Abstract
The lipooligosaccharide (LOS) of Neisseria meningitidis can be decorated with phosphoethanolamine (PEA) at the 4′ position of lipid A and at the O-3 and O-6 positions of the inner core of the heptose II residue. The biological role of PEA modification in N. meningitidis remains unclear. During the course of our studies to elucidate the pathogenicity of the ST-2032 (invasive) meningococcal clonal group, disruption of lptA, the gene that encodes the PEA transferase for 4′ lipid A, led to a approximately 10-fold decrease in N. meningitidis adhesion to four kinds of human endothelial and epithelial cell lines at an multiplicity of infection of 5,000. Complementation of the lptA gene in a ΔlptA mutant restored wild-type adherence. By matrix-assisted laser desorption ionization-time-of-flight mass spectrometry analysis, PEA was lost from the lipid A of the ΔlptA mutant compared to that of the wild-type strain. The effect of LptA on meningococcal adhesion was independent of other adhesins such as pili, Opc, Opa, and PilC but was inhibited by the presence of capsule. These results indicate that modification of LOS with PEA by LptA enhances meningococcal adhesion to human endothelial and epithelial cells in unencapsulated N. meningitidis.
Neisseria meningitidis is a gram-negative diplococcal pathogen that colonizes the nasopharynxes of humans as a unique host and may enter the bloodstream, where it can cause fulminant septicemia or induce acute meningitis after passing through the brain-blood barrier and gaining access to cerebrospinal fluid (CSF). Meningococcal factors such as Opa, Opc, pili, and PilC are known to participate in the adhesion to and invasion of human host cells (reviewed in reference 9). Nonprotein factors such as capsule and lipooligosaccharide (LOS) also play important roles in meningococcal infections in humans (30, 34, 42, 49). However, many questions about meningococcal pathogenicity remain unsolved (26-28).
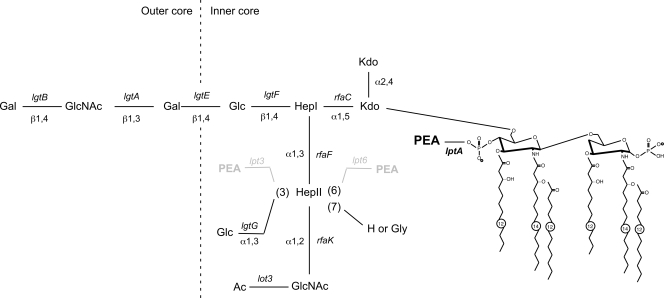
Meningococcal LOS, an important inducer of the host inflammatory response in meningococcal sepsis, is structurally distinct from lipopolysaccharide (LPS) of enteric gram-negative bacteria. Instead of the repeating O antigens of enteric LPS, meningococcal LOS consists of short variable oligosaccharide (OS) residues linked to two conserved core heptose molecules attached to lipid A via a ketodeoxyoctulonic acid molecule (Kdo I). The OS chains are the alpha chain branching from heptose I (Hep I) in a β(1,4) linkage and the beta and gamma and chains branching from heptose II (Hep II) in α(1,3) and α(1,2) linkages, respectively (Fig. 1) (44). Lipid A triggers the Toll-like receptor 4/MD-2-mediated inflammatory response. Changes in the configuration or the conformational structure of lipid A affect the biological response (31). Structural diversity of meningococcal LOS is due to variability of OS and inner core moieties and leads to immunological differences characterized as 12 distinct immunotypes (44). Diversity is due to the presence, absence or variability of expression of LOS biosynthesis-associated genes (3, 18, 20). Variation of the meningococcal LOS structure also mediates differences in host immune responses and bacterial virulence (32, 44).
FIG. 1.
Schematic representation of the LOS structure in strain HT1125 of N. meningitidis. The LOS structure of N. meningitidis HT1125 was determined by glycosyl composition, glycosyl linkage, nuclear magnetic resonance, and MALDI-TOF MS analyses of the intact LOS, de-O-acylated LOS, isolated lipid A, and isolated OSs from purified LOS (data not shown). The biosynthesis-associated genes are shown in italic at the respective positions. PEA groups at Hep II of the 3 and 6 positions, shown in gray, were absent in the HT1125 strain.
Meningococcal LOS is modified with one or possibly multiple phosphoethanolamine (PEA) groups at the 4′ lipid A moiety by a PEA transferase named LptA (PEA transferase specific for lipid A) (7). In addition to the modification at lipid A, the LOS may also be modified with PEA groups at Hep II by two kinds of other PEA transferases, named Lpt6 (50) and Lpt3 (25). Lpt6 and Lpt3 add a PEA group at the 6 or 3 position, respectively (50). A glucose residue can also be added at the 3 position by the lgtG gene product (α1,3-galactosyl transferase) (25). The presence of lpt6, lpt3, and lgtG varies among meningococcal isolates (50). The LOS modification by LgtG is phase variable due to slippage of a homopolymeric tract of cytidines (18). The Hep II LOS modification with PEA is dependent on the presence of lpt3 and/or lpt6 in a strain, but the addition of the glucose residue at the 3 position is dominant if the lgtG and lpt3 genes are both present in a strain (20). The modification by Lpt3, Lpt6, and LgtG changes the LOS inner core structure and influences the immunotype (3, 18). There is epidemiological evidence that specific kinds of immunotypes, such as L1,8 (33) and L3,7,9 (19), are correlated with meningococcal pathogenesis. Experimentally, an N. meningitidis strain with the L3,7,9 immunotype enhances colonization at the nasal region in an infant mouse intranasal infection model (24), and an L8 N. meningitidis strain adheres to human umbilical vein endothelial cells (HUVEC) more efficiently than an L3 immunotype strain (48). However, the precise role of LOS structure in meningococcal adherence and invasion remains unclear. An encapsulated LOS-deficient mutant shows impaired adherence to human epithelial cells (2), while encapsulated N. meningitidis pgm, rafK, or gmnB mutants, producing a gradual truncation of the outer and inner cores of the LOS, showed enhanced adhesion to Detroit 562 epithelial cells (29). An unencapsulated meningococcal strain enhanced the adherence to dendritic cells by the gradual truncation of meningococcal LOS with a mutation in the lst, lgt, galE, or pgm gene (22). However, on the whole, the relationship between adhesive ability and LOS structure, including the PEA modification, in N. meningitidis is still unclear (7).
We previously reported that a Japan-specific invasive clone, ST-2032, efficiently adheres to and invades human cultured endothelial and epithelial cell lines (36). During the course of our studies to elucidate the pathogenicity of the ST-2032 clone, we noted a relationship between LOS modifications and meningococcal adherence. In the present study, the role of PEA modification in meningococcal adhesion to human cells was examined, and it was found that the PEA modification by LptA enhanced meningococcal adhesion to human endothelial and epithelial cells.
MATERIALS AND METHODS
Bacterial growth conditions.
