Abstract
Tuberculosis (TB) caused by Mycobacterium tuberculosis remains a major world disease, with approximately 9 million new cases each year. Identification and treatment of active disease are essential for TB control. Serology may offer increased detection of active disease in patients with a positive tuberculin skin test (TST) or QuantiFERON-TB (QFT-G). The InBios Active TbDetect immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA), IBL M. tuberculosis IgG ELISA, and Anda Biologics TB ELISAs were evaluated for the ability to detect M. tuberculosis antibodies in patients with active disease. Agreement, sensitivity, and specificity for each ELISA were determined and compared to those for culture or amplified direct detection and M. tuberculosis low-risk control patients. The InBios Active TbDetect ELISA had an agreement of 96.2%, a sensitivity of 83.3%, and a specificity of 98.9%. The IBL M. tuberculosis ELISA had an agreement of 84.0%, a sensitivity of 5.6%, and a specificity of 100.0%. The agreement, sensitivity, and specificity of the Anda Biologics TB ELISA were 74.2%, 83.3%, and 72.0%, respectively. The sensitivity for detecting M. tuberculosis antibodies in human immunodeficiency virus-associated TB was 50% for both the InBios Active TbDetect ELISA and the Anda Biologics TB ELISA and 0% for the IBL M. tuberculosis ELISA. The positivity rates for InBios Active TbDetect ELISA, IBL M. tuberculosis ELISA, and Anda Biologics TB ELISA in latently infected individuals positive by TST and/or QFT-G were 5.1%, 0.0%, and 30.8%, respectively. It can be concluded that the InBios Active TbDetect IgG ELISA is superior to the other ELISAs in accurately detecting active TB.
Approximately nine million new cases of disease and over two million deaths result from tuberculosis (TB) each year (29, 56). It is estimated that over one-third of the world's population is infected, with ∼95% of all cases occurring in developing countries. Global measures attempting to reduce the transmission of TB are currently in place.
An essential component of TB control efforts is to identify and treat individuals with active TB disease. The ability to correctly identify individuals with latent TB infection who will progress to active TB disease is vital to this goal (9, 49). Current test procedures are inadequate to accurately detect and identify active TB disease (14, 27, 30, 31, 41, 44). These shortcomings result in the unnecessary treatment of many individuals who may not need it (3, 17, 32, 45). While the tuberculin skin test (TST) and the QuantiFERON-TB Gold (QFT-G), the traditional methods for latent TB infection screening, rely on the cell-mediated response, the humoral response appears to correlate with the progression of the infection to active TB disease (5, 6, 11, 15, 20, 21).
Many studies have been conducted to evaluate the utility of individual specific Mycobacterium tuberculosis antigens for detecting antibodies in patients with active TB disease (1, 7, 10, 11, 20, 21, 25, 26, 29, 38, 39, 45, 46). Several of these antigens have been developed into commercial assays capable of detecting M. tuberculosis antibodies (4, 28, 35, 53). This study evaluates three commercially available enzyme-linked immunosorbent assays (ELISAs) for their ability to detect immunoglobulin G (IgG) antibodies to M. tuberculosis in patients with active TB disease.
MATERIALS AND METHODS
Human sera.
The procedures followed were in accordance with the ethical standards established by the University of Utah and are in accordance with the Helsinki Declaration of 1975. This study was approved by the Institutional Review Board of the University of Utah, IRB 17152. All patient samples included in this study were deidentified to meet the Health Information Portability and Accountability Act (HIPAA) patient confidentiality guidelines.
Serum samples were stored at −70°C until testing commenced and were then stored at 2 to 8°C while testing was performed. The whole-blood samples were processed immediately after collection. Samples with discrepant results were tested again by each respective assay to ensure reproducibility. A total of 209 samples were used and divided into four groups.
Risk factors that were evaluated for exposure to TB included work in the health care field, laboratory work (especially in mycobacteriology laboratories and specimen processing), immigration from or travel to an country where TB is endemic, and exposure to persons with known active disease. Work in a mycobacteriology laboratory, exposure to a known active case, or immigration from a country where TV is endemic were considered high risk for exposure. Work in a health care field was considered moderate risk.
Group I serum samples consisted of 88 samples from healthy U.S.-born individuals who tested negative by QFT-G and had no risk factors for M. tuberculosis infection. All serum samples from group I were tested on each of the three commercially available ELISAs.
Group II serum samples included samples from 18 M. tuberculosis culture-positive and/or amplified direct detection (ADD)-positive patients. The samples in group II were tested for M. tuberculosis antibodies using the three commercial ELISA kits.
Group III serum samples were collected from 25 individuals who had received the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine. The majority of these individuals had been vaccinated in infancy or early childhood and obtained the vaccinations in Western and Eastern Europe, Central and South America, and Asia. All samples from group III were tested by the three antibody detection assays.
Group IV serum samples were from 78 individuals who were diagnosed with latent M. tuberculosis infection by TST and/or QFT-G. Tuberculin testing in the study subjects was performed within 1 year to 1 month before the blood samples were drawn. Fifty samples were positive by TST, 14 were positive by QFT, and 14 were positive for both TST and QFT. All 78 samples were assessed for anti-M. tuberculosis antibodies using the three commercial ELISAs.
M. tuberculosis culture and amplified direct detection.
Serum samples from group II were obtained from individuals found to be culture or ADD positive for M. tuberculosis through testing at ARUP Laboratories (Salt Lake City, UT).
