Abstract
During infection and inflammation, bacterial and inflammatory proteases break down extracellular matrices into macromolecular fragments. Fibronectin fragments are associated with disease severity in arthritis and periodontitis. The mechanisms by which these fragments contribute to disease pathogenesis are unclear. One likely mechanism is that fibronectin fragments induce apoptosis of resident cells, which can be further modulated by nitric oxide. Nitric oxide levels are increased at inflammatory sites in periodontitis patients. The aim of this study was to examine whether a proapoptotic fibronectin matrix (AFn) exerts its action by inducing nitric oxide and whether priming by bacterial and inflammatory components exacerbates this mechanism. Our data demonstrate that AFn increased the levels of nitric oxide and inducible nitric oxide synthase (iNOS) dose and time dependently in periodontal ligament (PDL) cells. These effects and apoptosis were inhibited by iNOS suppression and enhanced by iNOS overexpression. Nitric oxide and iNOS induction were paralleled by increased c-Jun N-terminal kinase 1 (JNK-1) phosphorylation. JNK-1 overexpression enhanced the expression of nitric oxide and iNOS, whereas inhibiting JNK-1 by small interfering RNA or a kinase mutant reversed these findings. Priming PDL cells with Porphyromonas gingivalis, its lipopolysaccharide (LPS), or gamma interferon (IFN-γ) further increased nitric oxide levels and apoptosis. Escherichia coli and Streptococcus mutans induced lesser effects. Gingival fibroblasts and neutrophils responded to a lesser degree to these stimuli, whereas keratinocytes were resistant to apoptosis. Thus, proapoptotic matrices trigger nitric oxide release via JNK-1, promoting further apoptosis in host cells. LPS and IFN-γ accentuate this mechanism, suggesting that during inflammation, the affected matrices and bacterial and inflammatory components combined exert a greater pathogenic effect on host cells.
Periodontal disease, a chronic bacterial infection that targets the supporting structures of teeth, including the bone and connective tissues of the gingiva and PDL, can lead to tooth loss, and thereby limited mastication, speech, and overall quality of life. Periodontal disease has also been associated with increased severity and/or risk for heart disease, respiratory infection, premature birth, and diabetes. Periodontal disease affects eighty percent of the adult population in the United States and an even higher percentage worldwide. Given its impact, it is crucial to understand the pathogenesis of periodontal disease. One important component in its pathogenesis is the bacterial insult on the ECM and resident cells of the PDL. The periodontal ECM consists of several proteins, including collagen and fibronectin, which play an important role in providing structural integrity to these tissues. The ECM also plays an important role in cell adhesion, migration, signaling, and survival. During infection and inflammation, there is proteolytic cleavage of the ECM molecules into fragments (22, 29, 74). These ECM fragments, in turn, have deleterious effects on the surrounding tissues (1, 14, 73). Specifically, the degraded ECM products can lead to aberrant signaling in the surrounding cells and signal programmed cell death or apoptosis (72). In periodontal disease, PDL cell function is compromised as bacterial proteases destroy the integrity of the ECM and release fragmented adhesion molecules, including fibronectin fragments that induce apoptosis, into the inflammatory milieu. In addition, the degraded matrix molecules, like those from fibronectin and hyaluronan, may stimulate the resident cells around the sites of inflammation to produce proinflammatory cytokines and nitric oxide (6, 28, 30, 31, 34, 56, 67, 76).
We previously identified a recombinant altered fibronectin molecule and a comparable disease-associated proteolytic fibronectin fragment that induce apoptosis in primary cells, including PDL cells (13, 68). This apoptotic process is unique since it is accompanied by a downregulation of p53 at both the transcriptional and the posttranslational level. Specifically, these AFn molecules decrease the phosphorylation of focal adhesion kinase but increase the phosphorylation of JNK-1. Results in other reports show that human monocytes, endothelial cells, macrophages, and gingival fibroblasts produce inflammatory cytokines, such as interleukin-1, -6, and -8 and nitric oxide, when stimulated with bacteria or bacterial components like LPS (3, 7, 9, 24). This cytokine and nitric oxide induction is mediated through mitogen-activated protein kinases, like JNK and p38 (20, 24, 36). Thus, there may be a convergence in signaling pathways triggered by bacterial components and matrix fragments.
Nitric oxide is a free radical with a short half-life that is synthesized by a family of three NOS isoenzymes from l-arginine, and it is produced by a variety of cells (8, 47, 70). Due to its small size and neutral charge, it can diffuse freely through the cell membrane and act as a signaling and effector molecule. iNOS is typically not present in a cell but can be induced when cells are stimulated by bacterial endotoxins and proinflammatory cytokines (12). Once induced, iNOS can produce near-micromolar amounts of nitric oxide for sustained periods of time, and this production is independent of calcium ions. Nitric oxide can function both as an apoptotic and as an antiapoptotic molecule depending on its concentration and its interactions with other cellular molecules (62, 64). Furthermore, at high levels, nitric oxide functions as a proinflammatory mediator (62).
Our study is the first to document that proapoptotic matrices induce high levels of nitric oxide in PDL cells through the activation of JNK-1, suggesting a novel mechanism for inflammation-associated matrix fragments in the pathogenesis of periodontal inflammation.
MATERIALS AND METHODS
Abbreviations.
AFn, proapoptotic fibronectin fragment; ATS, p-toluensulfonate; cAFn, control proapoptotic fibronectin fragment; DAPI, 4′,6′-diamidino-2-phenylindole; ECM, extracellular matrix; ELISA, enzyme-linked immunosorbent assay; FITC, fluorescein isothiocyanate; IFN-γ, gamma interferon; iNOS, inducible nitric oxide synthase; JNK-1, c-Jun N-terminal kinase 1; JNK-KD, kinase-deficient c-Jun N-terminal kinase 1; LPS, lipopolysaccharide; MOI, multiplicity of infection; NOS, nitric oxide synthase; PBS, phosphate-buffered saline; PDL, periodontal ligament; pJNK-1, phosphorylated c-Jun N-terminal kinase 1; siRNA, small interfering RNA; SMT, S-methylisothiourea; TBST, 25 mM Tris, 150 mM NaCl, 0.05% Tween-20, pH 8.0; 1400W, dihydrochloride.
Cell culture.
PDL cells were obtained from extracted third molars or premolars of healthy patients and cultured as described previously (38) in 100-mm tissue culture dishes in a minimal essential medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin and used from passages 2 to 6. The use of human PDL cells for these studies was approved by the University of Michigan Health Sciences Institutional Review Board. Human oral keratinocytes (Sciencell Research Laboratories, CA) were maintained in keratinocyte medium (Sciencell Research Laboratories) as suggested by the company. Human primary normal peripheral blood neutrophils (AllCells, CA) were maintained in Iscove's modified Dulbecco's medium containing 5% fetal bovine serum, 2 mM glutamine, 25 mM HEPES, 50 μM beta-mercaptoethanol, and 1% penicillin-streptomycin.
Recombinant fibronectin proteins.
The two recombinant fibronectin fragments used in these studies contain the alternatively spliced V region (V+) and either an intact (H+) or a mutated, nonfunctional (H−) high-affinity heparin binding domain (39). The V+ H− fragment is designated AFn in the text. The V+ H+ control is designated cAFn. The cells were treated with these fibronectin fragments in serum-free medium.
Growth conditions for Porphyromonas gingivalis, Escherichia coli, and Streptococcus mutans.
Porphyromonas gingivalis ATCC 33277 was maintained in brucella broth (Becton Dickinson and Co., Sparks, MD) or agar medium supplemented with hemin (5 μg ml−1) and vitamin K (5 μg ml−1). Escherichia coli ATCC 25922 was grown in Luria broth or agar medium. Streptococcus mutans UA159 was grown in brain heart infusion broth or agar medium (Difco). For challenge experiments, a single colony was inoculated into liquid medium and incubated under aerobic (E. coli) or anaerobic conditions (S. mutans and P. gingivalis) at 37°C for approximately 18 h.
Treatment of cells with Porphyromonas gingivalis, Escherichia coli, and Streptococcus mutans.
PDL cells were treated with the above bacteria at MOIs of 25, 50, and 100 in serum- and antibiotic-free minimal essential medium for 2 h, and then the bacteria were washed out with PBS. The cells were allowed to grow in the medium for an additional 5 h, and the conditioned medium was collected for nitric oxide measurement.
Treatment of cells with bacteria, IFN-γ, and AFn.
