Abstract
The impact of the interaction between excreted and/or secreted (ES) Necator americanus products and NK cells from Necator-infected individuals was analyzed. We investigated the binding of ES products to NK cells, the expression of NK cell receptors (CD56, CD159a/NKG2A, CD314/NKG2D, CD335/NKp46, and KLRF1/NKp80), the frequency of gamma interferon (IFN-γ)-producing NK cells after whole-blood in vitro stimulation, and the capacity of N. americanus ES products to induce NK cell chemotaxis. In contrast to those from noninfected individuals, NK cells from Necator-infected individuals demonstrated no binding with N. americanus ES products. This phenomenon was not due to alterations in NK cell receptor expression in infected subjects and could not be reproduced with NK cells from uninfected individuals by incubation with immunoregulatory cytokines (interleukin-10/transforming growth factor β). Further, we found that a significantly greater percentage of NK cells from infected subjects than NK cells from uninfected individuals spontaneously produced IFN-γ upon ex vivo culture. Our findings support a model whereby NK cells from Necator-infected individuals may interact with ES products, making these cells refractory to binding with exogenous ES products. During N. americanus infection, human NK cells are attracted to the site of infection by chemotactic ES products produced by adult Necator worms in the gut mucosa. Binding of ES products causes the NK cells to become activated and secrete IFN-γ locally, thereby contributing to the adult hookworm's ability to evade host immune responses.
Human hookworms infect up to 740 million individuals (11), mostly people living in rural areas of the developing world. Necator americanus is the most prevalent of the hookworms, with an extensive geographic distribution, including sub-Saharan Africa, the Americas, and Asia. Hookworm infection causes more disability than death (20), with hookworm disease occurring when infection-associated blood loss exceeds host nutritional reserves, resulting in iron deficiency anemia (IDA). In children, IDA from chronic hookworm infection can cause impairment in physical, intellectual, and cognitive development. IDA arising from hookworm infection during pregnancy can result in adverse consequences for the mother, her unborn fetus, and the neonate (7, 19). These significant and long-term consequences of hookworm infection account for the loss of 22 million disability-adjusted life years annually (9). Current control efforts rely on the administration of anthelmintic drugs, although significant concern regarding the sustainability of this strategy has prompted the search for new approaches for control, including a hookworm vaccine (13).
There is little evidence that humans acquire a protective immune response to hookworm infection. N. americanus can parasitize the proximal small intestine for 5 to 7 years, with recent evidence indicating that the intensity of these infections increases with age (5, 22). N. americanus worms pass through a succession of developmental stages in the human host, with stage-specific immune evasion mechanisms finely tuned to each environment through which they migrate and establish infection. Many of the stage-specific molecules secreted by N. americanus have immunomodulatory properties (26). Our group has shown that excreted and/or secreted (ES) products of adult N. americanus worms selectively bind to and induce high levels of gamma interferon (IFN-γ) production in human NK cells (21), with the ES protein(s) mediating this effect referred to as NKBP (natural killer cell binding protein). These findings led us to hypothesize that the local production of proinflammatory cytokines, such as IFN-γ, during infection might interfere with the effector function of human host Th2 responses or possibly promote a decrease in the epithelial “escalator” function of gut mucosa (10), resulting in an environment beneficial to adult hookworms.
The aim of these studies was to investigate the interaction between ES products of adult N. americanus worms and NK cells from three types of individuals: (i) uninfected individuals with no history of exposure to helminth infection, (ii) uninfected individuals resident in an area of high Necator transmission, and (iii) Necator-infected individuals from an area of high Necator transmission. Interestingly, our data reveal that in contrast to those from noninfected individuals, NK cells from Necator-infected individuals failed to bind ES products of adult N. americanus worms. Studies were conducted to test several potential explanations for the failure of NK cells from Necator-infected individuals to bind ES products of adult N. americanus worms. Our findings fit with the hypothesis that NK cells from Necator-infected subjects may have already interacted with ES products of adult N. americanus worms/NKBP generated during infection, making these cells refractory to interaction with exogenous ES products.
MATERIALS AND METHODS
The study was conducted in Americaninhas in the state of Minas Gerais, Brazil. Details of the study area and study sample are reported in several other articles (6, 8, 14, 23). Briefly, individuals between the ages of 6 and 65 were recruited to the study if they met the criteria previously outlined (14). The presence of N. americanus infection was determined by formalin-ether sedimentation followed by a Kato-Katz thick smear (24). Only individuals found to be infected with N. americanus by these tests were enrolled in the study; individuals coinfected with any other helminth or intestinal protozoan were excluded from the study; the controls from both areas of endemicity and areas of nonendemicity were negative for all helminths and intestinal protozoa by the above-mentioned fecal-exam techniques. Fecal samples from a subset of Necator-infected individuals were obtained following treatment with albendazole (400 mg) and examined for expelled worms. Worms were clarified in a 50% phenol solution and their buccal capsules analyzed under a light microscope (×100 to ×400 magnification); of the 150 male and female adult worms examined, all were identified as N. americanus. Approximately 20 ml of blood was collected in heparinized tubes for separation of peripheral blood mononuclear cells (PBMCs). The protocol and informed consent for individuals recruited from areas of N. americanus endemicity in Brazil were approved by the ethical review committees of The George Washington University, the Centro de Pesquisas René Rachou, and the Brazilian National Committee for Ethics in Research. The protocol and informed consent for individuals recruited from areas without active Necator transmission (in the United States) were approved by the ethical review committee of The George Washington University.
Collection and labeling of hookworm ES products.
