Abstract
We conducted a double-blind, vehicle-controlled, dose escalation safety and immunogenicity trial of a candidate herpes simplex virus type 2 (HSV-2) surface glycoprotein D2 (gD2) DNA vaccine administered by use of a needle-free device. Sixty-two healthy adults were randomized using a 4:1 vaccine-to-placebo ratio. Half of the participants were HSV-1 seronegative, and all were HSV-2 seronegative. Vaccine doses included 100 μg, 300 μg, 1,000 μg or 3,000 μg of a plasmid expressing the gD2 protein. Subjects received vaccine at 0, 4, 8, and 24 weeks. Some subjects received an additional 1,000-μg boost at 52 weeks. We found that the vaccine was safe and well tolerated, with most adverse events being local site reactions. No dose-limiting toxicities were observed. gD2-specific cytotoxic T-lymphocyte and lymphoproliferation responses were detected 2 weeks after the third vaccine injection in one of four HSV-1-seronegative, HSV-2-seronegative participants who received 3,000 μg of vaccine. A DNA-based vaccination strategy against HSV-2 appears to be safe and may generate a vaccine-specific cellular immune response, but high vaccine doses are likely needed to elicit an immune response in most vaccinees.
Herpes simplex virus type 2 (HSV-2) infection is one of the most prevalent sexually transmitted infections worldwide and is a major cause of genital ulcer disease in developed and developing countries (Ahmed et al. 2003, Chen et al. 2000, Mertz et al. 1998, Fleming et al. 1997, Xu et al. 2006). In addition, a growing body of literature suggests that HSV-2 increases the risk of human immunodeficiency virus type 1 (HIV-1) acquisition and transmission (5, 7). HSV-2 also causes neonatal herpes, which is a rare but serious condition that often results in permanent neurodevelopmental sequelae (14). Development of an HSV vaccine is a high priority.
Two HSV-2 subunit vaccines have completed phase III trials and provided insight into the immunology of HSV infection. The first contained two major HSV-2 surface glycoproteins, glycoproteins D and B (gB and gD), with an adjuvant, MF59, previously shown to elicit a Th2-type response (31). No protection against HSV-2 acquisition or genital herpes disease was demonstrated, despite elicitation of high HSV-2-specific neutralizing antibody titers (6). The second vaccine contained a truncated form of gD with another adjuvant, aluminum hydroxide plus 3-O-deacylated monophosphoryl lipid A (alum-MPL), previously shown to elicit a Th1-type response (24, 31). This candidate vaccine elicited partial protection against genital herpes disease in women who were seronegative for HSV-1 and -2. The difference in efficacy between these subunit vaccines was attributed to the adjuvants (32). Animal models also suggest that cellular immunity is important in defending against HSV-2 infection (22, 23). These data highlight the likelihood that a robust cellular immune response in addition to neutralizing antibodies is critical in protection against HSV-2 infection.
DNA vaccination is an attractive approach toward HSV vaccine design because in vivo immunogen expression theoretically allows engagement of both the major histocompatibility complex class I and II pathways, thus generating specific CD8 and CD4 T-cell responses and antibody production (29). Here we describe the results of a human safety and immunogenicity trial of a DNA plasmid vaccine encoding full-length gD2 formulated with bupivacaine. This candidate vaccine previously showed protection against clinical disease and reduced HSV-2 viral replication in animal models (3). The vaccine was administered by use of a needle-free device which was shown to increase the immunogenicity of a DNA vaccine in a primate model (8).
MATERIALS AND METHODS
Study description.
We conducted a randomized, double-blind, vehicle-controlled, dose escalation safety and immunogenicity trial with healthy adults. Study participants were enrolled at the University of Washington Virology Research Clinic from September 1996 through February 2001. All participants provided written informed consent. The study protocol was approved by the University of Washington Institutional Review Board.
Volunteers were randomized to receive vaccine or placebo, using a 4:1 ratio for all dose groups. The decision to escalate to the next dose was made by a review of safety data by the principal investigator and study sponsor after all subjects in a given dose subgroup had received one injection at the planned dose and completed the study through day 14. Vaccine doses tested were 100 μg, 300 μg, 1,000 μg, and 3,000 μg. Twenty participants (10 HSV-seronegative and 10 HSV-seropositive patients) were entered into each lower dose group, and 10 (5 HSV-seronegative and 5 HSV-seropositive patients) were entered into each higher dose group. One additional person each received the 300-μg and 1,000-μg doses because of participant dropout in these dose groups early during the study.
Injections were administered to the deltoid muscle, using a Biojector needle-free device. Vaccine or placebo was given on weeks 0, 4, 8, and 24. All participants were monitored for 52 weeks. For participants in dose groups receiving 100 μg or 300 μg, an additional 1,000-μg boost was administered at week 52 as part of a protocol amendment.
Participants.
