Abstract
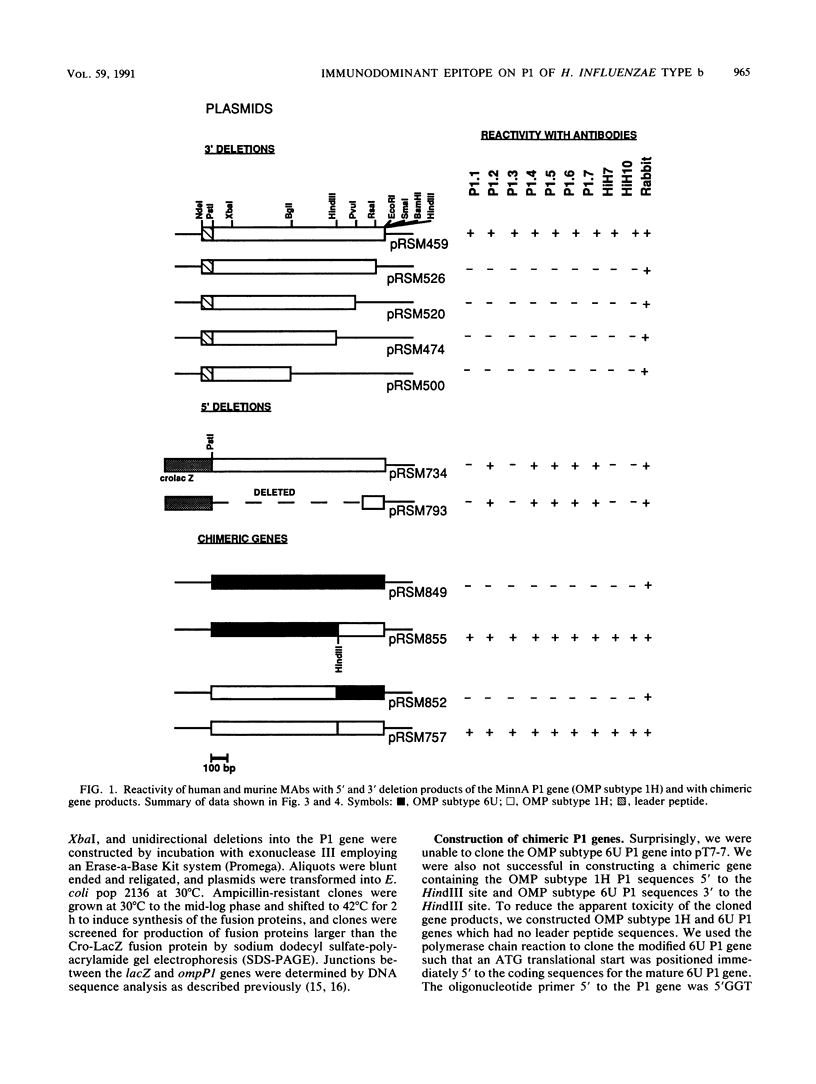
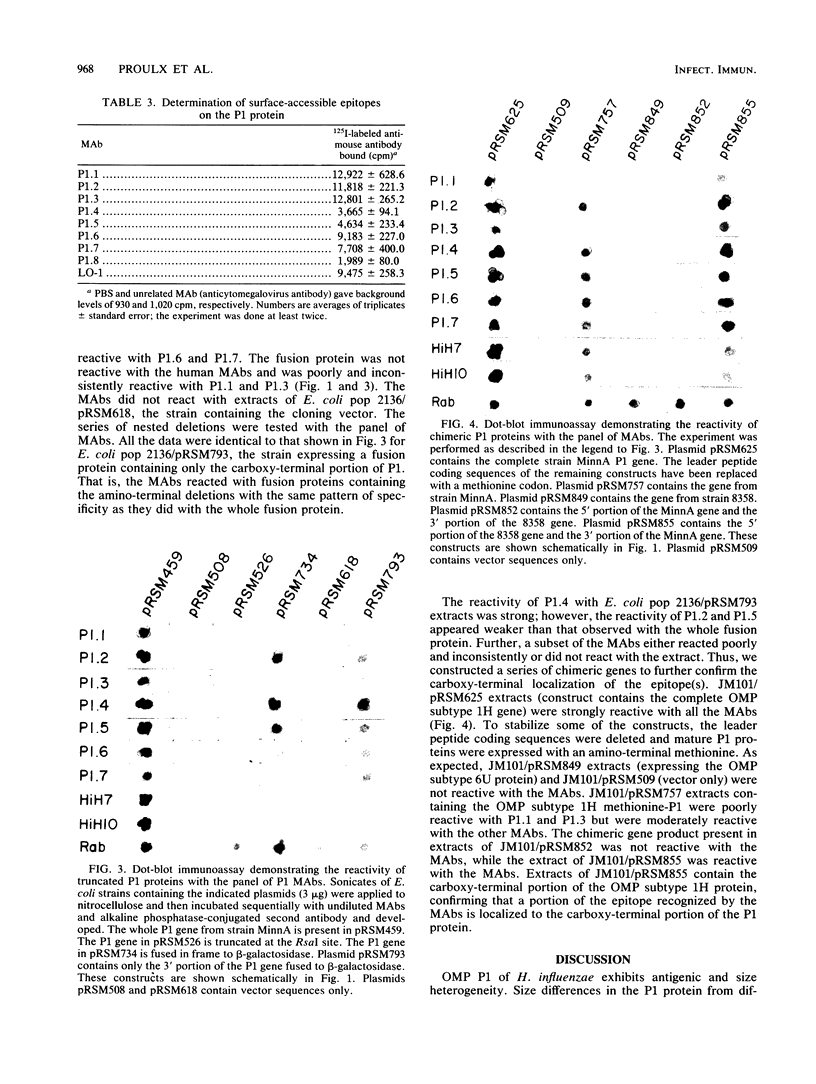
Eight murine monoclonal antibodies (MAbs) directed against outer membrane protein P1 of Haemophilus influenzae type b were generated and characterized. Seven of the eight MAbs reacted with recombinant P1 and purified P1 protein from H. influenzae type b strains MinnA and 1613; MAb P1.8 was specific for the latter strain. A panel of 32 nontypeable and 140 encapsulated Haemophilus strains recovered worldwide representing the major clonal families of serotypes a, b, and d was used to evaluate the distribution among Haemophilus strains of the epitopes identified by the P1-specific MAbs. The epitope reactive with the seven MAbs which recognized P1 from strains MinnA and 1613 was shared by 92% of the encapsulated Haemophilus isolates tested. The epitope is present in the H. influenzae type b strains from clonal families commonly recovered from cases of invasive disease in North America and Europe. A series of nested 5' and 3' deletions of the P1 gene were constructed and analyzed to localize the determinants on P1 recognized by the MAbs. MAbs P1.2, P1.4, P1.5, P1.6, and P1.7 recognized an epitope localized to the carboxy-terminal portion of P1. Murine MAbs P1.1 and P1.3 and two human MAbs, HiH-7 and HiH-10, recognized a complex epitope which was partially localized to the carboxy-terminal portion of the P1 protein. These data indicate that an immunodominant surface-exposed epitope is present on the carboxy-terminal portion of the P1 protein of type b Haemophilus isolates responsible for the majority of invasive disease in North America.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Granoff D. M., Munson R. S., Jr Outer-membrane protein subtypes of Haemophilus influenzae type b and spread of disease in day-care centers. J Infect Dis. 1981 Sep;144(3):210–217. doi: 10.1093/infdis/144.3.210. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Larose Y., Tsang P., Hamel J., Ashton F., Ryan A. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect Immun. 1985 Nov;50(2):510–516. doi: 10.1128/iai.50.2.510-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales F. R., Leachman S., Norgard M. V., Radolf J. D., McCracken G. H., Jr, Evans C., Hansen E. J. Cloning and expression in Escherichia coli of the gene encoding the heat-modifiable major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1987 Dec;55(12):2993–3000. doi: 10.1128/iai.55.12.2993-3000.