N. americanus was maintained in hamsters (32), and Ancylostoma caninum was maintained in experimental beagles (18). Adult worms were removed from the intestines of euthanized animals, washed in phosphate-buffered saline (PBS), and then cultured overnight in RPMI 1640 containing 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B (all reagents from Sigma-Aldrich, St. Louis, MO). ES products were concentrated using microconcentrators with a 10-kDa-cutoff membrane (Millipore, Bedford, MA). Aliquots of ES products were biotinylated by amine coupling using NHSLC-biotin (Pierce, Rockford, IL) according to the manufacturer's instructions. Biotinylated ES products (bioES) were then dialyzed against 1,000 volumes of PBS overnight at 4°C. A mixture of protease inhibitors {AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride], bestatin, pepstatin A, E-64 [trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane], and phosphoramidon; Invitrogen Life Technologies, Carlsbad, CA} was then added to the dialyzed bioES, which was then stored at 4°C until needed.
Monoclonal antibodies.
Monoclonal antibodies against cellular markers were purchased from Caltag Laboratories (Burlingame, CA) (TriColor [TC] anti-CD3), Becton Dickinson (Mountain View, CA) (fluorescein isothiocyanate [FITC] anti-CD16, FITC anti-CD56, phycoerythrin [PE] anti-CD3, peridinin chlorophyll protein anti-CD3, and allophycocyanin [APC] anti-CD56), R&D systems (Minneapolis, MN) (PE anti-NKG2A [CD159a], APC anti-NKG2A, PE anti-NKG2D [CD314], PE anti-NKp46 [CD335], and PE anti-NKp80 [KLRF1]), and e-Bioscience (San Diego, CA) (APC anti-NKG2D). Antibodies used for intracellular-cytokine staining (PE-labeled mouse anti-human IFN-γ and PE-labeled mouse immunoglobulin G2a isotype control) were purchased from Becton Dickinson (San Diego, CA).
Binding of ES products to cells and staining for FACS analysis.
PBMCs were enriched from whole blood by centrifugation over lymphocyte separation medium (ICN Biomedicals, Costa Mesa, CA). Enriched PBMCs (1 × 106 to 5 × 106) were resuspended in 1 ml PBS containing 1.0 μg of bioES from N. americanus and incubated for 1 h on a rocking platform at 37°C. Enriched PBMCs were also incubated with PBS alone as a control. The cells were then washed twice with PBS containing 5% fetal calf serum and stained on ice with FITC-conjugated streptavidin (for detection of bioES) plus PE-labeled anti-CD16 and anti-CD56 and TC-labeled anti-CD3 (to exclude any CD56+ CD3+ cells during analysis). In some studies, PBMCs were cultured overnight with/without various doses (1 to 125 ng/ml) of recombinant human interleukin-10 (IL-10) (PeproTech, Rocky Hill, NJ), followed by three washes in PBS and then incubation with bioES. For some studies of receptor blocking, PBMCs were incubated with APC anti-NKG2A or anti-NKG2D for 1 h on ice prior to incubation with bioES. Effective NKG2 receptor blocking was confirmed by assessment of the presence of fluorescence-tagged anti-NKG2A and -NKG2D antibodies on the cell surface by fluorescence-activated cell sorting (FACS) analysis both before and after bioES binding.
Intracellular-cytokine staining.
Whole-blood samples from individuals were collected and incubated for 24 h at 37°C and 5% CO2 in a humidified chamber in the presence of 10 μg/ml of brefeldin A, with/without phorbol myristate acetate (PMA; 25 ng/ml) and ionomycin (1 μg/ml), purchased from Sigma-Aldrich (St. Louis, MO). On the next day, samples were lysed with FACS lysing solution (Becton Dickinson, San Diego, CA) to remove red blood cells and then stained with fluorochrome-conjugated antibodies to CD56/CD16 and CD3. Stained cells were washed and then treated with permeabilization solution (PBS containing 0.5% bovine serum albumin, 0.5% saponin, and 0.1% sodium azide) for 20 min at 4°C, followed by incubation with PE-labeled anti-IFN-γ or an isotype control for 30 min at 4°C. Final data were expressed as frequencies of IFN-γ-expressing NK cells.
Chemotaxis assays.
NK cells were purified from PBMCs by using an NK cell isolation kit (Miltenyi Biotec, Inc., Auburn, CA), in which non-NK cells are depleted using a cocktail of biotin-conjugated antibodies against CD3, CD14, CD19, CD36, and anti-immunoglobulin E, followed by incubation with anti-biotin microbeads and retention on a magnetic field-activated cell sorting LS column. The purity of the recovered effluent cells was routinely 95 to 99% CD56+/CD16+ by FACS analysis. Bound cells were recovered from the magnetic field-activated cell sorting column by flushing and used as a source of non-NK cells. The chemotaxis of NK and non-NK cells (60% T cells, 30% monocytes, and 10% B cells) was assessed using 48-well modified Boyden chambers (NeuroProbe, Gaithersburg, MD), with the upper and lower chambers separated by a 5-μm polycarbonate membrane (NeuroProbe). The following were placed in the lower chambers, with six wells per test group: (i) medium only (1% bovine serum albumin fraction V in RPMI 1640), (ii) twofold-increasing doses (0.5 to 4 μg/ml) of N. americanus ES products, (iii) 1 μg/ml A. caninum ES products, and (iv) 1 ng/ml recombinant human RANTES (PeproTech). Cells were added to the upper chambers at 104 per well. The chambers were incubated at 37°C for 1 h, after which the membrane was removed, the nonmigrated cells were scraped off, and the membrane was stained with Wright-Giemsa to discriminate trapped cells. A chemotactic index was determined by dividing the number of migrated cells for each well by the mean number of migrated cells from the wells containing medium only.
