Abstract
The obligate intracellular parasite Toxoplasma gondii infects warm-blooded animals throughout the world and is an opportunistic pathogen of humans. As it invades a host cell, Toxoplasma forms a novel organelle, the parasitophorous vacuole, in which it resides during its intracellular development. The parasite modifies the parasitophorous vacuole and its host cell with numerous proteins delivered from rhoptries and dense granules, which are secretory organelles unique to the phylum Apicomplexa. For the majority of these proteins, little is known other than their localization. Here we show that the dense granule protein GRA7 is phosphorylated but only in the presence of host cells. Within 10 min of invasion, GRA7 is present in strand-like structures in the host cytosol that contain rhoptry proteins. GRA7 strands also contain GRA1 and GRA3. Independently of its phosphorylation state, GRA7 associates with the rhoptry proteins ROP2 and ROP4 in infected host cells. This is the first report of interactions between proteins secreted from rhoptries and dense granules.
The single-celled eukaryote Toxoplasma gondii infects warm-blooded animals throughout the world. Although the complex life cycle of this obligate intracellular parasite includes sexual and asexual stages, Toxoplasma can propagate asexually indefinitely because sexual reproduction is not required for transmission. The parasite has the capacity to invade any nucleated cell of its host and employs an array of proteins to facilitate invasion and to alter host cell physiology. These proteins are secreted from micronemes, rhoptries, and dense granules, which are specialized secretory organelles unique to organisms of the phylum Apicomplexa.
Concomitantly with invasion, Toxoplasma forms the parasitophorous vacuole (PV), its intracellular residence, from host plasma membrane (36). The delimiting membrane of the PV contains only a select group of host plasma membrane proteins as a consequence of a poorly understood molecular-sieving process (8, 30). Numerous rhoptry proteins (ROPs) and dense granule proteins (GRAs), including ROP2, ROP4, ROP5, ROP7, ROP18, GRA3, GRA5, GRA7, and GRA8, localize to the PV membrane, the major interface between host and parasite (1, 3, 6, 7, 14-16, 19, 23, 26). These proteins presumably have an important role in host-parasite interactions, but their specific functions are generally unknown.
GRA7 is expressed by all infectious forms of Toxoplasma (18), and anti-GRA7 antibodies are generated during murine and human infection (23, 31). GRA7 appears to be capable of inducing the tubulation of artificial liposomes and has been implicated in nutrient acquisition by the parasite via a mechanism that involves sequestration of host endolysosomes (10). Mutant parasites lacking GRA7 (Δgra7) exhibit slow growth and pronounced morphological defects when cultured under nutrient-limiting conditions (10).
GRA7 has been detected on the surface of infected host cells (31). Within the infected host cell, GRA7 localizes to the PV lumen, the PV membrane, and strands extending from the PV membrane into the host cytosol (10, 19, 23). The function and composition of the GRA7-containing strands are unknown, and the trafficking events culminating in the incorporation of GRA7 have not been characterized. The strands have been observed as early as 10 min after host cell invasion (10). Similar strands that contain ROPs and extend from the PV membrane into the host cytosol have also been observed within 10 min of invasion (21). ROPs in these strands are secreted into the host cell in vesicle-like structures prior to, and even in the absence of, PV formation (7, 14-16, 33). Whether the same strands comprise ROPs and GRAs has not been reported.
The electrophoretic mobility of GRA7 depends on whether samples are prepared from extracellular parasites or infected host cells. Only one form of GRA7, which migrates as if it were 26 kDa, is detectable in parasite lysates (23, 31). Infected host cell lysates also contain a modified form of GRA7 that migrates as if it were approximately 32 kDa (31). The identity of this mobility-altering modification has not been reported previously. Given GRA7's intra- and extravacuolar localization, host or parasite factors could be responsible for the modification.
In this study, we show that phosphorylation produces the modified forms of GRA7 and that GRA7 can be secreted into the host cell prior to PV formation. During invasion, extravacuolar GRA7 assembles into punctate strands that contain ROPs and additional GRAs. Moreover, data presented here demonstrate that ROP2 and ROP4 stably associate with GRA7 in lysates produced from infected human foreskin fibroblasts (HFFs); this is the first example of an interaction between rhoptry and dense-granule proteins.
MATERIALS AND METHODS
Parasite and host cell culture.
Toxoplasma gondii tachyzoites of the RH strain and Δgra7 tachyzoites (10) were maintained by serial passage in HFF monolayers. For some experiments, parasites were grown in African green monkey kidney (Vero) cells. HFFs and Vero cells were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen, Carlsbad, CA), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Antibodies.
The following monoclonal antibodies were used: DG52 (anti-SAG1); Tg17-179 (anti-GRA2) and Tg17-43 (anti-GRA1), generous gifts from Marie-France Cesbron-Delauw; 2H11 (anti-GRA3), Tg49 (anti-ROP1), and T3 4A7 (anti-ROP2/4), generous gifts from Jean-Francois Dubremetz; and 12B6 (anti-GRA7), a generous gift from Peter Bradley. Rabbit antibodies recognizing human intracellular adhesion molecule 1 (ICAM-1) (Santa Cruz Biotechnology, Santa Cruz, CA) and sera from rabbits immunized with SAG1 or with GRA7 (see below) were also used.
To generate rabbit anti-GRA7 antibodies, a portion of GRA7 was PCR amplified from genomic DNA and TA-cloned in pCR2.1-TOPO (Invitrogen, Carlsbad, CA). Primers used were 5′-ccggatccGCCACCGCGTCGGATGACGAA-3′ and 5′-ttctgcagCTACTGGCGGGCATCCTCCCC-3′ (the genomic sequence is in uppercase letters, and lowercase letters indicate a sequence added for cloning purposes). After sequencing to confirm error-free amplification, cloned GRA7 was excised from the TOPO vector using BamHI and PstI and then ligated into pMAL-p2X (New England Biolabs, Ipswich, MA) in frame with and downstream region of malE, the gene that encodes Escherichia coli maltose binding protein (MBP). Diagnostic restriction digests were performed to confirm the ligation.
The E. coli expression strain BL21(DE3) (Stratagene, La Jolla, CA) was transformed with the MBP-GRA7 expression construct. Bacteria were grown to an A600 of 0.6, and expression was induced with 0.3 mM isopropyl β-d-1-thiogalactopyranoside for 1 hour. The MBP fusion was purified using amylose beads (New England Biolabs, Ipswich, MA) under nondenaturing conditions according to the manufacturer's instructions and eluted with 100 mM maltose and 0.1% sodium dodecyl sulfate (SDS). The purified protein was dialyzed against phosphate-buffered saline (PBS) and sent to Covance Research Products (Denver, PA) for immunization of rabbits and production of rabbit antibodies.
PNGase F assay.
