Abstract
The pathogenesis of Staphylococcus aureus infections is influenced by multiple virulence factors that are expressed under variable conditions, and this has complicated the design of an effective vaccine. Clinical trials that targeted the capsule or clumping factor A (ClfA) failed to protect the recipients against staphylococcal infections. We passively immunized lactating mice with rabbit antibodies to S. aureus capsular polysaccharide (CP) serotype 5 (CP5) or CP8 or with monoclonal antibodies to ClfA. Mice immunized with antibodies to CP5 or CP8 or with ClfA had significantly reduced tissue bacterial burdens 4 days after intramammary challenge with encapsulated S. aureus strains. After several passages in mice passively immunized with CP-specific antiserum, increasing numbers of stable unencapsulated variants of S. aureus were cultured from the infected mammary glands. Greater numbers of these unencapsulated S. aureus variants than of the corresponding encapsulated parental strains were internalized in vitro in MAC-T bovine cells. Furthermore, small-colony variants (SCVs) were recovered from the infected mammary glands after several passages in mice passively immunized with CP-specific antiserum. A combination of antibodies effectively sterilized mammary glands in a significant number of passively immunized mice. More importantly, passive immunization with antibodies to both CP and ClfA fully inhibited the emergence of unencapsulated “escape mutants” and significantly reduced the appearance of SCVs. A vaccine formulation comprising CP conjugates plus a surface-associated protein adhesin may be more effective than either antigen alone for prevention of S. aureus infections.
Staphylococcus aureus is a highly prevalent, opportunistic, multifactorial pathogen that causes both community-acquired and life-threatening nosocomial infections (28). The control of staphylococcal infections is threatened by the emergence of community-acquired methicillin-resistant S. aureus strains (12, 20), as well as strains that show reduced susceptibility to vancomycin (3). Rational design of a staphylococcal vaccine is a logical approach for preventing the morbidity and mortality associated with S. aureus infections and for decreasing the medical costs associated with severe staphylococcal disease. Numerous targets have been identified for inclusion in vaccines to prevent S. aureus infections in humans, but most of these vaccines are still in the preclinical development stage (35). S. aureus capsular polysaccharide (CP) serotype 5 (CP5) and CP8 are produced by ∼75% of human isolates, and it has been shown that antibodies to the CPs have some protective efficacy for preventing staphylococcal infections in experimental animals (8, 22). However, two phase III clinical trials of a combined CP5-CP8 conjugate vaccine failed to show a cumulative reduction in episodes of S. aureus bacteremia in patients undergoing hemodialysis (38; http://www.nabi.com/pipeline/clinicaltrials.php#1).
There were probably numerous factors that were responsible for the failed CP vaccine trials, and these factors are poorly understood. Certainly, a vaccine that targets only encapsulated S. aureus does not protect against the 20 to 25% of clinical isolates that lack a capsule. Previous studies have demonstrated that CP is produced in vivo in animal models of staphylococcal infection (19, 23). However, the expression of S. aureus CP5 and CP8 is highly sensitive to several environmental signals, including nutrient and iron availability (32). In addition, CP is not expressed by S. aureus in the logarithmic phase of growth (32) or in the presence of ≥1% CO2 (15, 26). Furthermore, CP expression has been shown to be downregulated during chronic staphylococcal lung infection in cystic fibrosis patients (14) and in a guinea pig model of S. aureus implant infection (10). We have shown that loss of CP expression facilitates S. aureus internalization into bovine epithelial cells (2, 44) and contributes to the persistence of S. aureus in infected mammary glands of mice (44). Therefore, development of a vaccine for S. aureus requires addition of other bacterial components, as suggested previously (42), to ensure effectiveness with capsule-negative S. aureus.
Clumping factor A (ClfA) is a cell wall-anchored S. aureus surface protein that has been shown to enhance staphylococcal virulence in animal infection models (27, 49). ClfA has been suggested as a vaccine potential candidate (17, 18, 31) and as a target for passive immunization approaches (6, 33, 46). In a phase III clinical trial, 1,983 neonates received either a placebo or INH-A21 (Veronate), a pooled human immunoglobulin preparation from donors selected on the basis of high antibody titers against staphylococcal S. aureus ClfA and Staphylococcus epidermidis SdrG. There was no difference between the rates of late-onset sepsis caused by S. aureus or coagulase-negative staphylococci in the two groups (5). To date, vaccine trials targeting S. aureus have been performed only with immunocompromised patient populations (hemodialysis patients and premature neonates).
The present study was designed to determine whether antibodies to the S. aureus CPs or ClfA, given separately or together, prevent staphylococcal infection in a mouse model of mastitis. This model represents a well-characterized, sublethal, and localized S. aureus infection that mimics ruminant mastitis due to similarities between mice and cows (30). We also assessed the phenotype of the staphylococci cultured from the passively immunized animals to determine whether antibodies to CP selected in vivo for the emergence of escape mutants of S. aureus that lacked a capsule. Recovery of S. aureus small-colony variants (SCVs) from the infected animals passively immunized with CP antiserum was an unanticipated finding.
MATERIALS AND METHODS
Bacterial strains and antibodies.
