Abstract
Mixed-parasite infections are common in many parts of the world, but little is known of the effects of concomitant parasite infections on the immune response or on disease progression. We have investigated the in vivo effects of a chronic gastrointestinal nematode infection on the infectivity and development of the immune response against the common trematode helminth Schistosoma mansoni. The data show that mice carrying an established chronic Trichuris muris infection and coinfected with S. mansoni, had significantly higher S. mansoni worm burdens than mice without coinfection. The increase in S. mansoni worm burden was accompanied by a higher egg burden in the liver. Kinetic analysis of S. mansoni establishment indicate reduced trapping of S. mansoni larvae during skin-to-lung migration, with T. muris-induced alterations in lung cytokine expression and inflammatory foci surrounding lung-stage schistosomula, suggesting that the immunomodulatory effects of chronic T. muris infection elicited at the gut mucosal surface extend to other organs and perhaps specifically to other mucosal surfaces. The data show that a preexisting chronic gastrointestinal nematode infection facilitates the survival and migration of S. mansoni schistosomula to the portal system, and as a result, increases the egg burden and associated pathology of S. mansoni infection.
Helminth infections are among the most common infections of humans (2, 7, 26). Helminth infections are characteristically chronic in nature, and in order to achieve such long-lasting infections, these parasites have developed sophisticated survival strategies such as secretion of immunomodulatory substances and/or the induction of regulatory immune responses (35). These immunomodulatory activities may alter immune responsiveness to third party antigens such as vaccines (9, 10, 18, 42) and concurrent infections (reviewed in references 4 and 27). Intriguingly, recent studies have also demonstrated that the immunomodulatory effects of helminth infections may have beneficial effects, such as controlling allergy and inflammatory diseases (19, 53).
Individuals living in areas where helminths are endemic often carry more than one species of worm infection; egg output for an individual helminth species is often higher in individuals carrying mixed infections than in individuals carrying single-species infections (3, 5, 20, 38, 45), which may reflect higher intensities of infection and so a higher risk of morbidity. Geographical and environmental factors are known to be of significance in facilitating this type of polyparasitism: for example, similarities in the transmission pathways for certain soil-transmitted nematodes are likely to account for some of the observed associations. However, significant associations have also been demonstrated between helminth infections that do not share obvious transmission pathways and some of these associations are not explained solely by household or environmental effects (20). Furthermore, the intensity of helminth infections alters the risk of multiple-species infection (20, 31, 45) indicating that immunological and/or genetic factors may be involved in regulating resistance and susceptibility to helminth-helminth coinfections. In order to investigate how a chronic helminth infection affects the establishment and outcome of a second concurrent helminth infection, we have used the mouse models of the gastrointestinal nematode Trichuris muris (the murine equivalent of human Trichuris trichiura [whipworm] infection) together with the trematode Schistosoma mansoni (a common human infection in tropical countries). Mice that fail to develop an early protective Th2 response against T. muris go on to develop long-term chronic infections (8). However, as the infection progresses beyond the chronic stage, the initial Th1 response is downregulated and a subsequent increase in Th2 response is seen (21, 22). Moreover, production of the regulatory cytokine interleukin-10 (IL-10) has been shown to be critical for host survival (43). Similarly, mice infected with S. mansoni respond with a Th1 response in the early phase of infection, switching to a Th2-dominated response once the adult worms commence egg production (41). Some of the eggs produced by the female S. mansoni worms are trapped in the microvasculature of the liver, where they induce a strong granulomatous response. Most of the pathology associated with S. mansoni infection in both humans and mice is caused by these granulomas.
The data presented here demonstrate that mice with an established chronic T. muris infection and challenged with S. mansoni developed significantly higher S. mansoni worm burdens than mice without a concurrent T. muris infection. The higher S. mansoni burden resulted in significantly higher egg and granuloma burden in the liver. This is the first experimental demonstration that a chronic intestinal nematode infection can exacerbate S. mansoni infection.
MATERIALS AND METHODS
Animals and infections.
Six- to eight-week-old AKR mice were obtained from Harlan Olac, Ltd. (Bicester, United Kingdom). All experiments were performed under the regulations of the Home Office Scientific Procedures Act (1986). All experiments were performed at least three times. Experimental animals (5 to 10 per group) were infected with 150 embryonated T. muris eggs on day 0 by oral gavage and 40 days later infected with 50 or 200 S. mansoni cercariae percutaneously. Maintenance, infection, and recovery of the parasites were performed as described previously (17, 28). Briefly, S. mansoni worms were recovered by portal perfusion with perfusion buffer (phosphate-buffered saline with 0.02 U/ml heparin). The worms were washed free of erythrocytes and counted using a dissecting microscope. For estimation of the liver egg burden, livers were removed and weighed. Eggs were recovered by incubation of the tissue in 5% KOH overnight at 37°C, and the numbers of eggs in 50-μl aliquots were counted in triplicates. T. muris worms in ceca were counted under a dissection microscope.