N. meningitidis strains stored at −80°C were routinely grown on GC agar plates, consisting of GC medium agar (Becton-Dickinson) supplemented with 1% IsoVitaleX enrichment (Becton-Dickinson), at 37°C in 5% CO2 (37). For selecting kanamycin-resistant N. meningitidis strains, brain heart infusion agar (Becton-Dickinson) agar containing 3% defibrinated horse blood (Nihon Biotest, Japan) was used. Escherichia coli was grown on L plates or in L broth liquid culture at 37°C. When required, antibiotics were added at the following concentrations: kanamycin at 150 μg/ml, erythromycin at 4 μg/ml, chloramphenicol at 5 μg/ml, and spectinomycin at 75 μg/ml for N. meningitidis, and kanamycin at 50 μg/ml, erythromycin at 150 μg/ml, chloramphenicol at 10 μg/ml, ampicillin at 50 μg/ml, and spectinomycin at 75 μg/ml for E. coli. All of the strains used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant properties | Reference(s) or source |
|---|---|---|
| N. meningitidis strains | ||
| Wild-type parent strains (ST, serogroup, immunotype) | ||
| NIID280 (2032, untypeable, L5) | lpt3 lpt6 lgtG+lot3+ | 36 |
| H44/76 (32, B, L3) | lpt3+lpt6 lgtG lot3a | 18, 36, 37 |
| NIID311 (5, A, L2) | lpt3 lpt6+lgtG+lot3+ | 36 |
| Mutants | ||
| NIID280 derivatives | ||
| HT1125 | NIID280 ΔsiaB-D::kan | 36 |
| HT1219 | NIID280 ΔlptA::spc | This study |
| HT1222 | NIID280 ΔsiaB-D::kan ΔlptA::spc | This study |
| HT1277 | NIID280 ΔsiaB-D::kan ΔlptA::spc ggt::lptA+-cat | This study |
| HT1278 | NIID280 ΔsiaB-D::kan ΔlptA::spc ggt::cat | This study |
| HT1238 | NIID280 ΔsiaB-D::kan pilE::ermC | This study |
| HT1249 | NIID280 ΔsiaB-D::kan pilE::ermC ΔlptA::spc | This study |
| HT1178 | NIID280 pilC1::spc | 36 |
| HT1182 | NIID280 ΔpilC2::ermC | 36 |
| HT1186 | NIID280 pilC1::spc ΔpilC2::ermC | 36 |
| H44/76 derivatives | ||
| HT1034 | H44/76 ΔsiaB-D::kan | 36 |
| HT1378 | H44/76 ΔlptA::spc | This study |
| HT1243 | H44/76 siaB-D::kan ΔlptA::spc | This study |
| HT1235 | H44/76 siaB-D::kan pilE::ermC | This study |
| HT1245 | H44/76 siaB-D::kan ΔlptA::spc pilE::ermC | This study |
| HT1234 | H44/76 siaB-D::kan Δopc::cat | This study |
| HT1244 | H44/76 siaB-D::kan ΔlptA::spc Δopc::cat | This study |
| HT1236 | H44/76 siaB-D::kan pilE::ermC Δopc::cat | This study |
| HT1246 | H44/76 siaB-D::kan ΔlptA::spc Δopc::cat pilE::ermC | This study |
| HT1295 | H44/76 lptA+-FLAG-cat | This study |
| HT1015 | H44/76 pilE::ermC | This study |
| HT1002 | H44/76 Δopc::cat | This study |
| NIID311 derivatives | ||
| HT1336 | NIID311 ΔsacAB::kan | 36 |
| HT1380 | NIID311 ΔlptA::spc | This study |
| HT1359 | NIID311 sacAB::kan ΔlptA::spc | This study |
| E. coli strains | ||
| JM109 | endA1 gyrA96 hsdR17(rK− mK+) mcrB+recA1 relA1 supE44 thi-1 Δ(lac-proAB) F′ [traD36 proAB lacIqZΔM15] | Takara Bio |
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Novagen |
| Plasmids | ||
| pET24a | Expression vector; Kmr | Novagen |
| pHT463 | Derivative of pET24a expressing lptA gene; Kmr | This study |
| pMW118 | Cloning vector; Apr | Nippon Gene |
| pHT432 | Derivative of pMW118 carrying an lptA gene; Apr | This study |
| pHT433 | Derivative of pHT432 carrying a ΔlptA::Spcr gene; Apr Spcr | This study |
| pHT473 | Derivative of pHT432 carrying a cat gene at the SphI site; Apr Cmr | This study |
| pTWV228 | Cloning vector; Apr | Takara Bio |
| pHT195 | Derivative of pTWV228 carrying a ggt gene; Apr | 38 |
| pHT507 | Derivative of pHT195 carrying lptA+- cat genes at the BstXI site; Apr Cmr | This study |
| pHT508 | Derivative of pHT195 carrying a cat gene at the BstXI site; Apr Cmr | This study |
| pGEM-T | PCR product cloning vector; Apr | Promega |
| pHT515 | Derivative of pGEM-T carrying a 1-kb downstream region of the lptA gene; Apr | This study |
| pHT516 | Derivative of pGEM-T carrying a 3′ part of the lptA gene with a FLAG epitope; Apr | This study |
| pHT518 | Derivative of pHT516 carrying a 1-kb downstream region of the lptA gene; Apr | This study |
| pHT519 | Derivative of pHT518 carrying a cat gene at the HpaI site; Apr Cmr | This study |
| pUC118 | Cloning vector; Ampr | Takara Bio |
| pHT148 | Derivative of pUC118 carrying a Δopc::ermC gene; Apr Ermr | 37 |
| pHT10 | Derivative of pHT148 carrying a Δopc::cat gene; Apr Cmr | This study |
| pHT434 | Derivative of pHT432 deleted of 0.7 kb of the NruI-SphI DNA fragment; Apr Spcr | This study |
| pGSS33 | Broad-host-range vector; Apr Cmr Strr Tetr | 37 |
| pSTV28 | Cloning vector; Cmr | Takara Bio |
| pHT261 | Derivative of pGSS33 carrying a cat gene and multicloning site of pSTV28 at the SacI and SalI sites; Cmr | This study |
| pHT435 | Derivative of pHT261 carrying an lptA gene; Cmr | This study |
Production of anti-LptA rabbit antiserum.
A 1.6-kb DNA fragment containing the coding region of the lptA gene was amplified with a set of primers (NMB1638-pET24a-5 and NMB1638-pET24a-2 [Table 2]) by PCR. The DNA fragment was digested with BamHI and XhoI and cloned into the same cutting sites of the expression vector pET24a (Novagen), resulting in pHT463. Plasmid pHT463 was transformed into E. coli strain BL21(DE3) (Novagen). The purification and raising of a polyclonal rabbit serum to LptA were performed as described previously (38).
TABLE 2.
Oligonucleotides used in this study
| Primer | Target gene | Nucleotide sequence (5′→3′)a |
|---|---|---|
| NMB1638-pET24a-5 | lptA | CGggatccTACGCCTCGTTTTTCCGCAACAATAAATCA |
| NMB1638-pET24a-2 | lptA | CCGctcgagTGCGCGGACGGCGGCAGGCTGCCAA |
| lptA-1 | lptA | ACGCAGGGTTCAAGATGAGTTGAA |
| lptA-2 | lptA | CCCAGCAAACCTGCCTATCCGAAA |
| lptA-13 | lptA | GCCGGATATGCCGTCTGAAGCCTTCGG |
| lptA-14 | lptA | AActgcagCCGGATACCGCTACCACAACTGGGG |
| lptA-15 | lptA | GCCGCTGCTTGCCGCGCGCGGCGA |
| lptA-16-FLAG | lptA | GGGgttaacTTACTTGTCGTCATCGTCTTTGTAGTCGCGCGGGCAGGCTGCCAATATATC |
Lowercase indicates additional nucleotides used to create the restriction sites. The underlined nucleotides were originally added to attach the FLAG epitope (see Materials and Methods).
Construction of meningococcal mutants.
An N. meningitidis mutant with lptA deleted was constructed as follows. A 4-kb DNA fragment containing lptA was amplified with primers lptA-1 and lptA-2 (Table 2) from HT1125 chromosomal DNA and cloned in the SmaI site of pMW118 (Nippon Gene, Japan) to construct pHT432. A 1-kb SmaI-HincII spectinomycin resistance gene (spe) was inserted into the EcoRV and NruI sites of pHT432, which are located inside and outside the coding region of the lptA gene, respectively, to generate pHT433. Five hundred nanograms of linearized pHT433 was transformed into NIID280 and HT1125, and spectinomycin-resistant (Spcr) clones were selected, resulting in lptA deletion mutants named HT1219 and HT1222, respectively.
The transgenic meningococcal mutant that contained a wild-type lptA gene ectopically complemented in the ΔlptA mutant at the ggt locus was constructed as follows. a 1.2-kb fragment containing a chloramphenicol resistance gene (cat) was inserted in a blunted SphI site of pHT432, resulting in pHT473. A SacI-HindII fragment containing lptA+-cat genes was inserted in a blunted BstXI site, which is located in the middle of the coding region of ggt, of pHT195 (38) to construct pHT507. The negative control was also constructed by inserting only a cat gene in the BstXI site and was named pHT508. Five hundred nanograms of linearized pHT507 was transformed into HT1222 and chloramphenicol-resistant (Cmr) clones were selected, resulting in the ectopic complementation of the lptA+ gene at the ggt loci in the lptA deletion mutant of NIID280; this strain was named HT1277. The negative control mutant HT1278 was constructed by the same procedure by transforming HT1222 with linearized pHT508.
HT1002 (Δopc::cat) and HT1015 (pilE::ermC) were constructed by transforming linearized pHT10, which is a derivative of pHT148 (37) with the ermC gene replaced with a cat gene, and linearized pHT72 (37) into H44/76 and selecting Cmr and erythromycin-resistant (Ermr) clones, respectively.
The ΔlptA::spc (HT1219), pilE::ermC (HT1015), or Δopc::cat (HT1002) mutation was transferred to another genetic background by transforming an N. meningitidis strain with the purified chromosomal DNA and selecting Spcr, Ermr, or Cmr clones, respectively (Table 1).
Construction of an lptA-FLAG epitope-tagged fusion N. meningitidis strain.