PDL cells were pretreated with the various bacteria (MOI of 50) or IFN-γ (20 U/ml) or both for 2 h in serum- or antibiotic-free medium and then treated with 20 μg/ml AFn for 5 h. For cells challenged with bacteria, the bacteria were washed out with PBS before AFn was added to the medium. Nitric oxide was measured in the conditioned medium.
Transient transfection.
PDL cells were plated a day before transfection at 60 to 80% confluence in 6-well tissue culture plates and generally transfected with 100 ng of cDNA (human JNK-1 and a human JNK-KD) (68) (human iNOS cDNA was a kind gift from David A. Geller, University of Pittsburgh, Pittsburgh, PA) and control vectors using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions. At 36 h posttransfection, cells were washed with PBS, treated with the recombinant fibronectin fragments, and processed for Western blotting, nitric oxide determination, or apoptosis assays.
Antisense experiments.
The siRNAs for JNK-1 and iNOS (Dharmacon) were prepared by using Lipofectamine 2000 (Invitrogen), incubated for 20 min, and added to the cells in serum-free medium. After 4 h of incubation, the serum was replenished and the cells cultured for 36 h before being treated with the fibronectin fragments in serum-free medium. Stealth interference RNA (Invitrogen) was used as the negative control for these transfections.
Immunofluorescence.
To view iNOS inside the PDL fibroblasts, the cells were fixed with 3% paraformaldehyde solution in PBS, pH 7.2, and permeabilized with 0.1% Triton X-100 in PBS for 20 min. Nonspecific staining was blocked by incubating the fixed cells in 5% bovine serum albumin in TBST for 1 h at room temperature. The cells were then incubated with mouse anti-iNOS (Santa Cruz, CA) overnight at 4°C, washed with TBST, and then treated with FITC-conjugated goat anti-mouse antibody for 1 h at room temperature and washed with TBST. Nuclear staining was performed to assess the quality of the DNA. After fixation, permeabilization, and immunofluorescence staining for iNOS, the cells were stained with DAPI (Sigma) for 10 min, rinsed with calcium- and magnesium-free PBS, mounted on glass slides with Vectashield (Vector Labs, Burlingame, CA), and examined with a Nikon TS100 photomicroscope fitted with a DAPI and FITC filter.
Western blot analysis.
For Western blot analyses, cells were lysed in ice-cold radioimmunoprecipitation buffer containing a mixture of protease inhibitors, and the protein concentration was determined by using a bicinchoninic acid protein assay kit from Pierce. Equal amounts of protein were loaded into each well, resolved by 4 to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Novex; Invitrogen), and electroblotted onto polyvinylidene difluoride membranes (Immobiline-P; Millipore, Billerica, MA) by a semidry transfer blot method (Bio-Rad) according to the manufacturer's instructions. The membranes were blocked with 5% nonfat dry milk in TBST for 1 h at room temperature and then incubated with primary and horseradish peroxidase-conjugated secondary antibodies in blocking buffer for 2 h at room temperature or overnight at 4°C, washed with TBST, and developed by using a West-Pico ECL kit from Pierce.
Nitric oxide determination.
The conditioned media from cells treated with recombinant fibronectin fragments or medium alone were collected, and equal volumes of Griess reagent (Sigma) were added to the conditioned media in 96-well clusters and incubated at room temperature for 10 min before the absorbance at 540 nm was measured. Sodium nitrite was used as a standard to measure the amount of nitric oxide released into the medium. The treated cells were lysed, and their protein concentrations determined by using a bicinchoninic acid kit (Pierce, Rockford, IL). The level of nitric oxide released by treated cells was expressed relative to that in untreated control cells (100%).
ELISA for apoptosis.
The cells were lysed after treatment with the AFn or control serum-free medium, and equal amounts of cell lysate protein were processed to quantitate apoptosis by using a cell death detection ELISAPLUS kit (Roche) as recommended by the manufacturer. Cell death was measured in terms of the amount of DNA fragmentation relative to that in control cells by measuring the optical density at 495 nm as suggested in the assay kit.
Other reagents.
The chemical inhibitors used for iNOS were 1400W, 1-amino-2-hydroxyguanidine, ATS, and SMT (Calbiochem), and these were dissolved as recommended by the manufacturer. LPS from P. gingivalis (ATCC 33277) was from InvivoGen, CA. All antibodies were from Santa Cruz Biotechnology, CA, and other reagents were from Sigma unless stated otherwise.
Data analysis.
All the experiments were done at least three times, and representative blots are shown. Data were collected and are expressed as means ± standard deviations. Significance tests (Student's t test) were done by using Sigma Plot.
RESULTS
AFn molecule induces nitric oxide in primary human fibroblasts.
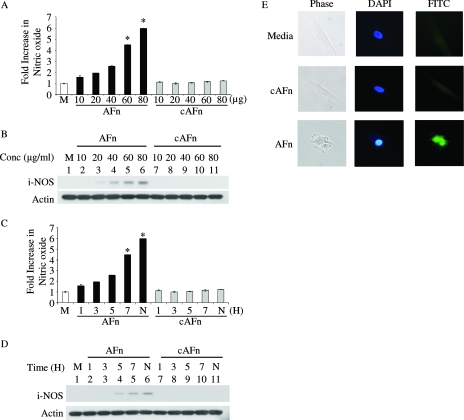
Human primary PDL cells were treated with increasing doses of AFn for 5 h, and then the cell culture medium was examined for the presence of nitric oxide by using Griess reagent. There was a gradual increase in nitric oxide in the medium with increasing doses of AFn (Fig. 1A), whereas cAFn was unable to induce nitric oxide in these cells.
FIG. 1.
AFn induced nitric oxide generation in primary cells in a dose- and time-dependent manner. (A) PDL cells were treated with increasing concentrations of AFn or cAFn for 5 h, and the nitric oxide generated in the medium was measured and expressed compared to the level released by cells treated with serum-free medium (M), which was taken as 100%. *, P value of <0.05 compared with results for medium-treated cells. (B) After removal of the medium for measuring nitric oxide, the cells used in the experiment described for panel A were lysed and analyzed by Western blotting for the expression of iNOS. Actin was used as a loading control. (C) PDL cells were treated with 40 μg/ml of AFn for different times, and the medium was assayed for the release of nitric oxide. H, hours; N, cells treated overnight with AFn; *, P value of <0.05 compared with results for medium-treated cells. (D) The cells used in the experiment described for panel C were lysed, and the cell lysate was analyzed by Western blotting to determine the level of expression of iNOS. Actin was used as a loading control. (E) PDL cells were treated with serum-free medium or AFn or cAFn, and iNOS was visualized by immunofluorescence using an FITC-conjugated secondary antibody. The nuclei were stained with DAPI. The cells shown are representative of cells from approximately 20 fields.
The generation of nitric oxide in response to a stimulant is usually mediated by iNOS. Therefore, using specific antibodies for inducible nitric oxide, we examined the expression of iNOS in primary cells incubated with AFn. As shown by the Western blot in Fig. 1B, AFn induced the expression of iNOS in these cells, and its expression increased with increasing doses of AFn. At the same time, the control molecule cAFn did not induce the expression of iNOS in these cells. Actin was used as a loading control, as shown in the Western blot (Fig. 1B).
We next treated the primary cells with 40 μg/ml of AFn over time, measured the generation of nitric oxide in the medium, and examined iNOS expression in the treated cells. As seen by the results in Fig. 1C, there was approximately a sixfold increase in nitric oxide generation by these cells when treated with AFn over time, which coincided with increased expression of iNOS in the cells (Fig. 1D). We confirmed the induction of iNOS in the primary cells upon AFn treatment by immunofluorescence. When treated with AFn, but not in the presence of the control fragment, cells underwent a change in morphology from a well-spread to a rounded appearance, with a simultaneous appearance of iNOS and a brighter, more-condensed nucleus (Fig. 1E), consistent with an apoptotic phenotype.
Inhibition of iNOS attenuates nitric oxide production and apoptosis induced by AFn in primary cells.
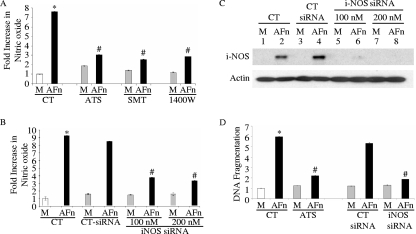
In cells pretreated with iNOS inhibitors, including ATS, SMT, and 1400W, the induction of nitric oxide usually triggered by AFn was significantly reduced (Fig. 2A). This suggested that the induction of nitric oxide produced in the presence of AFn was likely through the activity of iNOS and not due to other NOS isoenzymes.