Tuberculin skin test.
Some participants received a standard dose of 5 tuberculin units intradermally. Trained health care professionals interpreted the results 48 to 72 h after application according to the American Thoracic Society/Centers for Disease Control and Prevention guidelines (8). The positive interpretation of a TST is an induration area of ≥10 mm in individuals with increased risk factors and an induration area of ≥15 mm in individuals without known risk factors for M. tuberculosis infection (16). Other participants were not tested due to a history of a positive TST in the past.
QFT-G testing.
Sodium heparin blood tubes were collected from groups I and IV and tested using QuantiFERON-TB Gold (Cellestis Inc., Victoria, Australia) according to the manufacturer's guidelines. Results were interpreted as positive, negative, or indeterminate.
M. tuberculosis antibody detection by commercial ELISAs.
Anti-M. tuberculosis antibody levels were assessed using three commercially available ELISAs. The three kits evaluated included the InBios Active TbDetect IgG ELISA (InBios International, Seattle, WA), the IBL M. tuberculosis IgG ELISA (IBL-Hamburg, Hamburg, Germany), and the Anda Biologicals TB ELISA (Anda Biologicals, Strasbourg, France). Each assay follows standard ELISA format. For the InBios Active TbDetect ELISA, serum samples were tested according to the manufacturer's guidelines. The assay utilizes several antigens, including Mtb81, Mtb8, Mtb48, DPEP (MPT32), 38-kDa protein, and two additional proprietary antigens. Results are expressed in optical density (OD), with an OD of ≥0.500 indicating a positive result.
The IBL M. tuberculosis IgG ELISA was evaluated using the protocol supplied by the manufacturer. The microtiter wells are coated with 18-, 36-, and 40-kDa recombinant antigens. The results are expressed in units per milliliter and are calculated in reference to a cutoff standard provided by the manufacturer. A value of >12 U/ml is representative of a positive result. Because of the initial low sensitivity observed with the IBL kit, samples were also tested with a second different lot number of the IBL kit to control for the possibility that the low sensitivity was due to variability in the lots. The outcomes were identical with the second lot.
The Anda Biologicals TB ELISA was evaluated using A60 antigen-sensitized microtiter wells obtained from the manufacturer following the recommended protocol. Serum samples were diluted 1:100 for testing. One hundred microliters of each diluted sample was added to the sensitized microtiter well and incubated for 60 min at room temperature (18 to 24°C). The wells were washed, and 100 μl of horseradish peroxidase-conjugated goat anti-human IgG solution was added and incubated for 30 min at room temperature. Following another wash step, tetramethylbenzidine (TMB) was added and incubated at room temperature for 20 min. The enzymatic reaction results in the formation of a blue color, which intensifies in proportion to the amount of anti-M. tuberculosis antibodies that is present. The reaction was stopped by adding 1 N H2SO4, which results in a color change from blue to yellow, and the absorbance was read. Positive- and negative-control sera were analyzed with each run to ensure test function. Sample results were compared to a reference standard and reported in units per millliliter. A value of ≥1.2 U/ml was considered a positive value.
HIV serology.
Human immunodeficiency virus (HIV) status was determined for serum samples from group II by serology testing performed at Associated Regional and University Pathologists (ARUP) Laboratories. Samples were screened using the Bio-Rad GS HIV type 1 (HIV-1)/HIV-2 PLUS O enzyme immunoassay (Hercules, CA) with positive samples undergoing confirmation by Bio-Rad GS HIV-1 Western blot kit (Hercules, CA).
Statistical analysis.
The data were analyzed for agreement, sensitivity, and specificity with 95% confidence intervals (95% CI) using a two-way contingency table analysis with Yates-corrected chi-squared test (19). Equivocal results were excluded from the calculations. Receiver operator characteristic (ROC) curves were generated using R version 2.7.2 (R Development Core Team). Area under the curve (AUC) analysis was performed using Statistical Analysis Software version 9.1.3 (SAS Institute Inc., Cary, NC). Scatter plots were generated using GraphPad Prism version 5.01 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Comparison of the InBios Active TbDetect IgG ELISA against culture/ADD and a healthy control group.
When serum samples from group I (healthy normal controls) and group II (culture positive or ADD positive) were evaluated by the InBios Active TbDetect IgG ELISA, an overall agreement of 96.2% (agreement with positive and negative results) was observed. The clinical sensitivity and clinical specificity were 83.3% (95% CI, 68.6 to 87.8%) and 98.9% (95% CI, 95.8 to 99.8%), respectively (Table 1). One sample from group I gave a positive test result, while 87 samples gave negative results by the InBios assay. No clinical history could be collected on the positive individual to explain the presence of M. tuberculosis antibodies. Fifteen samples from group II tested positive, and three tested negative.
TABLE 1.
