Abstract
Leptospirosis is a zoonosis of multisystem involvement caused by pathogenic strains of the genus Leptospira. In the last few years, intensive studies aimed at the development of a vaccine have provided important knowledge about the nature of the immunological mechanisms of the host. The purpose of this study was to analyze the immune responses to two recombinant proteins, MPL17 and MPL21 (encoded by the genes LIC10765 and LIC13131, respectively) of Leptospira interrogans serovar Copenhageni in individuals during infection. The recombinant proteins were expressed in Escherichia coli as six-His tag fusion proteins and were purified from the soluble bacterial fraction by affinity chromatography with Ni2+-charged resin. The recombinant proteins were used to evaluate their ability to bind to immunoglobulin G (IgG) (and IgG subclass) or IgM antibodies in serum samples from patients in the early and convalescent phases of leptospirosis (n = 52) by enzyme-linked immunosorbent assays. The prevalences of total IgG antibodies against MPL17 and MPL21 were 38.5% and 21.2%, respectively. The titers achieved with MPL17 were statistically significantly higher than those obtained by the reference microscopic agglutination test. The specificity of the assay was estimated to be 95.5% for MPL17 and 80.6% for MPL21 when serum samples from individuals with unrelated febrile diseases and control healthy donors were tested. The proteins are conserved among Leptospira strains that cause human and animal diseases. MPL17 and MPL21 are most likely new surface proteins of leptospires, as revealed by liquid-phase immunofluorescence assays with living organisms. Our results demonstrate that these recombinant proteins are highly immunogenic and, when they are used together, might be useful as a means of diagnosing leptospirosis.
Leptospirosis is an acute febrile disease caused by pathogenic spirochetes of the genus Leptospira. It is considered an important reemerging infectious disease that affects populations worldwide (15). The disease is endemic in tropical and subtropical regions, while the disease is associated with occupational and recreational activities in temperate climates (5). Leptospiral serovar diversity results from structural heterogeneity in the carbohydrate component of lipopolysaccharides (12). Many serovars are adapted for specific mammalian reservoir hosts, which harbor the organisms in their renal tubules and shed them in their urine. Humans are accidental hosts that become infected through contact with wild or domestic animals or exposure to contaminated soil or water (27). Due to the broad spectrum of symptoms, the disease largely remains underdiagnosed. If it is not treated in due course, patients may develop renal damage or liver failure, and in some cases death may occur (2, 5, 22). Although leptospirosis can be treated, early diagnosis is critical for the establishment of effective antibiotic therapy. The “gold standard” reference method for serological diagnosis of the disease is the microscopic agglutination test (MAT), in which sera from patients are reacted with suspensions of antigens from live leptospiral serovars (15, 25). In addition to the complexities with the control and interpretation of MAT, serological diagnosis by MAT has a low sensitivity during the early phase of the disease, since it relies on antibodies to leptospiral antigens not detected in the first few days after exposure (2, 11, 22, 37). Although we have identified a leptospiral protein, Lp29, that was capable of reacting with sera from patients diagnosed with leptospirosis during the early phase of the disease (32), its poor conservation among pathogenic leptospires restricts the utility of this protein.
We have investigated two leptospiral proteins, MPL17 (encoded by LIC10765 and MPL21 (encoded by LIC13131), which have previously been identified in the genome sequences of Leptospira interrogans serovar Copenhageni (30, 31) and which have been shown to be reactive with serum from patients diagnosed with leptospirosis during the convalescent phase (19). These 2 proteins were selected for further study from among the 16 recombinant proteins that have been described because they showed low levels of reactivity with serum samples from healthy individuals and we were able to obtain them in a soluble form in an Escherichia coli expression system. These recombinant proteins were evaluated for their reactivities with sera from patients in the early and the convalescent phases of the illness by using a selection of serum samples that was larger than that employed in our earlier studies (19) and that included sera from patients with other febrile diseases.
MATERIALS AND METHODS
Characterization of the proteins in silico.
The predicted coding sequences of MPL17 and MPL21 from the L. interrogans serovar Copenhageni genome were selected on the basis of the prediction of their cellular localization by the PSORT program (http://psort.nibb.ac.jp/). The SMART (http://smart.embl-heidelberg.de/), PFAM (http://www.sanger.ac.uk/Software/Pfam/), and LipoP (http://www.cbs.dtu.dk/services/LipoP/) web servers were used to search for predicted functional and structural domains within the amino acid sequences of the selected sequences. The predicted sequence of the lipobox was evaluated by use of the SpLip program, as described by Setubal et al. (42). The PSIPRED server (http://bioinf.cs.ucl.ac.uk/psipred/) was employed for prediction of the protein secondary structure.
Sera and bacteria strains.
Serum samples from patients confirmed to have leptospirosis were obtained from the collection of the Instituto Adolfo Lutz; serum samples from patients with unrelated infectious diseases were obtained from the collections of the Laboratorio Imunoepidemiologia, SUCEN, São Paulo, Brazil; Laboratorio Protozoologia, IMT/USP, Brazil (sera from patients with Chagas' disease); Laboratorio Virologia, IMT/USP, Brazil (sera from patients with human immunodeficiency virus [HIV] infection and dengue); and the Nucleo Estudos em Malária, SUCEN/IMT/USP, Brazil (sera from patients with malaria). Human sera were from the collections of the Instituto Adolfo Lutz, SUCEN, and IMT (São Paulo, Brazil) and were donated for use for research purposes. Strains of L. interrogans serovars Bratislava (Berlin 406), Autumnalis (Akiyami A), Pyrogenes (Salinem), Canicola (Hond Utrechet IV), Copenhageni (M20), Hardjo (Hardjoprajtino), Icterohaemorrhagiae (RGA), and Pomona (Pomona) and L. biflexa serovar Patoc (Patoc 1) were from the Faculdade de Medicina Veterinária da Universidade de São Paulo, São Paulo, Brazil. The leptospires were cultured at 29°C under aerobic conditions in liquid Elinghausen-McCullough-Johnson-Harris medium (Difco) with 10% rabbit serum enriched with l-asparagine (0.015%, wt/vol), sodium pyruvate (0.001%, wt/vol), calcium chloride (0.001%, wt/vol), magnesium chloride (0.001%, wt/vol), peptone (0.03%, wt/vol), and meat extract (0.02%, wt/vol).
DNA recombinant techniques.
