Abstract
Non-sorbitol-fermenting (NSF) Escherichia coli O157:H7 is the primary Shiga toxin-producing E. coli (STEC) serotype associated with human infection. Since 1988, sorbitol-fermenting (SF) STEC O157:NM strains have emerged and have been associated with a higher incidence of progression to hemolytic-uremic syndrome (HUS) than NSF STEC O157:H7. This study investigated bacterial factors that may account for the increased pathogenic potential of SF STEC O157:NM. While no evidence of toxin or toxin expression differences between the two O157 groups was found, the SF STEC O157:NM strains adhered at significantly higher levels to a human colonic cell line. Under the conditions tested, curli were shown to be the main factor responsible for the increased adherence to Caco-2 cells. Notably, 52 of 66 (79%) European SF STEC O157:NM strains tested bound Congo red at 37οC and this correlated with curli expression. In a subset of strains, curli expression was due to increased expression from the csgBAC promoter that was not always a consequence of increased csgD expression. The capacity of SF STEC O157:NM strains to express curli at 37οC may have relevance to the epidemiology of human infections as curliated strains could promote higher levels of colonization and inflammation in the human intestine. In turn, this could lead to increased toxin exposure and an increased likelihood of progression to HUS.
Shiga toxin-producing Escherichia coli (STEC) strains of serogroup O157 are associated with human disease, including diarrhea, hemorrhagic colitis (HC), and hemolytic-uremic syndrome (HUS). While non-sorbitol-fermenting (NSF) STEC O157:H7 strains are important human pathogens worldwide (37), sorbitol-fermenting (SF) STEC O157:NM strains have emerged as important pathogens in continental Europe (28). SF STEC O157:NM strains were first recognized causing HUS in children in Bavaria, Germany, in 1988 (28), and since then, these strains have been isolated from patients throughout Germany (20, 29), including a further outbreak in Bavaria that resulted in 28 cases of HUS and three fatalities (2). The first isolation of SF STEC O157:NM strains outside Germany occurred in the Czech Republic in 1995 (7). There have since been reports of this pathogen causing diarrhea or HUS in Hungary (28), Finland (30), another region of the Czech Republic (8), Austria (41), and Sweden (S. Löfdahl, personal communication). Infection with SF STEC O157:NM was not reported outside continental Europe until 2002, when it was isolated in Australia (4) and Scotland (1). It was subsequently isolated from patients in England (24) and Ireland (18). Between April and May 2006, 18 cases of SF STEC O157:NM infection were identified in Scotland, 13 of which were associated with a nursery (13). A further two cases of SF STEC O157:NM infection were identified in August 2006.
There is some evidence to suggest that SF STEC O157:NM strains are more frequently associated with HUS than NSF STEC O157:H7 strains. For example, in Germany the probability of development of HUS after infection with SF STEC O157:NM is 1:2, whereas after infection with NSF STEC O157:H7, this ratio is 1:6 (M. Bielaszewska and H. Karch, personal communication). A similar situation appears to be the case in Scotland, where 10 out of 20 cases of SF STEC O157:NM infection identified between April and August 2006 progressed to HUS. While host susceptibility between outbreaks may account for such differences, it is also possible that SF STEC O157:NM strains are more virulent than NSF STEC O157:H7 strains. Shiga toxins are responsible for the serious consequences of STEC O157 infection, including HC and HUS. Increased toxicity could be accounted for by (i) a more potent toxin variant, (ii) increased toxin expression, or (iii) colonization of the human intestine at higher levels leading to a greater exposure to toxin. Recently published work has indicated that there are no significant differences in the Stx2 proteins encoded by SF STEC O157:NM and NSF STEC O157:H7 strains (6), and a report indicates that the toxicity levels may be similar (20).
In terms of intestinal colonization, there are multiple factors that may contribute to STEC O157 adherence. Both SF and NSF STEC O157 strains possess a type III secretion system that is responsible for intimate attachment and attaching and effacing lesion formation. In contrast to NSF STEC O157:H7, SF STEC O157:NM strains contain a complete efa1 gene (26), which encodes the enterohemorrhagic E. coli factor for adherence (Efa1) (39). Attachment may also be mediated by surface-expressed appendages such as fimbriae. SF STEC O157:NM uniquely possess the sfp gene cluster which encodes novel Sfp fimbriae (SF enterohemorrhagic E. coli O157 fimbriae, plasmid encoded) (9). At least 16 putative fimbrial operons have been identified in NSF STEC O157:H7 (22, 36, 44), including the well-characterized adhesins type 1 fimbriae and curli. While the function (25) or capacity to express many of these fimbrial adhesins appears to have been lost by NSF STEC O157:H7 strains (36), SF STEC O157:NM strains may be different.
The objective of this study was to compare both phenotypic and genotypic characteristics of SF STEC O157:NM and NSF STEC O157:H7 strains isolated from cases of infection in Scotland. In particular, the research addressed whether SF STEC O157:NM strains could be considered more virulent as a result of increased toxin expression or enhanced colonization potential.
MATERIALS AND METHODS
Bacterial strains.
The STEC O157 strains used in this study are detailed in Table 1. Wild-type SF STEC O157:NM strains H8824, H8432, H8489, H8757, and H8478 were isolated during the 2006 Scottish outbreak cluster and shared an indistinguishable pulsed-field gel electrophoresis profile. A single SF STEC O157:NM isolate (H2687) was obtained from an isolated case in Scotland (35) and was associated with bloody diarrhea. Four NSF STEC O157:H7 strains were included for comparison. Three strains (H77, H511, and 1477/AI) were isolated from separate Scottish outbreaks (11, 42, 47), and the remaining NSF STEC O157:H7 isolate was the sequenced strain EDL933 (44). Two representative SF STEC O157:NM strains (H2687 and H8824) and two NSF STEC O157:H7 strains (EDL933 and 1477/AI) were analyzed in the phenotypic assays. A further 66 SF STEC O157:NM strains, isolated in Europe, were also analyzed for their potential to express curli.
TABLE 1.
STEC O157 strains used in this study
| Serotype | Strain(s) | Country of origin | Source or reference |
|---|---|---|---|
| NSF STEC O157:H7 | EDL933 | United States | 44 |
| H77, H511, 1477/AI | Scotland | 11, 42, 47 | |
| SF STEC O157:NM | H2687 | Scotland | 35 |
| H8824, H8432, H8489, H8757, H8478 | Scotland | 2006 Scottish outbreak cluster | |
| 493/89, 2260/96, 3072/96, 340/97, 1527/97, 3873/98, 4368/98, 0355/99, 2696/99, DN940/00, 2518/00, 3317/00, 080/01, 164/01, 689/01, E02/16, E02/115, E02/129, E02/149, E02/172, E02/207, E02/456, E02/679, E02/774, E02/793, E02/809, E02/813, E02/817, E02/819, E02/829, E02/848, E02/852, E02/870, E02/878, E02/879, E03/1, E03/23, E03/49, E03/53, E03/55, E03/71, E03/86, E03/88, E03/100, E03/104, E03/106, E03/108, E03/198, E03/200, E04/1278, E05/15, E05/18, E05/22, E05/114, E06/443, E06/449, E06/481, E06/484, E06/486, E06/494, E06/537, E07/50, E07/59, E07/64, E07/65, E07/67 | Germany | H. Karch, Muenster University, Germany |
Characterization PCRs.
Multiplex PCR for the detection of genes encoding Shiga toxin 1 (stx1), Shiga toxin 2 (stx2), intimin (eae), and enterohemolysin (hlyA) was performed as described previously (43). The gene encoding the flagellin subunit (fliC) was detected and characterized using the fliC restriction fragment length polymorphism method (16). PCR to detect the presence of the 16-bp deletion in the fim switch that controls type 1 fimbriae expression was performed as previously described (34).
Nucleotide sequencing.
Nucleotide sequencing was performed using an ABI Prism BigDye terminator cycle sequencing kit, version 3.1 (Applied Biosystems), and reactions were analyzed on an ABI 3730 DNA sequencer. The nucleotide sequences of stx2 from the six Scottish SF STEC O157:NM strains were determined following PCR amplification of stxA2 and stxB2 using a Taq PCR core kit (Qiagen) and primer pairs stx2Aseq_F1 and stx2Aseq_R2 and stx2Bseq_F and stx2Bseq_R, respectively. (Table 2 details the primer sequences and cycling conditions for the PCRs.) The amplicons were purified and sequenced directly with the PCR primers and internal stxA2 primers stx2Aseq_F2 and stx2Aseq_R1 (Table 2). For sequence analysis of the region required for curli formation (csgBAC and csgDEFG operons and the csgB-to-csgD intergenic region), the entire region was amplified with five separate PCRs using iProof high-fidelity DNA polymerase (Bio-Rad) and primer pairs curli1F and curli1R, curli2F and curli2R, curli3F and curli3R, curli4F and curli4R, and curli5F and curli5R (Table 2). PCR products were purified and ligated into the StrataClone PCR cloning vector, pSC-B, with a StrataClone blunt PCR cloning kit (Stratagene); transformed following the manufacturer's instructions; and sequenced with primers T3 and T7.
