Abstract
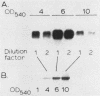
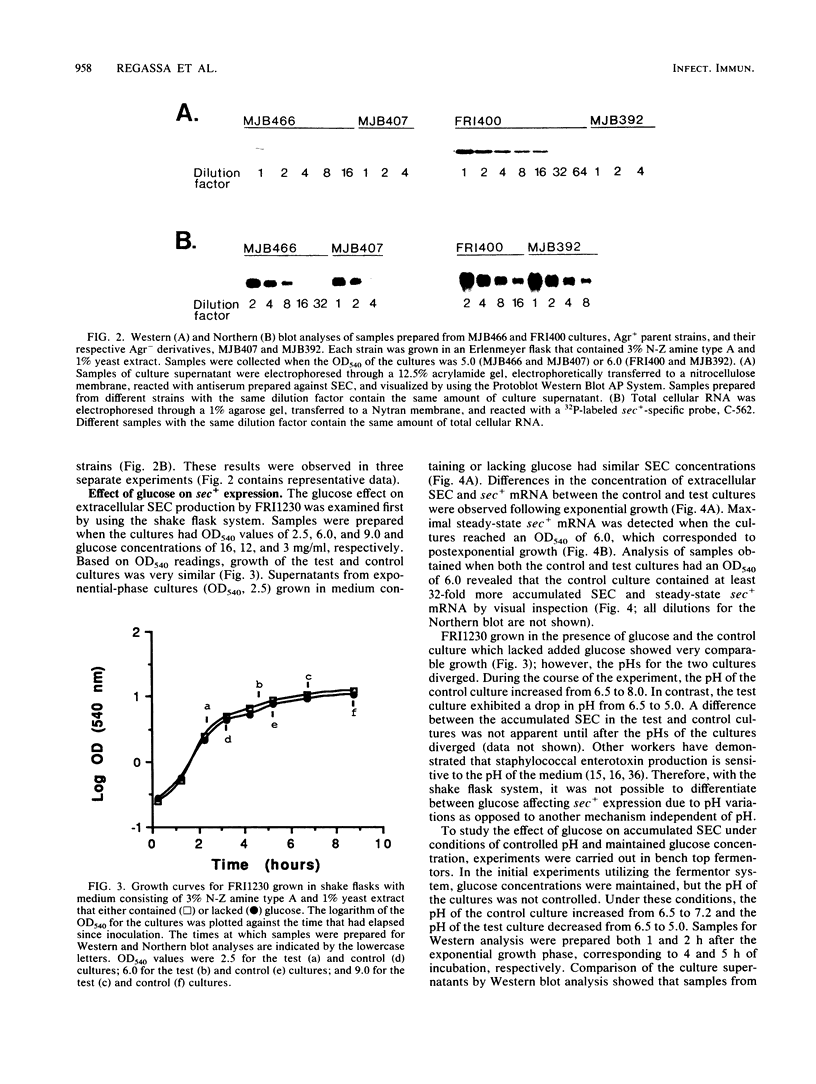
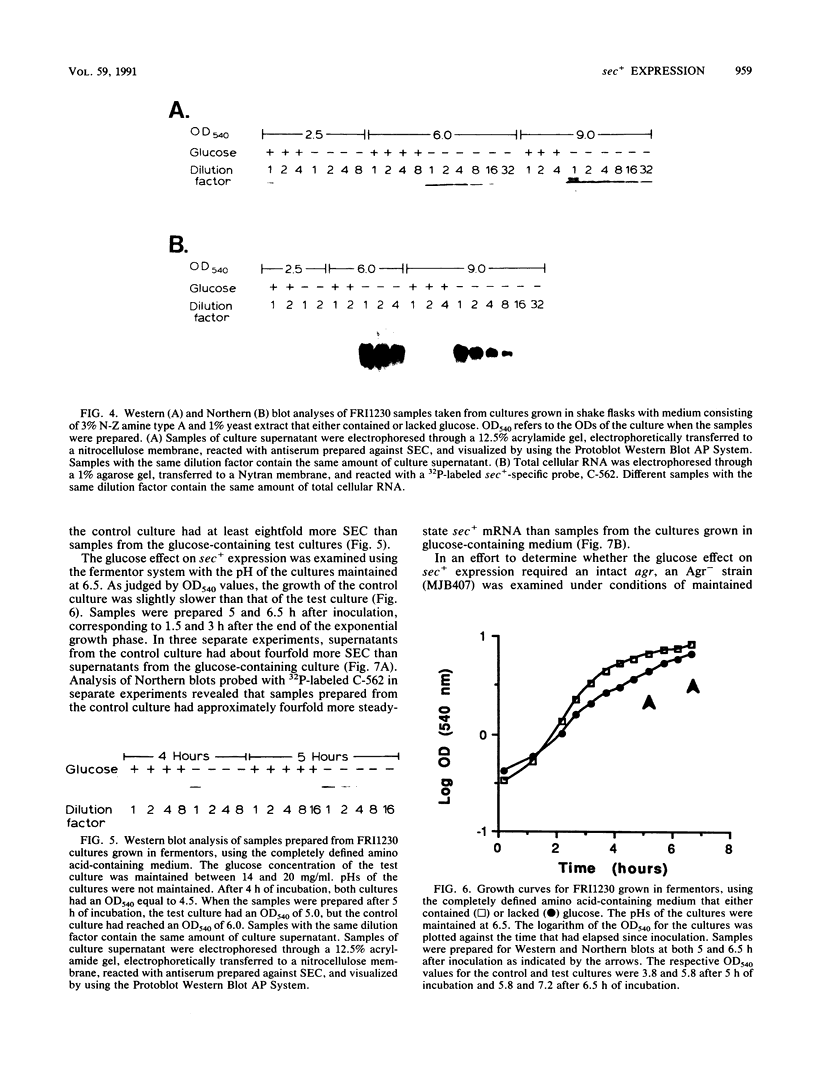
The effects of the accessory gene regulator (agr) and glucose on staphylococcal enterotoxin type C (SEC) gene (sec+) expression were examined. For the agr studies, a Tn551 insertionally inactivated agr was transferred into two different sec+ Staphylococcus aureus strains. Western blot (immunoblot) analysis showed that each of the sec+ Agr- derivatives produced less extracellular SEC than their Agr+ parent strains. Analysis of Northern (RNA) blots was consistent with at least part of the agr effect being at the level of steady-state sec+ mRNA. We examined the glucose effect on sec+ expression by utilizing both a fermentor system with a completely defined amino acid-containing medium in which the pH of the medium was maintained at 6.5 and a shake flask system with a complex medium in which the pH was allowed to fluctuate during bacterial growth. In both systems, samples from the cultures containing glucose had less extracellular SEC and less steady-state sec+ mRNA compared with the control cultures which lacked glucose. An intact agr was not required for the glucose effect on sec+ expression; MJB407, an Agr- sec+ strain, produced more SEC and had more steady-state sec+ mRNA when grown in medium that lacked glucose compared with medium that contained glucose.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayles K. W., Iandolo J. J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Conservation of the biologically active portions of staphylococcal enterotoxins C1 and C2. Infect Immun. 1989 Jul;57(7):2249–2252. doi: 10.1128/iai.57.7.2249-2252.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987 Aug;209(1):15–20. doi: 10.1007/BF00329830. [DOI] [PubMed] [Google Scholar]
- Couch J. L., Betley M. J. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J Bacteriol. 1989 Aug;171(8):4507–4510. doi: 10.1128/jb.171.8.4507-4510.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch J. L., Soltis M. T., Betley M. J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988 Jul;170(7):2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crass B. A., Bergdoll M. S. Toxin involvement in toxic shock syndrome. J Infect Dis. 1986 May;153(5):918–926. doi: 10.1093/infdis/153.5.918. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Bergdoll M. S. Staphylococcal enterotoxin synthesis during the exponential, transitional, and stationary growth phases. Infect Immun. 1974 Feb;9(2):229–235. doi: 10.1128/iai.9.2.229-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Cho G. J. Production of staphylococcal alpha toxin. II. Glucose repression of toxin formation. Infect Immun. 1972 Nov;6(5):689–694. doi: 10.1128/iai.6.5.689-694.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast D. J., Schlievert P. M., Nelson R. D. Nonpurulent response to toxic shock syndrome toxin 1-producing Staphylococcus aureus. Relationship to toxin-stimulated production of tumor necrosis factor. J Immunol. 1988 Feb 1;140(3):949–953. [PubMed] [Google Scholar]
