Abstract
Monoclonal antibodies (MAbs) to the Chlamydia trachomatis mouse pneumonitis (MoPn) major outer membrane protein (MOMP) were characterized for their ability to neutralize the infectivity of this organism in vitro and in vivo. One of the MAbs (MoPn-23) recognizes a nonlinear epitope in the MOMP, MAb MoPn-40 binds to a linear epitope in the variable domain 1 (VD1), and MAb MoPn-32 recognizes the chlamydial lipopolysaccharide. MAb MoPn-23 neutralized 50% of the infectivity of Chlamydia, as measured in vitro by using HAK (FcγIII−) and HeLa-229 (FcγIII+) cells at a concentration 100 times lower than MAb MoPn-40. MAb MoPn-32 had no neutralizing ability. In comparison to the control normal mouse immunoglobulin G, passive immunization of BALB/c mice with MAb MoPn-23 resulted in a highly significant protection against an intranasal (i.n.) challenge as determined by the change in body weight, the weight of the lungs, and the yield of Chlamydia inclusion-forming units (IFU) from the lungs. Passive immunization with MAb MoPn-40 resulted in a lower degree of protection, and MAb MoPn-32 afforded no protection. MAb MoPn-23 was also tested for its ability to protect wild-type (WT) and severe combined immunodeficient (SCID) C.B-17 mice against an i.n. challenge. Protection based on total body weight, lung weight, and yield of Chlamydia IFU was as effective in SCID as in WT C.B-17 mice. In conclusion, antibodies to MOMP can protect mice against a chlamydial infection in the presence or absence of T and B cells.
Chlamydia trachomatis infections have a worldwide distribution and can affect individuals of all ages (14, 31, 33). At birth, newborns can become infected in the eyes and lungs if the mother has a genital tract infection at the time of delivery. In young individuals, C. trachomatis is the most common sexually transmitted bacterial pathogen (14). Many infections remain asymptomatic, but others can produce acute symptomatology and, particularly in women, long-term sequelae, such as infertility and ectopic pregnancy, can develop (36). In countries with poor hygienic conditions, young children can have multiple ocular infections that result in the development of trachoma later on in life (31, 33). In addition, the lymphogranuloma venereum serovars of C. trachomatis can produce severe medical complications due to scarring and stenosis of the lymphatics (31, 33). Antibiotic therapy is available, but many individuals go untreated, and even patients that are treated may develop chronic sequelae as a result of this pathogen inducing a persistent infection. A better understanding of the immunopathogenesis of these infections is required in order to implement preventive measures that will eventually eradicate C. trachomatis.
The role that the cell-mediated and the humoral immune responses play in the control of chlamydial infections is still under investigation. Transfer experiments of immune cells and antibodies to mice and work with knockout (KO) animals have identified a critical role for CD4+ Th1 cells in protection against chlamydial infections (3, 12, 16, 18, 29, 38). The contribution to protection that B cells and antibodies have during an infection has been the subject of intense investigation, and opposing results have been reported. For example, while in the guinea pig model serum antibodies appear to be protective, in the mouse model contradictory findings have been reported in the literature (4, 8, 17, 29, 30, 37, 39). Williams et al. (37) showed that sera from mice infected with Chlamydia delivered intravenously could protect athymic nude mice against an intranasal (i.n.) challenge with C. trachomatis mouse pneumonitis (MoPn). Interestingly, when hyperclean mice, animals born from germfree mice and subsequently colonized with a limited nonpathogenic flora, were given antibodies intravenously, no protection was observed (39). In contrast, if the immune serum was delivered i.n. shortly before a nasal challenge the mice were protected (39). Based on these findings the authors concluded that a background of stimulated cell-mediated immunity (CMI) was necessary for antibodies to be systemically effective, while high levels of local antibodies at the time of the infection could also be protective.
Recent publications appear to support the concept that for antibodies to be protective they need to interact with immune cells. Moore et al. (15), based on the results they obtained using Fc receptor KO mice, proposed that chlamydial antibodies facilitate a Th1 response via FcR-mediated mechanisms that involve dendritic cells. Also, Morrison and Morrison (17), using antibody-deficient mice, found that animals vaginally challenged, followed by the passive transfer of antichlamydial immune sera or monoclonal antibody (MAb), were protected against reinfection but not against a primary infection. Based on these findings these authors concluded that antibody protection is dependent on CD4+ T-cell-mediated adaptive changes occurring during the primary infection. In the present study, to help clarify the role that antibodies may play in protection, we passively immunized wild-type (WT) and severe combined immunodeficient (SCID) mice with MAb to the C. trachomatis MOMP, before they were i.n. challenged. MOMP is highly antigenic and, when formulated in a vaccine, it has been shown to induce protection in mice against a genital challenge (1, 5, 23, 25, 34, 35).
MATERIALS AND METHODS
Growth of C. trachomatis MoPn.
