Abstract
MgrA is a pleiotropic regulator that controls autolysis, virulence, and efflux pump activity in Staphylococcus aureus. We recently found that mgrA mutants of strains RN6390, SH1000, and MW2 also displayed enhanced biofilm formation compared with their respective parents. The biofilms formed by mgrA mutants of RN6390 and MW2 are independent of sigB and ica loci, two genetic elements that have been previously associated with biofilm formation in S. aureus. Biofilms formed by mgrA mutants are dependent on the expression of surface proteins mediated by the sortase gene srtA. Extracellular DNA was also a crucial component of the early biofilm of mgrA mutants. Genetic analysis indicated that biofilm formation in mgrA mutants is mediated in part by agr RNAIII, a genetic locus regulated by mgrA. Additionally, SarA is important to biofilm formation in mgrA mutants since the double sarA mgrA mutants failed to form biofilms compared to single mgrA mutants of RN6390 and MW2. However, the SarA-mediated effect is independent of agr and proteases such as V8 protease and aureolysin. Collectively, our data showed MgrA to be a repressor of biofilm formation, and biofilms formed by mgrA mutants have features that are distinct from other reported biofilm types in S. aureus.
Staphylococcus aureus is a major human pathogen that is a common cause of community- and hospital-acquired infections (22). These infections have been problematic due in part to a dramatic increase in antibiotic resistance (e.g., methicillin-resistant S. aureus [MRSA]). The problem with antibiotic resistance can be traced in part to S. aureus's ability to produce biofilms (14, 26). Biofilms constitute a protected environment of growth that enables the bacteria to proliferate by restricting antibiotic access and shielding the bacterial pathogen from host immune defenses (11). The matrices of bacterial biofilms generally consist of polysaccharides, surface proteins, and, in some cases, extracellular DNA (13, 35). However, the expression and specific composition of individual biofilms can differ among bacterial species and are dependent on the metabolic state and environmental conditions (13).
Bacterial biofilms are purported to be important in chronic infections (13). Despite their clinical significance, the expression and regulation of S. aureus biofilms remain poorly defined. Several S. aureus genes have been shown to be crucial to biofilm formation, including ica, arlRS, agr, and sarA (25, 38, 39, 43). The extracellular polysaccharide adhesin PIA/PNAG, (polysaccharide intracellular adhesion/poly-N-acetylglucosamine) encoded by the icaADBC locus (25), has been shown to be responsible for some of the biofilm-positive phenotypes (adhesion, microaggregation, and macroaggregation) in S. aureus and, to a greater extent, in Staphylococcus epidermidis (43). However, recent studies have shown that S. aureus can produce an alternative ica-independent biofilm (9, 10), especially in the absence of the two-component regulatory system arlRS, thus indicating that PIA/PNAG is not an essential component of all biofilm matrices in S. aureus (38).
Another two-component regulatory system linked to biofilm formation is the accessory gene regulator (agr), which entails a complex quorum-sensing scheme. The agr locus consists of two divergent transcripts, RNAII and RNAIII, which carry agrDBCA and hld, respectively (31). RNAIII is the agr effector molecule that enhances the expression of exoprotein genes while downregulating genes encoding surface proteins. The inactivation of agr leads to an enhanced biofilm phenotype, in part due to the increased expression of surface adhesive proteins (32). The agr locus is also activated in part by SarA, a DNA binding protein that belongs to a family of transcription factors in S. aureus called the SarA protein family (5). The sarA locus, encoding the 14.5-kDa SarA protein, is required for optimal ica expression and also for the controlled expression of Bap, a surface protein essential for biofilm formation in bovine S. aureus isolates (39). Additionally, a recent study divulged that cidA, encoding a putative holin molecule, may mediate DNA release by triggering cell lysis during biofilm formation in S. aureus (35). However, the exact role of DNA release in the different stages of biofilm formation has not been defined.
In this study, we demonstrate that the inactivation of mgrA, a negative regulator of autolysin genes, enhances biofilm formation via a sigB- and ica-independent manner. This effect is likely mediated by surface proteins regulated by srtA. Additionally, extracellular DNA released within the biofilm is also an essential matrix component in early biofilms formed by mgrA mutants. Genetic analyses have implicated agr and sarA as playing important roles in biofilm formation in mgrA mutants.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Phages φ11 and 80α were used to transduce S. aureus strains. S. aureus cells were grown at 37°C with aeration in CYGP or 03GL broth (30) or tryptic soy broth (TSB) supplemented with antibiotics when necessary. Luria-Bertani broth was used for cultivating Escherichia coli. Antibiotics were used at the following concentrations: for S. aureus, erythromycin at 5 μg/ml, tetracycline at 3 μg/ml, chloramphenicol at 10 μg/ml, and kanamycin at 50 μg/ml and, for E. coli, ampicillin at 50 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| RN4220 | Mutant strain of 8325-4 that accepts foreign DNA | 30 |
| RN6911 | agr mutant of RN6390 with complete deletion of RNAII and RNAIII (Δagr::tetM) | 31 |
| SA113 | Common laboratory isolate | 10 |
| RN6390 | agr+ laboratory strain related to 8325-4 but with a defective rsbU | 33 |
| SH1000 | Functional rsbU derivate of 8325-4 | 15 |
| MW2 | Community-associated methicillin-resistant strain | 2 |
| ALC4006 | RN6390 ΔmgrA | This study |
| ALC6226 | RN6390 ΔmgrA pCL84-mgrA | 19,28 |
| ALC6214 | RN6390 ΔmgrA ΔsrtA | This study |
| ALC2057 | RN6390 ΔsarA::kan | 16 |
| ALC5408 | RN6390 ΔmgrA ΔsarA::kan | This study |
| ALC6216 | RN6390 ΔmgrA ΔsarA::kan Δaur::ermB | This study |
