Abstract
Despite routine vaccination with Mycobacterium bovis bacillus Calmette-Guérin (BCG) soon after birth, tuberculosis in babies and adults remains epidemic in South Africa. The immune responses of the naïve newborn child and how they are affected by vaccination with BCG are as yet not fully understood. Immunity during pregnancy and in healthy human newborns may be skewed toward type 2 cytokine production; however, it is type 1 cytokines that are required for protection against M. tuberculosis infection. To better understand neonatal cytokine responses prior to and following exposure to mycobacteria, we have collected cord blood and peripheral blood samples and evaluated the cytokine response following ex vivo incubation with BCG. Gamma interferon (IFN-γ), interleukin 10 (IL-10), IL-12, and low levels of IL-13 and IL-5 but no IL-4 were secreted into the culture supernatant of cord blood mononuclear cells. Intracellular staining showed that IL-10 and IL-12 were produced by monocytes and that IFN-γ was produced by natural killer (NK) cells but not by CD4+ or CD8+ T cells. In contrast, in the peripheral blood samples collected from babies 13 weeks post-BCG vaccination, IFN-γ was detected within CD4+ and CD8+ cells. Taken together, the data suggest a central role for Th1 cytokines in naïve as well as BCG-vaccinated neonates in the protective immune response to tuberculosis. NK cell-derived IFN-γ produced in naïve neonates likely plays a key protective role via monocyte activation and the priming of a subsequent adaptive Th1 response.
Mycobacterium bovis BCG was introduced as an antituberculosis (anti-TB) vaccine over 80 years ago and has since become one of the most widely used of all vaccines (11, 40). Approximately 100 million children worldwide are vaccinated with BCG each year (40). BCG vaccination of infants affords 80% protection against TB meningitis and against disseminated or miliary TB. However, it is variably effective against pulmonary disease at all ages or against the reactivation of latent pulmonary infection in adults (10, 37, 40). Consequently, in spite of extensive BCG vaccination in many parts of the world, TB rates are high, with about 8 million cases and 2 million deaths annually (32). South Africa ranks eighth in the world for TB incidence, with a rate of approximately 600 cases per 100,000 population (39). Because TB is endemic in South Africa, infants are vaccinated with BCG (Danish strain) shortly after birth. Even with >95% BCG vaccination coverage, the rate of TB disease in babies remains very high, exceeding 2% per year among children under 2 years old in some areas (13).
Little is known about the immune response of human neonates to mycobacteria prior to vaccination with BCG. Ota and coworkers have reported low levels of gamma interferon (IFN-γ), interleukin 5 (IL-5), and IL-13 following the stimulation of cord blood cells with a purified protein derivative of tuberculin (28). Another study that assessed the responses of cord blood to mycobacteria found low levels of secreted IFN-γ, IL-10, and IL-5 following incubation with BCG (16). However, it has not been clear which cells produce these cytokines and how IFN-γ is induced in the naïve host prior to exposure to mycobacterial antigens and the stimulation of an acquired T-cell response. To further explore the infant immune response prior to vaccination with BCG, we incubated cord blood mononuclear cells (CBMC) and purified CD14+ cells in vitro with the Danish strain of BCG and quantified the IFN-γ, IL-12, IL-10, IL-4, IL-5, and IL-13 secreted into the culture supernatants. Furthermore, to identify the cell types producing these cytokines, intracellular CD4+ and CD8+ T-cell IFN-γ and IL-10, intracellular CD56+ NK cell IFN-γ, and intracellular CD14+ monocyte IFN-γ and IL-10 were evaluated after the ex vivo incubation of whole cord blood with Danish BCG. The results were compared to the responses of the same cellular subsets in peripheral blood samples from 13-week-old babies vaccinated with BCG at birth. The data indicate that the Th1 cytokine response of the naïve host prior to BCG vaccination was confined to cells of the innate immune system and did not involve T-cell responses. However, post-BCG vaccination, while a similar profile of cytokines was produced, it was T cells that produced IFN-γ, thus providing a larger and more sustainable source of immune stimulation.
MATERIALS AND METHODS
Participants.
Umbilical cord blood was collected from elective caesarean donors at full term (38 to 39 weeks) to avoid any possible effects of labor. A total of 23 infant cord blood samples were studied. Exclusion criteria included fetal distress, maternal diabetes mellitus, maternal human immunodeficiency virus infection, maternal infection, and preeclampsia. The TB history of the mothers was not known, but none of the mothers had active TB at the time of caesarean section. Different assays for cord blood samples were carried out, depending on the volume of cord blood obtained. The median age of the mothers was 31 years (interquartile range [IQR], 26 to 33 years), and the median gestation time was 38 weeks (three babies had a gestation time of 39 weeks, and all others had a gestation period of 38 weeks). Twelve infants (52%) were female. The median weights of the neonates and of the placentas were 2,978 g (IQR, 2,845 to 3,340 g) and 620 g (IQR, 600 to 760 g), respectively. All infants had immediate Apgar scores, which assess newborn health, of ≥8 and 5-min Apgar scores of 9 or 10 (10 being the best). In addition, from six healthy infants from whom cord blood had been obtained, peripheral blood was collected at a median of 92 days (89 to 95 days) postvaccination with Danish BCG by the intradermal route at birth. The median birth weight of these infants was 2,928 g (IQR, 2,830 to 3,063 g). The ratio of female to male infants was 5:1. Human participation was consistent with the U.S. Department of Health and Human Services and good clinical practice guidelines. These standards included protocol approval by the University of Cape Town Research Ethics Committee. Written informed consent for the collection of cord blood was obtained from the mothers prenatally and that for the collection of peripheral blood from 13-week-old infants, where relevant, was obtained prior to sample collection.
Cord blood collection.