The C. trachomatis MoPn biovar (strain Nigg II; also called C. muridarum) was purchased from the American Type Culture Collection (Rockville, MD) and grown in HeLa-229 cells (21). Elementary bodies (EB) were purified as described by Caldwell et al. (5). The number of inclusion-forming units (IFU) was determined by using McCoy cells (22).
Animals.
BALB/c (H-2d), SCID and WT C.B-17(H-2d) mice were purchased from Charles River Laboratories (Wilmington, MA). SCID C.B-17 inbred mice are congenic to the BALB/c mice but lack T and B cells (9, 13). Animals were housed in isolation cubicles at a constant temperature of 24°C with a cycle of 12 h of light and 12 h of darkness and were fed mouse chowder ad libitum. For the i.n. challenge, animals were anesthetized and, while lying on their backs, the inoculum (104 IFU) was placed in the nostrils (20 μl/nostril) (22). Mice were kept on their backs until the inoculum was observed to disappear. The University of California, Irvine, Animal Care and Use Committee approved the animal protocol.
Preparation and characterization of MAb.
For the production of MAb, 4- to 6-week-old female BALB/c mice were inoculated i.n. with 104 IFU C. trachomatis MoPn and, 3 days before harvesting splenocytes, 107 IFU of MoPn were inoculated intravenously (27, 28). Isolation and screening of the hybridomas was performed as described previously (27, 28). Epitope mapping of the MAb was performed using synthetic octameric peptides. The peptides, corresponding to the MoPn MOMP, were synthesized by using a commercial kit (Cambridge Biochemical, Cambridge, MA) (11).
In vitro and in vivo neutralization assays.
The in vitro neutralization assay was run according to the protocol described by Peterson et al. (27). C. trachomatis MoPn (104 IFU) were added to twofold serial dilutions of the MAb made with or without 5% guinea pig sera in Ca2+- and Mg 2+-free phosphate-buffered saline. After incubation at 37°C for 45 min, the mixture was used to inoculate HeLa-229 and HAK cells (American Type Culture Collection) by centrifugation. The cells were fixed with methanol 30 h after infection, stained as previously described, and the numbers of IFU were counted. Neutralization was defined as ≥50% inhibition of the number of IFU using normal mouse immunoglobulin G (IgG) (NL-IgG) as a control (Sigma, St. Louis, MO).
To test the ability of the MAb to protect in vivo, 7- to 8-week-old BALB/c and SCID and WT C.B-17 mice were inoculated intraperitoneally with 50 μg of each MAb 1 and 2 days before the i.n. challenge and on days 1, 2, and 4 after the challenge as described by Pal et al. (24). Mice were infected i.n. with 104 IFU of C. trachomatis MoPn in Eagle minimal essential medium and weighed daily after the challenge. At 10 days postinfection the mice were euthanized, and the lungs were weighed and cultured. Tissues were inoculated in McCoy cells seeded in 48-well plates and centrifuged at 1,000 × g for 1 h at 24°C. At 30 h postinfection the monolayers were fixed and stained. The experiments were done in duplicate with five animals in each group.
Western blots.
For immunoblotting, C. trachomatis MoPn EB were resolved by Tricine-sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (32). Approximately 250 μg of purified EB was loaded onto a 7.5-cm-wide slab gel. After transfer to nitrocellulose membranes, the nonspecific sites were blocked with BLOTTO (Bovine Lacto Transfer Technique Optimizer; 5% [wt/vol] nonfat dried milk, 2 mM CaCl2, and 50 mM Tris-HCl [pH 8.0]). The serum samples, diluted 1:100 in phosphate-buffered saline, were incubated overnight at 4°C with the nitrocellulose membranes. Antibody binding was detected using horseradish peroxidase-conjugated goat anti-mouse antibody developed with 0.01% hydrogen peroxide and 4-chloro-1-naphthol (23).
Measurement of cytokines.
Single cell suspensions were produced by grinding the spleens and passing them through a metal mesh. The spleen cells were washed and resuspended in RPMI 1640 supplemented with 10% fetal bovine serum. Spleen cells were plated in 48-well flat bottom trays at a concentration of 5 × 106 cells/ml. Cells were stimulated with EB, concanavalin A (ConA), or medium. The levels of gamma interferon (IFN-γ) were determined at 48 h poststimulation by using a commercial kit (BD Bioscience Pharmingen, San Diego, CA) (2, 23).
RESULTS
In vitro neutralization of C. trachomatis MoPn by the MAbs.
The three MAbs selected—MoPn-23, MoPn-32, and MoPn-40—recognized chlamydial inclusions when screened by immunofluorescence using C. trachomatis MoPn-infected McCoy cells (data not shown). On dot blots, using heated and nonheated EB, MoPn-40 and MoPn-32 recognized both forms, while MAb MoPn-23 bound only to the nonheated EB. On Western blots, using boiled denatured EB, MoPn-40 bound the 40-kDa band corresponding to MOMP, while MoPn-23 did not bind. MoPn-32 recognized the lipopolysaccharide (LPS). On Western blots of purified nondenatured MOMP, both MAb MoPn-40 and MAb MoPn-23 bound to the nondenatured trimeric form of MOMP. Using synthetic peptides MAb MoPn-40 recognized the linear epitope TGDADLTTAPTP in VD1, whereas MoPn-23 did not bind (data not shown).
