Abstract
Production of interleukin-10 (IL-10) by C57BL/6 mice following infection with Borrelia burgdorferi has been proposed as a mechanism whereby resistance to the development of experimental Lyme arthritis is maintained. In the current study, we sought to determine the role of IL-10 during infection of arthritis- and carditis-susceptible C3H mice. Infection of C3H IL-10−/− mice led to increased joint swelling and arthritis severity scores over those of wild-type C3H mice. Measurement of B. burgdorferi numbers in joints or disseminated tissues indicated a more efficient clearance of spirochetes in the absence of IL-10, similar to that reported in C57BL/6 IL-10−/− mice. However, in contrast to previous in vitro work, infection of C3H IL-10−/− mice led to decreased in vivo expression of the cytokines KC, IL-1β, IL-4, and IL-12p70 in the infected joints. Finally, adenoviral expression of IL-10 in the infected joints of C3H mice was unable to modulate the development of severe Lyme arthritis and had no effect on spirochete clearance or Borrelia-specific antibody production. Development of Lyme carditis appeared to be independent of modulation by IL-10. These results suggest that IL-10 limits the development of joint inflammation in both arthritis-resistant and -susceptible mouse strains infected with B. burgdorferi and that increased IL-10 production cannot rescue genetic susceptibility to development of pathology in this model.
Experimental inoculation of mice with the spirochete Borrelia burgdorferi results in the development of Lyme borreliosis and recapitulates much of the disease spectrum seen in naturally infected humans (54). As in human Lyme disease, arthritis is the primary disease sequela occurring in B. burgdorferi-infected mice, the severity of which appears to be regulated by murine genetics and mediated primarily by innate immunity (4, 6, 12, 45). Arthritis severity does not typically correlate with spirochete loads in joint tissues, and arthritis-susceptible C3H/He (C3H) and arthritis-resistant DBA/2 (DBA) or C57BL/6 (B6) mice can harbor similar numbers of spirochetes in their joints and yet maintain their distinct disease phenotypes (11, 36). In mice, arthritis severity peaks around 3 weeks postinfection and then spontaneously resolves, a process thought to be mediated by spirochete clearance from the joint tissue by Borrelia-specific antibodies (4, 5). Thus, while spirochete presence within the joint is required for the development and maintenance of arthritis pathology, polymorphisms within the host immune response appear to drive the extent of arthritis severity.
Like other animal models of arthritis, experimental Lyme arthritis is thought to be mediated primarily by the production of proinflammatory chemokines and cytokines, which drives the development of pathology (41, 46, 51). Spirochete colonization of joint tissues activates innate immune cells to secrete proinflammatory chemokines and cytokines that recruit and activate inflammatory cells, leading to joint damage. Thus, the balance between pro- and anti-inflammatory mediators produced in response to B. burgdorferi infection has been suggested to regulate the extent of arthritis development. Indeed, several studies have demonstrated modulation of arthritis severity by treating mice with a specific anti-cytokine antibody, for example, gamma interferon (IFN-γ) or interleukin-4 (IL-4) (31, 38). However, to date, manipulation of no single cytokine alone has been able to completely alter the genetic phenotype of the mouse, which suggests that a complex balance of both pro- and anti-inflammatory mediators is ultimately responsible for arthritis resistance or susceptibility.
Recently, a number of investigators have focused on the role of IL-10 during the infection of mice with B. burgdorferi. IL-10 is a pleiotropic cytokine that has received much interest due to its dominant suppressive effects on the production of proinflammatory cytokines (7, 14, 18, 19, 28). During murine collagen-induced arthritis (CIA), antibody-mediated depletion of IL-10 resulted in accelerated onset of disease and increased arthritis severity (29), while treatment with recombinant IL-10 was able to inhibit the development or progression of CIA (27, 47, 49) and streptococcal cell wall (SCW)-induced arthritis (35). In vitro, B. burgdorferi antigens can stimulate the production of IL-10 from isolated human blood mononuclear cells (23, 25, 43, 44), synovial fluid (24, 55), or microglial cells (15). Stimulation of murine bone marrow macrophages with the B. burgdorferi lipoprotein OspA demonstrated higher production of IL-10 from B6 than from C3H macrophages (13), suggesting that this increased production of IL-10 from B6 macrophages might contribute to the arthritis-resistant phenotype of the mouse strain. Infection of B6 IL-10−/− mice resulted in an intermediate arthritis phenotype, more severe than that of the wild-type B6 mice but less severe than that of C3H mice, demonstrating that IL-10 does play a role in regulating the severity of experimental Lyme arthritis (13). However, other investigators measured IL-10 directly from the infected tibiotarsal joint and showed that, early during the infection (up to day 7 postinfection), C3H mice had 50% higher production of IL-10 in their joints than the B6 mice (10). These results suggested that IL-10 might be limiting the overall inflammatory response in both strains of mice rather than mediating their phenotypic differences.
In the current study, we infected C3H IL-10−/− mice and C3H mice treated with a human IL-10 (hIL-10)-expressing adenoviral vector (AdIL-10) to more fully explore the role of IL-10 during murine Lyme borreliosis. We found that IL-10 deficiency in C3H mice increased their peak ankle swelling and arthritis severity scores and decreased the overall numbers of B. burgdorferi spirochetes in tissues, similar to what was described in B6 mice. In contrast, however, increased IL-10 production in the AdIL-10-treated mice had little effect on the development of Lyme arthritis or carditis.
MATERIALS AND METHODS
Animals.
Male and female C3Bir.129P2(B6)-Il10tm1Cgn/Lt (C3H IL-10−/−) mice and control C3Bir.129P2(B6)-Il110C3Bir/LtJ (C3H wild type [wt]) mice and female C3H/HeJ mice 4 to 6 weeks of age were purchased from the Jackson Laboratory. The mice were housed in a specific-pathogen-free facility and given food and water ad libitum. All experimental protocols were approved by the Animal Use and Care Committee of the University of Missouri.