- Garbe P. L., Arko R. J., Reingold A. L., Graves L. M., Hayes P. S., Hightower A. W., Chandler F. W., Broome C. V. Staphylococcus aureus isolates from patients with nonmenstrual toxic shock syndrome. Evidence for additional toxins. JAMA. 1985 May 3;253(17):2538–2542. [PubMed] [Google Scholar]
- Gaskill M. E., Khan S. A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J Biol Chem. 1988 May 5;263(13):6276–6280. [PubMed] [Google Scholar]
- Genigeorgis C., Foda M. S., Mantis A., Sadler W. W. Effect of sodium chloride and pH on enterotoxin C production. Appl Microbiol. 1971 May;21(5):862–866. doi: 10.1128/am.21.5.862-866.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genigeorgis C., Sadler W. W. Effect of sodium chloride and pH on enterotoxin B production. J Bacteriol. 1966 Nov;92(5):1383–1387. doi: 10.1128/jb.92.5.1383-1387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovde C. J., Hackett S. P., Bohach G. A. Nucleotide sequence of the staphylococcal enterotoxin C3 gene: sequence comparison of all three type C staphylococcal enterotoxins. Mol Gen Genet. 1990 Jan;220(2):329–333. doi: 10.1007/BF00260504. [DOI] [PubMed] [Google Scholar]
- Iandolo J. J., Shafer W. M. Regulation of staphylococcal enterotoxin B. Infect Immun. 1977 May;16(2):610–616. doi: 10.1128/iai.16.2.610-616.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., van der Meer J. W., Dinarello C. A. Induction by toxic-shock-syndrome toxin-1 of a circulating tumor necrosis factor-like substance in rabbits and of immunoreactive tumor necrosis factor and interleukin-1 from human mononuclear cells. J Infect Dis. 1988 Nov;158(5):1017–1025. doi: 10.1093/infdis/158.5.1017. [DOI] [PubMed] [Google Scholar]
- Janzon L., Löfdahl S., Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989 Nov;219(3):480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- Jarvis A. W., Lawrence R. C., Pritchard G. G. Glucose repression of enterotoxins A, B and C and other extracellular proteins in staphlyococci in batch and continuous culture. J Gen Microbiol. 1975 Jan;86(1):75–87. doi: 10.1099/00221287-86-1-75. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallonee D. H., Glatz B. A., Pattee P. A. Chromosomal mapping of a gene affecting enterotoxin A production in Staphylococcus aureus. Appl Environ Microbiol. 1982 Feb;43(2):397–402. doi: 10.1128/aem.43.2.397-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Brodsky R. Studies on plasmid replication. I. Plasmid incompatibility and establishment in Staphylococcus aureus. J Mol Biol. 1972 Jul 21;68(2):285–302. doi: 10.1016/0022-2836(72)90214-8. