Abstract
The United Kingdom introduced meningococcal serogroup C conjugate (MCC) vaccines in 1999, resulting in substantial declines in serogroup C disease and carriage. Here, we measured the age-specific prevalence of serum bactericidal antibodies (SBA) to Neisseria meningitidis serogroup C and immunoglobulin G (IgG) concentrations to serogroups A, C, W-135, and Y in 2,673 serum samples collected in England between 2000 and 2004. We compared the seroprevalence of SBA titers of ≥8 in the postvaccination era with results from an earlier prevaccination study conducted using the same methods. We found that the percentages of individuals with protective SBA titers were higher in 2000 to 2004 in all of the age groups targeted for MCC vaccination. In the postvaccine era, the prevalence of protective titers was high (75%) in children who had recently been offered routine immunization, but this fell to 36% more than 18 months after scheduled immunization. In the cohorts targeted in the catch-up campaign, the percentage achieving SBA titers of ≥8 was higher in children offered the vaccine at ages 5 to 17 years than in children offered the vaccine at ages 1 to 4 years. The geometric mean concentration (GMC) IgG for serogroup C followed a similar pattern, corresponding to the age at and time since scheduled MCC vaccination. Serogroup-specific IgG GMCs for W-135 and Y were low and showed little variation by age. Serogroup A IgG GMCs were higher, possibly reflecting exposure to cross-reacting antigens. Although the incidence of serogroup C disease remains low due to persisting herd effects, population antibody levels to serogroup C meningococci should be monitored so that potentially susceptible age groups can be identified should herd immunity wane.
The United Kingdom was the first country to introduce meningococcal serogroup C conjugate (MCC) vaccination, in 1999, incorporating MCC vaccines into the routine infant immunization schedule at 2, 3, and 4 months of age and implementing an extensive catch-up campaign targeting children and young adults up to the age of 18 years (later extended to 24 years) (19). Since then, the incidence of serogroup C disease has declined markedly as a result of high short-term vaccine effectiveness (29) and a reduction in serogroup C carriage leading to herd immunity (15, 16, 24). The MCC vaccines were licensed on the basis of immunogenicity and safety trials in the absence of a large phase III efficacy trial. This was possible because well-defined immunologic correlates of protection for serogroup C disease existed. It is widely accepted that serum bactericidal antibody (SBA) titers of ≥4 (with human complement) and ≥8 (with baby rabbit complement) are indicative of protection from meningococcal disease (1-3, 7). As well as providing the basis for prelicensure evaluation of vaccines (19), such surrogates of protection are also useful for postlicensure surveillance.
Serologic surveillance has been used in the United Kingdom to inform vaccine policy for several diseases, for example, measles (6) and Haemophilus influenzae type b (Hib) (31). Continued meningococcal disease surveillance is obviously crucial to monitor the long-term impact of the MCC vaccine program, but serologic surveillance can be used to complement this. In England, we observed that the effectiveness of the MCC vaccine waned rapidly in children immunized with a 2-, 3-, and 4-month schedule (29). In response to this, the vaccine schedule was changed in September 2006 so that now children in the United Kingdom are offered MCC vaccines at 3, 4, and 12 months of age (5). The later dose at 12 months (given in combination with Hib) is expected to improve and extend direct protection in those children born after September 2005. The number of serogroup C cases remains low for all ages, including the cohort of children immunized according to a 2-, 3-, and 4-month schedule, probably as a result of low transmission and continued herd immunity. It is uncertain how long these herd effects may last. Seroprevalence studies can be used to improve our understanding of population immunity and to identify groups who are likely to be susceptible to serogroup C meningococcal infection should herd protection wane.
We previously reported the seroprevalence of SBAs to serogroup C meningococci in the prevaccine era (28). This earlier serosurvey used the same laboratory methods to test residual sera from the same populations as the present study and thus provides a baseline measurement of “natural” immunity against which we can compare population immunity in the presence of MCC vaccination. Here, we examined the age-specific seroprevalence profile for bactericidal serogroup C antibodies by using sera collected in the postvaccination era between 2000 and 2004. In addition, we measured immunoglobulin G (IgG) antibodies against Neisseria meningitidis serogroups A, C, W-135, and Y.