TABLE 2.
Primers and PCR conditions used in this study
| Primer | Nucleotide sequence (5′→3′)a | PCR conditions (30 cycles)
|
Amplicon size (bp) | ||
|---|---|---|---|---|---|
| Denaturing | Annealing | Extension | |||
| stx2Aseq_F1 | TACCAGGCTCGCTTTTGCGG | 94°C, 30 s | 60°C, 45 s | 72°C, 80 s | 1,110 |
| stx2Aseq_R2 | CGCCATTGCATTAACAGAAGC | ||||
| stx2Aseq_F2 | CCATGACAACGGACAGCAG | ||||
| stx2Aseq_R1 | CTGTATCTGCCTGAAGCGTAAGGC | ||||
| stx2Bseq_F | CCAGAATGTCAGATAACTGGC | 94°C, 30 s | 58°C, 45 s | 72°C, 30 s | 476 |
| stx2Bseq_R | GGCAACTGTCAACTGACTG | ||||
| curli1F | CGCTTAAACAGTAAATGCCG | 98°C, 10 s | 60°C, 30 s | 72°C, 35 s | 1,046 |
| curli1R | CCGCATGGTGACCAACGA | ||||
| curli2F | TTCCTTATGAAGCTGGGGC | 98°C, 10 s | 60°C, 30 s | 72°C, 35 s | 1,054 |
| curli2R | CGGAATCAGCCCTCCTTAC | ||||
| curli3F | CGCTGATGAACAACGAACG | 98°C, 10 s | 60°C, 30 s | 72°C, 35 s | 1,028 |
| curli3R | CCCGTCGCTGATTGCTGC | ||||
| curli4F | CTCCACACCACCGTGGAC | 98°C, 10 s | 60°C, 30 s | 72°C, 35 s | 1,047 |
| curli4R | GCTTGCAGAGCAAGTGCAG | ||||
| curli5F | GGCAGGTGTTGTTCCTCAG | 98°C, 10 s | 60°C, 30 s | 72°C, 35 s | 886 |
| curli5R | CCTTGAGGGTTGTGTTATCC | ||||
| csgBACprom_F | cgcggatccGTTGTACATTTGGTTTTTATTGCAC | 94°C, 15 s | 60°C, 30 s | 72°C, 60 s | 524 |
| csgBACprom_R | cggggtaccCAATTTGTTTTTCATGTTGTCACC | ||||
| F1upCsgBA | aagagctcGCACACCTGACAGCTGCC | 94°C, 30 s | 60°C, 30 s | 72°C, 60 s | 739 |
| F1downCsgBA | aaggatccTCACCCTGGACCTGGTCG | ||||
| F2upCsgBA | aaggatccCGCGACCGCTCATCAGTAC | 94°C, 30 s | 60°C, 30 s | 72°C, 60 s | 960 |
| F2downCsgBA | aactgcagTTCATAACGCCTCCTTACACC | ||||
| CsgBAC1 | aaggatccGTTGCGTTAACAACCAAGTTG | 94°C, 30 s | 60°C, 30 s | 72°C, 90 s | 1,016 |
| CsgBAC2 | aaggtaccGCTGATTCGCAGCAGACCA | ||||
The lowercase portions of some sequences are nonmatching, and the underlined bases show the incorporation of restriction sites.
Vero cell cytotoxicity assay.
Bacterial strains were cultured in minimal essential medium-HEPES, supplemented with glucose (final concentration of 0.2%) and 0.25 μM Fe(NO3)3, at 37°C at 200 rpm for 24 h. Mitomycin C (0.5 μg/ml) was added when required to cultures at an optical density at 600 nm (OD600) of 0.3. Bacterial cultures were centrifuged (4,000 × g for 20 min), and supernatants were filter sterilized through low-protein-binding 0.22-μm filters. Culture filtrates were added to Vero cells (seeded in 96-well plates), and the cytotoxicity assay was performed at 37°C in 5% CO2 for 72 h. Following the 72-h incubation, Vero cells were washed with phosphate-buffered saline (PBS) and fixed with 2% formalin in PBS. Quantification of viable Vero cells was determined by staining with crystal violet solution (0.13% crystal violet, 0.065% phenol, 5% ethanol, 2% formaldehyde in PBS) for 1 h. Plates were rinsed with distilled water and dried. Crystal violet was released from the cells with 10% acetic acid. Absorbance measurements were taken at 590 nm.
Adherence assays to Caco-2 cells.
Caco-2 cells were seeded into eight-chamber microscope slides at a density of 1 × 105 cells/well and incubated at 37°C in 5% CO2 for 48 h prior to adherence assays. Bacteria were cultured for 24 h at 37°C on CFA agar plates (1% Casamino Acids, 0.15% yeast extract, 0.005% MgSO4, 0.0005% MnCl2, 1.5% agar) and in CDMT broth [13 mM K2HPO4, 6 mM KH2PO4, 8 mM (NH4)2SO4, 2 mM sodium citrate, 0.4 mM MgSO4, 0.2% Casamino Acids, 0.2% glucose, 5 μM CaCl2, 0.01% tryptone], which have both been used previously to promote fimbrial expression (9, 15). Bacteria were harvested from CFA plates in PBS. Bacterial cultures were diluted to an OD600 of 0.5, and 300 μl was added to the Caco-2 cells. Adherence assays were performed at 37°C in 5% CO2 for 2 h. This time point was chosen because we wanted to study initial attachment and avoid the complication of adherence due to induction of type III secretion. The bacterial suspension was removed, and the cells were washed with PBS three times prior to fixation with 4% paraformaldehyde. Bacteria attached to cells were labeled by indirect immunofluorescence using rabbit anti-O157 antisera (1:100 [Mast Assure]) and Alexa Fluor 568 secondary antibody (1:1,000 [Molecular Probes]). Slides were examined by fluorescence microscopy, and the number of bacteria per field was counted for randomly selected fields in each well for at least 20 fields. Images were captured with Leica software.
Biofilm assays.
Bacteria were cultured statically overnight at 28°C in LB, CFA, and CDMT broths. Cultures were diluted 1:100 in fresh medium, and 200 μl/well was added to 96-well polystyrene plates. Plates were incubated at 28°C for 24 h and 48 h. Bacterial adherence to microtiter well surfaces was determined by crystal violet staining, as described earlier, except that washes were done with PBS. Sterile medium was included as a control, and the A590 was subtracted from the other values.
Yeast cell agglutination.
A single colony of the strain to be tested was inoculated into 5 ml LB broth and incubated statically at 37°C for 24 h. The culture was subcultured into a further 5 ml LB broth and incubated statically for another 24 h. Subculturing was repeated once more. Yeast cell agglutination was carried out by mixing 15 μl bacterial culture with an equal volume of baker's yeast suspension (10 mg/ml) on glass slides, and the degree of clumping was assessed. Mannose inhibition of agglutination was confirmed using 3% α-d-mannose in the yeast suspension.
fim switch orientation assay.
Orientation of the fim switch was carried out as described previously (23, 33, 45). Briefly, PCR was used to amplify a 603-bp region that incorporates the invertible fimA promoter element. Template DNA for the PCR was prepared by adding 50 μl of bacterial culture (following 3 days of consecutive subculture in LB broth) or a colony from cultures grown on CFA agar to 50 μl MilliQ H2O and boiling at 100°C for 15 min. The PCR-amplified product was digested asymmetrically with HinfI, and the fragments were separated on a 4% polyacrylamide Tris-borate-EDTA gel. Fragments were visualized after staining with ethidium bromide.
Immunostaining of surface curli.
Bacteria were cultured on CFA agar and in CFA and CDMT broths. Bacterial cultures were fixed in 4% paraformaldehyde. Curli fimbriae were labeled using mouse anti-SEF17 monoclonal antibody (1:100) and fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin secondary antibody (1:500 [Sigma-Aldrich]). Slides were examined by fluorescence microscopy, and the images were captured with Leica software.
Construction and measurement of plasmid-based promoter-GFP fusions.