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Hamel J., Brodeur B. R., Larose Y., Tsang P. S., Belmaaza A., Montplaisir S. A monoclonal antibody directed against a serotype-specific, outer-membrane protein of Haemophilus influenzae type b. J Med Microbiol. 1987 Mar;23(2):163–170. doi: 10.1099/00222615-23-2-163. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laing P. Luminescent visualization of antigens on blots. J Immunol Methods. 1986 Sep 27;92(2):161–165. doi: 10.1016/0022-1759(86)90161-4. [DOI] [PubMed] [Google Scholar]
- Loeb M. R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987 Nov;55(11):2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier M., Brodeur B. R., Winston S. Detection of Neisseria gonorrhoeae by dot-enzyme immunoassay using monoclonal antibodies. J Immunoassay. 1989;10(4):373–394. doi: 10.1080/01971528908053248. [DOI] [PubMed] [Google Scholar]
- Martin D., Hamel J., Brodeur B. R., Musser J. M. Antigenic relationships among the porin proteins of encapsulated Haemophilus influenzae clones. J Clin Microbiol. 1990 Aug;28(8):1720–1724. doi: 10.1128/jcm.28.8.1720-1724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Larose Y., Hamel J., Lagacé J., Brodeur B. R. Heterohybridomas secreting human monoclonal antibodies against Haemophilus influenzae type b. Eur J Immunol. 1988 Apr;18(4):601–606. doi: 10.1002/eji.1830180417. [DOI] [PubMed] [Google Scholar]
- Munson R., Jr, Grass S., Einhorn M., Bailey C., Newell C. Comparative analysis of the structures of the outer membrane protein P1 genes from major clones of Haemophilus influenzae type b. Infect Immun. 1989 Nov;57(11):3300–3305. doi: 10.1128/iai.57.11.3300-3305.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Grass S. Purification, cloning, and sequence of outer membrane protein P1 of Haemophilus influenzae type b. Infect Immun. 1988 Sep;56(9):2235–2242. doi: 10.1128/iai.56.9.2235-2242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Hunt A. Isolation and characterization of a mutant of Haemophilus influenzae type b deficient in outer membrane protein P1. Infect Immun. 1989 Mar;57(3):1002–1004. doi: 10.1128/iai.57.3.1002-1004.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Tolan R. W., Jr Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989 Jan;57(1):88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]