Genes without signal peptides were amplified from the genomic DNA of L. interrogans serovar Copenhageni by PCR with specific primers (primers LIC10765 F [CACC GAA AGT CCC GTA AGG TTC AAA], LIC10765R [TGC AGG AGT TCC CAC ATT TTA], LIC13131F [CACC ACG TCT CAA AGT TAC GCT TCA G], and LIC13131R [TTC TCA CCA TCC AGC TCG G]), and the conditions used were those previously described by Gamberini et al. (19). The E. coli Gateway cloning (pENTR) and expression (pDEST17) system (Invitrogen) was used. The pDEST17 vector allows the expression of recombinant proteins with a six-His tag at the N terminus.
Expression and purification of recombinant proteins.
E. coli BL21(DE3) (45) and E. coli BL21 SI (4) containing recombinant plasmids pDEST17-LIC10765 and pDEST17-LIC13131, respectively, were grown at 37°C and 30°C in Luria-Bertani broth with or without NaCl and with 100 μg/ml ampicillin with continuous shaking until an optical density at 600 nm (OD600) of 0.6 to 0.8 was reached. Recombinant protein synthesis was induced by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) or 3 mM NaCl. After 3 h, the bacterial suspensions were pelleted by centrifugation and resuspended in lysis buffer (20 mM Tris-HCl, pH 8.0, 200 mM NaCl, 200 μg/ml of lysozyme, 1% Triton X-100, 2 mM phenylmethylsulfonyl fluoride [PMSF]). The bacterial cell pellets were lysed on ice with the aid of a sonicator (Ultrasonic Processor; GE). The soluble and insoluble fractions were separated by centrifugation at 3,000 × g for 30 min at 4°C. Recombinant proteins were purified from the supernatants of the bacterial cell lysates by using Ni2+-charged Sepharose beads (GE). After the proteins were extensively washed with increasing concentrations of imidazole (20 to 60 mM), the proteins were eluted in 20 mM Tris-HCl (pH 8.0)-200 mM NaCl containing 500 mM imidazole. The efficiency of purification of the recombinant proteins was evaluated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Fractions containing high concentrations of purified recombinant proteins were pooled and extensively dialyzed against phosphate-buffered saline (PBS) containing 1 mM PMSF. The concentration of the recombinant proteins was estimated by the method of Bradford (6).
CD spectroscopy.
Measurements were obtained by circular dichroism (CD) spectroscopy at 20°C in a Jasco J-810 spectropolarimeter (Japan Spectroscopic, Tokyo, Japan) equipped with a Peltier unit for temperature control. CD spectroscopy of the far-UV spectrum for 20 μM recombinant proteins in 10 mM sodium phosphate buffer was performed. The spectra were measured at 0.5-nm intervals by using a cell with a 1-mm path length and are presented as the averages of five scans recorded from 190 to 260 nm. Spectral data were analyzed with Dicroprot software (http://dicroprot-pbil.ibcp.fr/) by using the K2D (Kohonen neural network with a two-dimensional output layer) method for estimation of the secondary structure content (1, 13).
MAT.
MAT was performed as described by Faine et al. (15) by using a battery of the following 22 serovars of Leptospira spp. as antigens: Andaman, Australis, Autumnalis, Bataviae, Butembo, Canicola, Castellonis, Celledoni, Copenhageni, Cynopteri, Djasiman, Grippotyphosa, Hebdomadis, Icterohaemorrhagiae, Javanica, Panama, Patoc, Pomona, Pyrogenes, Shermani, Tarassovi, and Wolffi. All the strains were maintained in liquid Elinghausen-McCullough-Johnson-Harris medium (Difco) at 29°C. A laboratory-confirmed case of leptospirosis was defined by the demonstration of a fourfold rise in the microagglutination titer between paired serum samples. The serovar with a titer with 50% agglutination of the highest sample dilution was considered the probable predominant serovar. Serum was considered MAT negative if no agglutination was detected when it was tested with the 22 serovars listed above.
ELISA for detection of human IgG and IgM antibodies.
Human immunoglobulin G (IgG) antibodies against MPL17 and MPL21 were detected by enzyme-linked immunosorbent assay (ELISA), as described previously (39), but in this work the plates were covered with 250 ng/well of each protein. All samples were diluted 1:100 and were evaluated for total IgG and IgM by using peroxidase-conjugated anti-human IgG and IgM antibodies (1:5,000; Sigma). Serial dilutions (1:100 to 1:6,400) of sera positive for recombinant proteins were subsequently analyzed. We utilized individual titers that represented the highest dilution of serum that presented an OD492 greater than 0.1. The results were expressed as log10, and the cutoff points were set at 3 standard deviations above the mean OD492 for sera from six individuals from the city of São Paulo, Brazil, who had not been exposed to leptospirosis. Sera from patients diagnosed with unrelated febrile infection diseases and healthy donors were employed, as follows: dengue (n = 13), malaria (n = 15), HIV infection (n = 15), Chagas' disease (n = 20), and healthy donors (n = 6). The sera were used at 1:100 dilutions.
Detection of antigen-specific antibodies of distinct IgG subclasses by ELISA.
The ELISA used to detect IgG subclasses was performed as described previously (33). The serum dilution used was 1:25; and the mouse monoclonal antibodies to human IgG subclasses (Sigma) used were diluted 1:400 (anti-IgG1), 1:2,500 (anti-IgG2), 1:125 (anti-IgG3), and 1:500 (anti-IgG4). Monoclonal antibody binding was detected with peroxidase-conjugated anti-mouse Ig (Sigma) at 1:1,000 dilutions.
Production of serum with antibodies against the recombinant proteins.
Female BALB/c mice (weight, 18 to 22 g) were immunized subcutaneously with 10 μg of recombinant protein MPL17 or MPL21 containing complete Freund adjuvant (1:1, vol/vol). A booster injection of the protein preparations, but with incomplete Freund adjuvant, was given after 2 weeks. One week after each immunization, the mice were bled from the retroorbital plexus, and the pooled sera were analyzed by ELISA for determination of the antibody titers. The control group was inoculated with PBS and adjuvant. All animal studies were approved by the Ethics Committee of the Instituto Butantan, São Paulo, Brazil.
Liquid-phase immunofluorescence assay (L-IFA).