| ALC6215 | RN6390 ΔmgrA ΔsarA::kan ΔsspA::ermB | This study |
| ALC5413 | SH1000 ΔmgrA | This study |
| ALC6225 | SH1000 ΔmgrA pCL84-mgrA | This study |
| ALC2892 | SH1000 ΔsarA::kan | This study |
| ALC5414 | SH1000 ΔmgrA ΔsarA::kan | This study |
| ALC6228 | SH1000 ΔmgrA ΔsarA::kan Δaur::ermB | This study |
| ALC6229 | SH1000 ΔmgrA ΔsarA::kan ΔsspA::ermB | This study |
| ALC5419 | MW2 ΔmgrA | This study |
| ALC6230 | MW2 ΔmgrA pCL84-mgrA | This study |
| ALC6218 | MW2 ΔmgrA ΔsrtA | This study |
| ALC6259 | MW2 Δagr RNAII/III::tetM | This study |
| ALC5415 | MW2 ΔsarA::kan | This study |
| ALC5420 | MW2 ΔmgrA ΔsarA::kan | This study |
| E. coli | ||
| XL1-Blue | Host strain for cloning | |
| Plasmids | ||
| pALC2464 | pCL84 with the complete mgrA gene (pCL84-mgrA) | This study |
| pMAD | E. coli-S. aureus shuttle vector with a thermosensitive origin of replication for gram-positive bacteria and the bgaB gene encoding β-galactosidase | 1 |
| pALC3371 | pMAD plasmid containing the mutant allele for deletion of mgrA (pMAD::ΔmgrA) | This study |
| pALC6199 | pMAD plasmid containing the mutant allele for deletion of the srtA gene (pMAD::ΔsrtA) | This study |
| pRN6735 | Contains promoterless RNAIII under the control of the blaZ promoter | 31 |
| pALC1484 | Derivate of pSK236, containing the recombinant gfpuvr gene | 20 |
| pALC6211 | pALC1484 with a 194-bp promoter fragment of ica fused with the gfpuvr reporter gene at the KpnI/EcoRI sites | This study |
| pALC1743 | pALC1484 with the 229-bp agr RNAIII promoter fragment fused with the gfpuvr reporter gene at the EcoRI/XbaI sites | 17 |
Genetic manipulations in E. coli and S. aureus.
Construction of the recombinant plasmids was performed in E. coli XL1-Blue with standard molecular biology and recombinant DNA techniques as described by Maniatis et al. (27). Restriction enzymes were purchased from New England Biolabs and used according to the manufacturer's instructions. Primers were obtained from Operon Technology. Staphylococcus aureus strain RN4220, a restriction-deficient derivative of strain 8325-4, was used as the initial recipient of the plasmid constructs by electroporation (36).
The sarA, aur, and sspA mutants of S. aureus were constructed by transducing the sarA::kan, aur::ermB, and sspA::ermB mutations from 8325-4 to recipient strains, using phage φ11.
To construct the mgrA mutant, we amplified by PCR two fragments that flanked the left and right sides of the gene sequence targeted for deletion. The two resulting PCR fragments have a 16-base complementary region to facilitate annealing followed by a second round of PCR amplification with outside primers to obtain a single fragment. The fusion product was purified, digested with SmaI, and ligated into shuttle vector pMAD. A similar strategy was also used to construct deletion mutants of srtA and agr in S. aureus strains. The resulting recombinant pMAD plasmids (Table 1) were transformed first into S. aureus RN4220 and then into the target strain by electroporation. The allelic exchange in the absence of the selection marker was performed as previously described (41). Briefly, the plasmid pMAD, containing a temperature-sensitive S. aureus origin of replication, an erythromycin resistance cassette, and a β-galactosidase gene (bga), was integrated into the host chromosome at the nonpermissive temperature (44°C), resulting in a light-blue erythromycin-resistant colony on X-Gal (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid; 150 μg/ml) plates. One to five light-blue colonies were picked onto 10 ml of TSB and incubated overnight at 30°C without any antibiotic. Tenfold serial dilutions of this culture were plated onto Trypticase soy agar plates containing X-Gal. White and erythromycin-sensitive colonies, which no longer contained the pMAD plasmid, were then selected as plausible allelic replacement mutants, which were confirmed by PCR and Southern blots with the appropriate probes.
Transcriptional fusion studies of the ica and the RNAIII promoters linked to the gfpuvr reporter gene.
To confirm the effect of the ΔmgrA mutation on the promoter activity of ica and RNAIII, we used the shuttle plasmids pALC6211 and pALC1743 containing the icaA and the agr RNAIII promoters, respectively, driving the gfpuvr reporter gene (Table 1). In brief, the 164-bp region between icaR and icaA, representing the ica promoter driving icaADBC expression (19), was cloned upstream of the gfp reporter in shuttle plasmid pALC1484 to yield pALC6211. Similarly, the agr RNAIII promoter was also cloned upstream of pALC1484 to yield pALC1743 (17). The plasmids pALC6211 and pALC1743 were transformed into RN4220, purified from RN4220 transformants, and then electroporated into RN6390 and/or MW2 and its isogenic ΔmgrA mutants. To detect promoter activity, overnight cultures of S. aureus strains harboring either pALC6211 or pALC1743 were diluted 1:50 in TSB containing chloramphenicol (10 μg/ml) and grown at 37°C with shaking. Aliquots (200 μl) were transferred in triplicate every 30 min to microtiter plates to assay for cell density (optical density at 595 nm [OD595]) and fluorescence for a 6- to 10-h period in an FL600 microplate fluorescence reader (BioTek Instruments, Winooski, VT). Promoter activation was plotted as the mean fluorescence/OD595 ratio to minimize variations from different cell densities, using the average values from triplicate readings.
Biofilm formation.
S. aureus strains were grown overnight and diluted 1:40 in TSB supplemented with 0.25% glucose (TSB-glucose). Two hundred microliters each of this cell suspension was used to inoculate sterile 96-well polystyrene microtiter plates (Costar) in triplicate. After 16 h of incubation at 37°C, the wells were washed three times with sterile water, stained with 0.1% crystal violet for 1 min, and washed again three times with water. Photographs of the inverted plate were taken with a scanner. To quantitate the biofilms, the crystal violet stain was solubilized with 30% glacial acetic acid for 15 min. The relative biofilm formation was determined by reading the OD562, using a Bio-Tek microplate reader (Bio-Tek FL600).