Infected HFFs were collected 12 hours after parasite addition. Monolayers of infected cells were washed three times with cold PBS, collected in PBS, and centrifuged at 2,000 × g for 5 min at 4°C. The resulting pellet was flash frozen in a bath of dry ice and ethanol, thawed in hypotonic lysis buffer (10 mM Tris-HCl [pH 7.5], 5 mM sodium chloride), and incubated on ice for 20 min. The membrane fraction was collected by centrifugation at 10,000 × g for 10 min at 4°C, resuspended in 1× glycoprotein denaturing buffer (0.5% SDS, 40 mM dithiothreitol), boiled for 10 min at 90°C, and cooled at room temperature. The buffer composition was adjusted with the appropriate volumes of 10× stocks to give final concentrations of 1% NP-40 and 50 mM sodium phosphate, pH 7.5. Aliquots containing ∼3 × 106 parasites were incubated with 250, 50, or 0 units of peptide:N-glycosidase F (PNGase F) (New England Biolabs, Ipswich, MA) for 60 min at 37°C. Samples were prepared for SDS-polyacrylamide gel electrophoresis (SDS-PAGE) by addition of one sample volume of 2× reducing sample buffer (125 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 200 mM dithiothreitol, 0.01% bromophenol blue) prior to boiling for 5 min.
AP assay.
Alkaline phosphatase (AP) assays were performed on denatured lysates generated from infected HFFs 12 to 16 h after parasite addition. Monolayers of infected cells were washed thrice with cold PBS and once with cold phosphorylation buffer (50 mM HEPES, 100 mM sodium fluoride, 2 mM sodium orthovanadate, 2 mM EDTA, 1× Complete protease inhibitor cocktail [Roche Applied Science, Indianapolis, IN]) prior to collection in phosphorylation buffer and Dounce homogenization. The homogenate was boiled for 20 min at 95°C to inactivate endogenous phosphatases and then cooled on ice. The boiled homogenate was centrifuged at 10,000 × g for 10 min at 4°C to pellet membranes. The membrane pellet was washed once in PBS prior to solubilization in AP-compatible buffer (50 mM Tris-HCl [pH 8], 100 mM sodium chloride, 10 mM magnesium chloride, 1 mM dithiothreitol, 0.2% Triton X-100, 1× Complete protease inhibitor cocktail) for 1 hour on ice with periodic vortexing. The solubilized pellet was centrifuged at 10,000 × g for 10 min at 4°C to pellet the insoluble fraction. Samples of the resulting supernatant, each containing ∼3 × 106 parasite equivalents, were incubated with 20 units of calf intestinal AP (New England Biolabs, Ipswich, MA) or no AP for 10 or 30 min at 37°C. After collection, samples were flash frozen in a bath of dry ice and ethanol and then boiled in an equal volume of 2× reducing sample buffer for 5 min.
Western blot analysis.
Protein samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. Typically, membranes were blocked for 1 hour in PBS-0.1% Tween 20 (PBS-T) containing 5% (wt/vol) milk, incubated for 1 hour with primary antibodies, washed thrice with PBS-T, incubated for 1 hour with secondary antibodies, and washed thrice with PBS-T. Primary and secondary antibodies were diluted in PBS-T plus 5% (wt/vol) milk. Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse antibodies (Bio-Rad, Hercules, CA) were used as secondary antibodies. Horseradish peroxidase activity was visualized using the SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Parasite purification.
Gel filtration was used to purify parasites from lysed cultures (17). After 24 h of infection, monolayers were rinsed twice with cold invasion medium (Dulbecco's modified Eagle's medium supplemented only with 2 mM glutamine), collected in invasion medium, and mechanically lysed by six passages through a 27-gauge needle. Large debris was removed by centrifugation at 100 × g for 5 min at 10°C. The resulting supernatant was then centrifuged at 300 × g for 10 min at 10°C. The 300 × g pellet, which contains intact parasites and membranous material from infected HFFs, was resuspended in 2.5 ml of invasion medium and loaded onto a PD-10 desalting column (GE Healthcare, Piscataway, NJ) preequilibrated with 30 ml invasion medium. An additional 3.5 ml of invasion medium were added to the column, and the displaced buffer was collected as the elution fraction. The elution fraction is enriched for intact parasites, while secreted proteins and debris from the infected HFF lysate remain on the column. Invasion medium was used at 4°C, and the gel filtration was done in a 4°C cold room. To avoid clogs, no more than 2.5 × 107 infected HFF equivalents were added to each column.
Time course of GRA7 modification.
Parasites eluted from a PD-10 column were centrifuged at 300 × g for 10 min at 4°C and then resuspended at 5 × 107 parasites/ml in invasion medium chilled to 4°C. Six 35-mm tissue culture dishes containing Vero cell monolayers were chilled on ice. The medium covering each monolayer was removed and replaced with 200 μl of the purified parasites. To synchronize invasion, parasites were allowed to settle on the monolayers for 20 min on wet ice. Five samples were transferred to 37°C simultaneously to allow invasion, and a sample was collected after 15, 30, 60, 120, and 240 min. The sample kept on ice was collected as the 0-minute time point. Six additional 200-μl aliquots of purified parasites were taken through the time course in the absence of host cells. At the time of collection, samples were boiled for 5 min after addition of an approximately equal volume of 2× reducing sample buffer prewarmed to 90°C. The medium was not removed prior to sample buffer addition.
Immunofluorescence.
For the localization of GRA7 during the early stages of infection, HFF monolayers on glass coverslips were infected with parasites at a nominal multiplicity of infection of ≥20 for 30 min prior to fixation. For the colocalization of GRA7 with ROP2/4, HFF monolayers were pulsed with parasites at a nominal multiplicity of infection of ≥40 for 5 min prior to washes in PBS and then incubation in Dulbecco's modified Eagle's medium for 15 min. For the colocalization of ROP1 and GRA7, samples were fixed after synchronized invasion. To synchronize invasion, parasites were allowed to attach to HFF monolayers seeded on coverslips for 20 min at ∼1°C, a temperature that prevents invasion. The coverslips were then shifted to 37°C, an invasion-permissive temperature, for 15 min before fixation. Infected cells were washed thrice in PBS and fixed in PBS plus 2.5 to 4.0% (wt/vol) formaldehyde for 20 min. The formaldehyde fixative was quenched with PBS plus 100 mM glycine for 5 min, and then the coverslips were washed in PBS and blocked in PBS plus 3% bovine serum albumin (BSA) for a minimum of 30 min. Fixed cells were permeabilized in PBS supplemented with 3% BSA and 0.01% (vol/vol) saponin for 20 min. At this concentration, saponin permeabilizes membranes of the infected host cell (including the membrane of the PV) but does not permeabilize the parasite plasma membrane (34). Fixed cells were incubated for an hour with primary antibodies diluted in PBS-BSA-saponin. After three to five washes in PBS, the samples were incubated for 1 hour with secondary antibodies diluted in PBS plus 3% BSA. After three to five washes in PBS, samples were mounted in Vectashield (Vector Laboratories, Burlingame, CA) on microscope slides. For the costaining with GRA7 and GRA1 and for the strand formation assay with the Δgra7 parasites, invasion was synchronized using the temperature shift as described above, and cells were fixed with formaldehyde after 6 min of invasion. Samples were processed as described above with the exceptions that fixed cells were permeabilized with 0.2% (vol/vol) Triton X-100 and that 0.01% (vol/vol) Triton X-100 was included in the incubations with antibodies. For the 24-hour time point, monolayers seeded on glass coverslips were infected with parasites for 24 h before fixation. Fixed cells were processed as described above with the exceptions that for GRA7 and GRA3 costaining, permeabilization was done in acetone for 5 min at −20°C prior to blocking and the primary antibody dilutions did not contain saponin. For the GRA7 and GRA3 costaining at 15 min postinvasion, invasion was synchronized using the Endo buffer method (24). Infected monolayers were fixed after 15 min in invasion-permissive buffer and processed as described for the 24-hour time point.
