Abstract
The role of CD4+ T-cell interleukin-4 (IL-4) receptor alpha (IL-4Rα) expression in T helper 2 (TH2) immune responses has not been defined. To examine this role, we infected CD4+ T-cell IL-4Rα knockout (KO) mice with the parasitic nematode Nippostrongylus brasiliensis, which induces strong host TH2 responses. Although N. brasiliensis expulsion was not affected in CD4+ T-cell IL-4Rα KO mice, the associated lung pathology was reduced. Infected CD4+ T-cell IL-4Rα KO mice showed abrogation of airway mucus production. Furthermore, CD4+ T-cell IL-4Rα KO mouse lungs contained reduced numbers of lymphocytes and eosinophils. Restimulation of pulmonary region-associated T-cell populations showed that TH2 cytokine responses were disrupted. Secretion of IL-4, but not secretion of IL-13 or IL-5, from mediastinal lymph node CD4+ T cells was reduced in infected CD4+ T-cell IL-4Rα KO mice. Restimulation of tissue-derived CD4+ T cells resulted in equivalent levels of IL-4 and IL-13 on day 7 postinfection (p.i.) in control and CD4+ T-cell IL-4Rα KO mice. By day 10 p.i. the TH2 cytokine levels had significantly declined in CD4+ T-cell IL-4Rα KO mice. Restimulation with N. brasiliensis antigen of total lung cell populations and populations with CD4+ T cells depleted showed that CD4+ T cells were a key TH2 cytokine source. These data demonstrated that CD4+ T-cell IL-4 responsiveness facilitates eosinophil and lymphocyte recruitment, lymphocyte localization, and TH2 cytokine production in the allergic pathology associated with N. brasiliensis infections.
T helper type 2 (TH2) immune effector responses are characterized by interleukin-4 (IL-4)- and IL-13-dependent signaling through heterodimeric receptors containing an IL-4 receptor alpha (IL-4Rα) subunit (3). These effector responses are particularly associated with the resolution of helminth infections (16, 32, 34) and the induction of allergic reactions (12). IL-4Rα signaling results in activation and upregulation of the transcription factors STAT-6 and GATA-3, which stabilize the TH2 program in polarized CD4+ T cells (3, 27). CD4+ T-cell IL-4Rα-dependent TH2 differentiation is considered to be specific to IL-4, as T cells lack functional IL-13 receptors (43). The resulting TH2 response is characterized by B-cell immunoglobulin E (IgE) and IgG1 antibody production (4, 37), goblet cell hyperplasia (17), and secretion of the TH2 cytokines IL-4, IL-13, IL-5, IL-10, and IL-9 by a number of hematopoietic cells (26).
CD4+ T-cell populations and IL-4Rα expression are essential for the optimal development of TH2 responses (16). However, no requirement for IL-4Rα expression in T-cell subpopulations for the development of a TH2 response has been demonstrated. Indeed, the interesting observation that there is CD4+ T-cell IL-4Rα-independent IL-4 and IL-13 production has been widely reported (8, 15, 23, 29, 31, 40). Thus, IL-4-responsive CD4+ T cells may not be essential for TH2 polarization (20). In order to examine this possibility, we generated CD4+ T-cell IL-4Rα transgenic mice with 95.5% ± 5.8% disruption of IL-4Rα expression on CD4+ T cells (CD4+ T-cell IL-4Rα knockout [KO] mice) (30). Infection of these mice with Leishmania major indicated that non-IL-4-responsive CD4+ T cells can launch aspects of a TH2 response (30). Additionally, in ovalbumin-induced anaphylaxis (a TH2-associated pathology) T-cell TH2 cytokine secretion appears to be unaffected by IL-4Rα CD4+ T-cell expression (28). Together, these findings demonstrate that IL-4Rα expression on CD4+ T cells is not essential for generation of a TH2 immune response.
In the study described here we further defined the roles of IL-4-responsive CD4+ T cells by infecting CD4+ T-cell IL-4Rα KO mice with the nematode Nippostrongylus brasiliensis. N. brasiliensis infections are characterized by a striking IL-4Rα-driven TH2 polarized host immune response which is essential for expulsion of adult worms from the host intestine (35). Importantly, N. brasiliensis infections are analogous to human hookworm infections (e.g., Necator americanus and Ancylostoma duodenale infections) and thus provide an excellent model to study the host-parasite relationships in hookworm disease (11). Infection is initiated by cutaneous larval invasion. The larvae then migrate via the circulatory and bronchial systems to the small intestine. The larval movement through the lungs generates a severe asthmalike pulmonary pathology characterized by airway mucus production and localized immune cell recruitment (19, 22, 33). In experimental asthma this pathology is strongly associated with IL-4Rα-dependent T-cell recruitment to the lungs and localization to the airways (36). Once established in the small intestine, the larvae mature to adults, producing large numbers of eggs which are passed in the host feces. The infection is resolved by the host via currently undefined TH2-dependent mechanisms, which may include intestinal contractions (42) and goblet cell hyperplasia (35).