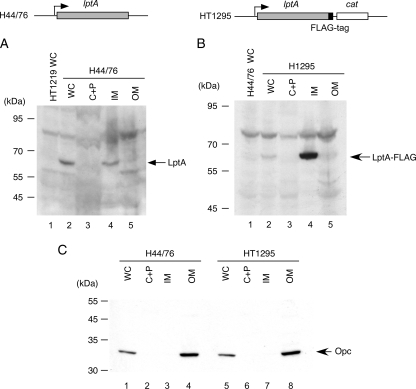
An N. meningitidis strain with a FLAG epitope-tagged lptA gene was constructed as follows. A 1-kb DNA fragment of the downstream region of the lptA gene amplified by PCR with primers lptA-13 and lptA-14 (Table 2) was cloned into the pGEM-T vector (Promega) to construct pHT515. A 0.9-kb DNA fragment containing the 3′ part of the lptA gene was amplified with primers lptA-15 and lptA-16-FLAG (Table 2) and cloned into the pGEM-T vector to construct pHT516. A 1-kb SpeI-PstI fragment of pHT515 was inserted in the same site of pHT516 to construct pHT518. A blunted 1.2-kb fragment containing a cat gene was inserted in an HpaI site of pHT518, resulting in pHT519. Five hundred nanograms of linearized pHT519 was transformed into the H44/76 N. meningitidis strain and Cmr clones were selected, resulting in the lptA-FLAG epitope-tagged strain of H44/76, named HT1295 (see Fig. 4).
FIG. 4.
Localization of the LptA protein in N. meningitidis. Whole-cell lysates (WC) and the cytoplasmic and periplasmic fraction (C+P), the inner-membrane-enriched fraction (IM), and the outer-membrane-enriched fraction (OM) prepared by sonication from N. meningitidis strain H44/76 (A and C) or HT1295 (B and C) were separated and analyzed by Western blotting. (A and B) Five microliters of each sample was analyzed by Western blotting with anti-LptA rabbit antiserum (A) or with anti-FLAG monoclonal antibody (mAb) (B). (C) One microliter of each sample was analyzed to detect the Opc protein by Western blotting with monoclonal antibody B306 (1) as an outer membrane protein marker.
SDS-polyacrylamide gel electrophoresis and Western blotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and Western blotting were performed as described previously (36, 37).
Transmission electron microscopy.
N. meningitidis, grown on GC agar plates overnight, was scraped from the top of colony and dispensed in 1 drop of HEPES-minimal essential medium. A 400-mesh Formvar-coated copper grid (Electron Microscopy Sciences) was inversely put on the bacterial suspension and left for 1 min. The grid was then fixed with 1 drop of 1% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) for 2 min, followed by washing in 2 drops of sterile H2O for a few seconds. The fixed grid was stained with 1 drop of 1% sodium phosphotanguistate (pH 6.0) for 40 s. After removal of the residual solution, the grids was completely dried and stored until observation. Electron microscopy was performed with a Hitachi H-7500 transmission electron microscope.
Preparation of cellular protein fractions.
Cellular fractionation was performed as described previously (38). In brief, bacterial cells in suspension in ice-cold Dulbecco's phosphate-buffered saline (PBS) were disrupted by sonication and centrifuged at 1,000 × g for 5 min to remove undisrupted cells. The supernatant was transferred to a new tube and centrifuged again at 15,000 × g for 10 min. Proteins in the supernatant fraction (cytoplasmic and periplasmic proteins) were precipitated with trichloroacetic acid, and the resultant pellet was dissolved in 20 μl of 2 M Tris base and an equal volume of 2× SDS buffer. The pellet fraction (membrane fractions) was further fractionated by suspension in 20 μl of 4% Triton X-100 in Dulbecco's PBS and, after incubation at 37°C for 30 min, centrifuged at 15,000 × g for 10 min. The Triton-soluble supernatant (inner membrane-enriched fraction) was mixed with an equal volume of 2× SDS buffer, and the Triton-insoluble pellet (outer membrane-enriched fraction) was dissolved in 40 μl of SDS buffer.
Tissue culture.
Human brain microvascular endothelial cells (HBMEC), HUVEC (Toyobo, Japan), and epithelial cells (larynx carcinoma cell line Hep-2 and lung carcinoma cell line A549) were cultivated as described previously (36). A549 cells were also cultivated in serum-free medium (XTen GO; Thermo Trace) supplemented with 1 mg/ml of streptomycin and penicillin for experiments performed under serum-free condition (see Fig. 9C).
FIG. 9.
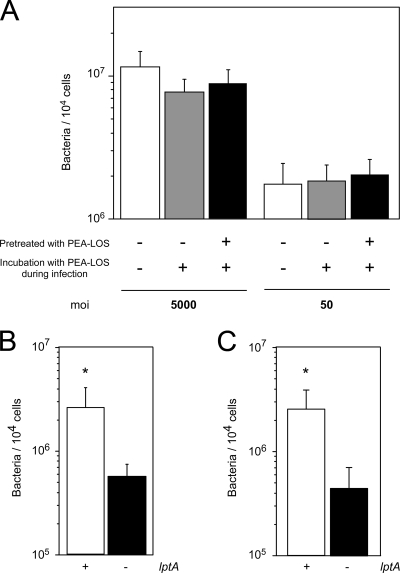
Influence of the presence of purified PEA-LOS and bovine serum albumin on LptA-mediated meningococcal adherence to human cultured cells. (A) Adhesion of N. meningitidis to HBMEC in the presence of 100 μg of PEA-LOS (purified from wild-type N. meningitidis strain HT1125) during infection at MOIs of 5,000 and 50. Gray and open bars indicate the bacterial number with or without PEA-LOS, respectively. Solid bars indicate the number of bacteria adhering to HBMEC pretreated with 100 μg of PEA-LOS for 2 h prior to the bacterial infection. (B and C) Adhesion of N. meningitidis to HBMEC (B) and to A549 cells grown in serum-free medium (C) in serum-free AM during the infection assay. Opened and filled bars indicate the numbers of wild-type (HT1125) and ΔlptA (HT1222) N. meningitidis bacteria, respectively, represented as CFU. Mean CFU of adherent bacteria per 104 cells in at least three experiments are shown, and error bars represent the standard errors of the means. *, P < 0.001 compared to the ΔlptA strain.
Determination of bacteria associated with and internalized in host cells.
Infection assays using tissue culture were performed as described previously (36, 45). Endothelial or epithelial cells were seeded on gelatin-coated (for endothelial cells) or noncoated (for epithelial cells) 96-well tissue culture plates (Iwaki) (104 cells) and incubated at 37°C with 5% CO2 for 1 day. At 2 h prior to the bacterial infection, the culture medium was replaced with assay medium (AM), which was MCDB131 supplemented with 10% fetal bovine serum, 90 μg ml−1 of heparin, and 3 mM glutamine unless otherwise indicated. In a standard experiment, the bacterial suspension was prepared in AM at an optical density at 600 nm (OD600) of 0.5, which corresponded to approximately 5 × 108 CFU ml−1. If a reduced multiplicity of infection (MOI) was indicated, the bacterial suspension was prepared by further dilution. One hundred microliters of the bacterial suspension was inoculated onto host cell monolayers in duplicate for each assay and incubated at 37°C with 5% CO2 for 4 h. To determine bacterial adherence, the monolayers were washed with AM four times to remove nonadherent bacteria. Adherent bacteria were released by addition of PBS (Nissui, Japan) containing 2% saponin, and CFU were determined on GC agar plates with appropriate dilution to count cell-adherent bacteria. To determine internalized bacteria, AM containing 150 μg ml−1 of gentamicin was added and further incubated for 1 h to kill all extracellular bacteria. It was confirmed that 5 × 107 extracellular meningococci were almost completely (>99.999%) killed under these experimental conditions (data not shown). Internalized bacteria that were not killed by the gentamicin treatment were determined by addition of PBS containing 2% saponin and plating on GC agar after appropriate dilutions to count the bacterial numbers as CFU. Results are expressed as means ± standard deviations, and bacterial numbers were statistically compared using a two-tailed Student t test.
Observation of infected cells by Giemsa staining.
HBMEC were seeded on one well of two-well Lab-Tek II chamber slides (Nalge Nunc International) (105 cells) and incubated at 37°C with 5% CO2 for 1 day. One milliliter of bacterial suspension containing 5 × 108 bacteria was inoculated onto cell monolayers and incubated at 37°C with 5% CO2 for 4 h. The monolayer was washed four times with AM, fixed with absolute methanol for 1 min, and stained with Giemsa staining solution (Wako, Japan). After the stained cells were washed with H2O, the bacterial adhesion was observed under oil immersion.
Purification of meningococcal LOS and isolation of the LOS OSs and lipid A.