To assess GRA7 delivery into host cells by extracellular parasites, HFF monolayers were challenged with 108 parasites/ml that had been pretreated with 1 μM cytochalasin D (CytD) for 10 min at room temperature. Following challenge for 6 min at 37°C in the presence of 1 μM CytD, the monolayers were rinsed in PBS prior to fixation. Infected monolayers were fixed in 4% (wt/vol) formaldehyde in PBS for 20 min at room temperature and blocked by incubation for 2 h in PBS plus 3% BSA. After staining with anti-SAG1 antibody, monolayers were permeabilized using PBS containing 3% BSA and 0.2% (vol/vol) Triton X-100 and then incubated with anti-GRA7 antibodies. For ROP1 and GRA7 costaining, monolayers were permeabilized prior to antibody addition. Incubation with secondary antibodies and subsequent processing were as described above.
Phase and fluorescence images were captured on a Hamamatsu Orca100 charge-coupled device camera coupled to an Olympus BX60 microscope and were processed using Image-Pro Plus 2.0 (MediaCybernetics, Silver Spring, MD) and Photoshop 9.0.2 and Photoshop CS3 (Adobe Systems, San Jose, CA). Image processing was limited to cropping, pseudo-coloring, merging of individual color channels into single images, and adjusting of tonal ranges to reproduce observations faithfully.
IP.
Lysates used in the immunoprecipitations (IPs) were generated from HFFs infected with Δgra7 or wild-type parasites. For GRA7 IPs, polyclonal antibodies from rabbits immunized with the GRA7-MBP fusion were used. For ROP2/4 IPs, monoclonal antibody T3 4A7 was used. Antibodies were directly coupled to protein A-Sepharose FF (GE Healthcare, Piscataway, NJ) using dimethylpimelimidate as described previously (22).
After 4 to 5 h of infection with parasites at a nominal multiplicity of infection of 12 to 17, confluent monolayers seeded on 150-mm dishes were washed 10 times with 10 ml chilled Tris wash buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl) and collected/solubilized in 1 ml radioimmunoprecipitation assay buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1% [vol/vol] NP-40, 0.5% [wt/vol] sodium deoxycholate, 0.02% [wt/vol] SDS) supplemented with Complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and HALT phosphatase inhibitor cocktail (Pierce, Rockford, IL). After centrifugation at 10,000 × g for 20 min to pellet insoluble material, the cleared lysate was incubated with bead-coupled antibodies for 2 hours at 4°C. Beads were washed twice with 5 column volumes of RIPA buffer and three or four times with 5 column volumes of IP wash buffer II (50 mM Tris-HCl [pH 7.4], 300 mM NaCl, 5 mM EDTA, 0.1% [vol/vol] NP-40, 0.02% sodium azide). Prior to elution, beads were washed once with 10 column volumes of 10 mM sodium phosphate, pH 8. Bound proteins were eluted with high-pH buffer (100 mM triethylamine, pH 11.7) in 100-μl fractions. To neutralize elution fractions, 5 μl of 1 M sodium phosphate (pH 6.8) was added. Samples were boiled in an equal volume of 2× reducing sample buffer in preparation for SDS-PAGE.
RESULTS
GRA7 is phosphorylated.
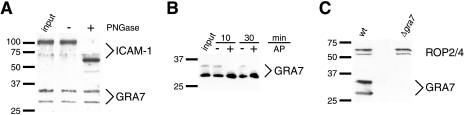
An additional form of GRA7 was detected in lysates produced from infected HFFs (Fig. 1). In contrast to the form of GRA7 associated with extracellular parasites, which migrates as if it were 26 kDa, this modified GRA7 migrates as if it were 32 kDa. Given the large mobility shift resulting from the modification and the observation that GRA7 has a potential N-linked glycosylation site (19), glycosylation of secreted GRA7, possibly by the host cell, was investigated. Lysates from infected HFFs were treated with PNGase F prior to Western blot analysis. PNGase F removes N-linked glycans from glycoproteins and thus reduces the molecular weight of a glycoprotein. PNGase F treatment decreased the size of the positive control ICAM-1 but had no effect on the 32-kDa GRA7 band (Fig. 1A). Thus, the forms of GRA7 with decreased mobility do not appear to be a result of N-linked glycosylation.
FIG. 1.
GRA7 is phosphorylated. (A and B) Infected HFF lysates were incubated with PNGase F (A) for 1 hour or with AP (B) for the indicated times to determine if N-linked glycosylation or phosphorylation, respectively, produces the modified forms of GRA7. Effects of enzymatic treatment on electrophoretic mobility were assessed by Western blotting with rabbit anti-GRA7 and anti-ICAM-1 antibodies. ICAM-1 is a positive control for glycosidase activity. (C) To assess the specificity of the rabbit anti-GRA7 antibody, lysates of cells infected with either the wild type (wt) or a gra7 deletion mutant (Δgra7) were subjected to Western blot analysis. As a loading control, the blot was probed with anti-ROP2/4 antibodies. Bars to the left of each blot indicate positions of standards, and numbers indicate the molecular masses (kDa).
Phosphorylation can also alter the electrophoretic mobility of a protein. The PV-localized proteins ROP2, ROP4, and GRA6 are phosphorylated in host cells, and phosphorylation decreases the mobility of GRA6 (7, 25). To determine whether the mobility of GRA7 decreases due to phosphorylation, infected HFF lysates were treated with AP prior to Western blot analysis. After a 10-minute incubation with AP, the 32-kDa GRA7 band was no longer detectable, and the 26-kDa band displayed an increase in intensity in addition to the appearance of a fuzzy signal extending from the 26-kDa band to approximately 28 kDa (Fig. 1B). After 30 minutes, the fuzzy signal, exhibited a decrease in intensity with respect to the 10-minute time point (Fig. 1B). This fuzzy signal is most likely indicative of an assortment of incompletely dephosphorylated GRA7 species but might indicate an additional GRA7 modification. In the absence of AP, no change in the relative abundance of the 32 kDa GRA7 band was observed (Fig. 1B). No bands similar in size to modified GRA7 were detected by the rabbit anti-GRA7 antibody in Western blots of lysates generated from cells infected with Δgra7 parasites (Fig. 1C). These results demonstrate that phosphorylation produces forms of GRA7 exhibiting decreased electrophoretic mobility.
GRA7 phosphorylation requires the host cell.