The promoter for csgBAC (curli main subunit) was amplified by PCR from strain H8824 using primer pair csgBACprom_F and csgBACprom_R (Table 2). The promoter was cloned in frame to gfp in pAJR70 (46), creating translational fusions. The promoter for csgD (positive transcriptional regulator of the csgBAC operon) cloned into pAJR70 was constructed previously (36). The fusions were transformed into the appropriate STEC O157 background, and single transformants were cultured on CFA agar and in CDMT broth (both containing 25 μl ml−1 chloramphenicol [CAM]). Bacteria were harvested from CFA-CAM agar plates in CFA-CAM broth. Total green fluorescent protein (GFP) produced by the population was determined by analyzing 100-μl aliquots of culture (OD600 of 0.5) in a FLUOstar Optima fluorimeter. Promoterless plasmid pAJR70 in each strain background acted as a control for background fluorescence and was subtracted from the other values.
Inhibition adherence assays.
Bacteria were cultured on CFA agar as described previously and prior to adding bacteria to Caco-2 cells, aliquots of bacterial cultures were incubated in the presence or absence of anticurli monoclonal antibody (1:100). Adherence to Caco-2 cells and subsequent staining, visualization, and enumeration of results were carried out as described previously.
Detection of curli expression.
To screen for surface curli expression bacteria were cultured on CFA-Congo red (CR) indicator plates (0.01% CR) for 24 h at 37°C. Curliated bacteria are able to bind CR dye and stain red. The ability of selected strains to bind CR was also assessed quantitatively as described previously (17). Briefly, bacteria harvested from CFA agar plates were incubated in the presence of 100 μM CR at 37°C in an orbital shaker for 30 min. Bacterial suspensions were centrifuged (10,000 × g for 10 min) and the absorbance of free CR in the supernatant was measured at 490 nm.
Construction of a csgBA deletion and complementation.
The csgA and csgB genes were deleted from SF O157:NM strain H8824 by allelic exchange (45, 46). Briefly, regions flanking csgBA were amplified using primer pairs F1upCsgBA/F1downCsgBA and F2upCsgBA/F2downCsgBA (Table 2) and cloned into pIB307 (45, 46) to produce pTD06. A sacB kan cassette was then cloned between the two flanking regions to create pTD08. This was then used to replace csgBA following electrotransformation of H8824. The clean deletion was then produced by exchanging in the region from pTD06 into this intermediate strain to create ZAP1125. The csgBA deletion was verified by its resistance profile and PCR screening. To complement the deletion, the csgBAC genes were amplified from H8824 using primer pair CsgBAC1/CsgBAC2 (Table 2) and this fragment was cloned into pWSK29 (54) to make pTD10. csgBAC can be induced from the lac promoter of this plasmid using 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Following induction, pTD10 complemented the csgBA deletion strain for CR binding and curli expression (data not shown).
Statistical analysis.
For the initial Caco-2 adherence assays and curli inhibition assays, bacteria per field counts were log10 transformed and analyzed with mixed models using the REML (restricted maximum likelihood) directive, dropping nonsignificant terms for fixed effects. A difference between predicted means was considered significant if it was greater than twice the standard error of difference. For graphical representation, predicted means and confidence intervals were back-transformed. These statistical calculations were performed in Genstat, 10th ed. The Vero cell cytotoxicity and biofilm assay data were analyzed by analysis of variance in Genstat, 10th ed. Caco-2 adherence assays with the curli deletion mutant and the European SF STEC O157:NM strains and the promoter expression data were analyzed using the Student's t test in Excel.
Nucleotide sequence accession numbers.
Nucleotide sequences from this study have been deposited in the GenBank database under the accession numbers listed in Table 3.
TABLE 3.
Details and accession numbers of nucleotide sequences determined in this study
| Strain | Sequence description | Sequence length (bp) | Accession no. |
|---|---|---|---|
| H2687 | stx2 | 1,248 | EU526759 |
| H8824 | stx2 | 1,248 | EU526760 |
| H8432 | stx2 | 1,248 | EU526761 |
| H8489 | stx2 | 1,248 | EU526762 |
| H8757 | stx2 | 1,248 | EU526763 |
| H8478 | stx2 | 1,248 | EU526764 |
| 340/97 | csgBA and csgDEFG operons and csgB-to-csgD intergenic region | 4,544 | EU554557 |
| 080/01 | csgBA and csgDEFG operons and csgB-to-csgD intergenic region | 4,544 | EU554558 |
| E06/486 | csgBA and csgDEFG operons and csgB-to-csgD intergenic region | 4,544 | EU554559 |
| H8824 | csgBA and csgDEFG operons and csgB-to-csgD intergenic region | 4,544 | EU554560 |
RESULTS
Characterization of STEC O157 isolates.
The six SF STEC O157:NM strains isolated from infections in Scotland (Materials and Methods) were found to possess an H7 fliC restriction fragment length polymorphism pattern. Multiplex PCR for the detection of genes encoding Shiga toxins 1 and 2, intimin, and enterohemolysin demonstrated that the SF STEC O157:NM isolates were positive for stx2, eae, and hlyA and negative for stx1. The three NSF STEC O157:H7 isolates from Scottish outbreaks were also positive for stx2, eae, and hlyA and negative for stx1. STEC O157:H7 EDL933 was confirmed to be positive for stx1, stx2, eae, and hlyA.
Sequencing of stx2.
The nucleotide sequences of stx2 were determined for the six Scottish SF STEC O157:NM isolates. The sequences from the five isolates obtained from the 2006 Scottish cluster (with GenBank accession numbers detailed in Table 3) were identical and the same as those published for three previously characterized SF STEC O157:NM strains (6). Their A and B subunit genes differed from the published E. coli EDL933 sequence by seven nucleotides and one nucleotide, respectively, and encode a Stx2 protein which differed from Stx2 from EDL933 by a single amino acid residue in each of the subunits. The stx2 sequence from SF STEC O157:NM isolate H2687 (GenBank accession no. EU526759) differed from EDL933 by a single nucleotide in the A subunit, and the B subunits were identical. This SF STEC O157:NM isolate had a predicted protein sequence for Stx2 identical to that of EDL933.
Vero cell cytotoxicity assays.
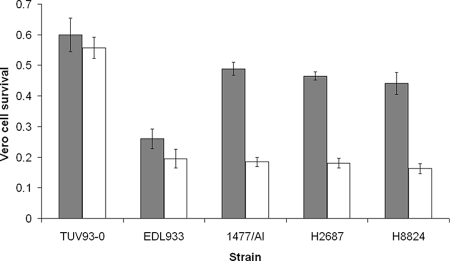
To determine if Stx2 expression levels could vary between the two STEC O157 groups, standard Vero cell cytotoxicity assays were carried out with culture filtrates of strains without and after induction with mitomycin C. A single SF STEC O157:NM isolate from the 2006 Scottish outbreak cluster (H8824) was analyzed along with SF STEC O157:NM strain H2687 isolated from a single case in 2003 and compared with NSF STEC O157:H7 strains EDL933 and 1477/AI (Fig. 1). One-way analysis of variance analysis of Vero cell survival following exposure to basal levels of toxin in cultures filtrates from the four STEC O157 uninduced cultures demonstrated significant variation (P < 0.001). The source of this variation is almost entirely due to EDL933, which has a different Shiga toxin background (Stx1 and Stx2) from the other three STEC O157 isolates (Stx2 alone). Induction with mitomycin C reduced Vero cell survival for all toxin-positive strains tested, but there was no significant difference in Vero cell survival rates between the four STEC O157 strains examined following induction (P = 0.723). E. coli O157:H7 TUV93-0, a toxin-negative derivative of EDL933, was included as a negative control. Therefore, under the in vitro conditions tested there was no evidence of increased toxin expression and activity from the SF STEC O157:NM isolates in comparison with the NSF STEC O157:H7 isolates.
FIG. 1.
Vero cell cytotoxicity of culture filtrates from NSF STEC O157:H7 strains (EDL933 and 1477/AI) and SF STEC O157:NM strains (H2687 and H8824) with (unshaded bars) and without (shaded bars) mitomycin C (0.5 μg/ml). TUV93-0, a VT-negative derivative of EDL933, serves as a negative control. Viable Vero cells, following 72 h of exposure to culture filtrates, were quantified by crystal violet staining and subsequent absorbance measurement of crystal violet in solution. Vero cell survival is expressed relative to untreated controls not exposed to bacterial culture filtrates. Error bars indicate standard errors of the means.
Adherence assays.