Bovine S. aureus strain RA9 is a capsule serotype 5 strain obtained from a cow with mastitis and was provided by L. Calvinho, Estación Experimental INTA, Santa Fé, Argentina. Bovine strain MBC212 (CP8 positive) and strains Reynolds (CP5) and Reynolds (CP8), derived from serotype 5 human isolate Reynolds, have been described elsewhere (4, 48). S. aureus was cultured overnight on tryptic soy agar (TSA) plates (Difco, Detroit, MI). Bacterial suspensions were prepared in phosphate-buffered saline (PBS) and diluted to obtain suspensions containing 2 × 107 CFU/ml. The species of variants obtained in animal experiments were confirmed by PCR amplification of species-specific sequences as described by Martineau et al. (25).
Antibodies to CP5 or CP8 were obtained by immunization of rabbits with killed encapsulated bacteria as described previously (21), followed by absorption of the sera with unencapsulated mutant strains to make them specific for CP5 or CP8. Preimmune rabbit serum was nonreactive as determined by immunodiffusion with bacterial extracts containing CP5 or CP8. Monoclonal antibodies (MAbs) to ClfA (immunoglobulin G1; Aurexis 904-A1 INH-H06048; lot 27E001-4E7042) were kindly provided by Inhibitex Inc. (Alpharetta, GA) (13, 36).
Mouse models.
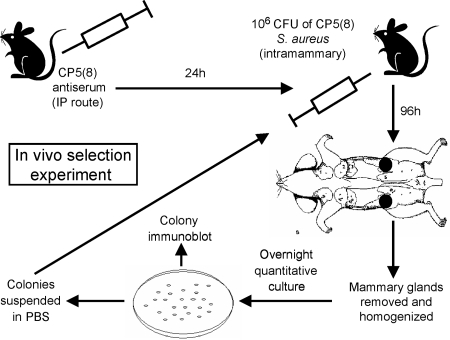
CF1 outbred mice were maintained in the vivarium of the Department of Microbiology, School of Medicine, University of Buenos Aires, Buenos Aires, Argentina, in accordance with the guidelines of the National Institutes of Health (29). The mouse model of mastitis has been described previously (11, 44). Groups of 8 to 10 mice were passively immunized by the intraperitoneal (i.p.) route with either 0.5 ml of anti-CP5 serum, 0.5 ml of anti-CP8 serum, 0.5 ml of preimmune rabbit serum, or 10 mg/kg of ClfA MAbs. Additional groups of mice received antibodies to both CP5 and ClfA. After 24 h the mice were challenged by injection of 106 CFU S. aureus in 50 μl into the left fourth and right fourth mammary glands. After 4 days the left fourth and right fourth mammary glands were excised from each animal and homogenized separately in 2 ml of tryptic soy broth. Dilutions of the homogenates were plated quantitatively on TSA plates. CP production was evaluated by colony immunoblotting (21) on TSA plates with 30 to 150 S. aureus colonies. Staphylococcal colonies were harvested from duplicate plates and suspended in PBS, and 106 CFU was injected into another group of lactating mice (Fig. 1). This cycle of in vivo passage and bacterial retrieval was repeated up to 16 times. To determine whether the unencapsulated (also known as nontypeable [NT]) (4) S. aureus variants recovered from mice with mastitis reverted to production of CP in the bloodstream, we challenged groups of 6 to 10 mice by the i.p. route with 200 μl of a suspension containing 108 CFU of an NT variant of serotype 5 strain RA9. Mice were euthanized 24 h after challenge, and blood was extracted by cardiac puncture and plated in duplicate on TSA plates. Additional mice were challenged with bacteria recovered from the TSA plate, and this cycle was repeated up to 10 times. The production of capsule by colonies plated from the blood after each cycle was assessed by colony immunoblotting.
FIG. 1.
Experimental regimen consisting of consecutive cycles of passive immunization with rabbit antiserum or MAbs followed by challenge with in vivo-passaged S. aureus. The capsule phenotype of colonies recovered from each infection cycle was assessed by a colony immunoblot method. IP, intraperitoneal.
Cell culture assays.
The MAC-T bovine mammary epithelial cell line (16) was generously provided by Nexia Biotechnologies (Quebec, Canada). MAC-T cells were grown in Dulbecco's modified Eagle's medium (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Gibco BRL), insulin (5 μg/ml), hydrocortisone (5 μg/ml), penicillin (100 U/ml), and streptomycin sulfate (100 μg/ml) (Sigma Chemical Co., St. Louis, MO). Prior to each experiment, MAC-T cells were seeded at a concentration of 1.5 × 105 cells/well in 24-well tissue culture plates and grown for 1 day at 37°C with 5% CO2. Confluent MAC-T cell monolayers (∼2 × 105 to 2.5 × 105 cells/well) were washed four times with sterile PBS, and cell viability was evaluated by trypan blue exclusion. MAC-T cell monolayers were then inoculated with S. aureus strains suspended in fresh growth medium without antibiotics (invasion medium) using a multiplicity of infection of 40. The tissue culture plates were subjected to centrifugation at 1,000 × g for 20 min to deposit S. aureus cells on the monolayer surface. After incubation for 1 h at 37°C under 5% CO2, the wells were washed with PBS, and 1 ml of invasion medium supplemented with 25 μg/ml of lysostaphin (Sigma) was added to each well to kill the extracellular bacteria. After 2 h of incubation at 37°C with 5% CO2, the culture supernatants were collected and plated on TSA. No growth was detected in any cell culture supernatant, indicating that 100% of the extracellular S. aureus cells were lysed by lysostaphin. The monolayer was washed four times with sterile PBS, treated for 5 min at 37°C with 100 μl of 0.25% trypsin-0.1% EDTA (Gibco BRL), and lysed by addition of 900 μl of 0.025% Triton X-100 (USB, Cleveland, OH) in sterile distilled water to release intracellular staphylococci. The numbers of CFU/ml in the cell lysates were determined by plating serial dilutions on TSA plates.