FIG. 2.
Inhibition of iNOS decreased nitric oxide production and apoptosis in PDL cells treated with AFn. (A) PDL cells were treated with 40 μg/ml AFn for 5 h after an initial pretreatment of the cells with the specific iNOS inhibitor ATS (25 μM), SMT (2 mM), or 1400W (10 μM), and then nitric oxide release from the medium was measured. M, serum-free medium; CT, control cells; *, P value of <0.05 compared with results for medium-treated cells under control conditions; #, P value of <0.05 compared to results for AFn-treated cells under control conditions. (B) PDL cells were transfected with siRNA for iNOS (iNOS siRNA) or control siRNA (CT-siRNA) at 100 nM and 200 nM concentrations for 36 h and then treated with AFn, and the nitric oxide released into the medium was measured. *, P value of <0.05 compared with results for medium (M)-treated cells under control conditions; #, P value of <0.05 compared to results for AFn-treated cells under control conditions. (C) The cells used in the experiment described for panel B were lysed and analyzed by Western blotting to determine the level of expression of iNOS. Actin was used as a loading control. Abbreviations are as defined for panels A and B. (D) PDL cells were pretreated with ATS, the iNOS inhibitor, as described for panel A or were transfected with 200 nM siRNA for iNOS (iNOS siRNA) or control siRNA (CT siRNA) and then treated with AFn. The cells were then lysed, and the amount of apoptosis in the treated cells relative to that in untreated or untransfected control cells was determined by ELISA. The absorbance of the serum-free medium (M)-treated control cell lysates at 495 nm was arbitrarily defined as 1 unit, and the absorbances of other cell lysates were expressed as levels of change with respect to the absorbance of the control cell lysate. *, P value of <0.05 compared with results for medium-treated cells under control conditions; #, P value of <0.05 compared to results for AFn-treated cells under control conditions.
To confirm that nitric oxide production in primary cells treated with AFn resulted from induction of iNOS and was not due to the activities of other NOS isoenzymes, we inhibited the expression of iNOS by transfecting cells with iNOS siRNA. When iNOS siRNA-transfected cells were treated with AFn, these cells generated a significantly reduced level of nitric oxide (Fig. 2B). Western blot analysis confirmed the suppression of iNOS with siRNA at 100 nM and its complete abrogation with a higher concentration of siRNA (Fig. 2C).
To directly examine whether iNOS is involved in apoptosis mediated by AFn, cells were treated with iNOS pharmacologic inhibitors and siRNA for iNOS under AFn conditions before apoptosis was assessed. DNA fragmentation was significantly diminished under AFn conditions when iNOS was suppressed (Fig. 2D), demonstrating that iNOS is a required step in this apoptotic mechanism. Control treatments were not able to overcome the elevated levels of DNA fragmentation or apoptosis induced by AFn.
Overexpression of iNOS increased generation of nitric oxide from AFn-treated cells.
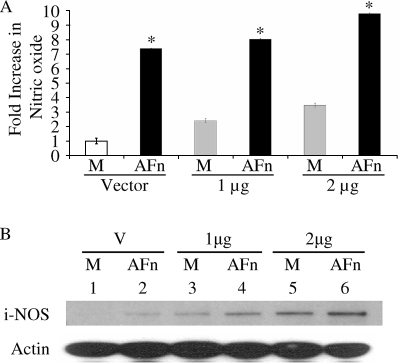
We next investigated whether the overexpression of iNOS could enhance the production of nitric oxide from cells treated with AFn. Cells transfected with increasing doses of iNOS and treated with AFn produced higher levels of nitric oxide than cells that were transfected with the control vector (Fig. 3A). Moreover, increased amounts of iNOS cDNA led to even higher levels of nitric oxide production in the presence of AFn. The results shown in Fig. 3A were confirmed by Western blot analyses for iNOS (Fig. 3B).
FIG. 3.
Overexpression of iNOS in PDL cells increased AFn-mediated nitric oxide production. (A) PDL cells were transfected with cDNA for iNOS at two different doses or with a vector control and then treated with AFn for 5 h, and the nitric oxide generated into the medium was measured. M, cells treated with serum-free medium only; *, P value of <0.05 compared with results for vector-transfected and medium-treated cells. (B) The cells used in the experiment described for panel A were lysed, and the lysate used to analyze iNOS by Western blotting. Actin was used as a loading control. M, cells treated only with serum-free medium; V, vector-transfected cells.
AFn mediates nitric oxide production by activating JNK-1.
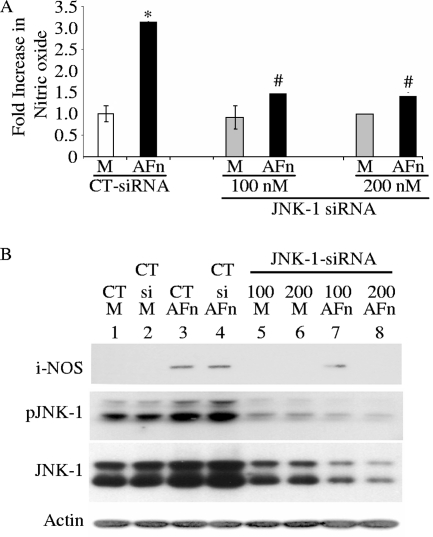
In previous studies, we demonstrated that AFn mediates apoptosis by inducing JNK phosphorylation in primary cells. In addition, other reports suggest that JNK is upstream of nitric oxide production under bacterial LPS challenge (9, 16, 32, 48, 49, 55, 60). Therefore, we examined whether the activation of JNK-1 was required for the generation of nitric oxide in these cells in the presence of AFn. As shown by the results in Fig. 4A, inhibiting the expression of JNK-1 with siRNA led to a significant reduction in nitric oxide production in the cells in the presence of AFn. Western blot results (Fig. 4B) confirmed that suppressing JNK-1 with siRNA blocked iNOS expression even in the presence of AFn (Fig. 4B, lane 8). Western blots also confirmed the robust suppression of JNK-1 expression by siRNA in these cells (Fig. 4B, lanes 5 and 6). Note that the double bands present in the Western blots for pJNK and total JNK reveal the cross-reactivity of this antibody with JNK-2 (also noticeable in Fig. 5B).
FIG. 4.
Inhibition of JNK-1 inhibited nitric oxide generation in PDL cells treated with AFn fragment. (A) PDL cells were transfected with two different doses of siRNA for JNK-1 or a control siRNA (CT-siRNA) and then treated with AFn or serum-free medium (M), and the nitric oxide in the medium was assayed by using Griess reagent. *, P value of <0.05 compared with results for control siRNA-transfected and medium-treated cells; #, P value of <0.05 compared with results for control siRNA-transfected and AFn-treated cells. (B) The cells used for the experiment described for panel A were lysed, and the levels of iNOS, pJNK-1, and JNK-1 were determined by Western blot analysis. Actin was used as a loading control. CT, control untransfected cells; CT si, control siRNA-transfected cells; M, serum-free medium.
FIG. 5.
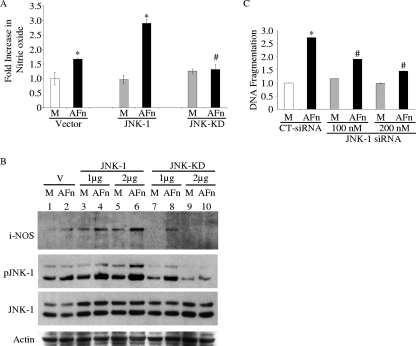
Overexpression of JNK-1 increased nitric oxide generation in the presence of AFn, whereas cells transfected with JNK-KD attenuated nitric oxide production in the presence of AFn. (A) PDL cells were transfected with 2 μg cDNA for JNK-1, JNK-KD, or control vector and then treated with AFn or serum-free medium (M), and nitric oxide was measured in the medium. *, P value of <0.05 compared with results for vector-transfected and medium-treated cells; #, P value of <0.05 compared to results for JNK-1-transfected and AFn-treated cells. (B) PDL cells were treated under conditions similar to those described for panel A except that two different doses of cDNA for JNK-1 and JNK-KD were used to transfect the cells. After treatment, the cells were lysed for Western blot analyses to determine the levels of iNOS, pJNK, and JNK-1. M, cells treated only with serum-free medium; V, cells transfected with vector control. (C) PDL cells were transfected with an siRNA for JNK-1 (JNK-1 siRNA) at concentrations of 100 and 200 nM or with a control siRNA (CT-siRNA) and then treated with AFn. The cells were lysed, and the lysate used for measuring apoptosis by ELISA. The level of absorbance of the serum-free medium (M)-treated control cell lysates at 495 nm was arbitrarily defined as 1 unit, and the levels of absorbance of other cell lysates were expressed as the level of change with respect to the absorbance of the control cell lysate. *, P value of <0.05 compared with results for medium-treated control siRNA-transfected cells; #, P value of <0.05 compared to results for control siRNA-transfected and AFn-treated cells.