Bacteria and infections.
A low-passage, virulent clonal isolate of the B. burgdorferi N40 strain (a kind gift from Janis Weis, University of Utah) was used for all experiments. For all infections, frozen aliquots were grown in 7.5 ml of Barbour-Stoenner-Kelly II medium supplemented with 6% rabbit serum (Sigma-Aldrich) for 5 days at 32°C. The viable spirochetes were then enumerated using a Petroff-Hauser counting chamber and dark-field microscopy. The spirochetes were diluted using sterile Barbour-Stoenner-Kelly II medium, and mice were inoculated in both hind footpads with 5 × 103 B. burgdorferi organisms contained in 50 μl of medium. In some experiments, mice were given an inoculum of 1 × 105 spirochetes with similar results, except for attenuated arthritis severity. For IL-10 add-back experiments, mice were inoculated in each footpad on day −1 of Borrelia infection with 5 × 108 PFU of a recombinant adenovirus vector expressing hIL-10 (Ad CMV hIL10 no. 1) or control vector [AdCMVpLpA(−)loxP] purchased from the Vector Core Laboratory at the University of Michigan.
Assessment of pathology.
The development of arthritis was monitored by measuring ankle thickness through the thickest anteroposterior portion of both tibiotarsal joints using a metric caliper (Ralmike's Tool-A-Rama). Initial baseline measurements were made immediately prior to infection and then weekly thereafter. Ankle diameter increases were determined by subtracting the initial baseline measurement from the weekly measurement. For arthritis severity scores, one ankle from each mouse was chosen at random at sacrifice and was excised by removing the skin and cutting just above and below the tibiotarsal joint. The excised joint was placed in 10% buffered zinc-formalin, decalcified, and embedded in paraffin, and sections were stained with hematoxylin and eosin (H&E). The sections were scored on a scale from 0 to 4 by two independent experienced observers in a blinded manner. Grade 0 represented no inflammation in the sample, grade 1 indicated minimal inflammatory infiltration affecting <5% of the tissue, grade 2 represented multiple focally extensive areas of inflammation covering 5 to 25% of the sample, grade 3 represented confluent inflammation with multiple (>3) structures affected with mild distention of tissues by inflammatory cells involving 25 to 50% of the sample, and grade 4 represented confluent inflammation with all structures involved and severely distended tissues containing large numbers of inflammatory cells involving >50% of the sample. The arthritis was characterized by neutrophil and monocyte infiltration into the joints, tendons, and ligament sheaths with hyperplasia and hypertrophy of the synovium and fibrin exudates. Independent-observer severity scores were averaged for each sample. For carditis, the hearts were bisected sagittally through both atria and ventricles and fixed in 10% buffered zinc-formalin, mounted, and stained with H&E. The histological samples were then evaluated on a scale from 0 to 4 for four separate areas: ventricular inflammation, atrial inflammation, valvulitis, and vasculitis. Grade 0 represented no change from controls, grade 1 represented minimal scattered inflammation covering <1% of the area, grade 2 represented mild multifocal inflammation over 1 to 25% of the area, grade 3 represented moderate focally extensive inflammation covering 25 to 50% of the area, and grade 4 represented marked confluent areas of inflammation over 50% of the sample.
Quantitative assessment of B. burgdorferi numbers in tissues.
To analyze the numbers of spirochetes present in various target tissues from B. burgdorferi-infected mice, we performed quantitative multiplex real-time PCR using the Applied Biosystems 7300 Real-Time PCR System as described previously (10). Briefly, following mouse sacrifice, ankles were excised as described above and a portion of the ear was also removed, and the samples were frozen separately in liquid nitrogen. All tissue samples were homogenized in Trizol (Invitrogen), and DNA was extracted according to the manufacturer's instructions. The extracted DNA was diluted to 50 ng/ml using Tris-EDTA buffer, and 1 microliter was used in PCRs. The mouse nidogen gene was used as an endogenous control and was amplified using primers and probe as described previously (39). Quantification of B. burgdorferi DNA in samples was done by detection of the flagellin gene using primers and probe as described previously (42). Quantitative multiplex real-time PCR was performed in triplicate for flagellin and normalized to copies of nidogen in the same tube. B. burgdorferi DNA within each sample was quantified by comparing it to a standard curve consisting of known numbers of B. burgdorferi organisms. Similarly, normalization of mouse nidogen DNA within each sample was completed by comparing it to a standard curve of dilutions of mouse DNA from the same tissue (ear or ankle).
Ex vivo quantification of cytokines from tissues.
Cytokines were measured directly from tissue extracts as described previously (10). Briefly, knee or heart tissues were excised as described above and immediately snap-frozen in liquid nitrogen. Individual samples were wrapped in aluminum foil and pulverized with a hammer. The still-frozen powder was then immediately placed into 1 ml of ice-cold homogenization buffer consisting of Hanks balanced salt solution containing 0.2% protease inhibitor (Sigma-Aldrich) and 0.4% Triton X-100. The samples were then sonicated (three times for 20 s each time) on ice, centrifuged at 2,000 × g for 20 min at 4°C, and filtered through a 0.45-μm filter. The levels of cytokines were then determined by enzyme-linked immunosorbent assay (ELISA) kits for murine IL-1β, tumor necrosis factor alpha, IL-12p70, IL-4, IFN-γ, KC, and monocyte chemoattractant protein 1 (MCP-1) (OptEIA kits; BD Pharmingen) and hIL-10 (DuoSet; R&D Systems). The total protein concentration was measured using the bicinchoninic acid kit (Pierce), and cytokine concentrations were expressed in picograms per milligram protein.
Immunohistochemical detection of hIL-10 in joint tissues.