Statistical analyses.
Quantitative culture data for tissue homogenates were compared by using the Mann-Whitney test for nonparametric data. P values of <0.05 were considered significant. The Prism 4.0 software (GraphPad) was used for all calculations.
RESULTS
Passive immunization with CP-specific antiserum.
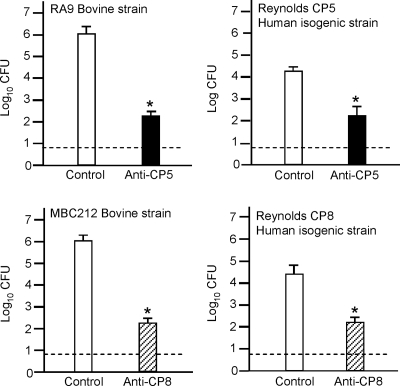
We passively immunized mice i.p. with CP-specific antiserum or nonimmune serum 24 h before intramammary challenge with 106 CFU S. aureus (Fig. 1). The challenge strains included bovine isolates RA9 (CP5 positive) and MBC212 (CP8 positive) and human strains Reynolds (CP5) and Reynolds (CP8). Compared to animals given preimmune serum, mice given antibodies to CP5 or CP8 showed significant reductions in the numbers of S. aureus CFU recovered from the infected glands after 4 days (Fig. 2). The protective effect of CP antibodies was most striking for bovine isolates MBC212 and RA9 (4-log10 reductions in the bacterial burden compared to mice given preimmune serum). Similarly, a 2-log10 reduction in the number of CFU/gland was observed for mice passively immunized with CP antibodies and challenged with human isolate Reynolds (CP5) or Reynolds (CP8). In vitro experiments performed with strains RA9 and MBC212 indicated that rabbit antiserum to CP5 and rabbit antiserum to CP8, respectively, had no direct effect on S. aureus viability (data not shown).
FIG. 2.
Passive immunization with CP antibodies reduced the bacterial burden in the mammary glands of lactating mice. Each bar indicates the mean log10 CFU/gland obtained in cycle 1, and the error bars indicate the standard errors of the means (six to eight mice/group). The dotted line indicates the limit of detection (0.7 log CFU/gland). *, significant difference in the number of CFU at 96 h after challenge of mice inoculated with immune or nonimmune serum. The levels of significance were as follows: for CP5-positive strain RA9, P < 0.001; for CP8-positive strain MBC212, P < 0.001; for Reynolds (CP5), P = 0.014; and for Reynolds (CP8), P = 0.001.
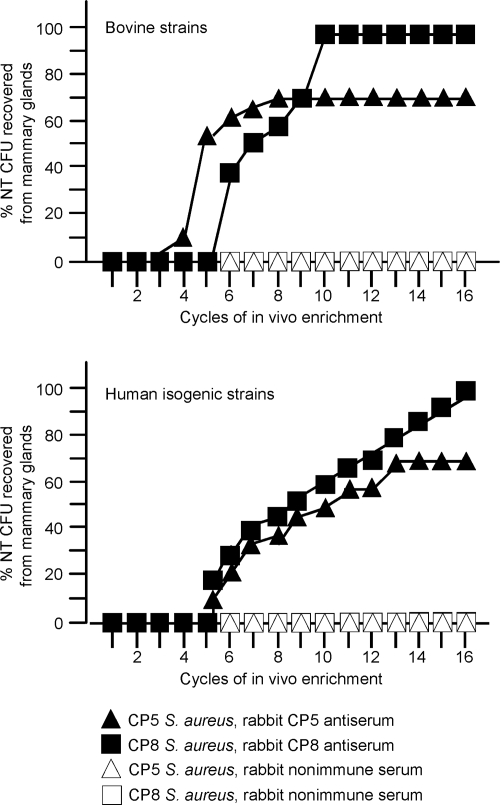
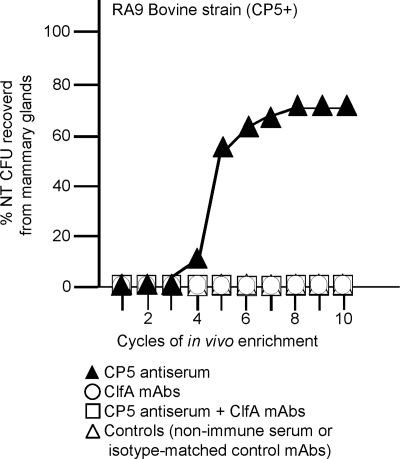
S. aureus cells recovered after 4 days from mice given antibodies specific to CP5 or CP8 were injected into another group of passively immunized mice in the second cycle of the experiment (Fig. 1). The cycle of challenge and recovery from the mammary glands was repeated 16 times, and S. aureus colonies were tested after each cycle for CP production. All of the colonies recovered from mice given preimmune or immune serum during the first three cycles of passage were CP positive. By the fourth cycle, however, a few colonies of S. aureus RA9 cultured from the mammary tissue homogenates of passively immunized mice showed a loss of CP5 production (Fig. 3, upper panel). The percentage of NT S. aureus colonies recovered from the infected glands increased rapidly thereafter and reached a plateau (∼70%) by the eighth cycle. Similarly, NT S. aureus emerged after five or six mouse passages from passively immunized animals challenged with bovine isolate MBC212 (Fig. 3, upper panel) or the isogenic CP5-positive and CP8-positive derivatives of strain Reynolds (Fig. 3, lower panel). With NT derivatives obtained in vivo there was positive PCR amplification for S. aureus-specific sequences. In addition, the SmaI pulsed-field gel electrophoresis band patterns of NT strains recovered from different mice coincided with those of the encapsulated strains inoculated into the mice in the first cycle of the experiment (not shown). NT S. aureus was obtained from mammary gland homogenates of none of the control mice followed for up to 16 passages, and staphylococci were not recovered from the liver, kidneys, spleens, or lungs of mice in either group 4 days after bacterial inoculation. It is noteworthy that the protection afforded by passive immunization with CP-specific antibodies (Fig. 2) was apparent only when most of the S. aureus cells recovered from the infected glands were CP positive (i.e., after fewer than six infection cycles) (Fig. 3). The CP antibody-mediated reduction in the bacterial burden decreased thereafter, and there was no decrease in the number of CFU/gland when all of the S. aureus cells recovered from the glands were NT (data not shown).