LOS samples were prepared from N. meningitidis strains grown on 120 GC agar plates. The bacteria were scraped and put into a 200-ml glass flask. Fifty milliliters of fresh extraction solvent (6.7 ml of 90% phenol, 16.7 ml of chloroform, and 26.7 ml of petroleum ether) was added to the flask, and the bacteria were homogenized with a PT1300D homogenizer (Polytoron) five times for 30 s each with 30-s intervals in an ice bath at 15,000 rpm. The bacterial suspension was stirred for 2 h at room temperature and then centrifuged at 10,000 × g for 15 min at 4°C. The supernatant was transferred to a new flask, and the resultant pellet was again extracted with a new 50 ml of fresh extraction solvent by the same procedure. The total 100 ml of supernatant obtained by this procedure was evaporated in a water bath at 40°C for 3 min; the evaporation condenser was cooled at 4°C and vacuumed at 670 hPa, resulting in approximately 14 ml of solvent. The solvent was precipitated by adding 14 ml of diethyl ether and 70 ml of acetone and centrifuged at 10,000 × g at 4°C for 15 min. The precipitated LOS was dried for overnight under reduced pressure. The dried LOS was dissolved in 5 ml of H2O and stored at −70°C before use. Samples were then subjected to mild acid hydrolysis in 1% (vol/vol) aqueous acetic acid for 1.5 h at 100°C with constant stirring. The lipid A fractions were separated from OS by centrifugation at 3,500 × g for 15 min at 4°C, suspended in water, and centrifuged again; this water washing was repeated one more time. The lipid A sediment fractions were saved for analysis. The original and wash supernatants were combined and lyophilized for each LOS. They contained the released OSs. The OSs were further purified by gel filtration chromatography using a Bio-Gel P-4 (Bio-Rad) column (120 by 1 cm) and eluted with deionized water. The eluting fractions were recorded with a Shimadzu refractive index detector (RID-10A).
MS analysis of lipid A fractions.
The lipid A fraction was analyzed by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) on an Applied Biosystems 4700 proteomics analyzer. Lipid A samples were dissolved in chloroform-methanol (3:1, vol/vol) and mixed with 0.5 M 2,4,6-trihydroxyacetophoenone matrix in methanol. The lipid A analyses were performed in a negative-reflectron mode.
Construction of the meningococcal lptA gene on a multicopy plasmid and introduction into N. meningitidis.
To isolate the DNA region containing lptA, pHT432 was digested with NruI and SalI, followed by blunting and self-ligation to obtain pHT434. EcoRI-SphI 2.4-kb DNA fragments of pHT434 were cloned into the same sites of pHT261, which was a derivative of the IncQ broad-host-range vector pGSS33 (37) (Table 1), resulting in pHT435. Introduction of the plasmids into N. meningitidis was performed as described previously (37).
RESULTS
Disruption of lptA in N. meningitidis reduces meningococcal adherence to HBMEC at any MOI.
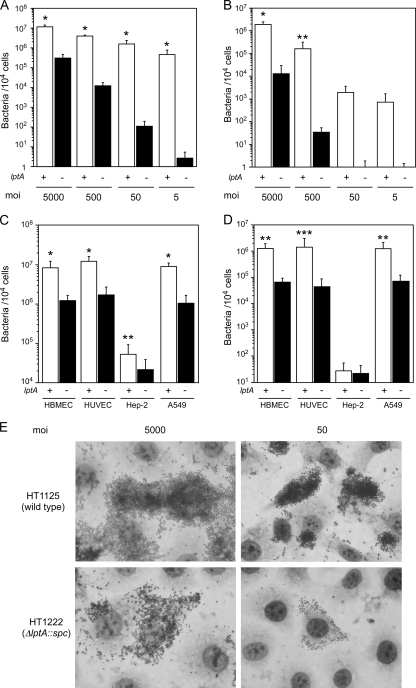
To study the biological role of LptA in N. meningitidis, a ΔlptA mutation was introduced into the ST-2032 strain, and adherence and internalization were examined by in vitro infection assays using HBMEC at MOIs of 5,000, 500, 50, and 5 (Fig. 2A). The growth rate of the ΔlptA mutant (HT1222) in AM (see Materials and Methods) was not different from that of the wild-type strain HT1125 (data not shown). At an MOI of 5,000, the ΔlptA mutant HT1222 adhered to and invaded HBMEC approximately 10 times less efficiently than the wild-type strain HT1125 (Fig. 2A and B). The inefficiency of adhesive and invasive activities of the ΔlptA mutant was more apparent in proportion to the reduction of the MOI (Fig. 2A and B). These results suggested that the mutation in the lptA gene influenced the meningococcal adhesion to HBMEC even at an MOI of 5,000. Thereafter, to maximize the number of internalized bacteria and to exclude the overestimation of adhesion defectiveness of the ΔlptA mutant compared to the wild-type strain, bacteria associated with and internalized in host cells were determined at an MOI of 5,000 unless otherwise indicated.
FIG. 2.
Adherence and internalization of the ΔlptA N. meningitidis mutant to human endothelial and epithelial cells. (A and B) Adhesion (A) and internalization (B) of N. meningitidis to HBMEC at MOIs of 5,000, 500, 50, and 5. (C and D) Adhesion (C) and internalization (D) of N. meningitidis to human endothelial cells (HBMEC and HUVEC) and human epithelial cells (HEp-2 and A549) at an MOI of 5,000. Bacterial numbers are represented as CFU. Internalized bacteria were determined as the gentamicin-resistant bacterial number. Mean CFU of adherent bacteria (A and C) or gentamicin-resistant bacteria (B and D) per 104 cells in at least three experiments are shown, and error bars represent the standard errors of the means. Open and filled bars indicate the numbers of wild-type (HT1125) and ΔlptA (HT1222) N. meningitidis cells, respectively. *, P < 0.001; **, P < 0.05; ***, P = 0.06 (compared to the ΔlptA strain). (E) Meningococcal adhesion to HBMEC was observed by light microscopy with Giemsa staining. Infection with wild-type (HT1125) and ΔlptA (HT1222) N. meningitidis strains at MOIs of 5,000 and 50 is shown.
Disruption of lptA in N. meningitidis reduces meningococcal adherence to human endothelial and epithelial cell lines.
The defectiveness of adhesion of the ΔlptA mutant was also examined with the other endothelial cell line (HUVEC) and two kinds of epithelial cell lines (A549 and HEp-2). The HT1222 strain adhered to all of the human cell lines approximately 10 times less efficiently than the HT1125 strain (Fig. 2C). The number of internalized HT1222 bacteria also decreased to approximately 1/10 of that of HT1125 (Fig. 2D). All these results suggested that the lptA gene affected the adhesion of N. meningitidis to human cells.
Confirmation of the adhesion defect of the ΔlptA mutant by microscopy.
The adhesion of the ΔlptA N. meningitidis mutant to HBMEC was further examined by light microscopy. At an MOI of 5,000, the ΔlptA mutant HT1222 adhered less to HBMEC than the wild-type strain HT1125 (Fig. 2E, left panels). At an MOI of 50, decreased adherence of the ΔlptA mutant was also observed (Fig. 2E, right panels). These data confirmed that disruption of the lptA gene in N. meningitidis reduced meningococcal adhesion to human cells.
Complementation with the wild-type lptA gene in the ΔlptA mutant restores meningococcal adhesion to HBMEC.
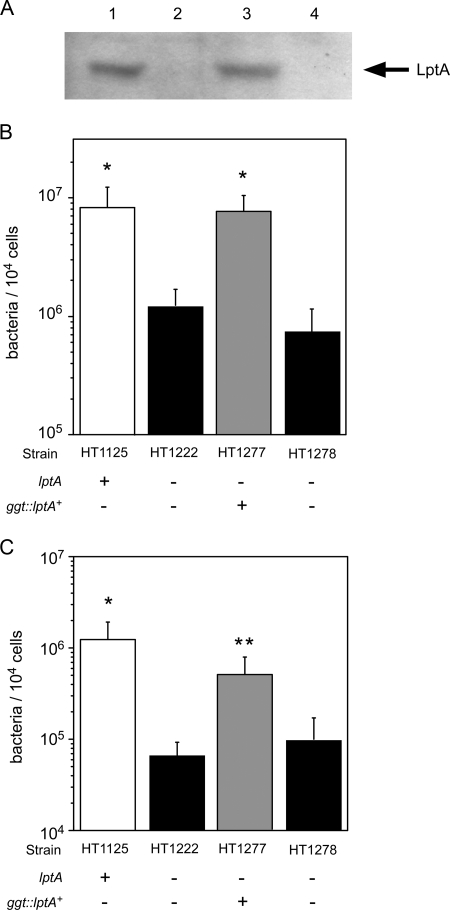
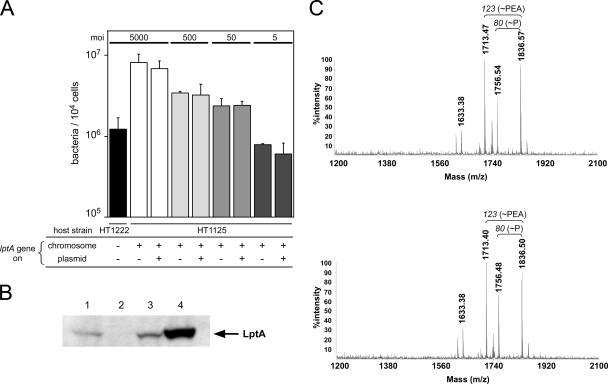
To confirm the effect of ΔlptA N. meningitidis, the wild-type lptA gene was ectopically complemented in ggt of the ΔlptA mutant. The insertion at the ggt locus does not affect adhesive activity (35). The LptA protein was sufficiently expressed in the lptA+-complemented ΔlptA mutant HT1277 but not in the isogenic strain HT1278, which was not complemented (Fig. 3A). The adhesive activity of strain HT1277 was completely recovered to the level of the wild-type strain HT1125, but recovery was not observed in the HT1278 strain (Fig. 3B). The same results were obtained for meningococcal bacterial internalization (Fig. 3C). These results suggested that the lptA gene product affects the adhesion of N. meningitidis to human cells.