A standard blood donation bag (Sabax, Johannesburg, South Africa) was prepared for the collection of the cord blood by aseptically adding 2,000 U of sodium heparin (Sigma-Aldrich, Steinham, Germany). Immediately following the delivery of the infant, the umbilical cord was clamped and not detached from the placenta. After removal from the uterus, the placenta was placed in a sterile bowl and the umbilical cord was cleaned with 70% ethanol. The cord was then punctured with a 16-gauge needle connected to the heparinized blood donor bag, and cord blood from the umbilical vein was allowed to flow by gravity. During this time, the contents of the blood donor bag were gently mixed to prevent clotting (3, 5, 30, 43). The cord blood sample was then immediately transferred to the laboratory at ambient temperature.
Isolation of CBMC.
The cord blood was diluted with an equal volume of phosphate-buffered saline (PBS) without calcium or magnesium (Bio Whittaker, Walkersville, MD) and carefully layered onto Ficoll-Hypaque (half the volume of the cord blood-PBS mixture) (Sigma-Aldrich, Steinham, Germany). The gradient was centrifuged at 1,200 × g for 30 min at room temperature in a centrifuge with a swing-out rotor. The opaque interface containing the mononuclear cells was removed and washed twice with PBS by centrifugation at 300 × g for 10 min. Cells were resuspended in PBS to the original volume of cord blood, and the procedure was repeated with another round of Ficoll-Hypaque density gradient centrifugation to ensure the removal of erythroid precursors from CBMC. This step was followed by three washes in PBS (30, 31, 44). The purity of cord blood established via the assessment of CD45+ and CD45− cells to determine the percentage of erythroid precursors (CD45− cells) after a single round compared to that after two rounds of Ficoll-Hypaque gradient centrifugation was 40% CD45+ cells (median; IQR, 32 to 52%) and 94% CD45+ cells (IQR, 83 to 97%), respectively.
Purification of CD14+ cells.
A portion of the CBMC was further used to purify CD14+ monocytes using anti-CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and a MiniMacs magnetic multistand (Miltenyi Biotec, Bergisch Gladbach, Germany) as recommended by the manufacturer.
CBMC and CD14+ monocyte cultures.
CBMC were dually stained with CD45-allophycocyanin (Caltag, Burlingame, CA) and CD14-phycoerythrin (BD Biosciences, San Jose, CA) to determine the percentage of CD14+ monocytes present among CBMC; CBMC cultures were adjusted to a concentration of 3 × 105 monocytes per ml. CBMC in complete medium containing 10% AB human serum (Western Province Blood Transfusion Service, South Africa) in RPMI medium (Bio Whittaker, Walkersville, MD) were then plated into 96-well U-bottom plates (Costar; Corning Incorporated, New York, NY). Cultures of purified CD14+ monocytes (following magnetic bead separation) were set up in parallel at 3 × 105/ml.
Antigen stimulation for secreted cytokine and intracellular cytokine (ICC) evaluation.
CBMC and CD14+ monocytes were stimulated with the Danish BCG vaccine strain (Statens Serum Institut, Copenhagen, Denmark) resuspended in RPMI medium to an average multiplicity of infection (MOI) of 5 BCG cells per monocyte (5:1). Endotoxin was not detectable in the BCG preparations as determined by the BioVac Institute, South Africa. Staphylococcal enterotoxin B (SEB [0.1 μg/ml; Sigma-Aldrich, Steinham, Germany]) was used as a positive control. Antigens were added to the culture to a final volume of 200 μl per well. Microtiter plates were incubated at 37°C in 95% humidity containing 5% CO2. After the optimal duration of incubation (2 days for IL-12 and IL-10 and 6 days for IFN-γ, IL-5, IL-4, and IL-13), culture supernatant was removed and stored at −80°C for cytokine evaluation. These incubation periods were identified as optimal following preliminary kinetic experiments (data not shown). To validate the enzyme-linked immunosorbent assay, IL-5 production by peripheral blood cells from an allergic adult volunteer was confirmed (data not shown). Cytokine levels in supernatants were determined for IL-12 (Pierce Biotechnology, Rockford, IL) and IL-10, IFN-γ, IL-5, IL-4, and IL-13 (all from Pharmingen, BD Biosciences, San Jose, CA) by a sandwich enzyme-linked immunosorbent assay as recommended by the manufacturers. The limits of detection for the cytokines were as follows: IL-12, ≤5 pg/ml; IL-10 and IFN-γ, ≤15 pg/ml; and IL-5, IL-4, and IL-13, ≤10 pg/ml. Recombinant cytokines were used as standards as recommended by the manufacturer. The amounts of secreted cytokines present in the culture supernatants of CBMC and CD14+ purified monocytes were expressed as medians after the deduction of cytokine levels (in picograms per milliliter) in unstimulated cultures. All tests were conducted in triplicate.
ICC levels in whole cord blood samples and peripheral blood samples from 13-week-old infants were determined as described previously (14). Whole cord blood and not CBMC was used for ICC evaluation, because in optimization assays it was difficult to obtain a sufficient number of gated events when CBMC were used and the stimulation of CBMC with BCG caused monocyte death, which did not occur in whole cord blood (data not shown). Furthermore, for the 13-week-old infants, there was a limit of the volume of peripheral blood that could be drawn, so a whole-blood ICC assay was used at 13 weeks. Whole cord blood or peripheral blood (0.5 to 2 ml) was stimulated with BCG at an average MOI of 3.6:1. SEB (10 μg/ml; Sigma-Aldrich, Steinham, Germany) was used as a positive control. Cord blood was added to tubes containing antigen or mitogen, and the tubes were placed in a 37°C water bath for 7 h. Brefeldin A (10 μg/ml; Sigma-Aldrich, Steinham, Germany) was added, and the tubes were then placed in a programmable water bath to incubate for a further 5 h (i.e., a total of 12 h at 37°C) as described previously (14). Anti-CD49d and anti-CD28 costimulatory antibodies (1 μg/ml; BD Biosciences, San Jose, CA) were added to enhance the T-cell and NK cell IFN-γ responses (36).