In vitro neutralization was performed with HAK (FcγIII−) and HeLa (FcγIII+) cells in the presence or absence of complement. NL-IgG was utilized as a control to calculate the neutralizing ability of the MAb. As shown in Table 1, in the presence of complement, the concentrations of MoPn-23 required to achieve 50% inhibition in HAK and HeLa cells were 0.01 and 0.001 μg/ml, respectively. The amount of MAb MoPn-40 required to achieve the same level of neutralization was 100-fold higher in both cell lines. No in vitro neutralizing activity by any of the MAbs was detected in the absence of complement. The MAb MoPn-32 had no significant neutralizing activity in the presence or absence of complement.
TABLE 1.
In vitro neutralization of C. trachomatis MoPn
| Antibody | Isotype | Antigen/type of epitope | Concn of MAb (μg/ml)a
|
|||
|---|---|---|---|---|---|---|
| With complementb
|
Without complement
|
|||||
| HAK cell (FcγIII−) | HeLa cell (FcγIII+) | HAK cell (FcγIII−) | HeLa cell (FcγIII+) | |||
| MoPn-23 | IgG2b | MOMP/nonlinear | 0.01 | 0.001 | >10 | >10 |
| MoPn-40 | IgG3 | MOMP/linear | 1.0 | 0.1 | >10 | >10 |
| MoPn-32 | IgG2a | LPS | >10 | >10 | ND | ND |
| NL-IgG | IgG | None | Control | Control | Control | Control |
The concentration of MAb that resulted in 50% inhibition in the number of chlamydial IFU compared to NL-IgG. ND, not determined.
The assay was performed in the presence of 5% guinea pig complement.
Ability of the Chlamydia-specific MAb to protect BALB/c mice against an i.n. challenge.
To assess the ability of the three Chlamydia-specific MAb to protect against an i.n. challenge with C. trachomatis MoPn, BALB/c mice were passively immunized with the MAb. As a control, a fourth group of mice was passively immunized with NL-IgG. Serum specimens were collected from each group of animals before the i.n. challenge, and the samples were tested to determine the Chlamydia neutralizing activity. A neutralizing titer of 250 was obtained with the serum sample from the group of mice passively immunized with MoPn-23, whereas no neutralization was observed with the serum samples from the other three groups of mice (Table 2).
TABLE 2.
Passive immunization of BALB/c mice with MAbs to the C. trachomatis MoPn MOMP
| Antibody | Serum neutralizing titera | Median (range)b
|
|
|---|---|---|---|
| Lung wt (g) | No. of IFU recovered from the lungs (106) | ||
| MoPn-23 | 250 | 0.28 (0.19-0.34)*† | 6 (0.2-155)*† |
| MoPn-40 | <50 | 0.34 (0.17-0.43)*‡ | 73 (0.6-246)* |
| MoPn-32 | <50 | 0.36 (0.21-0.39) | 133 (32-1,255) |
| NL-IgG | <50 | 0.38 (0.26-0.43) | 235 (62-1,675) |
The serum neutralizing titer was determined the day of the challenge.
*, P < 0.05 as determined by the Mann-Whitney U test compared to the mice inoculated with NL-IgG; †, P < 0.05 as determined by the Mann-Whitney U test compared to the mice inoculated with MAb MoPn-40; ‡, P > 0.05 as determined by the Mann-Whitney U test compared to the mice inoculated with NL-IgG.
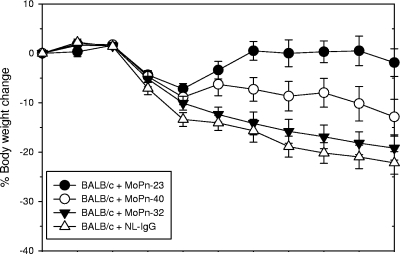
After the challenge the animals were weighed daily and were then euthanized at 10 days after the challenge. As shown in Fig. 1, as a consequence of the infection, the three groups of mice lost weight up to 4 days after the challenge. Mice passively immunized with NL-IgG lost ca. 23% of their total body weight by day 10 (P < 0.05). Similarly, animals passively immunized with the MAb MoPn-32 to the LPS lost 21% of their body weight by 10 days after the challenge (P < 0.05). Mice passively immunized with MoPn-40 lost 12% (P < 0.05) of their body weight by day 10 postchallenge, while mice immunized with MAb MoPn-23 had gained the weight they had initially lost (P > 0.05).
FIG. 1.