Participants were adults of 18 to 60 years of age. Half of the participants were HSV-1 seronegative and HSV-2 seronegative (HSV seronegative), and half were HSV-1 seropositive and HSV-2 seronegative, as determined by Western blotting (2). All participants received a screening physical examination and laboratory testing. Exclusion criteria included prior immunization with any experimental vaccine directed against HSV or any experimental agent within 30 days prior to enrollment, known or suspected abnormality in immune function, current use of any medication which may affect immune function, acute infection, chronic infection, abnormal baseline laboratory values, including elevated total serum creatine phosphokinase (CPK), antinuclear antibody (ANA), or anti-DNA antibodies, prior malignancy, positive HIV serology, evidence of active hepatitis B or C, and a history or presence of HSV lesions consistent with genital herpes within 4 weeks of screening. Persons who reported acyclovir use within 4 weeks of enrollment were also excluded. For the HSV-negative subgroup, exclusion criteria also included the history or presence of oral-labial HSV lesions within 4 weeks of screening to avoid enrolling people with recent HSV-1 infection prior to development of HSV-1 antibodies.
Vaccine.
The Genevax-HSV vaccine is the double-stranded plasmid pAPL-gD2 (26). The HSV-2 sequences are the gD gene from a clinical isolate of HSV-2 (strain 12) and several hundred bases of 5′- and 3′-flanking regions. A cytomegalovirus promoter and respiratory syncytial virus enhancer were added, as were a simian virus 40 polyadenylation signal, bacterial origin, and aminoglycoside resistance gene for bacterial selection. Plasmid was isolated from transformed Escherichia coli K-12 strain DH10B bacteria and purified by physiochemical and chromatographic techniques. Expression was confirmed by transfection of eukaryotic cells with pAPL-gD2 followed by immunofluorescence with anti-gD2 monoclonal antibody. Specific staining localized to the plasma membrane was seen (26).
Each vial of purified plasmid vaccine was formulated in an isotonic citrate buffer solution at a concentration of 600 μg/ml (for the 100-μg and 300-μg doses) or 1,000 μg/ml (for the 1,000-μg and 3,000-μg doses) containing 0.25% (wt/vol) bupivacaine [2-piperidinecarboxamide, 1-butyl-N-(2,6-demethylphenyl)-monohydrochloride]. Inactive ingredients included sodium citrate, citric acid monohydrate, sodium chloride, and EDTA disodium. The diluent was a similar bupivacaine solution. Vaccine was held at 2 to 8°C until administration. Diluent (for 100-μg dose only) was added immediately prior to use. Vaccine was administered at 500 μl/injection.
Safety assessment.
After each injection, participants were monitored for 1 hour. Serial vital signs were obtained, and local and systemic symptoms were assessed. Participants returned to the clinic at 24 h postinjection and were contacted by telephone at 48 h postinjection for assessment of local and systemic symptoms. Diary cards were also provided for a week following each injection. Unsolicited symptoms were recorded throughout the study. All adverse events were recorded in standard medical terminology. The intensity of the adverse event was graded as 1 (mild), 2 (moderate), 3 (severe), or 4 (life-threatening/fatal), consistent with standardized toxicity criteria (25). The relationship of each adverse event to the injection was classified as probable, possible, remote, or unrelated based on criteria including temporal sequence, participants' clinical data, including other medication use, and response to rechallenge.
Blood and urine samples for safety evaluation were collected at baseline and weeks 2, 4, 6, 8, 10, 12, 23, 24, 26, 28, 40, and 52 for all participants. For participants who received a booster, blood and urine samples were collected 4 weeks prior to the booster, on the day of booster administration, and postbooster, at 24 h and weeks 2, 4, 8, 16, 24, and 52.
Immunologic testing.
Blood samples for immunological assays were collected at baseline and on days 1, 14, 28 (week 4), 29, 42, 56 (week 8), 57, 70, 84 (week 12), 161, 168 (week 24), 169, 182, 196, 280, and 364 (week 52) for all participants. For participants who received a booster at week 52, blood samples were collected at weeks 52, 54, and 60. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque centrifugation and cryopreserved as previously described (16). gD2 (provided by Eric Mishkin, Wyeth, Pearl River, NY) was a baculovirus-derived recombinant protein (19).
(i) CD4 T-cell testing.
To measure gD2-specific CD4 T-cell responses, we performed bulk lymphoproliferation assays using 105 PBMC/well in triplicate 96-well U-bottom plates, with gD2 antigen (1 μg/ml), medium (negative control), and phytohemagglutinin P (PHA-P) (0.8 μg/ml; positive control). A lymphoproliferation response was considered positive if the net proliferation (counts per minute [cpm] for gD2 antigen minus cpm for medium) was >5,000 cpm. Next, from specimens with activity in the previous assay, HSV-specific CD4+ T-cell clones were established from PBMC as previously described (16). Cryopreserved PBMC (2 × 106/well) were thawed and stimulated with HSV-2 antigen (UV-inactivated HSV-2 strain 333, diluted 1:1,000 from a preinactivation titer of 109 PFU per ml) in T-cell medium (16, 17). Growth was supported with human natural interleukin-2 as previously described (15). After 10 to 12 days, bulk cultures were cloned at 1 cell/well as described previously (16). Clones were expanded using PHA and interleukin-2 as described previously (16). Clones were tested in a triplicate lymphoproliferation assay for reactivity to HSV-1 antigen (UV-inactivated HSV-1 strain E115), HSV-2 antigen, gD2 (1 μg/ml), tetanus toxin (negative control), and PHA-P (0.8 μg/ml; positive control). Assays used autologous gamma-irradiated PBMC (105/well) as antigen-presenting cells. In addition, T-cell clones were tested in a 24-h gamma interferon-specific enzyme-linked immunospot assay (ELISPOT) assay as described previously, with the modifications of using T-cell clones instead of PBMC and purified protein instead of infected dendritic cells (13). Clones were tested using gD2 (1 μg/ml), gB2 (1 μg/ml; provided by Rae Lyn Burke, Chiron, Inc.), medium (negative control), and PHA-P (0.8 μg/ml; positive control). ELISPOT assays were scored as positive if the spots were too numerous to count. Selected T-cell clones with activity in these assays were analyzed for cell surface CD4 and CD8 expression by flow cytometry (16).