FIG. 3.
Effect of complementation of the lptA+ gene in ΔlptA N. meningitidis on bacterial adhesion to HBMEC. (A) Western blotting for LptA protein. Bacterial extracts equivalent to an OD600 of 0.05 were analyzed. Lane 1, HT1125 (wild type); lane 2, HT1222 (ΔlptA); lane 3, HT1277 (ΔlptA ggt::lptA+-cat); lane 4, HT1278 (ΔlptA ggt::cat). (B and C) Adhesion (B) and internalization (C) of the ΔlptA N. meningitidis mutant in which the lptA+ gene was ectopically complemented. The internalized bacteria were determined as the number of gentamicin-resistant bacteria. Mean CFU of adherent bacteria (B) or gentamicin-resistant bacteria (C) per 104 cells of HBMEC in at least three experiments are shown, and error bars represent the standard errors of the means. *, P < 0.01; **, P < 0.05 (compared to the strain not complemented with the lptA+ gene).
LptA does not directly act as an adhesin molecule.
The LptA protein might act as a direct adhesion molecule on the meningococcal surface. In fact, a signal peptide sequence was found in the deduced amino acid sequence of the LptA protein (data not shown). To assess the location of meningococcal LptA in N. meningitidis, the cellular proteins were fractionated and analyzed by Western blotting with an anti-meningococcal LptA rabbit antiserum (Fig. 4A). The typical outer membrane protein in N. meningitidis, the Opc protein, was not detected in the cytosolic fraction (Fig. 4C, lanes 2 and 6), indicating that separation of the total membrane fraction from the soluble (cytosolic and periplasmic) fraction was successful. Furthermore, since the Opc protein was detected only in the outer membrane fraction isolated from the total membrane fraction with 4% Triton X-100 (Fig. 4C, lanes 4 and 8), it was confirmed that the inner and outer membranes were also successfully separated from the total membrane fraction in this study. The LptA protein was detected in the inner membrane fraction (Fig. 4A, lane 4) but not in the outer membrane fraction (Fig. 4A, lane 5). To confirm this result, we further examined the LptA cellular localization by using the LptA protein fused to a FLAG tag at the C terminus (see Materials and Methods). The LptA-FLAG protein was also detected only in the inner membrane fraction (Fig. 4B, lanes 4 and 5). These data suggested that the LptA protein was not exposed on the meningococcal surface, excluding the possibility that the LptA protein itself could function as an adhesin molecule in N. meningitidis (see Discussion).
Structural analyses of meningococcal LOS.
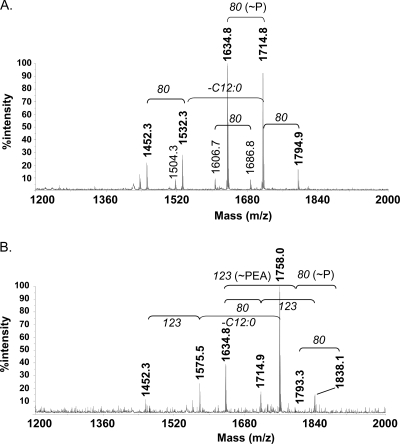
To examine the structural change in the meningococcal LOSs due to the ΔlptA mutation, the lipid A moieties of the LOSs from N. meningitidis strains HT1125 (wild type) and HT1222 (ΔlptA) were analyzed by MALDI-TOF MS (Fig. 5). The ions observed in the lipid A of HT1125 were not different from those of HT1222, except for the ions of PEA-containing molecules (Fig. 1 and 5 and Table 3). No other structural changes in the OS moieties were observed by gas chromatography-MS, MALDI-TOF MS, or nuclear magnetic resonance analyses (Fig. 1 and data not shown). These results indicated that only the PEA group at the 4′ position of lipid A was lost in the LOS of the ΔlptA N. meningitidis mutant, which is consistent with a previous report (7), and suggested that the loss of the PEA group led to the decrease in adhesion of the ΔlptA N. meningitidis mutant to human cells.
FIG. 5.
MALDI-TOF spectra of lipid A purified from strains HT1222 (ΔlptA::spc) (A) and HT1125 (wild type) (B). The masses of the abundant fragments are indicated at the tops of the corresponding peaks. The ions of molecules containing a PEA group in HT1125 lipid A (m/z = 1838, 1758, and 1575) (Table 3) are not detected in those of HT1222 lipid A.
TABLE 3.
MALDI-TOF MS analysis (negative mode) of lipid A fractions of LOSs from N. meningitidis HT1222 and HT1125 and proposed compositions for observed ionsa
| Strain | Observed [M − H]− ionb | Proposed composition |
|---|---|---|
| HT1222 (ΔlptA::spc) | 1794.9 | P3GlcN2C12:023OHC14:023OH(C12:0)2 |
| 1714.8 | P2GlcN2C12:023OHC14:023OH(C12:0)2 | |
| 1634.8 | P1GlcN2C12:023OHC14:023OH(C12:0)2 | |
| 1532.3 | P2GlcN2C12:013OHC14:023OH(C12:0)2 | |
| 1452.3 | P1GlcN2C12:013OHC14:023OH(C12:0)2 | |
| HT1125 (wild type) | 1838.1 | P2PEAGlcN2C12:023OHC14:023OH(C12:0)2 |
| 1758.0 | P1PEAGlcN2C12:023OHC14:023OH(C12:0)2 | |
| 1793.3 | P3GlcN2C12:023OHC14:023OH(C12:0)2 | |
| 1714.9 | P2GlcN2C12:023OHC14:023OH(C12:0)2 | |
| 1634.8 | P1GlcN2C12:023OHC14:023OH(C12:0)2 | |
| 1575.5 | P1PEAGlcN2C12:013OHC14:023OH(C12:0)2 | |
| 1452.3 | P1GlcN2C12:013OHC14:023OH(C12:0)2 |
Influence of pili, Opc, Opa, and PilC on the adhesion defect of the ΔlptA N. meningitidis mutant.
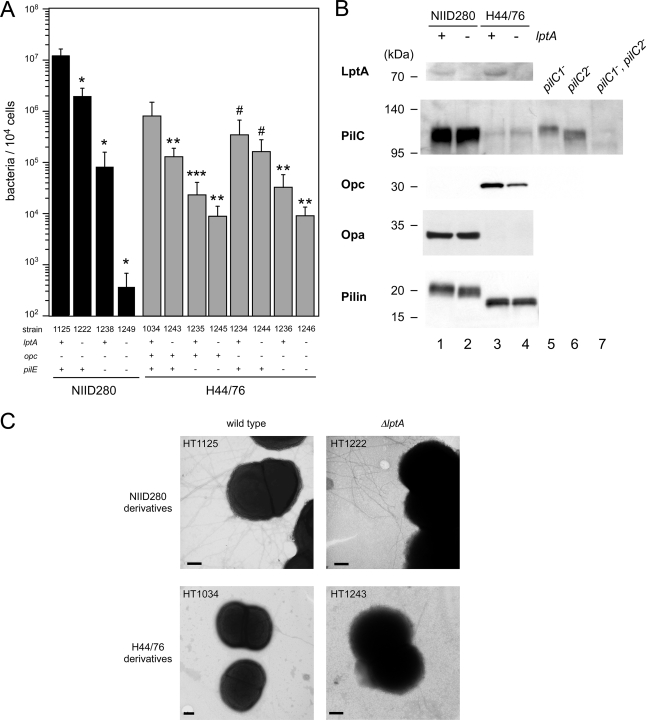
We sought to determine whether the reduction of the adhesive activity in the ΔlptA mutant was dependent on or associated with changes in known adhesive molecules such as pili, Opc, Opa, and PilC. In the NIID280 (ST-2032) genetic background, pilE::ermC (HT1238) and ΔlptA::spc (HT1222) mutations reduced the adhesive activity to approximately 1/100 and 1/10 of the level in the wild-type strain (HT1125), respectively (Fig. 6A). The adhesive ability of the pilE ΔlptA double mutant (HT1249) was decreased to approximately 1/10,000 of the level in the wild-type strain (Fig. 6A). In an H44/76 (ST-32) genetic background, pilE (HT1235) and ΔlptA (HT1243) mutations also reduced the adhesive activity to approximately 1/100 and 1/10 of the level in the wild-type strain (HT1034), respectively (Fig. 6A). The cooperative reduction of the adhesive ability was also observed in the pilE ΔlptA double mutant (HT1245) (Fig. 6A). The cooperative reduction of adhesive ability caused by the pilE and ΔlptA mutations was also observed in a Δopc::cat H44/76 genetic background (HT1234, HT1244, HT1236, and HT1246) (Fig. 6A). The results genetically suggested that the reduction of adhesive activity in the ΔlptA mutant was independent of pili regardless of the presence of Opc. We examined the effect of the ΔlptA mutation on PilC, Opc, Opa, and pilin production by Western blotting. The expression of Opc appeared to be slightly reduced in the ΔlptA mutant, while the amounts of pili and PilC proteins were not significantly different in wild-type and ΔlptA N. meningitidis strains (Fig. 6B, lanes 3 and 4). The opc gene was absent in the NIID280 strain (Fig. 6B, lanes 1 and 2) (36), and PilC and Opa proteins were faintly detected in the H44/76 strains (Fig. 6B, lanes 3 and 4) (36). It was further confirmed by transmission electron microscopy that ∼6 to 12 diplococcus and pilus bundles were found in both HT1125 (wild type) and HT1222 (ΔlptA) in the NIID280 (ST-2032) genetic background, while ∼0 to 4 pili per diplococcus and a few pilus bundles were expressed in both the wild-type HT1034 and ΔlptA HT1243 strains in the H44/76 (ST-32) genetic background (Fig. 6C). The reduction in adhesion produced by the ΔlptA mutation was observed even in an opc-negative strain (ST-2032) and in an Opa-negative strain expressing little PilC (H44/76) (Fig. 6A and B). All the results suggested that the Opc, Opa, and PilC proteins and pili appear not to be directly related to the reduction of adhesive activity in the ΔlptA mutant (see Discussion).