Percent change in mean body weight of BALB/c mice after an i.n. challenge with C. trachomatis MoPn. The animals were passively immunized with antibodies as indicated in the figure.
Ten days after challenge the mice were euthanized, their lungs were harvested and weighed. The weight of the lungs increases due to the inflammatory reaction resulting from the infection. As shown in Table 2, the mean weight (0.28 g) of the lungs of the BALB/c mice passively immunized with MoPn-23 was significantly lower than the mean weight of the lungs from the group of animals inoculated with NL-IgG (0.38 g; P < 0.05). The lungs of the mice passively immunized with MoPn-40 weighed significantly less than the lungs of the control group. In addition, the number of IFU recovered from the lungs was 40-fold lower in the mice passively immunized with MAb MoPn-23 compared to the group treated with NL-IgG (P < 0.05). The number of IFU recovered from the lungs of the mice immunized with MAb MoPn-40 was threefold lower than in the control animals (P < 0.05). Compared to control mice, no significant decrease in the number of IFU recovered from the lungs was observed in the mice inoculated with MoPn-32 (P > 0.05).
Passive immunization of WT and SCID C.B-17 mice with Chlamydia-specific MAb.
To determine the ability of the MAb to neutralize Chlamydia in vivo in the absence of T and B cells, WT C.B-17 and the C.B-17 SCID mice were passively immunized with MAb MoPn-23 or normal mouse IgG. After passive immunization, the animals were challenged i.n. with 104 IFU of C. trachomatis MoPn.
As shown in Fig. 2, WT C.B-17 and SCID C.B-17 mice passively immunized with MAb MoPn-23 lost weight for the first 4 days after the i.n. challenge. However, 10 days after the challenge the WT C.B-17 type and SCID C.B-17 animals inoculated with the NL-IgG had lost more than 35% of their weight, while the WT and SCID animals passively immunized MAb MoPn-23 had lost <3% of their body weight (P < 0.05).
FIG. 2.
Percent change in mean body weight of C.B-17 WT and SCID mice after an i.n. challenge with C. trachomatis MoPn. The mice were passively immunized with antibodies as marked in the figure.
Ten days after the i.n. challenge the mice were euthanized, serum samples were collected, and the lungs were harvested. As shown in Table 3, WT C.B-17 and SCID C.B-17 mice passively immunized with MAb MoPn-23 had serum neutralization titers of 6,250 at 10 days after the challenge. Of the two groups of C.B-17 mice passively immunized with NL-IgG, only the serum from the WT had a significant Chlamydia neutralization titer (1,250), while the serum sample from SCID did not have in vitro neutralizing activity.
TABLE 3.
Passive immunization of C.B-17-WT and C.B-17-SCID mice with MAb MoPn-23 to the C. trachomatis MoPn MOMPa
| Antibody | Serum neutralizing titerb
|
Median (range)c
|
||||
|---|---|---|---|---|---|---|
| Lung wt (g)
|
No. of IFU recovered/lungs (106)
|
|||||
| C.B-17-WT | C.B-17-SCID | C.B-17-WT | C.B-17-SCID | C.B-17-WT | C.B-17-SCID | |
| MoPn-23 | 6,250 | 6,250 | 0.21 (0.18-0.30)*† | 0.22 (0.16-0.31)* | 3.3 (0.1-47)*† | 9.7 (0.9-94)* |
| NL-IgG | 1,250 | <50 | 0.35 (0.28-0.52)‡ | 0.40 (0.32-0.58) | 22,969 (654-61,683)‡ | 15,651 (697-68,277) |
Mice were euthanized at day 10 postinfection.
The serum neutralizing titer measured at day 10 postinfection.
*, P < 0.05 by the Mann-Whitney U test compared to the mice inoculated with NL-IgG; †, P > 0.05 as determined by the Mann-Whitney U test compared to the C.B-17-SCID mice receiving MoPn MAb 23; ‡, P > 0.05 as determined by the Mann-Whitney U test compared to the C.B-17-SCID mice receiving NL-IgG.
The lungs from the WT C.B-17 and SCID C.B-17 mice passively immunized with MAb MoPn-23 weighed significantly less than the lungs from the animals immunized with NL-IgG (median weights of 0.21 and 0.22 g, respectively, versus 0.35 and 0.40 g, respectively; P < 0.05) (Table 3). Quantitation of Chlamydia from the lungs showed that in WT C.B-17 and SCID C.B-17 mice passively immunized with MoPn-23 there was a >1,000-fold lower yield of IFU compared to the corresponding groups of mice immunized with NL-IgG (P < 0.05).
Western blot of serum from mice passively immunized with MAb.