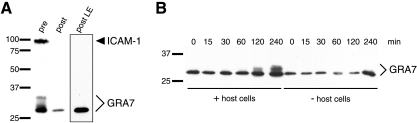
To determine the context and timing of GRA7 phosphorylation, an invasion time course was performed using purified parasites. Suspensions of intact parasites and host cell debris, generated by mechanical lysis of infected HFF monolayers, were applied to gel filtration columns. The parasite suspensions added to the columns contain the host plasma membrane protein ICAM-1 and a ladder of GRA7 bands extending from 26 kDa to 32 kDa (Fig. 2A). Neither ICAM-1 nor any modified forms of GRA7 were detected in the column eluate, which contains intact parasites (Fig. 2A).
FIG. 2.
GRA7 phosphorylation requires the host cell. Infected HFF monolayers were collected as cell suspensions, mechanically lysed, and passed over a gel filtration column to purify parasites from infected host cell debris. (A) The presence of GRA7 and of ICAM-1 in samples taken before purification and after purification was assessed by Western blot analysis. (B) After purification, parasites were incubated with or without host cells for up to 4 hours. GRA7 modification was assessed by Western blot analysis. pre, sample taken prior to gel filtration. post, sample taken after gel filtration. post LE, longer exposure of sample taken after gel filtration. Bars to the left of each blot indicate positions of standards, and numbers indicate the relative masses (kDa).
Column-purified parasites were incubated with Vero cell monolayers at ∼0°C to allow attachment but not invasion and then shifted to 37°C for up to 4 hours to facilitate synchronized invasion. GRA7 modification was assessed by Western blot analysis. Sixty minutes after the shift to 37°C, only the nonphosphorylated form of GRA7 was detected (Fig. 2B). After 2 hours in the presence of host cells, GRA7 bands with decreased mobility were visible, and after 4 hours, an additional GRA7 band migrating even more slowly was observed (Fig. 2B). The GRA7 band migrating most slowly after 4 hours appears to be the fully phosphorylated form; however, given gel-to-gel variation in migration, it remains possible that this band is not the 32-kDa band and that additional phosphorylation will occur. A mock infection time course, in which parasites were incubated in medium only, was performed in parallel. Because dense granule secretion occurs constitutively (9), samples of extracellular parasites were processed without removing the medium in order to include internal and secreted GRA7. In the absence of host cells, no modified forms of GRA7 were detected at any time point (Fig. 2B).
GRA7 associates with ROP-containing structures inside the host cell.
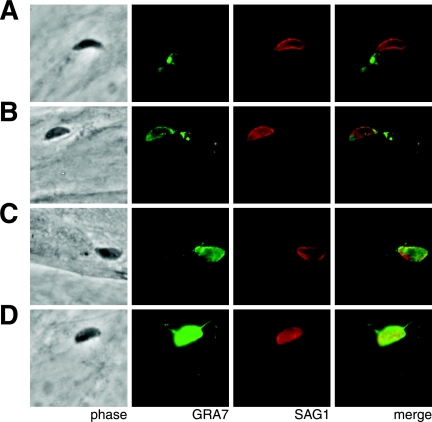
In an attempt to delineate the early stages of GRA7 trafficking, indirect immunofluorescence assays (IFAs) were performed on invading and recently invaded parasites. Parasites were added to host cell monolayers, fixed after 30 min of asynchronous invasion, and stained with anti-GRA7 antibodies and antibodies that recognize surface antigen 1 (SAG1), the major protein of the parasite surface. Infected cells were permeabilized with 0.01% saponin to permeabilize the host plasma membrane and the PV membrane but not the parasite plasma membrane (35). Under these conditions GRA7 is accessible to antibodies only after it is secreted into the host cell; consequently, anti-GRA7 antibodies do not stain extracellular parasites, while anti-SAG1 antibodies stain both extra- and intracellular parasites.
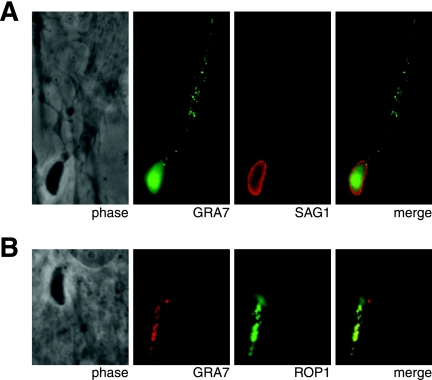
Unexpectedly, a bolus of GRA7 signal was observed at a low frequency inside host cells immediately adjacent to a parasite that is extracellular based on the lack of GRA7 signal around it (Fig. 3A). The parasite's elongated shape indicates that it is likely to be in the process of invading the host cell. After invasion, GRA7 staining was detected in the nascent PV, which is only slightly larger than the parasite, and in the host cytosol (Fig. 3B to D). Two patterns of cytosolic staining were observed: a cluster of vesicle-like structures distinct from the PV (Fig. 3B) and the previously detected punctate strands extending into the host cytosol from the PV membrane (Fig. 3C) (10, 23). Some vacuoles exhibited multiple, sometimes branching strands (Fig. 3D).
FIG. 3.
GRA7 is secreted directly into host cells. After 30 min of asynchronous invasion, infected HFF monolayers were fixed, permeabilized with 0.01% saponin, and stained for GRA7 and the Toxoplasma surface protein SAG1.
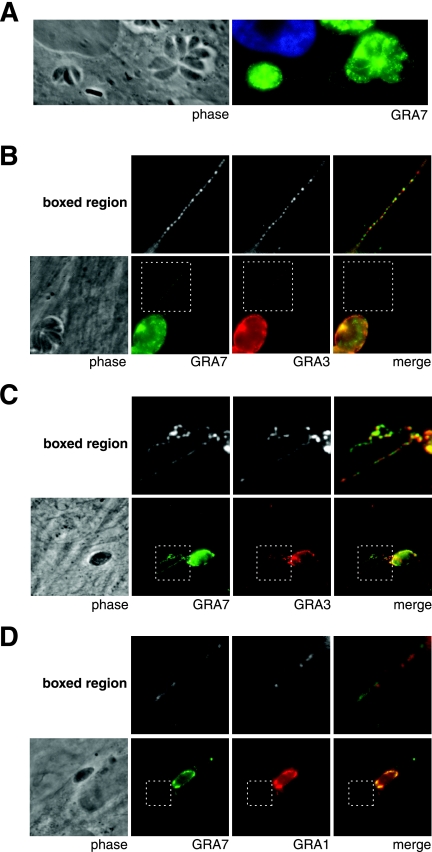
At 24 h postinvasion, GRA7 strands were observed forming intracellular connections between vacuoles (Fig. 4A). GRA3 has also been localized to strand-like structures that form intracellular connections between vacuoles (12). To determine if individual strands contain both GRA3 and GRA7, infected HFFs were fixed, permeabilized with acetone, and stained with anti-GRA7 and anti-GRA3 antibodies. GRA7 and GRA3 are present in the same strands 24 h after parasite addition and tend to be in the same punctae (Fig. 4B). Strands containing both GRA3 and GRA7 were detected as early as 15 min postinvasion (Fig. 4C).