FIG. 3.
CP antiserum selects for NT S. aureus in the mammary glands of mice challenged with CP-positive S. aureus. Each symbols indicates the percentage of NT S. aureus variants obtained after a cycle of enrichment. (Upper panel) Strains RA9 (CP5) and MBC212 (CP8). (Lower panel) Strains Reynolds (CP5) and Reynolds (CP8).
In vivo reversion of NT S. aureus.
NT S. aureus recovered from the mammary glands of mice given CP antiserum did not revert to CP production after 10 in vitro passages on Columbia salt agar, a medium that supports optimal CP expression. To determine whether reversion might occur in vivo, we challenged groups of 6 to 10 nonlactating, naïve mice by the i.p. route with 108 CFU of a stable NT derivative of strain RA9. Twenty-four hours later, ∼103 CFU S. aureus/ml was recovered from the mouse blood samples. No CP5-positive colonies were detected after the first or second passages. By the third passage, however, 11% of the colonies isolated from the blood expressed CP5. The percentage of CP5-positive S. aureus colonies increased to 25% by the fourth cycle and remained unchanged through the 10th cycle of the experiment.
Passive immunization with MAbs to S. aureus ClfA.
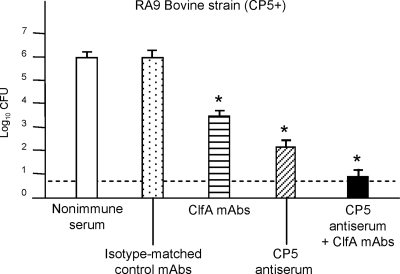
Additional groups of lactating mice were passively immunized i.p. with MAbs to S. aureus ClfA or with isotype-matched control MAbs. Administration of the ClfA MAbs to mice prior to challenge with bovine strain RA9 (CP5 positive) resulted in a 3-log10 reduction in the tissue bacterial burden compared to that in mice given control MAbs (Fig. 4). Passive immunization with antibodies to both CP5 and ClfA had an additive effect, effectively sterilizing 17 (42%) of 40 mammary glands infected with S. aureus RA9 during experimental cycles 1 through 4. CP5 and ClfA antibody-mediated protection against infection of the mammary glands persisted for up to 10 infection cycles (data not shown). Moreover, NT S. aureus colonies were not recovered from the mammary glands of mice passively immunized with ClfA MAbs alone or in combination with CP5 antibodies for up to 10 infection cycles (Fig. 5).
FIG. 4.
Passive immunization with antibodies to CP5 and ClfA reduced the intramammary bacterial load 96 h after intramammary challenge. Each bar indicates the mean log10 CFU S. aureus/gland, and the error bars indicate the standard errors of the means (six to eight mice/group). The dotted line indicates the limit of detection by culture (0.7 log CFU/gland). *, significant difference in the number of CFU at 96 h after challenge between mice inoculated with immune serum and the corresponding controls. The levels of significance were as follows: for mice passively immunized with ClfA MAbs versus the control, P < 0.01; for mice passively immunized with CP5 antiserum versus the control, P < 0.01; and for mice passively immunized with ClfA MAbs plus CP5 antiserum versus either control group, P < 0.001.
FIG. 5.
Emergence of NT S. aureus in the mammary glands of mice challenged with S. aureus strain RA9 (CP5 positive) did not occur in mice inoculated with ClfA MAbs (with or without CP5 antibodies) up to the 10th experimental cycle. Each symbol indicates the percentage of NT S. aureus colonies obtained after a cycle of enrichment.
Internalization in MAC-T cells.