We demonstrated in this study that resolution of N. brasiliensis infection was independent of IL-4-responsive CD4+ T cells. Importantly, a striking decrease in N. brasiliensis-induced pulmonary pathology was found in CD4+ T-cell IL-4Rα KO mice. The decreased pathology was associated with disrupted T-cell localization and recruitment and cytokine production in the lungs. Our data suggest that although IL-4Rα expression on T cells may not be essential for development of a TH2 response, it does play an important role in the pathology associated with tissue T-cell responses.
MATERIALS AND METHODS
Mice.
Eight- to 12-week-old mice were obtained from the University of Cape Town specific-pathogen-free animal facility. All experiments were approved by the University of Cape Town Animal Ethics Committee. CD4+ T-cell IL-4Rα KO mice were generated as previously described (30), and hemizygous IL-4Rα−/lox mice (control mice) and homozygous IL-4Rα−/− mice (IL-4Rα KO mice) were used as controls. All mice used had a BALB/c background.
Infection studies.
Mice were inoculated subcutaneously with 750 N. brasiliensis L3 larvae (kindly provided by Klaus Erb, Wurzburg, Germany). An analysis of parasite eggs in feces was carried out using the modified McMaster technique (10). Adult worm burdens were determined as previously described (1). Briefly, intestines were removed from infected mice, and each lumen was exposed by dissection. The intestines were then incubated at 37°C for 4 h in 0.65% NaCl. Intestinal tissue was then removed, and the adult worms in the remaining saline solution were counted.
BAL.
Canulas (18 gauge) were inserted into the tracheae of euthanized mice, and the lungs were lavaged with 1 ml of phosphate-buffered saline (PBS). Bronchoalveolar lavage (BAL) samples were centrifuged at 1,200 rpm for 5 min and subjected to red cell lysis, and the numbers of cells were determined. Cells were then centrifuged at 500 rpm for 5 min (Shandon Cytospin4; Thermo Scientific, Runcorn, United Kingdom), dried at room temperature overnight, and stained with RapidDiff (Clinical Research Science, South Africa) prior to analysis.
Histology.
Tissue samples were fixed in a neutral buffered formalin solution. Following embedding in paraffin, samples were cut into 5-μm sections. Sections were stained with periodic acid-Schiff reagent (PAS) for quantification of intestinal and pulmonary goblet cell hyperplasia, which was carried out as previously described (6, 14). Briefly, intestinal goblet cell hyperplasia in individual mice was determined by determining the number of positive goblet cells per five villi from the small intestine. The histological mucus index (HMI) was used to quantify pulmonary goblet cell hyperplasia in individual mice. Airways were photographed at a magnification of ×100 and overlaid with a standard grid. The total number of epithelial cells was divided by the number of mucus-positive squares to determine the HMI. All samples were randomized and counted by a blinded observer. For CD3 staining, sections were incubated at 56°C overnight and rehydrated using zylols, alcohol, and water. Sections were then blocked with 3% H2O2 in methanol, blocked again with 5% goat serum, incubated with anti-CD3 antibody (A0452; Dako) followed by anti-rabbit Envision secondary antibody (K4003; Dako), and then visualized with the 1,4-diamino-2-butanone substrate (K3466; Dako) and counterstained with Mayer's hematoxylin.
Isolation of lung CD4+ T-cell populations.
PBS-perfused lungs were removed from euthanized mice. The lungs were finely cut and digested in Dulbecco modified Eagle medium (Invitrogen) with 50 U/ml collagenase type I (Invitrogen) and 13 μg/ml DNase I (Roche) at 37°C for 90 min. Samples were pushed through a 70-μm cell strainer and subjected to red blood cell lysis. Lung CD4+ T cells were detected with anti-CD4 phycoerythrin-conjugated monoclonal antibody (MAb) GK1.5 (BD Pharmingen) before they were purified by cell sorting using a FACSVantage cell sorter (Becton Dickinson).
Generation of enriched CD4+ T-cell populations.
CD4+ T cells were enriched (purity, >94%) from mediastinal lymph nodes at days 7 and 10 postinfection (p.i.). Single-cell suspensions were stained with anti-CD8 MAb 53.6.72, anti-CD11b MAb M1/70, anti-GR-1 MAb RB68C5, and anti-B220 MAb RA36B2, all of which were rat derived. Stained cells were depleted using goat anti-rat IgG-coated magnetic beads (Biomag beads; Qiagen, Germany). MAbs were purified from hybridoma supernatants.
Flow cytometry.
T lymphocytes were stained with anti-CD3 fluorescein isothiocyanate-conjugated antibody 145-2C11 or anti-CD4 fluorescein isothiocyanate-conjugated antibody GK1.5 before they were analyzed with a FACSCalibur (Becton Dickinson). Dead cells were excluded based on 7-amino-actinomycin D staining.
Enzyme-linked immunosorbent assay (ELISA) analysis.