To further examine the upstream regulation of nitric oxide by JNK-1 in this mechanism, JNK-1 was overexpressed in these cells in the presence of AFn. The overexpression of JNK-1 heightened the response to AFn and increased the levels of nitric oxide (Fig. 5A) and iNOS (Fig. 5B) significantly. As the results for iNOS expression show, its response to AFn increased with increasing doses of JNK-1 cDNA. Furthermore, the phosphorylation of JNK-1 was also increased by the dual actions of AFn and JNK-1 overexpression, showing its important role in this mechanism (Fig. 5B). To confirm the importance of the requirement for JNK-1 phosphorylation in regulating nitric oxide expression in this pathway, a JNK-KD, which is incapable of being phosphorylated, was tested. Cells overexpressing JNK-KD and treated with AFn produced significantly lower levels of nitric oxide with increasing doses of the JNK-KD cDNA (Fig. 5B). The Western blot results (Fig. 5B) also confirmed the higher levels of JNK-1 expression achieved with transfection and the increased pJNK levels exhibited for JNK-1- but not for JNK-KD-transfected cells. Taken together, these results (Fig. 4 and 5) demonstrate that pJNK is required for AFn's ability to generate nitric oxide in these cells.
As a reconfirmation of JNK-1's role in this apoptotic mechanism, suppressing JNK-1 with siRNA revealed that DNA fragmentation could be suppressed when JNK-1's actions were silenced in these cells (Fig. 5C). Some of the apoptosis noticed with JNK-1 suppression is likely due to residual JNK-1 activity (Fig. 4B).
Bacteria, bacterial endotoxin, and IFN-γ prime cells to release nitric oxide under AFn conditions.
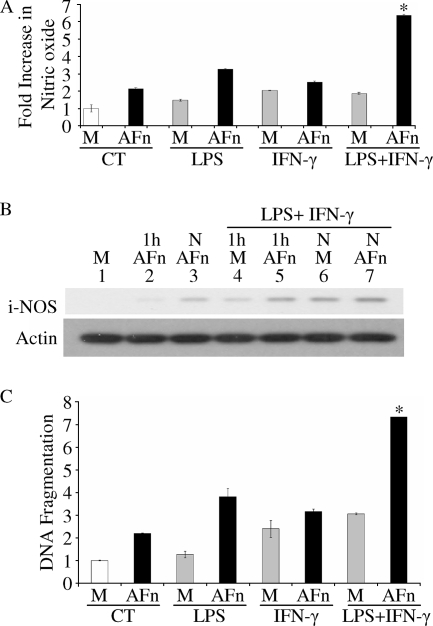
Bacterial components not only degrade the ECM into molecules like AFn but also induce cell signaling in resident cells. Gram-negative bacteria contain LPS, a potent proinflammatory molecule that induces iNOS, on their cell surface. Moreover, at sites of infection, IFN-γ is secreted to stimulate the immune system to eradicate these pathogens, and IFN-γ also induces iNOS (41-43). Therefore, the roles of LPS and IFN-γ in the production of nitric oxide by AFn were investigated. Cells pretreated with both LPS and IFN-γ and then treated with AFn exhibited a significant increase in nitric oxide generation (Fig. 6A). However, there was only a modest increase in nitric oxide production when cells were pretreated with either LPS or IFN-γ and then treated with AFn. The treatment of cells with LPS or IFN-γ alone resulted in a small increase in nitric oxide production.
FIG. 6.
LPS and IFN-γ primed PDL cells toward AFn-mediated nitric oxide production and exacerbated apoptosis. (A) Cells were treated with various combinations of LPS (20 μg/ml), IFN-γ (20 U/ml), and AFn or serum-free medium (M), and the level of nitric oxide generated was measured in the medium. CT, control; *, P value of <0.05 compared with results for AFn-treated cells under control conditions. (B) PDL cells were treated with different combinations of LPS (20 μg/ml), IFN-γ (20 U/ml), and AFn or serum-free medium (M) for 1 h or overnight (N), and the level of expression of iNOS was determined in the cell lysates of the treated cells by Western blot analysis. (C) The cell lysates from the experiment described for panel A were used to determine the level of apoptosis in cells treated with LPS, IFN-γ, and AFn in different combinations. The absorbance of the serum-free medium (M)-treated control cell lysates at 495 nm was arbitrarily defined as 1 unit, and the levels of absorbance of other cell lysates were expressed as the levels of change with respect to the result for the control cell lysate (CT). *, P value of <0.05 compared with result for AFn-treated cells under control conditions.
We further investigated the levels of iNOS in cells treated with LPS and IFN-γ alone or in various combinations with AFn. We found that the levels of iNOS were higher in cells pretreated with the combination of LPS and IFN-γ and then treated with AFn (Fig. 6B). In addition, the levels of iNOS were higher in cells treated overnight with AFn than in those treated for 1 h. We confirmed the role of nitric oxide in mediating this apoptotic mechanism under the modulating effects of LPS and IFN-γ by showing that DNA fragmentation was significantly higher under combined treatments with AFn, LPS, and IFN-γ (Fig. 6C).
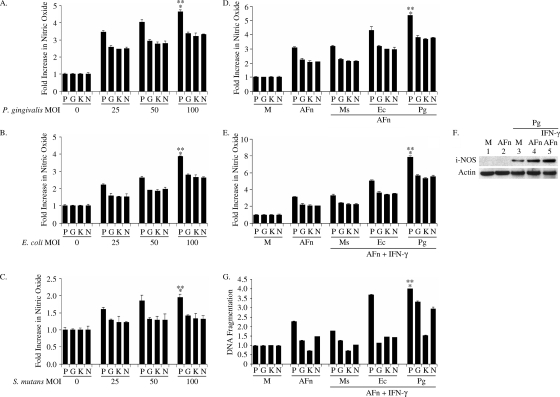
Finally, to more generally examine these mechanisms, we evaluated the effects of live P. gingivalis, E. coli, and S. mutans bacteria on nitric oxide generation in PDL cells, human gingival fibroblasts, human oral keratinocytes, and human neutrophils. Unlike S. mutans, a gram-positive bacteria that lacks LPS on its cell surface, the gram-negative bacteria P. gingivalis and E. coli contain abundant levels of LPS on their cell surface which can activate cells. When PDL cells were treated with P. gingivalis, there were significant increases in nitric oxide production (Fig. 7A) with increasing MOIs compared to the levels observed for cells treated with E. coli and S. mutans (Fig. 7B and C). Although the levels were lower than the levels for PDL cells, human gingival fibroblasts, human oral keratinocytes, and human neutrophils also produced higher levels of nitric oxide in the presence of P. gingivalis than that of E. coli and S. mutans. We further observed dose-dependent increases in nitric oxide production by PDL cells with increasing MOIs of E. coli that were greater than those of human gingival fibroblasts, human oral keratinocytes, and human neutrophils (Fig. 7B). With increasing MOIs, S. mutans induced modest increases in nitric oxide production in PDL cells and slight increases in nitric oxide production in gingival fibroblasts, human oral keratinocytes, and human neutrophils (Fig. 7C).
FIG. 7.