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Schwesinger M. D., Gruss A. D., Swanson E. C., Pattee P. A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B., Stewart G. C. Repression and catabolite repression of the lactose operon of Staphylococcus aureus. J Bacteriol. 1990 Jul;172(7):3804–3812. doi: 10.1128/jb.172.7.3804-3812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Hickman R. K., Eardley D. D., Pier G. B. Induction of human interleukin-1 by toxic-shock-syndrome toxin-1. J Infect Dis. 1985 Mar;151(3):514–522. doi: 10.1093/infdis/151.3.514. [DOI] [PubMed] [Google Scholar]
- Pattee P. A., Glatz B. A. Identification of a chromosomal determinant of enterotoxin A production in Staphylococcus aureus. Appl Environ Microbiol. 1980 Jan;39(1):186–193. doi: 10.1128/aem.39.1.186-193.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recsei P., Kreiswirth B., O'Reilly M., Schlievert P., Gruss A., Novick R. P. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986 Jan;202(1):58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- Reiser R. F., Robbins R. N., Noleto A. L., Khoe G. P., Bergdoll M. S. Identification, purification, and some physicochemical properties of staphylococcal enterotoxin C3. Infect Immun. 1984 Sep;45(3):625–630. doi: 10.1128/iai.45.3.625-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser R. F., Weiss K. F. Production of staphylococcal enterotoxins A, B, and C in various media. Appl Microbiol. 1969 Dec;18(6):1041–1043. doi: 10.1128/am.18.6.1041-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler P., Weisblum B. Erythromycin-induced stabilization of ermA messenger RNA in Staphylococcus aureus and Bacillus subtilis. J Mol Biol. 1988 Oct 20;203(4):905–915. doi: 10.1016/0022-2836(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986 May 17;1(8490):1149–1150. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- Schroeder C. J., Pattee P. A. Transduction analysis of transposon Tn551 insertions in the trp-thy region of the Staphylococcus aureus chromosome. J Bacteriol. 1984 Feb;157(2):533–537. doi: 10.1128/jb.157.2.533-537.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. L., Bencivengo M. M., Kunsch C. A. Enterotoxin A synthesis in Staphylococcus aureus: inhibition by glycerol and maltose. J Gen Microbiol. 1986 Dec;132(12):3375–3380. doi: 10.1099/00221287-132-12-3375. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Bergdoll M. S. Stimulation of Enterotoxin B Production II. Synthetic Medium for Staphylococcal Growth and Enterotoxin B Production. Infect Immun. 1971 Jun;3(6):784–792. doi: 10.1128/iai.3.6.784-792.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]