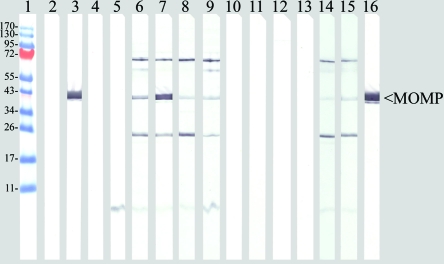
To establish the structural integrity and functionality of the passively transferred MAb, blood was collected from mice, and the sera were tested by Western blotting with boiled-denatured EB as an antigen. As shown in Fig. 3, sera collected before the challenge from WT and SCID mice passively immunized with MAb MoPn-40 and MAb- MoPn-32 reacted only with MOMP and LPS, respectively, their corresponding antigens. As expected, MoPn-23 did not react with the denatured MOMP. Serum collected at 10 days postinfection showed a different pattern for the BALB/c and C.B-17 WT compared to the SCID mice. Sera from WT BALB/c and C.B-17 mice, in addition to reacting to MOMP or LPS, depending on what MAb the animals were passively immunized with, also had antibodies that recognized bands in the 60-, 70-, and 23-kDa regions. No reactivity was detected with the sera from the SCID mice passively immunized with NL-IgG or MAb MoPn-23.
FIG. 3.
Western blots of purified C. trachomatis MoPn MOMP reacted with various MAbs. Lane 1, EZ-run prestained recombinant protein ladder (Fisher Scientific; Pittsburg, PA). Sera were collected the day of the challenge from BALB/c mice passively immunized with the antibodies NL-IgG (lane 2), MoPn-40 (lane 3), MoPn-23 (lane 4), and MoPn-32 (lane 5). Sera were also collected on day 10 after challenge from BALB/c mice passively immunized with NL-IgG (lane 6), MoPn-40 (lane 7), MoPn-23 (lane 8), and MoPn-32 (lane 9). Sera were collected before passive immunization from SCID mice (lane 10) and C.B-17 mice (lane 11). Sera were collected at day 10 after challenge from SCID C.B-17 mice passively immunized with MoPn-23 (lane 12) and SCID C.B-17 mice immunized with NL-IgG (lane 13). Sera were collected at day 10 after challenge from WT C.B-17 mice passively immunized with MoPn-23 (lane 14) and WT C.B-17 mice immunized with control NL-IgG (lane 15). Lane 16, control MAb MoPn-40.
Cytokine response in splenocytes.
To ascertain whether passive immunization with the antibodies affected the levels of cytokines involved in defense against C. trachomatis MoPn infection, the spleens from the four groups of animals were harvested 10 days after the i.n. challenge. Splenocytes were stimulated with EB, and the levels of IFN-γ were determined in the supernatants. ConA and tissue culture media were used as positive and negative stimulation controls, respectively. As shown in Table 4, the amounts of IFN-γ were not significantly different for any of the groups when comparing the mice passively immunized with MAb MoPn-23 to those receiving NL-IgG, indicating that this cytokine could not account for the differences in protection. As expected, the levels of IFN-γ in the supernatants from splenocytes stimulated with ConA or EB were very low in the two groups of SCID mice passively immunized with MoPn-23 and NL-IgG compared to the levels of IFN-γ in the corresponding WT C.B-17 mice (P < 0.05).
TABLE 4.
Cytokine response of splenocytes from C.B-17-WT and C.B-17-SCID mice at day 10 postinfection
| Mouse | Treatment | IFN-γ (pg/ml)
|
||
|---|---|---|---|---|
| EBa | ConAb | Medium | ||
| C.B-17-WT | MoPn-23 | 17,663 ± 1,590*† | 18,722 ± 1,987 | <0.03 |
| C.B-17-SCID | MoPn-23 | 675 ± 15 | 52 ± 110 | <0.03 |
| C.B-17-WT | NL-IgG | 17,476 ± 760*† | 15,907 ± 158 | <0.03 |
| C.B-17-SCID | NL-IgG | 766 ± 180 | <0.03 | <0.03 |
UV-inactivated C. trachomatis MoPn EB were added at a 10:1 ratio to the antigen-presenting cells. *, P < 0.05 as determined by the Student t test compared to the C.B-17-SCID mice receiving MoPn MAb 23; †, P < 0.05 as determined by the Student t test compared to the C.B-17-SCID mice receiving NL-IgG.
ConA was added at a concentration of 5 μg/ml.
DISCUSSION
Data using KO mice and passive transfer experiments has established a critical function for T cells in the resolution of a genital infection with C. trachomatis (3, 12, 16). B cells and or antibodies also appear to play a significant role in the control of a Chlamydia infection (8, 15, 17, 18). Here, to gain a better understanding of the role of antibodies, we tested in vitro and in vivo several MAbs for their ability to control Chlamydia. Our data show that passive immunization with MAbs that recognize nonlinear epitopes of the MOMP can protect immunocompetent naive WT and SCID mice against an i.n. challenge with C. trachomatis MoPn. Of the two MAbs that we tested, MoPn-23 that binds to the trimer of MOMP neutralized in vitro 50% of the chlamydial infectivity at a concentration 100 times lower than MoPn-40 that recognizes a linear epitope in the VD-1 of MOMP. MAb MoPn-23 was also able to protect SCID mice against an i.n. challenge. These results demonstrate that antibodies can protect against chlamydial infections independent of effector T and B cells.