Colony morphology on CRA.
Colony morphology on Congo red agar (CRA) was determined as previously described (7). Briefly, a biofilm-positive strain is linked with rough colonies, while a biofilm-negative strain is associated with smooth colonies.
Biofilm detachment assays.
Biofilms were grown for 16 h in the wells of 96-well microtiter plates as described above. The biofilms were washed with water and then treated for 2 h at 37°C with 100 μg of proteinase K per ml (Sigma) in 20 mM Tris buffer (pH 7.5) or 0.14 units of DNase I in 20 mM Tris buffer (pH 7.5). After treatment, the biofilms were washed with water, stained with crystal violet, and quantitated as described above. Biofilm detachment assays were performed three times with similar results. In some assays, proteinase K and DNase I were added to the TSB-glucose culture medium in the microtiter wells before formation of the biofilm.
PIA/PNAG detection.
PIA/PNAG production in S. aureus strains was detected as described by Cramton et al. (8). Briefly, cells were grown in TSB-glucose, and the same number of cells from each culture was resuspended in 50 μl of 0.5 M EDTA (pH 8.0), incubated for 5 min at 100°C, and centrifuged. Forty microliters of the supernatant was incubated with 10 μl of proteinase K (20 mg/ml; Sigma) for 30 min at 37°C. One microliter was then spotted on a nitrocellulose filter using a Bio-Dot microfiltration apparatus (Bio-Rad), blocked overnight with 5% skimmed milk in phosphate-buffered saline with 0.1% Tween 20, and incubated for 2 h with rabbit anti-S. aureus PIA/PNAG antibody diluted 1:5,000 (4). Bound antibodies were detected with a peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody (Jackson ImmunoResearch Laboratories) diluted 1:10,000 and developed with the GE Health Care ECL kit.
RESULTS
mgrA represses biofilm formation in S. aureus.
In previous studies, we have shown that MgrA is a negative regulator of autolysis in S. aureus (16). Specifically, the mgrA mutant has a mild growth defect during the late postexponential phase and exhibited increased sensitivity to lysis upon exposure to Triton X-100 and penicillin (16). Since an enhanced autolytic phenotype has been associated with biofilm formation (12), we wanted to determine if an mgrA mutant also exhibited an enhanced biofilm phenotype in various S. aureus strains.
We initially chose to study RN6390, a methicillin-sensitive S. aureus (MSSA) laboratory isolate with an impaired rsbU gene, and SH1000, a derivative of 8325-4 in which the rsbU mutation has been repaired (15). As shown in Fig. 1, both mgrA mutants, irrespective of their rsbU status, were able to produce more biofilm on polystyrene plates than their respective parental controls. To verify that this effect is not limited to MSSA strains, we constructed an mgrA deletion mutant of MW2, a community-acquired MRSA strain, which also displayed enhanced biofilm formation with an mgrA mutation (Fig. 1). The enhanced biofilm phenotype correlated with the presence of rough mgrA mutant colonies on Congo red agar, while the parental strains displayed a smooth-colony phenotype (Fig. 1, bottom panel). The biofilm phenotype and colonial morphology on CRA in mgrA mutants were restored to those of the respective parents upon complementation (Fig. 1). Collectively, these findings indicated that biofilm formation in mgrA mutants is rsbU independent and can occur in both MSSA and MRSA strains.
FIG. 1.
Biofilm formation by mgrA mutants. Top panel, biofilm formation of the mgrA mutants, wild types (wt), and their respective complemented strains (c) was quantified by solubilizing the crystal violet stain in 30% glacial acetic acid and measuring the absorbance at 562 nm. As assessed by the Student t test, the significance of the data was as follows: the mgrA mutant of RN6300 versus the parent and complement, P < 0.0004; the mgrA mutant of SH1000 versus the parent and complement, P < 0.03; the mgrA mutant of MW2 versus the parent and mutant, P < 0.0001. Bottom panel, biofilm formation of mgrA mutants and their complemented strains on microtiter plates grown in TSB supplemented with glucose (0.25%) at 37°C over 16 h. A characterization of the colony morphology of different mgrA mutants and their respective complemented strains on CRA plates is presented below.
The ica locus is not required for biofilm formation in mgrA mutants.
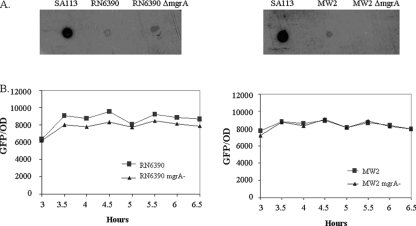
Previous studies have shown that S. aureus can develop PIA-dependent and PIA-independent biofilms (8, 38, 41). To determine if the biofilm in an mgrA mutant is reliant on PIA, a dot blot analysis of cell surface extracts from overnight cultures of RN6390 and MW2 and their isogenic mgrA mutants was performed on nitrocellulose membranes as described in Materials and Methods. PIA (PNAG), encoded by the icaADBC locus, was detected with anti-PIA polyclonal antibody (a gift of Gerald Pier). As shown in Fig. 2A, the mgrA mutants of RN6390 and MW2, with an enhanced biofilm phenotype, displayed a small reduction in PIA expression compared with the isogenic parents, while the positive-control S. aureus strain SA113 expressed PIA at a high level. To verify the dot blot analysis, we conducted transcriptional fusion assays of an ica promoter driving the gfp reporter gene in pALC1484 in strains RN6390 and MW2 and their isogenic mgrA mutants. This 164-bp ica promoter fragment which spans the entire region between the icaR and icaA open reading frames has previously been shown by another research group to be active in ica transcription (19). The fluorescence attributable to the ica promoter activity was ranging from ∼6,000 to 8,000 fluorescence units/OD595 in these strains and did not differ significantly between the mgrA mutants and their respective parents (Fig. 2B). Notably, the baseline fluorescence for this assay with the shuttle plasmid pALC1484 alone without the ica promoter was ∼200 fluorescence units, thus indicating that the ica promoter was active in both parental strains and their isogenic mgrA mutants.