MATERIALS AND METHODS
Serum samples.
Sera were obtained from the Health Protection Agency (HPA) Seroepidemiology Unit. This collection is described fully elsewhere (22), but briefly, participating laboratories submit residual sera from routine diagnostic testing to the collection. Samples are made anonymous and a unique identity number is assigned, and details regarding the age and sex of the donor, year of collection, and participating laboratory are collated on a database. The reason why the blood sample was taken is not documented, but immunocompromised individuals are excluded.
Sera collected between 2000 and 2004 (up to 5 years after the introduction of MCC vaccination) were selected for analysis. For each year, samples were selected randomly within the age group and region strata. Sample-size targets were about 40 per year (2001 to 2004) for 0, 1, 2, 3, 4, 5, 6, 7, 8/9, 10/11, 12/13, 14/15, 16/17, and 18/19 years old. This sample size was chosen to enable reasonable precision of proportions with SBA titers of ≥8 within strata of interest (e.g., combining two age groups gives a total of 80 per age group, which would enable a proportion of 0.5 to be estimated with a 95% confidence interval [CI] of 0.39 to 0.61). For some age groups, there were insufficient samples available to meet these targets. Since the MCC catch-up campaign was rolled out during 2000, we did not select any sera from individuals less than 20 years old that year, so all sera from 0- to 19-year-olds are from 2001 to 2004. For adults (20 or more years old), samples were tested from those available in the year 2000. A total of 2,673 samples were chosen across the age range from individuals between 0 and 93 years of age. Some results were not obtained, due to insufficient volumes; the numbers of serum samples by age group for which results were obtained are shown in Table 1.
TABLE 1.
Number of serum samples analyzed for SBAs and serogroup-specific IgG antibodies per age group from the HPA Seroepidemiology Unit collection for England from 2000 to 2004a
| Age group | No. of SBA samples | No. of serogroup-specific IgG samples |
|---|---|---|
| Mo | ||
| <6 | 49 | 48 |
| 6-11 | 35 | 38 |
| Yr | ||
| 1 | 138 | 168 |
| 2 | 151 | 186 |
| 3 | 162 | 185 |
| 4 | 159 | 179 |
| 5 | 179 | 202 |
| 6 | 133 | 157 |
| 7 | 105 | 119 |
| 8-9 | 159 | 175 |
| 10-11 | 116 | 129 |
| 12-13 | 107 | 121 |
| 14-15 | 98 | 107 |
| 16-17 | 112 | 119 |
| 18-19 | 138 | 143 |
| 20-24 | 86 | 88 |
| 25-34 | 62 | 62 |
| 35-44 | 111 | 112 |
| 45-54 | 79 | 81 |
| 55-64 | 86 | 95 |
| ≥65 | 126 | 144 |
| Total | 2,391 | 2,658 |
All samples from individuals less than 20 years old are from 2001 to 2004.
SBA assay.
Sera were tested using a standardized complement-mediated SBA assay against O-acetylated serogroup C strain C11 (C:16:P1.7-1,1) as previously described (17). The complement source was pooled with 3- to 4-week-old baby rabbit serum (Pel-Freeze Biologicals, WI). SBA titers were expressed as the reciprocal of the final serum dilution killing ≥50% at 60 min. SBA titers of <4 were assigned a value of 2 for computational purposes. Results were obtained for 2,391 samples.
Serum IgG concentrations.
Serogroup-specific IgG concentrations were determined in serum by use of a tetraplex IgG bead assay for serogroups A, C, W-135, and Y as previously described (14). The calibration factors of the standard reference serum CDC1992 for N. meningitidis-specific IgG used were 91.8 μg/ml for serogroup A, 24.1 μg/ml for serogroup C (10), 25.23 μg/ml for serogroup W-135, and 28.92 μg/ml for serogroup Y (12). The lower limits of detection were 0.08, 0.06, 0.065, and 0.075 μg/ml for serogroups A, C, W-135, and Y, respectively. For any values recorded below this threshold, a value equal to half the lower limit of detection was assigned for computational purposes. Results were obtained for 2,658 samples.