CP-positive S. aureus strains avoid internalization by professional phagocytes, as well as by other types of mammalian cells (2, 24, 43, 44, 48). Consistent with this observation, the numbers of cells of NT S. aureus variants (derived from mice passively immunized with CP5 antiserum before challenge with strain RA9) internalized in vitro in MAC-T cells were greater than the numbers of cells of the corresponding encapsulated parental strains internalized (for NT variants, 1.96 × 106 ± 0.02 × 106 CFU/ml of cell lysate; for RA9, 1.37 × 106 ± 0.02 × 106 CFU/ml of cell lysate [arithmetic means ± standard errors of the means]). Each CFU value is the average for 11 wells from a representative experiment. The well-to-well dispersion was extremely low, and the experiment was performed three times with similar results. Likewise, increased internalization of NT S. aureus was observed in experiments performed with an NT S. aureus variant derived from mice passively immunized with CP8 antiserum before challenge with strain MBC212 (for the NT variant, 1.23 × 105 ± 0.02 × 105 CFU/ml of cell lysate; for MBC212, 0.39 × 105 ± 0.01 × 105 CFU/ml cell of lysate). The results of the present study are consistent with previous results obtained in our laboratory with strains Reynolds (CP5) and Reynolds (CP8) and the isogenic derivative Reynolds (CP−) (44).
Selection of SCVs by passive immunization with CP-specific antiserum.
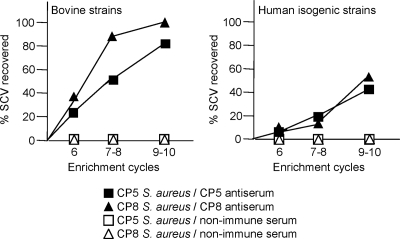
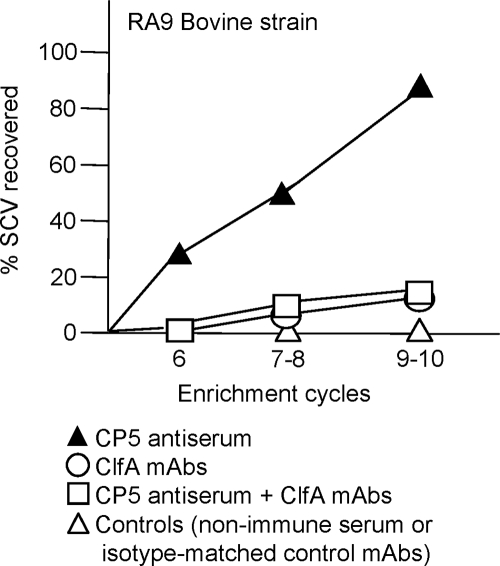
All of the mammary glands (8 to 10 glands per enrichment cycle) from mice treated with either CP5- or CP8-specific antiserum and inoculated with bovine strain RA9 (CP5 positive) or MBC212 (CP8 positive) yielded SCVs by the sixth enrichment cycle (Fig. 6, left panel). SCVs also emerged in mice challenged with human strain Reynolds (CP5) or Reynolds (CP8) (Fig. 6, right panel) but not from mice given nonimmune serum. Most SCVs obtained in the sixth cycle reverted to a normal colony phenotype after one or two in vitro subcultures, whereas some SCVs recovered after the ninth cycle exhibited a more stable phenotype that did not revert after seven passages. SCVs were detected only after 48 h of incubation at 37°C, and the small colonies showed little pigmentation, hemolysis, or coagulase activity (positive only after 18 h of incubation at 37°C). The CP phenotype was assessed for SCVs that emerged in vivo from mice infected with strains RA9 (n = 78), MBC212 (n = 80), Reynolds (CP5) (n = 93), and Reynolds (CP8) (n = 89); all 340 SCVs were CP positive and exhibited positive PCR amplification of S. aureus-specific sequences. The auxotrophies and genetic lesions responsible for the SCV phenotypes of strains recovered from mice with mastitis were not characterized, but they have been described in previous reports and were summarized in a recent review (34). Only a few SCVs were detected in the mammary glands of mice given both CP5 antibodies and ClfA MAbs, and these SCVs were not apparent until the seventh infection cycle (Fig. 7).
FIG. 6.
Recovery of SCVs from the mammary glands of passively immunized mice during the enrichment experiment. The SCVs were isolated from mice treated with CP antibodies and challenged with bovine isolate RA9 or MBC212 (left panel) or human isogenic strain Reynolds (CP5) or Reynolds (CP8) (right panel). SCVs were recovered from the majority of mice inoculated with the bovine RA9 or MBC212 strain but from only one-half of the mice challenged with Reynolds (CP5) or Reynolds (CP8). SCVs were not detected in tissues from mice inoculated with nonimmune rabbit serum.
FIG. 7.
Passive immunization with CP5 antiserum, as shown in Fig. 1, resulted in emergence of S. aureus SCVs. Injection of ClfA MAbs plus CP5 antiserum significantly (P < 0.001) reduced the emergence of S. aureus SCVs in the mammary glands of mice challenged with S. aureus strain RA9 (CP5 positive) up to the 10th infection cycle. Passive immunization with ClfA MAbs prevented the emergence of S. aureus SCVs. Each symbol indicates the percentage of S. aureus SCV derivatives obtained for a cycle(s) of enrichment.