Whole cord blood and peripheral blood cytokine detection.
Fluorescent dye-conjugated antibodies against CD3, CD4, CD8, CD14, CD56, IFN-γ, and IL-10 were obtained from BD Biosciences (San Jose, CA). The quantification of IFN-γ and IL-10 ICC in CD4+ and CD8+ T cells and CD14+ monocytes was performed, and a minimum of 20,000 gated events were acquired. In CD3− CD56+ NK cells, only intracellular IFN-γ was evaluated, and a minimum of 15,000 gated events were acquired. A larger volume of blood was required for NK cell ICC evaluation than for T-cell ICC evaluation due to the lower frequencies of NK cells in cord blood. Thus, the volume of blood available was the rate-limiting factor for NK cell cytokine analysis. The results are presented as the median percentage of positively staining cells exposed to the stimulant (BCG or SEB) minus the percentage of stained control cells not stimulated with BCG or SEB. A result of >0.1% was considered positive, as this value is the threshold of detection for this assay.
Statistical analysis.
Results are reported as medians and IQR. The Mann-Whitney U two-tailed test for nonparametric data was used for group comparison. All statistical analyses were carried out using InStat software (version 3.06; GraphPad Software, Inc.).
RESULTS
BCG-induced CBMC cytokine secretion into culture supernatants.
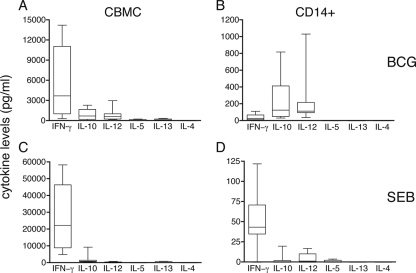
To assess the profile and levels of cytokine production in cord blood in response to stimulation with BCG, CBMC were incubated with BCG for 2 days (IL-10 and IL-12) or 6 days (IFN-γ, IL-13, IL-5, and IL-4) and cytokines released into the culture supernatants were quantified. High levels of IFN-γ, lower levels of IL-10 and IL-12, and minimal amounts of IL-13 and IL-5 were detected (Fig. 1A), whereas IL-4 was not measurable. When CD14+ cells were purified from the CBMC and incubated with BCG, high levels of both IL-10 and IL-12 and only very low levels of IFN-γ were measured (Fig. 1B). IL-5, IL-13, and IL-4 were not produced by these cells. Thus, the high levels of IFN-γ produced by BCG-stimulated CBMC derived primarily from populations other than CD14+ monocytes, whereas IL-10 and IL-12 were produced mostly by these cells. When CBMC were stimulated with the polyclonal superantigen SEB as a positive control for general T-cell reactivity in newborns, very high levels of IFN-γ (22,427 pg/ml; IQR, 9,027 to 46,470 pg/ml) and lower levels of IL-10 (984 pg/ml; IQR, 709 to 1,595 pg/ml) were detected (Fig. 1C). Similar quantities of IL-12 (328 pg/ml; IQR, 117 to 594 pg/ml) and IL-13 (280 pg/ml; IQR, 208 to 816 pg/ml) were measured, and negligible levels of IL-5 and IL-4 were detected. In contrast, purified CD14+ cells exposed to SEB produced very little IFN-γ and essentially no IL-10, IL-12, IL-5, IL-13, or IL-4 (Fig. 1D). Thus, CBMC responded both to BCG and to SEB, while monocytes responded only to BCG.
FIG. 1.
(A and B) CBMC and cord blood purified CD14+ cells were stimulated with Danish BCG (MOI, 5:1), the supernatants were harvested, and cytokine levels in picograms per milliliter were determined. IL-10 and IL-12 cytokines were measured after 2 days of in vitro stimulation, and IFN-γ, IL-5, IL-13, and IL-4 were measured after 6 days of stimulation. The unstimulated response (median levels for CBMC and CD14+ cells were 73 and 57 pg/ml for IFN-γ, 121 and 85 pg/ml for IL-10, 0 and 0 pg/ml for IL-12, 2 and 2 pg/ml for IL-5, 0 and 0 pg/ml for IL-13, and 0 and 0 pg/ml for IL-4) has been deducted from all results. (C and D) CBMC and cord blood purified CD14+ cells were stimulated with SEB (0.1 μg/ml), the supernatants were harvested after the incubation periods indicated for panels A and B, and cytokine levels in picograms per milliliter were determined. Medians and IQR calculated following the deduction of unstimulated responses are indicated by the horizontal lines and the boxes, respectively. Minimum and maximum results are indicated by whiskers. n = 6 to 9 independent cord blood evaluations. Please note the use of different scales for the graphs.
BCG-induced whole cord blood ICC production.
