Abstract
In areas where levels of transmission of Plasmodium falciparum are high and stable, the age-related acquisition of high-level immunoglobulin G (IgG) antibodies to preerythrocytic circumsporozoite protein (CSP) and liver-stage antigen 1 (LSA-1) has been associated with protection from clinical malaria. In contrast, age-related protection from malaria develops slowly or not at all in residents of epidemic-prone areas with unstable low levels of malaria transmission. We hypothesized that this suboptimal clinical and parasitological immunity may in part be due to reduced antibodies to CSP or LSA-1 and/or vaccine candidate blood-stage antigens. Frequencies and levels of IgG antibodies to CSP, LSA-1, thrombospondin-related adhesive protein (TRAP), apical membrane antigen 1 (AMA-1), erythrocyte binding antigen 175 (EBA-175), and merozoite surface protein 1 (MSP-1) were compared in 243 Kenyans living in a highland area of unstable transmission and 210 residents of a nearby lowland area of stable transmission. Levels of antibodies to CSP, LSA-1, TRAP, and AMA-1 in the oldest age group (>40 years) in the unstable transmission area were lower than or similar to those of children 2 to 6 years old in the stable transmission area. Only 3.3% of individuals in the unstable transmission area had high levels of IgG (>2 arbitrary units) to both CSP and LSA-1, compared to 43.3% of individuals in the stable transmission area. In contrast, antibody levels to and frequencies of MSP-1 and EBA-175 were similar in adults in areas of stable and unstable malaria transmission. Suboptimal immunity to malaria in areas of unstable malaria transmission may relate in part to infrequent high-level antibodies to preerythrocytic antigens and AMA-1.
Areas of unstable low malaria transmission such as the highlands of East Africa are characterized by a persistent risk of clinical malaria in older children and adults (13), whereas in areas with stable high-level transmission, the risk of clinical malaria decreases markedly after the age of 3 to 5 years (30). Immunoglobulin G (IgG) antibodies to a number of vaccine candidate antigens including the preerythrocytic antigens circumsporozoite protein (CSP) (19), liver-stage antigen 1 (LSA-1) (19, 23), and thrombospondin-related adhesive protein (TRAP) (29); the blood-stage antigen merozoite surface protein 1 (MSP-1) (5, 9, 26, 27); and the blood-stage and preerythrocytic antigen apical membrane antigen 1 (AMA-1) (24, 26) have been associated with protection from clinical malaria in areas of stable transmission. The acquisition of IgG antibodies to these antigens also strongly correlates with increasing age (6, 12, 15, 25, 27, 29). However, the development of antimalarial antibodies in relation to age and protection from clinical disease in areas of unstable transmission has not been well characterized.
We have recently demonstrated that in an area of Kenya with stable transmission, the presence of high-level IgG antibodies to three preerythrocytic antigens, CSP, LSA-1, and TRAP, correlates positively with protection from infection in adults (15) and from clinical malaria in children (19). The association with protection from infection and disease was attributable largely to antibodies to CSP and LSA-1. We therefore hypothesize that one reason for the higher risk of clinical malaria in older children and adults in areas of unstable transmission might be a relatively low frequency and/or level of IgG antibodies to CSP and LSA-1. To test this hypothesis, we measured IgG antibody frequencies and levels of IgG antibody to CSP and LSA-1 in individuals aged 2 to 84 years with divergent malaria exposure in Kenya. In addition, we quantified and compared in the two populations of IgG antibodies to other Plasmodium falciparum antigens under consideration as vaccine candidates, including the preerythrocytic-stage antigen TRAP and blood-stage antigens erythrocyte binding antigen 175 (EBA-175), MSP-1, and AMA-1.
MATERIALS AND METHODS
Study population and recruitment.
Individuals 2 years of age and older were recruited from the sublocations of Kanyawegi (population of ∼3,000) and Kipsamoite (population of ∼3,500) in western Kenya. Kanyawegi is located in Kisumu District, a holoendemic lowland area with stable and intense malaria transmission, where entomological inoculation rates exceed 300 infectious bites per person per year (3). In contrast, Kipsamoite in Nandi District is located in an epidemic-prone highland area characterized by unstable malaria transmission during the prolonged interepidemic periods (10), with an estimated entomological inoculation rate of <1 infectious bite per person per year (C. C. John, unpublished data). This cross-sectional study was conducted in August 2001 at a time of relatively high malaria incidence in the highland area (14) and stable malaria incidence in the lowland area. Blood was collected by venipuncture from adults (10 to 20 ml) and children (5 ml). Microscopy was performed to determine blood-stage infection, as previously described (16).
Individuals were recruited through local barasas or meetings across the study sites, where information about the study was provided. Individuals who had attended the barasa or discussed the study with field assistants were eligible to join the study. Field assistants were asked to enroll specific numbers of individuals from each village in each site to allow a proportional geographic representation of each overall site. Field assistants went to households chosen randomly from a list of households in each village and asked if individuals were interested in participating in the study. Enrollment for each village was completed when the required numbers from each village were enrolled. Written informed consent was obtained from the study participants or the parents or guardians of individuals under 18 years of age prior to sample collection. Ethical approval was obtained from the Ethical Review Committee at the Kenya Medical Research Institute and the Human Investigations Institutional Review Board at Case Western Reserve University and the University Hospital of Cleveland.
Antigens and peptides.