Tibiotarsal joints were removed from mice inoculated with adenoviral vectors and infected with B. burgdorferi 14 days earlier. The samples were processed as described above for assessment of pathology. Paraffin sections (5 μm) were fixed in xylene and rehydrated through graded ethanol solutions. Endogenous peroxidases were quenched in 3% H2O2, and nonspecific binding was blocked using normal goat serum. An anti-hIL-10 polyclonal antibody (R&D Systems, Minneapolis, MN) was used as the primary antibody at a 1:200 dilution. The secondary antibody was a biotinylated rabbit anti-goat polyclonal antibody (Dako, Carpentaria, CA) used at 1:400 dilution. Staining was visualized using streptavidin-horseradish peroxidase and diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO). The slides were counterstained with Meyer's hematoxylin solution (Fisher Scientific, Fair Lawn, NJ).
Statistical analysis.
Results are expressed as means ± standard errors of the mean. Data were analyzed by using Student's t test or analysis of variance, followed by the Tukey test for multiple comparisons using SigmaStat software (SPSS, Inc). Significance levels were set at an α value of 0.05.
RESULTS
Development of pathology in C3H IL-10-deficient mice.
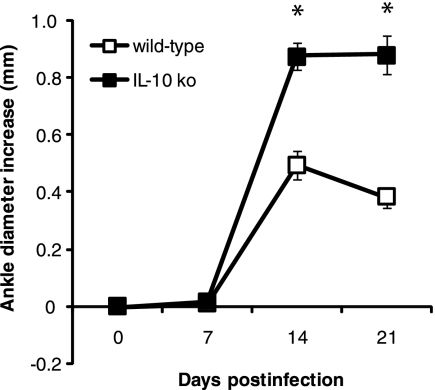
A previous study reported exacerbated Lyme arthritis in resistant B6 mice deficient in IL-10 (13). We have previously shown that C3H mice actually produce higher levels of IL-10 in their tibiotarsal joints than do B6 mice during the first week of infection with B. burgdorferi (10). Thus, it was of interest to determine the effect of IL-10 deficiency in the arthritis-susceptible C3H background. C3H (wt) and C3H IL-10−/− (IL-10 knockout [ko]) mice were infected in both hind footpads with 5 × 103 spirochetes of the virulent N40 strain of B. burgdorferi, and the development of arthritis was followed for 3 weeks (Fig. 1). As is typical for this experimental model, C3H mice developed increased ankle swelling during the second week of infection, which began to resolve slightly by day 21 postinfection. The IL-10 ko mice developed ankle swelling during the same time period as the wt mice, except the ankle swelling was significantly greater in the IL-10 ko mice at days 14 and 21 postinfection (P < 0.05).
FIG. 1.
Development of experimental Lyme arthritis in control C3H (wt) and C3H IL-10−/− (IL-10 ko) mice following infection with B. burgdorferi. Ankle swelling was determined weekly as described in Materials and Methods and was compared to ankle diameter measurements taken immediately prior to infection. The data are pooled means ± standard errors from two separate experiments; n = 10. An asterisk indicates that the IL-10 ko value is significantly different from the wt value at the P < 0.001 level.
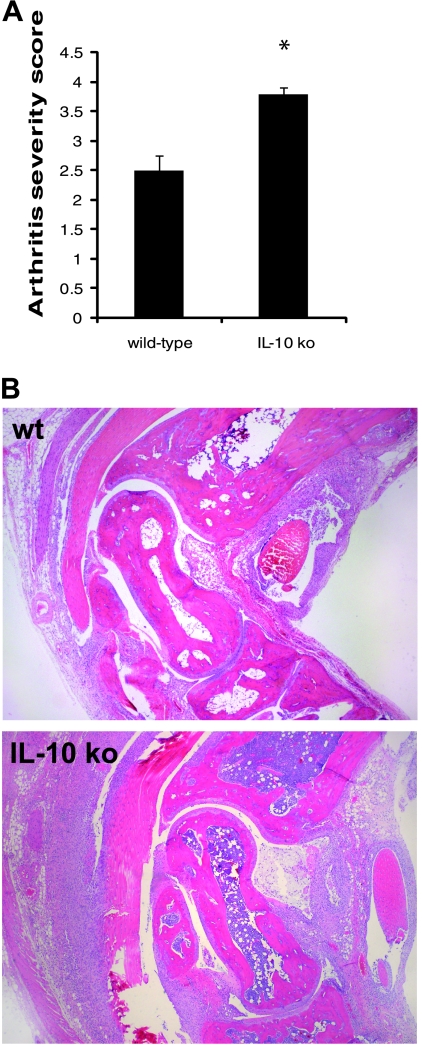
Ankle swelling during Lyme arthritis is generally correlative with the underlying inflammatory response but has been reported to be differentially regulated (52). Therefore, the arthritis severity was determined from lesion scores in histological sections of tibiotarsal joints from mice sacrificed at day 21 postinfection (Fig. 2A). Both mouse strains developed severe arthritis; however, the IL-10 ko mice had significantly higher arthritis severity scores (P < 0.001) than the wt C3H mice at this time point. These results were exemplified by a greater level of inflammatory-cell recruitment into the infected joint, with greater numbers of neutrophils present in the joints from IL-10 ko mice than in those of wt mice (Fig. 2B). Morphometric evaluation of neutrophil numbers present in the tibiotarsal joints demonstrated a significant increase (P < 0.04) in neutrophils in IL-10 ko mice (147) compared to C3H mice (81). Taken together, these results demonstrate a significant increase in the development of joint pathology in the absence of IL-10 in C3H mice infected with B. burgdorferi.
FIG. 2.
Assessment of pathology in ankle joints of control C3H (wt) and C3H IL-10−/− (IL-10 ko) mice 21 days after infection with B. burgdorferi. (A) Arthritis severity scores were assigned by two independent, blinded observers. The data are pooled means plus standard errors from two separate experiments; n = 10. The asterisk indicates that the IL-10 ko value is significantly different from the wt value at the P < 0.001 level. (B) Representative examples of H&E-stained histological sections from C3H wt and IL-10 ko mice. Magnification, ×100.