DISCUSSION
Passive immunization with antibodies to CP5 and CP8 significantly reduced the bacterial load in S. aureus infections in a mouse model of mastitis. Encapsulated staphylococci, opsonized with antibodies to CP, were likely cleared more efficiently from the mammary glands through phagocytic uptake and killing by professional phagocytes recruited to the infection site. This is the first report that documents that antibodies to CP8 protect against staphylococcal infection, since previous studies focused on protection against infections caused by serotype 5 S. aureus isolates (9, 22). Our findings underscore the efficacy of CP antibody-mediated prevention of S. aureus infection of the mammary gland and confirm previous suggestions that CP5 and CP8 are critical components of an S. aureus vaccine (7, 39). Passive administration of MAbs to ClfA also resulted in a significant decrease in the intramammary bacterial load in mice challenged with CP-positive S. aureus isolates. Whether these antibodies are opsonic or block staphylococcal adherence to mammalian tissues has not been determined yet. It is noteworthy that with passive immunization with both ClfA MAbs and CP5 antiserum there was an additive effect and that the mammary glands were effectively cleared of viable staphylococci. Thus, antibodies targeting a surface polysaccharide and a cell wall-associated protein provided better protection than either of the antibodies alone. Similar findings were reported by Stranger-Jones et al., who showed that a vaccine comprising multiple S. aureus adhesins provided enhanced protection against mouse lethality compared to any of the individual proteins (42).
Our results also provide evidence that antibodies specific to CP5 or CP8 promoted selection and emergence of NT S. aureus in vivo. Although several mechanisms can explain the loss of CP expression by S. aureus, point mutations in essential cap5 and cap8 genes are most prevalent in NT human and bovine isolates (4, 41, 45). Alternatively, loss of CP expression by S. aureus in mice passively immunized with antibodies to CP5 or CP8 may result from mutations in regulatory genes like agr or arlRS. Most of the NT derivatives obtained in our study were hemolytic, suggesting that they were not agr mutants. In the absence of the selective pressure provided by antibodies to CP, the CP5-positive phenotype was restored in the bloodstream of nonimmune mice challenged with the experimental NT variants.
Thakker et al. (43) demonstrated the critical role played by CP in staphylococcal bloodstream infection since CP5-positive S. aureus resulted in a higher level of mouse bacteremia than unencapsulated mutants. In contrast, CP5 and CP8 attenuated virulence in a mouse model of staphylococcal mastitis and reduced internalization by bovine mammary epithelial cells in vitro (2, 44). Thus, S. aureus readily adapts to its microenvironment; CP-expressing bacterial cells survive in the bloodstream, whereas unencapsulated variants adhere, invade, and selectively persist within infected tissues. Our findings suggest that antibodies to CP5 and CP8 may enhance the clearance of encapsulated S. aureus from infected mice but, at the same time, select for a bacterial subpopulation (unencapsulated) that can be internalized within epithelial cells, thereby avoiding further immune clearance.
S. aureus SCVs have been implicated in chronic and persistent staphylococcal infections (1) and in S. aureus intracellular survival (9, 40, 47). Our study demonstrated that selective pressure exerted in vivo by antibodies to CP5 and CP8 led to the emergence of NT variants and SCVs. Whereas it is logical that NT “escape mutants” might emerge in the presence of antibodies to CP5 or CP8, it is not apparent how administration of these antibodies resulted in the emergence in vivo of SCVs. It is possible that NT variants of S. aureus were internalized within epithelial cells and that this facilitated evolution of SCVs in the intracellular milieu. However, our SCVs expressed CP5, consistent with previous investigations that showed that there was upregulation of capsule genes in S. aureus SCVs (37). The emergence of stable NT S. aureus variants and the emergence of SCVs in our mouse infection model seemed to be independent phenomena, since such variants were isolated simultaneously from the infected glands of lactating mice. The environmental factors that trigger SCV formation are poorly understood. It is possible that downregulation of CP expression in vivo may generate unstable NT phenotypes that are internalized within epithelial cells. Once in the intracellular milieu, the NT S. aureus variants may evolve into SCVs and regain CP expression. This scenario might explain the concomitant emergence of stable NT S. aureus variants and CP-positive SCVs in our mastitis infection model.
We conclude that a vaccine formulation comprising CP conjugates plus a surface-associated protein adhesin may be more effective than either antigen alone for prevention of S. aureus infections. We showed that antibodies to ClfA enhanced the protection against infection provided by antibodies to the CPs. Furthermore, administration of antibodies to CP and ClfA abrogated the emergence of NT S. aureus and decreased the recovery of SCVs from the infected mouse tissues. Whether this combination of antibodies protects against other types of staphylococcal infections merits further investigation.
Acknowledgments
This study was partially supported by grants from the NIH Fogarty Foundation (grant FIRCA 1-R03-TW006264) and the NIAID (grant RO1 AI29040) to J.C.L., from the Agencia Nacional de Promoción de la Ciencia y la Tecnología, Argentina (grants ANPCyT PICT 05-10648 and ANPCyT PICT 05-32577), and from the Secretaría de Ciencia y Técnica, Universidad de Buenos Aires, Argentina (grant UBACyT M-070).