CD4+ T-cell, whole lung, and lymph node preparations were restimulated for 48 h with either 20 μg/ml anti-CD3 antibody 145-2C11 or 10 μg/ml N. brasiliensis excretory-secretory proteins (13). Supernatants were then collected and stored at −80°C until they were analyzed. Cytokines in supernatants and serum antibody isotype levels for infected animals were determined as previously described (24). Briefly, flat-bottom 96-well plates were coated overnight with the appropriate capturing antibody diluted in PBS. The plates were then washed and incubated in PBS containing 2% milk for 1 h at 37°C. Following this, the plates were washed, and samples and standards were loaded overnight at 4°C. Appropriate biotinylated secondary antibodies were then added following further washing and incubated overnight at 4°C. The plates were then washed, and antibody and cytokine levels were determined using streptavidin-coupled horseradish peroxidase. The plates were developed with a 3,3′,5,5′-tetramethylbenzidine microwell peroxidase substrate system, and the reaction was stopped with 1 M H3PO3. The absorbance at 450 nm was determined with a Versamax microplate spectrophotometer (Molecular Devices, Germany).
Statistics.
Values are expressed below as means ± standard deviations or means ± standard errors of the means, and significant differences were determined using the Mann-Whitney U test or an unpaired two-tailed Student t test (GraphPad Prism4).
RESULTS
N. brasiliensis fecundity and expulsion are independent of IL-4-responsive CD4+ T cells.
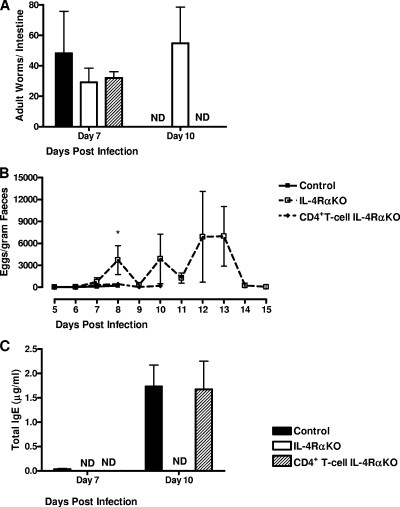
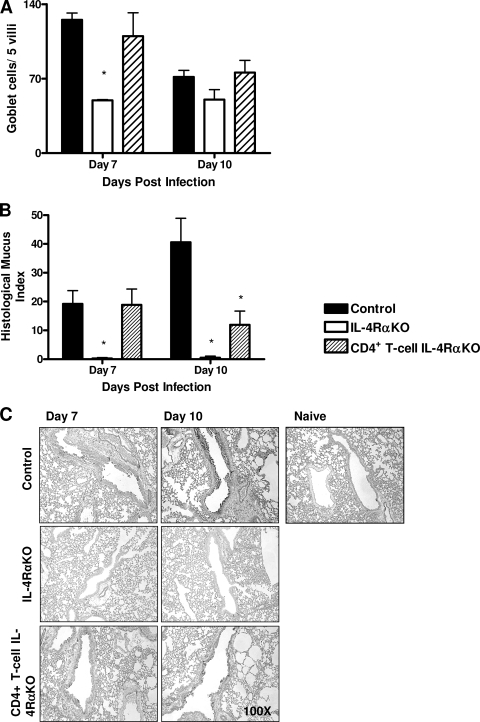
To investigate the possible role of IL-4Rα expression on CD4+ lymphocytes in resolving N. brasiliensis infections, control, IL-4Rα KO, and CD4+ T-cell IL-4Rα KO mice were infected with 750 L3 N. brasiliensis larvae. Worm burdens were determined at days 7 and 10 p.i. (Fig. 1A). At day 7 p.i. all mouse groups had comparable worm burdens. As previously shown (14), the intestinal infection in global IL-4Rα KO mice was significantly prolonged compared to that in the controls (the global IL-4Rα KO mice were still infected at day 10 p.i.). However, CD4+ T-cell IL-4Rα KO mice cleared the infection like their littermate controls. These results were comparable to the results for worm fecundity, as demonstrated by fecal egg counting (Fig. 1B), which showed that no eggs were present in either control or CD4+ T-cell IL-4Rα KO mice after day 10 p.i. Systemic IgE levels were also not affected in CD4+ T-cell IL-4Rα KO mice compared to control mice (Fig. 1C), indicating that the type 2 B-cell responses were normal. TH2-dependent host responses to adult N. brasiliensis are characterized by increased intestinal goblet cell hyperplasia and mucus production. We found that the levels of intestinal mucus production were comparable for control and CD4+ T-cell IL-4Rα KO mice (Fig. 2A), while in global IL-4Rα KO mice the level of intestinal mucus production was significantly lower (P < 0.01). Together, these results (Fig. 1 and 2A) demonstrate that neither expulsion nor intestinal goblet cell hyperplasia is dependent on IL-4-promoted TH2 responses.
FIG. 1.