Natural infection, or immunization with whole organisms or subunit vaccines, induces a highly complex and diverse response in the host. Optimally, all components of the host response should help to control and limit the damage by the foreign antigen. However, the heterogeneity of the host response is such that not every component of the immune response is protective. For example, some antibodies may have a protective effect, others may be functionally irrelevant, and some of them may even have a negative effect on the host (7, 20, 28). Therefore, when doing passive transfer experiments with immune polyclonal serum, the effect on the host is the aggregate of the multiple types and functions of the individual antibodies present in the sample. The feasibility in the last three decades of generating MAbs with well-defined structural and functional characteristics has now opened the possibility of gaining a better understanding of the role of individual components of the adaptive immune response.
Antibodies can mediate protection against pathogens directly or by interaction with cellular and noncellular components of the innate and adaptive immune responses (7). For instance, an antibody can directly neutralize the infectivity of a pathogen by agglutination. Lysis of a pathogen can also occur from the combined action of an antibody with complement. A similar protective role can be the result of the cooperation of an antibody with cellular elements leading to opsonization or to antibody-dependent cellular cytotoxicity. Therefore, the functional activity of an antibody that depends on the cooperation with other immune components will be affected by the environmental conditions and by the isotype of the antibody. For example, Peterson et al. (28) showed that MAb E-4, an IgG2b antibody to the C. trachomatis E MOMP, could, in the presence of complement, neutralize chlamydial infectivity in HeLa 229 cells. In contrast, when MAb E-4 was tested in HeLa 229 cells without complement, there was an enhancement of infectivity. However, the same MAb E-4 neutralized Chlamydia infectivity in HaK cells in a complement-independent manner. These results suggest that MAb E-4 enhanced infectivity in HeLa 229 cells by binding to FCγRIII receptors, thus facilitating entry of Chlamydia into the host cells. In contrast, in HaK cells that do not have FCγRIII receptors the infectivity of Chlamydia could be neutralized by MAb E-4 without complement.
The greater ability of MoPn-23 (IgG2b) versus MoPn-40 (IgG3) to protect mice against an i.n. challenge could be due to differences in the capacity of the two IgG isotypes to access the lung tissues. However, most likely, this is not the case, since Palladino et al. (26) have shown that MAbs belonging to the four different mouse IgG isotypes—IgG1, IgG2a, IgG2b, and IgG3—inoculated intraperitoneally were equally effective at protecting SCID mice against an i.n. challenge with influenza.
Passive transfer experiments of immune sera to WT and immunodeficient mice have shown that in several bacterial, fungal, and viral infections the efficacy of antibodies is dependent on CMI (7, 17, 19). Nevertheless, in the case of other microbial infections, antibodies have been shown to be effective in the absence of an intact CMI (10, 20). In addition, at least for certain pathogens, depending on the antibody there may or may not be a requirement for CMI. For example, Morrison et al. (19) showed that protection by polyclonal immune sera against a genital challenge with herpes simplex virus (HSV) required an intact immune system. In contrast, Eis-Hubinger et al. (10) demonstrated protection by passively immunizing T-cell-depleted mice with an MAb to the glycoprotein B of HSV. The results we report here show that, as in the case of HSV, passive immunization with antibodies can neutralize the infectivity of Chlamydia in the absence of T and B cells.
Several reports have concluded that protection with antibodies against a primary chlamydial infection requires the presence of an intact CMI. For example, Morrison and Morrison (17) showed that Chlamydia immune sera could not protect antibody-deficient, CD4-depleted mice from a primary genital infection. A small, but measurable, protective effect was noted in naive mice not depleted of CD4+ T cells. In contrast, the same immune serum conferred significant protection to a chlamydial reinfection in CD4- and CD8-depleted, antibody-deficient, mice. Morrison and Morrison (17) also did passive immunization experiments with three MAbs: MP-33b that binds to the MOMP of C. trachomatis MoPn; MAb EVI-HI that recognizes the chlamydial LPS; and A57-B9, an MAb to the hsp-60 of Chlamydia. With the MAb to the MOMP and to the LPS these researchers obtained the same results as with the polyclonal immune serum. Importantly, none of these three MAbs have been shown to neutralize Chlamydia in vitro, although Cotter et al. (8) previously reported that MP-33b could protect mice against a primary intravaginal challenge with a low dose of C. trachomatis MoPn. Here, we obtained protection with the two MAbs to the MOMP but not with the MAb to the LPS. Based on their findings, Morrison and Morrison (17) concluded that the protective efficacy of antibodies is dependent on CD4+ T-cell-mediated adaptive changes that occur locally in the genital tissue during a primary infection. These authors proposed that CD4+ T cells activate or recruit some type of effector cells, possibly dendritic cells, to the genital tract that interact with antibodies to resolve the chlamydial reinfection.