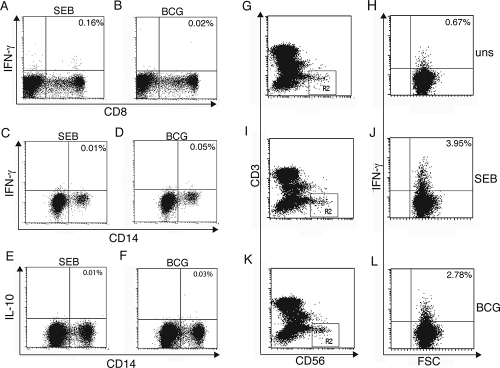
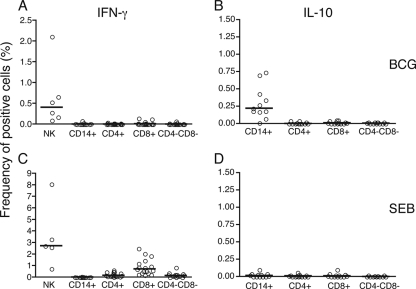
To directly determine which cell populations produced IFN-γ and IL-10, we assessed cell-specific cytokine production in whole cord blood by staining for ICC using the assay described in Materials and Methods. Figure 2 shows representative examples of dot plots generated; analyses of ICC IFN-γ within CD8+ T cells (Fig. 2A and B), ICC IFN-γ within CD14+ cells (Fig. 2C and D), ICC IL-10 within CD14+ cells (Fig. 2E and F), and ICC IFN-γ within unstimulated CD3− CD56+ NK cells (Fig. 2G and H) and of ICC following SEB (Fig. 2I and J) or BCG (Fig. 2K and L) stimulation of whole cord blood are shown. The response of unstimulated cells was subtracted from the response of stimulated cells. The background frequency of IFN-γ in NK cells was markedly higher than that in T cells (Fig. 2G and H). The frequencies of CD4+ or CD8+ T cells, CD4− CD8− cells, CD14+ monocytes, and CD3− CD56+ NK cells expressing intracellular IFN-γ after the ex vivo stimulation of cord blood with BCG are shown in Fig. 3A. Clearly, the IFN-γ produced by CBMC exposed to BCG was predominantly NK cell derived, with a very minor contribution from CD8+ T cells in 2 of 17 donor blood samples analyzed. IL-10 was produced by CD14+ monocytes (Fig. 3B) and not by CD4+ or CD8+ T cells. When SEB was used to stimulate cytokine production by whole cord blood, low levels of IFN-γ were produced by CD4+ and CD8+ T cells, as well as CD4− CD8− cells, compared to those produced by CD3− CD56+ NK cells (Fig. 3C). Similar to those in BCG-stimulated whole cord blood, CD8+ T cells were more responsive in terms of IFN-γ production than the other T-cell subsets. IL-10 was not induced by stimulation with SEB (Fig. 3D).
FIG. 2.
(A to F) Dot plots for whole cord blood samples showing intracellular IFN-γ responses (y axis) within CD8+ T cells (x axis) (A and B) and CD14+ cells (x axis) (C and D) and intracellular IL-10 responses (y axis) within CD14+cells (x axis) (E and F) following SEB stimulation or BCG stimulation. (G to L) Dot plots for the assessment of ICC IFN-γ production (y axis) within NK cells. Panels G, I, and K are dot plots for whole cord blood samples with CD3 (y axis) and CD56 (x axis) to enable the evaluation of NK cells (CD3− CD56+); only 1% of cells are represented, as 8 to 10 million cells were acquired for >15,000 gated events to be obtained. (H, J, and L) NK cells were selected in gate R2, and the ICC IFN-γ responses (y axis) within NK cells are shown; 100% of cells are represented. The unstimulated (uns) ICC IFN-γ NK cell response (H) and the responses to stimulation with SEB (J) and BCG (L) are indicated. The percentages in the upper right corners of the dot plots are frequencies of positive cells. FSC, forward scatter.
FIG. 3.
(A and B) IFN-γ ICC and IL-10 ICC in whole unseparated cord blood samples stimulated ex vivo with Danish BCG (MOI, 3.6:1). (C and D) Cord blood IFN-γ ICC responses to stimulation with SEB (10 μg/ml) and cord blood IL-10 ICC responses to stimulation with SEB (10 μg/ml) are shown. Results are expressed as frequencies (in percentages) of positive cells, following the deduction of the unstimulated response. Medians are indicated by horizontal lines. n = 6 to 17 independent cord blood evaluations. Please note the use of different scales in the graphs.
ICC production by whole-blood cells of neonates vaccinated with BCG at birth.
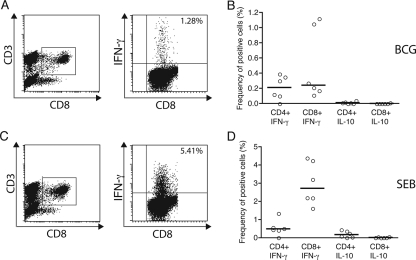
To evaluate ICC induction following BCG vaccination at birth, peripheral blood samples from six infants at 13 weeks of age were stimulated ex vivo with BCG and cytokine-producing cells were identified. Figure 4A and C show results from dot plot analyses of the staining of peripheral blood cells from a 13-week-old infant for ICC; the ICC IFN-γ response in CD8+ T cells following either BCG or SEB stimulation is indicated. When the blood samples of BCG-vaccinated infants were studied, we observed that T cells had acquired the ability to produce IFN-γ in response to ex vivo stimulation with BCG. Significant increases in the frequencies of CD4+ (P < 0.01) and CD8+ (P < 0.0001) T cells producing intracellular IFN-γ compared with those in cord blood was observed (Fig. 4B). CD4+ and CD8+ T-cell IL-10 production was not induced by the ex vivo stimulation of the cells with BCG. Figure 4D shows the ICC IFN-γ and IL-10 production by peripheral blood cells obtained from 13-week-old vaccinated infants following ex vivo stimulation with SEB. IFN-γ expression in CD4+ and CD8+ T cells was increased. In addition, CD4+ T cells from several vaccinated infants had acquired the ability to produce IL-10 in response to ex vivo stimulation with SEB (Fig. 4D). Since no more than 8 ml of blood could be collected from each of these infants, it was not possible to determine the NK cell ICC response postvaccination, as these assays required 2 ml per treatment condition to ensure that 15,000 gated events were obtained.
FIG. 4.