Summary of InBios Active TbDetect IgG ELISA, IBL M. tuberculosis IgG ELISA, and Anda Biologicals TB ELISA results to culture and/or ADD results in a healthy control group
| Assay and result or parameter | No. of samples with the following culture/ADD result:
|
% Agreement (95% CI) | % Sensitivity (95% CI) | % Specificity (95% CI) | |
|---|---|---|---|---|---|
| Positivea | Negativeb | ||||
| InBios Active TbDetect IgG ELISA | 96.2 (91.1-97.7) | 83.3 (68.6-87.8) | 98.9 (95.8-99.8) | ||
| Positive | 15 | 1 | |||
| Negative | 3 | 86 | |||
| Equivocal | 0 | 1 | |||
| Total | 18 | 88 | |||
| IBL M. tuberculosis IgG ELISA | 84.0 (82.5-84.0) | 5.6 (1.2-5.6) | 100.0 (99.1-100) | ||
| Positive | 1 | 0 | |||
| Negative | 17 | 88 | |||
| Equivocal | 0 | 0 | |||
| Total | 18 | 88 | |||
| Anda Biologicals TB ELISA | 74.2 (65.5-78.3) | 83.3 (63.5-94.0) | 72.0 (67.2-74.6) | ||
| Positive | 15 | 21 | |||
| Negative | 3 | 54 | |||
| Equivocal | 0 | 13 | |||
| Total | 18 | 88 | |||
Samples are positive by culture and/or ADD. The 18 positive samples are comprised of 12 that are confirmed serologically to be HIV negative and 6 that are serologically HIV positive.
Negative samples are from low-risk U.S.-born individuals (group I).
Because of previous reports of the poor sensitivity of M. tuberculosis antibody tests in HIV-associated TB, HIV status was determined for the group II culture/ADD-positive patients (48). Of the eighteen samples from group II, six were HIV positive. The three samples that tested negative by the InBios assay were from patients with HIV-associated TB. If the HIV-associated TB samples are removed from analysis, the clinical sensitivity is 100% (all 12 samples). The sensitivity of the InBios antibody test for HIV-associated TB is 50.0% (three out of six samples).
Comparison of the IBL M. tuberculosis IgG ELISA to culture/ADD and a healthy control group.
The IBL M. tuberculosis IgG ELISA was evaluated using serum samples from groups I and II. The agreement, sensitivity, and specificity were determined to be 84.0%, 5.6% (95% CI, 1.2 to 5.6%), and 100.0% (99.1 to 100.0%), respectively (Table 1). All 88 samples from group I tested negative, and 17 of the 18 samples from group II also tested negative by the IBL assay. All six of the sera from HIV-associated TB patients were negative for M. tuberculosis antibodies by the IBL assay; the remaining 11 negative samples were HIV negative.
Comparison of the Anda Biologicals TB ELISA against culture/ADD and a healthy control group.
Serum samples from group I and group II were analyzed using Anda Biologicals TB ELISA. The overall agreement was found to be 74.2%, the clinical sensitivity was 83.3% (95% CI, 63.5 to 94.0%), and the clinical specificity was 72.0% (95% CI, 67.2 to 74.6%) (Table 1). Twenty-one of 88 samples from group I were positive for a positivity rate of 24% in healthy QFT-G-negative individuals. Three of the 18 samples from group II were negative. All three of the negative samples from group II were from patients with HIV-associated TB. The sensitivity of the Anda assay for HIV-associated TB was 50.0% (three out of six samples).
Comparison of the ELISA tests by scatter plot.
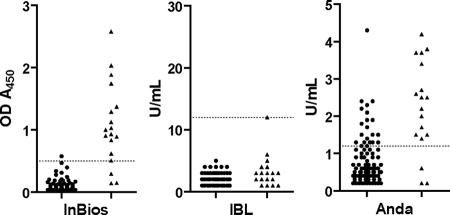
Quantitative ELISA results (OD at 450 nm [OD450] readings or U/ml values) of healthy patients and active TB disease patients were graphed in a scatter plot (Fig. 1). The Anda Biologicals TB ELISA showed the greatest range of results for active TB disease patients, from 0.2 to 4.2 U/ml. The range of values for healthy patients for the Anda ELISA overlapped into the positive range to 2.4, with one result at 4.3. The InBios Active TbDetect IgG ELISA showed a range of results for active TB disease patients, from 0.144 to 2.582 OD450, with a tight distribution of OD450 values for the healthy patients below the cutoff value. Most of the IBL M. tuberculosis IgG ELISA values were in the negative range for both healthy and active TB disease patients, with the only positive patient having a result just above the cutoff at 12 U/ml.
FIG. 1.
Scatter plots of values for healthy patients (solid circles) and patients with active TB disease (solid triangles) on each of the tested ELISA kits. The dotted line on each graph represents the cutoff value for the respective kit (0.500 OD450 for the InBios Active TbDetect IgG ELISA kit, 12 U/ml for the IBL M. tuberculosis IgG ELISA kit, and 1.2 U/ml for the Anda Biologicals TB ELISA kit).
ROC curves.
Receiver operator characteristic curves for the InBios Active TbDetect IgG, IBL M. tuberculosis IgG, and Anda Biologicals TB ELISAs were constructed by plotting the true-positive rate and false-positive rate with each unique value of the indicator variable (i.e., commercial test kit) for healthy and active TB disease patients (Fig. 2). An indicator variable with high discriminatory ability will have a curve with an area under the curve near 1, and an indicator variable with low discriminatory ability will have an AUC near 0.5. The InBios test demonstrated a significantly higher discriminating power than the IBL and Anda tests, with a high AUC value of 0.911 close to a value of 1. The IBL test had very poor discriminatory ability, with an AUC of 0.528 close to a value of 0.5.
FIG. 2.