FIG. 6.
(A) Adhesion of pilE::ermC and Δopc::cat mutants with NIID280 (ST-2032) and H44/76 (ST-32) genetic backgrounds to HBMEC. Mean CFU of adherent bacteria per 104 cells of HBMEC in at least three experiments are shown, and error bars represent the standard errors of the means. *, P < 0.01; **, P < 0.05; ***, P < 0.06; and #, P > 0.1 (compared to the isogenic wild-type strain). (B) Western blotting for LptA, PilC, Opc, Opa, and pilin proteins. Bacterial extracts equivalent to an OD600 of 0.05 (for LptA and PilC), 0.01 (for Opc and Opa), or 0.0025 (for pilin) were analyzed by Western blotting. Lane 1, HT1125 (ST-2032 wild type); lane 2, HT1222 (ST-2032 ΔlptA); lane 3, HT1034 (ST-32 wild type); lane 4, HT1243 (ST-32 ΔlptA). The pilC1 mutant (HT1178, lane 5), the pilC2 mutant (HT1182, lane 6), and the double mutant (HT1186, lane 7) were used only as controls for anti-PilC rabbit serum. (C) Transmission electron micrographs showing piliation of meningococcal strain HT1125 and the ΔlptA mutant HT1222 and of meningococcal strain HT1034 and the ΔlptA mutant HT1243. Bars, 200 nm.
Influence of capsule on LptA-mediated meningococcal adhesion.
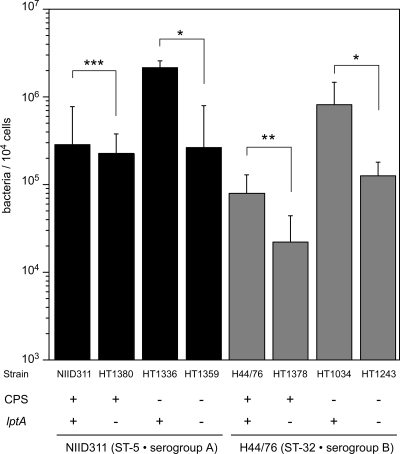
We further examined whether the LOS modification by LptA and the effect on adhesion was affected by the presence of capsule, because the strains examined so far were all derived from capsule-negative strains (Table 1). As shown in Fig. 7, the ΔlptA mutation reduced the adhesive activities of both the NIID311 and H44/76 unencapsulated N. meningitidis strains (P < 0.001 for HT1336 versus HT1359 and for HT1034 versus HT1243) (Fig. 7). However, the lptA mutation did not affect the reduced adhesive ability in an encapsulated N. meningitidis NIID311 genetic background (P = 0.78, NIID311 versus HT1380) (Fig. 7), while the ΔlptA mutation slightly reduced the adhesive activity in the H44/76 genetic background (P < 0.05, H44/76 versus HT1378). Adhesion of encapsulated meningococcal strains was less affected by the ΔlptA mutation than that of unencapsulated strains with the same NIID311 and H44/76 genetic backgrounds. This suggested that LptA-mediated adhesion may be masked when meningococci are encapsulated (see Discussion).
FIG. 7.
The enhancement of bacterial adhesion to HBMEC with LOS modification by LptA is not prominent in encapsulated N. meningitidis. NIID311 (ST-5) and H44/76 (ST-32) genetic backgrounds were studied. Mean CFU of adherent bacteria per 104 cells of HBMEC in at least three experiments are shown, and error bars represent the standard errors of the means. *, P < 0.001; **, P < 0.05; ***, P = 0.78 (compared to the isogenic ΔlptA strain). CPS, capsular polysaccharide.
Influence of a multicopy lptA gene in a wild-type strain.
Increased expression or copy number of the lptA gene in wild-type N. meningitidis could lead to the enhancement of the number of PEAs in the LOS molecule as observed for some meningococcal strains (7). This could result in increased adhesive activity of N. meningitidis. To examine this possibility, the lptA gene was introduced on a plasmid into the wild-type N. meningitidis strain HT1125, and adhesion was examined using HBMEC. While LptA protein expression increased in HT1125 harboring the pHT435 plasmid (HT1125/pHT435) (Fig. 8B, lane 4), the adhesion was not significantly different in the HT1125/pHT435 transformant compared to the HT1125/pHT261 transformant (Fig. 8A). MALDI-TOF MS analysis of lipid A preparations from these N. meningitidis transformants revealed that the ions of the PEA-containing molecules in the LOS from HT1125/pHT435 (m/z = 1836 and 1756) (Fig. 8C, lower panel) did not change compared to those in the LOS from HT1125/pHT261 (Fig. 8C, upper panel). The results suggested that the overexpression of LptA in wild-type N. meningitidis did not affect the PEA of meningococcal LOS and adhesion to human cells.
FIG. 8.
Introduction of the lptA gene on a multicopy plasmid into wild-type N. meningitidis does not enhance LOS modification with the PEA group or bacterial adhesion to HBMEC. (A) Adhesion of HT1125 harboring a plasmid containing the lptA gene (pHT435) or a vector plasmid (pHT261) to HBMEC. The bacterial adhesion was examined at MOIs of 5,000, 500, 50, and 5. Mean CFU of adherent bacteria per monolayer of HBMEC in at least three experiments are shown, and error bars represent the standard errors of the means. (B) Western blotting for LptA protein. Bacterial extracts equivalent to an OD600 of 0.05 were analyzed by Western blotting. Lane 1, HT1125 (ST-2032 wild type); lane 2, HT1222 (ST-2032 ΔlptA); lane 3, HT1125 harboring pHT261 (vector); lane 4, HT1125 harboring pHT435 (lptA+ on a plasmid). (C) MALDI-TOF spectra of lipid A purified from HT1125 harboring pHT261 (upper panel) and from HT1125 harboring pHT435 (lower panel). The masses of the major fragments are indicated at the tops of the corresponding peaks. The fragments and peaks of these ions were nearly identical in the two N. meningitidis transformants.
Influence of the presence of purified PEA-LOS and bovine serum albumin on meningococcal infection.
To obtain some clues about the possibility that LOS modified with PEA (PEA-LOS) could act as an adhesin molecule, the meningococcal adhesion to HBMEC was examined in the presence of the LOS purified from wild-type N. meningitidis strain HT1125 during the infection assay. The meningococcal adhesion was not inhibited by as much as 100 μg of purified PEA-LOS, and the inhibitory effect was not also observed even when HBMEC were preincubated with the purified PEA-LOS for 2 h prior to the bacterial infection (Fig. 9A). These results suggested that PEA-LOS could not directly act as an adhesin molecule (see Discussion). We also examined the effect of serum on the LptA-mediated meningococcal adhesion. Under infection conditions without serum (serum-free AM), it was still observed that the ΔlptA mutant HT1222 adhered to HBEMC approximately 10 times less efficiently than the wild-type strain HT1125 (Fig. 9B). To completely exclude the presence of serum or serum factors, bacterial adhesion was also examined with serum-free AM and A549 epithelial cells grown in serum-free medium (see Materials and Methods). Approximately 10 times less efficient adherence by the ΔlptA mutant was also observed under the completely serum-free conditions (Fig. 9C). These results suggested that LptA-mediated meningococcal adhesion was not related to serum (see Discussion).
DISCUSSION
The modification of the 4′ position of the lipid A moiety of LOS (or LPS) with PEA is known to confer resistance to cationic antimicrobial peptides such as polymyxin B on N. meningitidis (41), Salmonella enterica (23), and E. coli (21). However, other biological roles of the modification have not been elucidated. In the present study, we demonstrated that the modification of LOS by LptA can enhance meningococcal adhesion to human cells.