The presence of IgG antibodies to CSP and LSA-1 was tested using previously observed immunogenic central repeat sequence peptides: the (NANP)5 repeat peptide (7) and LAKEKLQGQQSDLEQERLAKEKLQEQQ-SDLEQERLAKEKLQ (LSA-Rep) (11) for CSP and LSA-1, respectively. The presence of IgG antibodies to TRAP, AMA-1, EBA-175, and MSP-1 was tested using recombinant antigens. Recombinant P. falciparum TRAP (3D7) was expressed in Escherichia coli and provided by one of the authors (D. E. Lanar). The gene fragment encoding amino acids D48 to K394 was PCR amplified from the 3D7 strain of parasite genomic DNA using gene-specific sense and antisense primers. Recombinant AMA-1 (ectodomain, nonglycosylated) and EBA-175 (nonglycosylated) were expressed in Pichia pastoris and provided by one of the authors (D. E. Lanar). Recombinant MSP-119 protein corresponding to the E-KNG variant was expressed in Saccharomyces cerevisiae (21) and provided by the Malaria Research and Reference Reagent Resource Center (Manassas, VA). In previous testings in this area, IgG antibodies from adult sera most frequently recognized the E-KNG variant (C. C. John, unpublished data).
Antibody measurements.
IgG antibodies were measured by enzyme-linked immunosorbent assay (ELISA). The CSP and LSA-1 peptides were dissolved in 0.01 M phosphate-buffered saline (PBS) to a concentration of 10 μg/ml, and recombinant antigens were dissolved in 0.01 M PBS to concentrations of 0.1 μg/ml (AMA-1 and EBA-175), 0.2 μg/ml (MSP-1), and 0.5 μg/ml (TRAP). Fifty microliters of antigen solution was added to Immulon-4 plates (Dynex Technologies, Chantilly, VA). Following overnight incubation at 4°C, washing with PBS-0.05% Tween 20, and blocking in 5% (wt/vol) nonfat powdered milk in PBS, duplicate 50-μl samples of serum diluted 1:100 in 5% powdered milk were added to wells and incubated for 2 h at room temperature. After washing with PBS-0.05% Tween 20, 50 μl of alkaline phosphatase-conjugated goat anti-human IgG (Jackson ImmunoResearch, West Grove, PA) diluted 1:1,000 in 5% powdered milk was added and removed after 1 h. After extensive washing with PBS-0.05% Tween 20, p-nitrophenylphosphate was added in accordance with the manufacturer's instructions (Sigma Chemical Co., St. Louis, MO). The optical density (OD) was measured at 405 nm. Previous titrations of serum at concentrations of 1:100, 1:200, and 1:1,000 showed that for all antigens, ODs were highest at a concentration of 1:100.
Antibody values were expressed in arbitrary units (AU), which were calculated by dividing the OD generated by the test sample by the mean OD plus three standard deviations of samples from 40 North Americans never exposed to malaria. Individual sera from nine North American control subjects with OD values representative of the 40 North American malaria-naïve samples were used on each plate. OD values greater than 1.0 AU were considered to be positive. OD values corresponding to 1.0 AU for CSP, LSA-1, TRAP, AMA-1, EBA-175, and MSP-1 were 0.144, 0.079, 0.110, 0.183, 0.089, and 0.091, respectively.
Statistical analysis.
To determine the significance of antibody frequencies and levels in each age group across the two transmission sites, χ2 analysis and the Wilcoxon rank-sum test were used, respectively. To determine the significance of the trend of antibody frequencies and levels in populations across the four age groups for each site, the χ2 test for trend and a nonparametric test for trend across ordered groups, an extension of the Wilcoxon rank-sum test (Stata command nptrend), were used, respectively. The Wilcoxon rank-sum test was also used to compare antibody levels in individuals with and without parasitemia by blood smear microscopy testing. Multiple comparisons were adjusted for with the correction of Bonferroni. All statistical analysis was done using Stata 10.0 software (Stata Corporation, College Station, TX).
RESULTS
Study demographics and prevalence of parasitemia.
A total of 210 and 243 serum samples were collected from asymptomatic individuals in areas of stable and unstable malaria transmission, respectively. Individuals from the two areas were divided into four age groups: 2 to 5 years, 6 to 15 years, 15 to 40 years, and >40 years. Microscopy results were available for 193 and 227 of the individuals in the areas of stable and unstable transmission, respectively. In the area of stable malaria transmission, 126 of 193 individuals (65.3%) were infected with P. falciparum by microscopic examination of blood smear, while in the area of unstable transmission, 3 of 227 asymptomatic individuals (1.3%) were infected with P. falciparum. Sixteen of 193 individuals (8.3%) in the area of stable transmission were infected with Plasmodium malariae; in 13 of these 16 individuals (81.2%), this was a coinfection with P. falciparum. No individuals in the area of unstable transmission were infected with Plasmodium malariae. No P. vivax or Plasmodium ovale infections were seen at either site.
Antibody frequency and levels in an area of stable malaria transmission.
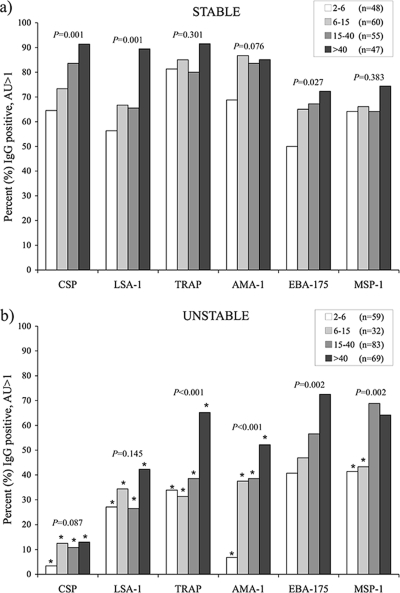
In the area of stable malaria transmission, IgG antibodies to all antigens (CSP, LSA-1, TRAP, AMA-1, EBA-175, and MSP-1) were present in a majority (≥50%) of individuals from all age groups when the cutoff for positive responses was set at >1 AU (Fig. 1a). At least 80% of individuals 15 years of age or older had positive responses to CSP, TRAP, and AMA-1, and antibodies to all six antigens were present in >72% of individuals older than 40 years of age. Antibody frequencies increased with age for all antigens, with approximately 90% of adults older than 40 years of age possessing antibodies to the preerythrocytic antigens CSP, LSA-1, and TRAP and with 85% of adults having antibodies to AMA-1. However, the increase with age was statistically significant only for CSP, LSA-1, and EBA-175 because of the high proportion of children already antibody positive for TRAP, AMA-1, and MSP-1 (Fig. 1a).