Statistical analysis.
Results from the serologic analyses were linked to information on the age and sex of the donor. The age of the serum donor and the year of sample collection were used to infer the age and time at which MCC vaccination is likely to have been offered according to the routine and catch-up immunization schedules (19). Since all samples were made anonymous, it was not possible to collect information on immunization status. The percentage of individuals, by age, with SBA titers of ≥8 was calculated together with 95% CI. For individuals less than 20 years old, the age-specific prevalences of SBA titers of ≥8 against serogroup C meningococci were compared according to the age at vaccination/schedule that the group was eligible to have received and the time since vaccination was offered (within 2 years or after 2 or more years). A two-sample, two-sided z-test was used to assess the equality of proportions. The geometric mean concentrations (GMCs) of serogroup-specific IgG were calculated, by age, with corresponding 95% CI. The correlation between serogroup C IgG and SBA titers in individuals was assessed using the Spearman's rho statistic. Data were analyzed using Stata version 10.0.
Comparison with prevaccine study.
The age-specific prevalence of SBA to serogroup C between 2000 and 2004 was compared with the results of a previously published seroprevalence study from the prevaccine era (28). This earlier study used the same source of sera (the HPA Seroepidemiology Unit collection, described above) and the same standardized laboratory methods performed by the same laboratory.
Ethical approval.
The HPA Seroepidemiology Unit has ethical approval from the Joint University College London/University College London Hospitals Committees on the Ethics of Human Research (REC reference number 05/Q0505/U5) for serological surveillance of the National Immunisation Programme for England and Wales.
RESULTS
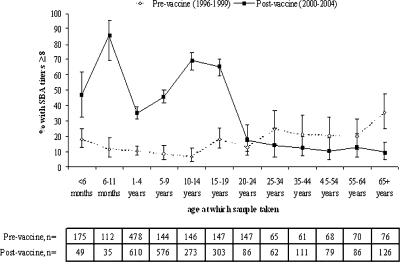
The cross-sectional, age-specific prevalence of SBA titers of ≥8 in the postvaccine era is shown in Fig. 1 and compared with the seroprevalence profile before the introduction of MCC vaccines. In individuals less than 20 years old (the main age range targeted in the MCC campaign), the percentage with SBA titers of ≥8 is higher in the postvaccine era than in the prevaccine era (P < 0.001 for all age groups). In sera collected from infants less than 1 year old, 63.1% (95% CI, 51.9 to 73.4%) achieved protective titers in 2001 to 2004, with a higher proportion (P < 0.001) with SBA titers of ≥8 in those aged 6 to 11 months than in those <6 months old (Fig. 1). In children aged 1 to 4 years, the percentage with protective titers is lower than those observed in infants, at 35.4% (95% CI, 31.6 to 39.4%; P < 0.001). In older children, the percentage with protective titers increases, and in 10- to 14- and 15- to 19-year-olds, the prevalence of SBA titers of ≥8 is around 67%. In sera collected from individuals aged 25 to 64 years old, prevalence is similar to that in the prevaccine era (as shown by overlapping CIs in Fig. 1). In those aged 65 years or older, however, the prevalence of protective titers is significantly lower after vaccine introduction (P < 0.001).
FIG. 1.
Comparison of the percentages of serum samples with serogroup C SBA titers of ≥8 (with 95% CI) by age in the pre- and postvaccine eras.
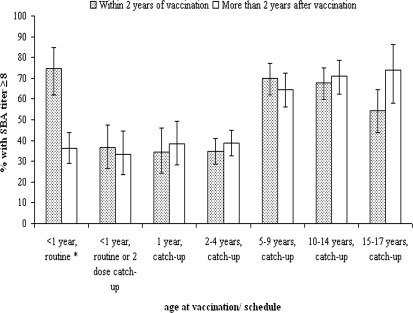
The percentages of children with protective titers, stratified by both age at and time since scheduled vaccination, are shown in Fig. 2. In children scheduled for immunization in infancy at 2, 3, and 4 months old, the percentage with SBA titers of ≥8 declines from 74.6% within 18 months to 36.2% 18 months or more after vaccination was scheduled (P < 0.001). For children offered the vaccine at older ages, no significant differences are observed according to time since immunization. The proportion of children achieving (and maintaining) SBA titers of ≥8 is higher in those offered the vaccine at older ages (5 years old and above) than in those offered the vaccine at 1 to 4 years of age (as shown by nonoverlapping CIs in Fig. 2).