The localization of MPL17 and MPL21 by immunofluorescence was performed by a protocol modified from that of Costa et al. (8). In brief, suspensions of 2.5 ml live leptospires were harvested at 10,000 rpm for 15 min, washed twice with PBS (with 50 mM NaCl), resuspended in 200 μl of PBS with 6 μg/ml propidium iodide to stain the nuclei, and incubated for 45 min at 37°C. After incubation, the leptospires were washed gently with PBS and incubated for 30 min at 4°C with polyclonal mouse antiserum against recombinant MPL17, recombinant MPL21, recombinant LipL32, or recombinant GroEL at a 1:50 dilution. The leptospires were washed and incubated with goat anti-mouse IgG antibodies conjugated to fluorescein isothiocyanate (FITC; Sigma) at a dilution of 1:50 for 30 min at 4°C. After incubation with secondary antibody, the leptospires were washed twice and resuspended in PBS-antifading solution (ProLong Gold; Molecular Probes). The immunofluorescence-labeled leptospires were examined by use of a confocal LSM 510 META immunofluorescence microscope (Zeiss, Germany).
DNA and RNA isolation and PCR analysis.
Leptospira cultures were harvested by centrifugation at 11,500 × g for 30 min and were gently washed twice in sterile PBS. The genomic DNA was isolated from the pellets with a guanidine-and-detergent lysing solution (DNAzol reagent; Invitrogen), according to the manufacturer's instructions. For reverse transcription-PCR (RT-PCR), total RNA was isolated from leptospires cultured as described above by the acid guanidinium thiocyanate phenol-chloroform method with the TRIzol reagent (Invitrogen), according to the manufacturer's recommendations. Two micrograms of RNA from each sample was treated with 1 U of DNase I (Invitrogen) for 15 min at room temperature. DNase I was inactivated by the addition of 1 μl of 25 mM EDTA solution, followed by incubation at 65°C for 10 min. DNase-treated RNAs were reverse transcribed by using the SuperScript III first-strand synthesis system (Invitrogen) for RT-PCR. Two-microliter aliquots of the cDNA were used to amplify cDNA-specific genes by PCR. Sample integrity was verified by amplification of a 1,042-bp 16S ribosomal cDNA fragment by using oligomers 16R and 16F.
Statistical analysis.
The differences between the proportions of responders were analyzed by the chi-square test (version 3.0; GraphPad Prism, San Diego, CA). The unpaired t test was used to compare the mean values of the antibody titers between the two tests. A P value less than 0.05 was considered statistically significant. The values for specificity were determined by the method of Galen and Gambino (18).
RESULTS
Features of the proteins.
In silico analysis predicts that both MPL17 and MPL21 encode membrane proteins (28); have signal peptide tags with a signal cleavage site between amino acids 35/36 and 21/22, respectively (3); and have no assigned function (40). Putative lipoprotein signal peptide sequences (SpII) in both coding sequences were not identified by the use of either the LipoP server (24) or the SpLip lipoprotein prediction algorithm described by Setubal et al. (42). The sequence encoding MPL17 in L. interrogans serovar Copenhageni was present with 100% similarity to that in the genome sequence of L. interrogans serovar Lai strain 56601 (38) and with 80% similarity to that in the genome sequences of L. borgpetersenii serovar Hardjo-bovis strains L550 and JB197 (7), while a lower level of sequence similarity (40%) was found in both strains of L. biflexa serovar Patoc sequenced (36). The MPL21 sequence of L. interrogans serovar Copenhageni has a similar counterpart (98%) in L. interrogans serovar Lai strain 56601 (38) and 45% similarity to the sequences in the genomes of both strains of L. biflexa serovar Patoc (36), but it is absent from the genome sequences of L. borgpetersenii serovar Hardjo-bovis strains L550 and JB197 (7).
Expression, purification, and characterization of MPL17 and MPL21 recombinant proteins.
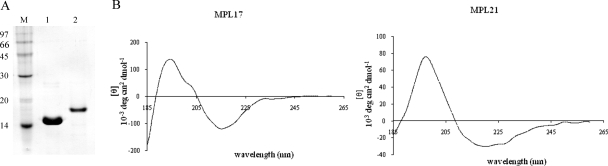
The proteins were expressed in a soluble form by the addition of 0.1 mM IPTG to E. coli BL21(DE3) for MPL17 and 3 mM NaCl to E. coli BL21 SI for MPL21. The recombinant proteins were purified by Ni2+-charged chromatography under nondenaturing conditions. Aliquots of the purified MPL17 and MPL21 proteins were analyzed by 15% SDS-PAGE (Fig. 1A), by which the proteins appeared as single major bands (Fig. 1A). Because certain recombinant proteins tend to lose their structure either as a result of the purification process or during storage, we assessed their secondary structures by CD spectroscopy. As demonstrated in Fig. 1B, both proteins present a mixture of secondary structures, as none of the spectra showed a flat line, characteristic of the denatured nonstructured form. The spectrum of MPL21 depicts a positive band at 198 nm and a minimum broad band at 215 to 225 nm; these consist of predominantly β strands and α helices, respectively. For MPL17, the spectrum shows a negative ellipticity of approximately 215 nm and a positive band near 192 nm; these consist of predominantly β strands and α helices, respectively. The estimation of the secondary structure content resulted in 37% and 26% α helix and β strands, respectively, for both recombinant proteins by the K2D method (1, 13). These data corroborate the predicted secondary structures of the proteins, which have shown a mixture of α helices and β strands for both proteins (23).
FIG. 1.
SDS-PAGE analysis of purified recombinant proteins and CD spectra. (A) Recombinant proteins MPL17 and MPL21 were expressed in E. coli BL21(DE3) and BL21 SI, respectively, and purified from the supernatant of the bacterial cell lysate by using Ni2+-charged chelating Sepharose columns. Lane 1, MPL17; lane 2, MPL21; lane M, protein molecular mass marker. (B) Far-UV spectrum by CD spectroscopy of 20 μM recombinant proteins obtained in 10 mM sodium phosphate buffer. The y axes indicate ellipticity values (to be multiplied by 103) in degrees cm2 dmol−1.
Reactivities of MPL17 and MPL21 with sera from confirmed and unconfirmed cases of leptospirosis.