(ii) CD8 T-cell testing.
PBMC were stimulated for 10 days with HSV-2-infected autologous PHA blasts. NK cell-depleted bulk cultures were tested for lytic activity in a 4-h 51Cr release assay as previously described (28). Lymphoblastoid cell lines (LCL) were created by Epstein-Barr virus transformation of PBMC as described previously (18). NK cell-depleted bulk cultures were tested for gD2-specific cytotoxic T-lymphocyte (CTL) activity against autologous LCL infected with vaccinia virus wild-type strain WR or a vaccinia virus recombinant expressing gD2 (vgD2) (34), and the net gD2-specific lysis was measured. Effector-to-target ratios were 100:1, 50:1, and 25:1 or 50:1, 25:1, and 12:1. A CTL response was considered positive if the net specific lysis (lysis of vgD2 LCL minus lysis of wild-type vaccinia virus LCL) was ≥10% at two or more effector-to-target ratios.
(iii) Serological testing.
The humoral response to plasmid-expressed gD2 was determined using an enzyme-linked immunosorbent assay. Briefly, 100 μl of gD2 protein at 0.6 μg/ml was used to coat a 96-well flat-bottom microtiter plate (Dyna Tech Immulon) in a pH 9.1 to 9.4 carbonate buffer. After being incubated overnight at 4°C, plates were washed three times and blocked with 3% bovine serum albumin (Sigma, St. Louis, MO) in Tris-buffered saline for 1 h at room temperature. Blocking reagent was removed, and 100 μl of appropriately diluted test sample, blank, or positive control serum was added to the plate in triplicate and incubated at room temperature for 2 to 4 h. Plates were then washed four times, 100 μl of secondary antibody (goat anti-human immunoglobulin G [IgG] conjugated with alkaline phosphatase; Sigma) diluted in blocking buffer was added, and the plate was incubated at 37°C for 1 h. Plates were washed four times before the addition of 200 μl of substrate (Sigma) solution and incubation for 30 min at room temperature. The reaction was stopped with 50 μl of 3 N NaOH, and the absorption was read at 405 nm.
RESULTS
Study subjects.
A total of 62 subjects were enrolled in the study and received at least one dose of study vaccine. The mean age of the subjects was 34 years (range, 19 to 60 years). Thirty-five (56%) of the subjects were men, and 27 (44%) were women. Fifty-nine subjects (95%) were Caucasian, one (2%) was African-American, and two (3%) reported mixed race.
Protocol adherence.
Table 1 shows the number of participants who enrolled and completed the study protocol by dose group and HSV-1 status. Ten, 11, 6, and 5 participants who were HSV-1 seronegative and 10, 10, 5, and 5 participants who were HSV-1 seropositive received at least one dose at 100, 300, 1,000, and 3,000 μg, respectively. Ten, nine, five, and four participants who were HSV-1 seronegative and nine, nine, five, and five participants who were HSV-1 seropositive and in the 100-, 300-, 1,000-, and 3,000-μg dose groups, respectively, completed the protocol. Among participants in the 100- and 300-μg dose groups completing the initial study protocol, seven and nine participants who were HSV-1 seronegative and six and four participants who were HSV-1 seropositive received a 1,000-μg booster.
TABLE 1.
Numbers of participants who enrolled, completed the study protocol, and received a booster by HSV-1 serostatus and dose group
| Dose group (μg)a | No. of HSV-1-seronegative participants
|
No. of HSV-1-seropositive participants
|
||||
|---|---|---|---|---|---|---|
| Enrolled | Competed protocol | Received boosterb | Enrolled | Completed protocol | Received boosterb | |
| 100 | 10 | 10 | 7 | 10 | 9e | 6 |
| 300 | 11 | 9c | 9 | 10 | 9f | 4 |
| 1,000 | 6 | 5d | 5 | 5 | ||
| 3,000 | 5 | 4e | 5 | 5 | ||
A 4:1 ratio of vaccine recipients to placebo recipients was used for all dose groups.
A 1,000-μg booster was offered to the 100- and 300-μg dose groups as part of a protocol amendment.
One placebo recipient and one vaccine recipient dropped out after three and one study injection, respectively.
One vaccine recipient dropped out after two study injections.
One vaccine recipient dropped out after three study injections.
One placebo recipient dropped out after three study injections.