Effects of gram-positive and gram-negative bacteria on nitric oxide generation in primary cells. (A to C) Cells were treated with various MOIs of P. gingivalis, E. coli, and S. mutans; nitric oxide was measured in each conditioned medium, and the level compared to the level generated by cells which were treated with serum-free medium. *, P value of <0.05 compared with result for serum-free-medium-treated cells; **, P value of <0.05 compared to results for gingival fibroblasts, keratinocytes, and neutrophils at an MOI of 100. (D, E) Cells were pretreated with P. gingivalis, S. mutans, or E. coli at an MOI of 50 or with IFN-γ (20 U/ml) or both and then treated with 20 μg/ml AFn. The nitric oxide generated was measured in each conditioned medium and compared to the level generated by cells which were treated with serum-free medium (M). *, P value of <0.05 compared with result for serum-free medium-treated cells; **, P value of <0.05 compared to results for gingival fibroblasts, keratinocytes, and neutrophils treated with P. gingivalis and AFn shown in panel D and for P. gingivalis, IFN-γ, and AFn shown in panel E, respectively. (F) The PDL cells pretreated with P. gingivalis for which results are shown in panel E were lysed, and their cell lysates immunoblotted for iNOS detection. Actin was used as a loading control. (G) The PDL cells which were treated as described for panel E were lysed, and the lysates were used to determine the levels of apoptosis relative to the levels in cells which were treated with serum-free medium (M). The absorbance of the serum-free medium-treated control cell lysates at 495 nm was arbitrarily defined as 1 unit, and the levels of absorbance of other cell lysates were expressed as levels of change with respect to the result for the control cell lysate. *, P value of <0.05 compared with result for serum-free medium-treated cells; **, P value of <0.05 compared to results for keratinocytes treated with P. gingivalis, IFN-γ, and AFn; P, PDL cells; G, human gingival fibroblasts; K, human oral keratinocytes; N, human neutrophils; Pg, P. gingivalis; Sm, S. mutans; Ec, E. coli.
Next, we pretreated the various cells with P. gingivalis, E. coli, and S. mutans at an MOI of 50 before challenging the cells with AFn or a combination of AFn and IFN-γ. We used a lower dose of AFn for these pretreated cells in order to clearly differentiate the effects of AFn alone versus those with the various bacteria and IFN-γ. We observed that the pretreatment of cells with AFn and P. gingivalis resulted in a greater increase in nitric oxide production (Fig. 7D and E) than pretreatment with AFn and E. coli or S. mutans, especially in PDL cells. The greater magnitude of nitric oxide production by PDL cells in the presence of P. gingivalis was due to robust induction of iNOS, as seen by the results in Fig. 7F. We further observed increased apoptosis in PDL cells (Fig. 7G) in the presence of P. gingivalis and IFN-γ and AFn. A combination of AFn and IFN-γ induced almost a fourfold increase in apoptosis, the highest level among the cells tested, in PDL cells infected with P. gingivalis over the level in uninfected controls. Human gingival fibroblasts and neutrophils exhibited slightly increased apoptosis in the presence of AFn, but this was further augmented with the combination of AFn, IFN-γ, and P. gingivalis. S. mutans or E. coli only caused a slight increase in apoptosis in the gingival fibroblasts and neutrophils when combined with AFn and IFN-γ treatment. In contrast, the apoptosis of oral keratinocytes was not augmented by P. gingivalis treatment when combined with AFn and IFN-γ treatment.
In our initial prescreening experiments (data not shown), we infected various cells with bacteria at MOIs of 25, 50, and 100 and observed that with increasing MOIs, there were increased levels of apoptosis of PDL cells in the presence of E. coli and P. gingivalis, but not with S. mutans, and these levels were highest with P. gingivalis. The same was noted with neutrophils, although to a lesser extent. The apoptosis of human gingival fibroblasts was slightly increased at an MOI of 25 for E. coli but then decreased at MOIs of 50 and 100, whereas in the presence of P. gingivalis and S. mutans, the gingival fibroblasts underwent a slight increase (about a 1.4- and 1.5-fold increase, respectively) in apoptosis compared to the level in uninfected cells (data not shown). There have been conflicting reports in the literature about the effects of P. gingivalis on apoptosis of epithelial cells. In our experiments with human oral keratinocytes, P. gingivalis increased their apoptosis slightly, to 1.3-fold, at an MOI of 50 but then apoptosis at an MOI of 100 was suppressed back to control levels (data not shown). Also, neither S. mutans nor E. coli induced apoptosis of oral keratinocytes with increasing MOIs. These data strongly suggest that during bacterially induced periodontal disease and inflammation, the combined effects of an altered matrix, bacterial components, and inflammatory mediators are more deleterious to host cells by inducing a higher level of nitric oxide and apoptosis.
DISCUSSION
The macromolecules that comprise the ECM play important roles in providing structural integrity to cells, tissues, and organs and also serve as a conduit for cell-cell signaling. However, in diseases caused by infection and inflammation, like periodontitis, and diseases of the joints, like arthritis, the ECM undergoes degradation by bacterial proteases or host-derived inflammatory proteases (37, 73, 75). Thus, breakdown fragments of the ECM indicate a state of dissolution, and they trigger deleterious cellular events, including apoptosis. In our earlier studies, we characterized one such breakdown product of the ECM that arises from the fragmentation of fibronectin. This breakdown product of fibronectin originates from the proteolytic cleavage of intact fibronectin, and it is found in the gingival crevicular fluid of patients affected by periodontal disease and in joint fluids from arthritic patients (29, 33). Our earlier studies showed that this proteolytic fragment and its recombinant counterpart, AFn, trigger aberrant cell signaling in primary cells that ultimately leads to apoptosis (13, 40, 68). In addition, the breakdown products of macromolecules generate nitric oxide in cells present in inflamed sites. Nitric oxide is an instrumental signaling molecule in many apoptotic, as well as antiapoptotic, events (8). The aim of this study was to examine whether AFn induces the generation of nitric oxide in primary cells and whether the inhibition of nitric oxide generation could prevent apoptosis of these cells. Furthermore, since our previous studies showed that AFn phosphorylates JNK as part of the apoptotic mechanism, we investigated whether JNK was necessary for the generation of nitric oxide.
Our data demonstrate that nitric oxide and its regulatory enzyme iNOS are both upregulated under proapoptotic matrix conditions, suggesting a key role for these processes in the pathogenesis of periodontal disease and inflammation. High levels of nitric oxide and iNOS have been reported in patients with periodontal disease (5, 18, 23, 44, 58, 65) and in rodent models of periodontal disease (15, 25, 26, 46, 50, 51). However, the mechanisms by which these molecules mediate their pathogenic effects have not been fully delineated. Reports indicate that periodontopathic bacteria and bacterial components, including LPS, stimulate local gingival fibroblasts and inflammatory cells, including macrophages, to produce nitric oxide (2, 7, 12, 17, 41, 46, 61, 66). In agreement with these findings, our data show for the first time that PDL cells also respond to an LPS challenge by producing increased levels of nitric oxide and iNOS. Our data further showcase a novel matrix-mediated mechanism by which nitric oxide might be induced under conditions of periodontal inflammation.
Moreover, sites of infection and inflammation often contain immunomodulators like IFN-γ which can facilitate cellular release of nitric oxide (10). Therefore, in the present study, we examined the effects of LPS and IFN-γ on the ability of AFn to generate nitric oxide in primary PDL cells. We observed that neither LPS nor IFN-γ on their own nor a combination of the two increased the generation of nitric oxide from these primary cells beyond twofold compared to the level generated by untreated cells (Fig. 6A). When cells were treated with AFn and either LPS or IFN-γ, there was approximately a 2.5- to 3-fold increase in nitric oxide production from these cells. However, there was a dramatic increase in the production of nitric oxide when cells were treated with AFn in the presence of LPS and IFN-γ. Similarly, the induction of iNOS was high in the presence of LPS and IFN-γ, and this induction was greater when cells were further treated with AFn.
To examine these mechanisms more generally and to ascertain the differences in the involvement of different types of bacteria for nitric oxide production, we used live gram-positive (S. mutans) and gram-negative (P. gingivalis and E. coli) bacteria in our experiments. We observed that S. mutans, a gram-positive bacterium, was able to induce small amounts of nitric oxide generation in PDL cells. Although S. mutans lacks LPS, other cell wall components, like lipoteichoic acid and polyosides, might trigger the release of nitric oxide from PDL cells. The treatment of PDL cells with E. coli, a gram-negative bacteria with abundant LPS, resulted in the production of higher levels of nitric oxide than those observed with S. mutans. However our experiments with live P. gingivalis prompted PDL cells to release nitric oxide at much higher levels (Fig. 7E) than were observed with LPS purified from P. gingivalis (Fig. 6A). This is likely attributable to the fact that live bacteria can provide a more-active conformation of the LPS than the purified form, and they also possess other virulence factors, which are more potent and distinct from those of E. coli. Moreover, PDL cells primed with P. gingivalis and IFN-γ required much lower concentrations of AFn to release significant amounts of nitric oxide and to undergo apoptosis. We also tested the abilities of other cells, including human gingival fibroblasts, human oral keratinocytes, and human neutrophils, to respond to gram-negative and gram-positive bacteria and AFn. The results of our experiments revealed that among the cells tested, PDL cells were the most responsive toward nitric oxide generation in response to either the gram-positive or the gram-negative bacteria. Also, PDL cells produced much higher levels of nitric oxide in the presence of AFn and even higher levels of nitric oxide in combination with IFN-γ than the other primary cells that were used to mimic in vivo conditions.