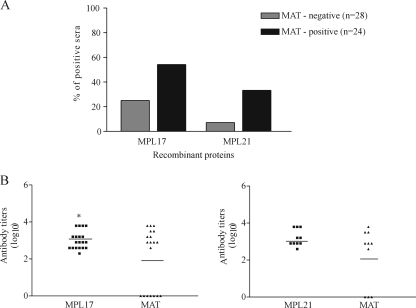
Sera from patients with confirmed early- and convalescent-phase leptospirosis were evaluated for the presence of total IgG antibodies by ELISA. The cutoff values were defined as the mean plus 3 standard deviations obtained with serum samples from six healthy individuals. Our results show that MPL17 IgG and MPL21 IgG antibodies were detected by ELISA both in samples from patients with early-phase disease who were negative by MAT and in samples from patients with convalescent-phase disease who were positive by MAT (Fig. 2A). No statistically significant difference between the frequency of sera positive for the recombinant MPL17 and MPL21 proteins was detected in the different groups of individuals. Since MAT is the reference test used to diagnose the disease, we evaluated the IgG antibody titers of the recombinant proteins by MAT. The titers achieved with MPL17 were statistically significant higher than those achieved by MAT (Fig. 2B), but comparable values were obtained when MPL21 and MAT were used (Fig. 2B). IgM antibodies to recombinant protein MPL21 were found in sera from individuals diagnosed with leptospirosis. Total IgM antibodies was detected only with MPL21, and the frequency of responders was statistically significant higher when sera from patients in the convalescent phase than when sera from patients in the early phase of leptospirosis were used (Fig. 3A). The results were considered positive when the mean values at OD492 were above the mean plus 3 standard deviations for sera from six uninfected controls. Compared to the results of MAT (Fig. 3B), no statistically significant difference in the antibody levels obtained was observed between the two tests.
FIG. 2.
Recognition of recombinant proteins MPL17 and MPL21 by IgG antibodies in sera from individuals diagnosed with leptospirosis. (A) Percentage of positive sera (responders) determined by ELISA with recombinant proteins MPL17 and MPL21 and serum samples from patients with both phases of the disease tested at a dilution of 1:100. Serum was considered MAT positive or MAT negative if agglutination was detected when the sera were tested for their reactivities with isolates of the 22 serovars (see Materials and Methods). The cutoff values were defined as the mean plus 3 standard deviations obtained for sera from six healthy individuals. No statistically significant difference in the frequency of positive sera for recombinant proteins MPL17 and MPL21 in the different groups of individuals was observed. (B) Comparison of the antibody titers of serum samples that recognize recombinant proteins MPL17 (left) and MPL21 (right) and that were obtained by MAT. The symbols represent the titer of each serum sample. The solid lines represent the geometric mean antibody titers in each group. Significance was assessed by comparison with antibody titers to MPL17 and those obtained by MAT by use of the unpaired t test (*, P < 0.05).
FIG. 3.
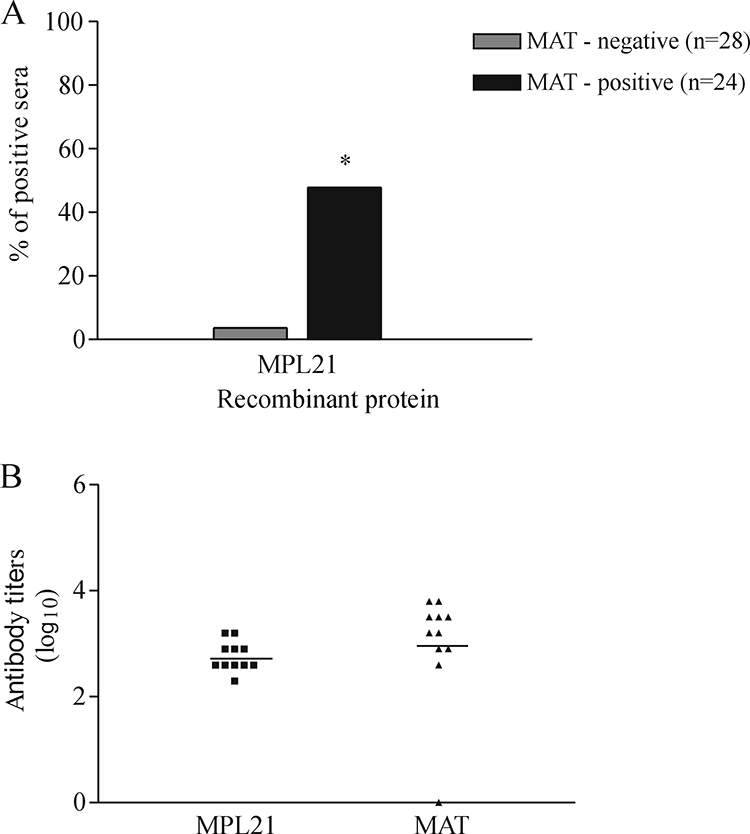
Prevalence of IgM antibodies to recombinant protein MPL21 in sera from individuals diagnosed with leptospirosis as determined by ELISA. (A) Serum samples diluted 1:100 were considered positive when the mean OD492 values were above the mean plus 3 standard deviations for six uninfected control serum samples. *, a significant increase in the frequency of responders containing IgM antibodies in convalescent-phase serum (P < 0.05). (B) The antibody titers of each individual represent the last dilution that had an OD492 greater than 0.1. The horizontal lines represent the geometric mean antibody titers for each group. No statistically significant difference in antibody levels was observed between the two tests.
Specificities of the recombinant proteins with serum samples from individuals with unrelated diseases and healthy controls.
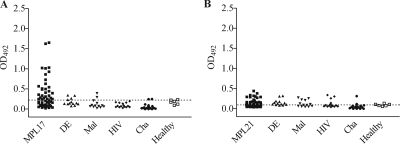
Due to the nonspecific clinical symptoms of leptospirosis, we analyzed the reactivities of the recombinant proteins with serum samples from patients with other febrile illnesses, including dengue (n = 13), malaria (n = 13), Chagas' disease (n = 20), and HIV infection (n = 15), and control sera from healthy donors (n = 6). The reactivities obtained with MPL17 and these serum samples from patients with other febrile diseases were similar to the ones obtained with serum samples from healthy donors, but they were much lower than those obtained with serum samples from patients with leptospirosis (Fig. 4A). MPL21 showed a behavior similar to that of MPL17, but it presented a reactivity lower than that of MPL17 with sera from patients with leptospirosis (Fig. 4B). The specificities of MPL17 and MPL21 were calculated to be 95.5% and 80.6%, respectively.
FIG. 4.