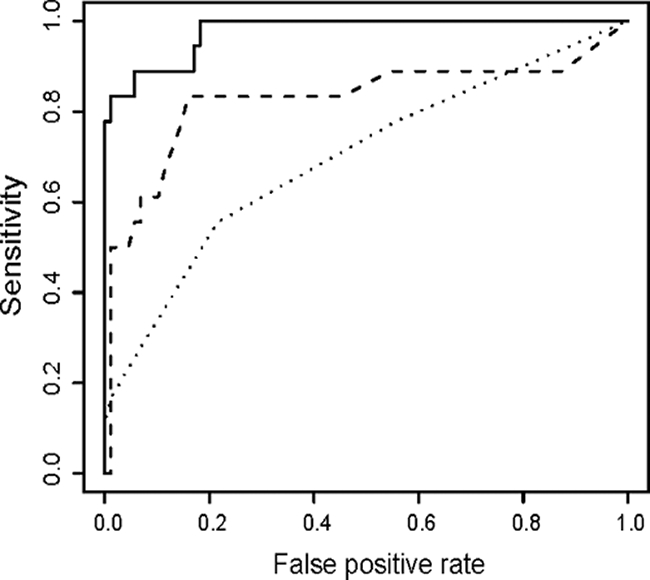
ROC curves for the InBios Active TbDetect IgG ELISA (solid), IBL M. tuberculosis IgG ELISA (dotted), and Anda Biologicals TB ELISA (dashed) for healthy and active TB disease patients. The AUCs for InBios, IBL and Anda are 0.911, 0.528, and 0.797, respectively. Compared to InBios, the P values for IBL and Anda are both <0.0001, indicating a significant difference between InBios and both the IBL and Anda kits.
Measurement of Mycobacterium tuberculosis antibodies in individuals receiving the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine.
Twenty-five serum samples from group III (BCG-vaccinated individuals) were evaluated for the presence of M. tuberculosis antibodies by each ELISA (Table 2). Antibodies were detected in one sample by the InBios Active TbDetect ELISA, giving a positivity rate of 4.0%. The IBL M. tuberculosis IgG ELISA detected no antibodies in this group. The Anda Biologicals TB ELISA detected antibodies in 14 samples, resulting in a 56.0% positivity rate.
TABLE 2.
Summary of positive results of InBios Active TbDetect IgG ELISA, IBL M. tuberculosis IgG ELISA, and Anda Biologicals TB ELISA from latently infected individuals and BCG-vaccinated individuals
| Commercial kit | No. of samples (% positive) with a positive result from:
|
|
|---|---|---|
| Latently infected individuals (n = 78) | BCG-vaccinated individuals (n = 25) | |
| InBios Active TbDetect ELISA | 4 (5.1) | 1 (4) |
| IBL M. tuberculosis IgG ELISA | 0 (0) | 0 (0) |
| Anda Biologicals TB ELISA | 24 (30.8) | 14 (56.0) |
Presence of M. tuberculosis antibodies in patients with evidence of latent M. tuberculosis infection.
Seventy-eight patients who exhibited positive results by the QFT and/or TST were evaluated for the presence of M. tuberculosis antibodies (Table 2). Fourteen positive samples were positive by QFT (17.9%), 50 samples were positive by TST (64.1%), and 14 samples tested by both TST and QFT were positive for both (17.9%). All samples were evaluated using each of the three commercial ELISAs evaluated in this study for the presence of antibodies to M. tuberculosis.
Testing by the InBios Active TbDetect IgG ELISA yielded positive results for 4 of the 78 samples from group IV, resulting in a positivity rate of 5.1% (Table 2). Three of the four samples that were positive by the InBios Active TbDetect IgG ELISA were from patients who had moderate to high risk of exposure to TB (Table 3). One of the positive serum samples was from a medical technician with diabetes who worked in the specimen-processing department of a large diagnostic laboratory. The patient had shown negative results in the past by TST but received 6 months of isoniazid prophylaxis following his recent positive QFT-G result. The second positive serum sample was from a medical technologist who was a long-term employee in a mycobacteriology laboratory. This technologist was given 6 months of isoniazid prophylaxis in 1988 for conversion to a positive TST result and is positive by QFT-G. The third positive serum sample was from a patient who had emigrated from Bolivia with positive results by both TST and QFT-G. The fourth patient had no known risk factors for exposure to TB and was positive by TST but negative by QFT-G.
TABLE 3.
Antibody-positive patients with expected latent TB infectiona
| Age (yr) | Genderb | Test resultc
|
Travel or risk factor | Treatment | ||
|---|---|---|---|---|---|---|
| QFT-G | TST | InBiosd | ||||
| 25 | M | Pos. | Neg. | Pos. | Medical technician diabetic | Treated for 6 mo in 2007 |
| 52 | M | Pos. | Pos. | Pos. | Mycobacterium lab technician | Treated in 1988 |
| 40 | M | Pos. | Pos. | Pos. | Immigrated from Bolivia in 2000 | No history of treatment |
| 21 | F | Neg. | Pos. | Pos. | No known travel or risk factors | No history of treatment |
All antibody-positive patients had negative chest X-ray results.
M, male; F, female.
Pos., positive; Neg., negative.
InBios Active TbDetect IgG ELISA.
The IBL M. tuberculosis IgG ELISA failed to detect M. tuberculosis antibodies in any of the serum samples from group IV. The Anda Biologicals TB ELISA detected 24 positive samples from this group, giving a positivity rate of 30.8%, about 10 times higher than the expected conversion rate (3 to 5%) from latent TB infection to active TB disease that has been cited in several studies on TB infection rates (3, 24).