Statistical analysis.
When a continuous variable was normally distributed and had an adequate sample size (n > 30), analysis of variance was used to test whether a difference existed among groups (>2 groups). This was followed by pairwise multiple comparisons between each group by Tukey's honestly significant difference test, with the alpha level set at 0.05 and adjusted for multiple comparisons. When a variable was either (i) nonnormally distributed, (ii) dichotomously distributed, (iii) categorically distributed, or (iv) lacking in adequate sample size (n < 30), the Kruskal-Wallis test was used for among-group comparisons, followed by a Dunn test for pairwise multiple comparisons, with alpha set at 0.05 and adjusted for multiple comparisons.
RESULTS
Study population. (i) Areas of nonendemicity.
Seven male volunteers, ranging in age from 25 to 45 years and having no history of hookworm infection (as determined by interviews), were enrolled in the study and are referred to as “egg-negative individuals from areas of nonendemicity.”
(ii) Areas of endemicity.
Seventeen individuals, between the ages of 6 and 66, from an area of N. americanus endemicity in northeastern Minas Gerais state, Brazil, were determined to be “egg negative” for any intestinal helminth or intestinal protozoan infection by ether sedimentation and a Kato-Katz fecal thick smear; these individuals are referred to as “egg-negative individuals from areas of endemicity.” Thirty-six individuals, between the ages of 6 and 65, from an area of Necator endemicity in northeastern Minas Gerais state, Brazil, were determined to be monoinfected with N. americanus by ether sedimentation and a Kato-Katz fecal thick smear, with a mean number of eggs per gram of 1,163 and an intensity of infection between 36 and 3,540 eggs per gram; these individuals are referred to as “egg-positive individuals from areas of endemicity” or “Necator-infected individuals.”
Differential binding of N. americanus ES products to NK cells from infected and noninfected subjects.
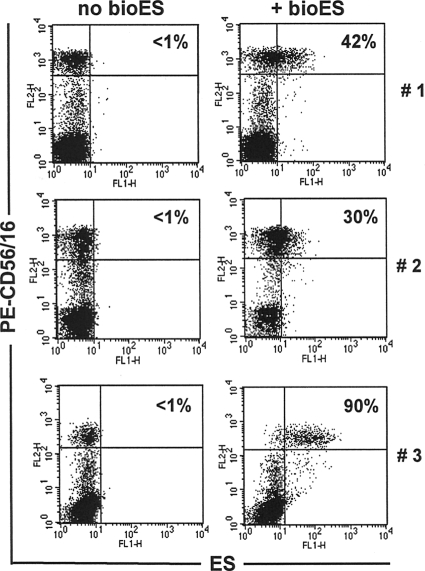
Previously, we reported that ES products from adult N. americanus worms bound selectively to NK cells from individuals from an area of nonendemicity (21). Figure 1 shows a binding profile of bioES on PBMCs from three egg-negative donors from areas of nonendemicity. Even though the extents of staining varied among the three individuals, positive staining was observed only on CD56/CD16+ NK cells. We then compared the binding profiles of bioES on NK cells from Necator-infected and egg-negative individuals from the same area of endemicity. As shown in Fig. 2, NK cells from egg-negative individuals from both areas of endemicity and areas of nonendemicity demonstrated bioES binding. In contrast, NK cells from Necator-infected individuals showed negligible binding to ES products. It should be noted that no other leukocyte subset was observed to bind bioES (data not shown).
FIG. 1.
N. americanus ES products bind selectively to NK cells from donors from areas of nonendemicity. PBMCs were isolated from blood samples of three egg-negative individuals from areas of nonendemicity (in the United States) (labeled 1 to 3) and were incubated with/without bioES from N. americanus, followed by staining with FITC-streptavidin, PE-labeled anti-CD56 and anti-CD16, and TC anti-CD3. FACS dot plots show the distribution of ES (FL1) versus NK (FL2) cells, after exclusion of CD3+ cells, in cells incubated with or without bioES. The percentage of NK cells (CD56+ CD16+) staining positive for bioES is shown in the upper right quadrant for each donor.
FIG. 2.
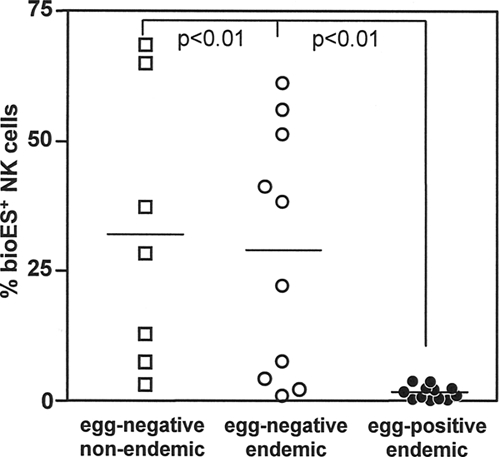
Binding of N. americanus ES products to NK cells from hookworm-infected and uninfected subjects. PBMCs were isolated from egg-negative individuals from areas of nonendemicity (in the United States), egg-negative individuals from areas of endemicity (in Brazil), and N. americanus-infected individuals (from Brazil). PBMCs from individual donors were incubated with bioES and then costained with FITC-streptavidin, PE-labeled anti-CD56 and anti-CD16, and TC anti-CD3. Data show the percentages of NK cells (CD56+ CD16+ CD3−) staining positive for bioES for each donor group. The bars represent group medians. The connected lines represent statistically significant differences, with P values indicated.