T-cell-specific IL-4Rα-deficient mice control N. brasiliensis infection in the intestine. (A) Mice were infected with 750 N. brasiliensis L3 larvae, and at days 7 and 10 p.i. the worm burdens in the small intestine were assessed to determine the expulsion kinetics. (B) Feces were collected from day 5 to day 13 p.i., and egg production by N. brasiliensis was calculated using the modified McMaster technique. (C) Antibody production in the serum was assessed by an ELISA at days 7 and 10 p.i. An asterisk indicates that there is a significance difference between KO and control mice (P < 0.05; four or five mice per group). The data are representative of the results of three separate experiments. ND, not detected.
FIG. 2.
Reduced pulmonary pathology in T-cell-specific IL-4Rα-deficient mice. (A) Intestinal mucus production was assessed by determining the total number of PAS-positive goblet cells per five villi in histological sections of the small intestine at days 7 and 10 p.i. (B and C) Lung tissue was removed at days 7 and 10 p.i., fixed in formalin, and stained with PAS to examine mucus production. The HMI (B) was determined using PAS-stained lung sections (C) in order to compare mucus production by airway goblet cells. An asterisk indicates that there is a significance difference between KO and control mice (P < 0.05; four or five mice per group). The data are representative of the results of three separate experiments.
Reduced pulmonary pathology in CD4+ T-cell-specific IL-4Rα-deficient mice.
To determine if impaired IL-4-promoted TH2 cell differentiation affected N. brasiliensis-induced allergy like goblet cell hyperplasia in the lungs of CD4+ T-cell IL-4Rα KO mice, airway mucus production was examined by using PAS and was quantified by determining the HMI. Significantly less mucus production was apparent at day 10 p.i. in CD4+ T-cell IL-4Rα KO mice than in mice in control groups (Fig. 2B and 2C). As expected, the airway mucus production in IL-4Rα KO mice was significantly reduced at days 7 and 10 p.i.
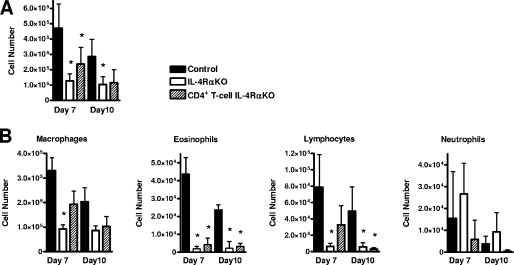
Pulmonary allergic responses can also be defined by increased bronchial cellular infiltration by a range of immune cells. BAL analysis of the cellular infiltrate in infected mice demonstrated that there were significant reductions in the total numbers of cells in both IL-4Rα KO and CD4+ T-cell IL-4Rα KO mice compared to control mice (Fig. 3A). Furthermore, differential cell counting demonstrated that there were significant reductions in the eosinophil and lymphocyte populations in IL-4Rα KO and CD4+ T-cell IL-4Rα KO mice (Fig. 3B).
FIG. 3.
Reduced bronchial immune cell infiltration in N. brasiliensis-infected T-cell-specific IL-4Rα-deficient mice. (A) Total BAL cell counts at days 7 and 10 p.i. (B) Differential BAL cell counts for infiltrating macrophages, eosinophils, lymphocytes, and neutrophils at days 7 and 10 p.i. An asterisk indicates that there is a significance difference between KO and control mice (P < 0.05; four or five mice per group). The data are representative of the results of two separate experiments.
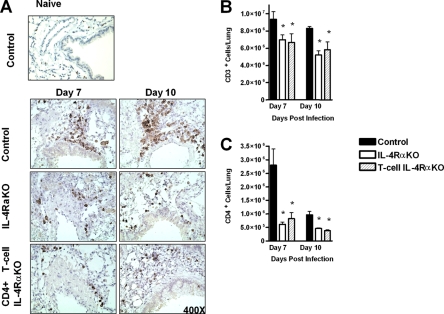
A further indicator of allergic pathology is the formation of peribronchial lymphocyte foci. Immunohistochemical analysis of CD3+ T cells revealed foci associated with vascular systems and airways in control mice (Fig. 4A, upper panel). Such foci were not apparent in either IL-4Rα KO or CD4+ T-cell IL-4Rα KO mice (Fig. 4A, middle and lower panels). We found that in these mice CD3+ T cells were dispersed throughout the pulmonary tissue. Furthermore, flow cytometric analysis of whole lungs showed that, in agreement with the disrupted CD3+ T-cell localization and reduced numbers of lymphocytes in the BAL fluid, there were significantly lower numbers of CD3+ T cells in the lungs of both IL-4Rα KO and CD4+ T-cell IL-4Rα KO mice (Fig. 4B). Further flow cytometric analysis demonstrated that there were lower numbers of CD4+ T cells in both IL-4Rα KO and CD4+ T-cell IL-4Rα KO mice (Fig. 4C).
FIG. 4.
Reduced T-cell recruitment to the lungs and localization in N. brasiliensis-infected T-cell-specific IL-4Rα-deficient mice. (A) Lung tissue removed at days 7 and 10 p.i., fixed, and stained with anti-CD3, showing the formation of lymphocyte foci around airways and vascular systems in control mice. Anti-CD3 staining is dispersed throughout the tissue of CD4+ T-cell IL-4Rα KO mice and IL-4Rα KO mice. (B) Single-cell suspensions of whole lungs from individual mice were analyzed by flow cytometry to determine the numbers of CD3+ cells present in the various types of mice. (C) Single-cell suspensions of whole lungs from individual mice were analyzed by flow cytometry to determine the numbers of CD4+ cells present in the various types of mice. An asterisk indicates that there is a significance difference between KO and control mice (P < 0.05; four or five mice per group). The data are representative of the results of two separate experiments.