Control groups receiving single infections were infected in parallel for each experiment.
Cell culture and cytokine analysis.
Mesenteric lymph nodes (MLN) and spleens were removed from uninfected and infected animals, and single-cell preparations were resuspended in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.05 mM β-mercaptoethanol (all from Invitrogen, Paisley, United Kingdom). Cells were cultured at 37°C and 5% CO2 in flat-bottom 96-well plates (Nunc, Roskilde, Denmark) at a final concentration of 5 × 106/ml in a final volume of 0.2 ml/well. Cells were stimulated with T. muris ES antigen (25 μg/ml), S. mansoni egg antigen (SEA) (25 μg/ml), S. mansoni worm antigen (25 μg/ml), or plate-bound anti-CD3 antibody (mAb145-2C11, 10 μg/ml [ATCC]). Cell-free supernatants were harvested after 48 h and stored at −80°C.
Cytokine ELISA.
Cytokine analyses were carried out using commercially available sandwich enzyme-linked immunosorbent assays (ELISAs) for IL-4, gamma interferon (IFN-γ) (Mabtech AB, Nacka, Sweden), and IL-13 and IL-10 (R&D Systems, Abingdon, United Kingdom).
Histopathological analyses.
Tissues were fixed in neutral buffered formalin and histologically processed by standard methods, and sections were stained with hematoxylin and eosin. Liver granulomas surrounding eggs containing visible miracidia were assessed for size, and inflammatory foci in the lungs were assessed for size and cellular composition. The transverse and longitudinal diameters of granulomas and foci were measured using an ocular micrometer, and the mean diameter was calculated. At least 15 granulomas and 20 foci were measured per tissue per mouse, and the mean values were calculated. Individual animal means were then used to derive the group mean values. The cellular composition of lung foci was determined using an eyepiece graticule by counting all of the cells in a rectangular grid spanning the whole diameter of the foci. The percentages of the predominant cell types (eosinophils, fibroblasts, and macrophages) were determined from at least 20 foci per mouse and used to determine the group mean values.
Real-time PCR.
Tissues were harvested and stored in RNAlater (Qiagen, Crawley, United Kingdom) at −80C until processing. RNA was purified using an RNeasy minikit from Qiagen according to the manufacturer's instructions, with an additional DNase treatment step (Qiagen). Reverse transcription was performed using the Omniscript reverse transcription kit (Qiagen). Real-time PCR was performed in an ABI 7000 sequence detection system (Applied Biosystems, Warrington, United Kingdom) using Sybr green PCR Master Mix (Qiagen). Primers for hypoxanthine phosphoribosyltransferase (5′-GTTGGATACAGGCCAGACTTTGTTG and 3′-GATTCAACCTTGCGCTCATCTTAGGC), IL-4 (5′-CCTCACAGCAACGAAGAACA and 3′-TGGACTCATTCATGGTGCAG), IL-10 (5′-AGGGTTACTTGGGTTGCCAA and 3′-CACAGGGGAGAAATCGATGA), and IL-13, IL-5, inducible nitric oxide synthase (iNOS), IFN-γ, and tumor necrosis factor (TNF) (40) were obtained from Invitrogen. Results were normalized to the housekeeping gene coding for hypoxanthine phosphoribosyltransferase and expressed as increase (fold) compared to tissue from naïve, uninfected controls (given an arbitrary value of 1).
Statistical analyses.
Significant differences (P < 0.05) between experimental groups were determined using the Mann-Whitney U test for worm counts and Students t test for all other analyses.
RESULTS
A preexisting chronic T. muris infection result in increased S. mansoni worm burden.