The LptA-mediated effect on meningococcal adhesion seemed to be masked by capsular polysaccharides, since the modification of LOS by LptA did not significantly affect meningococcal adhesion to HBMEC in encapsulated N. meningitidis (Fig. 7). The meningococcal capsule confers serum resistance (14, 49) and decreases meningococcal adhesion to and invasion of the cultured human cells in in vitro experiments (Fig. 7) (47). Although N. meningitidis clinical isolates from blood or CSF are encapsulated (5), N. meningitidis can undergo frequent phase variation (16) to unencapsulated variants, which may be selected at the surface of the human nasopharynx. Unencapsulated meningococci efficiently enter the epithelial and endothelial cells and subsequently disseminate into blood or CSF after the reversion to the encapsulated state (15, 42, 51). LptA-mediated meningococcal adhesion may play an important role in the unencapsulated state of N. meningitidis in the natural reservoir of the human nasopharynx.
The exact mechanism of LptA-mediated meningococcal adhesion is interesting but at present unknown. It seems unlikely that LOS modification by LptA affects meningococcal adhesion by altering the overall charge of the LOS molecule; disruption of lpt3 or lpt6, which encode proteins adding PEA to the inner core heptose II, in immunotype L2 (lpt6+) or L3 (lpt3+) N. meningitidis, respectively, did not affect the meningococcal adhesion to HBMEC (data not shown). Another possibility is that the PEA-LOS itself acts as an adhesin molecule. It is known that gonococcal LOS directly mediates interactions with host cells; N. gonorrhoeae adheres to primary human cervical epithelial cells by the interaction between gonococcal lipid A and a human C3 acceptor molecule (CR3) (10-12), and gonococcal LOS binds to an asialoglycoprotein receptor on HepG2 epithelial cells (30) and on human sperm (17). However, it is less likely that N. meningitidis PEA-LOS interacts with human cells via the CR3, since the LptA-mediated meningococcal adhesion with A549 epithelial cells, which do not produce any CRs themselves (8), could be observed under completely serum-free conditions (Fig. 9C). We also tried to examine the direct interaction between purified PEA-LOS and HBMEC by immunostaining and flow cytometry with Alexa Fluor 488-labeled PEA-LOS (39), but we could not detect adherence (data not shown). In addition, meningococcal adhesion to HBEMC was not inhibited by the addition of the PEA-LOS (Fig. 9A). However, meningococcal attachment to human cells is a multistep process involving initial pilus-mediated adherence followed by close adherence with Opc and Opa. PEA-LOS may be involved in this close adherence through an unknown mechanism.
Considering that the modulation of LOS can expose or induce conformational changes of certain proteins in N. gonorrhoeae (13) and affect Opc-mediated meningococcal interactions with HUVEC (46), LOS modification with PEA by LptA may also induce configurational or conformational changes on the meningococcal surface, which could result in the exposure of a specific adhesin molecule in N. meningitidis. Since the reduction of adhesive activity by the ΔlptA mutation was observed in all N. meningitidis strains examined in this study (Fig. 2, 6, 7, and 8) and in ST-11 and ST-1011 strains (data not shown), the PEA-LOS-mediated adherence mechanism is generally present among N. meningitidis strains. We showed in this study that meningococcal adhesion enhanced by the LptA was independent of pili and Opc and probably of Opa and PilC (Fig. 6), which was consistent with a previous report by Unkmeir et al. that meningococcal adhesion to HBMEC is not mediated by Opc and human fibronectin (43). Further, it is unlikely that meningococcal adhesion enhanced by LptA is mediated by recently reported new meningococcal adhesins such as NadA (4) and MspA (40), because NadA facilitates adherence to epithelial cells but not to endothelial cells (4) and both proteins are not expressed in all N. meningitidis strains (6, 40). Enhancement of meningococcal adhesion by LptA appears to be mediated by unidentified adhesin molecules, which was also an implication of our previous study (36). This concept is consistent with the observation that LptA-mediated adhesion to HBMEC and Opc-mediated meningococcal adhesion to HUVEC are masked by capsule (Fig. 7) (47). However, further experiments are required to elucidate the precise mechanism.
Since growth conditions affect the lipid A phosphorylation pattern in N. meningitidis (7), PEA levels on the LOS may also vary among N. meningitidis strains that show different adhesive activities (36). However, the LptA protein was found equally among all N. meningitidis strains examined (data not shown) (7). The amount of PEA-LOS did not increase even with the overexpression of the lptA gene in wild-type N. meningitidis (Fig. 8) and in the noninvasive ST-2046 strain (data not shown) (36). Considering all these results, the PEA level on lipid A of the LOS might be constant in N. meningitidis.
We found that the LOS modification by LptA enhanced the adhesion of N. meningitidis to human epithelial or endothelial cells. Since homologues of lptA are found in other neisserial genomes (N. gonorrhoeae, N. lactamica, and N. elongata) (unpublished data), as well in those of other bacteria (23), enhancement of adhesion by LptA may be observed in other bacteria. The general ability and the mechanism of enhancement of bacterial adhesion by LptA will be further examined.
Acknowledgments
We thank Makoto Ohnishi for helpful discussion.
This work was supported by grants from the Ministry of Health, Welfare and Labor of Japan. H.T. was also supported by a grant from the Ministry of Education, Science and Culture of Japan (grant no. 16790265), D.S.S. and R.W.C. were supported by NIH grant R01 AI-33517. R.W.C. was partly supported by the U.S. Department of Energy-funded (DE-FG09-93ER-20097) Center for Plant and Microbial Complex Carbohydrates.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 29 September 2008.
REFERENCES
- 1.Achtman, M., B. Kusecek, G. Morelli, K. Eickmann, J. F. Wang, B. Crowe, R. A. Wall, M. Hassan-King, P. S. Moore, and W. Zollinger. 1992. A comparison of the variable antigens expressed by clone IV-1 and subgroup III of Neisseria meningitidis serogroup A. J. Infect. Dis. 16553-68. [DOI] [PubMed] [Google Scholar]
- 2.Albiger, B., L. Johansson, and A. B. Jonsson. 2003. Lipooligosaccharide-deficient Neisseria meningitidis shows altered pilus-associated characteristics. Infect. Immun. 71155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrington, A. W., Y. C. Tan, Y. Srikhanta, B. Kuipers, P. van der Ley, I. R. Peak, and M. P. Jennings. 2002. Phase variation in meningococcal lipooligosaccharide biosynthesis genes. FEMS Immunol. Med. Microbiol. 34267-275. [DOI] [PubMed] [Google Scholar]
- 4.Capecchi, B., J. Adu-Bobie, F. Di Marcello, L. Ciucchi, V. Masignani, A. Taddei, R. Rappuoli, M. Pizza, and B. Arico. 2005. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 55687-698. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright, K. A., J. M. Stuart, D. M. Jones, and N. D. Noah. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comanducci, M., S. Bambini, D. A. Caugant, M. Mora, B. Brunelli, B. Capecchi, L. Ciucchi, R. Rappuoli, and M. Pizza. 2004. NadA diversity and carriage in Neisseria meningitidis. Infect. Immun. 724217-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox, A. D., J. C. Wright, J. Li, D. W. Hood, E. R. Moxon, and J. C. Richards. 2003. Phosphorylation of the lipid A region of meningococcal lipopolysaccharide: identification of a family of transferases that add phosphoethanolamine to lipopolysaccharide. J. Bacteriol. 1853270-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Astorza, B., G. Cortes, C. Crespi, C. Saus, J. M. Rojo, and S. Alberti. 2004. C3 promotes clearance of Klebsiella pneumoniae by A549 epithelial cells. Infect. Immun. 721767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 1998. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6489-495. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, J. L., and M. A. Apicella. 2002. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell. Microbiol. 4585-598. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, J. L., E. J. Brown, K. A. Ault, and M. A. Apicella. 2001. The role of complement receptor 3 (CR3) in Neisseria gonorrhoeae infection of human cervical epithelia. Cell. Microbiol. 3611-622. [DOI] [PubMed] [Google Scholar]
- 12.Edwards, J. L., E. J. Brown, S. Uk-Nham, J. G. Cannon, M. S. Blake, and M. A. Apicella. 2002. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell. Microbiol. 4571-584. [DOI] [PubMed] [Google Scholar]
- 13.Elkins, C., N. H. Carbonetti, V. A. Varela, D. Stirewalt, D. G. Klapper, and P. F. Sparling. 1992. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol. Microbiol. 62617-2628. [DOI] [PubMed] [Google Scholar]