FIG. 4.
GRA7 strands connect vacuoles and contain additional GRAs. (A and B) At 24 h postinvasion, infected HFFs were fixed, permeabilized with 0.2% Triton X-100, and stained with anti-GRA7 antibodies and Hoechst stain (A) or were permeabilized with acetone and stained for GRA3 and GRA7 (B). (C) At 15 min postinvasion, infected HFFs were fixed, permeabilized with 0.01% saponin, and stained for GRA7 and GRA3. (D) At 6 min postinvasion, infected HFFs were fixed, permeabilized with 0.2% Triton X-100 and stained for GRA7 and GRA1.
Immediately after invasion, an additional GRA, GRA1, was detected in punctae arranged into strands. Parasites were allowed to settle onto HFF monolayers at 4°C, transferred to 37°C to permit invasion, fixed in formaldehyde, and permeabilized with Triton X-100 prior to antibody staining. Faint strands containing GRA1 punctae and GRA7 punctae were observed 6 minutes after invasion (Fig. 4D). The GRA1-containing strands were not readily detectable beyond 30 minutes postinvasion.
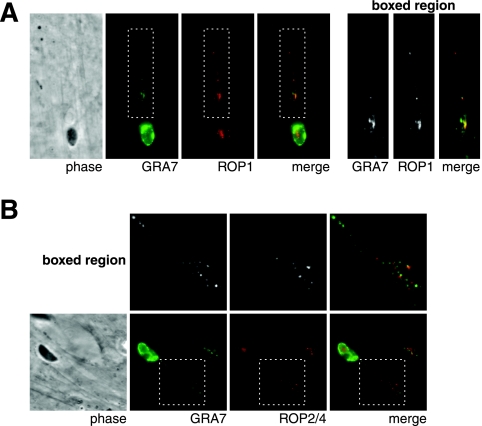
The punctate strands of GRA7 present during invasion are reminiscent of the strand-like structures containing ROPs that are secreted into the host cytosol during Toxoplasma invasion (21). To determine the relationship between the ROP-containing strands and the GRA7 strands, infected monolayers were fixed 15 minutes after parasite addition, permeabilized with 0.01% saponin, and costained with anti-GRA7 antibodies and with antibodies that recognize ROPs previously reported to be present in rhoptry-derived structures (21). As shown in Fig. 5A, the patterns produced by staining with anti-GRA7 and anti-ROP1 are strikingly similar but not identical. GRA7-staining punctae are present in the same strands as ROP1-containing punctae, and a portion of the punctae stain with both antibodies. Similarly, in the patterns produced from staining with monoclonal T3 4A7 (anti-ROP2/4) and anti-GRA7, GRA7 and ROP2/4 are present in the same strands and some of the same punctae (Fig. 5B).
FIG. 5.
Secreted GRA7 partially colocalizes with ROP-containing structures. After 15 min of invasion, infected HFF monolayers were fixed, permeabilized with 0.01% saponin, and stained for GRA7 and either ROP1 (A) or ROP2/4 (B).
To confirm that GRA7 is secreted into host cells by extracellular Toxoplasma, IFAs were performed on parasites added to host cells in the presence of CytD, a mycotoxin that disrupts actin filaments and blocks parasite entry (11, 32). CytD-treated parasites attach to host cells and deliver ROPs into the host cell either directly into the cytosol (20) or in vesicle-like structures referred to as evacuoles (21). These evacuoles are of unknown structure and, although they appear to contain membranous material, there is no evidence that they are membrane delimited. For this study, parasites were treated with 1 μM CytD, added to host cells in the presence of CytD, incubated with anti-SAG1 antibodies, permeabilized, and finally stained with anti-GRA7 antibodies. Incubation with anti-SAG1 antibodies prior to permeabilization enables selective staining of extracellular parasites. Punctate, strand-like structures containing GRA7 were indeed observed in host cells adjacent to the apical end of parasites that are attached to but, apparently largely if not entirely, still outside of the host cell (Fig. 6A). To determine the relationship between GRA7 structures and ROP-containing structures secreted by CytD-arrested parasites, cells were costained with anti-ROP1 and anti-GRA7 antibodies. The GRA7 signal exhibits extensive colocalization with the ROP1-positive punctae in the host cell (Fig. 6B). The parasites imaged in Fig. 6A, but not those in Fig. 6B, express cytosolic green fluorescent protein.
FIG. 6.
GRA7 is secreted into host cells by invasion-inhibited parasites. Parasites were incubated with 1 μM CytD for 10 min prior to incubation with host cells for 6 min in the presence of 1 μM CytD. Cells were fixed in formaldehyde and either incubated with anti-SAG1 antibodies prior to permeabilization with 0.2% Triton X-100 and staining for GRA7 (A) or permeabilized prior to staining for ROP1 and GRA7 (B). Note that parasites expressing cytosolic green fluorescent protein were used for panel A.
GRA7 is not required for strand formation.
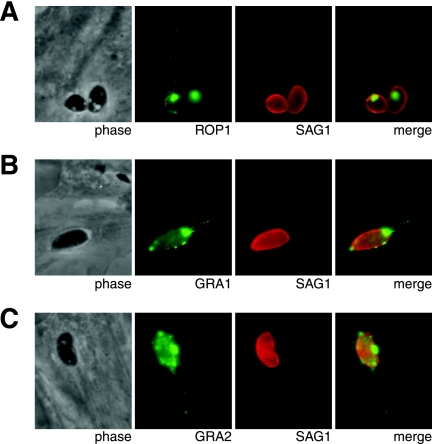
Based on the ability of GRA7 to induce tubule formation when added to artificial liposomes (10) and the fact that the rhoptry-derived strands with which GRA7 associates appear to be membranous (21), it seemed possible that GRA7 might be involved in the formation of these strands. The ROP-containing strands have been proposed to play a role in the delivery of ROPs to the PV (21); thus, if GRA7 is needed for strand formation, then its absence might affect final ROP localization. To test these possibilities, Δgra7 parasites, which lack GRA7 due to a targeted deletion (10), were used in IFAs. The results showed that within 6 min of invasion with the Δgra7 parasites, ROP1 is present within the PV and on strands indistinguishable from those seen with wild-type parasites (Fig. 7A and data not shown). Typical GRA1-staining strands were detected (Fig. 7B), and GRA2-staining strands were also observed (Fig. 7C). Thus, GRA7 does not appear to be necessary for strand formation or trafficking of ROPs to the PV.
FIG. 7.
GRA7 is not necessary for formation of strands containing ROPs or GRAs. After 6 min of invasion with Δgra7 parasites, infected HFFs were fixed with formaldehyde, permeabilized with 0.2% Triton X-100, and stained for SAG1 and ROP2 (A), GRA1 (B), or GRA2 (C).
GRA7 interacts with ROPs.