The results reported here expand previous experiments using IgA MAb to the C. trachomatis MoPn MOMP. Pal et al. (24) compared the ability of two IgA MAbs to the C. trachomatis MoPn MOMP to protect immunocompetent mice against a genital challenge. MAb MoPn 4-2 binds to a nonlinear epitope of MOMP, whereas MAb 13-2 recognizes a linear epitope in VD4. Of the two MAbs tested, MoPn 4-2 had a 20-fold higher in vitro neutralizing activity than did MoPn 13-2. Furthermore, passive immunization with MAb MoPn 4-2 resulted in significant protection against a genital challenge, as determined by a decrease in the number of mice infected, and in the intensity and duration of vaginal shedding. No significant protection was observed in the group receiving MAb MoPn 13-2. Here, by passively immunizing SCID mice we have excluded a role for effector T and B cells. In addition, the low levels of IFN-γ in the supernatants of EB-stimulated splenocytes from C.B-17 SCID mice suggests that this cytokine did not play a role in controlling the infection in the animals passively immunized with the MAb.
In conclusion, it has previously been shown that an IgA MAb can protect mice against a primary genital challenge with Chlamydia (24). Here, we have demonstrated that an IgG MAb can confer protection against a primary respiratory infection independent of the presence of T and B cells. Therefore, antibodies can protect against both mucosal and systemic chlamydial primary infections. From the point of view of developing a subunit vaccine against C. trachomatis, the challenge will be to formulate an immunization protocol that induces protective cell-mediated and humoral immune responses. The findings previously reported using immunizations with live or nonviable whole Chlamydia are certainly valuable but cannot be used as the only guide for the development of a vaccine. As discussed by Casadevall and Pirofski (6), most vaccines protect by eliciting “unnatural” immunity an important concept to take into consideration since vaccination of humans with live Chlamydia is not a realistic alternative. The antigen itself, the adjuvant, and the route of delivery of a subunit vaccine are going to determine whether the immune response elicited is protective or not. Previously, it was shown that CD4+ Th1 cells are critical for protection. Here, we have shown that antibodies can also play an important role in protection. Therefore, to control Chlamydia infections, we should direct our efforts at engineering a vaccine that elicits both protective cell-mediated and humoral immune responses.
Acknowledgments
This study was supported by Public Health Service grant AI-67888 from the National Institute of Allergy and Infectious Diseases.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Baehr, W., Y. X. Zhang, T. Joseph, H. Su, F. E. Nano. K. D. E. Everett, and H. D. Caldwell. 1988. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc. Natl. Acad. Sci. USA 854000-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bancroft, G. J., K. C. F. Sheehan, R. D. Schreiber, and E. R. Unanue. 1989. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in SCID mice. J. Immunol. 143127-130. [PubMed] [Google Scholar]
- 3.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5149-161. [DOI] [PubMed] [Google Scholar]
- 4.Buzoni-Gatel, D., F. Bernard, A. Andersen, and A. Rodolakis. 1990. Protective effect of polyclonal and monoclonal antibodies against abortion in mice infected by Chlamydia psittaci. Vaccine 8342-346. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 311161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall, A., and L. A. Pirofski. 2003. Exploiting the redundancy in the immune system: vaccines can mediate protection by eliciting “unnatural” immunity. J. Exp. Med. 1971401-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall, A., and L. A. Pirofski. 2004. New concepts in antibody-mediated immunity. Infect. Immun. 726191-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter, T. W., Q. Meng, Z. L. Shen, Y. X. Zhang, H. Su, and H. D. Caldwell. 1995. Protective efficacy of major outer membrane protein specific immunoglobulin A (IgA) and IgG monoclonal antibodies in a murine model of Chlamydia trachomatis genital tract infection. Infect. Immun. 634704-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorshkind, K., G. M. Keller, R. A. Philips, R. G. Miller, G. C. Bosma, M. O'Toole, and M. J. Bosma. 1984. Functional status of cells form lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J. Immunol. 1321804-1808. [PubMed] [Google Scholar]
- 10.Eis-Hubinger, A. M., D. S. Schmidt, and K. E. Schneweis. 1993. Anti-glycoprotein B monoclonal antibody protects T cell-depleted mice against herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membranes. J. Gen. Virol. 74379-385. [DOI] [PubMed] [Google Scholar]
- 11.Geysen, H. M., S. J. Rodda, T. J. Mason, G. Tribbick, and P. Schoofs. 1987. Strategies for epitope analysis using peptide synthesis. J. Immunol. Methods 102259-274. [DOI] [PubMed] [Google Scholar]
- 12.Igietseme, J. U., K. H. Ramsey, D. M. Magee, D. M. Williams, T. J. Kincy, and R. G. Rank. 1993. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific Th1 lymphocyte clone. Reg. Immunol. 5317-324. [PubMed] [Google Scholar]