Reasons for dropout included two serious adverse events felt not to be study vaccine related. At the 100-μg level, one participant was discontinued after three doses due to neutropenia. Subsequent medical history review revealed a prior diagnosis of neutropenia of unknown etiology. At the 1,000-μg level, one participant was discontinued after two doses due to diagnosis of a schwannoma requiring surgical resection. The diagnosis was made after biopsy of an intramedullary spinal mass noted as an incidental finding on computed tomography scan during workup of a right-sided parotid mass. Four other persons prematurely discontinued participation in the study for reasons not related to an adverse event or lack of tolerability.
Vaccine safety.
All subjects who received at least one study injection were included in the safety analysis. The numbers of local and systemic symptoms reported within 7 days of each study injection are shown in Table 2. Placebo and vaccine recipients had similar adverse event profiles. Local site reactions were the most commonly experienced symptoms in both groups. The majority of participants experienced tenderness/pain and erythema/induration over all time points. Headache was the most common systemic symptom, occurring in 42% and 28% of placebo and vaccine recipients, respectively, after the first injection. There was not an increasing trend in headache with dose escalation, with occurrence in four, five, two, and three persons in the 100-, 300-, 1,000-, and 3,000-μg groups, respectively. The occurrence of headache appeared to decrease over time, with 2% and 0% of vaccine and placebo recipients, respectively, experiencing this symptom after the fourth injection. Other systemic symptoms, including fatigue, malaise, nausea, diarrhea, light headedness, dizziness, and low-grade fever, were uncommon.
TABLE 2.
Local and systemic symptoms experienced in the 7 days following each injection
| Group and symptom | No. (%) of participants with symptom at weeka:
|
||||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 24 | 52 | |
| Placebo group | |||||
| Tenderness/pain | 8 (67) | 9 (75) | 7 (58) | 6 (60) | 2 (40) |
| Erythema/induration | 9 (75) | 7 (58) | 8 (67) | 7 (70) | 4 (80) |
| Ecchymosis | 2 (17) | 2 (17) | 1 (8) | 0 | 0 |
| Injection site edema | 4 (33) | 1 (8) | 2 (17) | 1 (10) | 1 (20) |
| Headache | 5 (42) | 4 (33) | 2 (17) | 0 | 0 |
| Fatigue/malaise | 1 (8) | 0 | 2 (17) | 0 | 0 |
| Hematoma | 2 (17) | 0 | 0 | 0 | 0 |
| Injection site hypersensitivity | 0 | 1 (8) | 0 | 0 | 0 |
| Nausea/diarrhea | 1 (8) | 0 | 0 | 1 (10) | 0 |
| Paraesthesia | 3 (25) | 1 (8) | 1 (8) | 0 | 0 |
| Light headedness/dizziness | 0 | 0 | 0 | 0 | 0 |
| Low-grade fever | 0 | 0 | 0 | 0 | 0 |
| Total | 35 | 25 | 23 | 15 | 7 |
| Vaccine groups | |||||
| Tenderness/pain | 38 (76) | 41 (84) | 34 (71) | 34 (74) | 17 (63) |
| Erythema/induration | 33 (66) | 31 (63) | 33 (69) | 35 (76) | 15 (56) |
| Ecchymosis | 10 (20) | 10 (20) | 9 (19) | 10 (22) | 6 (22) |
| Injection site edema | 7 (14) | 7 (14) | 10 (21) | 7 (15) | 4 (15) |
| Headache | 14 (28) | 6 (12) | 7 (15) | 4 (9) | 3 (11) |
| Fatigue/malaise | 7 (14) | 3 (6) | 3 (6) | 2 (4) | 2 (7) |
| Hematoma | 1 (2) | 2 (4) | 3 (6) | 4 (9) | 0 |
| Injection site hypersensitivity | 4 (8) | 1 (2) | 1 (2) | 2 (4) | 1 (4) |
| Nausea/diarrhea | 2 (4) | 3 (6) | 1 (2) | 1 (2) | 0 |
| Paraesthesia | 3 (6) | 1 (2) | 1 (2) | 1 (2) | 0 |
| Light headedness/dizziness | 1 (2) | 3 (6) | 0 | 0 | 1 (4) |
| Low-grade fever | 0 | 1 (2) | 0 | 0 | 0 |
| Total | 120 | 109 | 102 | 100 | 49 |
The number of participants in the placebo group was 12, 12, 12, 10, and 5 at weeks 0, 4, 8, 24, and 52, respectively; the number of participants in the vaccine groups was 50, 49, 48, 46, and 27 at weeks 0, 4, 8, 24, and 52, respectively.
Six participants, all in the vaccine group, experienced serious adverse events. None of these were considered related to vaccination. Two of these events led to study discontinuation and are described above. One participant in the 3,000-μg dose group required hospitalization for treatment of pneumonia 48 days after the third dose of study vaccine. This was followed by a full recovery and continuation in the study, including receipt of the fourth vaccination. One participant in the 1,000-μg dose group had an elective hysterectomy 187 days after her fourth dose of study vaccine. One participant in the 300-μg dose group had a hysterectomy and bilateral oophorectomy for uterine fibroids. She had previous history of myomectomy 6 years earlier for uterine fibroids. One participant in the 100-μg dose group was hospitalized with acute appendicitis at week 13. This was followed by a full recovery and completion of the study protocol.