Evaluation of the specificities of the recombinant proteins with serum samples from individuals with unrelated diseases and healthy controls. The specificities of the reactivities of the proteins to serum samples from patients with other febrile illnesses and healthy donors were determined by ELISA. The symbols represent the reactivities against recombinant proteins MPL17 (A) and MPL21 (B) of each serum sample tested in duplicate at a 1:100 dilution. DE, individuals with dengue (n = 13); Mal, individuals with Plasmodium vivax or P. falciparum malaria (n = 13), HIV, individuals infected with HIV (n = 15); Cha, individuals with Chagas' disease (n = 20); healthy, healthy individuals (n = 6). The dashed lines represent the cutoff values (0.325 and 0.168 for MPL17 and MPL21, respectively).
IgG subclasses specific for recombinant protein MPL17 or MPL21.
To study the type of immune response triggered by these proteins, we performed the ELISA using subclass-specific anti-human IgG1, IgG2, IgG3, or IgG4 antibodies and serum samples at a dilution of 1:25. Only serum samples that had total IgG specific for recombinant protein MPL17 or MPL21 were tested by this assay. For MPL17, the proportions of the IgG1, IgG2, IgG3, and IgG4 subclasses detected were 89.5%, 94.7%, 100.0%, and 94.7%, respectively, whereas for MPL21 the proportions were 0%, 10.0%, 10.0%, and 10.0%, respectively.
Localization of leptospiral antigens by L-IFA.
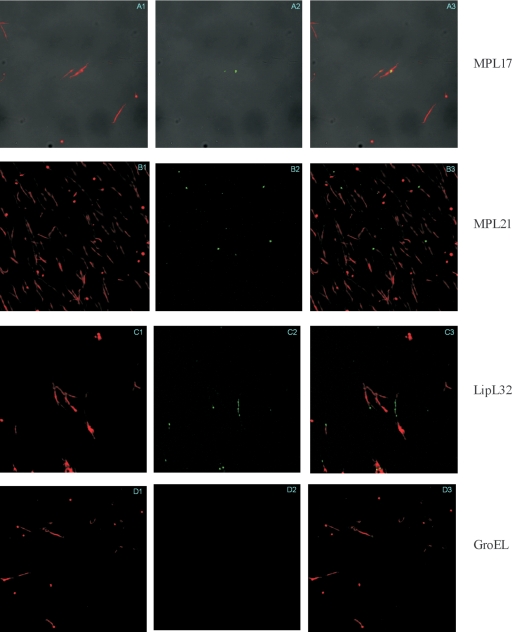
To evaluate whether MPL17 and MPL21 are located in the bacterial membrane, we set out to analyze their localization by using living organisms and the L-IFA method. Leptospires were visualized by propidium iodide staining (Fig. 5A1, B1, C1, and D1), followed by protein detection with polyclonal mouse antiserum raised against each protein in the presence of anti-mouse IgG antibodies conjugated to FITC. Green fluorescence could be observed for MPL17 (Fig. 5A2), MPL21 (Fig. 5B2), and LipL32 (an outer membrane protein used as a positive control; Fig. 5C2) (29) but not with GroEL (Fig. 5D2), a protoplasmic cylinder marker used as a negative control (21). The localization within the leptospires of the green fluorescence indicating the protein was achieved by superimposing both fields; and the results obtained are shown in Fig. 5A3, B3, C3, and D3 for MPL17, MPL21, LipL32, and GroEL, respectively.
FIG. 5.
Protein recognition by L-IFA. Proteins in live L. interrogans isolates were recognized by antibodies against MPL17, MPL21, LipL32 (a surface-exposed lipoprotein), and GroEL (a protoplasmic cylinder marker) under a confocal microscope. The leptospires were identified by using the DNA counterstain propidium iodide. Panels 1 to 3, propidium iodide-stained, FITC-stained, and composite images, respectively.
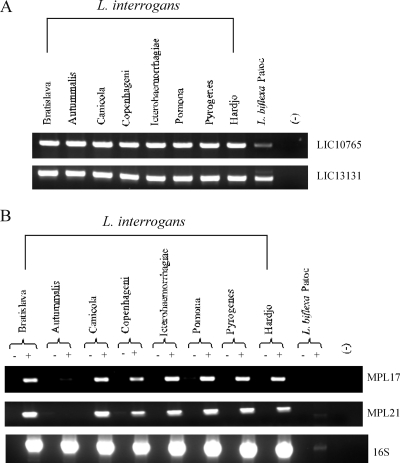
Conservation of protein antigens among leptospiral strains.
Protein conservation in the most prevalent pathogenic serovars of L. interrogans is an important requirement for leptospiral antigens. To characterize the conservation of the coding sequences selected, we evaluated several L. interrogans serovars (Bratislava, Autumnalis, Canicola, Copenhageni, Icterohaemorrhagiae, Pomona, Pyrogenes, and Hardjo) and a nonpathogenic strain (L. biflexa serovar Patoc) for the presence of DNA (Fig. 6A) and the corresponding transcript (Fig. 6B). The presence of the MPL17 and MPL21 genes was confirmed in all strains tested (Fig. 6A), and their transcripts could also be detected in all serovars except serovar Autumnalis, in the case of MPL21 (Fig. 6B). These data were confirmed by the absence of reactivity of the MPL21 protein with serum from a patient infected with this serovar. Although a weak band was seen for the MPL17 transcript, MPL17 was able to react with serum samples from patients infected with serovar Autumnalis. Transcripts were barely noticed for both MPL17 and MPL21 for nonpathogenic strain L. biflexa, consistent with the data obtained by BLAST analysis, which showed partial sequence similarity.
FIG. 6.
MPL17 and MPL21 gene and transcript conservation in different strains of Leptospira. (A) Genomic DNA from L. biflexa Patoc and eight serovars belonging to the pathogenic species L. interrogans was subjected to PCR analysis with primers that were specific for MPL17 and MPL21 and that were designed according to the L. interrogans serovar Copenhageni genome sequence. No DNA was added to the negative control reaction, as indicated by (−). (B) RT-PCR analysis of the MPL17 and MPL21 transcripts along with those of different serovars of L. interrogans strains and the saprophytic strain L. biflexa serovar Patoc strain. Reactions were performed with the same primer pairs mentioned above. Sample quantity and integrity were verified by amplification of a 1,042-bp 16S ribosomal cDNA fragment. +, reverse transcriptase present; −, reverse transcriptase omitted.