C3H mice also develop carditis in response to B. burgdorferi infection; however, the inflammatory lesion is dominated by a macrophage, rather than a neutrophil, inflammatory infiltrate (3). Thus, it was of interest to determine if the development of carditis would be altered in IL-10 ko animals. Table 1 shows the histopathology scores of heart tissue following infection with B. burgdorferi. At day 21 postinfection, there was no difference in cardiac severity scores for any of the parameters evaluated. Therefore, the development of Lyme carditis in C3H mice does not appear to be affected by IL-10 deficiency.
TABLE 1.
Histopathology scores for heart tissue following infection with B. burgdorferi
| Mouse | Day | Scorea
|
|||
|---|---|---|---|---|---|
| Inflammation
|
Valvulitis | Vasculitis | |||
| Ventricular | Atrial | ||||
| wt | 21 | 0.1 ± 0.4 | 3.4 ± 0.8 | 2.3 ± 0.4 | 1.5 ± 1.4 |
| IL-10 ko | 21 | 0.6 ± 0.5 | 3.0 ± 1.0 | 1.8 ± 1.0 | 1.1 ± 1.2 |
Values are means ± standard deviation (n = 7) and are representative of two separate experiments.
Effect of IL-10 deficiency on spirochete loads in tissue.
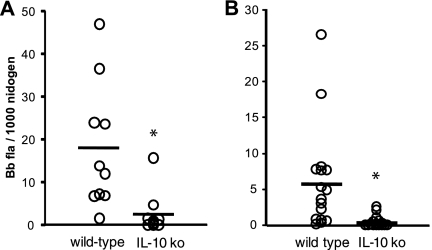
IL-10 is a potent inhibitor of proinflammatory immune responses, and as such, it is capable of inhibiting the efficient clearance of microbial pathogens (9). Infection of B6 IL-10-deficient mice led to an enhanced clearance of spirochetes from joint, heart, and ear tissues by 4 weeks of infection (13). In the current study, infection of C3H IL-10 ko mice also led to the more efficient clearance of spirochetes from the ankle joints and ears (Fig. 3). By day 21 postinfection, the IL-10 ko mice contained significantly fewer spirochetes in their ankle joints than the wt mice (Fig. 3A) (P < 0.05). Ear tissue is representative of spirochete dissemination from the initial inoculation site in the hind footpad. By day 21, the levels of spirochetes in ear tissue were significantly below those of the wt mice (Fig. 3B) (P < 0.05).
FIG. 3.
Levels of B. burgdorferi (Bb) in tissues from control C3H (wild-type) and C3H IL-10−/− (IL-10 ko) mice 21 days after infection with B. burgdorferi. B. burgdorferi DNA copy numbers in the ankle joint (A) or in ear tissue (B) were determined using real-time PCR and normalized using the single-copy mouse gene nidogen. The bars represent pooled means from two separate experiments; n = 10. An asterisk indicates that the IL-10 ko value is significantly different from the wt value at the P < 0.001 level. fla, flagellin.
Cytokine production in joints of C3H IL-10-deficient mice.
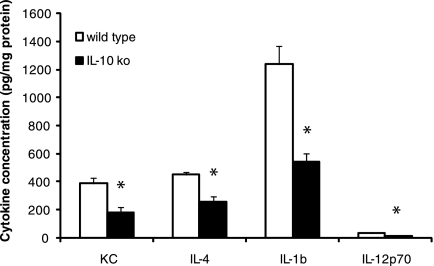
As stated above, IL-10 is a powerful regulator of proinflammatory cytokine production by several innate immune cell types, including macrophages and neutrophils. While several studies have examined the role of IL-10 in regulating cytokine production in vitro, few have examined in vivo cytokine production in mouse models of arthritis. We were interested in how the IL-10 deficiency might alter the production of cytokines in the arthritic joint. Knee joints were isolated from C3H wt or IL-10 ko mice, and cytokines were directly isolated and measured as described previously (10, 28). Knee joints were harvested at the time of sacrifice, and the levels of IL-1β, IL-4, IL-12p70, IFN-γ, IL-17, MCP-1, and KC were determined (Fig. 4). Levels of IFN-γ, IL-17, and MCP-1 did not reach the threshold of detection in any of the samples (data not shown). We found that the levels of the other four cytokines were significantly lower in joints from the IL-10 ko mice than in those from the wt mice (P < 0.002). These results indicate that there may be significant differences in the actions of IL-10 on isolated cells in vitro and those from in vivo sites of inflammation.
FIG. 4.
Ex vivo levels of cytokines in ankle tissue from control C3H (wild type) and C3H IL-10−/− (IL-10 ko) mice following infection with B. burgdorferi. The mice were sacrificed on day 21 postinfection, and one knee joint was removed immediately upon sacrifice and frozen in liquid nitrogen as described in Materials and Methods. Samples were homogenized, and the levels of IL-1β, IL-12p70, IL-4, and KC were determined by ELISA. The data are means plus standard errors expressed as pg cytokine/mg total protein and are pooled data from two separate experiments; n = 10. An asterisk indicates that the IL-10 ko value is significantly different from the wt value at the P < 0.002 level.
Development of Lyme borreliosis in wt mice treated with AdIL-10.