We thank Joseph M. Patti, Inhibitex, Inc., Alpharetta, GA, for providing ClfA MAbs (Aurexis). We also thank Lorena Medina for her dedicated technical assistance and Sabrina Soldavini for her assistance with animal experiments.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Brouillette, E., A. Martinez, B. J. Boyll, N. E. Allen, and F. Malouin. 2004. Persistence of a Staphylococcus aureus small-colony variant under antibiotic pressure in vivo. FEMS Immunol. Med. Microbiol. 4135-41. [DOI] [PubMed] [Google Scholar]
- 2.Buzzola, F. R., L. P. Alvarez, L. P. N. Tuchscherr, M. S. Barbagelata, S. M. Lattar, L. Calvinho, and D. O. Sordelli. 2007. Differential abilities of capsulated and noncapsulated Staphylococcus aureus isolates from diverse agr groups to invade mammary epithelial cells. Infect. Immun. 75886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb. Mortal. Wkly. Rep. 51565-567. [PubMed] [Google Scholar]
- 4.Cocchiaro, J. L., M. I. Gómez, A. Risley, R. Solinga, D. O. Sordelli, and J. C. Lee. 2006. Molecular characterization of the capsule locus from nontypeable Staphylococcus aureus. Mol. Microbiol. 59948-960. [DOI] [PubMed] [Google Scholar]
- 5.DeJonge, M., D. Burchfield, B. Bloom, M. Duenas, W. Walker, M. Polak, E. Jung, D. Millard, R. Schelonka, F. Eyal, A. Morris, B. Kapik, D. Roberson, K. Kesler, J. Patti, and S. Hetherington. 2007. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J. Pediatr. 151260-265. [DOI] [PubMed] [Google Scholar]
- 6.Domanski, P. J., P. R. Patel, A. S. Bayer, L. Zhang, A. E. Hall, P. J. Syribeys, E. L. Gorovits, D. Bryant, J. H. Vernachio, J. T. Hutchins, and J. M. Patti. 2005. Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus. Infect. Immun. 735229-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fattom, A., S. Fuller, M. Propst, S. Winston, L. Muenz, D. He, R. Naso, and G. Horwith. 2004. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23656-663. [DOI] [PubMed] [Google Scholar]
- 8.Fattom, A. I., J. Sarwar, A. Ortiz, and R. Naso. 1996. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect. Immun. 641659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garzoni, C., P. Francois, A. Huyghe, S. Couzinet, C. Tapparel, Y. Charbonnier, A. Renzoni, S. Lucchini, D. P. Lew, P. Vaudaux, W. L. Kelley, and J. Schrenzel. 2007. A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics 8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goerke, C., and C. Wölz. 2004. Regulatory and genomic plasticity of Staphylococcus aureus during persistent colonization and infection. Int. J. Med. Microbiol. 294195-202. [DOI] [PubMed] [Google Scholar]
- 11.Gómez, M. I., V. E. García, M. M. Gherardi, M. C. Cerquetti, and D. O. Sordelli. 1998. Intramammary immunization with live-attenuated Staphylococcus aureus protects mice from experimental mastitis. FEMS Immunol. Med. Microbiol. 2021-27. [DOI] [PubMed] [Google Scholar]
- 12.Haley, C. C., D. Mittal, A. Laviolette, S. Jannapureddy, N. Parvez, and R. W. Haley. 2007. Methicillin-resistant Staphylococcus aureus infection or colonization at hospital admission: multivariable risk factor screening to increase efficiency of surveillance culturing. J. Clin. Microbiol. 453031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall, A. E., P. J. Domanski, P. R. Patel, J. H. Vernachio, P. J. Syribeys, E. L. Gorovits, M. A. Johnson, J. M. Ross, J. T. Hutchins, and J. M. Patti. 2003. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect. Immun. 716864-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbert, S., S. W. Newell, C. Lee, K. P. Wieland, B. Dassy, J. M. Fournier, C. Wölz, and G. Döring. 2001. Regulation of Staphylococcus aureus type 5 and type 8 polysaccharides by CO2. J. Bacteriol. 1834309-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert, S., D. Worlitzsch, B. Dassy, A. Boutonnier, J. M. Fournier, G. Bellon, A. Dalhoff, and G. Döring. 1997. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J. Infect. Dis. 176431-438. [DOI] [PubMed] [Google Scholar]
- 16.Huynh, H. T., G. Robitaille, and J. D. Turner. 1991. Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp. Cell Res. 197191-199. [DOI] [PubMed] [Google Scholar]
- 17.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 1841572-1580. [DOI] [PubMed] [Google Scholar]
- 18.Josefsson, E., J. Higgins, T. J. Foster, and A. Tarkowski. 2008. Fibrinogen binding sites P336 and Y338 of clumping factor A are crucial for Staphylococcus aureus virulence. PLoS ONE 3e2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiser, K. B., J. M. Cantey-Kiser, and J. C. Lee. 1999. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect. Immun. 675001-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kluytmans-Vandenberg, M. F. Q., and J. A. J. W. Kluytmans. 2006. Community-acquired methicillin-resistant Staphylococcus aureus: current perspectives. Clin. Microbiol. Infect. 12(Suppl 1)9-15. [DOI] [PubMed] [Google Scholar]
- 21.Lee, J. C., M. J. Liu, J. Parsonnet, and R. D. Arbeit. 1990. Expression of type-8 capsular polysaccharide and production of toxic shock syndrome toxin-1 are associated among vaginal isolates of Staphylococcus aureus. J. Clin. Microbiol. 282612-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, J. C., J. S. Park, S. E. Shepherd, V. Carey, and A. Fattom. 1997. Protective efficacy of antibodies to the Staphylococcus aureus capsular polysaccharide type 5 in a modified model of endocarditis in rats. Infect. Immun. 654146-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, J. C., S. Takeda, P. J. Livolsi, and L. C. Paoletti. 1993. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect. Immun. 611853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luong, T. T., and C. Y. Lee. 2002. Overproduction of type 8 capsular polysaccharide augments Staphylococcus aureus virulence. Infect. Immun. 703389-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martineau, F., F. J. Picard, P. H. Roy, M. Ouellette, and M. G. Bergeron. 1998. Species-specific and ubiquitous-DNA-based assays for rapid identification of Staphylococcus aureus. J. Clin. Microbiol. 36618-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Döring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 2841523-1527. [DOI] [PubMed] [Google Scholar]
- 27.Moreillon, P., J. M. Entenza, P. Francioli, D. McDevitt, T. J. Foster, P. François, and P. Vaudaux. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 634738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreillon, P., Y. I. Que, and M. P. Glauser. 2005. Staphylococcus aureus, p. 2321-2351. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious disease. Elsevier-Churchill Livingstone, Philadelphia, PA.