ANA and anti-DNA titers.
Seventeen of 50 vaccine recipients had positive ANA titers detected after vaccination. All values were low titers (<1:160) and considered not clinically significant. One vaccine participant had a positive anti-DNA antibody which was not considered clinically significant.
CPK.
There were 27 (23 vaccine and 4 placebo) subjects with elevated CPK values (>160 U/liter) observed during the study. None of these were clinically significant. Four subjects had grade 1 (fourfold increase) or greater elevations. Of these, only one participant, from the placebo group, had a grade 4 (>20 times the upper limit of normal) elevation (5,400 U/liter), which was observed at baseline. Subsequent to this visit, a grade 1 elevation was observed at study week 6. All other elevated values in this participant were low level (less than grade 1).
Cell-mediated immunity.
Six of the participants in the HSV-seronegative group have previously been described as immune seronegative (IS; HSV-seronegative with HSV-specific T-cell responses) subjects (28). These persons had cellular immune responses to HSV-2 at baseline and were eliminated from further analysis. Among participants not identified as IS subjects, significant gD2-specific CTL lytic activity and lymphoproliferation were observed in one subject in the HSV-negative, 3,000-μg dose group (Table 3 and Fig. 1). HSV-specific CD4+ clones were isolated and expanded from this participant's PBMC after stimulation with UV-inactivated HSV-2. Clones were tested for reactivity to HSV-1 and HSV-2. Table 4 shows that six of the seven clones were HSV-2 type specific because they proliferated in response to HSV-2 only. Clone 42 was HSV type common. Notably, every HSV-2-reactive clone from this subject proliferated in response to gD2. In addition, the four clones available for testing produced gamma interferon in response to gD2, but not gB2, in an ELISPOT assay.
TABLE 3.
gD2-specific cellular immune responses in PBMC from HSV-seronegative vaccineesa
| Subject | Initial dose (μg) | gD-2 specific CTL response
|
gD-2 specific lymphoproliferation
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After first vaccine
|
After 1,000-μg boost
|
After first vaccine
|
After 1,000-μg boost
|
||||||||||
| 0 | 70 | 182 | 0 | 14 | 56 | 0 | 70 | 182 | 0 | 14 | 56 | ||
| 1* | 0 | − | − | − | − | − | − | − | − | − | − | − | − |
| 2 | 0 | − | NA | − | − | − | − | − | NA | − | − | − | − |
| 3* | 0 | − | − | + | − | − | − | − | + | − | − | + | − |
| 4* | 0 | + | − | NA | No boost | − | − | NA | No boost | ||||
| 5 | 0 | − | − | − | No boost | − | − | − | No boost | ||||
| 6 | 0 | − | − | − | No boost | − | − | − | No boost | ||||
| 7 | 100 | − | NA | − | No boost | − | − | − | No boost | ||||
| 8 | 100 | − | NA | − | No boost | − | − | − | No boost | ||||
| 9 | 100 | − | NA | − | − | − | − | − | − | − | − | − | − |
| 10 | 100 | − | NA | − | − | − | − | − | NA | − | − | − | − |
| 11 | 100 | − | NA | − | − | − | − | − | NA | − | − | − | − |
| 12 | 100 | − | NA | − | No boost | − | NA | − | No boost | ||||
| 13 | 100 | − | NA | − | − | − | − | − | NA | − | − | − | − |
| 14 | 100 | − | NA | − | − | − | − | − | NA | − | − | − | − |
| 15 | 300 | + | − | − | − | − | − | − | − | − | − | − | − |
| 16 | 300 | − | − | − | − | − | − | − | − | − | − | − | − |
| 17 | 300 | − | − | − | − | − | − | − | − | − | − | − | − |
| 18 | 300 | − | − | − | − | − | − | − | − | − | − | − | − |
| 19* | 300 | − | − | − | + | − | + | − | − | − | − | − | − |
| 20 | 300 | − | − | − | − | − | − | − | − | − | − | − | − |
| 21 | 300 | − | − | − | − | − | − | − | − | − | − | − | − |
| 22* | 300 | − | − | − | − | − | − | − | − | − | − | − | − |
| 23 | 1,000 | − | − | − | No boost | − | − | − | No boost | ||||
| 24 | 1,000 | − | − | − | No boost | − | − | − | No boost | ||||
| 25 | 1,000 | − | − | − | No boost | − | − | − | No boost | ||||
| 26 | 1,000 | − | − | − | No boost | − | − | − | No boost | ||||
| 27 | 3,000 | − | − | − | No boost | − | − | − | No boost | ||||
| 28 | 3,000 | − | − | − | No boost | − | − | − | No boost | ||||
| 29 | 3,000 | − | + | NA | No boost | − | + | NA | No boost | ||||
| 30* | 3,000 | + | + | + | No boost | + | + | + | No boost | ||||
HSV-seronegative subjects were vaccinated with vehicle only or with doses of HSV-2 gD2 DNA vaccine ranging from 100 to 3,000 μg per vaccination. In some cases, subjects were boosted with 1,000 μg of vaccine 1 year after the start of the first vaccine series. gD2-specific CTL activity and gD2-specific lymphoproliferation were measured at various times before and after the first vaccine injection and pre- and postboosting, as described in Materials and Methods. Time points after the first injection correspond to 2 weeks after the third and fourth infections. +, positive response; −, negative response; NA, sample not available. Subjects with asterisks are immune seronegative (positive CTL and/or lymphoproliferative response to HSV-2 in the absence of detectable HSV antibodies [28]).