Analyses of whole peripheral blood from 13-week-old infants. (A and C) Flow cytometric dot plots obtained following BCG stimulation (A) and SEB stimulation (C) showing the gating strategy for CD8+ cells (x axis) and CD3+ cells (y axis) and the evaluation of intracellular IFN-γ (y axis). The percentages in the upper right corners of the dot plots are frequencies of positive cells. (B and D) Scatter plots of IFN-γ ICC and IL-10 ICC expression in whole unseparated peripheral blood samples from 13-week-old infants vaccinated with Danish BCG at birth and stimulated ex vivo with Danish BCG (MOI, 3.6:1) (B) or SEB (10 μg/ml) (D) are shown. Results are expressed as frequencies (in percentages) of positive cells, following the deduction of the unstimulated response. Medians are indicated by horizontal lines. By the Mann-Whitney test (two tailed), statistically significant differences in the CD4+ IFN-γ+ (P < 0.01) and CD8+ IFN-γ+ (P < 0.0001) responses between the cord blood samples (Fig. 3A) and samples from 13-week-old infants (Fig. 4B) were demonstrated.
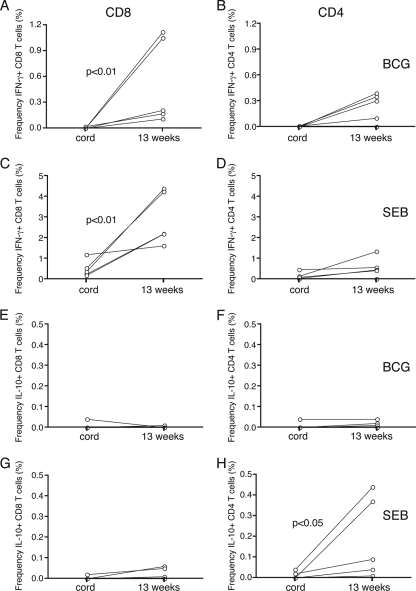
Figure 5 shows the matched ICC responses in the cord blood samples and in the peripheral blood samples from the same infants at 13 weeks of age. After ex vivo BCG stimulation, there was a statistically significant increase in ICC IFN-γ in CD8+ T cells (P < 0.01) in the blood samples from vaccinated infants compared with that in cord blood (Fig. 5A). However, there was a clear difference in the extent of the increase seen among the individual infants. When CD4+ T cells were examined, a small increase in ICC IFN-γ-producing cells in samples from four of the six neonates was noted. Results for the group were not statistically significant (Fig. 5B). IL-10 expression in either the CD4+ or CD8+ T cells of any of the vaccinated neonates tested was not induced in response to ex vivo BCG stimulation (Fig. 5E and F). When SEB-induced T-cell IFN-γ was examined, a significant increase in the percentage of CD8+ cells that were IFN-γ+ in peripheral blood (P < 0.01) compared to that in cord blood was seen (Fig. 5C). Similar to those stimulated with BCG, CD4+ T cells from vaccinated infants displayed a smaller increase in IFN-γ production upon SEB stimulation than CD8+ T cells; consequently, the results were not statistically significant (Fig. 5D). In contrast, ex vivo stimulation with SEB resulted in a statistically significant increase in the frequency of CD4+ IL-10+ T cells (P < 0.05) in the blood samples of vaccinated neonates compared with that in the cord blood samples (Fig. 5H), which was not seen for CD8+ T cells (Fig. 5G).
FIG. 5.
IFN-γ ICC and IL-10 ICC responses in the cord blood and the peripheral blood samples obtained from the same infants at 13 weeks of age. (A, B, E, and F) The IFN-γ responses in CD8+ T cells (A) and CD4+ T cells (B) and the IL-10 responses in CD8+ T cells (E) and CD4+ T cells (F) in peripheral blood samples compared to those in cord blood samples following stimulation with Danish BCG (MOI, 3.6:1) are indicated. (C, D, G, and H) The IFN-γ responses in CD8+ T cells (C) and CD4+ T cells (D) and the IL-10 responses in CD8+ T cells (G) and CD4+ T cells (H) compared to those in cord blood samples following stimulation with SEB (10 μg/ml) are similarly indicated. There was a significant difference between cord blood and 13-week blood samples for CD8+ IFN-γ+ cells (P < 0.01) following BCG stimulation (A) and for CD8+ IFN-γ+ cells (P < 0.01) (C) and CD4+ IL-10+ cells (P < 0.05) (H) following SEB stimulation, as indicated. Results are expressed as frequencies (in percentages) of cells expressing cytokine, following the deduction of the unstimulated response. Please note the use of different scales in the graphs.
DISCUSSION
In this study, we have shown that IFN-γ, IL-10, and IL-12 were produced by CBMC when the cells were stimulated in vitro with BCG. IL-5, IL-13, and IL-4 were barely detectable after BCG stimulation. Furthermore, IFN-γ appeared to be produced predominantly by NK cells and only to a very limited extent by CD8+ T cells, but not by CD4+ T cells. In contrast, IL-10 was produced by CD14+ monocytes but not by T cells. These novel results clearly show that immunologically naïve neonates have the capacity to respond to BCG with a Th1 cytokine response prior to T-cell activation and the induction of acquired immunity. However, at this early stage, it is clearly not a T-cell response but rather an NK cell response. The ability of T cells to produce IFN-γ in response to SEB stimulation closely mirrors BCG-induced responses, i.e., naïve CD4+ T cells not previously exposed to mycobacterial antigen did not produce IFN-γ even in response to the superantigen. The immune suppression associated with pregnancy or the immature nature of the immune function of the leukocytes in cord blood may account for the absent CD4+ T-cell IFN-γ response and the very low level of the CD8+ T-cell IFN-γ response following exposure to SEB. Upon SEB stimulation, a higher frequency of CD8+ T cells than of CD4+ T cells in cord blood produced IFN-γ. In line with our observations with SEB, White et al. report a cord blood CD4+ T-cell IFN-γ response lower than that in naïve adult peripheral blood cells but a CD8+ T-cell IFN-γ response similar to that in naïve adult peripheral blood cells following stimulation with phorbol 12-myristate 13-acetate and ionomycin (38).