Together, these data demonstrate that the profound allergic pulmonary pathology induced by N. brasiliensis is driven by IL-4Rα-responsive CD4+ T cells. Specifically, we found that CD4+ T-cell IL-4Rα expression is important in the bronchial recruitment of both lymphocyte and eosinophil populations.
Mediastinal lymph node-derived CD4+ T-cell production of IL-4, but not mediastinal lymph node-derived CD4+ T-cell production of IL-5 and IL-13, is diminished in infected CD4+ T-cell IL-4Rα KO mice.
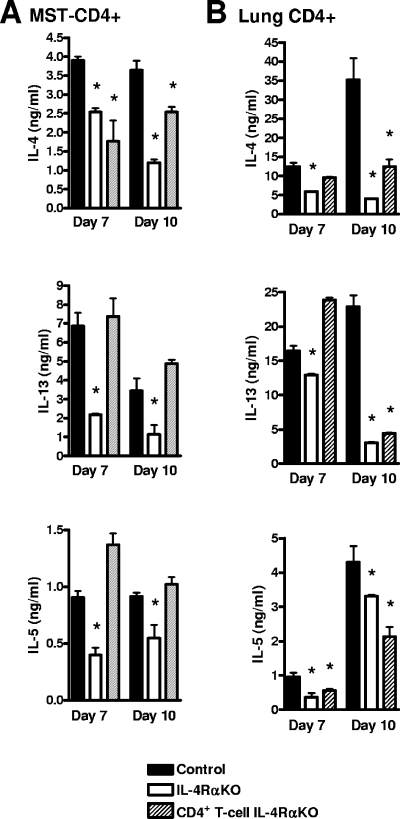
Central to the induction of allergic airway pathology is elevated secretion of TH2 cytokines, in particular IL-13, by CD4+ T cells. Analysis of pulmonary cytokine production in response to N. brasiliensis infection by anti-CD3 restimulated CD4+ lymphocytes isolated from mediastinal lymph nodes revealed significantly impaired IL-4 secretion in both IL-4Rα KO and CD4+ T-cell IL-4Rα KO mice. However, the IL-13 and IL-5 secretion in CD4+ T-cell IL-4Rα KO mice was consistently comparable to that in control mice (Fig. 5A). IL-4Rα-independent IL-13 production was confirmed by intracellular detection of IL-13 in restimulated mediastinal lymph node-derived cells (data not shown). Together, these data demonstrated that IL-4 secretion, but not IL-5 and IL-13 secretion, was dependent on IL-4Rα-expressing CD4+ T cells during N. brasiliensis infection.
FIG. 5.
Restimulated CD4+ T-cell cytokine secretion, showing reduced TH2 responses and IL-4Rα-independent IL-13 production. (A) Pooled supernatant cytokine levels for CD4+ sorted mediastinal lymph nodes (MST) restimulated with anti-CD3 for 48 h and detected by an ELISA. The data are representative of the results of two separate experiments. (B) Pooled supernatant cytokine levels for isolated CD4+ lung cells restimulated with anti-CD3 for 48 h and detected by an ELISA. The data are representative of the results of two separate experiments. An asterisk indicates that there is a significance difference between KO and control mice (P < 0.05; four mice per group).
IL-4Rα-responsive pulmonary CD4+ T cells are major producers of IL-4 and IL-13 during N. brasiliensis infection.
Similar to anti-CD3 restimulation of mediastinal lymph node-derived CD4+ T-cells, anti-CD3 restimulation of CD4+ T cells isolated from infected lungs resulted in an overall reduction in the levels of IL-4, IL-13, and IL-5 in IL-4Rα KO mice at both days 7 and 10 p.i. compared to control mice (Fig. 5B). Specifically, CD4+ T-cell IL-4Rα KO mice had levels of IL-4 and IL-13, but not levels of IL-5, equivalent to those of control animals at day 7 p.i., but by day 10 p.i. all TH2 cytokine levels were significantly reduced in CD4+ T-cell IL-4Rα KO mice compared to control mice. As expected, all TH2 cytokine levels were significantly reduced in IL-4Rα KO mice.