DISCUSSION
Assays for the serodiagnosis of TB have been regularly evaluated with very different results (1, 2, 12, 38, 43, 45, 51). Rarely is an acceptable sensitivity and specificity from a commercially available kit for routine laboratory testing reported. The increased availability, decreased price, and rapid reporting ability of serology testing have prompted continued investigation into these options.
Each of the three commercial ELISAs evaluated showed significant differences in their ability to detect M. tuberculosis infection. The InBios Active TbDetect IgG ELISA demonstrated a significantly higher discriminating power than the IBL M. tuberculosis IgG ELISA and the Anda Biologicals TB ELISA by both ROC and scatter plot. Most studies concluded that a combination of several specific antigens would be the most effective way to increase sensitivity while not significantly decreasing specificity (1, 36, 38, 51, 54). The Anda Biologicals TB ELISA relies on the use of only a single (A60) antigen for antibody detection. While the sensitivity (83.3%) of the assay appears to be acceptable, the 24% positivity rate in healthy low-risk individuals makes this kit unacceptable for use as a screen for active TB disease. Although the IBL M. tuberculosis IgG ELISA employs three separate antigens for detection and had excellent specificity (100%), it performed with a very poor sensitivity (5.6%), which also eliminates it as a viable tool for detecting active TB disease. The InBios Active TbDetect IgG ELISA uses seven separate antigens. It performed well in both sensitivity (83.3%) and specificity (98.9%). The InBios kit, however, was insensitive (50% sensitivity) in detecting active TB disease in HIV-positive patients.
The ability of an assay to differentiate between latent TB infection and active TB disease offers a significant improvement over current routine test methods. Studies on TB infection indicate an expected conversion rate from latent TB infection to active TB disease at 3 to 5% within 2 years of exposure (3, 24). The InBios Active TbDetect IgG ELISA positivity rate for the latently infected individuals in our study (5.1%) was significantly closer to the expected positivity rate than that from Anda Biologicals TB ELISA (30.8%) or IBL M. tuberculosis IgG ELISA (0.0%). Three of the four patients that tested positive for TB antibodies by the InBios kit indeed had a moderate to high risk of exposure to TB. The increased reactivity of the Anda assay is most likely due to the cross-reactivity of the A60 antigen used in the assay with many environmental strains of Mycobacterium. Additionally, the administration of the TST test to some of our study patients may even have had a booster effect on the antibody response to the A60 antigens used in the Anda ELISA. In this study, the InBios assay appeared to offer reliable data to aid differentiation between active TB disease and latent TB infection in HIV-negative individuals.
One obstacle in TB testing is the high positivity rate for TST in BCG-vaccinated individuals (13, 22, 50). As observed in other studies, the high reactivity (56.0%) of the Anda Biologicals assay in the BCG-vaccinated group gives the test little clinical utility in this population (12, 34).
A significant problem for effective TB disease detection is the increasing prevalence of HIV-associated TB (18, 40, 47, 55). Although some M. tuberculosis antigens have shown a propensity for detecting TB in individuals infected with HIV/AIDS, a reliable and effective assay has not been developed (1, 23, 25, 36, 52). Our study showed that the InBios and Anda ELISAs both showed reactivity with sera from only three of six HIV-associated TB patients. Both assays failed to detect antibodies in the same three samples.
Considering the stage of HIV/AIDS infection at the time of testing and exposure to M. tuberculosis, several significant differences in antibody detection may be observed (33, 37, 42). While reports vary greatly on the effectiveness of various antigens, it is clear that additional work is necessary before accurate claims about the effectiveness of M. tuberculosis serology in HIV patients can be made.
In conclusion, of the three commercial antibody detection ELISAs we evaluated, the InBios Active TbDetect IgG ELISA was found to be the best overall assay for detecting active M. tuberculosis infection. The application of this assay in various populations and with a range of TB disease states should be further studied. The good sensitivity and specificity of the InBios Assay in the detection of active M. tuberculosis suggest that it may be a valuable additional diagnostic tool in the diagnosis of M. tuberculosis infection when used in conjunction with clinical findings and additional M. tuberculosis testing.
Acknowledgments
This study was supported by the ARUP Institute for Clinical and Experimental Pathology. A special thanks to InBios International, Inc., IBL-Hamburg, and Anda Biologicals for supplying reagents for this study.
We also thank Andrew Wilson from ARUP for his aid in the ROC analysis.
Footnotes
Published ahead of print on 30 September 2008.