- 14.Hammerschmidt, S., C. Birkholz, U. Zahringer, B. D. Robertson, J. van Putten, O. Ebeling, and M. Frosch. 1994. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol. Microbiol. 11885-896. [DOI] [PubMed] [Google Scholar]
- 15.Hammerschmidt, S., R. Hilse, J. P. van Putten, R. Gerardy-Schahn, A. Unkmeir, and M. Frosch. 1996. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 15192-198. [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerschmidt, S., A. Muller, H. Sillmann, M. Muhlenhoff, R. Borrow, A. Fox, J. van Putten, W. D. Zollinger, R. Gerardy-Schahn, and M. Frosch. 1996. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol. Microbiol. 201211-1220. [DOI] [PubMed] [Google Scholar]
- 17.Harvey, H. A., N. Porat, C. A. Campbell, M. Jennings, B. W. Gibson, N. J. Phillips, M. A. Apicella, and M. S. Blake. 2000. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol. Microbiol. 361059-1070. [DOI] [PubMed] [Google Scholar]
- 18.Jennings, M. P., Y. N. Srikhanta, E. R. Moxon, M. Kramer, J. T. Poolman, B. Kuipers, and P. van der Ley. 1999. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiology 1453013-3021. [DOI] [PubMed] [Google Scholar]
- 19.Jones, D. M., R. Borrow, A. J. Fox, S. Gray, K. A. Cartwright, and J. T. Poolman. 1992. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb. Pathog. 13219-224. [DOI] [PubMed] [Google Scholar]
- 20.Kahler, C. M., S. Lyons-Schindler, B. Choudhury, J. Glushka, R. W. Carlson, and D. S. Stephens. 2006. O-Acetylation of the terminal N-acetylglucosamine of the lipooligosaccharide inner core in Neisseria meningitidis. Influence on inner core structure and assembly. J. Biol. Chem. 28119939-19948. [DOI] [PubMed] [Google Scholar]
- 21.Kim, S. H., W. Jia, V. R. Parreira, R. E. Bishop, and C. L. Gyles. 2006. Phosphoethanolamine substitution in the lipid A of Escherichia coli O157:H7 and its association with PmrC. Microbiology 152657-666. [DOI] [PubMed] [Google Scholar]
- 22.Kurzai, O., C. Schmitt, H. Claus, U. Vogel, M. Frosch, and A. Kolb-Maurer. 2005. Carbohydrate composition of meningococcal lipopolysaccharide modulates the interaction of Neisseria meningitidis with human dendritic cells. Cell. Microbiol. 71319-1334. [DOI] [PubMed] [Google Scholar]
- 23.Lee, H., F. F. Hsu, J. Turk, and E. A. Groisman. 2004. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J. Bacteriol. 1864124-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackinnon, F. G., R. Borrow, A. R. Gorringe, A. J. Fox, D. M. Jones, and A. Robinson. 1993. Demonstration of lipooligosaccharide immunotype and capsule as virulence factors for Neisseria meningitidis using an infant mouse intranasal infection model. Microb. Pathog. 15359-366. [DOI] [PubMed] [Google Scholar]
- 25.Mackinnon, F. G., A. D. Cox, J. S. Plested, C. M. Tang, K. Makepeace, P. A. Coull, J. C. Wright, R. Chalmers, D. W. Hood, J. C. Richards, and E. R. Moxon. 2002. Identification of a gene (lpt-3) required for the addition of phosphoethanolamine to the lipopolysaccharide inner core of Neisseria meningitidis and its role in mediating susceptibility to bactericidal killing and opsonophagocytosis. Mol. Microbiol. 43931-943. [DOI] [PubMed] [Google Scholar]
- 26.Nassif, X. 1999. Interaction mechanisms of encapsulated meningococci with eucaryotic cells: what does this tell us about the crossing of the blood-brain barrier by Neisseria meningitidis? Curr. Opin. Microbiol. 271-77. [DOI] [PubMed] [Google Scholar]
- 27.Nassif, X., S. Bourdoulous, E. Eugene, and P. O. Couraud. 2002. How do extracellular pathogens cross the blood-brain barrier? Trends Microbiol. 10227-232. [DOI] [PubMed] [Google Scholar]
- 28.Nassif, X., C. Pujol, P. Morand, and E. Eugene. 1999. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol. Microbiol. 321124-1132. [DOI] [PubMed] [Google Scholar]
- 29.Plant, L., J. Sundqvist, S. Zughaier, L. Lovkvist, D. S. Stephens, and A. B. Jonsson. 2006. Lipooligosaccharide structure contributes to multiple steps in the virulence of Neisseria meningitidis. Infect. Immun. 741360-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porat, N., M. A. Apicella, and M. S. Blake. 1995. Neisseria gonorrhoeae utilizes and enhances the biosynthesis of the asialoglycoprotein receptor expressed on the surface of the hepatic HepG2 cell line. Infect. Immun. 631498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rietschel, E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zahringer, U. Seydel, F. Di Padova, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8217-225. [DOI] [PubMed] [Google Scholar]
- 32.Schneider, H., J. M. Griffiss, R. E. Mandrell, and G. A. Jarvis. 1985. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect. Immun. 50672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scholten, R. J., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41236-243. [DOI] [PubMed] [Google Scholar]
- 34.Song, W., L. Ma, R. Chen, and D. C. Stein. 2000. Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J. Exp. Med. 191949-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi, H., K. Hirose, and H. Watanabe. 2004. Necessity of meningococcal γ-glutamyl aminopeptidase for the Neisseria meningitidis growth in rat cerebrospinal fluid (CSF) and CSF mimicking medium. J. Bacteriol. 186244-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi, H., K. S. Kim, and H. Watanabe. 2008. Differential in vitro infectious abilities of two common Japan-specific sequence-type (ST) clones of disease-associated ST-2032 and carrier-associated ST-2046 Neisseria meningitidis strains in human endothelial and epithelial cell lines. FEMS Immunol. Med. Microbiol. 5236-46. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi, H., and H. Watanabe. 2002. A broad-host-range vector of incompatibility group Q can work as a plasmid vector in Neisseria meningitidis: a new genetical tool. Microbiology 148229-236. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi, H., and H. Watanabe. 2004. Post-translational processing of Neisseria meningitidis γ-glutamyl aminopeptidase and its association with inner membrane facing to the cytoplasmic space. FEMS Microbiol. Lett. 23427-35. [DOI] [PubMed] [Google Scholar]
- 39.Triantafilou, K., M. Triantafilou, and N. Fernandez. 2000. Lipopolysaccharide (LPS) labeled with Alexa 488 hydrazide as a novel probe for LPS binding studies. Cytometry 41316-320. [PubMed] [Google Scholar]
- 40.Turner, D. P., A. G. Marietou, L. Johnston, K. K. Ho, A. J. Rogers, K. G. Wooldridge, and D. A. Ala'Aldeen. 2006. Characterization of MspA, an immunogenic autotransporter protein that mediates adhesion to epithelial and endothelial cells in Neisseria meningitidis. Infect. Immun. 742957-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzeng, Y. L., K. D. Ambrose, S. Zughaier, X. Zhou, Y. K. Miller, W. M. Shafer, and D. S. Stephens. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 1875387-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzeng, Y. L., and D. S. Stephens. 2000. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2687-700. [DOI] [PubMed] [Google Scholar]
- 43.Unkmeir, A., K. Latsch, G. Dietrich, E. Wintermeyer, B. Schinke, S. Schwender, K. S. Kim, M. Eigenthaler, and M. Frosch. 2002. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol. Microbiol. 46933-946. [DOI] [PubMed] [Google Scholar]
- 44.Verheul, A. F., H. Snippe, and J. T. Poolman. 1993. Meningococcal lipopolysaccharides: virulence factor and potential vaccine component. Microbiol. Rev. 5734-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virji, M., H. Kayhty, D. J. Ferguson, C. Alexandrescu, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 51831-1841. [DOI] [PubMed] [Google Scholar]
- 46.Virji, M., K. Makepeace, D. J. Ferguson, M. Achtman, and E. R. Moxon. 1993. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 10499-510. [DOI] [PubMed] [Google Scholar]
- 47.Virji, M., K. Makepeace, D. J. Ferguson, M. Achtman, J. Sarkari, and E. R. Moxon. 1992. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol. Microbiol. 62785-2795. [DOI] [PubMed] [Google Scholar]
- 48.Virji, M., K. Makepeace, I. R. Peak, D. J. Ferguson, M. P. Jennings, and E. R. Moxon. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18741-754. [DOI] [PubMed] [Google Scholar]
- 49.Vogel, U., and M. Frosch. 1999. Mechanisms of neisserial serum resistance. Mol. Microbiol. 321133-1139. [DOI] [PubMed] [Google Scholar]
- 50.Wright, J. C., D. W. Hood, G. A. Randle, K. Makepeace, A. D. Cox, J. Li, R. Chalmers, J. C. Richards, and E. R. Moxon. 2004. lpt6, a gene required for addition of phosphoethanolamine to inner-core lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae. J. Bacteriol. 1866970-6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yazdankhah, S. P., and D. A. Caugant. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53821-832. [DOI] [PubMed] [Google Scholar]