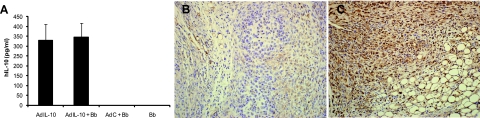
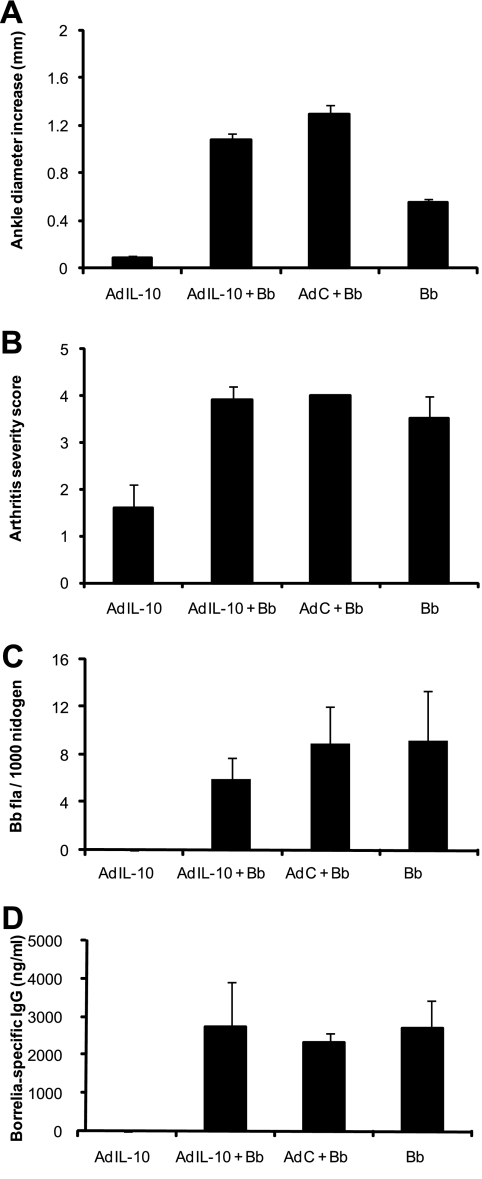
Previous experiments using the CIA model of arthritis demonstrated that treatment of mice with recombinant IL-10 (27, 29, 35, 47, 49), or with an IL-10-expressing adenovirus vector (2, 37, 53), could attenuate or inhibit the development of joint pathology. To determine the effect of increased IL-10 production on the development of Lyme arthritis or carditis, we treated mice with an adenoviral vector expressing hIL-10 and then infected them 1 day later with B. burgdorferi. hIL-10 is known to stimulate mouse cells in a manner similar to that of mouse IL-10 (48) and can be measured independently of murine IL-10 by ELISA. Mice were inoculated with the adenoviral vector in both hind footpads, since a previous report demonstrated production of IL-10 in the ankle joints for up to 3 weeks using a similar adenoviral delivery system (53). On day 14 postinfection, mice treated with the AdIL-10 vector had high levels of hIL-10 in their sera, indicating that the viral expression vector was working and that the mice were exposed to high levels of IL-10 (Fig. 5A). In addition, immunohistochemical staining of joint sections from mice treated with the control adenoviral vector (AdC) (Fig. 5B) or with the IL-10-expressing adenoviral vector (AdIL-10) (Fig. 5C) demonstrated specific expression of hIL-10 in the joints of AdIL-10 treated mice. Treatment of mice with AdIL-10 alone had a minimal effect on ankle swelling (Fig. 6A). Treatment of mice with the adenoviral vector followed by B. burgdorferi infection exacerbated the level of ankle swelling, an effect that was described previously by others (33, 37). More importantly, however, the expression of IL-10 in these animals did not significantly lower the levels of ankle swelling in the AdIL-10 plus B. burgdorferi compared with the AdC plus B. burgdorferi group (Fig. 6A). Thus, in contrast to CIA, the increased expression of IL-10 in B. burgdorferi-infected mice did not prevent or attenuate the development of arthritis in this model system. Arthritis severity scores were uniformly severe in all mice infected with B. burgdorferi regardless of the presence of additional IL-10 (Fig. 6B). The cellular makeup of the inflammatory infiltrate was also not altered by the presence of the adenoviral vector, primarily consisting of neutrophils and monocytes (data not shown). Finally, the development of Lyme carditis was unchanged in the mice receiving AdIL-10 or AdC in addition to B. burgdorferi infection (data not shown).
FIG. 5.
Expression of hIL-10 in C3H mice following infection with B. burgdorferi. Groups of mice (n = 5) were infected in the hind footpads on day −1 with adenoviral vectors expressing hIL-10 (AdIL-10) or empty (control) vector (AdC) or left uninfected. The following day, the mice were left alone or infected with 1 × 105 B. burgdorferi spirochetes in each hind footpad (Bb). (A) Blood was collected via cardiac puncture at sacrifice on day 14 postinfection, and levels of hIL-10 were determined by ELISA. The data are means plus standard errors and are representative of two separate experiments with similar results. (B and C) Immunohistochemistry for expression of hIL-10 in joints treated with AdC (B) or AdIL-10 (C). Magnification, ×200.
FIG. 6.
Development of arthritis and levels of B. burgdorferi in ankle tissue and Borrelia-specific IgG antibodies in C3H mice treated with adenoviral vectors. The mice were treated as described in the legend to Fig. 5. The mice were sacrificed on day 14 postinfection, and the increases in ankle diameters (A) and severity scores (B), as well as levels of B. burgdorferi in ankle joint tissue (C) and Borrelia-specific IgG levels in sera (D), were determined as described in Materials and Methods. The data are means plus standard errors and are representative of two separate experiments with similar results. n = 5 per group.
Effect of AdIL-10 expression on B. burgdorferi loads in tissues and antibody responses.
Infection of C3H IL-10 ko mice resulted in a significantly lower B. burgdorferi burden in joints and elevated production of Borrelia-specific immunoglobulin G (IgG) (data not shown). Thus, it was of interest to determine what effect increased expression of IL-10 might have on the immune response to Borrelia infection. In contrast to the changes in the immune response seen in the absence of IL-10, an excess of IL-10 appeared to have no effect on the host response to B. burgdorferi infection (Fig. 6). All groups infected with B. burgdorferi harbored similar numbers of spirochetes in their ankle joints (Fig. 6C), indicating the overexpression of IL-10 did not have a significant effect on the ability of the host to control spirochete replication. In addition, there was no effect of increased IL-10 expression on Borrelia-specific antibody production (Fig. 6D).