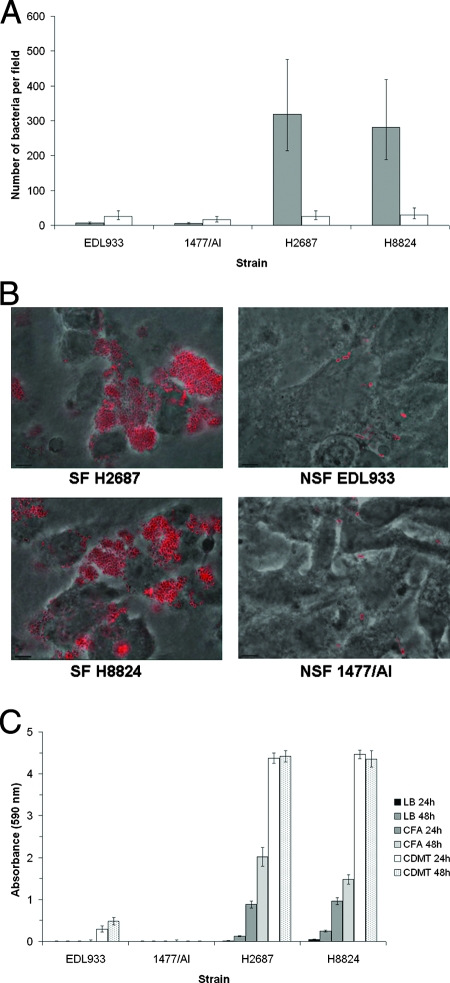
To investigate if there are differences in the capacities of the two O157 groups to adhere to human intestinal epithelial cells, NSF STEC O157:H7 strains EDL933 and 1477/AI and SF STEC O157:NM isolates H2687 and H8824, cultured under two different conditions, were tested for their capacity to bind to Caco-2 cells. Significantly higher levels of adherence (50-fold) were observed for the SF STEC O157:NM strains compared with the NSF STEC O157:H7 strains when cultured on CFA agar, whereas little difference was observed between the two groups when cultured in CDMT broth (Fig. 2A). Images showing the adherence of STEC O157 strains, cultured on CFA agar, to Caco-2 cells are provided in Fig. 2B. It was apparent that the SF STEC O157:NM strains, but not NSF STEC O157:H7 strains, autoaggregated following CFA culture, and as a result, biofilm formation was also tested. SF STEC O157:NM isolates demonstrated increased adherence to polystyrene microtiter plates in comparison with the NSF STEC O157:H7 isolates under the different conditions tested (Fig. 2C and data not shown). It was evident that culturing the two SF STEC O157:NM strains in CDMT broth promoted biofilm formation but did not increase adherence to Caco-2 cells. This indicates that the same combination of factors is not required for the two different adherence phenotypes.
FIG. 2.
Adherence of NSF STEC O157:H7 strains (EDL933 and 1477/AI) and SF STEC O157:NM strains (H2687 and H8824) cultured with different growth media. (A) Adherence to Caco-2 cells after 2 h of incubation at 37°C. The indicated strains were cultured on CFA agar (shaded bars) and in CDMT broth (unshaded bars). Microscopy was used to determine the number of bacteria per field for at least 20 fields. Data were log10 transformed and analyzed within mixed models using the REML directive. The graph represents back-transformed predicted means, and error bars define 95% confidence interval values. (B) Visualization of adherence to Caco-2 cells by immunofluorescence microscopy. The indicated strains were cultured on CFA agar at 37°C, and the micrographs show bacteria (stained red) adherent to Caco-2 cells after 2 h of incubation at 37°C. (C) Adherence to polystyrene microtiter plates after 24 h and 48 h of incubation at 28°C. Bacterial adherence was quantified by crystal violet staining of bacteria and subsequent absorbance measurement (590 nm) of released crystal violet. Background levels were determined for sterile media and were subtracted from the other values. Error bars indicate standard errors of the means.
Analysis of type 1 fimbria expression by NSF STEC O157:H7 and SF STEC O157:NM.
NSF E. coli O157:H7 strains have been shown to contain a 16-bp deletion in the fim switch that controls type 1 fimbria expression (34, 45), thus preventing inversion of the fim switch to the on orientation and the expression of type 1 fimbriae. A PCR designed to detect the presence of this deletion was used to analyze the two groups of STEC O157 strains. The SF STEC O157:NM strains did not contain the deletion (Fig. 3A, lanes 5 to 10), whereas the deletion was present in the NSF STEC O157:H7 strains tested (Fig. 3A, lanes 1 to 4). When the two groups of STEC O157 strains were cultured under conditions optimal for expression of type 1 fimbriae (3-day subculture in LB broth, statically at 37°C), only the SF STEC O157:NM strains exhibited mannose-sensitive agglutination of yeast cells and had the fim switch in both the off and on orientations (Fig. 3B). In comparison, when SF STEC O157:NM strains were cultured on CFA agar, as for the adherence assays, the majority of the bacterial population had the fim switch in the off orientation (Fig. 3C, lanes 3 and 4).
FIG. 3.
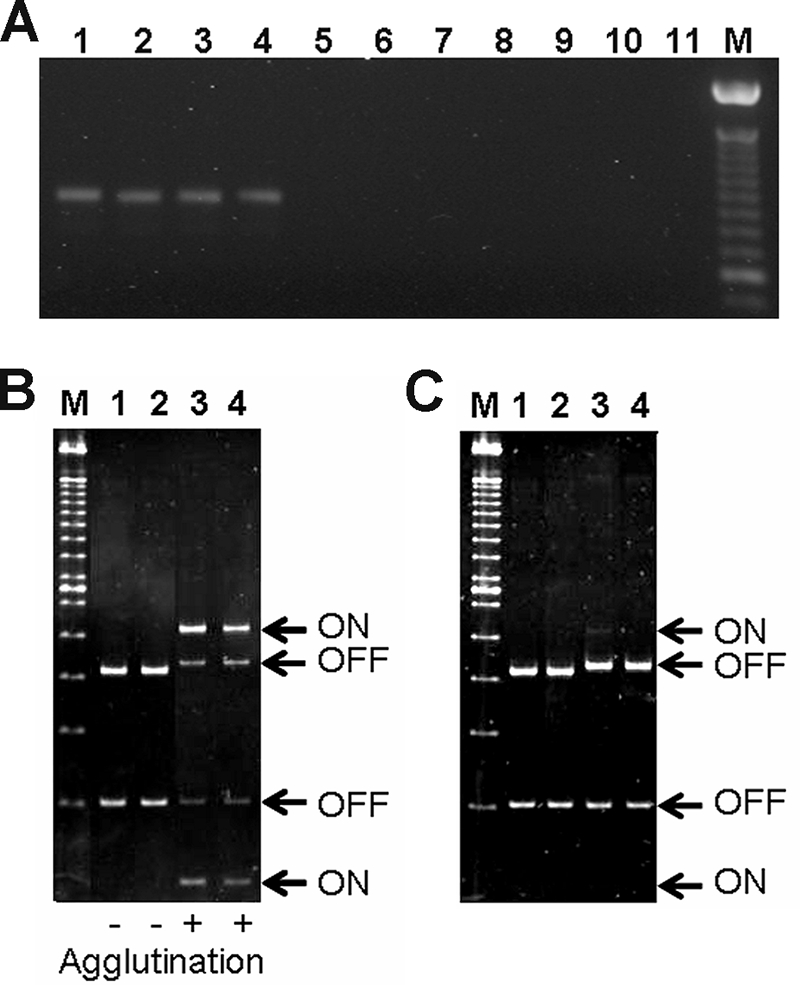
Analysis of type 1 fimbria expression by STEC O157 strains. (A) PCR analysis of the fim switch deletion using STEC O157:H7-specific primers. NSF STEC O157:H7 strains EDL933, H77, H511, and 1477/AI (lanes 1 to 4, respectively) generate a 943-bp product indicating the presence of the deletion that is absent in the SF STEC O157:NM strains H2687, H8824, H8432, H8489, H8757, and H8478 (lanes 5 to 10, respectively). Lane 11 contains a DNA-negative control, and lane M contains a 100-bp molecular size marker. (B and C) PCR and restriction digestion of the fim switch from isolates indicating the expression status within the bacterial population. (B) The isolates were cultured under conditions optimal for type 1 fimbria expression (3-day subculture in LB broth, statically at 37°C). The results of the yeast cell agglutination assay are summarized below the gel image. The fim switch was detected in both the on and off orientations for the two SF STEC O157:NM strains examined (H2687 and H8824, lanes 3 and 4, respectively) but only in the off orientation for the two NSF STEC O157:H7 strains analyzed (EDL933 and 1477/AI, lanes 1 and 2, respectively). (C) When cultured on CFA agar plates, the fim switch in the on orientation was only detected in one SF STEC O157:NM strain (H2687, lane 3) but not H8824 (lane 4) or the two NSF STEC O157:H7 strains (EDL933 and 1477/AI, lanes 1 and 2, respectively). Lane M contains a 100-bp molecular size marker.