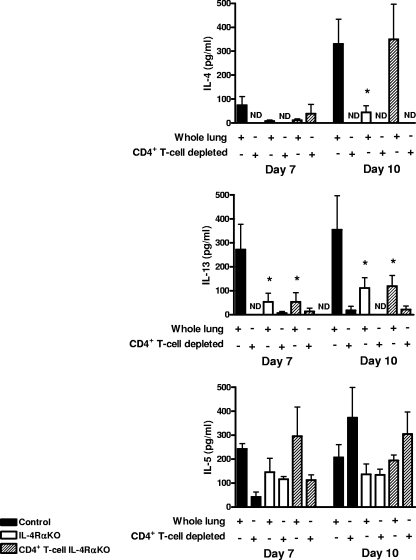
Restimulation with N. brasiliensis L3 larva secreted antigen of both total cell preparations derived from lung digests and preparations with CD4+ T cells depleted demonstrated that IL-4 and IL-13 cytokine production in the lung was dependent on resident CD4+ T-cell populations (Fig. 6). IL-5 production, however, appeared to be largely independent of the presence of CD4+ T-cell populations, and this indicates that there was a significant alternative cellular source of IL-5, such as mast cells (2) or eosinophils (9). TH2 cytokine levels were reduced in both IL-4Rα KO and CD4+ T-cell IL-4Rα KO mice at days 7 and 10 p.i. In particular, the striking reduction in IL-13 secretion in both IL-4Rα KO and CD4+ T-cell IL-4Rα KO mice would explain the abrogated host airway mucus production.
FIG. 6.
Pulmonary TH2 cytokine production is IL-4Rα-expressing CD4+ T cell dependent. Supernatant cytokine levels of whole-cell preparations from three individual mice and pooled cell preparations with CD4+ depleted from four mice were restimulated with N. brasiliensis excretory-secretory proteins for 48 h, and cytokine secretion was then detected by an ELISA. An asterisk indicates that there is a significance difference between KO and control mice (P < 0.05). The data are representative of the results of two separate experiments.
DISCUSSION
We demonstrated in this study that IL-4Rα-responsive CD4+ T cells are not essential for the resolution of N. brasiliensis infections. Interestingly, we found that equivalent levels of the key cytokine for worm clearance, IL-13 (21), were secreted in mediastinal lymph node CD4+ T-cell IL-4Rα KO and control mice, demonstrating that IL-13 production by CD4+ T cells can be independent of signaling via IL-4Rα. IL-4Rα-independent IL-13 production in CD4+ T cells has been demonstrated previously for ovalbumin-induced airway hyperreactivity (40), and our data extend this observation to N. brasiliensis infections. Importantly, we also demonstrated that peripheral CD4+ T cells are able to produce levels of IL-13, independent of IL-4Rα, that are equivalent to the levels in control animals.
The absence of a measurable effect on worm expulsion in CD4+ T-cell IL-4Rα KO mice is in agreement with studies which showed that although CD4+ cells (14) and IL-4Rα (35) are required for expulsion of N. brasiliensis, signaling through the STAT-6 pathway in CD4+ cells is not required (38). Indeed, effective worm expulsion independent of IL-4Rα-responsive peripheral CD4+ T cells may be explained by our demonstration that the IL-4Rα-independent CD4+ T-cell production of IL-13 in CD4+ T-cell IL-4Rα KO mice is comparable to that in control mice.
The striking observation made in this study was that N. brasiliensis-induced pulmonary immunopathology was considerably reduced in CD4+ T-cell IL-4Rα KO mice. Here the significantly lower airway mucus responses were associated with severely disrupted T-cell recruitment and localization in the lungs of CD4+ T-cell IL-4Rα KO mice. Importantly, the reduction in TH2 cytokine secretion by T-cell populations in tissues corresponded with reduced pulmonary T-cell populations and airway mucus production in CD4+ T-cell IL-4Rα KO mice. These observations are in agreement with the results of previous work in which TH2 CD4+ T-cell recruitment to the lungs of N. brasiliensis infected mice was shown to depend on the expression STAT-6 (25, 39). Moreover, ovalbumin-induced TH2 CD4+ T-cell recruitment to the lungs also requires IL-4-responsive T cells (6). In further agreement with our data, ovalbumin-induced airway mucus production has also been shown to be not wholly dependent on IL-4 (7) but to absolutely require IL-13 (41) and IL-4Rα (5). Indeed, CD4+ T-cell IL-13/IL-4Rα-dependent interactions with airway epithelial cells can induce airway mucus production (41). These studies demonstrated that there is a common requirement for STAT-6/IL-4Rα and IL-13 (and to a lesser extent IL-4) for recruitment of T cells to the lungs and induction of airway mucus production. Our data supported and expanded these findings, demonstrating that there is a requirement for IL-4-responsive peripheral CD4+ T-cell populations for effective T-cell and eosinophil recruitment and localization in the lung. Disruption of IL-4Rα expression on CD4+ T cells eliminated the ability to produce CD4+ T-cell TH2 cytokines in the lungs but not in the draining lymph nodes. Together, these effects explain the decreased levels of airway mucus production reported in this study. The data reported here are important for understanding the roles played by IL-4Rα in chronic pulmonary diseases (18).
Acknowledgments
This work was supported by the Royal Society, United Kingdom, by the Medical Research Council, and by the National Research Foundation, South Africa. B.D. is a postdoctoral researcher of the Fonds National Belge de la Recherche Scientifique.