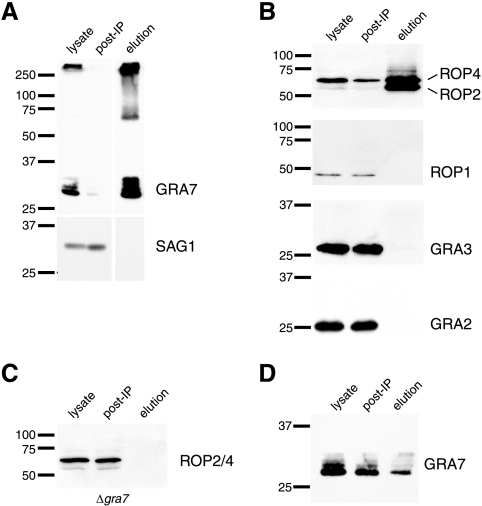
The association of some GRA7-staining punctae with rhoptry-derived structures during invasion and the localization of GRA7 and ROPs in the PV membrane suggest that GRA7 might interact with ROPs for function and/or trafficking after secretion into the host cell. Interactions between Toxoplasma proteins secreted from distinct organelles are not unprecedented: complexes comprising rhoptry and microneme proteins facilitate the invasion of host cells (2). To determine whether ROPs form complexes with GRA7, GRA7 was immunoprecipitated from infected HFF lysates generated after 4.5 h of infection. Under the conditions employed, GRA7 was severely depleted from the infected HFF lysate whereas SAG1 was not depleted and was not present in the elution fraction (Fig. 8A). In addition to the expected ladder of phosphorylated and nonphosphorylated GRA7 bands in the 26- to 32-kDa range, the elution fraction also contained a GRA7 band migrating as if it were ∼67 kDa and a smear extending from 67 kDa to the top of the lane (Fig. 8A). The IP fractions were probed for ROP1 and ROP2/4, ROPs present in the rhoptry-derived, strand-like structures (7, 21). ROP2 and ROP4 were detected in the fraction of proteins that coprecipitate with GRA7, while ROP1 was not (Fig. 8B). Interestingly, the ratio of signal intensities of ROP2 to ROP4 is increased in the precipitate relative to the starting lysate, which suggests enrichment for ROP2. The lack of ROP1 in the coprecipitating fraction indicates a specific direct or indirect interaction between GRA7 and ROP2/ROP4; the entire rhoptry-derived structures do not precipitate with GRA7. GRA2 and GRA3, which localize to some of the same PV subcompartments as GRA7 (12, 29), are not detectable in the precipitated fraction (Fig. 8B). To control for direct IP of ROP2 and ROP4 by the anti-GRA7 antibody, IPs were also performed on lysates generated from host cells infected with Δgra7 parasites. In the absence of GRA7, ROP2 and ROP4 were not detected in the coprecipitating fraction (Fig. 8C).
FIG. 8.
ROP2 and ROP4 associate with GRA7. Lysates generated from infected HFFs 4.5 h after parasite addition were subjected to IP with either rabbit anti-GRA7 (A to C) or murine anti-ROP2/4 (D). Fractions were probed for the proteins indicated to the right of each blot. With the exception of rabbit anti-SAG1, blots were probed with murine antibodies. For each blot, the elution fraction lane was loaded with 10 times the cellular equivalents loaded in the other lanes. post-IP, sample of infected HFF lysates collected after IP. Δgra7, IP performed on lysates generated from cells infected with the GRA7 deletion mutant. Bars to the left of each blot indicate positions of standards, and numbers indicate the molecular masses (kDa).
To confirm the specific association of GRA7 with ROP2/4, IPs were performed using monoclonal antibody T3 4A7, which recognizes ROP2/4, and the precipitating fraction was probed for GRA7. A ladder of GRA7 bands, including phosphorylated and nonphosphorylated forms, coprecipitated with ROP2/4 (Fig. 8D).
DISCUSSION
The role of secreted kinases, phosphatases, and phosphoproteins in Toxoplasma-host cell interactions has only recently been appreciated. ROP2 is the founding member of a family of at least 12 ROPs that contain protein-kinase-like domains (13). While some members of this family, such as ROP2, are predicted to have catalytically dead kinase domains, other members, such as ROP18, still possess kinase activity. ROP18, which is present in the punctate strands and the PV membrane, appears to be a major virulence determinant (14, 33, 37). Moreover, the parasite traffics a phosphatase and a kinase from the rhoptries to the host nucleus (20, 34).
Based on the data presented in this study, GRA7 can be added to the small but growing list of Toxoplasma proteins that are phosphorylated in the host cell. This list includes GRA6 in addition to ROP2 and ROP4 (7, 25). Currently, the processes regulated by phosphorylation of these proteins are unknown. Phosphorylation as a means to regulate membrane association of GRAs has been proposed (25, 28). For GRA7, however, association with membranes appears to be independent of its phosphorylation state: GRA7 secreted from extracellular parasites is not phosphorylated and associates with artificial liposomes (10), while a membranous fraction isolated from infected host cells preferentially contains phosphorylated forms of GRA7 (31). Phosphorylation does not appear to affect the association of GRA7 with ROP2 or ROP4, because phosphorylated and nonphosphorylated forms of GRA7 coprecipitate with ROP2/4 (Fig. 8D). Nevertheless, phosphorylation might regulate the association of GRA7 with other proteins.
The data in Fig. 2, in conjunction with data from Neudeck and colleagues (31), demonstrate that GRA7 phosphorylation requires the host cell, but they cannot determine whether a host or a Toxoplasma kinase is responsible. GRA7 might be phosphorylated by a parasite kinase immediately before or during secretion. Because GRA7 is not phosphorylated by extracellular Toxoplasma (Fig. 2), phosphorylation prior to delivery into the host cell posits that the parasite senses the host cell and modifies GRA7 accordingly. If GRA7 is phosphorylated after it is secreted into the host cell, then it could be the substrate of either a host or a parasite kinase because GRA7 is present throughout the infected host cell. NetPhos, a neural network-based method for predicting phosphorylation sites, predicts that GRA7 has 14 potential phosphorylation sites for mammalian kinases (http://www.cbs.dtu.dk/services/NetPhos/) (4). As yet, however, the predictive value of these and other algorithms does not allow the prediction of which, if any, host kinase might be responsible.
Inside the PV, GRA7 presumably encounters only parasite proteins, whereas outside the PV, it would be exposed to host kinases in addition to Toxoplasma kinases present in the host cytosol (14, 33, 34, 37). Due to extensive polymorphism and, in at least one instance, a major difference in the relevant promoter, the repertoire of secreted protein kinases differs between Toxoplasma strains (33, 37). This raises the possibility that if one of these kinases were involved in GRA7 phosphorylation, then strain-specific differences might be observed. This possibility was investigated and, at least as concerns the canonical type I, II, and III strains, no differences in the mobility shift for GRA7 were seen (data not shown), which indicates that the results reported here are not strain specific. Thus, the kinase responsible for phosphorylating GRA7 is unlikely to be a parasite kinase that varies in activity or expression among strains.