Analysis of curli expression by NSF STEC O157:H7 and SF STEC O157:NM.
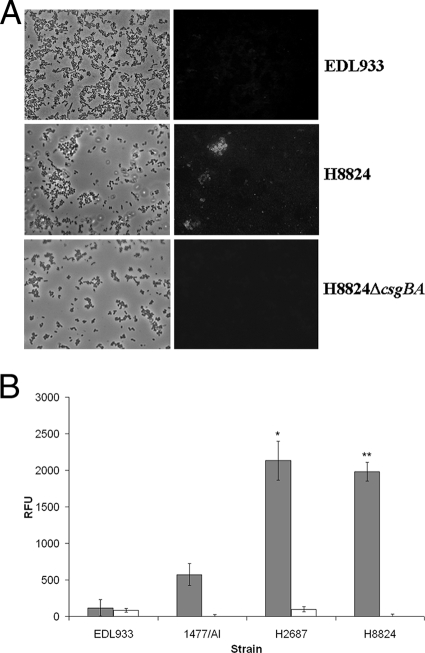
CR binding is a well-established method to demonstrate surface curli expression by enteric bacteria (3, 21, 52). The STEC O157 strains were cultured on CFA-CR indicator plates at 37°C, and while the NSF STEC O157:H7 strains, EDL933 and 1477/AI, were negative as expected (10, 52), the SF STEC O157:NM strains, H2687 and H8824, were positive (data not shown). Additional evidence for curli expression was provided by immunostaining with an anticurli monoclonal antibody. When the bacteria were cultured on CFA agar or in CFA broth, the majority of aggregated SF STEC O157:NM bacteria exhibited fluorescent surface staining (Fig. 4A, middle panels), while the NSF STEC O157:H7 strains were negative (Fig. 4A, top panels). When the bacteria were cultured in CDMT, there was no surface staining of either STEC O157 group (data not shown). Furthermore, the SF STEC O157:NM strains demonstrated significantly higher levels of expression from the csgBAC curli promoter compared with the NSF STEC O157:H7 strains when cultured on CFA agar (Fig. 4B). Taken together, these data indicate that the SF STEC O157:NM strains tested express curli at 37°C on CFA agar.
FIG. 4.
Analysis of curli expression by STEC O157 strains. (A) Curli detection by immunofluorescence microscopy. Phase-contrast and fluorescence micrographs are shown of NSF STEC O157:H7 EDL933 (top panel), SF STEC O157:NM strain H8824 (middle panel), and SF STEC O157:NM strain H8824ΔcsgBA (bottom panel) stained for curli using an anticurli monoclonal antibody following culture on CFA medium at 37°C as described in Materials and Methods. (B) csgBAC curli promoter activity in STEC O157 strains. Expression from the csgBAC promoter was determined in the indicated strains using a promoter-GFP fusion as described in Materials and Methods. Shaded and unshaded bars represent values obtained following culture of the bacteria on CFA agar and in CDMT broth, respectively. Background fluorescence levels were determined for the promoterless plasmid pAJR70 and subtracted. The data are expressed as relative fluorescence units (RFU). A single asterisk indicates that, following culture on CFA agar, expression from the csgBAC promoter for SF STEC O157:NM strain H2687 was significantly higher than that for NSF STEC O157:H7 strains EDL933 and 1477/AI (P < 0.001). Double asterisks indicate that, following culture on CFA agar, expression from the csgBAC promoter for SF STEC O157:NM strain H8824 was significantly higher than that for NSF STEC O157:H7 strains EDL933 and 1477/AI (P < 0.001). Error bars indicate standard errors of the means.
The contribution of curli to SF STEC O157:NM adherence.
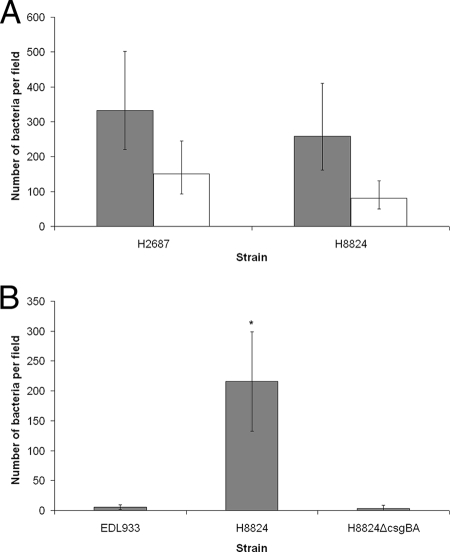
To evaluate the significance of curli in the adherence of SF STEC O157:NM strains to Caco2 cells, inhibition assays were carried out in the presence and absence of anticurli monoclonal antibody. The SF STEC O157:NM isolates cultured on CFA agar demonstrated a significant inhibition of adherence to Caco-2 cells in the presence of the antibody (Fig. 5A). To further substantiate the role of curli in the adherence, csgBA was deleted in SF STEC O157:NM strain H8824 to generate ZAP1125. This strain could be complemented for CR binding and curli expression using pTD10 (induced csgBAC [data not shown]). The csgBA deletion strain did not bind CR and was negative for curli expression as determined by immunofluorescence (Fig. 4A, bottom panel). The adherence of this strain to Caco-2 cells was significantly reduced (P < 0.001) compared to the parent strain and was equivalent to the non-curli-expressing NSF STEC O157:H7 strain EDL933 (Fig. 5B).
FIG. 5.
Contribution of curli to Caco-2 cell adherence. (A) Inhibition of adherence using anticurli antibody. SF STEC O157:NM strains H2687 and H8824 were cultured on CFA agar and incubated in the absence (shaded bars) and presence (unshaded) of anticurli monoclonal antibody. Microscopy was used to determine the number of bacteria per field for 30 fields. Data were log10 transformed and analyzed within mixed models using the REML directive. The graph represents back-transformed predicted means, and error bars define 95% confidence interval values. (B) Comparative binding studies of a curli deletion mutant and wild-type strains. Strain SF STEC O157:NM H8824 was deleted for csgBA (Materials and Methods) and was demonstrated not to bind CR or express curli (Fig. 4A, bottom panel). The adherence of this strain to Caco-2 cells was compared with its parent and NSF STEC O157:H7 EDL933. Microscopy was used to determine the number of bacteria per field for 30 fields. Error bars define standard deviations. The asterisk indicates that the CR-positive SF STEC O157:NM strain H8824 adhered to Caco-2 cells at significantly higher levels than the ΔcsgBA derivative of this strain (P < 0.001).
Analysis of curli expression by European SF STEC O157:NM.
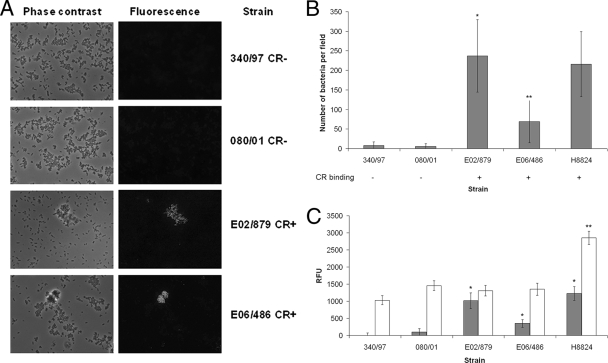
To determine whether curli expression at 37°C is a common attribute of SF STEC O157:NM strains, a collection of 66 SF STEC O157 strains collected by Helge Karch's group (Muenster, Germany) (9, 26, 28) were analyzed for their potential to bind CR on CFA-CR indicator plates. CR binding was confirmed for a subset of the strains by a quantitative method (Materials and Methods). A total of 52/66 (79%) were assessed as positive for CR binding. To further demonstrate the association of CR binding with curli expression and Caco-2 cell adherence, two of these CR-positive strains (E02/879 and E06/486) and two CR-negative strains (340/97 and 080/01) were analyzed by immunofluorescence using the anticurli monoclonal antibody and tested in adherence assays. Both CR-positive strains expressed curli and adhered at significantly higher levels to the Caco-2 cells compared with the CR-negative strains that did not express curli (Fig. 6A and B). Curli expression on CFA at 37°C is therefore a common property of SF STEC O157:NM strains.
FIG. 6.