We thank Lizette Fick for her assistance with histology and Ronald Dreyer, Reagon Peterson, Erica Smit, Wendy Green, and Zenaria Abbas for technical assistance.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Barner, M., M. Mohrs, F. Brombacher, and M. Kopf. 1998. Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr. Biol. 8669-672. [DOI] [PubMed] [Google Scholar]
- 2.Bradding, P., J. A. Roberts, K. M. Britten, S. Montefort, R. Djukanovic, R. Mueller, C. H. Heusser, P. H. Howarth, and S. T. Holgate. 1994. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am. J. Respir. Cell Mol. Biol. 10471-480. [DOI] [PubMed] [Google Scholar]
- 3.Brombacher, F. 2000. The role of interleukin-13 in infectious diseases and allergy. Bioessays 22646-656. [DOI] [PubMed] [Google Scholar]
- 4.Coffman, R. L., J. Ohara, M. W. Bond, J. Carty, A. Zlotnik, and W. E. Paul. 1986. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J. Immunol. 1364538-4541. [PubMed] [Google Scholar]
- 5.Cohn, L., R. J. Homer, H. MacLeod, M. Mohrs, F. Brombacher, and K. Bottomly. 1999. Th2-induced airway mucus production is dependent on IL-4Ralpha, but not on eosinophils. J. Immunol. 1626178-6183. [PubMed] [Google Scholar]
- 6.Cohn, L., R. J. Homer, A. Marinov, J. Rankin, and K. Bottomly. 1997. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 1861737-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohn, L., J. S. Tepper, and K. Bottomly. 1998. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J. Immunol. 1613813-3816. [PubMed] [Google Scholar]
- 8.Cunningham, A. F., K. Serre, K. M. Toellner, M. Khan, J. Alexander, F. Brombacher, and I. C. MacLennan. 2004. Pinpointing IL-4-independent acquisition and IL-4-influenced maintenance of Th2 activity by CD4 T cells. Eur. J. Immunol. 34686-694. [DOI] [PubMed] [Google Scholar]
- 9.Dubucquoi, S., P. Desreumaux, A. Janin, O. Klein, M. Goldman, J. Tavernier, A. Capron, and M. Capron. 1994. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J. Exp. Med. 179703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn, A., and A. Keymer. 1986. Factors affecting the reliability of the McMaster technique. J. Helminthol. 60260-262. [DOI] [PubMed] [Google Scholar]
- 11.Finkelman, F. D., T. Shea-Donohue, S. C. Morris, L. Gildea, R. Strait, K. B. Madden, L. Schopf, and J. F. Urban, Jr. 2004. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 201139-155. [DOI] [PubMed] [Google Scholar]
- 12.Holgate, S. T. 1999. The epidemic of allergy and asthma. Nature 402B2-4. [DOI] [PubMed] [Google Scholar]
- 13.Holland, M. J., Y. M. Harcus, A. Balic, and R. M. Maizels. 2005. Th2 induction by Nippostrongylus secreted antigens in mice deficient in B cells, eosinophils or MHC class I-related receptors. Immunol. Lett. 9693-101. [DOI] [PubMed] [Google Scholar]
- 14.Horsnell, W. G., A. J. Cutler, C. J. Hoving, H. Mearns, E. Myburgh, B. Arendse, F. D. Finkelman, G. K. Owens, D. Erle, and F. Brombacher. 2007. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog. 3e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jankovic, D., M. C. Kullberg, N. Noben-Trauth, P. Caspar, W. E. Paul, and A. Sher. 2000. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J. Immunol. 1643047-3055. [DOI] [PubMed] [Google Scholar]
- 16.Kopf, M., G. Le Gros, M. Bachmann, M. C. Lamers, H. Bluethmann, and G. Kohler. 1993. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 362245-248. [DOI] [PubMed] [Google Scholar]
- 17.Madden, K. B., J. F. Urban, Jr., H. J. Ziltener, J. W. Schrader, F. D. Finkelman, and I. M. Katona. 1991. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J. Immunol. 1471387-1391. [PubMed] [Google Scholar]
- 18.Marsland, B. J., M. Kurrer, R. Reissmann, N. L. Harris, and M. Kopf. 2008. Nippostrongylus brasiliensis infection leads to the development of emphysema associated with the induction of alternatively activated macrophages. Eur. J. Immunol. 38479-488. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda, S., Y. Tani, M. Yamada, K. Yoshimura, and N. Arizono. 2001. Type 2-biased expression of cytokine genes in lung granulomatous lesions induced by Nippostrongylus brasiliensis infection. Parasite Immunol. 23219-226. [DOI] [PubMed] [Google Scholar]
- 20.Mattes, J., M. Yang, A. Siqueira, K. Clark, J. MacKenzie, A. N. McKenzie, D. C. Webb, K. I. Matthaei, and P. S. Foster. 2001. IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung. J. Immunol. 1671683-1692. [DOI] [PubMed] [Google Scholar]
- 21.McKenzie, G. J., A. Bancroft, R. K. Grencis, and A. N. McKenzie. 1998. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 8339-342. [DOI] [PubMed] [Google Scholar]