FIG. 1.
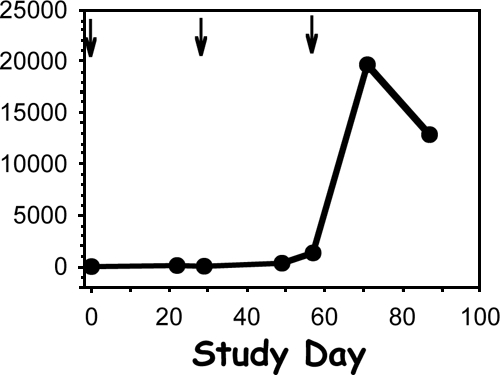
gD2-specific lymphoproliferation in subject 29 (see Table 3). Arrows represent vaccine injection time points.
TABLE 4.
| Clone | Net proliferation (dcpm) with antigen
|
IFN-γ production
|
Summary | |||||
|---|---|---|---|---|---|---|---|---|
| UV-inactivated HSV-1 | UV-inactivated HSV-2 | gD2 | Tetanus toxoid | PHA | gD2 | gB2 | ||
| 3 | 4 | 11,708 | 27,877 | −9 | 29,929 | + | − | gD2 specific |
| 11 | 1,052 | 98,388 | 57,007 | 0 | 129,489 | + | − | gD2 specific |
| 18 | −24 | 2,758 | 3,848 | −17 | 19,104 | + | − | gD2 specific |
| 25 | −4 | 101,210 | 82,191 | 190 | 73,372 | ND | ND | gD2 specific |
| 36 | 27 | 28,454 | 37,099 | −18 | 20,573 | ND | ND | gD2 specific |
| 40 | −108 | 75,249 | 55,756 | −186 | 78,186 | ND | ND | gD2 specific |
| 42 | 75,079 | 40,183 | 15,920 | −57 | 39,888 | + | − | gD type common |
Lymphoproliferation was measured in response to HSV-1, HSV- 2, and gD2. Tetanus toxoid was used as a negative control, and PHA was used as a positive control. Data are means of triplicate [3H]thymidine incorporation values and are net values compared to those for medium-only control wells. IFN-γ production was measured using a 24-h ELISPOT assay in response to gD2, gB2, medium (negative control), and PHA-P (positive control). +, spots were too numerous to count. ND, not done.
Significant boosts in HSV-2-specific CTL activity or lymphoproliferation were not observed in any HSV-1-seropositive, HSV-2-seronegative participants (data not shown), as measured by precursor frequency assays (27).
Serology.
Serologic assays of gD2 binding performed by enzyme-linked immunosorbent assay did not show elicitation of new responses in any HSV-seronegative individual and did not show significant boosts in preexisting binding antibodies in persons with preexisting HSV-1 immunity (data not shown).
DISCUSSION
We have shown that the plasmid gD2-based HSV-2 DNA candidate vaccine delivered using a Biojector needle-free device is safe and well tolerated in healthy adults. This is the first reported human study of a DNA-based vaccine aimed against HSV-2 infection. Adverse events were mostly local site reactions, and there were no dose-limiting toxicities. These data are consistent with several other trials showing that DNA-based vaccination strategies are safe (9, 11, 12, 20, 21, 30, 33). One participant was found to have positive anti-DNA antibody, but this was not considered clinically significant. The FDA has not required monitoring for systemic autoimmunity in trials of DNA vaccines since 2005, as these responses have not been clinically significant (4).
Vaccine-induced HSV-specific T-cell activity was detected in one of four HSV-seronegative participants in the highest (3,000 μg) dose group. Both cytolytic activity and proliferation were demonstrated in response to appropriate antigen-presenting cells expressing gD2. The same subject yielded a series of seven CD4+ T-cell clones reactive with recombinant gD2 protein after PBMC were restimulated with whole HSV-2. This subject was HSV-2 seronegative by Western blotting 1 month after receiving the final vaccine injection, so seroconversion to HSV-2 seems unlikely. While IgG responses to HSV-2 in the context of HSV-2 infection can occasionally be delayed (1), we believe that our data support the conclusion that these responses in this subject occurred in response to the vaccine. We previously studied naturally HSV-2-infected subjects, using the same whole HSV-2 stimulation protocol. We typically detected CD4 T-cell responses to a median of five of the nine proteins studied. While gD2-specific responses were observed in most subjects, responses to other glycoproteins and to tegument proteins were similar in prevalence and magnitude to the responses observed for gD2 (17). The fact that each of seven clones generated in response to whole HSV-2 was gD2 specific would be highly unusual in the context of such a polyclonal response. The CD4 clones available for testing failed to respond to gB2, reinforcing their specificity for gD2.