Analysis of curli expression and adherence among selected European SF STEC O157:NM strains. (A) Curli detection by immunofluorescence microscopy. Phase-contrast and fluorescence micrographs are shown for the indicated strains stained for curli using an anticurli monoclonal antibody following culture on CFA medium at 37°C as described in Materials and Methods. (B) Adherence of selected strains to Caco-2 cells. The indicated strains were cultured on CFA agar, and their adherence to Caco-2 cells was determined after 2 h of incubation at 37°C. SF STEC O157:NM strain H8824 was included as a positive control. Microscopy was used to determine the number of bacteria per field for 30 fields. CR binding which correlates with curli expression (CFA, 37°C) is indicated for the strains below the graph. Error bars define standard deviations. Single asterisks indicate that, following culture on CFA agar, the CR-positive SF STEC O157:NM strain E02/879 adhered to Caco-2 cells at significantly higher levels than the CR-negative SF STEC O157:NM strains 340/97 and 080/01 (P < 0.001). Double asterisks indicate that, following culture on CFA agar, the CR-positive SF STEC O157:NM strain E06/486 adhered to Caco-2 cells at significantly higher levels than the CR-negative SF STEC O157:NM strains 340/97 and 080/01 (P < 0.01). (C) csgBAC and csgD promoter activity in selected strains. The indicated strains were transformed with plasmid-based promoter-GFP fusion constructs, and expression of csgBAC::gfp (shaded bars) and csgD::gfp (unshaded bars) was measured following the culture of transformed strains on CFA-CAM agar plates. Background fluorescence levels were determined for the promoterless plasmid pAJR70 and subtracted. The data are expressed as relative fluorescence units (RFU). Error bars indicate standard errors of the means. Single asterisks indicate that, following culture on CFA-CAM agar, expression from the csgBAC promoter for CR-positive SF STEC O157:NM strains E02/879, E06/486, and H8824 was significantly higher than that for CR-negative SF STEC O157:NM strain 340/97 (P < 0.02). Expression from the csgBAC promoter for strains E02/879 and H8824 was also significantly higher than that for strain 080/01 (P ≤ 0.002). Double asterisks indicate that, following culture on CFA-CAM agar, expression from the csgD promoter for strain H8824 was significantly higher than that for strains 340/97, 080/01, E02/879, and E06/486 (P < 0.001).
Investigation of differential curli expression in SF STEC O157:NM strains.
To establish if sequence variation in curli loci could account for the differences in curli expression between strains, the nucleotide sequences of the relevant regions (csgBAC and csgDEFG operons and the csgB-to-csgD intergenic region) were determined for SF STEC O157:NM strains 340/97, 080/01, E06/486, and H8824. (GenBank accession numbers are detailed in Table 3.) The sequences of the entire region from strains 080/01, E06/486, and H8824 were identical, and strain 340/97 only differed from them by a single nucleotide in the csgD gene. The putative CsgG, CsgF, CsgE, CsgD, CsgB, CsgA, and CsgC proteins encoded by the four SF STEC O157:NM strains should be identical.
Given that the curli expression differences are not accounted for by mutations or changes in the curli operons themselves, it is likely that regulation acting on the csgBAC promoter must differ between these strains. This could be on the csgBAC promoter via differences in CsgD activator levels or on the csgBAC promoter independent of the CsgD level (3, 52). To investigate the expression of csgBAC and csgD in wild-type SF STEC O157:NM, promoter-GFP fusion constructs were transformed into different SF STEC O157:NM backgrounds. Following the culture of transformed strains on CFA-CAM agar plates, expression of csgBAC::gfp was found to be higher in strains E02/879, E06/486, and H8824 than in strains 340/97 and 080/01 (Fig. 6C). These results correlate with CR binding data and curli expression. In contrast, while csgD::gfp expression was significantly higher in one strain (H8824), which could account for the increase in csgBAC expression and curli production, it was equivalent in the four other strains tested, two of which express curli on CFA medium at 37°C (Fig. 6C). This indicates that curli expression in these strains may be driven by regulators other than CsgD acting directly on the csgBAC promoter.
DISCUSSION
SF STEC O157:NM strains have emerged as significant human pathogens in continental Europe, causing diarrheal disease often resulting in life-threatening HUS. Of particular concern is that 10 out of 20 Scottish patients infected with SF STEC O157:NM in 2006 progressed to HUS. This is consistent with findings in Germany, where humans infected by these strains are more likely to progress to HUS. This compares to NSF STEC O157:H7 infections that are typically associated with HUS at lower frequencies (5 to 10%) (19, 37). As clinical cases with SF STEC O157:NM are uncommon, host factors in these cases may be responsible for a bias toward HUS. These include age, clinical history, or treatment issues such as the administration of antibiotics and/or antimotility agents. However, the aim of this research was to investigate the hypothesis that bacterial factors are the cause of the increased association of SF STEC O157:NM with HUS.
Basic characterization of the Scottish SF STEC O157:NM strains showed that they possessed the genes encoding Shiga toxin 2, intimin, and hemolysin. This is in accord with all previous studies on SF STEC O157:NM strains (4, 7, 8, 14, 20, 30). It is possible that a higher HUS rate following SF STEC O157:NM infection compared to NSF STEC O157:H7 infection could be accounted for by a more active toxin or the release of more toxin during infection. In the present study, the predicted protein sequence for Stx2 produced by the SF STEC O157:NM strains was either identical to or differed by a single amino acid in the A and B subunits from Stx2 from NSF STEC O157:H7 EDL933. The levels of toxin released by strains under induced and uninduced in vitro conditions were examined, and there was no evidence of increased toxin expression from the SF STEC O157:NM isolates. Thus, toxin sequence variation and increased toxin release are unlikely to account for any differences in virulence between SF and NSF STEC O157 strains. However, it is appreciated that in vivo conditions may result in expression differences not observed in vitro.
Alternatively, SF STEC O157:NM strains may colonize the human intestine in higher numbers, resulting in increased exposure to toxin. To test this, we compared the potential of SF STEC O157:NM and NSF STEC O157:H7 strains to adhere to a human colon carcinoma cell line. Culture of the strains on CFA agar plates at 37°C resulted in up to 50 times more SF STEC O157:NM bacteria than NSF STEC O157:H7 bacteria adhering to Caco-2 cells within 2 h. While type III secretion-based intimate attachment is likely to be important for intestinal colonization (12, 49), type III secretion profiles and levels were similar for the strains analyzed in this study (data not shown). Consequently, initial adherence is more likely to involve surface-expressed factors such as fimbriae.
An initial observation in this study was that the SF STEC O157:NM strains tested could bind CR when cultured on CFA medium at 37°C. This assay is commonly applied at 28°C to detect expression of thin aggregative fimbriae called curli (3, 52). In the present study, curli expression by the strains was substantiated by antibody staining and promoter fusion analysis. Analysis of a larger collection of SF STEC O157:NM strains (n = 66) (Table 1) indicated that 79% could bind CR when cultured on CFA medium at 37°C. For the subset tested, this phenotype again correlated with curli expression (Fig. 6). In contrast, the majority of NSF E. coli O157:H7 strains are considered not to express curli under in vitro conditions (10, 52), and our data support this. Curli are an established adhesin, and so their contribution to the increased adherence of SF STEC O157:NM strains compared to NSF STEC O157:H7 strains was examined by construction of a curli mutant. The absence of curli expression reduced the binding capacity of the strain to a level comparable to that of the NSF STEC O157:H7 strains tested, indicating that, under these conditions, curli expression was the main factor responsible for adherence to cells. It is appreciated that these strains have multiple surface factors that can contribute to epithelial cell binding, and these are likely to play an important role in vivo. For example, we have confirmed in this study that SF STEC O157:NM strains can express type 1 fimbriae, in contrast to NSF STEC O157:H7 strains (34, 45), corroborating the recent findings of Shaikh et al. (48). In addition, SF E. coli O157:NM strains have been shown to possess a unique fimbrial cluster encoded on their large plasmid, pSFO157, which mediates the expression of Sfp fimbriae (9). Recently published work demonstrates that Sfp fimbriae are expressed by SF STEC O157:NM strains cultured under anaerobic conditions on solid media simulating the colonic environment and that the induction of Sfp fimbriae on wild-type SF STEC O157:NM strains correlated with increased adherence to Caco-2 and HCT-8 cells (38).