DISCUSSION
Leptospirosis is a zoonotic disease with a global distribution. Antibiotic treatment is available and can be effective when it is initiated early in the course of the disease (15). MAT, the reference method for the serological diagnosis of leptospirosis (2, 5), and other commercially available whole Leptospira-based assays (2, 26, 43) present low sensitivities during the early phase of the disease. As a result, there is an urgent need for an improved and accurate test for the diagnosis of leptospirosis. The search for conserved antigens that could be produced by recombinant technologies, thus avoiding the problems associated with whole-cell preparations, is under way (9, 14, 32, 34, 41, 44). Nevertheless, difficulties associated with a low sensitivity of detection during the early stage of the disease, poor protein conservation among Leptospira strains, and cross-reactivity with serum from individuals with unrelated febrile illness have, to date, hampered the identification of the ideal antigen to be used for diagnosis.
In the present work, we carried out further studies with two proteins, MPL17 and MPL21, that were previously shown to react with serum from a patient with leptospirosis during the convalescent phase (19). We succeeded in expressing the proteins in their soluble forms by employing such conditions as low IPTG and NaCl concentrations, depending on the E. coli strain used, and temperature induction. These conditions were found to favor the expression of their soluble forms. The maintenance of the secondary structures of the recombinant proteins after the purification process has been one of our concerns, because some denatured proteins may lose their immunogenicity and immunoprotection activities (35). CD spectroscopy showed a mixture of β strands and α helices in the secondary structure contents of both proteins, which validates their use for immunological studies.
The reactivities of the recombinant proteins against sera from patients with confirmed early and convalescent phases of leptospirosis were evaluated by ELISA. MPL17 and MPL21 were reactive with IgG antibodies present both in the sera of patients with early-phase leptospirosis who were negative by MAT and in the sera of patients with late-phase leptospirosis who were positive by MAT, but a higher sensitivity was revealed with MPL17. These results confirm and extend our previous data for MPL17 and MPL21, which have shown the reactivities of these proteins with IgG antibodies during the convalescent phase of the disease (19). Interestingly, a higher statistically significant sensitivity was observed with MPL17 compared to that obtained by MAT, but this was not the case for MPL21, which showed results similar to those obtained by MAT.
When we assessed sera from patients with early-phase leptospirosis who were negative by MAT and patients with late-phase leptospirosis who were positive by MAT for anti-MPL17 IgM and anti-MPL21 IgM antibodies, only anti-MPL21 IgM antibodies were detected. The reason for the absence of anti-MPL17 IgM antibodies is unknown. The strong IgG antibody response and the absence of IgM antibodies have been shown for other leptospiral proteins and possibly relate to a memory response due to a previous contact with the bacterium (9, 17, 20). Comparable data were achieved for anti-MPL21 IgM antibodies and the antibody titers determined by MAT.
The nonspecific symptoms of leptospirosis and their similarity to those of other tropical febrile diseases make the diagnosis of the disease difficult (26). We thus evaluated the specificities of MPL17 and MPL21 for their reactivities with the IgG antibodies present in serum samples of patients with unrelated febrile diseases. The specificity of the IgG antibody response to MPL17 was 95.5% with sera from patients with febrile diseases such as dengue, malaria, HIV infection, and Chagas' disease and serum samples from healthy individuals. An 80.6% specificity was detected with MPL21 when the same serum samples were tested. The specificities of recombinant LipL41 by the latex agglutination test and a flowthrough assay were reported to be 90.45% and 77.70%, respectively (41). The specificities of the IgG antibody responses to recombinant LigB were 100%, 94%, and 88% with sera from healthy individuals from the United States, from residents of Salvador, Brazil, and from individuals in high-risk slum communities in Salvador, Brazil (9). Even though the specificity of the LigA IgG ELISA was estimated to be 100%, only serum samples from patients with serologically confirmed scrub typhus and dengue were employed (44). Although distinct methodologies were employed, the specificity of the IgG antibody response to MPL17 was similar to the specificity of the IgG antibody response to recombinant LigB. This finding must be considered in light of the fact that in our study healthy individuals were from the city of São Paulo, Brazil, who are comparable to residents from the city of Salvador (Ministry of Health, MS, Brazil, 2007 [http://www.saude.gov.br/svs]).
The frequencies of responders to antibodies of the distinct IgG subclasses obtained with recombinant proteins MPL17 or MPL21 were similar and suggest a combination of Th1 and Th2 responses. Vernel-Pauillac and Merien (46) have demonstrated that pathogenic leptospires can stimulate a Th1 response, together with antilipopolysaccharide antibodies, in an animal model. The immunoprotective mechanism evaluated in animals immunized with a LigA DNA vaccine was recently shown to be conferred by both humoral and cellular immunities (16). Although more studies are needed, our results are in agreement with the data reported in the literature.
We have employed for the first time live leptospires to assess the bacterial cell localization of the proteins by L-IFA. A similar method was used in the work of Cullen et al. (10); however, they used dried pellets and sonicates. Polyclonal serum against each recombinant protein showed reactivities (positive green fluorescence) with MPL17 and MPL21 that were similar but to less intense than the reactivities obtained with the LipL32 outer membrane protein, a major leptospiral antigen (21, 29). The fluorescence obtained with MPL17 and MPL21, in addition to the negative reactivity to the protoplasmic cylinder protein GroEL antiserum, suggests that these proteins are surface exposed.
An important requirement for the development of recombinant protein-based serological tests is the conservation of proteins among diverse leptospiral pathogenic strains. The transcripts of both MPL17 and MPL21 were detected in several Leptospira strains, including those that cause diseases in humans and animals.
Although MPL17 had a higher specificity than MPL21, only the latter detected IgM antibodies by ELISA. Thus, we presume that MPL17 and MPL21 together are good leptospiral antigens. A larger array of serum samples, in addition to immune protection studies, will be used to evaluate the suitability of the use of these proteins for the diagnosis of leptospirosis, as well as their potentials as vaccine candidates.
Acknowledgments
We are deeply indebted to Toshie Kawano and Alexsander Seixas de Souza (Departamento de Parasitologia, Instituto Butantan, São Paulo, Brazil) for the use of the confocal microscopy facilities and helpful discussions.
This research was supported by FAPESP, CNPq, and the Fundação Butantan. T. R. Oliveira is a postdoctoral recipient fellow of FAPESP.
Footnotes
Published ahead of print on 17 September 2008.
