tran, C. J. Miller, A. J. Ouellette, and M. E. Selsted. 1999. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 286:498-502. [DOI] [PubMed] [Google Scholar]
- 27.van Dijk, A., E. J. Veldhuizen, S. I. Kalkhove, J. L. Tjeerdsma-van Bokhoven, R. A. Romijn, and H. P. Haagsman. 2007. The beta-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob. Agents Chemother. 51:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Vinderola, C. G., M. Medici, and G. Perdigon. 2004. Relationship between interaction sites in the gut, hydrophobicity, mucosal immunomodulating capacities and cell wall protein profiles in indigenous and exogenous bacteria. J. Appl. Microbiol. 96:230-243. [DOI] [PubMed] [Google Scholar]
- 30.Wehkamp, J., J. Harder, K. Wehkamp, B. Wehkamp-von Meissner, M. Schlee, C. Enders, U. Sonnenborn, S. Nuding, S. Bengmark, K. Fellermann, J. M. Schroder, and E. F. Stange. 2004. NF-κB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect. Immun. 72:5750-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehkamp, J., J. Schauber, and E. F. Stange. 2007. Defensins and cathelicidins in gastrointestinal infections. Curr. Opin. Gastroenterol. 23:32-38. [DOI] [PubMed] [Google Scholar]
- 32.Xiao, Y., Y. Cai, Y. R. Bommineni, S. C. Fernando, O. Prakash, S. E. Gilliland, and G. Zhang. 2006. Identification and functional characterization of three chicken cathelicidins with potent antimicrobial activity. J. Biol. Chem. 281:2858-2867. [DOI] [PubMed] [Google Scholar]
- 33.Reference deleted.
- 34.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura, Y., H. Ohashi, K. Subedi, M. Nishibori, and N. Isobe. 2006. Effects of age, egg-laying activity, and Salmonella-inoculation on the expressions of gallinacin mRNA in the vagina of the hen oviduct. J. Reprod. Dev. 52:211-218. [DOI] [PubMed] [Google Scholar]
- 36.Zhao, C., T. Nguyen, L. Liu, R. E. Sacco, K. A. Brogden, and R. I. Lehrer. 2001. Gallinacin-3, an inducible epithelial β-defensin in the chicken. Infect. Immun. 69:2684-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]