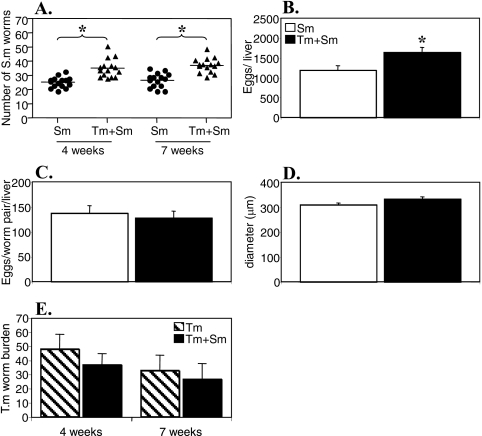
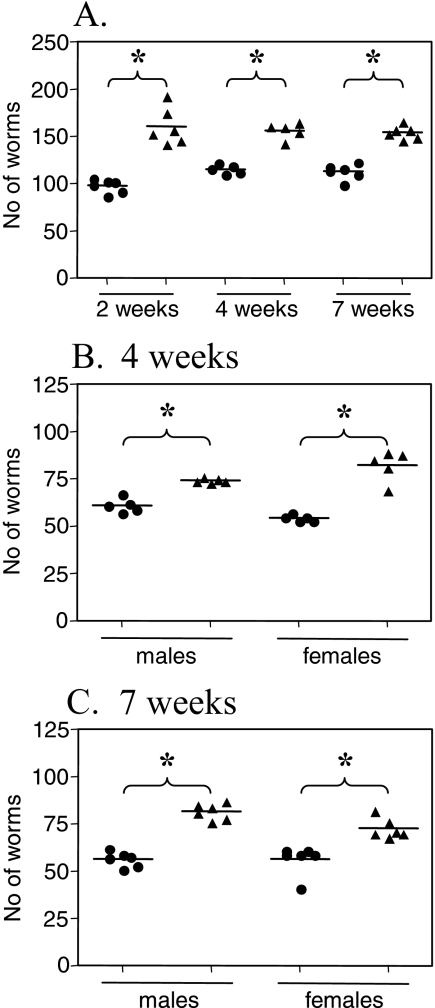
To investigate if a chronic intestinal nematode infection can alter the outcome of S. mansoni infection in vivo, we infected female AKR mice (10 to 20 per group) with 150 embryonated T. muris eggs by oral gavage. Forty days later, the mice were infected with 50 S. mansoni cercariae percutaneously. Age-matched control animals receiving single infections were infected in parallel for each experiment. Four and 7 weeks post-S. mansoni infection, the animals were sacrificed and the livers were perfused to obtain S. mansoni worm counts. The results in Fig. 1A show that mice with a preexisting T. muris infection had significantly higher S. mansoni worm burdens than mice infected with only S. mansoni at both 4 and 7 weeks post-S. mansoni infection (P < 0.05 at both time points). The T. muris worm burdens were not affected by S. mansoni infection (Fig. 1E).
FIG. 1.
Schistosoma mansoni (Sm) worm burden (A), total egg burden in the liver (B), S. mansoni female fecundity (C), mean liver granuloma diameter (D), and T. muris (Tm) worm burden during T. muris-S. mansoni coinfection. AKR mice were infected orally with 150 T. muris eggs, percutaneously with 50 S. mansoni cercariae, or both. S. mansoni infection was started 40 days after T. muris infection. Worm burdens were analyzed 4 and 7 weeks post-S. mansoni infection, and liver pathology was analyzed 7 weeks post-S. mansoni infection. Means and standard errors are shown. Data were pooled from three separate experiments. *, statistically significant difference between groups of mice (P < 0.05).
Increased liver egg burden in coinfected mice.
Analysis of the S. mansoni liver egg burdens in singly infected and coinfected mice 7 weeks post-S. mansoni infection revealed that the higher number of adult schistosomes found in T. muris-coinfected animals was reflected in a significant increase in the total number of S. mansoni eggs in the liver (Fig. 1B). When the egg output per female worm was calculated, the data show that the fecundities of female schistosomes were comparable between the two groups, confirming that the egg output is directly correlated to the number of worms (Fig. 1C). Furthermore, the mean diameters of liver granulomas were similar between the two groups (Fig. 1D).
Concurrent S. mansoni infection modulates the cytokine response to T. muris antigen in the spleen but not in local lymph nodes.