DISCUSSION
Experimental Lyme arthritis is an inflammatory disease caused in susceptible mice by the colonization of the joints by B. burgdorferi. Early spirochete recognition most likely occurs through Toll-like receptors, which results in the production of cytokines and chemokines that recruit and activate phagocytic inflammatory cells and mediate spirochetal clearance (1, 10, 50, 56). The balance of pro- and anti-inflammatory cytokines is thought to underlie disease resistance and susceptibility (54), and indeed, treatment modalities that alter cytokine activity can modulate disease severity (30, 31, 38). Thus, a more complete understanding of the roles of various cytokines and chemokines in the pathogenesis of Lyme arthritis may lead to better treatments for this and other inflammatory diseases.
IL-10 acts as a potent inflammatory mediator by inhibiting the production of proinflammatory cytokines, especially those produced by macrophages and neutrophils, cells that make up the majority of the inflammatory infiltrate during Lyme arthritis (19, 28). To determine the role of IL-10 during the immune response to B. burgdorferi infection, we used both add-back and take-away experiments in arthritis-susceptible C3H mice. Infection of C3H IL-10 ko mice resulted in significantly increased ankle swelling at the peak of arthritis and increased arthritis severity. Infection of arthritis-resistant B6 mice deficient in IL-10 with B. burgdorferi resulted in an intermediate arthritis phenotype, indicating that other inflammatory mediators in addition to IL-10 are involved in determining arthritis severity (13). In the CIA model, treatment of mice with neutralizing anti-IL-10 antibodies or inoculation of IL-10 ko mice resulted in acceleration of the onset and an increase in the severity of arthritis (26, 29). These results suggest that IL-10 plays an important regulatory role during the development of arthritis by limiting the magnitude of the inflammatory response. Removal or inhibition of this immune regulator results in exacerbation of the inflammatory response and an increase in arthritis severity. On the other hand, boosting IL-10 levels, through either administration of recombinant IL-10 (27, 35, 47, 49) or adenoviral-gene therapy (2, 37, 53), inhibits the onset and severity of CIA and SCW-induced arthritis. Similarly, systemic recombinant IL-10 treatment of mice infected with the relapsing fever spirochete, Borrelia turicatae, reduced clinical disease (22). In the current study, however, we found no benefit of increased IL-10 on the development of experimental Lyme arthritis in susceptible C3H mice. Experimental Lyme arthritis is an inflammatory arthritis and is independent of T- and B-cell activity, whereas CIA is dependent upon breaking T-cell tolerance and the development of anti-collagen antibodies. Thus, the effect of additional IL-10 upon arthritis severity might be due to the mechanism of disease manifestation and might explain the failure of IL-10 therapy in human clinical trials (8).
Genetic ablation of IL-10 has been linked to increased cellular immune responses and efficient clearance of bacterial and viral pathogens (9, 13, 22). In the current study, we found that infection of C3H IL-10 ko mice resulted in more efficient clearance of spirochetes from both the joints and disseminated sites (ears) than from wt C3H mice. This was similar to results reported for B. burgdorferi infection of arthritis-resistant B6 mice (13). During B. burgdorferi infection of mice, spirochetal clearance is thought to be due mainly to the production of Borrelia-specific antibodies (4, 5). In agreement with Brown et al. (13), we found that levels of Borrelia-specific IgG were increased in the sera of IL-10 ko mice compared with wt mice (data not shown). However, it was recently reported that B. burgdorferi clearance in IL-10 ko mice was mediated predominantly through enhanced innate immune responses, rather than through the increased antibody response (32). In the current study, adenoviral delivery of IL-10 had no effect on the levels of spirochetes in tissues or on the production of Borrelia-specific IgG. In contrast to our findings, Gelderblom et al. (22) reported that administration of IL-10, rather than IL-10 ablation, resulted in more efficient clearance of B. turicatae. Clearance of relapsing fever spirochetes is mediated by complement-independent bactericidal antibodies (16); thus, the effect of IL-10 on pathogen clearance might again be dependent upon the specific mechanism used, especially innate versus adaptive responses.
IL-10 was first described as a factor that could inhibit the production of proinflammatory cytokines by macrophages and other cell types (14-17). In vitro, a number of cell types can produce IL-10 in response to stimulation by B. burgdorferi or B. burgdorferi antigens (24-30). Also, addition of IL-10 to culture supernatants can alter the production of cytokines in response to Borrelia antigens (17, 20, 21, 34, 40); however, no previous studies have examined the in vivo cytokine responses in the joint following IL-10 ablation or therapy during B. burgdorferi infection. We found that IL-10 ablation during B. burgdorferi infection of C3H mice resulted in decreased production of IL-1β, IL-4, KC, and IL-12. Adenoviral delivery of IL-10 to B. burgdorferi-infected mice had no effect on the production of cytokines in the joints of C3H mice (data not shown). Based upon in vitro responses, we expected an increase in the production of proinflammatory cytokines in the joints of B. burgdorferi-infected C3H IL-10 ko mice. One possible explanation for our results might be the more efficient clearance of B. burgdorferi in the IL-10 ko mice, leading to an attenuated cytokine response or a more rapid decrease in cytokine expression. During SCW-induced arthritis, the levels of tumor necrosis factor alpha and IL-1β were decreased in patellar washouts from mice treated with recombinant IL-10 (35). Conversely, during CIA, joint expression of MIP-1a and MIP-2 was increased in mice receiving anti-IL-10 antiserum (29). These results demonstrate that care must be taken when extrapolating in vitro results to in vivo and that the role of Il-10 might vary depending upon the model system used.
In summary, our results suggest that IL-10 regulates arthritis severity in both arthritis-resistant and -susceptible mouse strains following infection with B. burgdorferi. However, increased expression of IL-10 in C3H mice does not attenuate the development of pathology in this inflammatory-arthritis model, and other inflammatory mediators are likely to play important roles in regulating arthritis severity.
Acknowledgments
We thank Lauren Lewis, Jenniffer Stetler, Melitza Bonet, Sangita Pal, Kawasi Lett, and Chanakya Das for their excellent technical support.
This work was supported by National Institutes of Health Grant R01 AI059292.
We have no financial conflict of interest.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 29 September 2008.