Differential expression of NK cell receptors in Necator-infected individuals.
To provide a potential explanation for the lack of binding of ES products to NK cells from Necator-infected individuals, we examined whether the subsets of NK cells expressing different receptors, including CD16, CD56, CD159a (NKG2A), CD314 (NKG2D), CD335 (NKp46), and KLRF1 (NKp80), might be reduced due to patent hookworm infection. Table 1 is a summary of different subsets of NK cells for egg-negative individuals (from both areas of endemicity and areas of nonendemicity) and Necator-infected individuals. Strikingly, Necator-infected individuals had higher numbers of all circulating NK cell subsets examined. However, the levels of expression of the individual markers show that levels on NK cells from egg-positive individuals were slightly decreased for NKG2A and NKG2D receptors (Table 1).
TABLE 1.
The distribution of NK cell subsets is altered during N. americanus infectiona
| NK cell marker | Mean ± SD for indicated group of patients from areas of endemicity
|
|||
|---|---|---|---|---|
| Absolute no. of cells/mm3
|
Frequency (%)
|
|||
| Egg negative | Egg positive | Egg negative | Egg positive | |
| CD56+ | 168.0 ± 32.5 | 389.4 ± 258.7† | 6.72 ± 1.3 | 14.3 ± 9.5† |
| CD56+ CD16+ | 58.0 ± 28.5 | 204.2 ± 65.3† | 34.5 ± 17.0 | 52.7 ± 16.9 |
| NKG2A+ | 122.5 ± 10.0 | 182.4 ± 68.1 | 72.2 ± 0.5 | 46.7 ± 17.6† |
| NKG2D+ | 157.5 ± 10.0 | 321.3 ± 46.3† | 93.1 ± 6.4 | 82.4 ± 11.9§ |
| NKp46+ | 23.7 ± 9.0 | 51.3 ± 18.9† | 14.1 ± 5.4 | 13.9 ± 4.9 |
| NKp80+ | 47.1 ± 17.3 | 96.9 ± 41.7† | 28.0 ± 10.3 | 24.9 ± 10.7 |
PBMCs were isolated and stained with various combinations of antibodies against the indicated NK cell markers. Data show the numbers or frequencies of cells expressing each marker for each patient group after gating on CD56+ CD3− lymphocytes. CD56+ cells were gated on CD3− lymphocytes only. † indicates a significant difference between egg-negative (n = 17) and egg-positive (n = 36) groups at a P value of 0.05 by Tukey's test; § indicates a significant difference between the same groups at a P value of 0.10.
To establish whether the decreased expression levels of NKG2A and NKG2D might account for the failure of N. americanus ES products to bind to NK cells from Necator-infected individuals, we conducted in vitro binding studies in which these receptors on NK cells from egg-negative individuals were blocked prior to incubation with bioES. As shown in Fig. 3, blocking these receptors had no impact on the subsequent binding of bioES to NK cells, suggesting that NKG2A and NKG2D are unlikely to be the principal receptors for interaction of NK cells with N. americanus ES products.
FIG. 3.
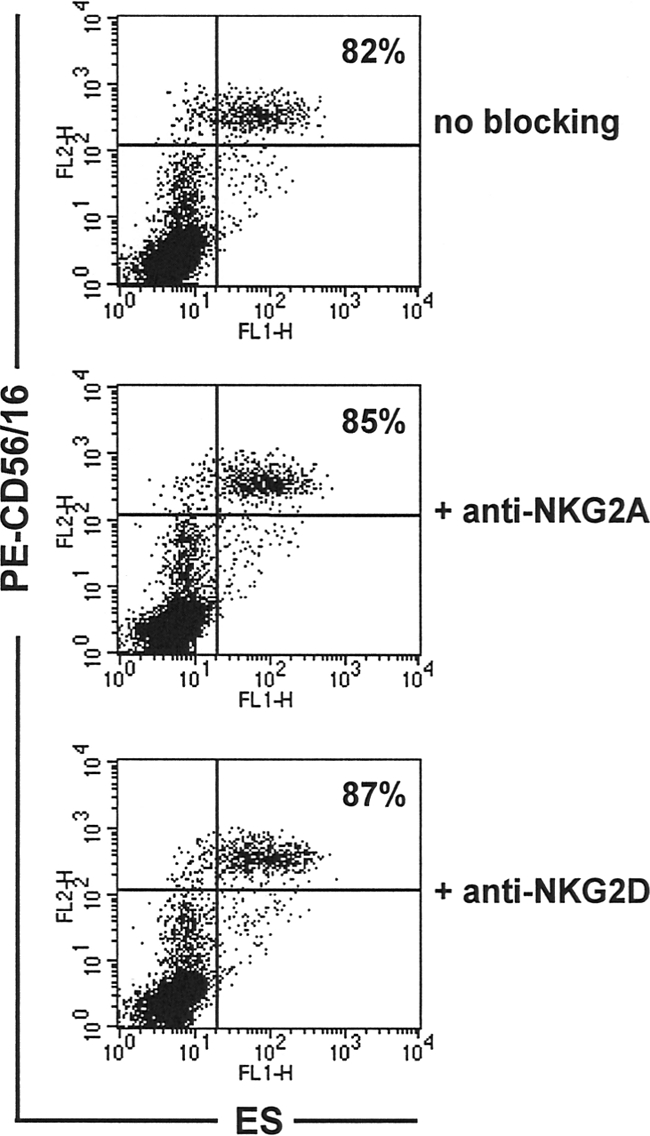
Blocking NKG2A or NKG2D receptors does not affect binding of N. americanus ES products to NK cells. PBMCS from egg-negative donors from areas of nonendemicity in the United States (n = 3) were incubated with/without anti-NKG2A or -NKG2D, followed by incubation with bioES and then costaining with FITC-streptavidin and PE anti-CD56/CD16. Dot plots show representative distributions of ES binding relative to CD56/CD16 staining for cells with no blocking, anti-NKG2A blocking, and anti-NKG2D blocking. The percentage of NK cells (CD56+ CD16+) staining positive for bioES is shown for each group.