The reason for the lack of immunogenicity in the other 3,000-μg dose group participants is not clear. Possibly, even higher vaccine doses would have elicited a higher rate of immune response. This candidate vaccine did not appear to elicit an antibody response. Animal models suggest that vaccination with plasmids encoding truncated gD2 generates a stronger humoral immune response, while full-length gD2 generates a stronger cellular immune response (10). This study is consistent with these findings, but more work in this area is needed. Future clinical studies using higher vaccine doses or combining plasmids with truncated and full-length glycoproteins may improve vaccine immunogenicity.
In summary, prior clinical trials and animal models suggest that cellular immunity is key in protecting against HSV-2 infection. This study has shown that a plasmid-based DNA vaccination strategy against HSV-2 is safe and capable of generating a vaccine-specific T-cell response. Further clinical trials using this approach are warranted.
Acknowledgments
Wyeth-Lederle, Inc., was the sponsor of the clinical trial. Additional support for the preparation of the manuscript was provided by NIH/NIAID grants AI030731, AI50132, K24-AI071113, and UL1 RR025014.
David Koelle, Larry Corey, and Christine Posavad are coinventors on patents owned by the University of Washington concerning HSV-2 vaccines that are unrelated to the subject of this submission. Anna Wald has received grant support from GlaxoSmithKline, Antigenics, and Astellas. She has been a consultant for Novartis, Powdermed, Aicuris, and Medigene and a speaker for Merck Vaccines. David Koelle has received grant support from GlaxoSmithKline, 3M, and Antigenics and has been a consultant for Amgen.
Footnotes
Published ahead of print on 10 September 2008.
We dedicate this study to the memory of Richard Ginsberg, who provided intellectual camaraderie and constant medical guidance for the clinical conduct of the trial, and to the memory of Jennifer Moses, who performed the T-cell immunogenicity assays.
REFERENCES
- 1.Ashley, R., J. Benedetti, and L. Corey. 1985. Humoral immune response to HSV-1 and HSV-2 viral proteins in patients with primary genital herpes. J. Med. Virol. 17:153-166. [DOI] [PubMed] [Google Scholar]
- 2.Ashley, R., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J. Clin. Microbiol. 26:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein, D., E. Tepe, J. Mester, R. Arnold, L. Stanberry, and T. Higgins. 1999. Effects of DNA immunization formulated with bupivacaine in murine and guinea pig models of genital herpes simplex virus infection. Vaccine 17:1964-1969. [DOI] [PubMed] [Google Scholar]
- 4.Center for Biologics Evaluation and Research, U.S. FDA. 2007. Guidance for industry considerations for plasmid DNA vaccines for infectious disease indications. U.S. FDA, Washington, DC.
- 5.Corey, L. 2007. Synergistic copathogens—HIV-1 and HSV-2. N. Engl. J. Med. 356:854-856. [DOI] [PubMed] [Google Scholar]
- 6.Corey, L., A. G. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, and S. E. Straus. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331-340. [DOI] [PubMed] [Google Scholar]
- 7.Corey, L., A. Wald, C. L. Celum, and T. C. Quinn. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35:435-445. [DOI] [PubMed] [Google Scholar]
- 8.Davis, H. L., and R. G. Whalen. 1995. DNA-based immunization. Mol. Cell. Biol. Hum. Dis. Ser. 5:368-387. [DOI] [PubMed] [Google Scholar]
- 9.Eller, M. A., L. A. Eller, M. S. Opollo, B. J. Ouma, P. O. Oballah, L. Galley, C. Karnasuta, S. R. Kim, M. L. Robb, N. L. Michael, H. Kibuuka, F. Wabwire-Mangen, B. S. Graham, D. L. Birx, M. S. de Souza, and J. H. Cox. 2007. Induction of HIV-specific functional immune responses by a multiclade HIV-1 DNA vaccine candidate in healthy Ugandans. Vaccine 25:7737-7742. [DOI] [PubMed] [Google Scholar]
- 10.Fló, J. 2003. Co-immunization with plasmids coding the full length and a soluble form of glycoprotein D of HSV-2 induces protective cellular and humoral immune response in mice. Vaccine 21:1239-1245. [DOI] [PubMed] [Google Scholar]
- 11.Graham, B. S., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, J. E. Martin, M. M. McCluskey, B. K. Chakrabarti, L. Lamoreaux, C. A. Andrews, P. L. Gomez, J. R. Mascola, and G. J. Nabel. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 194:1650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hejdeman, B., A. C. Bostrom, R. Matsuda, S. Calarota, R. Lenkei, E. L. Fredriksson, E. Sandstrom, G. Bratt, and B. Wahren. 2004. DNA immunization with HIV early genes in HIV type 1-infected patients on highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 20:860-870. [DOI] [PubMed] [Google Scholar]