FIG. 2.
Percentages of serum samples with serogroup C SBA titers of ≥8 (with 95% CI) by age targeted for vaccination, vaccine schedule, and time since vaccination. For the cohorts eligible for routine infant immunization, the columns reflect an offer of vaccination within 18 months and more than 18 months ago, respectively.
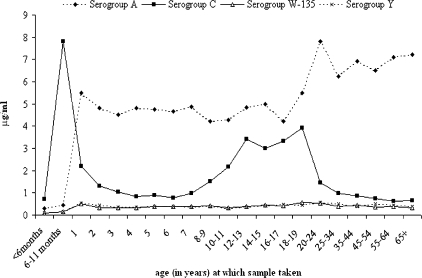
The serogroup- and age-specific IgG GMCs are shown in Fig. 3. For serogroup C, the distribution of GMCs by age is broadly similar to that for SBA titers and is consistent with coverage of and time since MCC immunization. There was evidence of a correlation between IgG GMC and SBA titers for serogroup C, with a Spearman's rho statistic of 0.6 (P < 0.001). The IgG GMC for individuals with an SBA titer of ≥8 was 4.2 μg/ml (95% CI, 3.8 to 4.7 μg/ml), compared to 0.61 μg/ml (95% CI, 0.57 to 0.65 μg/ml) in individuals with an SBA titer of <8.
FIG. 3.
Serogroup-specific IgG GMCs (in μg/ml) against serogroups A, C, W-135, and Y, by age group, in samples collected between 2000 and 2004.
Serogroup-specific IgG GMCs for serogroups W-135 and Y were low and showed little variation by age. For anti-serogroup A, we observed a sharp increase in IgG GMCs from age 0 to 1 year, followed by a plateau, with a further increase shown for older ages. The serogroup-specific IgG GMC for serogroup A was much higher than that for serogroups W-135 and Y and also significantly higher than that for serogroup C for all ages except <1-year-olds and 12- to 19-year-olds.
DISCUSSION
We have described the age-specific prevalence of SBAs and IgG antibodies to serogroup C meningococci in the English population following the introduction of MCC vaccines. As anticipated, a higher proportion of individuals achieved SBA titers of ≥8 in all age groups targeted for MCC immunization than in the prevaccine seroprevalence profile. We observed variations in the proportions achieving putative protective titers according to vaccine schedule offered, in particular the age at which vaccination was scheduled. In children targeted for routine immunizations at 2, 3, and 4 months of age, we observed that the prevalence of putative protective SBA titers declined with the time since vaccination. Previous studies have shown that antibody persistence is poor after MCC immunization in infancy (at 2, 3, and 4 months of age) and in children immunized at a young age (less than 2 years old) in the catch-up campaign (4, 26). This appears to be reflected in our results, where only 32% of children aged 1 to 4 years old had SBA titers of ≥8. For those targeted in the catch-up campaign, a higher percentage of children had SBA titers of ≥8 if they were offered vaccines at 5 to 17 years of age than if they were targeted at 1 to 4 years of age. This is consistent with estimates of vaccine effectiveness in England, which suggested that protection was higher and more persistent in those immunized above the age of 3 years than in children 1 to 2 years old (29) and which showed higher coverage in the school-based cohorts than in preschool children aged 2 to 5 years (32). Furthermore, studies of antibody persistence after vaccination have shown greater persistence when the vaccine was administered at older ages (26, 27).
The results of this cross-sectional study are compatible with those observed in studies that have followed cohorts of vaccinated children over time. Snape and colleagues reported that of 94 children who were immunized with a single dose of MCC around 2 years of age, 37% had an SBA titer of ≥8 a median of 1.8 years after immunization (26). In a further study, 987 children who had received 1 dose of MCC at age 6 to 15 years were followed up for a median of 5 years after immunization. Among these children, 84% had an SBA titer of ≥8 (27). This is slightly higher than that observed in our study, consistent with our study being an unselected population sample that would have included some unvaccinated children. These results further highlight the importance of age at the time of immunization in determining antibody persistence.