FIG. 2.
(A) Dot blot analysis of PIA/PNAG accumulation in mgrA mutants and wild-type strains. Cell surface extracts from overnight cultures of S. aureus wild-type strains RN6390 and MW2, their mgrA mutants, and the positive control SA113, treated as described in Materials and Methods, were placed onto nitrocellulose membranes. PIA/PNAG production was detected with an anti-PIA/PNAG polyclonal antibody. (B) Quantification of the activity of the 164-bp ica promoter (19) in the mgrA mutants and their corresponding wild types by transcriptional fusion studies. The expression of green fluorescent protein driven by the ica promoter was measured as a fluorescence/OD595 ratio using average values of triplicate readings. This experiment was repeated at least three times, with one representative experiment shown.
Biofilm formation in an mgrA mutant is dependent in part on srtA.
Surface proteins often play an important role in early adhesion and intercellular aggregation in the formation of biofilms (24). To examine the role of surface protein adhesins in the biofilms formed by mgrA mutants, we explored whether the sortase gene (srtA), which serves to anchor many cell wall proteins covalently to the staphylococcal cell wall (29), would affect biofilm formation in mgrA mutants. Accordingly, we deleted srtA in the mgrA mutants and found that the double mutants had significantly reduced biofilm formation compared with the respective single mgrA mutants while the parental strains RN6390 and MW2 were poor biofilm formers in this assay (Fig. 3). We thus deduced that surface proteins anchored by sortase A probably play a major role in biofilm formation in mgrA mutants, presumably due to the increased expression of surface adhesins and/or cellular aggregation.
FIG. 3.
Biofilm formation phenotype of the double ΔmgrA ΔsrtA mutants of RN6390 and MW2. Significant differences in biofilm formation capacity were found between the mgrA mutant and the ΔmgrA ΔsrtA double mutant. The significance data obtained with the Student t test were as follows: the mgrA mutant versus RN6390, P < 0.0001; the mgrA srtA mutant versus the mgrA mutant of RN6390, P < 0.0012; the mgrA mutant versus MW2, P < 0.0002; and the mgrA srtA mutant versus the mgrA mutant of MW2, P < 0.001. The microtiter plate results are shown below.
The mgrA mutant biofilm is composed of proteins and DNA.
The composition of S. aureus biofilms has been shown to vary with metabolic state, growth conditions, and genetic mutations (24). To ascertain if surface proteins are indeed important constituents of biofilms in mgrA mutants, preformed biofilms of mgrA mutants of RN6390 and MW2 after overnight growth in TSB-glucose were treated for 2 h with proteinase K (100 μg/ml), and the residual attached bacteria in the biofilm were then stained with crystal violet. As displayed in Fig. 4, treatment of the preformed biofilms of mgrA mutants with proteinase K eliminated the biofilms to the level of the respective parents. Similarly, the addition of proteinase K to TSB-glucose medium prior to overnight growth also abolished the formation of biofilms of mgrA mutants. Given that proteinase K cannot lyse S. aureus (data not shown), it is likely that surface proteins that mediate bacterial attachment or aggregation are eliminated as a result of proteinase K treatment.
FIG. 4.
Detachment of preformed biofilms by proteinase K. The biofilms of RN6390 and MW2 and their isogenic ΔmgrA mutant strains grown in TSB-glucose for 16 h at 37°C were treated for 2 h at 37°C with 100 μg of proteinase K/ml (striped bars). The bacteria that remained attached in the microtiter plate were stained with crystal violet. The dye was dissolved with 30% glacial acetic acid (200 μl per well) and measured by OD562. The untreated controls are represented by filled bars. The microtiter views are shown below.
Recent studies by Rice and colleagues (35) have also implicated released DNA from lysed cells in biofilm formation. To determine if DNA is an essential component of the early biofilms of mgrA mutants, DNase I was added to the TSB-glucose medium containing mgrA mutants of RN6390 or MW2. After overnight growth, the formation of a biofilm was quantified by measuring the OD562 of the released crystal violet stain. As shown in Fig. 5, the overnight treatment of cultures of mgrA mutants with DNase I (0.7 units/ml) has led to the eradication of biofilms compared with nontreated controls. Interestingly, the addition of DNase I at concentrations ranging from 1 to 64 units/ml to preformed biofilms of mgrA mutants did not abolish the biofilm (data not shown). This finding indicated that extracellular DNA is an important component of the early biofilms of mgrA mutants but not in preformed biofilms. However, with the concentrations of DNase I used in our study, we could not rule out a lack of access to DNA by DNase I in established biofilms.
FIG. 5.
Disruption of the early biofilm formation of mgrA mutants treated with DNase I. The biofilms of RN6390 and MW2 and their corresponding ΔmgrA mutant strains grown in 200 μl of TSB-glucose in microtiter wells for 16 h at 37°C in the presence of DNase I (0.7 units per ml) are represented by the striped bars. Biofilm formation was quantified by washing the biofilms three times with water, staining with crystal violet, and measuring the OD562. The filled bars are the untreated controls. The corresponding views of the microtiter plates are shown below.
Biofilm formation of the mgrA mutant is mediated by agr.
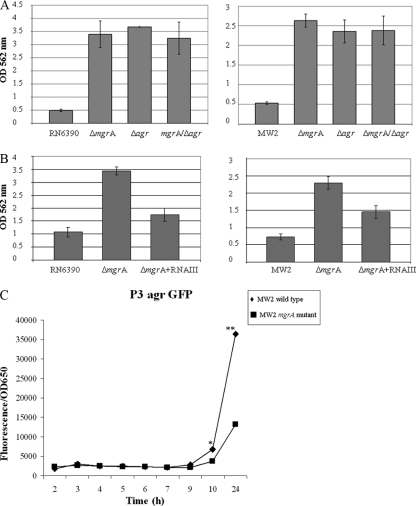
As agr represses the expression of surface proteins and its expression is reduced in mgrA mutants (17), we hypothesized that mgrA mutants may enhance biofilm formation by downregulating agr. The agr locus is composed of two divergent transcripts, RNAII and RNAIII, bearing agrDBCA and hld, respectively. Accordingly, we first examined biofilm formation in agr mutants lacking both RNAII and RNAIII in strains RN6390 and MW2 (Fig. 6A). As expected, a deletion in the agr operon including RNAII and RNAIII resulted in augmented biofilm formation in both RN6390 and MW2, thus emphasizing the importance of agr in repressing biofilm development. The additional deletion of mgrA in the agr mutants did not significantly alter biofilm formation in comparison to single agr mutants, consistent with the notion that mgrA and agr may modulate a similar pathway in biofilm formation.