FIG. 1.
Prevalence of total IgG antibodies, determined by ELISA, with AU values of >1 for various P. falciparum antigens in areas of stable (a) and unstable (b) malaria transmission across the following age groups: 2 to 5, 6 to 15, 16 to 40, and >40 years old. The number of individuals in each age group is indicated for all antigens, except MSP-1, where the numbers of individuals were 39, 56, 53, and 43, respectively, for stable and 58, 31, 81, and 67, respectively, for unstable transmission areas. An asterisk (*) indicates significant (P < 0.05) differences in age groups between areas by χ2 analysis. P values for trend for age by χ2 analysis are indicated.
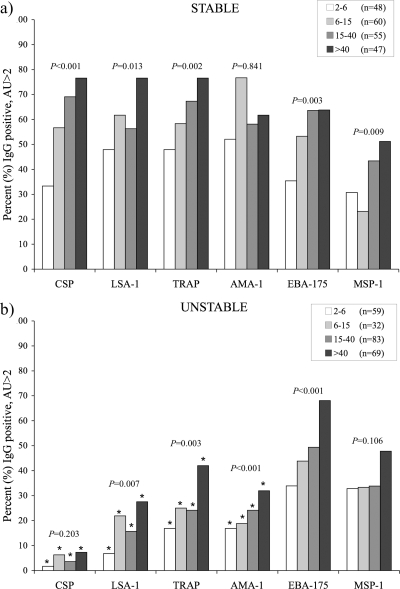
In earlier studies in the area of high transmission, we observed that IgG levels of >2 AU for both CSP and LSA-1 were associated with protection from clinical malaria in children (19). For this reason, levels of IgG of >2 AU for the preerythrocytic and blood-stage antigens were assessed in the two sites. The proportions of individuals with an IgG level of >2 AU was still greater than 30% for all antigens in the youngest age group, with more than 75% of individuals older than 40 years of age having IgG frequencies of >2 AU for each of the preerythrocytic antigens (CSP, LSA-1, and TRAP) (Fig. 2a). Ninety-one of 210 individuals (43.3%) in this area of stable transmission had IgG levels of >2 AU for both LSA-1 and CSP, including 30 of 47 individuals (63.8%) older than 40 years of age. Levels of IgG antibodies increased significantly with age for CSP, LSA-1, and TRAP but not MSP-1, AMA-1, or EBA-175 (Table 1).
FIG. 2.
Prevalence of total IgG antibodies, determined by ELISA, with AU values of >2 for various P. falciparum antigens in areas of stable (a) and unstable (b) malaria transmission across the following age groups: <6, 6 to 15, 16 to 40, and >40 years old. Numbers of individuals in each age group are indicated for all antigens, except MSP-1, where the numbers of individuals were 39, 56, 53, and 43, respectively, for stable and 58, 31, 81, and 67, respectively, for unstable transmission areas. An asterisk (*) indicates significant (P < 0.05) differences in age groups between areas by χ2 analysis. P values for trend for age by χ2 analysis are indicated.
TABLE 1.
Antibody levels across age groups in an area of stable malaria transmission
| Antigen | Median level (AU) (range) for age group:
|
P valuea | |||
|---|---|---|---|---|---|
| 2-5 yr (n = 48) | 6-15 yr (n = 60) | 16-40 yr (n = 55) | >40 yr (n = 47) | ||
| CSP | 1.37 (0.1-14) | 2.54 (0.1-21.4) | 3.21 (0.1-16.4) | 6.51 (0.2-27) | 0.000 |
| LSA-1 | 1.67 (0.0-42) | 3.46 (0.0-100.9) | 2.30 (0.0-61.5) | 6.26 (0.3-46) | 0.023 |
| TRAP | 1.81 (0.2-30) | 2.38 (0.1-15.8) | 3.56 (0.1-22.0) | 4.43 (0.6-17) | 0.002 |
| AMA-1 | 2.23 (0.0-18) | 4.87 (0.1-11.6) | 2.17 (0.1-9.1) | 2.47 (0.8-8.8) | 0.376 |
| EBA-175 | 0.91 (0.1-67) | 3.73 (0.0-86.7) | 3.60 (0.0-79.0) | 3.52 (0.1-116.6) | 0.238 |
| MSP-1 | 1.17 (0.1-36) | 1.39 (0.2-37.4) | 1.59 (0.1-33) | 2.05 (0.3-41.9) | 0.054 |
Determined by nonparametric test for trend (see Materials and Methods).
Levels of IgG antibody to LSA-1 were significantly correlated with levels of IgG antibody to CSP, TRAP, EBA-175, and MSP-1 (Table 2). In addition to LSA-1, levels of IgG antibody to EBA-175 were also significantly correlated with levels of antibodies to TRAP and AMA-1 (Τable 2).
TABLE 2.