NK cells have been shown previously to be required for host defense against several classes of extracellular pathogens, including bacteria, parasites, fungi, and yeasts (17, 26). NK cells have also been shown to play an important role in protective immunity against a number of intracellular bacterial pathogens, including mycobacteria, listeria, and salmonella (7, 9, 20, 41). Furthermore, IFN-γ has been reported to be produced by human NK cells but not by T cells during Staphylococcus aureus stimulation of peripheral blood mononuclear cells, and the early production of IFN-γ by NK cells was found previously to be essential in resistance to murine listeriosis (6, 45). Moreover, in murine studies using T-cell-deficient mice, resistance to M. tuberculosis was NK cell dependent and IL-12 was required for this innate response. In addition, T-cell-deficient mice were more resistant to M. tuberculosis than IFN-γ-deficient mice, and this result was shown to be due to NK cell-derived IFN-γ. The importance of NK cells for immunity against M. tuberculosis was further confirmed by the results of experiments using naïve splenocytes from T-cell-deficient mice stimulated with the bacilli, among which IFN-γ was found only within NK cells. However, when splenocytes from both T-cell- and NK cell-deficient mice were exposed to M. tuberculosis, no IFN-γ was detected (9). Thus, clearly NK cells are a very important source of IFN-γ production, especially in the absence of an acquired T-cell immune response. The production of IFN-γ by NK cells would serve both to enhance macrophage killing and to prime for the subsequent adaptive Th1 cytokine response. Thus, the findings in our study of cord blood NK cells producing IFN-γ in response to BCG stimulation support the idea that innate immunity plays a central role in neonatal responses to infections, including infection with pathogenic mycobacteria.
During pregnancy, the maternal cytokine milieu is reported to be dominated by type 2 cytokines (Th2), a profile considered essential to prevent the rejection of the fetus (21). For the first few months of life, neonates are protected against extracellular organisms to which the mother has developed immunity via maternal antibodies that cross the placenta. However, maternal lymphocytes do not cross the placenta, and therefore, newborn infants are not protected against intracellular organisms such as mycobacteria, which require T-cell-mediated protection (46). Thus, a rapid switch to the production of type 1 cytokines (Th1) at birth is required to provide protection against infectious organisms, particularly facultative organisms such as mycobacteria (12, 27). Since T cells are naïve and do not respond to mycobacterial antigens at birth, it is the role of NK cells to direct the host response toward a Th1 cytokine profile. BCG stimulation of IFN-γ production in human cells has previously been shown to require IL-12 and be dependent on CD40 costimulation (25). In the present study, IL-12 was produced by BCG-stimulated CD14+ cells. IL-12, which is produced by antigen-presenting cells in response to microorganisms, is an important cytokine that links innate and adaptive immunity (2, 34). Of note, the IL-12 receptor is found on both T cells and NK cells (2). IL-12 is critical in facilitating the differentiation of naïve CD4+ T cells into mature Th1 effector cells and stimulates both NK and CD8+ T cells to produce IFN-γ (2, 15, 18). IL-12 production would therefore be a prerequisite for the induction of T-cell-mediated protective immunity (18). However, it is possible that NK cell activation and the subsequent production of IFN-γ may be directly induced by the interaction of these cells with BCG. Two recent studies have reported the direct binding of BCG to NK cells, in one case via NKp44 and in the other via Toll-like receptor 2 (8, 23). Once the immune system is exposed to antigen, i.e., following BCG vaccination at birth, both CD4+ and CD8+ T cells acquire the ability to produce IFN-γ. T cells would be expected to produce higher levels of IFN-γ, as well as a more sustained response, than those produced by NK cells when exposed to BCG ex vivo. Our findings confirm the results of the study of Marchant et al., who reported a robust Th1 memory response in infants vaccinated with BCG at birth (24). Vekemans et al. report similar levels of IFN-γ production by CD4+ T cells in infants and adults following BCG vaccination and the restimulation of the cells with a purified protein derivative of tuberculin (35).
To avoid any influence that the mode of infant delivery may have on experimentally induced cytokine production, cord blood samples in the present study were collected from elective caesarean donors prior to the onset of labor. The mode of infant delivery (i.e., either caesarean section or vaginal delivery following labor) has been reported previously to be associated with differences in levels of cytokine production; cortisol, prostaglandins, and cytokines are secreted during labor, and this process may account for such differences (4, 29). During labor, the cytokine environment switches to Th1 to accelerate the inflammatory process which is required for successful labor and delivery. The cytokines that are increased during labor include IL-6, IL-8 (induces cervical ripening), IL-1β (stimulates the production of prostaglandin), IL-2, IL-15, IL-18, tumor necrosis factor alpha, and IFN-γ, whereas IL-10 levels are reduced (1, 19, 22, 42). The Th1 cytokines stimulate prostaglandins, which are responsible for the commencement of uterine contractions (42). In addition, labor has been found to cause an increase in the neonatal circulation of neutrophils, monocytes, and NK cells; neutrophils and T cells in vaginally delivered infants are activated compared to those in elective caesarean infants (22, 33). A surge of cortisol is also associated with labor, and this molecule is immunosuppressive, which may cause the inhibition of both cytokine production and T-cell function in cord blood (33). In addition, during normal vaginal delivery, the fetus is exposed to vaginal flora and lipopolysaccharide stimulation, whereas a caesarean section is conducted in a sterile environment without the aforementioned stimulation (22). Thus, labor as well as exposure to vaginal flora during delivery probably affects the cytokine response of cord blood cells, providing an explanation for any differences between the results of our studies and those reported by other investigators (15).