FIG. 6.
Effect of RNAIII on biofilm formation in mgrA mutants of RN6390 and MW2. (A) agr deletion mutants were compared with mgrA mutants and double mgrA agr mutants for biofilm formation. Biofilms were quantitated by solubilizing the crystal violet in 30% glacial acetic acid and measuring the OD562. Statistical values as determined by the Student t test were as follows: RN6390 versus the mgrA mutant, P < 0.0002; RN6390 versus the agr mutant, P < 0.000001; RN6390 versus the mgrA agr mutant, P < 0.00015; MW2 versus the mgrA mutant, P < 0.0001; MW2 versus the agr mutant, P < 0.004; and MW2 versus the mgrA agr mutant, P < 0.00002. There were no statistical differences among the mgrA, agr, and mgrA agr mutants in both RN6390 and MW2. (B) The plasmid pRN6735 with the blaZ promoter driving RNAIII was introduced into mgrA mutants of RN6390 and MW2 followed by quantitation of the biofilm formation as above, using parents and the mgrA mutant as controls. The P values were as follows: the mgrA mutant of RN6390 versus the mgrA mutant with RNAIII, P < 0.0004; RN6390 versus the mgrA mutant with RNAIII, P < 0.02; the mgrA mutant of MW2 versus the mgrA mutant with RNAIII, P < 0.005; MW2 versus the mgrA mutant with RNAIII, P < 0.003. (C) The plasmid pALC1743 with the RNAIII promoter driving the expression of GFPuvr was introduced into MW2 and its isogenic mgrA mutant. Promoter activity was measured as fluorescence units per OD562 over a 10-h period and then overnight. Each value represents the mean of three distinct clones from each genetic background. * and **, statistical significance by the Student t test when comparing fluorescence at 10 h (P < 0.03) and overnight (P < 0.05), respectively, between MW2 and the isogenic mgrA mutant.
To provide credence that biofilm formation in mgrA mutants is attributable to reduced agr expression, we provided RNAIII expression in trans in mgrA mutants of RN6390 and MW2 with the plasmid pRN6735, which expressed RNAIII under the control of a blaZ promoter (42). Remarkably, the provision of RNAIII in trans significantly reduced biofilm formation in mgrA mutants but not to the level of the respective parents (Fig. 6B). Collectively, these results indicated that enhanced biofilm formation in mgrA mutants is in large part due to the reduced expression of RNAIII of agr as well as to agr-independent but mgrA-mediated factors.
To verify further that mgrA indeed regulates agr in our strain, we introduced the shuttle plasmid pALC1743, a derivative of pSK236 containing the RNAIII promoter driving gfpuvr, into MW2 and its isogenic mgrA mutant. Fluorescence activities were then measured in these two isogenic strains over a 10-h period and then overnight. As shown in Fig. 6C, fluorescence attributable to agr RNAIII promoter activity was highest during the stationary phase of growth for both constructs, consistent with the postexponential nature of agr activation as described in previous studies (17, 31). More importantly, the level of fluorescence was significantly higher in the wild-type MW2 than in the mgrA mutant for the 10-h culture and the overnight samples. This finding, in conjunction with those of RN6390 in a previous study (17), confirmed the positive regulation of agr by mgrA.
The role of sarA in biofilm formation in mgrA mutants.
The global regulator SarA has been shown to be a positive regulator of biofilm formation in S. aureus (3, 41). As SarA is a positive regulator of agr (5) and mgrA negatively modulates biofilm formation via agr, we wanted to determine if sarA, which does not regulate mgrA and vice versa (16), would contribute independently to biofilm formation in mgrA mutants. Accordingly, we assayed for biofilm formation first in sarA mutants of RN6390 and MW2 (Fig. 7A). With RN6390, which is a relatively poor biofilm former, a mutation in sarA did not lead to an appreciable difference in biofilm formation between the mgrA mutant and the parent. In the background of MW2, which is a slightly better biofilm producer, biofilm formation was reduced in sarA mutants versus the respective parents in concordance with previous studies (41); in contrast, the corresponding mgrA mutants exhibited elevated levels of biofilm formation. When the sarA mutation was introduced into the mgrA mutant of strain RN6390, the biofilm phenotype was significantly reduced in the double mutant in comparison to the mgrA mutant, at an intermediate level between the sarA and mgrA mutant, while this effect was more pronounced in the MW2 background (Fig. 7A). This finding suggested that sarA is required for biofilm formation in mgrA mutants. Given the intermediate biofilm phenotype of the double mutant, the sarA-mediated effect on biofilm formation is probably independent of mgrA and also agr, since both the mgrA and sarA mutants have been shown to downregulate agr, but they displayed divergent biofilm phenotypes, with sarA being a positive regulator and agr being a negative regulator.
FIG. 7.
(A) Analysis of biofilm formation in wild-type strains RN6390 and MW2, isogenic mgrA mutants, sarA mutants, and the double ΔmgrA ΔsarA mutants. Significant differences were found between the mgrA mutants and the corresponding ΔmgrA ΔsarA mutants. P values were as follows: RN6390 versus the mgrA mutant, P < 0.002; the mgrA mutant of RN6390 versus the double mgrA sarA mutant, P < 0.02; MW2 versus the mgrA mutant, P < 0.004; the mgrA mutant of MW2 versus the double mgrA sarA mutant, P < 0.003. The results of the microtiter plates are shown below. (B) Biofilm formation in microtiter plates of mgrA mutants, sarA mutants, ΔmgrA ΔsarA mutants, and the ΔmgrA ΔsarA Δaur and ΔmgrA ΔsarA sspA triple mutants (top panels). The corresponding protease activity by these strains on skimmed-milk agar plates is shown in the bottom panels.