Antibody correlations between antigens for all individuals (n = 191) from a stable malaria transmission area
| Antigen | Antibody correlation (Spearman's rho)a
|
|||||
|---|---|---|---|---|---|---|
| CSP | LSA-1 | TRAP | AMA-1 | EBA-175 | MSP-1 | |
| CSP | ||||||
| LSA-1 | 0.3016* | |||||
| TRAP | 0.1878 | 0.4081* | ||||
| AMA-1 | −0.1056 | 0.0228 | 0.0700 | |||
| EBA-175 | 0.1943 | 0.4294* | 0.2567* | 0.2459* | ||
| MSP-1 | 0.0821 | 0.2416* | 0.1031 | 0.1249 | 0.1508 | |
* indicates statistical significance (P < 0.05).
Individuals with P. falciparum parasitemia had lower levels of IgG antibody to CSP (median levels [ranges] for P. falciparum parasitemic versus nonparasitemic individuals were 2.34 [0.12, 27.34] versus 5.40 [0.21, 21.38], respectively; P = 0.0005) and MSP-1 (median levels [ranges] for P. falciparum parasitemic versus nonparasitemic individuals were 1.25 [0.09, 41.96] versus 2.31 [0.29, 29.50], respectively; P = 0.008) than did those without parasitemia. Levels of IgG antibodies to LSA-1, TRAP, AMA-1, and EBA-175 did not differ according to the presence or absence of P. falciparum parasitemia (data not shown).
Antibody frequencies and levels in an area of unstable malaria transmission.
In the area of unstable transmission, the proportion of antibody-positive individuals at a cutoff of an AU of >1 was significantly lower in every age group for the preerythrocytic antigens (CSP, LSA-1, and TRAP) and the preerythrocytic/blood-stage antigen (AMA-1) (Fig. 1b) than those from the stable transmission area. This was particularly evident in the case of CSP, to which only 13% of adults over 40 years of age were antibody positive, whereas approximately 65% of children in the stable transmission area were antibody positive. Frequencies of IgG antibodies to MSP-1 were lower in the area of unstable transmission only in children younger than 15 years of age and similar between sites in adults, while frequencies of antibodies to EBA-175 did not differ for any age group between the two sites. The frequency of IgG antibodies to P. falciparum antigens in the unstable transmission area increased with age for all antigens except CSP and LSA-1 (Fig. 1b).
Frequencies of IgG levels of >2 AU were particularly low for LSA-1 and CSP, even in the oldest individuals. Less than 10% of individuals above the age of 40 years had IgG levels of >2 AU for CSP, and less than 30% had IgG levels of >2 AU for LSA-1 (Fig. 2b). Only 8 of 243 individuals (3.3%) in the unstable transmission area had IgG levels of >2 AU for both CSP and LSA-1, including 4 of 69 individuals (5.8%) over the age of 40 years.
Levels of antibody to the preerythrocytic antigens CSP, LSA-1, and TRAP and the preerythrocytic/blood-stage antigen AMA-1 were significantly lower in the area of unstable malaria transmission at all ages than those from the stable transmission area, whereas median levels of antibody to EBA-175 and MSP-1 were lower in children under 15 years of age but not adults (Table 3), and median levels of antibody to EBA-175 were marginally higher in adults greater than 40 years of age in the area of unstable transmission than in those in the area of stable transmission (P = 0.05) (Table 3).
TABLE 3.
Antibody levels across age groups in an area of unstable malaria transmission
| Antigen | Median level (AU) (range) for age groupa:
|
P valueb | |||
|---|---|---|---|---|---|
| 2-5 yr (n = 59) | 6-15 yr (n = 32) | 16-40 yr (n = 83) | >40 yr (n = 69) | ||
| CSP | 0.10 (0.0-2.8)* | 0.12 (0.0-3.7)* | 0.16 (0.0-5.3)* | 1.15 (0.0-4.4)* | 0.001 |
| LSA-1 | 0.24 (0.0-4.3)* | 0.43 (0.0-12)* | 0.41 (0.0-24)* | 0.54 (0.0-35)* | 0.002 |
| TRAP | 0.76 (0.0-7.8)* | 0.50 (0.0-15)* | 0.69 (0.0-26)* | 1.64 (0.0-13)* | 0.001 |
| AMA-1 | 0.12 (0.0-2.9)* | 0.38 (0.0-9.4)* | 0.62 (0.0-7.2)* | 1.04 (0.0-9.9)* | 0.000 |
| EBA-175 | 0.52 (0.0-20)* | 0.79 (0.0-130)* | 1.93 (0.0-63) | 5.92 (0.0-160) | 0.000 |
| MSP-1 | 0.80 (0.0-22) | 0.61 (0.0-28)* | 1.32 (0.1-14) | 1.52 (0.1-46) | 0.001 |
* indicates values significantly different from antibody levels for the same antigen and age group in the stable transmission area (P < 0.05 by Wilcoxon rank-sum test).
Determined by nonparametric test for trend (see Materials and Methods).
In the unstable transmission area, levels of IgG antibody to all antigens correlated significantly with each other except for antibodies to EBA-175 and CSP (Table 4). Too few individuals were P. falciparum parasitemic in this area to allow a comparison of levels of IgG antibodies to the various antigens in parasitemic versus nonparasitemic individuals.
TABLE 4.
Antibody correlations between antigens for all individuals (n = 232) from an unstable malaria transmission area
| Antigen | Antibody correlation (Spearman's rho)a
|
|||||
|---|---|---|---|---|---|---|
| CSP | LSA-1 | TRAP | AMA-1 | EBA-175 | MSP-1 | |
| CSP | ||||||
| LSA-1 | 0.2151* | |||||
| TRAP | 0.2818* | 0.3375* | ||||
| AMA-1 | 0.3848* | 0.3406* | 0.3096* | |||
| EBA-175 | 0.1810 | 0.5083* | 0.5615* | 0.5433* | ||
| MSP-1 | 0.2004* | 0.2655* | 0.3133* | 0.3815* | 0.4182* | |
* indicates statistical significance (P < 0.05).