In E. coli, two divergently transcribed operons, csgBAC and csgDEFG, are required for curli formation (21). The csgBAC operon encodes CsgA, the main subunit protein, and CsgB, the nucleator protein (3). CsgC, encoded by the third gene (csgC) in the csgBAC operon, has no reported role in curli formation (3). The csgDEFG operon encodes four accessory proteins required for curli assembly, where CsgD is the positive transcriptional regulator of the csgBAC operon (3). The curli loci from four SF STEC O157:NM strains—two curli-expressing strains and two that did not express curli under the conditions tested—were sequenced, and there were no changes detected that could account for the differences in curli expression. In particular, there were no sequence differences in the csgB-to-csgD intergenic region. This is significant as nucleotide changes in the csgD promoter region have been associated with variation in curli expression in E. coli O157:H7 (52). Transcription from the csgBAC promoter requires expression of the CsgD-positive regulator (21). In the present study, we have demonstrated that csgBAC expression was higher in strains expressing curli. In one case, this increased level coincided with a higher level of csgD expression, but in the other strains, levels of csgD expression were equivalent irrespective of curli expression phenotype. Therefore, it appears that curli expression in SF STEC O157:NM strains can be controlled either by increased csgD expression or by regulators acting directly on the csgBAC promoter with CsgD. Curli expression is controlled by osmolarity and various stress responses, in particular those that affect membrane integrity (27). Regulators controlled by these responses can act directly on the csgBAC promoter: for example, RcsB and CpxR, which are modified by phosphorylation. Further research will investigate differences in signaling pathways that control curli expression in SF STEC O157:NM strains.
Although previous studies have shown that in E. coli curli are generally expressed optimally at temperatures below 30°C (40), we have demonstrated that SF STEC O157:NM strains can express curli at 37°C. There is clear evidence that curli are an important virulence factor. For example, CR-binding variants of E. coli O157:H7 were more virulent in a mouse model (53) and expression of curli at 37°C has been reported for a significant number of human E. coli sepsis isolates (5). Curli expression may contribute to the virulence of the pathogen in several ways. First, curli can contribute to increased colonization through adherence to host cells (31, 32; this study) and extracellular matrix (3, 40) and through bacterial aggregation. Second, the interaction of curliated bacteria with certain host proteins may facilitate the spread of the bacteria through the host (3) and as bacteria expressing curli can bind to human contact-phase proteins, including fibrinogen, the clotting cascade may be inhibited (3). Third, curli can activate the innate immune system and have been shown to be a pathogen-associated molecular pattern recognized by TLR2 (51). This is in agreement with an earlier study which demonstrated that curli induce the proinflammatory cytokines tumor necrosis factor alpha, interleukin-6, and interleukin-8 (5). This final point may be critical in terms of toxin translocation during SF STEC O157:NM infections as there is evidence that toxin circulates on the surface of neutrophils (50). Therefore, the more inflammation that is induced by bacteria in the gastrointestinal tract, the more neutrophils are recruited that could traffic toxin into the bloodstream via the lymph system.
In conclusion, the emergence of SF STEC O157:NM strains is of concern since these pathogens appear to be associated with a higher incidence of HUS compared to the more common NSF STEC O157:H7 strains. We investigated potential factors which could account for increased virulence and found that, under the in vitro conditions investigated, SF STEC O157:NM can colonize a human colon cell line at higher levels than NSF STEC O157:H7. We have shown that curli are the major factor contributing to this increased adherence observed in vitro. However, the ability of SF STEC O157:NM cells to express functional type 1 fimbriae and Sfp fimbriae on their surface (38, 48; this study) suggests that these adhesins are also likely to contribute to the adherence of these pathogens in vivo. The ability of SF STEC O157:NM to express fimbrial adhesins and colonize the human intestine at higher levels could have serious implications for human health where the presence of greater bacterial numbers, and perhaps more persistent colonization, in the intestine leads to higher toxin levels and associated sequelae.
Acknowledgments
T.R. receives funding through the Food Standards Agency Postgraduate scholarship scheme. Aspects of this research were carried out with financial support from Scottish Government FSA grant B11013. The Scottish E. coli O157 Reference Laboratory (SERL) is funded by the National Services Division of the Common Services Agency, Scottish Office Department of Health.
The cooperation of colleagues throughout Scotland who submit samples to SERL is gratefully acknowledged, as is the contribution of everyone involved in the investigation of the 2006 cluster of cases, particularly Sheena Harding at SERL and Mary Locking and Kevin Pollock at Health Protection Scotland. We thank Helge Karch and Martina Bielaszewska for providing the European SF STEC O157:NM strains. We also acknowledge Ian Nevison of Biomaths and Statistics Scotland for statistical analysis and advice.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 13 October 2008.
REFERENCES
- 1.Allison, L. 2002. HUS due to a sorbitol-fermenting verotoxigenic E. coli O157 in Scotland. Euro. Surveill. Wkly. 6021031. http://www.eurosurveillance.org/ew/2002./021031.asp#2. [Google Scholar]
- 2.Ammon, A., L. R. Petersen, and H. Karch. 1999. A large outbreak of hemolytic uremic syndrome caused by an unusual sorbitol-fermenting strain of Escherichia coli O157:H−. J. Infect. Dis. 1791274-1277. [DOI] [PubMed] [Google Scholar]
- 3.Barnhart, M. M., and M. R. Chapman. 2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60131-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettelheim, K. A., M. Whipp, S. P. Djordjevic, and V. Ramachandran. 2002. First isolation outside Europe of sorbitol-fermenting verocytotoxigenic Escherichia coli (STEC) belonging to O group O157. J. Med. Microbiol. 51713-714. [DOI] [PubMed] [Google Scholar]
- 5.Bian, Z., A. Brauner, Y. Li, and S. Normark. 2000. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J. Infect. Dis. 181602-612. [DOI] [PubMed] [Google Scholar]
- 6.Bielaszewska, M., R. Prager, W. Zhang, A. W. Friedrich, A. Mellmann, H. Tschäpe, and H. Karch. 2006. Chromosomal dynamism in progeny of outbreak-related sorbitol-fermenting enterohemorrhagic Escherichia coli O157:NM. Appl. Environ. Microbiol. 721900-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielaszewska, M., H. Schmidt, M. A. Karmali, R. Khakhria, J. Janda, K. Bláhová, and H. Karch. 1998. Isolation and characterization of sorbitol-fermenting Shiga toxin (verocytotoxin)-producing Escherichia coli O157:H− strains in the Czech Republic. J. Clin. Microbiol. 362135-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielaszewska, M., H. Schmidt, A. Liesegang, R. Prager, W. Rabsch, H. Tschäpe, A. Cizek, J. Janda, K. Bláhová, and H. Karch. 2000. Cattle can be a reservoir of sorbitol-fermenting Shiga toxin-producing Escherichia coli O157:H− strains and a source of human diseases. J. Clin. Microbiol. 383470-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunder, W., A. S. Khan, J. Hacker, and H. Karch. 2001. Novel type of fimbriae encoded by the large plasmid of sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H−. Infect. Immun. 694447-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cookson, A. L., W. A. Cooley, and M. J. Woodward. 2002. The role of type 1 and curli fimbriae of Shiga toxin-producing Escherichia coli in adherence to abiotic surfaces. Int. J. Med. Microbiol. 292195-205. [DOI] [PubMed] [Google Scholar]
- 11.Cowden, J. 1997. Scottish outbreak of Escherichia coli O157, November-December 1996. Euro. Surveill. 21-2. http://www.eurosurveillance.org/em/v02n01/0201-221.asp. [DOI] [PubMed] [Google Scholar]
- 12.Donnenberg, M. S., C. O. Tacket, S. P. James, G. Losonsky, J. P. Nataro, S. S. Wasserman, J. B. Kaper, and M. M. Levine. 1993. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J. Clin. Investig. 921412-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Editorial Team. 2006. E. coli O157 infections in the UK. Euro. Surveill. 11E060601.2. http://www.eurosurveillance.org/ew/2006/060601.asp#2. [PubMed] [Google Scholar]
- 14.Eklund, M., M. Bielaszewska, U. M. Nakari, H. Karch, and A. Siitonen. 2006. Molecular and phenotypic profiling of sorbitol-fermenting Escherichia coli O157:H− human isolates from Finland. Clin. Microbiol. Infect. 12634-641. [DOI] [PubMed] [Google Scholar]
- 15.Evans, D. G., D. J. Evans, Jr., and W. Tjoa. 1977. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect. Immun. 18330-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields, P. I., K. Blom, H. J. Hughes, L. O. Helsel, P. Feng, and B. Swaminathan. 1997. Molecular characterization of the gene encoding H antigen in Escherichia coli and development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J. Clin. Microbiol. 351066-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman, L. E., B. N. de Rossi, M. T. Messina, and M. A. Franco. 2001. Phenotype evaluation of Bordetella bronchiseptica cultures by urease activity and Congo red affinity. Lett. Appl. Microbiol. 33285-290. [DOI] [PubMed] [Google Scholar]
- 18.Garvey, P., P. McKeown, A. Carroll, and E. McNamara. 2006. Epidemiology of Verotoxigenic E. coli in Ireland, 2005. EPI-Insight 72-3. http://www.ndsc.ie/hpsc/EPI-Insight/Volume72006/File,1936,en.pdf. [Google Scholar]
- 19.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 1360-98. [DOI] [PubMed] [Google Scholar]
- 20.Gunzer, F., H. Bóhm, H. Rússmann, M. Bitzan, S. Aleksic, and H. Karch. 1992. Molecular detection of sorbitol-fermenting Escherichia coli O157 in patients with hemolytic-uremic syndrome. J. Clin. Microbiol. 301807-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammar, M., A. Arnqvist, Z. Bian, A. Olsen, and S. Normark. 1995. Expression of two csg operons is required for production of fibronectin- and Congo red-binding curli polymers in Escherichia coli K-12. Mol. Microbiol. 18661-670. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 811-22. [DOI] [PubMed] [Google Scholar]
- 23.Holden, N. J., M. Totsika, E. Mahler, A. J. Roe, K. Catherwood, K. Lindner, U. Dobrindt, and D. L. Gally. 2006. Demonstration of regulatory cross-talk between P fimbriae and type 1 fimbriae in uropathogenic Escherichia coli. Microbiology 1521143-1153. [DOI] [PubMed] [Google Scholar]
- 24.HPA. 2006. Sorbitol-fermenting Vero cytotoxin-producing E. coli O157 (STEC O157). Commun. Dis. Rep. CDR Wkly. 16news. http://www.hpa.org.uk/cdr/archives/2006/cdr2106.pdf.