To further elucidate the contribution of sarA to the biofilms formed by mgrA mutants, we determined if decreased biofilm formation in mgrA sarA mutants may be due to the upregulation of protease activity typically found in sarA mutants. As displayed in Fig. 7B (bottom panels), protease production was increased in double mgrA sarA mutants compared with the isogenic parent RN6390. However, the introduction of an additional mutation in either the aureolysin (aur) or V8 protease gene (sspA) in the mgrA sarA mutant did not restore the biofilm phenotype to that of the single mgrA mutants (Fig. 7B, top panels), thus suggesting that the upregulation of these protease genes is not the major cause of diminished biofilm formation in sarA mgrA double mutants.
We also examined the role of sigB in biofilm formation in the mgrA sarA mutant of strain SH1000. In contrast to RN6390, SH1000 has a functional rsbU with intact sigB activity. Consistent with previous reports (6, 34), an intact sigB system in strain SH1000 increased biofilm production in comparison to RN6390 (Fig. 7B, right panels). Likewise, the mgrA mutant of SH1000 also exhibited enhanced biofilm formation compared to the parent. As with RN6390, a double mgrA sarA mutant of SH1000 almost completely eliminated biofilm production found in the single mgrA mutant. The inactivation of either aur or sspA in the double mutant did not restore biofilm formation to that of the single mgrA mutant in the SH1000 background. These results verified that the effect of sarA on biofilm formation in the mgrA mutant does not require full sigB activity.
DISCUSSION
MgrA is a 147-residue protein that belongs to a family of regulatory proteins called the SarA protein family by virtue of their homology to SarA of S. aureus (5). Contrary to other SarA protein family members, MgrA is more similar to MarR of E. coli than to SarA of S. aureus (16). Phenotypically, MgrA is a negative regulator of autolysis (16) and a positive regulator of virulence determinants by controlling agr expression (17). Recent studies have linked biofilm formation to cell lysis mediated by cidA (35) and also to autolysis in arlRS mutants (12, 38). Recognizing that an mgrA mutant has an augmented autolytic phenotype (16), we explored and confirmed that a mutation in mgrA led to enhanced biofilm formation in strains RN6390, SH1000, and MW2, while the complemented mutants in all three genetic backgrounds restored the parental biofilm phenotype (Fig. 1). In contrast to previous studies on S. aureus biofilms (6, 21, 34), the biofilm formed by mgrA mutants is not dependent on rsbU, a positive regulator of sigB activity, as evidenced by the comparable biofilm formation between mgrA mutants of the rsbU-defective RN6390 and the rsbU-repaired SH1000 in our study. Surprisingly, our observation on enhanced biofilm formation in mgrA mutants contradicted the findings of Tu Quoc et al., who found, in a forward genetic screen, diminished biofilm production in an mgrA mutant, among others, in a pediatric S. aureus clinical isolate from the University of Geneva Hospital collection called strain S30 (40). The difference between our finding and that of Tu Quoc et al. is not immediately apparent, but it may be due to strain differences. However, it should be emphasized that our studies incorporate both well-characterized laboratory and clinical isolates, while S. aureus S30 is a previously uncharacterized clinical strain.
In a transcriptional profiling study of the mgrA regulon of S. aureus strain Newman (23), Luong et al. found that MgrA upregulates 175 genes while downregulating 180 others. Among these are the positive regulation of lrgAB and the negative regulation of lytN and cidA by MgrA. LytN is a murein hydrolase, while CidA and LrgA are analogous to those of bacteriophage holins and antiholins, respectively. The bacteriophage holin molecule facilitates cell lysis while the antiholin opposes this effect. Accordingly, the negative regulation of CidA and the positive regulation of LrgA by MgrA would reduce cell lysis and the concomitant release of extracellular DNA, which can serve as a component of S. aureus biofilm (18, 35, 37). In particular, programmed cell lysis mediated by CidA has been shown to release extracellular DNA to promote biofilm formation (35) (Fig. 8). In our study, the addition of DNase I to media containing mgrA mutants of RN6390 and MW2 prior to biofilm formation resulted in the complete eradication of biofilms after overnight growth. However, DNase I treatment (0.7 to 64 units/ml) of preformed biofilms had no significant attenuation effect. This latter finding differs from that of Izano et al. (18), who showed that established S. aureus biofilms can be dispersed by a high concentration of DNase I (100 μg/ml or 40 units/ml). These differences cannot be explained by the DNase I dosage effect. However, it is conceivable that the biofilm formed by mgrA mutants may differ from the mature biofilms of wild-type S. aureus strains employed by Izano and colleagues (18). Nonetheless, extracellular DNA is probably an important part of the early biofilm in mgrA mutants of S. aureus. Once a biofilm of an mgrA mutant is formed, the efficacy of DNase I in disrupting the biofilm is less prominent, possibly due to a lack of access to DNA in the lower stratum of the biofilm. However, we did not entirely rule out the possibility that DNA may be a lesser component of the mature biofilm in mgrA mutants.
FIG. 8.

The proposed model of biofilm formation in mgrA mutants. The inactivation of mgrA would reduce agr expression, enhancing surface protein expression, reducing nuclease secretion (23), and hence augmenting biofilm formation. A mutation in mgrA also increases CidA expression as well as reduces LrgA expression (23), thus promoting autolysis and extracellular DNA release to facilitate biofilm formation. SrtA may also affect surface protein expression. The mode of interaction between MgrA and SarA in biofilm formation remains to be defined.