To assess whether saturation in binding might be occurring for antibodies to blood-stage antigens at a plasma dilution of 1:100, we performed additional testing on randomly chosen plasma samples from adults >40 years of age in the areas of stable (n = 40) and unstable (n = 40) transmission at a plasma dilution of 1:1,000. Adults >40 years of age were chosen because these individuals would be expected to have the highest antibody levels in both areas. Although the levels in AU varied somewhat between the samples diluted 1:100 and those diluted 1:1,000, the magnitude of differences in levels of IgG antibodies to AMA-1, EBA-175, and MSP-1 between individuals in areas of stable and unstable transmission was very similar at 1:100 and 1:1,000 dilutions (Table 5). In addition, the frequency of individuals in each site with IgG antibodies to a specific antigen did not differ significantly for any antigen at a 1:100 versus a 1:1,000 dilution (Table 6). In summary, similar levels and frequencies of antibodies to MSP-1 and EBA-175 were seen in adults in the areas of stable and unstable transmission whether assessed at a 1:100 or a 1:1,000 dilution (levels of antibodies to EBA-175 were actually marginally higher in adults in the area of unstable transmission at both dilutions). Thus, the data suggest that adults in these areas truly have similar frequencies of antibodies to EBA-175 and MSP-1 and that this finding is not an artifact of measuring these antibodies at a plasma dilution of 1:100.
TABLE 5.
Levels of IgG antibody to AMA-1, EBA-175, and MSP-1 at different plasma dilutions in adults >40 years of age in areas of stable (n = 40) and unstable (n = 40) transmission
| Antigen | Median IgG level (AU) (range) at a dilution of 1:100
|
P value for 1:100 dilution | Median IgG level (AU) (range) at a dilution of 1:1,000
|
P value for 1:1,000 dilution | ||
|---|---|---|---|---|---|---|
| Stable | Unstable | Stable | Unstable | |||
| AMA-1 | 2.39 (0.76, 7.47) | 0.64 (0.11, 9.86) | <0.001 | 2.13 (0.74, 12.43) | 0.99 (0.63, 8.22) | <0.001 |
| EBA-175 | 3.28 (0.13, 43.76) | 6.85 (0.01, 157.44) | 0.05 | 1.27 (0.88, 26.32) | 3.27 (0.87, 36.11) | 0.04 |
| MSP-1 | 2.23 (0.29, 41.96) | 1.61 (0.07, 24.47) | 0.81 | 1.44 (0.79, 8.74) | 1.58 (0.83, 10.02) | 0.95 |
TABLE 6.
Frequencies of IgG antibody (AU > 1) to AMA-1, EBA-175, and MSP-1 at different plasma dilutions in adults >40 years of age in areas of stable (n = 40) and unstable (n = 40) transmission
| Antigen | No. of adults with IgG antibodies (%) at a dilution of 1:100
|
P value for 1:100 dilution | No. of adults with IgG antibodies (%) at a dilution of 1:1,000
|
P value for 1:1,000 dilution | ||
|---|---|---|---|---|---|---|
| Stable | Unstable | Stable | Unstable | |||
| AMA-1 | 33 (82.5) | 17 (42.5) | <0.001 | 38 (95.0) | 20 (50.0) | <0.001 |
| EBA-175 | 27 (67.5) | 31 (77.5) | 0.32 | 28 (70.0) | 31 (77.5) | 0.45 |
| MSP-1 | 29 (72.5) | 26 (65.0) | 0.47 | 31 (77.5) | 30 (75.0) | 0.79 |
DISCUSSION
In areas of Africa with stable, high-level transmission, clinical immunity to malaria develops by the age of 3 to 5 years (30), with infrequent episodes of mild malaria and very few episodes of severe malaria occurring after this age. In highland areas of Africa where malaria transmission is low and stable, older children and adults continue to be at a significant risk for clinical malaria, although the risk is somewhat lower than that for young children (13). Our earlier studies documented that the presence of high-level IgG antibodies to three preerythrocytic-stage antigens, CSP, LSA-1, and TRAP, correlates with protection from P. falciparum infection in adults (15) and clinical malaria in children (19) and that the majority of this protection is associated with high-level IgG antibodies to both CSP and LSA-1. In the present study, we document that only 3.3% of those individuals living in an area of unstable malaria transmission had high-level IgG antibodies to both CSP and LSA-1, compared to 44.3% of those from an area with stable malaria transmission. Taken together, these findings suggest that the persistent risk of clinical malaria in older children and adults in areas of unstable transmission may relate in part to the absence of high-level IgG antibodies to CSP and LSA-1.
The immune correlates of protection from malaria that develop with age in areas of stable transmission are still incompletely characterized, but several studies demonstrated a correlation with protection from clinical malaria for IgG antibodies to antigens assessed in this study, including CSP and LSA-1 (19), TRAP (29), AMA-1 (24, 26), and MSP-1 (5, 9, 27), as well as antigens not tested in the present study, including GLURP (24), MSP-2 (2, 31), and MSP-3 (28). Those studies confirm an age-dependent acquisition of antimalaria antibodies associated with cumulative exposure in high-transmission areas. However, comparable data are sparse for people residing in areas of unstable, epidemic-prone malaria transmission. Two studies from Tanzania by Drakeley et al. and Tongren et al. assessed frequencies and levels of IgG antibodies to MSP-119 (8) and IgG1 and IgG3 antibodies to MSP-119, MSP-2, AMA-1, and glycosylphosphatidylinositol (32) across a wide gradient of ages and transmission intensities. Those studies included areas of very low, unstable transmission. Those studies suggested that IgG antibodies to MSP-119 can differentiate between short- and long-term trends in malaria transmission; that antibodies to MSP-119, once acquired, are very long-lived; and that antibody isotypes for the antigens tested relate to age and transmission intensity (8, 32).