REFERENCES
- 1.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8878-884. [DOI] [PubMed] [Google Scholar]
- 2.Apparailly, F., C. Verwaerde, C. Jacquet, C. Auriault, J. Sany, and C. Jorgensen. 1998. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J. Immunol. 1605213-5220. [PubMed] [Google Scholar]
- 3.Armstrong, A. L., S. W. Barthold, D. H. Persing, and D. S. Beck. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47249-258. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162133-138. [DOI] [PubMed] [Google Scholar]
- 5.Barthold, S. W., M. deSouza, and S. Feng. 1996. Serum-mediated resolution of Lyme arthritis in mice. Lab. Investig. 7457-67. [PubMed] [Google Scholar]
- 6.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47605-613. [DOI] [PubMed] [Google Scholar]
- 7.Bogdan, C., Y. Vodovotz, and C. Nathan. 1991. Macrophage deactivation by interleukin 10. J. Exp. Med. 1741549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan, F. M. 1999. Interleukin 10 and arthritis. Rheumatology 38293-296. [DOI] [PubMed] [Google Scholar]
- 9.Brooks, D. G., M. J. Trifilo, K. H. Edelmann, L. Teyton, D. B. McGavern, and M. B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 121301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, C. R., V. A. Blaho, and C. M. Loiacono. 2003. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171893-901. [DOI] [PubMed] [Google Scholar]
- 11.Brown, C. R., and S. L. Reiner. 1998. Clearance of Borrelia burgdorferi may not be required for resistance to experimental Lyme arthritis. Infect. Immun. 662065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, C. R., and S. L. Reiner. 1999. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect. Immun. 671967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 675142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassatella, M. A., L. Meda, S. Bonora, M. Ceska, and G. Constantin. 1993. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J. Exp. Med. 1782207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassiani-Ingoni, R., E. S. Cabral, J. D. Lunemann, Z. Garza, T. Magnus, H. Gelderblom, P. J. Munson, A. Marques, and R. Martin. 2006. Borrelia burgdorferi induces TLR1 and TLR2 in human microglia and peripheral blood monocytes but differentially regulates HLA-class II expression. J. Neuropathol. Exp. Neurol. 65540-548. [DOI] [PubMed] [Google Scholar]
- 16.Connolly, S. E., and J. L. Benach. 2001. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J. Immunol. 1673029-3032. [DOI] [PubMed] [Google Scholar]
- 17.Dennis, V. A., A. Jefferson, S. R. Singh, F. Ganapamo, and M. T. Philipp. 2006. Interleukin-10 anti-inflammatory response to Borrelia burgdorferi, the agent of Lyme disease: a possible role for suppressors of cytokine signaling 1 and 3. Infect. Immun. 745780-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1741209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiorentino, D. F., A. Zlotnik, T. R. Mosmann, M. Howard, and A. O'Garra. 1991. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1473815-3822. [PubMed] [Google Scholar]
- 20.Ganapamo, F., V. A. Dennis, and M. T. Philipp. 2000. Early induction of gamma interferon and interleukin-10 production in draining lymph nodes from mice infected with Borrelia burgdorferi. Infect. Immun. 687162-7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganapamo, F., V. A. Dennis, and M. T. Philipp. 2003. Differential acquired immune responsiveness to bacterial lipoproteins in Lyme disease-resistant and -susceptible mouse strains. Eur. J. Immunol. 331934-1940. [DOI] [PubMed] [Google Scholar]
- 22.Gelderblom, H., J. Schmidt, D. Londono, Y. Bai, J. Quandt, R. Hornung, A. Marques, R. Martin, and D. Cadavid. 2007. Role of interleukin 10 during persistent infection with the relapsing fever spirochete Borrelia turicatae. Am. J. Pathol. 170251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giambartolomei, G. H., V. A. Dennis, and M. T. Philipp. 1998. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect. Immun. 662691-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harjacek, M., S. Diaz-Cano, B. A. Alman, J. Coburn, R. Ruthazer, H. Wolfe, and A. C. Steere. 2000. Prominent expression of mRNA for proinflammatory cytokines in synovium in patients with juvenile rheumatoid arthritis or chronic Lyme arthritis. J. Rheumatol. 27497-503. [PubMed] [Google Scholar]
- 25.Haupl, T., S. Landgraf, P. Netusil, N. Biller, C. Capiau, P. Desmons, P. Hauser, and G. R. Burmester. 1997. Activation of monocytes by three OspA vaccine candidates: lipoprotein OspA is a potent stimulator of monokines. FEMS Immunol. Med. Microbiol. 1915-23. [DOI] [PubMed] [Google Scholar]
- 26.Johansson, A. C. M., A. S. Hansson, K. S. Nandakumar, J. Backlund, and R. Holmdahl. 2001. IL-10-deficient B10.Q. mice develop more severe collagen-induced arthritis, but are protected from arthritis induced with anti-type II collagen antibodies. J. Immunol. 1673505-3512. [DOI] [PubMed] [Google Scholar]
- 27.Joosten, L. A., E. Lubberts, P. Durez, M. M. Helsen, M. J. Jacobs, M. Goldman, and W. B. van den Berg. 1997. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 40249-260. [DOI] [PubMed] [Google Scholar]
- 28.Kasama, T., R. M. Strieter, N. W. Lukacs, M. D. Burdick, and S. L. Kunkel. 1994. Regulation of neutrophil-derived chemokine expression by IL-10. J. Immunol. 1523559-3569. [PubMed] [Google Scholar]
- 29.Kasama, T., R. M. Strieter, N. W. Lukacs, P. M. Lincoln, M. D. Burdick, and S. L. Kunkel. 1995. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J. Clin. Investig. 952868-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keane-Myers, A., C. R. Maliszewski, F. D. Finkelman, and S. P. Nickell. 1996. Recombinant IL-4 treatment augments resistance to Borrelia burgdorferi infections in both normal susceptible and antibody-deficient susceptible mice. J. Immunol. 1562488-2494. [PubMed] [Google Scholar]