REFERENCES
- 1.Abebe, F., C. Holm-Hansen, H. G. Wiker, and G. Bjune. 2007. Progress in serodiagnosis of Mycobacterium tuberculosis infection. Scand. J. Immunol. 66:176-191. [DOI] [PubMed] [Google Scholar]
- 2.Adjei, A. A., H. Armah, O. A. Duah, T. Adiku, and I. F. Hesse. 2003. Evaluation of a rapid serological chromatographic immunoassay for the diagnosis of pulmonary tuberculosis in Accra, Ghana. Jpn. J. Infect. Dis. 56:161-164. [PubMed] [Google Scholar]
- 3.American Thoracic Society. 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 161:221-247. [Google Scholar]
- 4.Araujo, Z., J. H. Waard, C. Fernandez de Larrea, D. Lopez, C. Fandino, A. Maldonado, E. Hernandez, Y. Ocana, R. Ortega, M. Singh, T. H. Ottenhoff, S. M. Arend, and J. Convit. 2004. Study of the antibody response against Mycobacterium tuberculosis antigens in Warao Amerindian children in Venezuela. Mem. Inst. Oswaldo Cruz 99:517-524. [DOI] [PubMed] [Google Scholar]
- 5.Arias-Bouda, L. M., S. Kuijper, A. Van der Werf, L. N. Nguyen, H. M. Jansen, and A. H. Kolk. 2003. Changes in avidity and level of immunoglobulin G antibodies to Mycobacterium tuberculosis in sera of patients undergoing treatment for pulmonary tuberculosis. Clin. Diagn. Lab. Immunol. 10:702-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzurri, A., G. V. Kanaujia, O. Y. Sow, B. Bah, A. Diallo, G. Del Prete, and M. L. Gennaro. 2006. Serological markers of pulmonary tuberculosis and of response to anti-tuberculosis treatment in a patient population in Guinea. Int. J. Immunopathol. Pharmacol. 19:199-208. [PubMed] [Google Scholar]
- 7.Beck, S. T., O. M. Leite, R. S. Arruda, and A. W. Ferreira. 2005. Combined use of Western blot/ELISA to improve the serological diagnosis of human tuberculosis. Braz. J. Infect. Dis. 9:35-43. [DOI] [PubMed] [Google Scholar]
- 8.Bellete, B., J. Coberly, G. L. Barnes, C. Ko, R. E. Chaisson, G. W. Comstock, and W. R. Bishai. 2002. Evaluation of a whole-blood interferon-gamma release assay for the detection of Mycobacterium tuberculosis infection in 2 study populations. Clin. Infect. Dis. 34:1449-1456. [DOI] [PubMed] [Google Scholar]
- 9.Bendayan, D., M. Raz, and M. R. Kramer. 2002. Latent tuberculosis infection: diagnosis and treatment. Harefuah 141:233-236, 316. [PubMed] [Google Scholar]
- 10.Bethunaickan, R., A. R. Baulard, C. Locht, and A. Raja. 2007. Antibody response in pulmonary tuberculosis against recombinant 27kDa (MPT51, Rv3803c) protein of Mycobacterium tuberculosis. Scand. J. Infect. Dis. 39:867-874. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia, A. S., S. Gupta, N. Shende, S. Kumar, and B. C. Harinath. 2005. Serodiagnosis of childhood tuberculosis by ELISA. Indian J. Pediatr. 72:383-387. [DOI] [PubMed] [Google Scholar]
- 12.Bukhary, Z. A. 2007. Evaluation of anti-A60 antigen IgG enzyme-linked immunosorbent assay for serodiagnosis of pulmonary tuberculosis. Ann. Thorac. Med. 2:47-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cascante, J., I. Pascal, V. Eguia, and J. Hueto. 2007. Diagnosis of tuberculosis infection. An. Sist. Sanit. Navar. 3(Suppl. 2):49-65. [PubMed] [Google Scholar]
- 14.Cesur, S., D. Saka, G. Tarhan, I. Ceyhan, H. Calisir, and M. Ogretensoy. 2005. Evaluation of a commercial enzyme immunoassay for the detection of interferon gamma levels in active tuberculosis patients and vaccinated healthy subjects. Mikrobiyol. Bul. 39:73-77. [PubMed] [Google Scholar]
- 15.Davidow, A., G. V. Kanaujia, L. Shi, J. Kaviar, X. Guo, N. Sung, G. Kaplan, D. Menzies, and M. L. Gennaro. 2005. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect. Immun. 73:6846-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desem, N., and S. L. Jones. 1998. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin. Diagn. Lab. Immunol. 5:531-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diel, R., A. Nienhaus, and R. Loddenkemper. 2007. Cost-effectiveness of interferon-gamma release assay screening for latent tuberculosis infection treatment in Germany. Chest 131:1424-1434. [DOI] [PubMed] [Google Scholar]
- 18.Diez, M., A. Diaz, M. J. Bleda, M. Aldamiz, M. Camafort, X. Camino, C. Cepeda, A. Costa, O. Ferrero, P. Geijo, J. A. Iribarren, S. Moreno, M. E. Moreno, P. Labarga, J. Pinilla, J. Portu, F. Pulido, C. Rosa, J. M. Santamaria, M. Telenti, L. Trapiella, M. Trastoy, and P. Viciana. 2007. Prevalence of M. tuberculosis infection and tuberculosis disease among HIV-infected people in Spain. Int. J. Tuberc. Lung Dis. 11:1196-1202. [PubMed] [Google Scholar]
- 19.Fleiss, J. L. 1981. Statistical methods for rates and proportions, 2nd ed. John Wiley and Sons, New York, NY.