In adults aged 25 years and older, the percentages of serum samples with SBA titers of ≥8 were similar in the pre- and postvaccine eras, with the exception of those aged 65 years and older, where significantly fewer individuals achieved protective SBA titers postvaccination. As shown with Hib, the reduction in natural boosting due to lower transmission after vaccine introduction could increase susceptibility in unvaccinated adults (18). However, since these samples were collected in 2000, less than 1 year after vaccine introduction, it seems unlikely that such an effect would be observed so quickly, and the reasons for this difference are unclear.
The SBA assay is the accepted surrogate of protection against meningococcal disease (3), but examination of serogroup-specific IgG levels can also be informative. In our study, serogroup C-specific IgG GMCs followed an age-specific pattern similar to that of SBAs in general, with higher GMCs observed in infants, lower GMCs in 1- to 9-year-olds, and higher concentrations in teenagers, consistent with age at and time since MCC vaccination. The low levels of serogroup-specific W-135 and Y IgG are unsurprising, since these serogroups are not common among cases (8) or carriers (16) in the United Kingdom. We noticed a steep increase in serogroup A-specific IgG at the age of 1 year, with no evidence of maternal immunity in infants. Thereafter, GMCs were higher for serogroup A than for serogroup C (significantly so, with the exception of 12- to 19-year-olds), despite MCC vaccination. Since serogroup A carriage and disease are also uncommon in this setting (8, 16) and the age distribution is flatter than would be expected from the age-specific patterns of meningococcal carriage, the high GMCs reported here may be attributable to the presence of cross-reacting antigens (9, 21, 25).
The sera used in this study were obtained through convenience, rather than random sampling, by collecting residual sera from routine diagnostic testing. This collection has been widely used in epidemiologic studies (see references 6, 11, 20, 31, 33, and 34 for examples). One disadvantage of this collection is that in order to preserve anonymity, few details regarding the individuals from whom the sera were collected are available. We were unable to ascertain the MCC immunization status of the individuals in this study, so we could infer likely vaccine coverage based only on national data. Routine MCC coverage remains high (over 90%), and coverage in the catch-up campaign was also high (84% in children aged 12 to 23 months, 76% in children aged 2 to 5 years, and 85% in school-aged children) (32). Selection bias in the serum collection is thought to be limited because all individuals in the United Kingdom have free access to comprehensive health care. Studies conducted elsewhere have shown that this type of convenience sampling gives results similar to those of random sampling (13).
This study shows that serologic surveillance may be a useful tool in the continued monitoring of the MCC vaccination program. The MCC vaccination program has been very successful to date in reducing morbidity and mortality from meningococcal disease. In September 2006, the United Kingdom routine infant immunization schedule was changed (5) in response to evidence from postlicensure surveillance studies showing that only short-term direct protection is conferred when children are routinely immunized at 2, 3, and 4 months of age with MCC (29) and Hib (23) vaccines. Now, two doses of MCC are offered in infancy at 3 and 4 months old, with a later dose incorporated at 12 months of age (in combination with Hib vaccine) to improve the level and duration of protection. Direct protection against meningococcal serogroup C disease is thought to depend not only on immune memory (which is induced by MCC vaccines) but also on persistence of circulating antibodies (4). The sustained high prevalence of protective titers in children immunized at older ages is reassuring. The lower prevalence of protective titers in children targeted for immunization at younger ages (either with the accelerated routine schedule or in the 1- to 4-year-old catch-up campaign) is of some concern, but it is important to note that the incidence of serogroup C disease remains very low. Indirect vaccine protection is clearly very important in this sustained reduction in serogroup C disease. Mathematical models suggest that herd immunity persists for several years and that serogroup C circulation will increase only slowly after reaching its nadir (30). These predictions are consistent with postlicensure disease surveillance to date; however, these models do not account for possible introductions of more-transmissible or more-virulent serogroup C strains from outside of the United Kingdom. Continued high-quality disease surveillance is essential, but repeat seroprevalence surveys will also be useful to inform vaccine policy in order to identify susceptible cohorts should herd immunity wane.