- 29.National Research Council. 1996. Guide for the care and use of laboratory animals (NIH guide, revised). National Research Council, Washington, DC.
- 30.Notebaert, S., and E. Meyer. 2006. Mouse models to study the pathogenesis and control of bovine mastitis. A review. Vet. Q. 282-13. [DOI] [PubMed] [Google Scholar]
- 31.Nour El-Din, A. N., L. Shkreta, B. G. Talbot, M. S. Diarra, and P. Lacasse. 2006. DNA immunization of dairy cows with the clumping factor A of Staphylococcus aureus. Vaccine 241997-2006. [DOI] [PubMed] [Google Scholar]
- 32.O'Riordan, K., and J. C. Lee. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17218-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti, J. M. 2004. A humanized monoclonal antibody targeting Staphylococcus aureus. Vaccine 22S39-S43. [DOI] [PubMed] [Google Scholar]
- 34.Proctor, R. A., C. von Eiff, B. C. Kahl, K. Becker, P. McNamara, M. Herrmann, and G. Peters. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4295-305. [DOI] [PubMed] [Google Scholar]
- 35.Projan, S. J., M. Nesin, and P. M. Dunman. 2006. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Sci. Direct. 6473-479. [DOI] [PubMed] [Google Scholar]
- 36.Reilley, S., E. Wenzel, L. Reynolds, B. Bennett, J. M. Patti, and S. Hetherington. 2005. Open-label, dose escalation study on the safety and pharmacokinetic profile of Tefibazumab in healthy volunteers. Antimicrob. Agents Chemother. 49959-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seggewiss, J., K. Becker, O. Kotte, M. Eisenacher, M. R. Yazdi, A. Fischer, P. McNamara, N. Al Laham, R. Proctor, G. Peters, M. Heinemann, and C. von Eiff. 2006. Reporter metabolite analysis of transcriptional profiles of a Staphylococcus aureus strain with normal phenotype and its isogenic hemB mutant displaying the small-colony-variant phenotype. J. Bacteriol. 1887765-7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shinefield, H., S. Black, A. Fattom, G. Horwith, S. Rasgon, J. Ordonez, H. Yeoh, D. Law, J. B. Robbins, R. Schneerson, L. Muenz, S. Fuller, J. Johnson, B. Fireman, H. Alcorn, and R. Naso. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346491-496. [DOI] [PubMed] [Google Scholar]
- 39.Shinefield, H. R. 2006. Use of a conjugate polysaccharide vaccine in the prevention of invasive staphylococcal disease: is an additional vaccine needed or possible? Vaccine 24(Suppl. 2)65-69. [DOI] [PubMed] [Google Scholar]
- 40.Sinha, B., and M. Herrmann. 2005. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb. Haemostasis 94266-277. [DOI] [PubMed] [Google Scholar]
- 41.Sordelli, D. O., F. R. Buzzola, M. I. Gómez, L. Steele-Moore, D. Berg, E. Gentilini, M. Catalano, A. J. Reitz, T. Tollersrud, G. Denamiel, P. Jeric, and J. C. Lee. 2000. Capsule expression by bovine isolates of Staphylococcus aureus from Argentina: genetic and epidemiologic analyses. J. Clin. Microbiol. 38846-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stranger-Jones, Y. K., T. Bae, and O. Schneewind. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 10316942-16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thakker, M., J. S. Park, V. Carey, and J. C. Lee. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 665183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuchscherr, L. P. N., F. R. Buzzola, L. Alvarez, R. Caccuri, J. C. Lee, and D. O. Sordelli. 2005. Capsule-negative Staphylococcus aureus induces chronic experimental mastitis in mice. Infect. Immun. 737932-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuchscherr, L. P. N., M. I. Gómez, F. R. Buzzola, L. F. Calvinho, J. C. Lee, and D. O. Sordelli. 2007. Characterization of a new variant of IS257 prevalent among bovine isolates of Staphylococcus aureus in Argentina. Infect. Immun. 755483-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vernachio, J., A. S. Bayer, T. Le, Y. L. Chai, B. Prater, A. Schneider, B. Ames, P. Syribeys, J. Robbins, and J. M. Patti. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 473400-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vesga, O., M. C. Groeschel, M. F. Otten, R. A. Proctor, and J. M. Vann. 1996. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J. Infect. Dis. 173739-742. [DOI] [PubMed] [Google Scholar]
- 48.Watts, A., D. Ke, Q. Wang, A. Pillay, A. Nicholson-Weller, and J. C. Lee. 2005. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect. Immun. 733502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wölz, C., C. Goerke, R. Landmann, W. Zimmerli, and U. Fluckiger. 2002. Transcription of clumping factor A in attached and unattached Staphylococcus aureus in vitro and during device-related infection. Infect. Immun. 702758-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]