- 13.Magee, D. M., J. U. Igietseme, J. G. Smith, C. A. Bleicker, B. G. Grubbs, J. Schachter, R. G. Rank, and D. M. Williams. 1993. Chlamydia trachomatis pneumonia in the severe combined immunodeficiency (SCID) mouse. Reg. Immunol. 5305-311. [PubMed] [Google Scholar]
- 14.Miller, W. C., C. A. Ford, M. Morris, M. S. Handcock, J. L. Schmitz, M. M., Hobbs, M. S. Cohen, K. M. Harris, and J. R. Udry. 2004. Prevalence of chlamydial and gonococcal infections among young adults in the United Sates. JAMA 2912229-2236. [DOI] [PubMed] [Google Scholar]
- 15.Moore, T., C. O. Ekworomadu, F. O. Eko, L. MacMillan, K. Ramey, G. A. Ananaba, J. W. Petrickson, P. R. Nagappan, D. Lyn, C. M. Black, and J. U. Igietseme. 2003. Rc receptor-mediated antibody regulation of T cells immunity against intracellular pathogens. J. Infect. Dis. 188617-624. [DOI] [PubMed] [Google Scholar]
- 16.Morrison, S. G., and R. P. Morrison. 2001. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ T cells. Infect. Immun. 692643-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison, S. G., and R. P. Morrison. 2005. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 1757536-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison, S. G., H. Su, H. D. Caldwell, and R. P. Morrison. 2000. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 686979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison, L. A., L. Zhu, and L. G. Thebeau. 2001. Vaccine-induced serum immunoglobulin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J. Virol. 751195-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukherjee, J., G. Nussbaum, M. D. Scharff, and A. Casadevall. 1995. Protective and non-protective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J. Exp. Med. 181405-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nigg, C. 1942. An unidentified virus which produces pneumonia and systemic infection in mice. Science 9949-50. [DOI] [PubMed] [Google Scholar]
- 22.Pal, S., T. J. Fielder, E. M. Peterson, and L. M. de la Maza. 1994. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 623354-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 738153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine 15575-582. [DOI] [PubMed] [Google Scholar]
- 25.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 696240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palladino, G., K. Mozdzanowska, G. Washko, and W. Gerhard. 1995. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 692075-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson, E. M., G. Zhong, E. Carlson, and L. M. de la Maza. 1988. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect. Immun. 65885-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson, E. M., X. Cheng, S. Pal, and L. M. de la Maza. 1993. Effects of antibody isotype and host cell type on in vitro neutralization of Chlamydia trachomatis. Infect. Immun. 61498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey, K. H., L. S. F. Soderberg, and R. G. Rank. 1988. Resolution of chlamydial genital infection in B-cell deficient mice and immunity to reinfection. Infect. Immun. 561320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rank, R. G., H. J. White, and A. L. Barron. 1979. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect. Immun. 26573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schachter, J., and C. Dawson. 1978. Human chlamydial infections. PSG Publishing Company, Inc., Littleton, MA.
- 32.Schagger, H., and G. Von Jagow. 1987. Tricine-sodium dodecyl sulphate polyacrylamide gel electrophoresis for the separation of protein range 1 to 100 kDa. Anal. Biochem. 166368-379. [DOI] [PubMed] [Google Scholar]
- 33.Stamm, W. E. 1999. Chlamydia trachomatis infections of the adult, p. 407-422. In K. K. Holmes et al. (ed.), Sexually transmitted diseases. McGraw-Hill Book Co., New York, NY.
- 34.Stephens, R. S., R. Sanchez-Pescador, E. A. Wagar, C. Inouye, and M. S. Urdea. 1987. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 1693879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, G., S. Pal, A. K. Sarcon, S. Kim, T. Sugawara, H. Nikaido, M. J. Cocco, E. M. Peterson, and L. M. de la Maza. 2007. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J. Bacteriol. 1896222-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westrom, L., R. Joesoef, G. Reynolds, A. Hagdu, and S. E. Thompson. 1992. Pelvic inflammatory disease and fertility: a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopy. Sex. Transm. Dis. 19185-192. [PubMed] [Google Scholar]
- 37.Williams, D. M., J. Schachter, B. Grubbs, and C. V. Sumaya. 1982. The role of antibody in host defense against the agent of mouse pneumonitis. J. Infect. Dis. 145200-205. [DOI] [PubMed] [Google Scholar]
- 38.Williams, D. M., J. Schachter, J. C. Coalson, and B. Grubbs. 1982. Cellular immunity to the mouse pneumonitis agent. J. Infect. Dis. 149630-639. [DOI] [PubMed] [Google Scholar]
- 39.Williams, D. M., J. Schachter, M. H. Weiner, and B. Grubbs. 1984. Antibody in host defense against mouse pneumonitis agent (murine Chlamydia trachomatis). Infect. Immun. 45674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]