- 20.Fujita, Y., H. Ogata, and I. Yano. 2005. Clinical evaluation of serodiagnosis of active tuberculosis by multiple-antigen ELISA using lipids from Mycobacterium bovis BCG Tokyo 172. Clin. Chem. Lab. Med. 43:1253-1262. [DOI] [PubMed] [Google Scholar]
- 21.Greenaway, C., C. Lienhardt, R. Adegbola, P. Brusasca, K. McAdam, and D. Menzies. 2005. Humoral response to Mycobacterium tuberculosis antigens in patients with tuberculosis in the Gambia. Int. J. Tuberc. Lung Dis. 9:1112-1119. [PubMed] [Google Scholar]
- 22.Harada, N., T. Mori, S. Shishido, K. Higuchi, and Y. Sekiya. 2004. Usefulness of a novel diagnostic method of tuberculosis infection, QuantiFERON TB-2G, in an outbreak of tuberculosis. Kekkaku 79:637-643. [PubMed] [Google Scholar]
- 23.Hendrickson, R. C., J. F. Douglass, L. D. Reynolds, P. D. McNeill, D. Carter, S. G. Reed, and R. L. Houghton. 2000. Mass spectrometric identification of Mtb81, a novel serological marker for tuberculosis. J. Clin. Microbiol. 38:2354-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horsburgh, C. R., Jr. 2004. Priorities for the treatment of latent tuberculosis infection in the United States. N. Engl. J. Med. 350:2060-2067. [DOI] [PubMed] [Google Scholar]
- 25.Houghton, R. L., M. J. Lodes, D. C. Dillon, L. D. Reynolds, C. H. Day, P. D. McNeill, R. C. Hendrickson, Y. A. Skeiky, D. P. Sampaio, R. Badaro, K. P. Lyashchenko, and S. G. Reed. 2002. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 9:883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imaz, M. S., M. A. Comini, E. Zerbini, M. D. Sequeira, M. J. Spoletti, A. A. Etchart, H. J. Pagano, E. Bonifasich, N. Diaz, J. D. Claus, and M. Singh. 2001. Evaluation of the diagnostic value of measuring IgG, IgM and IgA antibodies to the recombinant 16-kilodalton antigen of Mycobacterium tuberculosis in childhood tuberculosis. Int. J. Tuberc. Lung Dis. 5:1036-1043. [PubMed] [Google Scholar]
- 27.Janssens, J. P., P. Roux-Lombard, T. Perneger, M. Metzger, R. Vivien, and T. Rochat. 2007. Quantitative scoring of an interferon-gamma assay for differentiating active from latent tuberculosis. Eur. Respir. J. 30:722-728. [DOI] [PubMed] [Google Scholar]
- 28.Kalantri, Y., N. Hemvani, G. C. Bhatia, and D. S. Chitnis. 2005. ELISA kit evaluation for IgG and IgM antibodies to A-60 tubercular protein antigen. Indian J. Med. Sci. 59:337-346. [PubMed] [Google Scholar]
- 29.Khan, I. H., R. Ravindran, J. Yee, M. Ziman, D. M. Lewinsohn, M. L. Gennaro, J. L. Flynn, C. W. Goulding, K. DeRiemer, N. W. Lerche, and P. A. Luciw. 2008. Profiling antibodies to Mycobacterium tuberculosis by multiplex microbead suspension arrays for serodiagnosis of tuberculosis. Clin. Vaccine Immunol. 15:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, T. C., R. S. Blackman, K. M. Heatwole, T. Kim, and D. F. Rochester. 1984. Acid-fast bacilli in sputum smears of patients with pulmonary tuberculosis. Prevalence and significance of negative smears pretreatment and positive smears post-treatment. Am. Rev. Respir. Dis. 129:264-268. [PubMed] [Google Scholar]
- 31.Kudjawu, Y., V. Massari, O. Sow, B. Bah, B. Larouze, and J. F. Murray. 2006. Benefit of amoxicillin in differentiating between TB suspects whose initial AFB sputum smears are negative. Int. J. Tuberc. Lung Dis. 10:441-446. [PubMed] [Google Scholar]
- 32.Lalvani, A. 2007. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 131:1898-1906. [DOI] [PubMed] [Google Scholar]
- 33.Louie, L. G., W. E. Hartogensis, R. P. Jackman, K. A. Schultz, L. S. Zijenah, C. H. Yiu, V. D. Nguyen, M. Y. Sohsman, D. K. Katzenstein, and P. R. Mason. 2004. Mycobacterium tuberculosis/HIV-1 coinfection and disease: role of human leukocyte antigen variation. J. Infect. Dis. 189:1084-1090. [DOI] [PubMed] [Google Scholar]
- 34.Maekura, R., Y. Okuda, M. Nakagawa, T. Hiraga, S. Yokota, M. Ito, I. Yano, H. Kohno, M. Wada, C. Abe, T. Toyoda, T. Kishimoto, and T. Ogura. 2001. Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis. J. Clin. Microbiol. 39:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morimoto, T., S. Takanashi, Y. Hasegawa, K. Fujimoto, K. Okudera, A. Hayashi, K. Taima, and K. Okumura. 2006. Level of antibodies against mycobacterial glycolipid in the effusion for diagnosis of tuberculous pleural effusion. Respir. Med. 100:1775-1780. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee, S., N. Daifalla, Y. Zhang, J. Douglass, L. Brooks, T. Vedvick, R. Houghton, S. G. Reed, and A. Campos-Neto. 2004. Potential serological use of a recombinant protein that is a replica of a Mycobacterium tuberculosis protein found in the urine of infected mice. Clin. Diagn. Lab. Immunol. 11:280-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicol, M. Q., J. M. Mathys, A. Pereira, K. Ollington, M. H. Ieong, and P. R. Skolnik. 2008. Human immunodeficiency virus infection alters tumor necrosis factor alpha production via Toll-like receptor-dependent pathways in alveolar macrophages and U1 cells. J. Virol. 82:7790-7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raja, A., U. D. Ranganathan, and B. Ramalingam. 2006. Clinical value of specific detection of immune complex-bound antibodies in pulmonary tuberculosis. Diagn. Microbiol. Infect. Dis. 56:281-287. [DOI] [PubMed] [Google Scholar]