- 25.Hung, C. S., J. Bouckaert, D. Hung, J. Pinkner, C. Widberg, A. DeFusco, C. G. Auguste, R. Strouse, S. Langermann, G. Waksman, and S. J. Hultgren. 2002. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 44903-915. [DOI] [PubMed] [Google Scholar]
- 26.Janka, A., M. Bielaszewska, U. Dobrindt, and H. Karch. 2002. Identification and distribution of the enterohemorrhagic Escherichia coli factor for adherence (efa1) gene in sorbitol-fermenting Escherichia coli O157:H−. Int. J. Med. Microbiol. 292207-214. [DOI] [PubMed] [Google Scholar]
- 27.Jubelin, G., A. Vianney, C. Beloin, J.-M. Ghigo, J. C. Lazzaroni, P. Lejeune, and C. Dorel. 2005. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 1872038-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karch, H., and M. Bielaszewska. 2001. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157:H− strains: epidemiology, phenotypic and molecular characteristics, and microbiological diagnosis. J. Clin. Microbiol. 392043-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karch, H., H. Böhm, H. Schmidt, F. Gunzer, S. Aleksic, and J. Heesemann. 1993. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H−. J. Clin. Microbiol. 311200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keskimäki, M., M. Saari, T. Heiskanen, and A. Siitonen. 1998. Shiga toxin-producing Escherichia coli in Finland from 1990 through 1997: prevalence and characteristics of isolates. J. Clin. Microbiol. 363641-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kikuchi, T., Y. Mizunoe, A. Takade, S. Naito, and S. Yoshida. 2005. Curli fibers are required for development of biofilm architecture in Escherichia coli K-12 and enhance bacterial adherence to human uroepithelial cells. Microbiol. Immunol. 49875-884. [DOI] [PubMed] [Google Scholar]
- 32.Kim, S. H., and Y. H. Kim. 2004. Escherichia coli O157:H7 adherence to HEp-2 cells is implicated with curli expression and outer membrane integrity. J. Vet. Sci. 5119-124. [PubMed] [Google Scholar]
- 33.Leathart, J. B., and D. L. Gally. 1998. Regulation of type 1 fimbrial expression in uropathogenic Escherichia coli: heterogeneity of expression through sequence changes in the fim switch region. Mol. Microbiol. 28371-381. [DOI] [PubMed] [Google Scholar]
- 34.Li, B., W. H. Koch, and T. A. Cebula. 1997. Detection and characterization of the fimA gene of Escherichia coli O157:H7. Mol. Cell. Probes 11397-406. [DOI] [PubMed] [Google Scholar]
- 35.Locking, M., L. Allison, L. Rae, and M. Hanson. 2004. STEC in Scotland 2003: enhanced surveillance and reference laboratory data. Scott. Cent. Infect. Environ. Health Wkly. Rep. 38294-297. http://www.documents.hps.scot.nhs.uk/ewr/pdf2004/0449.pdf. [Google Scholar]
- 36.Low, A. S., N. Holden, T. Rosser, A. J. Roe, C. Constantinidou, J. L. Hobman, D. G. Smith, J. C. Low, and D. L. Gally. 2006. Analysis of fimbrial gene clusters and their expression in enterohaemorrhagic Escherichia coli O157:H7. Environ. Microbiol. 81033-1047. [DOI] [PubMed] [Google Scholar]
- 37.Mead, P. S., and P. M. Griffin. 1998. Escherichia coli O157:H7. Lancet 3521207-1212. [DOI] [PubMed] [Google Scholar]
- 38.Müsken, A., M. Bielaszewska, L. Greune, C. H. Schweppe, J. Müthing, H. Schmidt, M. A. Schmidt, H. Karch, and W. Zhang. 2008. Anaerobic conditions promote expression of Sfp fimbriae and adherence of sorbitol-fermenting enterohemorrhagic Escherichia coli O157:NM to human intestinal epithelial cells. Appl. Environ. Microbiol. 741087-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls, L., T. H. Grant, and R. M. Robins-Browne. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35275-288. [DOI] [PubMed] [Google Scholar]
- 40.Olsen, A., A. Jonsson, and S. Normark. 1989. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 338652-655. [DOI] [PubMed] [Google Scholar]
- 41.Orth, D., K. Grif, M. P. Dierich, and R. Wurzner. 2006. Sorbitol-fermenting Shiga toxin-producing Escherichia coli O157: indications for an animal reservoir. Epidemiol. Infect. 134719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Outbreak Control Team. 2001. Report on the outbreak of E. coli O157 at New Deer Millenium Scout camp, May/June 2000. Task Force on E. coli O157 (final report) June 2001. Annex 5, p. 127. http://www.food.gov.uk/multimedia/pdfs/ecolitaskfinreport.pdf.
- 43.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409529-533. [DOI] [PubMed] [Google Scholar]
- 45.Roe, A. J., C. Currie, D. G. Smith, and D. L. Gally. 2001. Analysis of type 1 fimbriae expression in verotoxigenic Escherichia coli: a comparison between serotypes O157 and O26. Microbiology 147145-152. [DOI] [PubMed] [Google Scholar]
- 46.Roe, A. J., H. Yull, S. W. Naylor, M. J. Woodward, D. G. E. Smith, and D. L. Gally. 2003. Heterogeneous surface expression of EspA translocon filaments by Escherichia coli O157:H7 is controlled at the posttranscriptional level. Infect. Immun. 715900-5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SCIEH. 2001. E. coli O157, Inverclyde. Scott. Cent. Infect. Environ. Health Wkly. Rep. 35153. http://www.documents.hps.scot.nhs.uk/ewr/pdf2001/0124.pdf. [Google Scholar]
- 48.Shaikh, N., N. J. Holt, J. R. Johnson, and P. I. Tarr. 2007. Fim operon variation in the emergence of enterohemorrhagic Escherichia coli: an evolutionary and functional analysis. FEMS Microbiol. Lett. 27358-63. [DOI] [PubMed] [Google Scholar]
- 49.Tacket, C. O., M. B. Sztein, G. Losonsky, A. Abe, B. B. Finlay, B. P. McNamara, G. T. Fantry, S. P. James, J. P. Nataro, M. M. Levine, and M. S. Donnenberg. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 683689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.te Loo, D. M., L. A. Monnens, T. J. van Der Velden, M. A. Vermeer, F. Preyers, P. N. Demacker, L. P. van Den Heuvel, and V. W. van Hinsbergh. 2000. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 953396-3402. [PubMed] [Google Scholar]
- 51.Tukel, C., M. Raffatellu, A. D. Humphries, R. P. Wilson, H. L. Andrews-Polymenis, T. Gull, J. F. Figueiredo, M. H. Wong, K. S. Michelsen, M. Akcelik, L. G. Adams, and A. J. Baumler. 2005. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol. Microbiol. 58289-304. [DOI] [PubMed] [Google Scholar]
- 52.Uhlich, G. A., J. E. Keen, and R. O. Elder. 2001. Mutations in the csgD promoter associated with variations in curli expression in certain strains of Escherichia coli O157:H7. Appl. Environ. Microbiol. 672367-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uhlich, G. A., J. E. Keen, and R. O. Elder. 2002. Variations in the csgD promoter of Escherichia coli O157:H7 associated with increased virulence in mice and increased invasion of HEp-2 cells. Infect. Immun. 70395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100195-199. [PubMed] [Google Scholar]