During invasion, some ROPs are secreted into the host cell prior to PV formation and then assemble in punctate strands extending to/from the nascent PV (7, 14-16, 21, 33). These strands also contain GRA7 punctae, a fraction of which overlap with the rhoptry punctae (Fig. 5). The trafficking events preceding incorporation of GRA7 into these strands is not clear. One possibility is that GRA7 initially secreted into the PV becomes part of extrusions extending from the PV membrane into the host cytosol. These extrusions then associate with ROP-containing structures already present in the cytosol. GRA7 can also be introduced into the host cell prior to PV formation (Fig. 3A and 6). These data lead to an alternative, non-mutually-exclusive model in which punctate strands assemble from GRA7 and ROPs first secreted into the host cytosol.
How GRA7 enters the host cell from parasites that are blocked from invasion using CytD is a mystery. Dense granules have previously been observed to secrete their contents only at the parasite surface in the subapical region that is posterior to the apical tip (12). In the experiments using CytD presented here, no portion of the parasite body was detected inside the host cell, but insertion of a very small region of the invading parasite cannot be excluded. Absent this, a completely novel mechanism for introduction of GRA proteins into the host cell must be invoked. The nature of such a mechanism awaits further study.
The ROP-containing strands have been proposed to function in delivery of ROPs to the PV membrane (21). GRA7 in these strands might also traffic to the PV membrane; however, given the constitutive release of GRAs into the PV (9, 27), multiple routes to the PV membrane seem likely. Moreover, the continued presence of the GRA7 strands at 24 h into infection suggests functions other than delivery to the vacuole (Fig. 4A and B). The strands might be involved in trafficking of GRA7 to the host surface or in the nutrient acquisition functions of GRA7 (10, 31).
Importantly, the relationship between the early ROP- and GRA7-containing strands and the strands observed at 24 h is not known. The GRA7 strands that persist might be distinct from the early strands that also contain ROPs. Alternatively, ROPs initially present in the same strands as GRA7 might traffic to the PV while GRA7 remains as part of a scaffold.
The underlying structure of these ROP- and GRA7-containing strands awaits determination. The original report on the ROP-containing strands indicated that they organize around microtubules and that the rhoptry punctae may or may not be membrane delimited (21). In contrast to the paucity of punctae that contain both GRA7 and ROP1 or ROP2/4 (Fig. 5), the ROPs found to be in the strands show near perfect colocalization with one another (7, 14-16, 33), which is consistent with secretion of preassembled rhoptry structures. Braun and colleagues recently found that GRA7 forms large (>1-MDa) complexes with other GRAs inside dense granules and hypothesized that complex formation facilitates secretion of transmembrane domain-containing proteins (5). The GRA7 punctae might be a secreted form of these complexes. A corollary of this hypothesis is that GRAs present in the oligomers with GRA7 are potentially in the GRA7/ROP strands as well. Indeed, costaining of individual punctae with GRA7 and GRA3 was observed (Fig. 4), and punctae containing only GRA7 or GRA3 could result from rearrangement or sorting of the GRA complexes upon secretion into the host cell.
Interestingly, despite their colocalization in punctae that might be GRA protein complexes, GRA3 does not coprecipitate with GRA7 under the conditions used in this study, and neither does GRA2 (Fig. 8B). These data are in contrast to the results of Braun and colleagues, who observed GRA3 and GRA7 coprecipitating with a tagged version of GRA2 (5). If the GRA complexes are sensitive to detergent, then this apparent conflict might result from differences in detergent concentrations in the IP buffers (1% NP-40, 0.5% sodium deoxycholate, and 0.02% SDS used in this study versus 0.5% NP-40 used by Braun et al.).
The coprecipitation of ROP2 and ROP4 with GRA7 (Fig. 8) provides the first evidence for interaction between dense granules and ROPs. The limited overlap of punctae containing ROP2, ROP4, and GRA7 (Fig. 5B) suggests that the strands are not the major location of GRA7-ROP2/4 association. The majority of ROP2, ROP4, and GRA7 localize to the PV membrane; thus, their association seems more likely to occur there.
The data presented here cannot distinguish between a single complex containing ROP2, ROP4, and GRA7 and separate GRA7-ROP2 and GRA7-ROP4 complexes. Carey and colleagues did not observe coprecipitation of ROP2 with ROP4 (7); these data suggest that GRA7 associates with each protein separately. ROP2 and ROP4 are closely related (13) and are likely to possess the same domain required for direct or indirect association with GRA7. Carey and colleagues observed three unidentified phosphorylated bands coprecipitating with ROP2 and ROP4 (7); based on their electrophoretic mobility, these bands could be the phosphorylated forms of GRA7. The presence of additional proteins in the complex, however, cannot be ruled out.
Intriguingly, ROP2 and ROP4 are also phosphorylated (7). Their association with GRA7 does not require GRA7 phosphorylation (Fig. 8D), but GRA7 phosphorylation might depend on association with ROP2 or ROP4. Complex formation might be important for kinase recruitment and/or proper orientation of the residues to be phosphorylated. Regardless of the function of this complex, the association of specific rhoptry and GRAs represents a new example of collaboration between otherwise distinct secretory organelles in Toxoplasma.
Acknowledgments
We are grateful to Jon P. Boyle, Jessica Tyler, and Linh Pham for critical reading of the manuscript; to Jean-Francois Dubremetz, Peter Bradley, and Marie-France Cesbron Delauw for provision of antibodies; and to members of the Boothroyd lab for helpful discussions.
This work was supported by the National Institutes of Health (grant AI21423), the Hughes Medical Institute (a predoctoral fellowship to J.D.D.), and Stanford University (a Stanford Graduate Fellowship to S.R.).