Since epithelial cells are the first line of defense against foreign invading pathogens, we tested their effectiveness against bacterial infection. We found that although these cells produced large amounts of nitric oxide in the presence of AFn or when combined with P. gingivalis or AFn plus IFN-γ, they were significantly less apoptotic than other cells under the same conditions. Current research on the fate of epithelial cells following infection with P. gingivalis is contradictory. In general, P. gingivalis induces apoptosis of fibroblasts (21, 35, 71), endothelial cells (59, 63), cardiac myoblasts (45), lymphocytes (19), monocytes (54), and polymorphonuclear neutrophils (27, 52, 57), whereas live P. gingivalis inhibits apoptosis of epithelial cells (53). For example, P. gingivalis inhibits apoptosis induced by camptothecin, a human topoisomerase I inhibitor used as an anticancer drug. In contrast, heat-killed P. gingivalis and protease-active extracellular components of P. gingivalis or its mutants induce apoptosis of epithelial cells (11). Interestingly, ex vivo studies revealed apoptosis at sites of bacterial-associated inflammation in human gingival tissues, especially near the epithelial layer (69). In these apoptotic sites, there are increased levels of caspase-3 and -7, the two effector caspases that are responsible for apoptosis (4). Nitric oxide can be anti- or proapoptotic (62), and thus it is possible that nitric oxide generated from oral keratinocytes infected with live P. gingivalis may contribute to their resistance to apoptosis.
Our experimental results show that under in vivo conditions, bacterial LPS and IFN-γ, by virtue of their presence at sites of infection and inflammation, can prime the neighboring cells to produce large amounts of nitric oxide in the presence of a degraded, proapoptotic ECM. This huge surge in nitric oxide production may be one potential mechanism behind tissue destruction and apoptosis at sites of infection and inflammation. We have also shown that inhibiting nitric oxide production by inhibiting iNOS and JNK-1 can help prevent apoptosis of these cells. Therefore, the use of such biological modifiers may have therapeutic benefits in the treatment of tissue-destructive diseases arising from disease-altered matrices (56).
Reported mechanisms in the activation of nitric oxide include the activation of mitogen-activated protein kinase family members, including p38 and the transcription factor NF-κB (24, 41, 42). However, there are no reports that have examined the role of the ECM in regulating nitric oxide and iNOS in inflammatory conditions, including periodontal disease. Our data indicate that JNK is critical to this mechanism, since specifically targeting its expression by transfection/overexpression or suppressing it with siRNA or a kinase mutant showed direct effects on nitric oxide production in PDL cells. Thus, these experimental observations indicate that the treatment of cells with AFn results in the activation of JNK-1 to pJNK, which in turn leads to the activation of iNOS, which ultimately results in the generation of nitric oxide by these primary cells. In summary, our collective data indicate that disease-altered matrices in combination with existing bacterial and inflammatory components prime resident connective tissue cells, especially PDL cells, to produce higher levels of nitric oxide into the inflammatory milieu, thus potentially exacerbating the extent of tissue destruction by apoptosis.
Acknowledgments
We thank Kenji Izumi, University of Michigan, for assistance with primary tissue preparation, Paul Johnson for recombinant fibronectin proteins, and David A. Geller, University of Pittsburgh, Pittsburgh, PA, for human iNOS cDNA.
This work was supported by NIH grant R01 DE 013725 to Y.L.K.
Editor: F. C. Fang
Footnotes
Published ahead of print on 6 October 2008.
REFERENCES
- 1.Adair-Kirk, T. L., and R. M. Senior. 2008. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 401101-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alayan, J., S. Ivanovski, E. Gemmell, P. Ford, S. Hamlet, and C. S. Farah. 2006. Deficiency of iNOS contributes to Porphyromonas gingivalis-induced tissue damage. Oral Microbiol. Immunol. 21360-365. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, M. B., and P. D. Damoulis. 1994. The role of cytokines in the pathogenesis of periodontal disease. Curr. Opin. Periodontol. 200839-53. [PubMed] [Google Scholar]
- 4.Bantel, H., T. Beikler, T. F. Flemmig, and K. Schulze-Osthoff. 2005. Caspase activation is involved in chronic periodontitis. FEBS Lett. 5795559-5564. [DOI] [PubMed] [Google Scholar]
- 5.Batista, A. C., T. A. Silva, J. H. Chun, and V. S. Lara. 2002. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis. 8254-260. [DOI] [PubMed] [Google Scholar]
- 6.Bewsey, K. E., C. Wen, C. Purple, and G. A. Homandberg. 1996. Fibronectin fragments induce the expression of stromelysin-1 mRNA and protein in bovine chondrocytes in monolayer culture. Biochim. Biophys. Acta 131755-64. [DOI] [PubMed] [Google Scholar]
- 7.Blix, I. J., and K. Helgeland. 1998. LPS from Actinobacillus actinomycetemcomitans and production of nitric oxide in murine macrophages J774. Eur. J. Oral Sci. 106576-581. [DOI] [PubMed] [Google Scholar]
- 8.Brune, B. 2003. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 10864-869. [DOI] [PubMed] [Google Scholar]
- 9.Chan, E. D., K. R. Morris, J. T. Belisle, P. Hill, L. K. Remigio, P. J. Brennan, and D. W. Riches. 2001. Induction of inducible nitric oxide synthase-NO· by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-κB signaling pathways. Infect. Immun. 692001-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, E. D., and D. W. Riches. 2001. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am. J. Physiol. Cell Physiol. 280C441-C450. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Z., C. A. Casiano, and H. M. Fletcher. 2001. Protease-active extracellular protein preparations from Porphyromonas gingivalis W83 induce N-cadherin proteolysis, loss of cell adhesion, and apoptosis in human epithelial cells. J. Periodontol. 72641-650. [DOI] [PubMed] [Google Scholar]
- 12.Choi, E. Y., Y. M. Hwang, J. Y. Lee, J. I. Choi, I. S. Choi, J. Y. Jin, J. S. Ko, and S. J. Kim. 2007. Lipid A-associated proteins from Porphyromonas gingivalis stimulate release of nitric oxide by inducing expression of inducible nitric oxide synthase. J. Periodontal Res. 42350-360. [DOI] [PubMed] [Google Scholar]
- 13.Dai, R., A. Iwama, S. Wang, and Y. L. Kapila. 2005. Disease-associated fibronectin matrix fragments trigger anoikis of human primary ligament cells: p53 and c-myc are suppressed. Apoptosis 10503-512. [DOI] [PubMed] [Google Scholar]
- 14.Ding, L., D. Guo, and G. A. Homandberg. 2008. The cartilage chondrolytic mechanism of fibronectin fragments involves MAP kinases: comparison of three fragments and native fibronectin. Osteoarthritis Cartilage 161253-1262. [DOI] [PubMed] [Google Scholar]
- 15.Di Paola, R., S. Marzocco, E. Mazzon, F. Dattola, F. Rotondo, D. Britti, M. De Majo, T. Genovese, and S. Cuzzocrea. 2004. Effect of aminoguanidine in ligature-induced periodontitis in rats. J. Dent. Res. 83343-348. [DOI] [PubMed] [Google Scholar]