The present study findings contrast with the Tanzanian study findings in important ways. The present study documents much higher frequencies of antibodies to MSP-119 in the area of unstable transmission than were documented by Drakeley et al. in areas with similar parasite prevalence (8), particularly in individuals aged 15 to 40 years. Since the antibody testing methods used were similar, differences might be due to the different expression vectors used for recombinant forms of MSP-119 in the two studies (S. cerevisiae in the present study and E. coli in the study by Drakeley et al.) (8) and the effects of these differing expression vectors on antigen conformation. Differences in the antigenic variants of MSP-119 tested are not likely to explain the differences in antibody frequency, as we previously documented that levels of IgG antibody to the four major variants of MSP-119 are highly correlated in both populations (C. C. John, unpublished data). The present study suggests that the prevalence of antibody to MSP-119 may be higher than previously estimated for sites of very low transmission. This finding will need to be confirmed in future studies. The present study also assessed antibodies to both preerythrocytic and blood-stage antigens, while the Tanzanian studies assessed only antibodies to blood-stage antigens. The key finding of the present study, that differences in levels of antibodies to preerythrocytic (CSP, LSA-1, and TRAP) or preerythrocytic/blood-stage (AMA-1) antigens between areas of differing transmission intensity were far more pronounced than were those to the blood-stage antigens MSP-119 and EBA-175, has not previously been reported. Further studies are required to document if these differences are antigen specific or are seen with many or all preerythrocytic compared to blood-stage antigens.
The present study results suggest that repeated, frequent infection appears to be the major determinant of high-level IgG antibodies to the preerythrocytic antigens and AMA-1 but that high-level IgG antibodies to the blood-stage antigens EBA-175 and MSP-1 may develop over time even with infrequent exposure. Additional evidence to support this contention is provided from previously reported studies by our group demonstrating that limited annual exposure did not lead to an increase in anti-CSP antibodies in an area of unstable transmission (20) and that high-level antibodies to LSA-1 were induced in young children in an area of high-level transmission (33) but were infrequent in children and adults in an area of unstable transmission (18).
The prior association of IgG antibodies to CSP and LSA-1 with protection from P. falciparum infection and disease in our stable transmission study site (15, 19) and the striking paucity of these high-level antibodies in the area of unstable transmission, even in adults, suggest that these responses may be important in protection from infection and disease. The success of the CSP-based RTS,S vaccine in inducing protection from clinical malaria due to P. falciparum in 35% of children over an 18-month period (1) supports the idea that an immune response to CSP is important for the development of clinical immunity to malaria. Indeed, studies of RTS,S in nonimmune volunteers (22) as well as immune individuals (4) documented that vaccine-inducted protection was associated with higher levels of antibodies to CSP. Similar patterns for the development of IgG antibodies to TRAP and AMA-1 coupled with previous studies documenting an association of these antibodies with protection from disease (24, 26, 29) suggest that lower levels of antibodies to these antigens in the area of unstable transmission could be part of the reason for impaired clinical immunity in adults in this area.
Although IgG antibody frequencies and levels were similar for MSP-119 and EBA-175 in adults in the stable and unstable transmission areas, it is still possible that antibodies to blood-stage antigens provide a degree of protection to adults in areas of unstable transmission but that the much greater degree of protection seen in adults in areas of stable transmission is due to protection associated with immunity to preerythrocytic and blood-stage antigens. Alternatively, it may be that the assessment of the functional activity of antibody to blood-stage antigens is a better marker for protection than antibody level. Our earlier studies documented that invasion-inhibitory antibodies to MSP-119 were associated with protection from P. falciparum infection in a different highland area (17), supporting the role of functional antibodies to blood-stage antigens in protection from infection. We are in the process of assessing the relationship of antibody levels and functional antibodies to the risk of clinical (symptomatic) malaria in this study area. Antibody subclasses may also be important for determining protection from clinical malaria, and we are currently assessing IgG subclasses in a subset of this population.
Study limitations include the number of study sites (two), which did not allow an assessment of antibodies across a full transmission gradient, and the cross-sectional study design, which does not allow an assessment of relationships between antibodies and malaria risk in the study populations. Furthermore, even in a longitudinal risk assessment study, the extreme paucity of individuals with high-level antibodies to CSP and LSA-1 (3.3%) in the area of unstable transmission would have precluded any estimates of risk association without a much larger sample size. For this reason, we can only indirectly infer that a lack of high-level antibodies to CSP and LSA-1 may be associated with an increased risk in this population based on our earlier findings for the population of stable transmission. Ongoing longitudinal studies with a larger sample size aim to assess the association between antibodies to multiple preerythrocytic and blood-stage antigens with protection from disease. Finally, the lack of complete randomization in recruitment may have led to a selection bias for individuals with recent malaria exposure in the two areas. Thus, we cannot exclude the possibility that the individuals studied may have higher levels of antibody to the antigens studied than the general population. If this were the case, it might in part explain the higher frequency of antibodies to MSP-119 seen in this study than what was seen in the study described previously by Drakeley et al. (8), but it also suggests that antibodies to CSP and LSA-1 in the general population were even lower than documented in this study.