S. aureus can form biofilms that are both dependent on and independent of PIA, a polymer of amino sugar (8, 38, 41). However, a dot blot analysis with anti-PIA antibody as well as transcriptional fusion with an ica promoter driving gfpuvr revealed that biofilms formed by the mgrA mutants are independent of the ica locus which encodes PIA. This was confirmed by the finding that ica expression seemed to be unchanged or slightly decreased in mgrA mutants of RN6390 and MW2.
Besides DNA and PIA, we also evaluated the contribution of surface proteins to the initiation of biofilms in mgrA mutants. Accordingly, we deleted srtA, the sortase A gene, in mgrA mutants, which anchors almost all of the surface proteins to the peptidoglycan chain in S. aureus (29). Interestingly, the deletion of srtA in mgrA mutants of RN6390 and also MW2 reduced the formation of biofilms, indicating that the effect of the mgrA mutation on biofilms is mediated in part by upregulating surface protein expression. As the double mgrA srtA mutant has an intermediate biofilm phenotype compared with the mgrA mutant (Fig. 3) and srtA and mgrA are positive and negative contributors to biofilm formation, respectively, these data indicate that MgrA also represses biofilm formation independently of srtA in S. aureus (Fig. 8).
As an additional assay to evaluate the contribution of surface proteins in early biofilm and preformed biofilm formation in mgrA mutants, we also exposed both sets of biofilms to proteinase K treatment. Treatment of both early and preformed biofilms with proteinase K reduced biofilm formation in mgrA mutants of RN6390 and MW2 to near-parental levels, thus confirming the importance of surface proteins in the initiation and maintenance of biofilms. Based on these studies, we theorize that surface proteins may be important for early adhesion as well as cell-cell aggregation in late biofilm architecture.
SarA is a global transcription factor that enhances biofilm formation, activates the expression of many surface protein adhesins, and also represses the secretion of proteases in S. aureus. In concordance with other studies (3, 41), the sarA mutants of RN6390 and MW2 failed to form biofilms. Interestingly, the introduction of a sarA mutation into mgrA mutants led to significantly lower biofilm formation compared with the single mgrA mutants. Given that sarA does not regulate mgrA and vice versa (16), it is likely that sarA impacts genes involved in the biofilm phenotype (e.g., surface protein genes) downstream of mgrA. As the sarA mutants also express elevated levels of proteases, we also ascertained the role of proteases in reducing biofilm formation in mgrA sarA double mutants of RN6390 and MW2. As anticipated, the double mgrA sarA mutant exhibited enhanced proteolytic activity (Fig. 7B, bottom panels), which theoretically should cleave surface proteins necessary for attachment and/or cell-cell aggregation. However, inactivation of the aureolysin (aur) or the V8 protease gene (sspA) in the double mgrA sarA mutant did not restore biofilm formation to the double mutant. These data make it less likely for proteases to be the prominent mediator of reduced biofilm formation in the mgrA sarA double mutant. However, it should be stressed that S. aureus possesses other protease gene products, including acidic proteases and metalloproteases. Indeed, residual protease activity was still present in the triple mgrA sarA aur and mgrA sarA sspA mutants of RN6390 (Fig. 7B, bottom panels). Therefore, a definitive role for protease in biofilms cannot be assessed until the plethora of protease genes in S. aureus is completely inactivated.
The positive regulation of agr by the mgrA locus (17) (Fig. 6C), coupled with enhanced biofilm formation in agr mutants (32, 44) and mgrA mutants in this study (Fig. 6A), led us to examine the role of agr in biofilm formation in mgrA mutants. As predicted, a double mgrA agr mutation in RN6390 and MW2 resulted in elevated biofilm production compared with the parents, similarly to what we have observed with the single mutants (Fig. 6A). The expression of RNAIII under the control of an exogenous blaZ promoter, which is not subjected to mgrA regulation, could reduce the biofilm formation of mgrA mutants but not to the level of the parents (Fig. 6B), thus suggesting that biofilms formed in mgrA mutants are attributable in part to reduced agr expression. This agr-mediated effect can be explained partially by the increased expression of surface protein adhesins attributable to reduced agr expression in early and established biofilms in mgrA mutants. In addition, recent transcriptional profiling studies have also demonstrated the reduced expression of nuclease (nuc) in the mgrA mutant (23). The reduction in secreted nuclease in mgrA mutants may conceivably expand the pool of extracellular DNA, a core component of early biofilms in these mutants. As agr is also a positive regulator of nuclease, it remains to be determined if the effect of mgrA on nuc is dependent on agr. Finally, the partial complementation by RNAIII in trans in mgrA mutants also implied that additional factors other than RNAIII likely play a role in biofilm formation in mgrA mutants.
In summary, MgrA has previously been shown to be a pleiotropic regulator of autolysis, virulence factors, and efflux activity in S. aureus. We have now added biofilm formation to that list. Based on our studies, it appeared that the biofilms formed by mgrA mutants are not dependent on PIA but instead rely on surface proteins (Fig. 8) and extracellular DNA for early formation and only on surface protein for the maintenance of mature biofilms. The biofilm formed by mgrA mutants is independent of SigB and selective proteases, such as V8 protease and aureolysin, but is reliant on agr and sarA (Fig. 8). Complementation data showed that the effect of mgrA on biofilms is likely mediated in part by regulating agr. However, the manner by which mgrA interfaces with sarA in biofilm formation is not due to agr, since SarA, as a positive regulator of agr (5), enhances biofilm formation while agr does not.
Acknowledgments
We thank George O'Toole, Jr., for critical reading of the manuscript.
This study was supported in part by NIH grant RO1 AI56114 to A.L.C.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 13 October 2008.