- 31.Keane-Myers, A., and S. P. Nickell. 1995. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 1552020-2028. [PubMed] [Google Scholar]
- 32.Lazarus, J. J., M. J. Meadows, R. E. Lintner, and R. M. Wooten. 2006. IL-10 deficiency promotes increased Borrelia burgdorferi clearance predominantly through enhanced innate immune responses. J. Immunol. 1777076-7085. [DOI] [PubMed] [Google Scholar]
- 33.Le, C. H., A. G. Nicolson, A. Morales, and K. L. Sewell. 1997. Suppression of collagen-induced arthritis through adenovirus-mediated transfer of a modified tumor necrosis factor α receptor gene. Arthritis Rheum. 401662-1669. [DOI] [PubMed] [Google Scholar]
- 34.Lisinski, T. J., and M. B. Furie. 2002. Interleukin-10 inhibits proinflammatory activation of endothelium in response to Borrelia burgdorferi or lipopolysaccharide but not interleukin-1β or tumor necrosis factor α. J. Leukoc. Biol. 72503-511. [PubMed] [Google Scholar]
- 35.Lubberts, E., L. A. Joosten, M. M. Helsen, and W. B. van den Berg. 1998. Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine 10361-369. [DOI] [PubMed] [Google Scholar]
- 36.Ma, Y., K. P. Seiler, E. J. Eichwald, J. H. Weis, C. Teuscher, and J. J. Weis. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma, Y., S. Thornton, L. E. Duwel, G. P. Boivin, E. H. Giannini, J. M. Leiden, J. A. Bluestone, and R. Hirsch. 1998. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. J. Immunol. 1611516-1524. [PubMed] [Google Scholar]
- 38.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 1811251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murthy, P. K., V. A. Dennis, B. L. Lasater, and M. T. Philipp. 2000. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect. Immun. 686663-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nandakumar, K. S., and R. Holmdahl. 2006. Antibody-induced arthritis: disease mechanisms and genes involved at the effector phase of arthritis. Arthritis Res. Ther. 8223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pahl, A., U. Kuhlbrandt, K. Brune, M. Rollinghoff, and A. Gessner. 1999. Quantitative detection of Borrelia burgdorferi by real-time PCR. J. Clin. Microbiol. 371958-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pohl-Koppe, A., K. E. Balashov, A. C. Steere, E. L. Logigian, and D. A. Hafler. 1998. Identification of a T cell subset capable of both IFN-γ and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J. Immunol. 1601804-1810. [PubMed] [Google Scholar]
- 44.Rasley, A., S. L. Tranguch, D. M. Rati, and I. Marriott. 2006. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia 53583-592. [DOI] [PubMed] [Google Scholar]
- 45.Schaible, U. E., M. D. Kramer, C. Museteanu, G. Zimmer, H. Mossmann, and M. M. Simon. 1989. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J. Exp. Med. 1701427-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szekanecz, Z., and A. E. Koch. 2007. Macrophages and their products in rheumatoid arthritis. Curr. Opin. Rheumatol. 19289-295. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka, Y., T. Otsuka, T. Hotokebuchi, H. Miyahara, H. Nakashima, S. Kuga, Y. Nemoto, H. Niiro, and Y. Niho. 1996. Effect of IL-10 on collagen-induced arthritis in mice. Inflamm. Res. 45283-288. [DOI] [PubMed] [Google Scholar]
- 48.Vieira, P., R. de Waal-Malefyt, M. N. Dang, K. E. Johnson, R. Kastelein, D. F. Fiorentino, J. E. deVries, M. G. Roncarolo, T. R. Mosmann, and K. W. Moore. 1991. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc. Natl. Acad. Sci. USA 881172-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walmsley, M., P. D. Katsikis, E. Abney, S. Parry, R. O. Williams, R. N. Maini, and M. Feldmann. 1996. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum. 39495-503. [DOI] [PubMed] [Google Scholar]
- 50.Wang, G., Y. Ma, A. Buyuk, S. McClain, J. J. Weis, and I. Schwartz. 2004. Impaired host defense to infection and Toll-like receptor 2-independent killing of Borrelia burgdorferi clinical isolates in TLR2-deficient C3H/HeJ mice. FEMS Microbiol. Lett. 231219-225. [DOI] [PubMed] [Google Scholar]
- 51.Weis, J. J. 2002. Host-pathogen interactions and the pathogenesis of murine Lyme disease. Curr. Opin. Rheumatol. 14399-403. [DOI] [PubMed] [Google Scholar]
- 52.Weis, J. J., B. A. McCracken, Y. Ma, D. Fairbairn, R. J. Roper, T. B. Morrison, J. H. Weis, J. F. Zachary, R. W. Doerge, and C. Teuscher. 1999. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162948-956. [PubMed] [Google Scholar]
- 53.Whalen, J. D., E. L. Lechman, C. A. Carlos, K. Weiss, I. Kovesdi, J. C. Glorioso, P. D. Robbins, and C. H. Evans. 1999. Adenoviral transfer of the viral IL-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws. J. Immunol. 1623625-3632. [PubMed] [Google Scholar]
- 54.Wooten, R. M., and J. J. Weis. 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr. Opin. Microbiol. 4274-279. [DOI] [PubMed] [Google Scholar]
- 55.Yin, Z., J. Braun, L. Neure, P. Wu, U. Eggens, A. Krause, T. Kamradt, and J. Sieper. 1997. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 4069-79. [DOI] [PubMed] [Google Scholar]
- 56.Yoder, A., X. Wang, Y. Ma, M. T. Philipp, M. Heilbrun, J. H. Weis, C. J. Kirschning, R. M. Wooten, and J. J. Weis. 2003. Tripalmitoyl-s-glyceryl-cysteine-dependent OspA vaccination of toll-like receptor 2-deficient mice results in effective protection from Borrelia burgdorferi challenge. Infect. Immun. 713894-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]