- 16.Ea, H. K., B. Uzan, C. Rey, and F. Liote. 2005. Octacalcium phosphate crystals directly stimulate expression of inducible nitric oxide synthase through p38 and JNK mitogen-activated protein kinases in articular chondrocytes. Arthritis Res. Ther. 7R915-R926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garlet, G. P., C. R. Cardoso, A. P. Campanelli, B. R. Ferreira, M. J. Avila-Campos, F. Q. Cunha, and J. S. Silva. 2007. The dual role of p55 tumour necrosis factor-alpha receptor in Actinobacillus actinomycetemcomitans-induced experimental periodontitis: host protection and tissue destruction. Clin. Exp. Immunol. 147128-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaspirc, B., A. Masera, and U. Skaleric. 2002. Immunolocalization of inducible nitric oxide synthase in localized juvenile periodontitis patients. Connect. Tissue Res. 43413-418. [DOI] [PubMed] [Google Scholar]
- 19.Geatch, D. R., J. I. Harris, P. A. Heasman, and J. J. Taylor. 1999. In vitro studies of lymphocyte apoptosis induced by the periodontal pathogen Porphyromonas gingivalis. J. Periodontal. Res. 3470-78. [DOI] [PubMed] [Google Scholar]
- 20.Gemba, T., J. Valbracht, S. Alsalameh, and M. Lotz. 2002. Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J. Biol. Chem. 277907-911. [DOI] [PubMed] [Google Scholar]
- 21.Graves, D. T., M. Oskoui, S. Volejnikova, G. Naguib, S. Cai, T. Desta, A. Kakouras, and Y. Jiang. 2001. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J. Dent. Res. 801875-1879. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths, A. M., K. E. Herbert, D. Perrett, and D. L. Scott. 1989. Fragmented fibronectin and other synovial fluid proteins in chronic arthritis: their relation to immune complexes. Clin. Chim. Acta 184133-146. [DOI] [PubMed] [Google Scholar]
- 23.Gullu, C., N. Ozmeric, B. Tokman, S. Elgun, and K. Balos. 2005. Effectiveness of scaling and root planing versus modified Widman flap on nitric oxide synthase and arginase activity in patients with chronic periodontitis. J. Periodontal. Res. 40168-175. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez-Venegas, G., S. Maldonado-Frias, A. Ontiveros-Granados, and P. Kawasaki-Cardenas. 2005. Role of p38 in nitric oxide synthase and cyclooxygenase expression, and nitric oxide and PGE2 synthesis in human gingival fibroblasts stimulated with lipopolysaccharides. Life Sci. 7760-73. [DOI] [PubMed] [Google Scholar]
- 25.Gyurko, R., G. Boustany, P. L. Huang, A. Kantarci, T. E. Van Dyke, C. A. Genco, and F. C. Gibson III. 2003. Mice lacking inducible nitric oxide synthase demonstrate impaired killing of Porphyromonas gingivalis. Infect. Immun. 714917-4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyurko, R., H. Shoji, R. A. Battaglino, G. Boustany, F. C. Gibson III, C. A. Genco, P. Stashenko, and T. E. Van Dyke. 2005. Inducible nitric oxide synthase mediates bone development and P. gingivalis-induced alveolar bone loss. Bone 36472-479. [DOI] [PubMed] [Google Scholar]
- 27.Hiroi, M., T. Shimojima, M. Kashimata, T. Miyata, H. Takano, M. Takahama, and H. Sakagami. 1998. Inhibition by Porphyromonas gingivalis LPS of apoptosis induction in human peripheral blood polymorphonuclear leukocytes. Anticancer Res. 183475-3479. [PubMed] [Google Scholar]
- 28.Homandberg, G. A., and F. Hui. 1996. Association of proteoglycan degradation with catabolic cytokine and stromelysin release from cartilage cultured with fibronectin fragments. Arch. Biochem. Biophys. 334325-331. [DOI] [PubMed] [Google Scholar]
- 29.Homandberg, G. A., C. Wen, and F. Hui. 1998. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthritis Cartilage 6231-244. [DOI] [PubMed] [Google Scholar]
- 30.Horton, M. R., C. M. McKee, C. Bao, F. Liao, J. M. Farber, J. Hodge-DuFour, E. Pure, B. L. Oliver, T. M. Wright, and P. W. Noble. 1998. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines mig and interferon-inducible protein-10 in mouse macrophages. J. Biol. Chem. 27335088-35094. [DOI] [PubMed] [Google Scholar]
- 31.Horton, M. R., S. Shapiro, C. Bao, C. J. Lowenstein, and P. W. Noble. 1999. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J. Immunol. 1624171-4176. [PubMed] [Google Scholar]
- 32.Huh, J. E., J. H. Yim, H. K. Lee, E. Y. Moon, D. K. Rhee, and S. Pyo. 2007. Prodigiosin isolated from Hahella chejuensis suppresses lipopolysaccharide-induced NO production by inhibiting p38 MAPK, JNK and NF-kappaB activation in murine peritoneal macrophages. Int. Immunopharmacol. 71825-1833. [DOI] [PubMed] [Google Scholar]
- 33.Huynh, Q. N., S. Wang, E. Tafolla, S. A. Gansky, S. Kapila, G. C. Armitage, and Y. L. Kapila. 2002. Specific fibronectin fragments as markers of periodontal disease status. J. Periodontol. 731101-1110. [DOI] [PubMed] [Google Scholar]
- 34.Iacob, S., and C. B. Knudson. 2006. Hyaluronan fragments activate nitric oxide synthase and the production of nitric oxide by articular chondrocytes. Int. J. Biochem. Cell Biol. 38123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imatani, T., T. Kato, K. Okuda, and Y. Yamashita. 2004. Histatin 5 inhibits apoptosis in human gingival fibroblasts induced by porphyromonas gingivalis cell-surface polysaccharide. Eur. J. Med. Res. 9528-532. [PubMed] [Google Scholar]
- 36.Ishikawa, T., and P. L. Morris. 2006. Interleukin-1beta signals through a c-Jun N-terminal kinase-dependent inducible nitric oxide synthase and nitric oxide production pathway in Sertoli epithelial cells. Endocrinology 1475424-5430. [DOI] [PubMed] [Google Scholar]
- 37.Jang, D., and G. A. Murrell. 1998. Nitric oxide in arthritis. Free Radic. Biol. Med. 241511-1519. [DOI] [PubMed] [Google Scholar]
- 38.Kapila, Y. L., S. Kapila, and P. W. Johnson. 1996. Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol. 15251-261. [DOI] [PubMed] [Google Scholar]
- 39.Kapila, Y. L., J. Niu, and P. W. Johnson. 1997. The high affinity heparin-binding domain and the V region of fibronectin mediate invasion of human oral squamous cell carcinoma cells in vitro. J. Biol. Chem. 27218932-18938. [DOI] [PubMed] [Google Scholar]
- 40.Kapila, Y. L., S. Wang, and P. W. Johnson. 1999. Mutations in the heparin binding domain of fibronectin in cooperation with the V region induce decreases in pp125(FAK) levels plus proteoglycan-mediated apoptosis via caspases. J. Biol. Chem. 27430906-30913. [DOI] [PubMed] [Google Scholar]
- 41.Kim, S. J., E. Y. Choi, Y. J. Cho, J. Y. Lee, J. I. Choi, and I. S. Choi. 2006. Surface-associated material from Porphyromonas gingivalis stimulates the release of nitric oxide by inducing expression of inducible nitric oxide synthase. Microbes Infect. 8470-477. [DOI] [PubMed] [Google Scholar]
- 42.Kim, S. J., M. S. Ha, E. Y. Choi, J. I. Choi, and I. S. Choi. 2005. Nitric oxide production and inducible nitric oxide synthase expression induced by Prevotella nigrescens lipopolysaccharide. FEMS Immunol. Med. Microbiol. 4351-58. [DOI] [PubMed] [Google Scholar]
- 43.Kim, S. J., M. S. Ha, E. Y. Choi, J. I. Choi, and I. S. Choi. 2004. Prevotella intermedia lipopolysaccharide stimulates release of nitric oxide by inducing expression of inducible nitric oxide synthase. J. Periodontal. Res. 39424-431. [DOI] [PubMed] [Google Scholar]
- 44.Lappin, D. F., M. Kjeldsen, L. Sander, and D. F. Kinane. 2000. Inducible nitric oxide synthase expression in periodontitis. J. Periodontal Res. 35369-373. [DOI] [PubMed] [Google Scholar]
- 45.Lee, S. D., C. C. Wu, W. W. Kuo, J. A. Lin, J. M. Hwang, M. C. Lu, L. M. Chen, H. H. Hsu, C. K. Wang, S. H. Chang, and C. Y. Huang. 2006. Porphyromonas gingivalis-related cardiac cell apoptosis was majorly co-activated by p38 and extracellular signal-regulated kinase pathways. J. Periodontal Res. 4139-46. [DOI] [PubMed] [Google Scholar]