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Achbarou, A., O. Mercereau-Puijalon, A. Sadak, B. Fortier, M. A. Leriche, D. Camus, and J. F. Dubremetz. 1991. Differential targeting of dense granule proteins in the parasitophorous vacuole of Toxoplasma gondii. Parasitology 103321-329. [DOI] [PubMed] [Google Scholar]
- 2.Alexander, D. L., J. Mital, G. E. Ward, P. Bradley, and J. C. Boothroyd. 2005. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 1e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckers, C. J., J. F. Dubremetz, O. Mercereau-Puijalon, and K. A. Joiner. 1994. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J. Cell Biol. 127947-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blom, N., S. Gammeltoft, and S. Brunak. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 2941351-1362. [DOI] [PubMed] [Google Scholar]
- 5.Braun, L., L. Travier, S. Kieffer, K. Musset, J. Garin, C. Mercier, and M. F. Cesbron-Delauw. 2008. Purification of Toxoplasma dense granule proteins reveals that they are in complexes throughout the secretory pathway. Mol. Biochem. Parasitol. 15713-21. [DOI] [PubMed] [Google Scholar]
- 6.Carey, K. L., C. G. Donahue, and G. E. Ward. 2000. Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol. Biochem. Parasitol. 10525-37. [DOI] [PubMed] [Google Scholar]
- 7.Carey, K. L., A. M. Jongco, K. Kim, and G. E. Ward. 2004. The Toxoplasma gondii rhoptry protein ROP4 is secreted into the parasitophorous vacuole and becomes phosphorylated in infected cells. Eukaryot. Cell 31320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charron, A. J., and L. D. Sibley. 2004. Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic 5855-867. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi, S., H. Qi, D. Coleman, A. Rodriguez, P. I. Hanson, B. Striepen, D. S. Roos, and K. A. Joiner. 1999. Constitutive calcium-independent release of Toxoplasma gondii dense granules occurs through the NSF/SNAP/SNARE/Rab machinery. J. Biol. Chem. 2742424-2431. [DOI] [PubMed] [Google Scholar]
- 10.Coppens, I., J. D. Dunn, J. D. Romano, M. Pypaert, H. Zhang, J. C. Boothroyd, and K. A. Joiner. 2006. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell 125261-274. [DOI] [PubMed] [Google Scholar]
- 11.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84933-939. [DOI] [PubMed] [Google Scholar]
- 12.Dubremetz, J. F., A. Achbarou, D. Bermudes, and K. A. Joiner. 1993. Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host-cell interaction. Parasitol. Res. 79402-408. [DOI] [PubMed] [Google Scholar]
- 13.El Hajj, H., E. Demey, J. Poncet, M. Lebrun, B. Wu, N. Galeotti, M. N. Fourmaux, O. Mercereau-Puijalon, H. Vial, G. Labesse, and J. F. Dubremetz. 2006. The ROP2 family of Toxoplasma gondii rhoptry proteins: proteomic and genomic characterization and molecular modeling. Proteomics 65773-5784. [DOI] [PubMed] [Google Scholar]
- 14.El Hajj, H., M. Lebrun, S. T. Arold, H. Vial, G. Labesse, and J. F. Dubremetz. 2007. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 3e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Hajj, H., M. Lebrun, M. N. Fourmaux, H. Vial, and J. F. Dubremetz. 2006. Characterization, biosynthesis and fate of ROP7, a ROP2 related rhoptry protein of Toxoplasma gondii. Mol. Biochem. Parasitol. 14698-100. [DOI] [PubMed] [Google Scholar]
- 16.El Hajj, H., M. Lebrun, M. N. Fourmaux, H. Vial, and J. F. Dubremetz. 2007. Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane. Cell. Microbiol. 954-64. [DOI] [PubMed] [Google Scholar]
- 17.Elsheikha, H. M., B. M. Rosenthal, A. J. Murphy, D. B. Dunams, D. A. Neelis, and L. S. Mansfield. 2006. Generally applicable methods to purify intracellular coccidia from cell cultures and to quantify purification efficacy using quantitative PCR. Vet. Parasitol. 135223-234. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson, D. J., D. Jacobs, E. Saman, J. F. Dubremetz, and S. E. Wright. 1999. In vivo expression and distribution of dense granule protein 7 (GRA7) in the exoenteric (tachyzoite, bradyzoite) and enteric (coccidian) forms of Toxoplasma gondii. Parasitology 119259-265. [DOI] [PubMed] [Google Scholar]
- 19.Fischer, H. G., S. Stachelhaus, M. Sahm, H. E. Meyer, and G. Reichmann. 1998. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol. Biochem. Parasitol. 91251-262. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert, L. A., S. Ravindran, J. M. Turetzky, J. C. Boothroyd, and P. J. Bradley. 2007. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot. Cell 673-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakansson, S., A. J. Charron, and L. D. Sibley. 2001. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 203132-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 23.Jacobs, D., J. F. Dubremetz, A. Loyens, F. Bosman, and E. Saman. 1998. Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol. Biochem. Parasitol. 91237-249. [DOI] [PubMed] [Google Scholar]
- 24.Kafsack, B. F., C. Beckers, and V. B. Carruthers. 2004. Synchronous invasion of host cells by Toxoplasma gondii. Mol. Biochem. Parasitol. 136309-311. [DOI] [PubMed] [Google Scholar]
- 25.Labruyere, E., M. Lingnau, C. Mercier, and L. D. Sibley. 1999. Differential membrane targeting of the secretory proteins GRA4 and GRA6 within the parasitophorous vacuole formed by Toxoplasma gondii. Mol. Biochem. Parasitol. 102311-324. [DOI] [PubMed] [Google Scholar]
- 26.Lecordier, L., C. Mercier, G. Torpier, B. Tourvieille, F. Darcy, J. L. Liu, P. Maes, A. Tartar, A. Capron, and M. F. Cesbron-Delauw. 1993. Molecular structure of a Toxoplasma gondii dense granule antigen (GRA 5) associated with the parasitophorous vacuole membrane. Mol. Biochem. Parasitol. 59143-153. [DOI] [PubMed] [Google Scholar]
- 27.Liendo, A., T. T. Stedman, H. M. Ngo, S. Chaturvedi, H. C. Hoppe, and K. A. Joiner. 2001. Toxoplasma gondii ADP-ribosylation factor 1 mediates enhanced release of constitutively secreted dense granule proteins. J. Biol. Chem. 27618272-18281. [DOI] [PubMed] [Google Scholar]
- 28.Mercier, C., K. D. Adjogble, W. Daubener, and M. F. Delauw. 2005. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites? Int. J. Parasitol. 35829-849. [DOI] [PubMed] [Google Scholar]
- 29.Mercier, C., L. Lecordier, F. Darcy, D. Deslee, A. Murray, B. Tourvieille, P. Maes, A. Capron, and M. F. Cesbron-Delauw. 1993. Molecular characterization of a dense granule antigen (Gra 2) associated with the network of the parasitophorous vacuole in Toxoplasma gondii. Mol. Biochem. Parasitol. 5871-82. [DOI] [PubMed] [Google Scholar]
- 30.Mordue, D. G., N. Desai, M. Dustin, and L. D. Sibley. 1999. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J. Exp. Med. 1901783-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neudeck, A., S. Stachelhaus, N. Nischik, B. Striepen, G. Reichmann, and H. G. Fischer. 2002. Expression variance, biochemical and immunological properties of Toxoplasma gondii dense granule protein GRA7. Microbes Infect. 4581-590. [DOI] [PubMed] [Google Scholar]
- 32.Ryning, F. W., and J. S. Remington. 1978. Effect of cytochalasin D on Toxoplasma gondii cell entry. Infect. Immun. 20739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saeij, J. P., J. P. Boyle, S. Coller, S. Taylor, L. D. Sibley, E. T. Brooke-Powell, J. W. Ajioka, and J. C. Boothroyd. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 3141780-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeij, J. P., S. Coller, J. P. Boyle, M. E. Jerome, M. W. White, and J. C. Boothroyd. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445324-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibley, L. D., I. R. Niesman, S. F. Parmley, and M. F. Cesbron-Delauw. 1995. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. J. Cell Sci. 1081669-1677. [DOI] [PubMed] [Google Scholar]
- 36.Suss-Toby, E., J. Zimmerberg, and G. E. Ward. 1996. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc. Natl. Acad. Sci. USA 938413-8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor, S., A. Barragan, C. Su, B. Fux, S. J. Fentress, K. Tang, W. L. Beatty, H. E. Hajj, M. Jerome, M. S. Behnke, M. White, J. C. Wootton, and L. D. Sibley. 2006. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 3141776-1780. [DOI] [PubMed] [Google Scholar]