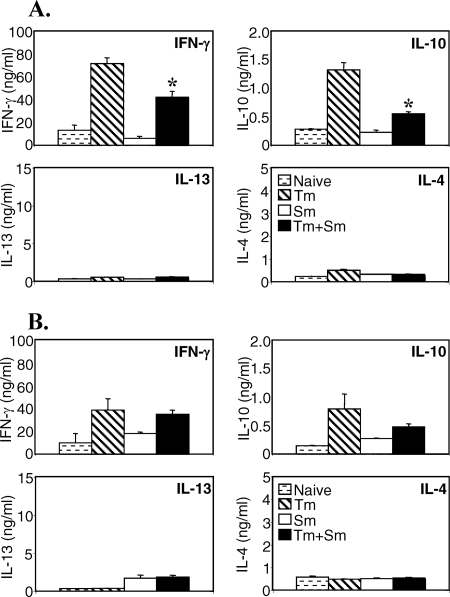
Previous studies have demonstrated that an established S. mansoni infection can markedly skew the cytokine response to unrelated antigens toward a Th2 profile. In order to investigate if this ability to skew cytokine responses could also alter an established T. muris response, we analyzed T. muris-specific cytokine production in single and coinfected animals. In vitro restimulations with T. muris antigen were performed on spleen and MLN cells, and cytokine secretion was analyzed by ELISA. The data in Fig. 2 show that spleen and MLN cells from mice singly infected with T. muris secreted high levels of IFN-γ and IL-10 and low levels of IL-4 and IL-13, in agreement with their susceptible phenotype (8). Spleen cells from mice that were coinfected with T. muris and S. mansoni, however, secreted significantly lower levels of T. muris-specific IFN-γ and IL-10 (Fig. 2A). Interestingly, the reduction in IFN-γ and IL-10 secretion was not associated with an increase in IL-4 and IL-13 secretion, indicating that the S. mansoni infection had not shifted the T. muris response toward Th2, but caused a general inhibition of the T. muris response. No significant difference in the cytokine response in the MLN could be detected in coinfected mice, showing that the T. muris-specific cytokine response nearest to the site of the nematode infection was intact.
FIG. 2.
Concurrent S. mansoni (Sm) infection modulates the cytokine response to T. muris (Tm) in the spleen. AKR mice were infected orally with 150 T. muris eggs, percutaneously with 50 S. mansoni cercariae, or both. S. mansoni infection was started 40 days after T. muris infection. Mice were sacrificed 7 weeks after S. mansoni infection. Spleen (A) and MLN (B) cells from uninfected (stippled bars), T. muris-infected (hatched bars), S. mansoni-infected (white bars), or S. mansoni- and T. muris-coinfected (black bars) mice were removed and stimulated in vitro with T. muris antigen. Supernatants were analyzed by sandwich ELISA for the presence of IFN-γ, IL-10, IL-13, and IL-4. Means of five mice per group and standard errors of the mean are shown. Data from one representative experiment out of three are shown. *, statistically significant difference between T. muris singly infected and coinfected groups (P < 0.05).
Chronic T. muris infection alters the response to SEA in the lymph nodes but not in the spleen.
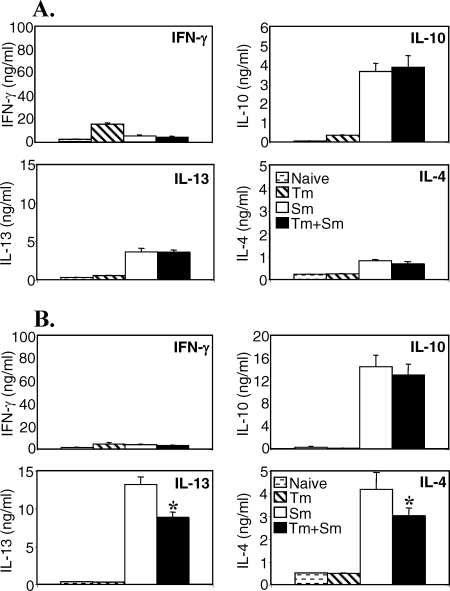
When we analyzed the cytokine secretion profile in response to SEA in vitro, we found that cells from mice singly infected with S. mansoni secreted high levels of IL-4, IL-13, and IL-10 and low levels of IFN-γ, as expected (Fig. 3). There were no differences in the levels of spleen cell cytokine secretion between mice with only S. mansoni infection and those that were coinfected with T. muris (Fig. 3A). In the MLN, however, coinfection resulted in a significant reduction in SEA-specific IL-13 and IL-4 secretion (P < 0.05), while the SEA-specific IL-10 and IFN-γ responses remained unaffected (Fig. 3B). Thus, a chronic T. muris infection is able to inhibit the SEA-specific Th2 response in the local lymph node but not in the spleen.
FIG. 3.