The presence of IL-10 does not affect binding of ES products to NK cells.
The second mechanism that we considered to explain the impaired interaction of ES products with NK cells from Necator-infected individuals was the presence of IL-10, a well-established immunomodulator during chronic helminth infections (4, 27). As reported by Geiger et al. (15, 16), IL-10 is highly elevated in individuals resident in areas of Necator transmission. To determine whether IL-10 might have the capacity to affect receptors important for binding of NK cells to ES products, we conducted in vitro binding experiments in which NK cells from egg-negative individuals were incubated for up to 72 h with different doses of IL-10 (1 to 125 ng/ml) prior to interaction with bioES. NK cells preincubated with IL-10 at all doses were observed to bind bioES to the same extent as untreated cells (Fig. 4B shows data for 25 ng/ml of IL-10). Similar studies conducted with cells preincubated with either transforming growth factor β (TGF-β) or a combination of IL-10/TGF-β also showed no impact on ES binding (data not shown). These findings suggest that the presence of cytokines typically associated with immune regulation during helminth infection, such as IL-10 and TGF-β, is unlikely to affect receptor expression for ES products on NK cells and is unlikely to explain the observed failure of NK cells from Necator-infected individuals to interact with bioES.
FIG. 4.
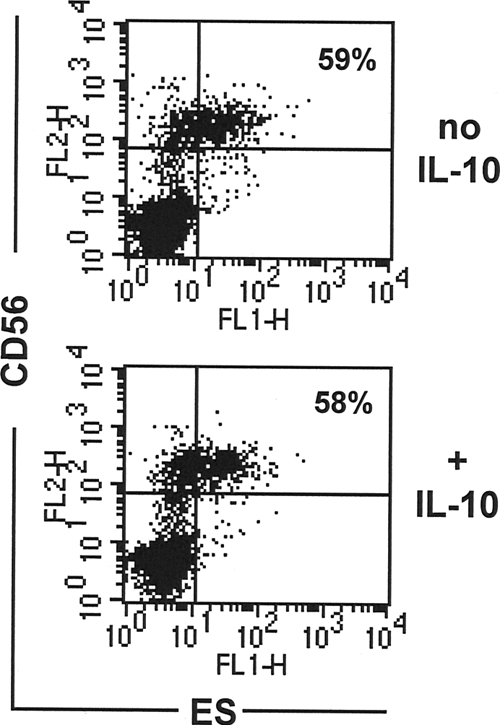
IL-10 is present during hookworm infection but does not affect binding of N. americanus ES products to NK cells. PBMCs from egg-negative donors from areas of nonendemicity (in the United States) were cultured overnight with/without recombinant human IL-10, followed by incubation with bioES and then costaining with FITC-streptavidin and PE anti-CD56/CD16. Dot plots show the distributions of ES binding relative to CD56/CD16 staining for cells incubated without IL-10 and with IL-10 (25 ng/ml). The percentage of NK cells (CD56+ CD16+) staining positive for bioES is shown for each group.
Increased frequency of IFN-γ-producing NK cells in Necator-infected individuals.
Previous data obtained by our group revealed that exposure of NK cells from egg-negative individuals to N. americanus ES products stimulates augmented production of IFN-γ (21). We postulated that interactions between ES products and NK cells in vivo might be beneficial for adult hookworms (26) because IFN-γ is known to cross-regulate Th2 effector responses (12, 30, 31) as well as to reduce the rate of gut epithelial cell turnover required for effective parasite expulsion (10). We investigated whether NK cells from Necator-infected individuals might demonstrate increased IFN-γ production due to their in vivo exposure to hookworms and/or hookworm-derived antigens. Strikingly, we found that a significantly greater percentage of NK cells from Necator-infected individuals than NK cells from egg-negative individuals produced IFN-γ when the cells were cultured directly ex vivo. These findings strongly suggest that during Necator infection, NK cells might interact with Necator-derived products and are activated to produce IFN-γ in situ (Fig. 5A). When the same groups of cells were stimulated with PMA, the NK cells from both egg-negative and egg-positive individuals from the area of Necator endemicity expressed higher levels of IFN-γ than NK cells from egg-negative individuals not resident in an area of hookworm endemicity (Fig. 5B). We suggest that the very potent and nonspecific stimulation induced by PMA likely obscures the true relationship between Necator infection and in vivo NK cell activation. Indeed, unlike culture in medium alone, stimulation with PMA causes all NK cells that have previously been activated in vivo to express IFN-γ, including NK cells not involved in Necator infection.
FIG. 5.