In conclusion, this study shows that similar profiles of cytokines are induced by the ex vivo exposure of mononuclear leukocytes to BCG prior to and after BCG vaccination of the neonate. The cytokines measured in response to BCG in cord blood were derived from cells of the innate immune response. This response is expected to be shorter lived and less robust than the subsequent acquired immune response mediated by antigen-specific T cells. Consequently, protective immunity to mycobacterial infection in immunologically naïve neonates is expected to be limited compared with the acquired immune response that follows the vaccine-induced activation of T cells. A better understanding of the contribution of the innate immune response to protective immunity against TB and its multiple effects on the induction of the adaptive response would facilitate the design of new and better vaccination strategies.
Acknowledgments
We thank John Anthony and staff in maternity theater and the pediatric neonatal staff at Groote Schuur Hospital, Cape Town, South Africa, for facilitating the collection of samples. The reagents for the evaluation of IL-4 and IL-13 were a generous gift from Barbara Nurse of the Department of Clinical Laboratory Sciences, University of Cape Town, Cape Town, South Africa.
This work was supported by a grant from the Medical Research Council Tuberculosis Vaccine Initiative (to S.R.R.) and by NIH grants AI 54631 and NO1-AI70022 (to G.K.). W.A.H. is supported by the Aeras Global Tuberculosis Vaccine Foundation, the NIH (grants NO1-AI70022 and RO1-HL55936), and the European and Developing Countries Trials Partnership.
Footnotes
Published ahead of print on 24 September 2008.
REFERENCES
- 1.Athanassakis, I., and S. Vassiliadis. 2002. Interplay between T helper type 1 and type 2 cytokines and soluble major histocompatibility complex molecules: a paradigm in pregnancy. Immunology 107:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastos, K. R., C. R. Marinho, R. Barboza, M. Russo, J. M. Alvarez, and M. R. D'Imperio Lima. 2004. What kind of message does IL-12/IL-23 bring to macrophages and dendritic cells? Microbes Infect. 6:630-636. [DOI] [PubMed] [Google Scholar]
- 3.Belvedere, O., C. Feruglio, W. Malangone, M. L. Bonora, A. M. Minisini, R. Spizzo, A. Donini, P. Sala, D. De Anna, D. M. Hilbert, and A. Degrassi. 2000. Increased blood volume and CD34+ CD38− progenitor cell recovery using a novel umbilical cord blood collection system. Stem Cells 18:245-251. [DOI] [PubMed] [Google Scholar]
- 4.Brown, M. A., P. Y. Rad, and M. J. Halonen. 2003. Method of birth alters interferon-gamma and interleukin-12 production by cord blood mononuclear cells. Pediatr. Allergy Immunol. 14:106-111. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson, C., R. Buchanan, J. Webster, V. Laundy, H. Horsley, C. Barron, N. Anderson, B. Bradley, and J. Hows. 2000. Development of a district cord blood bank: a model for cord blood banking in the National Health Service. Bone Marrow Transplant. 25:899-905. [DOI] [PubMed] [Google Scholar]
- 6.Dunn, P. L., and R. J. North. 1991. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect. Immun. 59:2892-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emoto, Y., M. Emoto, and S. H. Kaufmann. 1997. Transient control of interleukin-4-producing natural killer T cells in the livers of Listeria monocytogenes-infected mice by interleukin-12. Infect. Immun. 65:5003-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esin, S., G. Batoni, C. Counoupas, A. Stringaro, F. L. Brancatisano, M. Colone, G. Maisetta, W. Florio, G. Arancia, and M. Campa. 2008. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect. Immun. 76:1719-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, C. G., M. Kaviratne, A. G. Rothfuchs, A. Cheever, S. Hieny, H. A. Young, T. A. Wynn, and A. Sher. 2006. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J. Immunol. 177:7086-7093. [DOI] [PubMed] [Google Scholar]
- 10.Fine, P., and L. B. Reichman. 2000. BCG vaccines and vaccination, p. 503-524. In L. B. Reichman (ed.), Tuberculosis: a comprehensive international approach, 2nd ed. Marcel Decker, Inc., New York, NY.
- 11.Fine, P. E. M., I. A. M. Carneiro, J. B. Milstien, and C. J. Clements. 1999. Issues relating to the use of BCG in immunization programmes. World Health Organization, Geneva, Switzerland.