- 22.McNeil, K. S., D. P. Knox, and L. Proudfoot. 2002. Anti-inflammatory responses and oxidative stress in Nippostrongylus brasiliensis-induced pulmonary inflammation. Parasite Immunol. 2415-22. [DOI] [PubMed] [Google Scholar]
- 23.Mohrs, M., C. Holscher, and F. Brombacher. 2000. Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infect. Immun. 681773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohrs, M., B. Ledermann, G. Kohler, A. Dorfmuller, A. Gessner, and F. Brombacher. 1999. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 1627302-7308. [PubMed] [Google Scholar]
- 25.Mohrs, M., K. Shinkai, K. Mohrs, and R. M. Locksley. 2001. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15303-311. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7145-173. [DOI] [PubMed] [Google Scholar]
- 27.Nelms, K., A. D. Keegan, J. Zamorano, J. J. Ryan, and W. E. Paul. 1999. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 17701-738. [DOI] [PubMed] [Google Scholar]
- 28.Nieuwenhuizen, N., D. R. Herbert, A. L. Lopata, and F. Brombacher. 2007. CD4+ T cell-specific deletion of IL-4 receptor alpha prevents ovalbumin-induced anaphylaxis by an IFN-gamma-dependent mechanism. J. Immunol. 1792758-2765. [DOI] [PubMed] [Google Scholar]
- 29.Noben-Trauth, N., L. D. Shultz, F. Brombacher, J. F. Urban, Jr., H. Gu, and W. E. Paul. 1997. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl. Acad. Sci. USA 9410838-10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radwanska, M., A. J. Cutler, J. C. Hoving, S. Magez, C. Holscher, A. Bohms, B. Arendse, R. Kirsch, T. Hunig, J. Alexander, P. Kaye, and F. Brombacher. 2007. Deletion of IL-4Ralpha on CD4 T cells renders BALB/c mice resistant to Leishmania major Infection. PLoS Pathog. 3e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritz, S. A., M. J. Cundall, B. U. Gajewska, D. Alvarez, J. C. Gutierrez-Ramos, A. J. Coyle, A. N. McKenzie, M. R. Stampfli, and M. Jordana. 2002. Granulocyte macrophage colony-stimulating factor-driven respiratory mucosal sensitization induces Th2 differentiation and function independently of interleukin-4. Am. J. Respir. Cell Mol. Biol. 27428-435. [DOI] [PubMed] [Google Scholar]
- 32.Svetic, A., K. B. Madden, X. D. Zhou, P. Lu, I. M. Katona, F. D. Finkelman, J. F. Urban, Jr., and W. C. Gause. 1993. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J. Immunol. 1503434-3441. [PubMed] [Google Scholar]
- 33.Tomita, M., T. Kobayashi, H. Itoh, T. Onitsuka, and Y. Nawa. 2000. Goblet cell hyperplasia in the airway of Nippostrongylus brasiliensis-infected rats. Respiration 67565-569. [DOI] [PubMed] [Google Scholar]
- 34.Urban, J. F., Jr., I. M. Katona, W. E. Paul, and F. D. Finkelman. 1991. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. USA 885513-5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urban, J. F., Jr., N. Noben-Trauth, D. D. Donaldson, K. B. Madden, S. C. Morris, M. Collins, and F. D. Finkelman. 1998. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8255-264. [DOI] [PubMed] [Google Scholar]
- 36.Venkayya, R., M. Lam, M. Willkom, G. Grunig, D. B. Corry, and D. J. Erle. 2002. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am. J. Respir. Cell Mol. Biol. 26202-208. [DOI] [PubMed] [Google Scholar]
- 37.Vitetta, E. S., J. Ohara, C. D. Myers, J. E. Layton, P. H. Krammer, and W. E. Paul. 1985. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J. Exp. Med. 1621726-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voehringer, D., T. A. Reese, X. Huang, K. Shinkai, and R. M. Locksley. 2006. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 2031435-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voehringer, D., K. Shinkai, and R. M. Locksley. 2004. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 20267-277. [DOI] [PubMed] [Google Scholar]
- 40.Webb, D. C., S. Mahalingam, Y. Cai, K. I. Matthaei, D. D. Donaldson, and P. S. Foster. 2003. Antigen-specific production of interleukin (IL)-13 and IL-5 cooperate to mediate IL-4Ralpha-independent airway hyperreactivity. Eur. J. Immunol. 333377-3385. [DOI] [PubMed] [Google Scholar]
- 41.Whittaker, L., N. Niu, U. A. Temann, A. Stoddard, R. A. Flavell, A. Ray, R. J. Homer, and L. Cohn. 2002. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am. J. Respir. Cell Mol. Biol. 27593-602. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, A., J. McDermott, J. F. Urban, Jr., W. Gause, K. B. Madden, K. A. Yeung, S. C. Morris, F. D. Finkelman, and T. Shea-Donohue. 2003. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171948-954. [DOI] [PubMed] [Google Scholar]
- 43.Zurawski, G., and J. E. de Vries. 1994. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today 1519-26. [DOI] [PubMed] [Google Scholar]