REFERENCES
- 1.Arnaud, M., A. Chastanet, and M. Debarbouille. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 706887-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 3591819-1827. [DOI] [PubMed] [Google Scholar]
- 3.Beenken, K. E., J. S. Blevins, and M. S. Smeltzer. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 714206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerca, N., K. K. Jefferson, T. Maira-Litran, D. B. Pier, C. Kelly-Quintos, D. A. Goldmann, J. Azeredo, and G. B. Pier. 2007. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect. Immun. 753406-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung, A. L., A. S. Bayer, G. Zhang, H. Gresham, and Y.-Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Microbiol. Lett. 16491-9. [DOI] [PubMed] [Google Scholar]
- 6.Conlon, K. M., H. Humphreys, and J. P. O'Gara. 2004. Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J. Bacteriol. 1866208-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 2841318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Götz. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 675427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cucarella, C., C. Solano, J. Valle, B. Amorena, I. Lasa, and J. R. Penades. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 1832888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cucarella, C., M. A. Tormo, C. Úbeda, M. P. Trotonda, M. Monzón, C. Peris, B. Amorena, I. Lasa, and J. R. Penadés. 2004. Role of biofilm-associated protein Bap in the pathogenesis of bovine Staphylococcus aureus. Infect. Immun. 722177-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedtke, I., F. Gotz, and A. Peschel. 2004. Bacterial evasion of innate host defenses—the Staphylococcus aureus lesson. Int. J. Med. Microbiol. 294189-194. [DOI] [PubMed] [Google Scholar]
- 12.Fournier, B., and D. C. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 1823955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa, S., S. L. Kuchma, and G. A. O'Toole. 2006. Keeping their options open: acute versus persistent infections. J. Bacteriol. 1881211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 1872426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 1845457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingavale, S., W. Van Wamel, and A. L. Cheung. 2003. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol. Microbiol. 481451-1466. [DOI] [PubMed] [Google Scholar]
- 17.Ingavale, S., W. van Wamel, T. T. Luong, C. Y. Lee, and A. L. Cheung. 2005. Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus. Infect. Immun. 731423-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izano, E. A., M. A. Amarante, W. B. Kher, and J. B. Kaplan. 2008. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 74470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jefferson, K. K., S. E. Cramton, F. Gotz, and G. B. Pier. 2003. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol. Microbiol. 48889-899. [DOI] [PubMed] [Google Scholar]
- 20.Kahl, B., M. Goulian, W. Van Wamel, M. Herrmann, S. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line derived from a cystic fibrosis patient. Infect. Immun. 685385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, J. H., C. H. Kim, J. Hacker, W. Ziebuhr, B. K. Lee, and S. H. Cho. 2008. Molecular characterization of regulatory genes associated with biofilm variation in a Staphylococcus aureus strain. J. Microbiol. Biotechnol. 1828-34. [PubMed] [Google Scholar]
- 22.Lowy, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 23.Luong, T. T., P. M. Dunman, E. Murphy, S. J. Projan, and C. Y. Lee. 2006. Transcription profiling of the mgrA regulon in Staphylococcus aureus. J. Bacteriol. 1881899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mack, D., P. Becker, I. Chatterjee, S. Dobinsky, J. K. Knobloch, G. Peters, H. Rohde, and M. Herrmann. 2004. Mechanisms of biofilm formation in Staphylococcus epidermidis and Staphylococcus aureus: functional molecules, regulatory circuits, and adaptive responses. Int. J. Med. Microbiol. 294203-212. [DOI] [PubMed] [Google Scholar]
- 25.Mack, D., W. Fischer, A. Krokotsch, K. Leopold, R. Hartmann, H. Egge, and R. Laufs. 1996. The intracellular adhesin involved in biofilm accumulation of S. epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178175-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 934-39. [DOI] [PubMed] [Google Scholar]
- 27.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1989. Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 28.Manna, A. C., S. S. Ingavale, M. Maloney, W. Van Wamel, and A. L. Cheung. 2004. Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J. Bacteriol. 1865267-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 401049-1057. [DOI] [PubMed] [Google Scholar]
- 30.Novick, R. P. 1990. The staphylococcus as a molecular genetic system, p. 1-40. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH, New York, NY.
- 31.Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 481429-1449. [DOI] [PubMed] [Google Scholar]
- 32.Pratten, J., S. J. Foster, P. F. Chan, M. Wilson, and S. P. Nair. 2001. Staphylococcus aureus accessory regulators: expression within biofilms and effect on adhesion. Microbes Infect. 3633-637. [DOI] [PubMed] [Google Scholar]
- 33.Projan, S. J., and R. P. Novick. 1997. The molecular basis of pathogenicity, p. 55-81. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone, New York, NY.
- 34.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 1826824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rice, K. C., E. E. Mann, J. L. Endres, E. C. Weiss, J. E. Cassat, M. S. Smeltzer, and K. W. Bayles. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 1048113-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schenk, S., and R. A. Laddaga. 1992. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol. Lett. 94133-138. [DOI] [PubMed] [Google Scholar]
- 37.Spoering, A. L., and M. S. Gilmore. 2006. Quorum sensing and DNA release in bacterial biofilms. Curr. Opin. Microbiol. 9133-137. [DOI] [PubMed] [Google Scholar]
- 38.Toledo-Arana, A., N. Merino, M. Vergara-Irigaray, M. Debarbouille, J. R. Penades, and I. Lasa. 2005. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J. Bacteriol. 1875318-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trotonda, M. P., A. C. Manna, A. L. Cheung, I. Lasa, and J. R. Penades. 2005. SarA positively controls bap-dependent biofilm formation in Staphylococcus aureus. J. Bacteriol. 1875790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tu Quoc, P. H., P. Genevaux, M. Pajunen, H. Savilahti, C. Georgopoulos, J. Schrenzel, and W. L. Kelley. 2007. Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect. Immun. 751079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valle, J., A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa. 2003. SarA and not σB is essential for biofilm development by Staphylococcus aureus. Mol. Microbiol. 481075-1087. [DOI] [PubMed] [Google Scholar]
- 42.Vandenesch, F., J. Kornblum, and R. Novick. 1991. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J. Bacteriol. 1736313-6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vuong, C., S. Kocianova, J. M. Voyich, Y. Yao, E. R. Fischer, F. R. DeLeo, and M. Otto. 2004. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J. Biol. Chem. 27954881-54886. [DOI] [PubMed] [Google Scholar]
- 44.Vuong, C., H. L. Saenz, F. Gotz, and M. Otto. 2000. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 1821688-1693. [DOI] [PubMed] [Google Scholar]