In conclusion, the present study demonstrates that antibody levels and frequencies in areas of differing transmission vary according to antigen. The study specifically demonstrates low frequencies of high-level antibodies to preerythrocytic (CSP, LSA-1, and TRAP) or preerythrocytic/blood-stage (AMA-1) antigens in an area of unstable, very low transmission but similar frequencies of high-level antibodies to the blood-stage antigens MSP-1 and EBA-175 in very-low- and very-high-transmission settings. In light of the protection associated with high-level antibodies to CSP and LSA-1 in areas of stable transmission, it is possible that the persistent risk of clinical malaria in older children and adults in areas of unstable transmission may be due in part to a lack of these antibodies. However, further studies are required to evaluate the association of antibodies to preerythrocytic and blood-stage antigens with malaria risk in populations of unstable transmission and to elucidate the mechanisms underlying the differences in frequencies of antibody to these antigens in areas of differing transmission intensities.
Acknowledgments
This study was supported by grants from the National Institutes of Allergy and Infectious Diseases, AI01572 and AI056270 (C.C.J.) and AI43906 (J.W.K.).
We thank the study participants for their involvement in this study. We thank David Koech, Jackson Abuya, and Livingstone Wanyama for their work in the field collection of these samples and microscopy testing. The sample collection, field work, and initial testing for this study were performed while C.C.J. was at Case Western Reserve University.
This work is published with the permission of the Office of the Director of the Kenya Medical Research Institute.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 3662012-2018. [DOI] [PubMed] [Google Scholar]
- 2.al-Yaman, F., B. Genton, R. F. Anders, M. Falk, T. Triglia, D. Lewis, J. Hii, H. P. Beck, and M. P. Alpers. 1994. Relationship between humoral response to Plasmodium falciparum merozoite surface antigen-2 and malaria morbidity in a highly endemic area of Papua New Guinea. Am. J. Trop. Med. Hyg. 51593-602. [DOI] [PubMed] [Google Scholar]
- 3.Beier, J. C., P. V. Perkins, F. K. Onyango, T. P. Gargan, C. N. Oster, R. E. Whitmire, D. K. Koech, and C. R. Roberts. 1990. Characterization of malaria transmission by Anopheles (Diptera: Culicidae) in western Kenya in preparation for malaria vaccine trials. J. Med. Entomol. 27570-577. [DOI] [PubMed] [Google Scholar]
- 4.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 3581927-1934. [DOI] [PubMed] [Google Scholar]
- 5.Branch, O. H., V. Udhayakumar, A. W. Hightower, A. J. Oloo, W. A. Hawley, B. L. Nahlen, P. B. Bloland, D. C. Kaslow, and A. A. Lal. 1998. A longitudinal investigation of IgG and IgM antibody responses to the merozoite surface protein-1 19-kiloDalton domain of Plasmodium falciparum in pregnant women and infants: associations with febrile illness, parasitemia, and anemia. Am. J. Trop. Med. Hyg. 58211-219. [DOI] [PubMed] [Google Scholar]
- 6.Chelimo, K., A. V. Ofulla, D. L. Narum, J. W. Kazura, D. E. Lanar, and C. C. John. 2005. Antibodies to Plasmodium falciparum antigens vary by age and antigen in children in a malaria-holoendemic area of Kenya. Pediatr. Infect. Dis. J. 24680-684. [DOI] [PubMed] [Google Scholar]
- 7.Chougnet, C., J. P. Lepers, P. Astagneau, M. D. Rason, J. Savel, and P. Deloron. 1991. Lymphoproliferative responses to synthetic peptides from merozoite ring-infected erythrocyte surface antigen and circumsporozoite protein: a longitudinal study during a falciparum malaria episode. Am. J. Trop. Med. Hyg. 45560-566. [DOI] [PubMed] [Google Scholar]
- 8.Drakeley, C. J., P. H. Corran, P. G. Coleman, J. E. Tongren, S. L. McDonald, I. Carneiro, R. Malima, J. Lusingu, A. Manjurano, W. M. Nkya, M. M. Lemnge, J. Cox, H. Reyburn, and E. M. Riley. 2005. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc. Natl. Acad. Sci. USA 1025108-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173765-769. [DOI] [PubMed] [Google Scholar]
- 10.Ernst, K. C., S. O. Adoka, D. O. Kowuor, M. L. Wilson, and C. C. John. 2006. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar. J. 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidock, D. A., H. Gras-Masse, J. P. Lepers, K. Brahimi, L. Benmohamed, S. Mellouk, C. Guerin-Marchand, A. Londono, L. Raharimalala, and J. F. Meis. 1994. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J. Immunol. 153190-204. [PubMed] [Google Scholar]
- 12.Fruh, K., O. Doumbo, H. M. Muller, O. Koita, J. McBride, A. Crisanti, Y. Toure, and H. Bujard. 1991. Human antibody response to the major merozoite surface antigen of Plasmodium falciparum is strain specific and short-lived. Infect. Immun. 591319-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay, S. I., A. M. Noor, M. Simba, M. Busolo, H. L. Guyatt, S. A. Ochola, and R. W. Snow. 2002. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg. Infect. Dis. 8543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John, C. C., M. M. McHugh, A. M. Moormann, P. O. Sumba, and A. V. Ofulla. 2005. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Trans. R. Soc. Trop. Med. Hyg. 99780-786. [DOI] [PubMed] [Google Scholar]
- 15.John, C. C., A. M. Moormann, D. C. Pregibon, P. O. Sumba, M. M. McHugh, D. L. Narum, D. E. Lanar, M. D. Schluchter, and J. W. Kazura. 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am. J. Trop. Med. Hyg. 73222-228. [PubMed] [Google Scholar]