- 46.Leitao, R. F., R. A. Ribeiro, H. V. Chaves, F. A. Rocha, V. Lima, and G. A. Brito. 2005. Nitric oxide synthase inhibition prevents alveolar bone resorption in experimental periodontitis in rats. J. Periodontol. 76956-963. [DOI] [PubMed] [Google Scholar]
- 47.Li, C. Q., and G. N. Wogan. 2005. Nitric oxide as a modulator of apoptosis. Cancer Lett. 2261-15. [DOI] [PubMed] [Google Scholar]
- 48.Li, L., Z. Feng, and A. G. Porter. 2004. JNK-dependent phosphorylation of c-Jun on serine 63 mediates nitric oxide-induced apoptosis of neuroblastoma cells. J. Biol. Chem. 2794058-4065. [DOI] [PubMed] [Google Scholar]
- 49.Lin, M. W., L. T. Tsao, L. C. Chang, Y. L. Chen, L. J. Huang, S. C. Kuo, C. C. Tzeng, M. R. Lee, and J. P. Wang. 2007. Inhibition of lipopolysaccharide-stimulated NO production by a novel synthetic compound CYL-4d in RAW 264.7 macrophages involving the blockade of MEK4/JNK/AP-1 pathway. Biochem. Pharmacol. 731796-1806. [DOI] [PubMed] [Google Scholar]
- 50.Lohinai, Z., P. Benedek, E. Feher, A. Gyorfi, L. Rosivall, A. Fazekas, A. L. Salzman, and C. Szabo. 1998. Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature-induced periodontitis in the rat. Br. J. Pharmacol. 123353-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lohinai, Z., R. Stachlewitz, L. Virag, A. D. Szekely, G. Hasko, and C. Szabo. 2001. Evidence for reactive nitrogen species formation in the gingivomucosal tissue. J. Dent. Res. 80470-475. [DOI] [PubMed] [Google Scholar]
- 52.Murray, D. A., and J. M. Wilton. 2003. Lipopolysaccharide from the periodontal pathogen Porphyromonas gingivalis prevents apoptosis of HL60-derived neutrophils in vitro. Infect. Immun. 717232-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakhjiri, S. F., Y. Park, O. Yilmaz, W. O. Chung, K. Watanabe, A. El-Sabaeny, K. Park, and R. J. Lamont. 2001. Inhibition of epithelial cell apoptosis by Porphyromonas gingivalis. FEMS Microbiol. Lett. 200145-149. [DOI] [PubMed] [Google Scholar]
- 54.Ozaki, K., and S. Hanazawa. 2001. Porphyromonas gingivalis fimbriae inhibit caspase-3-mediated apoptosis of monocytic THP-1 cells under growth factor deprivation via extracellular signal-regulated kinase-dependent expression of p21 Cip/WAF1. Infect. Immun. 694944-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pawate, S., and N. R. Bhat. 2006. c-Jun N-terminal kinase (JNK) regulation of iNOS expression in glial cells: predominant role of JNK1 isoform. Antioxid. Redox Signal. 8903-909. [DOI] [PubMed] [Google Scholar]
- 56.Pichika, R., and G. A. Homandberg. 2004. Fibronectin fragments elevate nitric oxide (NO) and inducible NO synthetase (iNOS) levels in bovine cartilage and iNOS inhibitors block fibronectin fragment mediated damage and promote repair. Inflamm. Res. 53405-412. [DOI] [PubMed] [Google Scholar]
- 57.Preshaw, P. M., R. E. Schifferle, and J. D. Walters. 1999. Porphyromonas gingivalis lipopolysaccharide delays human polymorphonuclear leukocyte apoptosis in vitro. J. Periodontal Res. 34197-202. [DOI] [PubMed] [Google Scholar]
- 58.Reher, V. G., E. G. Zenobio, F. O. Costa, P. Reher, and R. V. Soares. 2007. Nitric oxide levels in saliva increase with severity of chronic periodontitis. J. Oral Sci. 49271-276. [DOI] [PubMed] [Google Scholar]
- 59.Roth, G. A., H. J. Ankersmit, V. B. Brown, P. N. Papapanou, A. M. Schmidt, and E. Lalla. 2007. Porphyromonas gingivalis infection and cell death in human aortic endothelial cells. FEMS Microbiol. Lett. 272106-113. [DOI] [PubMed] [Google Scholar]
- 60.Seminara, A. R., P. P. Ruvolo, and F. Murad. 2007. LPS/IFNgamma-induced RAW 264.7 apoptosis is regulated by both nitric oxide-dependent and -independent pathways involving JNK and the Bcl-2 family. Cell Cycle 61772-1778. [DOI] [PubMed] [Google Scholar]
- 61.Shapira, L., I. Frolov, A. Halabi, and D. Ben-Nathan. 2000. Experimental stress suppresses recruitment of macrophages but enhanced their P. gingivalis LPS-stimulated secretion of nitric oxide. J. Periodontol. 71476-481. [DOI] [PubMed] [Google Scholar]
- 62.Sharma, J. N., A. Al-Omran, and S. S. Parvathy. 2007. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 15252-259. [DOI] [PubMed] [Google Scholar]
- 63.Sheets, S. M., J. Potempa, J. Travis, C. A. Casiano, and H. M. Fletcher. 2005. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect. Immun. 731543-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shibata, K., M. L. Warbington, B. J. Gordon, H. Kurihara, and T. E. Van Dyke. 2001. Nitric oxide synthase activity in neutrophils from patients with localized aggressive periodontitis. J. Periodontol. 721052-1058. [DOI] [PubMed] [Google Scholar]
- 65.Skaleric, U., B. Gaspirc, N. McCartney-Francis, A. Masera, and S. M. Wahl. 2006. Proinflammatory and antimicrobial nitric oxide in gingival fluid of diabetic patients with periodontal disease. Infect. Immun. 747010-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sosroseno, W., E. Herminajeng, H. Susilowati, and S. Budiarti. 2002. Nitric oxide production by murine spleen cells stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans. Anaerobe 8333-339. [DOI] [PubMed] [Google Scholar]
- 67.Stanton, H., L. Ung, and A. J. Fosang. 2002. The 45 kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem. J. 364181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tafolla, E., S. Wang, B. Wong, J. Leong, and Y. L. Kapila. 2005. JNK1 and JNK2 oppositely regulate p53 in signaling linked to apoptosis triggered by an altered fibronectin matrix: JNK links FAK and p53. J. Biol. Chem. 28019992-19999. [DOI] [PubMed] [Google Scholar]
- 69.Tonetti, M. S., D. Cortellini, and N. P. Lang. 1998. In situ detection of apoptosis at sites of chronic bacterially induced inflammation in human gingiva. Infect. Immun. 665190-5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ugar-Cankal, D., and N. Ozmeric. 2006. A multifaceted molecule, nitric oxide in oral and periodontal diseases. Clin. Chim. Acta 36690-100. [DOI] [PubMed] [Google Scholar]
- 71.Urnowey, S., T. Ansai, V. Bitko, K. Nakayama, T. Takehara, and S. Barik. 2006. Temporal activation of anti- and proapoptotic factors in human gingival fibroblasts infected with the periodontal pathogen, Porphyromonas gingivalis: potential role of bacterial proteases in host signalling. BMC Microbiol. 626. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Vuolteenaho, K., T. Moilanen, R. G. Knowles, and E. Moilanen. 2007. The role of nitric oxide in osteoarthritis. Scand. J. Rheumatol. 36247-258. [DOI] [PubMed] [Google Scholar]
- 73.Xie, D. L., F. Hui, R. Meyers, and G. A. Homandberg. 1994. Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: stromelysin plays a major role in chondrolysis. Arch. Biochem. Biophys. 311205-212. [DOI] [PubMed] [Google Scholar]
- 74.Xie, D. L., R. Meyers, and G. A. Homandberg. 1992. Fibronectin fragments in osteoarthritic synovial fluid. J. Rheumatol. 191448-1452. [PubMed] [Google Scholar]
- 75.Yasuda, T., and A. R. Poole. 2002. A fibronectin fragment induces type II collagen degradation by collagenase through an interleukin-1-mediated pathway. Arthritis Rheum. 46138-148. [DOI] [PubMed] [Google Scholar]
- 76.Yasuda, T., M. Shimizu, T. Nakagawa, S. M. Julovi, and T. Nakamura. 2003. Matrix metalloproteinase production by COOH-terminal heparin-binding fibronectin fragment in rheumatoid synovial cells. Lab. Investig. 83153-162. [DOI] [PubMed] [Google Scholar]