Acknowledgments
We are grateful to the HPA Seroepidemiology Unit for providing the sera for this study. We thank Paul Balmer for data review and for serogroup-specific IgG concentrations, Louise Hesketh for maintaining the serum sample collection, and Andrew Vyse for assistance with sample selection.
Caroline Trotter is funded by a Personal Award Scheme Postdoctoral Award from the National Institutes of Health (Department of Health).
Caroline Trotter is an Honorary Research Fellow of the Immunization Department, HPA Centre for Infections.
Footnotes
Published ahead of print on 30 September 2008.
REFERENCES
- 1.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for the use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection-serum bactericidal antibody activity. Vaccine 23:2222-2227. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, R., and E. Miller. 2006. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev. Vaccines 5:851-857. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health. 8 February 2006. Planned changes to the routine childhood immunisation schedule. Department of Health, London, United Kingdom. http://www.dh.gov.uk/assetRoot/04/12/81/21/04128121.pdf/.
- 6.Gay, N. J., L. M. Hesketh, P. Morgan-Capner, and E. Miller. 1995. Interpretation of serological surveillance data for measles using mathematical models: implications for vaccine strategy. Epidemiol. Infect. 115:139-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray, S. J., C. L. Trotter, M. E. Ramsay, M. Guiver, A. J. Fox, R. Borrow, R. H. Mallard, and E. B. Kaczmarski. 2006. Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J. Med. Microbiol. 55:887-896. [DOI] [PubMed] [Google Scholar]
- 9.Guirguis, N., R. Schneerson, A. Bax, W. Egan, J. B. Robbins, J. Shiloach, I. Orskov, F. Orskov, and A. el Kholy. 1985. Escherichia coli K51 and K93 capsular polysaccharides are crossreactive with the group A capsular polysaccharide of Neisseria meningitidis. Immunochemical, biological, and epidemiological studies. J. Exp. Med. 162:1837-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holder, P. K., S. E. Maslanka, L. B. Pais, J. Dykes, B. D. Plikaytis, and G. M. Carlone. 1995. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin. Diagn. Lab. Immunol. 2:132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jit, M., A. Vyse, R. Borrow, R. Pebody, K. Soldan, and E. Miller. 2007. Prevalence of human papillomavirus antibodies in young female subjects in England. Br. J. Cancer 97:989-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph, H., P. Balmer, M. Bybel, T. Papa, R. Ryall, and R. Borrow. 2004. Assignment of Neisseria meningitidis serogroups A, C, W135, and Y anticapsular total immunoglobulin G (IgG), IgG1, and IgG2 concentrations to reference sera. Clin. Diagn. Lab. Immunol. 11:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly, H., M. A. Riddell, H. F. Gidding, T. Nolan, and G. L. Gilbert. 2002. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine 20:3130-3136. [DOI] [PubMed] [Google Scholar]
- 14.Lal, G., P. Balmer, H. Joseph, M. Dawson, and R. Borrow. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin. Diagn. Lab. Immunol. 11:272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maiden, M. C., A. B. Ibarz-Pavon, R. Urwin, S. J. Gray, N. J. Andrews, S. C. Clarke, A. M. Walker, M. R. Evans, J. S. Kroll, K. R. Neal, D. A. Ala'Aldeen, D. W. Crook, K. Cann, S. Harrison, R. Cunningham, D. Baxter, E. Kaczmarski, J. MacLennan, J. C. Cameron, and J. M. Stuart. 2008. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J. Infect. Dis. 197:737-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiden, M. C. J., and J. M. Stuart on behalf of the UK Meningococcal Carriage Group. 2002. Carriage of serogroup C meningococci one year after meningococcal C conjugate polysaccharide vaccination. Lancet 359:1829-1830. [DOI] [PubMed] [Google Scholar]