- 16.John, C. C., A. M. Moormann, P. O. Sumba, A. V. Ofulla, D. C. Pregibon, and J. W. Kazura. 2004. Gamma interferon responses to Plasmodium falciparum liver-stage antigen 1 and thrombospondin-related adhesive protein and their relationship to age, transmission intensity, and protection against malaria. Infect. Immun. 725135-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John, C. C., R. A. O'Donnell, P. O. Sumba, A. M. Moormann, T. F. de Koning-Ward, C. L. King, J. W. Kazura, and B. S. Crabb. 2004. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-1 19) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J. Immunol. 173666-672. [DOI] [PubMed] [Google Scholar]
- 18.John, C. C., J. H. Ouma, P. O. Sumba, M. R. Hollingdale, J. W. Kazura, and C. L. King. 2002. Lymphocyte proliferation and antibody responses to Plasmodium falciparum liver-stage antigen-1 in a highland area of Kenya with seasonal variation in malaria transmission. Am. J. Trop. Med. Hyg. 66372-378. [DOI] [PubMed] [Google Scholar]
- 19.John, C. C., A. J. Tande, A. M. Moormann, P. O. Sumba, D. E. Lanar, X. M. Min, and J. W. Kazura. 2008. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J. Infect. Dis. 197519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John, C. C., J. S. Zickafoose, P. O. Sumba, C. L. King, and J. W. Kazura. 2003. Antibodies to the Plasmodium falciparum antigens circumsporozoite protein, thrombospondin-related adhesive protein, and liver-stage antigen 1 vary by ages of subjects and by season in a highland area of Kenya. Infect. Immun. 714320-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaslow, D. C., G. Hui, and S. Kumar. 1994. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP1(19)) variants secreted from Saccharomyces cerevisiae. Mol. Biochem. Parasitol. 63283-289. [DOI] [PubMed] [Google Scholar]
- 22.Kester, K. E., J. F. Cummings, C. F. Ockenhouse, R. Nielsen, B. T. Hall, D. M. Gordon, R. J. Schwenk, U. Krzych, C. A. Holland, G. Richmond, M. G. Dowler, J. Williams, R. A. Wirtz, N. Tornieporth, L. Vigneron, M. Delchambre, M. A. Demoitie, W. R. Ballou, J. Cohen, and D. G. Heppner, Jr. 2008. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine 262191-2202. [DOI] [PubMed] [Google Scholar]
- 23.Migot-Nabias, F., P. Deloron, P. Ringwald, B. Dubois, J. Mayombo, T. N. Minh, N. Fievet, P. Millet, and A. Luty. 2000. Immune response to Plasmodium falciparum liver stage antigen-1: geographical variations within Central Africa and their relationship with protection from clinical malaria. Trans. R. Soc. Trop. Med. Hyg. 94557-562. [DOI] [PubMed] [Google Scholar]
- 24.Nebie, I., A. Diarra, A. Ouedraogo, I. Soulama, E. C. Bougouma, A. B. Tiono, A. T. Konate, R. Chilengi, M. Theisen, D. Dodoo, E. Remarque, S. Bosomprah, P. Milligan, and S. B. Sirima. 2008. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect. Immun. 76759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okenu, D. M., E. M. Riley, Q. D. Bickle, P. U. Agomo, A. Barbosa, J. R. Daugherty, D. E. Lanar, and D. J. Conway. 2000. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect. Immun. 685559-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osier, F. H., G. Fegan, S. D. Polley, L. Murungi, F. Verra, K. K. Tetteh, B. Lowe, T. Mwangi, P. C. Bull, A. W. Thomas, D. R. Cavanagh, J. S. McBride, D. E. Lanar, M. J. Mackinnon, D. J. Conway, and K. Marsh. 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun. 762240-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley, E. M., S. J. Allen, J. G. Wheeler, M. J. Blackman, S. Bennett, B. Takacs, H. J. Schonfeld, A. A. Holder, and B. M. Greenwood. 1992. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 14321-337. [DOI] [PubMed] [Google Scholar]
- 28.Roussilhon, C., C. Oeuvray, C. Muller-Graf, A. Tall, C. Rogier, J. F. Trape, M. Theisen, A. Balde, J. L. Perignon, and P. Druilhe. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 4e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarselli, E., R. Tolle, O. Koita, M. Diallo, H. M. Muller, K. Fruh, O. Doumbo, A. Crisanti, and H. Bujard. 1993. Analysis of the human antibody response to thrombospondin-related anonymous protein of Plasmodium falciparum. Infect. Immun. 613490-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snow, R. W., J. A. Omumbo, B. Lowe, C. S. Molyneux, J. O. Obiero, A. Palmer, M. W. Weber, M. Pinder, B. Nahlen, C. Obonyo, C. Newbold, S. Gupta, and K. Marsh. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 3491650-1654. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, R. R., S. J. Allen, B. M. Greenwood, and E. M. Riley. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58406-413. [DOI] [PubMed] [Google Scholar]
- 32.Tongren, J. E., C. J. Drakeley, S. L. McDonald, H. G. Reyburn, A. Manjurano, W. M. Nkya, M. M. Lemnge, C. D. Gowda, J. E. Todd, P. H. Corran, and E. M. Riley. 2006. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect. Immun. 74257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou, Z., L. Xiao, O. H. Branch, S. Kariuki, B. L. Nahlen, and A. A. Lal. 2002. Antibody responses to repetitive epitopes of the circumsporozoite protein, liver stage antigen-1, and merozoite surface protein-2 in infants residing in a Plasmodium falciparum-hyperendemic area of western Kenya. XIII. Asembo Bay Cohort Project. Am. J. Trop. Med. Hyg. 667-12. [DOI] [PubMed] [Google Scholar]