- 12.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 13.Hanekom, W. A. 2005. The immune response to BCG vaccination of newborns. Ann. N. Y. Acad. Sci. 1062:69-78. [DOI] [PubMed] [Google Scholar]
- 14.Hanekom, W. A., J. Hughes, M. Mavinkurve, M. Mendillo, M. Watkins, H. Gamieldien, S. J. Gelderbloem, M. Sidibana, N. Mansoor, V. Davids, R. A. Murray, A. Hawkridge, P. A. Haslett, S. Ress, G. D. Hussey, and G. Kaplan. 2004. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J. Immunol. Methods 291:185-195. [DOI] [PubMed] [Google Scholar]
- 15.Hunter, C. A. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5:521-531. [DOI] [PubMed] [Google Scholar]
- 16.Hussey, G. D., M. L. Watkins, E. A. Goddard, S. Gottschalk, E. J. Hughes, K. Iloni, M. A. Kibel, and S. R. Ress. 2002. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology 105:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jouanguy, E., R. Doffinger, S. Dupuis, A. Pallier, F. Altare, and J. L. Casanova. 1999. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11:346-351. [DOI] [PubMed] [Google Scholar]
- 18.Kastelein, R. A., C. A. Hunter, and D. J. Cua. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 25:221-242. [DOI] [PubMed] [Google Scholar]
- 19.Keelan, J. A., M. Blumenstein, R. J. Helliwell, T. A. Sato, K. W. Marvin, and M. D. Mitchell. 2003. Cytokines, prostaglandins and parturition—a review. Placenta 24(Suppl. A):33-46. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman, L. A., and C. A. Hunter. 2002. Regulatory pathways involved in the infection-induced production of IFN-gamma by NK cells. Microbes Infect. 4:1531-1538. [DOI] [PubMed] [Google Scholar]
- 21.Lin, H., T. R. Mosmann, L. Guilbert, S. Tuntipopipat, and T. G. Wegmann. 1993. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151:4562-4573. [PubMed] [Google Scholar]
- 22.Malamitsi-Puchner, A., E. Protonotariou, T. Boutsikou, E. Makrakis, A. Sarandakou, and G. Creatsas. 2005. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum. Dev. 81:387-392. [DOI] [PubMed] [Google Scholar]
- 23.Marcenaro, E., B. Ferranti, M. Falco, L. Moretta, and A. Moretta. 2008. Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC. Int. Immunol. 20:1155-1167. [DOI] [PubMed] [Google Scholar]
- 24.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 25.Mendez-Samperio, P., H. E. Ayala-Verdin, and A. Trejo-Echeverria. 1999. Interleukin-12 regulates the production of Bacille Calmette-Guerin-induced interferon-gamma from human cells in a CD40-dependent manner. Scand. J. Immunol. 50:61-67. [PubMed] [Google Scholar]
- 26.Morrison, B. E., S. J. Park, J. M. Mooney, and B. Mehrad. 2003. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J. Clin. Investig. 112:1862-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 28.Ota, M. O., J. Vekemans, S. E. Schlegel-Haueter, K. Fielding, M. Sanneh, M. Kidd, M. J. Newport, P. Aaby, H. Whittle, P. H. Lambert, K. P. McAdam, C. A. Siegrist, and A. Marchant. 2002. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 168:919-925. [DOI] [PubMed] [Google Scholar]
- 29.Protonotariou, E., A. Malamitsi-Puchner, D. Rizos, A. Sarandakou, E. Makrakis, and E. Salamolekis. 2003. Alterations in Thl/Th2 cytokine concentrations in early neonatal life. J. Matern.-Fetal Neonatal Med. 14:407-410. [DOI] [PubMed] [Google Scholar]
- 30.Regidor, C., M. Posada, D. Monteagudo, C. Garaulet, N. Somolinos, R. Fores, M. Briz, and M. N. Fernandez. 1999. Umbilical cord blood banking for unrelated transplantation: evaluation of cell separation and storage methods. Exp. Hematol. 27:380-385. [DOI] [PubMed] [Google Scholar]
- 31.Ridings, J., H. Weedon, C. Ioannou, L. Flego, P. J. Macardle, and H. Zola. 1996. Purification of cord blood lymphocytes. J. Immunol. Methods 195:43-48. [DOI] [PubMed] [Google Scholar]
- 32.Stop TB Partnership. 2006. Tuberculosis facts. Stop TB Partnership, Geneva, Switzerland. http://www.who.int/tb/publications/2006/tb_facts_2006.pdf.
- 33.Thornton, C. A., C. C. Capristo, L. L. Power, J. A. Holloway, E. J. Popplewell, N. D. Diaper, and J. O. Warner. 2003. The effect of labor on neonatal T-cell phenotype and function. Pediatr. Res. 54:120-124. [DOI] [PubMed] [Google Scholar]
- 34.van de Vosse, E., and T. H. Ottenhoff. 2006. Human host genetic factors in mycobacterial and Salmonella infection: lessons from single gene disorders in IL-12/IL-23-dependent signaling that affect innate and adaptive immunity. Microbes Infect. 8:1167-1173. [DOI] [PubMed] [Google Scholar]
- 35.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 36.Waldrop, S. L., K. A. Davis, V. C. Maino, and L. J. Picker. 1998. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J. Immunol. 161:5284-5295. [PubMed] [Google Scholar]
- 37.Walker, V., G. Selby, and I. Wacogne. 2006. Does neonatal BCG vaccination protect against tuberculous meningitis? Arch. Dis. Child. 91:789-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White, G. P., P. M. Watt, B. J. Holt, and P. G. Holt. 2002. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD. J. Immunol. 168:2820-2827. [DOI] [PubMed] [Google Scholar]
- 39.WHO. 2007. Global atlas of infectious diseases. World Health Organization, Geneva, Switzerland.
- 40.WHO. 2004. Weekly epidemiological record. World Health Organization, Geneva, Switzerland.
- 41.Wick, M. J. 2004. Living in the danger zone: innate immunity to Salmonella. Curr. Opin. Microbiol. 7:51-57. [DOI] [PubMed] [Google Scholar]
- 42.Wilczynski, J. 2005. Th1/Th2 cytokines balance—yin and yang of reproductive immunology. Eur. J. Obstet. Gynecol. Reprod. Biol. 122:136-143. [DOI] [PubMed] [Google Scholar]
- 43.Yamada, T., Y. Okamoto, H. Kasamatsu, Y. Horie, N. Yamashita, and K. Matsumoto. 2000. Factors affecting the volume of umbilical cord blood collections. Acta Obstet. Gynecol. Scand. 79:830-833. [PubMed] [Google Scholar]
- 44.Yang, M. H., and S. J. Lin. 2001. Effect of two-round Ficoll-Hypaque density gradient centrifugation on lymphocyte subsets and natural killer activity of umbilical cord blood mononuclear cells. Pediatr. Hematol. Oncol. 18:57-63. [DOI] [PubMed] [Google Scholar]
- 45.Yoshihara, R., S. Shiozawa, T. Fujita, and K. Chihara. 1993. Gamma interferon is produced by human natural killer cells but not T cells during Staphylococcus aureus stimulation. Infect. Immun. 61:3117-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zinkernagel, R. M. 2001. Maternal antibodies, childhood infections, and autoimmune diseases. N. Engl. J. Med. 345:1331-1335. [DOI] [PubMed] [Google Scholar]