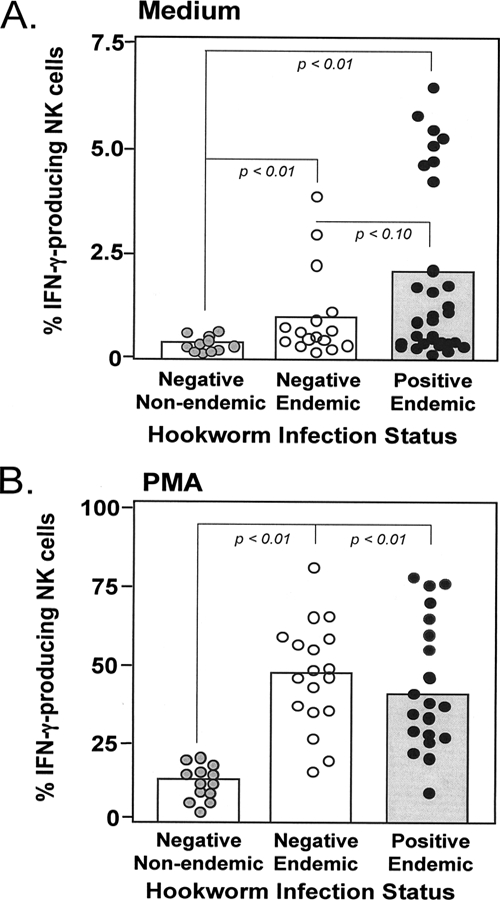
NK cells from subjects with N. americanus infection secrete IFN-γ. Whole-blood samples from egg-negative individuals from areas of nonendemicity (in the United States), egg-negative individuals from areas of endemicity (in Brazil), and egg-positive individuals from areas of endemicity (in Brazil) was incubated for 24 h with medium alone (A) or with PMA and ionomycin (B) in the presence of brefeldin A. Cells were then stained with antibodies to CD56/CD16 and CD3 and for intracellular IFN-γ. Data show the percentages of NK cells (CD56+ CD16+ CD3−) staining positive for IFN-γ. The boxes represent group means. The dotted lines represent pairwise comparisons between groups by Necator infection status; P values for each pairwise comparison are included where significant.
We also found that ES products from adult N. americanus worms can act as a recruitment factor for NK cells. As shown in Fig. 6A, N. americanus ES products caused NK cells to migrate, with the characteristic bell-shaped response of a chemokine. Interestingly, the chemotactic capacity of N. americanus ES products was specific for NK cells (Fig. 6B). ES products from the canine hookworm species, A. caninum, did not induce human NK cell migration, which fits well with our previous observations that A. caninum ES products do not bind to NK cells (21).
FIG. 6.
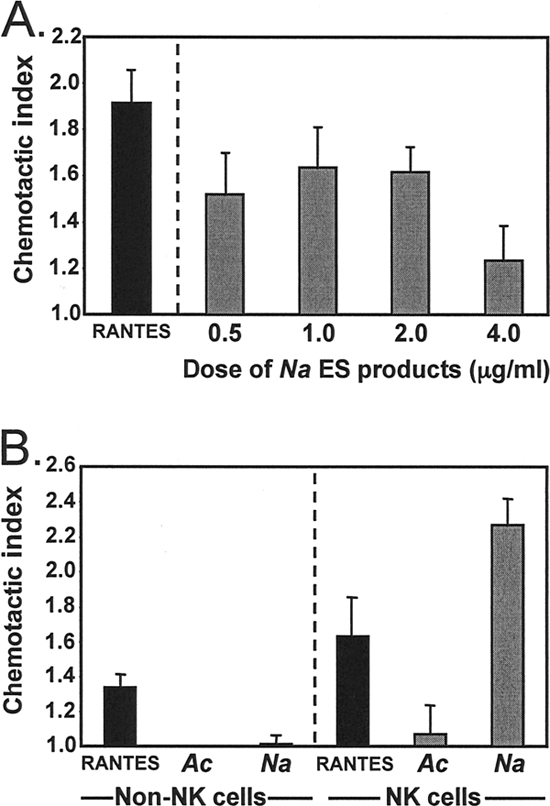
ES products from N. americanus worms induce NK cell chemotaxis. NK cells and non-NK cells were purified from PBMCS isolated from egg-negative donors from areas of nonendemicity. (A) NK cells were set up in Boyden chemotaxis chambers with different doses of N. americanus ES products or RANTES (a positive-control chemokine). Bar graphs show the mean (± standard error) indices for chemotaxis induced by the different stimulants. (B) NK cells and non-NK cells were set up in Boyden chemotaxis chambers with N. americanus (Na) or A. caninum (Ac) ES products at 1 μg/ml or RANTES. Bar graphs show the mean (± standard error) indices for chemotaxis induced by the different stimulants for non-NK cells and NK cells.