REFERENCES
- 1.Andrade, M. A., P. Chacon, J. J. Merelo, and F. Moran. 1993. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. 6:383-390. [DOI] [PubMed] [Google Scholar]
- 2.Bajani, M. D., D. A. Ashford, S. L. Bragg, C. W. Woods, T. Aye, R. A. Spiegel, B. D. Plikaytis, B. A. Perkins, M. Phelan, P. N. Levett, and R. S. Weyant. 2003. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J. Clin. Microbiol. 41:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari, P., and J. Gowrishankar. 1997. An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. J. Bacteriol. 179:4403-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757-771. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Bulach, D. M., R. L. Zuerner, P. Wilson, T. Seemann, A. McGrath, P. A. Cullen, J. Davis, M. Johnson, E. Kuczek, D. P. Alt, B. Peterson-Burch, R. L. Coppel, J. I. Rood, J. K. Davies, and B. Adler. 2006. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc. Natl. Acad. Sci. USA 103:14560-14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa, F. T., T. Fusai, D. Parzy, Y. Sterkers, M. Torrentino, J. B. Douki, B. Traore, S. Petres, A. Scherf, and J. Gysin. 2003. Immunization with recombinant duffy binding-like-gamma3 induces pan-reactive and adhesion-blocking antibodies against placental chondroitin sulfate A-binding Plasmodium falciparum parasites. J. Infect. Dis. 188:153-164. [DOI] [PubMed] [Google Scholar]
- 9.Croda, J., J. G. Ramos, J. Matsunaga, A. Queiroz, A. Homma, L. W. Riley, D. A. Haake, M. G. Reis, and A. I. Ko. 2007. Leptospira immunoglobulin-like proteins as a serodiagnostic marker for acute leptospirosis. J. Clin. Microbiol. 45:1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen, P. A., X. Xu, J. Matsunaga, Y. Sanchez, A. I. Ko, D. A. Haake, and B. Adler. 2005. Surfaceome of Leptospira spp. Infect. Immun. 73:4853-4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumberland, P., C. O. Everard, and P. N. Levett. 1999. Assessment of the efficacy of an IgM-ELISA and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am. J. Trop. Med. Hyg. 61:731-734. [DOI] [PubMed] [Google Scholar]
- 12.de la Pena-Moctezuma, A., D. M. Bulach, and B. Adler. 2001. Genetic differences among the LPS biosynthetic loci of serovars of Leptospira interrogans and Leptospira borgpetersenii. FEMS Immunol. Med. Microbiol. 31:73-81. [DOI] [PubMed] [Google Scholar]
- 13.Deleage, G., and C. Geourjon. 1993. An interactive graphic program for calculating the secondary structure content of proteins from circular dichroism spectrum. Comput. Appl. Biosci. 9:197-199. [DOI] [PubMed] [Google Scholar]
- 14.Doungchawee, G., U. Kositanont, A. Niwetpathomwat, T. Inwisai, P. Sagarasaeranee, and D. A. Haake. 2008. Early diagnosis of leptospirosis by immunoglobulin M immunoblot testing. Clin. Vaccine Immunol. 15:492-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faine, S., B. Adler, C. Bolin, and P. Perolat. 1999. Leptospira and leptospirosis, 2nd ed. MediSci, Melbourne, Australia.
- 16.Faisal, S. M., W. Yan, C. S. Chen, R. U. Palaniappan, S. P. McDonough, and Y. F. Chang. 2008. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine 26:277-287. [DOI] [PubMed] [Google Scholar]
- 17.Flannery, B., D. Costa, F. P. Carvalho, H. Guerreiro, J. Matsunaga, E. D. Da Silva, A. G. Ferreira, L. W. Riley, M. G. Reis, D. A. Haake, and A. I. Ko. 2001. Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. J. Clin. Microbiol. 39:3303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galen, R. S., and S. R. Gambino. 1975. Beyond normality: the predictive value and efficiency of medical diagnosis. John Wiley & Sons, Inc., New York, NY.
- 19.Gamberini, M., R. M. Gomez, M. V. Atzingen, E. A. Martins, S. A. Vasconcellos, E. C. Romero, L. C. Leite, P. L. Ho, and A. L. Nascimento. 2005. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol. Lett. 244:305-313. [DOI] [PubMed] [Google Scholar]
- 20.Guerreiro, H., J. Croda, B. Flannery, M. Mazel, J. Matsunaga, M. Galvao Reis, P. N. Levett, A. I. Ko, and D. A. Haake. 2001. Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect. Immun. 69:4958-4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haake, D. A., and J. Matsunaga. 2002. Characterization of the leptospiral outer membrane and description of three novel leptospiral membrane proteins. Infect. Immun. 70:4936-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hull-Jackson, C., M. B. Glass, M. D. Ari, S. L. Bragg, S. L. Branch, C. U. Whittington, C. N. Edwards, and P. N. Levett. 2006. Evaluation of a commercial latex agglutination assay for serological diagnosis of leptospirosis. J. Clin. Microbiol. 44:1853-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, D. T. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195-202. [DOI] [PubMed] [Google Scholar]
- 24.Juncker, A. S., H. Willenbrock, G. Von Heijne, S. Brunak, H. Nielsen, and A. Krogh. 2003. Prediction of lipoprotein signal peptides in gram-negative bacteria. Protein Sci. 12:1652-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levett, P. N. 2003. Usefulness of serologic analysis as a predictor of the infecting serovar in patients with severe leptospirosis. Clin. Infect. Dis. 36:447-452. [DOI] [PubMed] [Google Scholar]
- 26.Levett, P. N., and S. L. Branch. 2002. Evaluation of two enzyme-linked immunosorbent assay methods for detection of immunoglobulin M antibodies in acute leptospirosis. Am. J. Trop. Med. Hyg. 66:745-748. [DOI] [PubMed] [Google Scholar]
- 27.Levett, P. N., R. E. Morey, R. L. Galloway, D. E. Turner, A. G. Steigerwalt, and L. W. Mayer. 2005. Detection of pathogenic leptospires by real-time quantitative PCR. J. Med. Microbiol. 54:45-49. [DOI] [PubMed] [Google Scholar]
- 28.Nakai, K., and M. Kanehisa. 1991. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins 11:95-110. [DOI] [PubMed] [Google Scholar]