- 17.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. J. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. N. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. A. M. Peeters, S. Quatert, J. Y. Tai, G. M. Carlone, and the Multilaboratory Study Group. 1997. Standardisation and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McVernon, J., C. L. Trotter, M. P. Slack, and M. E. Ramsay. 2004. Trends in Haemophilus influenzae type b infections in adults in England and Wales: surveillance study. BMJ 329:655-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, E., D. M. Salisbury, and M. E. Ramsay. 2001. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 20:S58-S67. [DOI] [PubMed] [Google Scholar]
- 20.Morris, M. C., W. J. Edmunds, L. M. Hesketh, A. J. Vyse, E. Miller, P. Morgan-Capner, and D. W. Brown. 2002. Sero-epidemiological patterns of Epstein-Barr and herpes simplex (HSV-1 and HSV-2) viruses in England and Wales. J. Med. Virol. 67:522-527. [DOI] [PubMed] [Google Scholar]
- 21.Myerowitz, R. L., R. E. Gordon, and J. B. Robbins. 1973. Polysaccharides of the genus Bacillus cross-reactive with the capsular polysaccharides of Diplococcus pneumoniae type 3, Haemophilus influenzae type b, and Neisseria meningitidis group A. Infect. Immun. 8:896-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborne, K., N. J. Gay, L. Hesketh, P. Morgan-Capner, and E. Miller. 2000. Ten years of serological surveillance in England and Wales: methods, results, implications and action. Int. J. Epidemiol. 29:362-368. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay, M. E., J. McVernon, N. J. Andrews, P. T. Heath, and M. P. Slack. 2003. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J. Infect. Dis. 188:481-485. [DOI] [PubMed] [Google Scholar]
- 24.Ramsay, M. E., N. J. Andrews, C. L. Trotter, E. B. Kaczmarski, and E. Miller. 2003. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ 326:365-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins, J. B., L. Myerowitz, J. K. Whisnant, M. Argaman, R. Schneerson, Z. T. Handzel, and E. C. Gotschlich. 1972. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and III. Infect. Immun. 6:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snape, M. D., D. F. Kelly, B. Green, E. R. Moxon, R. Borrow, and A. J. Pollard. 2005. Lack of serum bactericidal activity in preschool children two years after a single dose of serogroup C meningococcal polysaccharide-protein conjugate vaccine. Pediatr. Infect. Dis. J. 24:128-131. [DOI] [PubMed] [Google Scholar]
- 27.Snape, M. D., D. F. Kelly, S. Lewis, C. Banner, L. Kibwana, C. E. Moore, L. Diggle, T. John, L. M. Yu, R. Borrow, A. Borkowski, C. Nau, and A. J. Pollard. 2008. Seroprotection against serogroup C meningococcal disease in adolescents in the United Kingdom: observational study. BMJ 336:1487-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trotter, C., R. Borrow, N. Andrews, and E. Miller. 2003. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine 21:1094-1098. [DOI] [PubMed] [Google Scholar]
- 29.Trotter, C. L., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365-367. [DOI] [PubMed] [Google Scholar]
- 30.Trotter, C. L., N. J. Gay, and W. J. Edmunds. 2005. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am. J. Epidemiol. 162:89-100. [DOI] [PubMed] [Google Scholar]
- 31.Trotter, C. L., J. McVernon, N. J. Andrews, M. Burrage, and M. E. Ramsay. 2003. Antibody to Haemophilus influenzae type b after routine and catch-up vaccination. Lancet 361:1523-1524. [DOI] [PubMed] [Google Scholar]
- 32.Trotter, C. L., M. E. Ramsay, and E. B. Kaczmarski. 2002. Meningococcal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Commun. Dis. Public Health 5:220-225. [PubMed] [Google Scholar]
- 33.Vyse, A. J., N. J. Andrews, L. M. Hesketh, and R. Pebody. 2007. The burden of parvovirus B19 infection in women of childbearing age in England and Wales. Epidemiol. Infect. 135:1354-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vyse, A. J., N. J. Gay, L. M. Hesketh, P. Morgan-Capner, and E. Miller. 2004. Seroprevalence of antibody to varicella zoster virus in England and Wales in children and young adults. Epidemiol. Infect. 132:1129-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]