DISCUSSION
The chronicity of a hookworm infection reflects the immune system's inability to eliminate the parasite under natural conditions. As with most eukaryotic parasites, hookworms pass through a succession of developmental stages within the host, each of which bears stage-specific antigens that may either aid in escape from immunity or predetermine the character of the immune response to subsequent stages. In our previous work, which points to the second alternative, ES products of adult N. americanus hookworms bound exclusively to NK cells from uninfected individuals, causing them to secrete augmented levels of IFN-γ (21), which would make a beneficial environment for both incoming and established hookworm infections. We now attempted to characterize the interactions between host leukocytes and N. americanus ES products in individuals with patent N. americanus infections. In contrast to our previous studies, we observed no significant binding of ES products to NK cells or to cells of any other leukocyte subset in Necator-infected individuals, leading us to question if the distribution of NK cell subsets might be altered during hookworm infection. If the specific NK subset and/or receptor for NKBP is missing, this would explain the lack of binding by ES products ex vivo. For example, malaria-infected erythrocytes preferentially activate certain subsets of NK cells (17), most notably those expressing high levels of the heterodimer CD94/NKG2A (1). Recent studies have also shown that different subsets of NK cells preferentially interact with filarial worms at different developmental stages (2). We observed that Necator-infected individuals had a greater number of cells of all NK subsets examined than did negative individuals, ruling out that the failure to bind NKBP is a result of having fewer NK cells during Necator infection. When the expression levels of the individual receptors were examined, we observed slight but significant decreases in the levels of NKG2A and NKG2D only. However, our antibody preblocking studies suggest that decreased expression of these two markers is unlikely to be responsible for a lack of NKPB binding. We also explored the possibility that immunoregulatory cytokines, such as IL-10 and TGF-β, which are documented to be elevated in individuals from areas of Necator endemicity (15, 16) and are known regulators of receptor expression on circulating leukocytes (27), might affect NKBP receptor expression. Our studies demonstrated that neither the presence of IL-10 nor that of TGF-β altered the capacity of NK cells from egg-negative individuals to bind ES products, suggesting that these cytokines have little impact on receptors for NKBP interaction. These observations are further supported by the findings of Geiger et al. (15, 16), showing that high levels of IL-10 are present in all individuals resident in areas of hookworm endemicity. Despite the presence of elevated IL-10 in egg-negative individuals from areas of endemicity, their NK cells were clearly not affected in their capacity to interact with Necator ES products (Fig. 2).
An alternative explanation for the lack of binding of ES products to NK cells from Necator-infected individuals is that their NK cells have already interacted with ES products/NKBP in vivo, making these cells refractory to further interaction with exogenous ES products. In support of this, we observed that NK cells isolated from Necator-infected patients and cultured directly ex vivo without further stimulation expressed significantly higher intracellular levels of IFN-γ than NK cells from egg-negative individuals from both areas of endemicity and areas of nonendemicity, suggesting that these cells could have been primed to secrete IFN-γ in vivo. Previous studies examining cytokine profiles in individuals infected with hookworms have shown a “mixed” cytokine production profile that includes the presence of IFN-γ (15, 16, 28, 29). Our findings that N. americanus ES products have the capacity to induce NK cell recruitment fits well with the idea that NK cells might be actively recruited by adult worms during infection, enabling them to interact with and be activated by ES products in situ. In a mouse model of filarial infection, strains that either lacked or had diminished numbers of NK cells were nonpermissive for infection, supporting the idea that NK cells contribute directly to the survival and/or immune evasion of these nematodes (3). In recent follow-up studies, third-stage larvae and microfilariae of Brugia malayi were shown to induce cytokine secretion in NK cells purified from human PBMCs (2). The authors concluded that such interactions between filarial parasites and NK cells might contribute to a cytokine milieu with the capacity to shape host immune responses to the pathogen's advantage. Thus, the presence of cytokines such as IFN-γ would provide a means to cross-regulate Th2 responses associated with worm expulsion.
Our findings support a model where, during N. americanus infection, NK cells are attracted to the site of infection by chemotactic ES products produced by adult worms in the gut mucosa. The binding of ES products causes these NK cells to become activated and secrete IFN-γ locally, contributing to the adult hookworm's ability to remain refractory to host immune responses for years. We also propose that NK cells from Necator-infected individuals may be refractory to binding exogenous NKBP, because their receptors have already interacted with in vivo-derived ES products. Thus, the receptors may already be saturated with ES products or may have been downregulated following interaction with ES products in vivo. Studies are currently under way to establish the presence of bound ES products on NK cells of hookworm-infected patients as well to as establish the identity of the NKBP protein(s) and its receptor on human NK cells.
Acknowledgments
We thank the people of Americaninhas for their cooperation in this study as well as Renata Caldeira Diniz for providing technical assistance in the field studies, Ifeanyi Okwumabua (The George Washington University) for generating the N. americanus ES antigen, and Bin Zhan (The George Washington University) for generating the A. caninum ES products. A.T.-C. and R.C.-O. are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq-Brasil for fellowships.
This study was supported by National Institutes of Health grant AI059280.
We declare that there are no potential conflicts of interest in the present work.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 6 October 2008.