- 13.Hosken, N., P. McGowan, A. Meier, D. Koelle, P. Sleath, F. Wagener, M. Elliott, K. Grabstein, C. Posavad, and L. Corey. 2006. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J. Virol. 80:5509-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimberlin, D. 2004. Neonatal herpes simplex infection. Clin. Microbiol. Rev. 17:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koelle, D., C. Posavad, G. Barnum, M. Johnson, J. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koelle, D. M., L. Corey, R. L. Burke, R. J. Eisenberg, G. H. Cohen, R. Pichyangkura, and S. J. Triezenberg. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68:2803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koelle, D. M., M. Schomogyi, C. McClurkan, S. N. Reymond, and H. B. Chen. 2000. CD4 T-cell responses to herpes simplex virus type 2 major capsid protein VP5: comparison with responses to tegument and envelope glycoproteins. J. Virol. 74:11422-11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koelle, D. M., M. A. Tigges, R. L. Burke, F. W. Symington, S. R. Riddell, H. Abbo, and L. Corey. 1993. Herpes simplex virus infection of human fibroblasts and keratinocytes inhibits recognition by cloned CD8+ cytotoxic T lymphocytes. J. Clin. Investig. 91:961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landolfi, V., C. D. Zarley, A. S. Abramovitz, N. Figueroa, S. L. Wu, M. Blasiak, S. T. Ishizaka, and E. M. Mishkin. 1993. Baculovirus-expressed herpes simplex virus type 2 glycoprotein D is immunogenic and protective against lethal HSV challenge. Vaccine 11:407-414. [DOI] [PubMed] [Google Scholar]
- 20.Lozano, M. A., O. J. Caycho, A. Antunez de Mayolo, D. Vera, and R. D. Posadas. 2003. Immunogenicity and efficacy of a new recombinant DNA vaccine for hepatitis B virus in Peru. Rev. Gastroenterol. Peru 23:259-264. [PubMed] [Google Scholar]
- 21.Martin, J. E., N. J. Sullivan, M. E. Enama, I. J. Gordon, M. Roederer, R. A. Koup, R. T. Bailer, B. K. Chakrabarti, M. A. Bailey, P. L. Gomez, C. A. Andrews, Z. Moodie, L. Gu, J. A. Stein, G. J. Nabel, and B. S. Graham. 2006. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin. Vaccine Immunol. 13:1267-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 23.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 24.Moore, A., L. McCarthy, and K. H. Mills. 1999. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine 17:2517-2527. [DOI] [PubMed] [Google Scholar]
- 25.National Cancer Institute. 1999. Reporting guidelines. National Cancer Institute, Bethesda, MD. http://ctep.cancer.gov/reporting/ctc_archive.html.
- 26.Pachuk, C. J., R. Arnold, K. Herold, R. B. Ciccarelli, and T. J. Higgins. 1998. Humoral and cellular immune responses to herpes simplex virus-2 glycoprotein D generated by facilitated DNA immunization of mice. Curr. Top. Microbiol. Immunol. 226:79-89. [DOI] [PubMed] [Google Scholar]
- 27.Posavad, C. M., D. M. Koelle, M. F. Shaughnessy, and L. Corey. 1997. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc. Natl. Acad. Sci. USA 94:10289-10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posavad, C. M., A. Wald, N. Hosken, M. L. Huang, D. M. Koelle, R. L. Ashley, and L. Corey. 2003. T cell immunity to herpes simplex viruses in seronegative subjects: silent infection or acquired immunity? J. Immunol. 170:4380-4388. [DOI] [PubMed] [Google Scholar]
- 29.Rajcani, J., T. Mosko, and I. Rezuchova. 2005. Current developments in viral DNA vaccines: shall they solve the unsolved? Rev. Med. Virol. 15:303-325. [DOI] [PubMed] [Google Scholar]
- 30.Roberts, L. K., L. J. Barr, D. H. Fuller, C. W. McMahon, P. T. Leese, and S. Jones. 2005. Clinical safety and efficacy of a powdered hepatitis B nucleic acid vaccine delivered to the epidermis by a commercial prototype device. Vaccine 23:4867-4878. [DOI] [PubMed] [Google Scholar]
- 31.Singh, M., J. R. Carlson, M. Briones, M. Ugozzoli, J. Kazzaz, J. Barackman, G. Ott, and D. O'Hagan. 1998. A comparison of biodegradable microparticles and MF59 as systemic adjuvants for recombinant gD from HSV-2. Vaccine 16:1822-1827. [DOI] [PubMed] [Google Scholar]
- 32.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 33.Tavel, J. A., J. E. Martin, G. G. Kelly, M. E. Enama, J. M. Shen, P. L. Gomez, C. A. Andrews, R. A. Koup, R. T. Bailer, J. A. Stein, M. Roederer, G. J. Nabel, and B. S. Graham. 2007. Safety and immunogenicity of a Gag-Pol candidate HIV-1 DNA vaccine administered by a needle-free device in HIV-1-seronegative subjects. J. Acquir. Immune Defic. Syndr. 44:601-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tigges, M., D. Koelle, K. Hartog, R. Sekulovich, L. Corey, and R. Burke. 1992. Human CD8+ herpes simplex virus-specific cytotoxic T-lymphocyte clones recognize diverse virion protein antigens. J. Virol. 66:1622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]