- 39.Raja, A., K. R. Uma Devi, B. Ramalingam, and P. J. Brennan. 2002. Immunoglobulin G, A, and M responses in serum and circulating immune complexes elicited by the 16-kilodalton antigen of Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 9:308-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranjbar, S., N. Ly, S. Thim, J. M. Reynes, and A. E. Goldfeld. 2004. Mycobacterium tuberculosis recall antigens suppress HIV-1 replication in anergic donor cells via CD8+ T cell expansion and increased IL-10 levels. J. Immunol. 172:1953-1959. [DOI] [PubMed] [Google Scholar]
- 41.Richeldi, L. 2006. An update on the diagnosis of tuberculosis infection. Am. J. Respir. Crit. Care Med. 174:736-742. [DOI] [PubMed] [Google Scholar]
- 42.Rosen, M. J. 2008. Pulmonary complications of HIV infection. Respirology 13:181-190. [DOI] [PubMed] [Google Scholar]
- 43.Rovatti, E., M. P. Corradi, M. Amicosante, P. L. Tartoni, W. Panini, A. Ancora, A. M. Cenci, L. Zucchi, L. Monno, G. Angarano, and C. Saltini. 1996. Evaluation of a Western blot serum test for the diagnosis of Mycobacterium tuberculosis infection. Eur. Respir. J. 9:288-292. [DOI] [PubMed] [Google Scholar]
- 44.Samb, B., D. Henzel, C. L. Daley, F. Mugusi, T. Niyongabo, N. Mlika-Cabanne, G. Kamanfu, P. Aubry, I. Mbaga, B. Larouze, and J. F. Murray. 1997. Methods for diagnosing tuberculosis among in-patients in eastern Africa whose sputum smears are negative. Int. J. Tuberc. Lung Dis. 1:25-30. [PubMed] [Google Scholar]
- 45.Silva, V. M., G. Kanaujia, M. L. Gennaro, and D. Menzies. 2003. Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int. J. Tuberc. Lung Dis. 7:478-484. [PubMed] [Google Scholar]
- 46.Sireci, G., F. Dieli, D. Di Liberto, S. Buccheri, M. P. La Manna, F. Scarpa, P. Macaluso, A. Romano, L. Titone, P. Di Carlo, M. Singh, J. Ivanyi, and A. Salerno. 2007. Anti-16-kilodalton mycobacterial protein immunoglobulin M levels in healthy but purified protein derivative-reactive children decrease after chemoprophylaxis. Clin. Vaccine Immunol. 14:1231-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobhani, R., A. Basavaraj, A. Gupta, A. S. Bhave, D. B. Kadam, S. A. Sangle, H. B. Prasad, J. Choi, J. Josephs, K. A. Gebo, S. N. Morde, R. C. Bollinger, Jr., and A. L. Kakrani. 2007. Mortality and clinical characteristics of hospitalized adult patients with HIV in Pune, India. Indian J. Med. Res. 126:116-121. [PubMed] [Google Scholar]
- 48.Steingart, K. R., M. Henry, S. Laal, P. C. Hopewell, A. Ramsay, D. Menzies, J. Cunningham, K. Weldingh, and M. Pai. 2007. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 4:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanir, G., A. Akin, C. Aydemir, C. Uner, and I. Ceyhan. 2005. The diagnosis of definitive or probable tuberculosis and latent tuberculosis infection in children with suspected tuberculosis. Tuberk. Toraks 53:259-264. [PubMed] [Google Scholar]
- 50.Teixeira, H. C., C. Abramo, and M. E. Munk. 2007. Immunological diagnosis of tuberculosis: problems and strategies for success. J. Bras. Pneumol. 33:323-334. [DOI] [PubMed] [Google Scholar]
- 51.Tiwari, R. P., N. S. Hattikudur, R. N. Bharmal, S. Kartikeyan, N. M. Deshmukh, and P. S. Bisen. 2007. Modern approaches to a rapid diagnosis of tuberculosis: promises and challenges ahead. Tuberculosis (Edinburgh) 87:193-201. [DOI] [PubMed] [Google Scholar]
- 52.Uma Devi, K. R., B. Ramalingam, and A. Raja. 2003. Antibody response to Mycobacterium tuberculosis 30 and 16kDa antigens in pulmonary tuberculosis with human immunodeficiency virus coinfection. Diagn. Microbiol. Infect. Dis. 46:205-209. [DOI] [PubMed] [Google Scholar]
- 53.van der Werf, T. S., P. K. Das, D. van Soolingen, S. Yong, T. W. van der Mark, and R. van den Akker. 1992. Sero-diagnosis of tuberculosis with A60 antigen enzyme-linked immunosorbent assay: failure in HIV-infected individuals in Ghana. Med. Microbiol. Immunol. 181:71-76. [DOI] [PubMed] [Google Scholar]
- 54.Wang, B. L., Y. Xu, Z. M. Li, Y. M. Xu, X. H. Weng, and H. H. Wang. 2005. Antibody response to four secretory proteins from Mycobacterium tuberculosis and their complex antigen in TB patients. Int. J. Tuberc. Lung Dis. 9:1327-1334. [PubMed] [Google Scholar]
- 55.Wang, Y., C. Collins, M. Vergis, N. Gerein, and J. Macq. 2007. HIV/AIDS and TB: contextual issues and policy choice in programme relationships. Trop. Med. Int. Health 12:183-194. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. 2008. Global tuberculosis control 2008: surveillance, planning, financing. World Health Organization, Geneva, Switzerland.