- 29.Nally, J. E., J. P. Whitelegge, S. Bassilian, D. R. Blanco, and M. A. Lovett. 2007. Characterization of the outer membrane proteome of Leptospira interrogans expressed during acute lethal infection. Infect. Immun. 75:766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nascimento, A. L., A. I. Ko, E. A. Martins, C. B. Monteiro-Vitorello, P. L. Ho, D. A. Haake, S. Verjovski-Almeida, R. A. Hartskeerl, M. V. Marques, M. C. Oliveira, C. F. Menck, L. C. Leite, H. Carrer, L. L. Coutinho, W. M. Degrave, O. A. Dellagostin, H. El-Dorry, E. S. Ferro, M. I. Ferro, L. R. Furlan, M. Gamberini, E. A. Giglioti, A. Goes-Neto, G. H. Goldman, M. H. Goldman, R. Harakava, S. M. Jeronimo, I. L. Junqueira-de-Azevedo, E. T. Kimura, E. E. Kuramae, E. G. Lemos, M. V. Lemos, C. L. Marino, L. R. Nunes, R. C. de Oliveira, G. G. Pereira, M. S. Reis, A. Schriefer, W. J. Siqueira, P. Sommer, S. M. Tsai, A. J. Simpson, J. A. Ferro, L. E. Camargo, J. P. Kitajima, J. C. Setubal, and M. A. Van Sluys. 2004. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186:2164-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nascimento, A. L., S. Verjovski-Almeida, M. A. Van Sluys, C. B. Monteiro-Vitorello, L. E. Camargo, L. A. Digiampietri, R. A. Harstkeerl, P. L. Ho, M. V. Marques, M. C. Oliveira, J. C. Setubal, D. A. Haake, and E. A. Martins. 2004. Genome features of Leptospira interrogans serovar Copenhageni. Braz. J. Med. Biol. Res. 37:459-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neves, F. O., P. A. Abreu, S. A. Vasconcellos, Z. M. de Morais, E. C. Romero, and A. L. Nascimento. 2007. Identification of a novel potential antigen for early-phase serodiagnosis of leptospirosis. Arch. Microbiol. 188:523-532. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira, T. R., C. Fernandez-Becerra, M. C. Jimenez, H. A. Del Portillo, and I. S. Soares. 2006. Evaluation of the acquired immune responses to Plasmodium vivax VIR variant antigens in individuals living in malaria-endemic areas of Brazil. Malar. J. 5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palaniappan, R. U., Y. F. Chang, F. Hassan, S. P. McDonough, M. Pough, S. C. Barr, K. W. Simpson, H. O. Mohammed, S. Shin, P. McDonough, R. L. Zuerner, J. Qu, and B. Roe. 2004. Expression of leptospiral immunoglobulin-like protein by Leptospira interrogans and evaluation of its diagnostic potential in a kinetic ELISA. J. Med. Microbiol. 53:975-984. [DOI] [PubMed] [Google Scholar]
- 35.Pertinhez, T. A., M. L. Sforca, A. C. Alves, C. R. Ramos, P. L. Ho, M. Tendler, N. I. Zanchin, and A. Spisni. 2004. 1H, 15N and 13C resonance assignments of the apo Sm14-M20(C62V) protein, a mutant of Schistosoma mansoni Sm14. J. Biomol. NMR 29:553-554. [DOI] [PubMed] [Google Scholar]
- 36.Picardeau, M., D. M. Bulach, C. Bouchier, R. L. Zuerner, N. Zidane, P. J. Wilson, S. Creno, E. S. Kuczek, S. Bommezzadri, J. C. Davis, A. McGrath, M. J. Johnson, C. Boursaux-Eude, T. Seemann, Z. Rouy, R. L. Coppel, J. I. Rood, A. Lajus, J. K. Davies, C. Medigue, and B. Adler. 2008. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE 3:e1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plank, R., and D. Dean. 2000. Overview of the epidemiology, microbiology, and pathogenesis of Leptospira spp. in humans. Microbes Infect. 2:1265-1276. [DOI] [PubMed] [Google Scholar]
- 38.Ren, S. X., G. Fu, X. G. Jiang, R. Zeng, Y. G. Miao, H. Xu, Y. X. Zhang, H. Xiong, G. Lu, L. F. Lu, H. Q. Jiang, J. Jia, Y. F. Tu, J. X. Jiang, W. Y. Gu, Y. Q. Zhang, Z. Cai, H. H. Sheng, H. F. Yin, Y. Zhang, G. F. Zhu, M. Wan, H. L. Huang, Z. Qian, S. Y. Wang, W. Ma, Z. J. Yao, Y. Shen, B. Q. Qiang, Q. C. Xia, X. K. Guo, A. Danchin, I. Saint Girons, R. L. Somerville, Y. M. Wen, M. H. Shi, Z. Chen, J. G. Xu, and G. P. Zhao. 2003. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422:888-893. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues, M. H., M. G. Cunha, R. L. Machado, O. C. Ferreira, Jr., M. M. Rodrigues, and I. S. Soares. 2003. Serological detection of Plasmodium vivax malaria using recombinant proteins corresponding to the 19-kDa C-terminal region of the merozoite surface protein-1. Malar. J. 2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaffer, A. A., L. Aravind, T. L. Madden, S. Shavirin, J. L. Spouge, Y. I. Wolf, E. V. Koonin, and S. F. Altschul. 2001. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 29:2994-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senthilkumar, T., M. Subathra, M. Phil, P. Ramadass, and V. Ramaswamy. 2008. Rapid serodiagnosis of leptospirosis by latex agglutination test and flow-through assay. Indian J. Med. Microbiol. 26:45-49. [DOI] [PubMed] [Google Scholar]
- 42.Setubal, J. C., M. Reis, J. Matsunaga, and D. A. Haake. 2006. Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smits, H. L., C. K. Eapen, S. Sugathan, M. Kuriakose, M. H. Gasem, C. Yersin, D. Sasaki, B. Pujianto, M. Vestering, T. H. Abdoel, and G. C. Gussenhoven. 2001. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin. Diagn. Lab. Immunol. 8:166-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srimanote, P., N. Wongdeethai, P. Jieanampunkul, S. Samonkiert, C. Leepiyasakulchai, T. Kalambaheti, and V. Prachayasittikul. 2008. Recombinant ligA for leptospirosis diagnosis and ligA among the Leptospira spp. clinical isolates. J. Microbiol. Methods 72:73-81. [DOI] [PubMed] [Google Scholar]
- 45.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 46.Vernel-Pauillac, F., and F. Merien. 2006. Proinflammatory and immunomodulatory cytokine mRNA time course profiles in hamsters infected with a virulent variant of Leptospira interrogans. Infect. Immun. 74:4172-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]