REFERENCES
- 1.Artavanis-Tsakonas, K., K. Eleme, K. L. McQueen, et al. 2003. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J. Immunol. 1715396-5405. [DOI] [PubMed] [Google Scholar]
- 2.Babu, S., C. P. Blauvelt, and T. B. Nutman. 2007. Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. J. Immunol. 1792445-2456. [DOI] [PubMed] [Google Scholar]
- 3.Babu, S., P. Porte, T. R. Klei, L. D. Shultz, and T. V. Rajan. 1998. Host NK cells are required for the growth of the human filarial parasite Brugia malayi in mice. J. Immunol. 1611428-1432. [PubMed] [Google Scholar]
- 4.Belkaid, Y., C. M. Sun, and N. Bouladoux. 2006. Parasites and immunoregulatory T cells. Curr. Opin. Immunol. 18406-412. [DOI] [PubMed] [Google Scholar]
- 5.Bethony, J., J. Chen, S. Lin, et al. 2002. Emerging patterns of hookworm infection: influence of aging on the intensity of Necator infection in Hainan Province, People's Republic of China. Clin. Infect. Dis. 351336-1344. [DOI] [PubMed] [Google Scholar]
- 6.Brooker, S., N. Alexander, S. Geiger, et al. 2006. Contrasting patterns in the small-scale heterogeneity of human helminth infections in urban and rural environments in Brazil. Int. J. Parasitol. 361143-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooker, S., J. Bethony, and P. J. Hotez. 2004. Human hookworm infection in the 21st century. Adv. Parasitol. 58197-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooker, S., A. Jardim-Botelho, R. J. Quinnell, et al. 2007. Age-related changes in hookworm infection, anaemia and iron deficiency in an area of high Necator americanus hookworm transmission in south-eastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 101146-154. [DOI] [PubMed] [Google Scholar]
- 9.Chan, M. S. 1997. The global burden of intestinal nematode infections—fifty years on. Parasitol. Today 13438-443. [DOI] [PubMed] [Google Scholar]
- 10.Cliffe, L. J., N. E. Humphreys, T. E. Lane, C. S. Potten, C. Booth, and R. K. Grencis. 2005. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 3081463-1465. [DOI] [PubMed] [Google Scholar]
- 11.de Silva, N. R., S. Brooker, P. J. Hotez, A. Montresor, D. Engels, and L. Savioli. 2003. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 19547-551. [DOI] [PubMed] [Google Scholar]
- 12.Dickensheets, H. L., and R. P. Donnelly. 1999. Inhibition of IL-4-inducible gene expression in human monocytes by type I and type II interferons. J. Leukoc. Biol. 65307-312. [DOI] [PubMed] [Google Scholar]
- 13.Diemert, D. J., J. M. Bethony, and P. J. Hotez. 2008. Hookworm vaccines. Clin. Infect. Dis. 46282-288. [DOI] [PubMed] [Google Scholar]
- 14.Fleming, F. M., S. Brooker, S. M. Geiger, et al. 2006. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop. Med. Int. Health 1156-64. [DOI] [PubMed] [Google Scholar]
- 15.Geiger, S. M., I. R. Caldas, B. E. McGlone, et al. 2007. Stage-specific immune responses in human Necator americanus infection. Parasite Immunol. 29347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiger, S. M., C. L. Massara, J. Bethony, P. T. Soboslay, and R. Correa-Oliveira. 2004. Cellular responses and cytokine production in post-treatment hookworm patients from an endemic area in Brazil. Clin. Exp. Immunol. 136334-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen, D. S., M. C. D'Ombrain, and L. Schofield. 2007. The role of leukocytes bearing Natural Killer Complex receptors and Killer Immunoglobulin-like Receptors in the immunology of malaria. Curr. Opin. Immunol. 19416-423. [DOI] [PubMed] [Google Scholar]
- 18.Hotez, P. J., J. Ashcom, Z. Bin, et al. 2002. Effect of vaccinations with recombinant fusion proteins on Ancylostoma caninum habitat selection in the canine intestine. J. Parasitol. 88684-690. [DOI] [PubMed] [Google Scholar]
- 19.Hotez, P. J., J. Bethony, M. E. Bottazzi, S. Brooker, and P. Buss. 2005. Hookworm: “the great infection of mankind.” PLoS Med. 2e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotez, P. J., S. Brooker, J. M. Bethony, M. E. Bottazzi, A. Loukas, and S. Xiao. 2004. Hookworm infection. N. Engl. J. Med. 351799-807. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh, G. C., A. Loukas, A. M. Wahl, et al. 2004. A secreted protein from the human hookworm necator americanus binds selectively to NK cells and induces IFN-gamma production. J. Immunol. 1732699-2704. [DOI] [PubMed] [Google Scholar]
- 22.Humphries, D. L., L. S. Stephenson, E. J. Pearce, P. H. The, H. T. Dan, and L. T. Khanh. 1997. The use of human faeces for fertilizer is associated with increased intensity of hookworm infection in Vietnamese women. Trans. R. Soc. Trop. Med. Hyg. 91518-520. [DOI] [PubMed] [Google Scholar]
- 23.Jardim-Botelho, A., S. Brooker, S. M. Geiger, et al. 2008. Age patterns in undernutrition and helminth infection in a rural area of Brazil: associations with ascariasis and hookworm. Trop. Med. Int. Health 13458-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz, N., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14397-400. [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Loukas, A., S. L. Constant, and J. M. Bethony. 2005. Immunobiology of hookworm infection. FEMS Immunol. Med. Microbiol. 43115-124. [DOI] [PubMed] [Google Scholar]
- 27.Pestka, S., C. D. Krause, D. Sarkar, M. R. Walter, Y. Shi, and P. B. Fisher. 2004. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 22929-979. [DOI] [PubMed] [Google Scholar]
- 28.Pit, D. S., A. M. Polderman, S. Baeta, H. Schulz-Key, and P. T. Soboslay. 2001. Parasite-specific antibody and cellular immune responses in human infected with Necator americanus and Oesophagostomum bifurcum. Parasitol. Res. 87722-729. [DOI] [PubMed] [Google Scholar]
- 29.Quinnell, R. J., D. I. Pritchard, A. Raiko, A. P. Brown, and M. A. Shaw. 2004. Immune responses in human necatoriasis: association between interleukin-5 responses and resistance to reinfection. J. Infect. Dis. 190430-438. [DOI] [PubMed] [Google Scholar]
- 30.Romagnani, S. 2006. Regulation of the T cell response. Clin. Exp. Allergy 361357-1366. [DOI] [PubMed] [Google Scholar]
- 31.Schoenborn, J. R., and C. B. Wilson. 2007. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 9641-101. [DOI] [PubMed] [Google Scholar]
- 32.Sen, H. G., and D. Seth. 1970. Development of Necator americanus in golden hamsters Mesocricetus auratus. Indian J. Med. Res. 581356-1360. [PubMed] [Google Scholar]