Concurrent T. muris (Tm) infection does not modulate the cytokine response to SEA in the spleen. AKR mice were infected orally with 150 T. muris eggs, percutaneously with 50 S. mansoni (Sm) cercariae, or both. S. mansoni infection was started 40 days after T. muris infection. Mice were sacrificed 7 weeks after S. mansoni infection. Spleen (A) and MLN (B) cells from uninfected (stippled bars), T. muris-infected (hatched bars), S. mansoni-infected (white bars), or S. mansoni- and T. muris-coinfected (black bars) mice were removed and stimulated in vitro with SEA. Supernatants were analyzed by sandwich ELISA for the presence of IFN-γ, IL-10, IL-13, and IL-4. Means of five mice per group and standard errors of the mean are shown. Data from one representative experiment out of three are shown. *, statistically significant difference between S. mansoni singly infected and coinfected groups (P < 0.05).
Altered cytokine mRNA expression in the livers of coinfected animals.
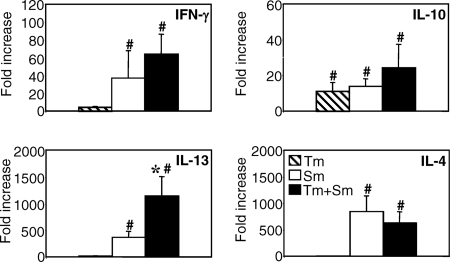
Cytokine mRNA expression in liver tissue was analyzed 7 weeks post-S. mansoni infection, by quantitative real-time RT-PCR. Mice with only S. mansoni infection had high expression of IFN-γ, IL-4, IL-10, and IL-13 in liver tissue compared to uninfected controls (Fig. 4). Mice with only T. muris infection had significantly increased expression of IL-10 compared to naïve tissue, but no increase in IFN-γ, IL-4, or IL-13. The levels of IL-10 mRNA in livers from T. muris-infected mice were comparable to that detected in livers from S. mansoni-infected mice. Mice coinfected with T. muris and S. mansoni had significantly higher expression of IL-13 compared to the group infected with S. mansoni only, while the levels of IFN-γ, IL-10, and IL-4 were comparable between the two groups. This piece of data shows that the coinfection resulted in increased liver mRNA levels of the profibrotic cytokine IL-13, correlating with the increase in liver egg burden. However, the increase in IL-13 was not accompanied by an increase in the levels of the antifibrotic cytokine IFN-γ, suggesting a possibility of more severe disease progression during coinfection.
FIG. 4.
Liver cytokine mRNA expression during T. muris-S. mansoni coinfection (Tm + Sm). AKR mice were infected as described above. RNA was prepared from freshly isolated liver tissue 7 weeks after S. mansoni infection and reverse transcribed, and cytokine mRNA expression was analyzed by real-time PCR. Means of four to six mice per group and standard errors of the mean are shown. Data from one representative experiment out of three are shown. *, statistically significant difference between S. mansoni singly infected and coinfected groups (P < 0.05); #, statistically significant difference between infected and uninfected groups (P < 0.05).
Chronic T. muris infection increases the success rate of S. mansoni larval migration.
Since the coinfected mice had significantly higher S. mansoni worm burdens at 4 and 7 weeks post-S. mansoni infection, we investigated if this was due to a higher survival rate of schistosomula during the migration phase. The S. mansoni larvae migrate from the skin, through the lungs, to the hepatic portal system during the first few weeks of infection, and it was possible that the higher worm burden observed in the coinfected group resulted from increased survival during the skin-to-lung migration or by increased survival of adult worms once established in the portal system. We therefore superinfected mice harboring a chronic T. muris infection with 200 S. mansoni cercariae percutaneously and perfused them for S. mansoni worm counts at 2, 4, and 7 weeks postinfection. Age-matched littermates were infected with S. mansoni alone in parallel. The data in Fig. 5 show that a significantly higher S. mansoni worm burden was already present in the hepatic portal system of coinfected mice at 2 weeks postinfection, demonstrating that the higher worm burden during coinfection was due to enhanced survival of schistosomula during the larval migration phase. The data also show that the increase in worm burden applied to both male and female worms at both 4 and 7 weeks postinfection (Fig. 5).
FIG. 5.
Kinetics of Schistosoma mansoni total worm burden (A) and sex-differentiated Schistosoma mansoni worm burden at 4 (B) and 7 (C) weeks post-S. mansoni infection in mice with only S. mansoni infection (circles) or T. muris-S. mansoni coinfection (triangles). AKR mice were infected orally with 150 T. muris eggs, percutaneously with 200 S. mansoni cercariae, or both. S. mansoni infection was started 40 days after T. muris infection. Worm burdens were analyzed 2, 4, and 7 weeks post-S. mansoni infection. Means of five or six mice per group and standard errors of the mean are shown. Data from one representative experiment out of three are shown. *, statistically significant difference between groups of mice (P < 0.05).
Lung histology during S. mansoni pulmonary migration.
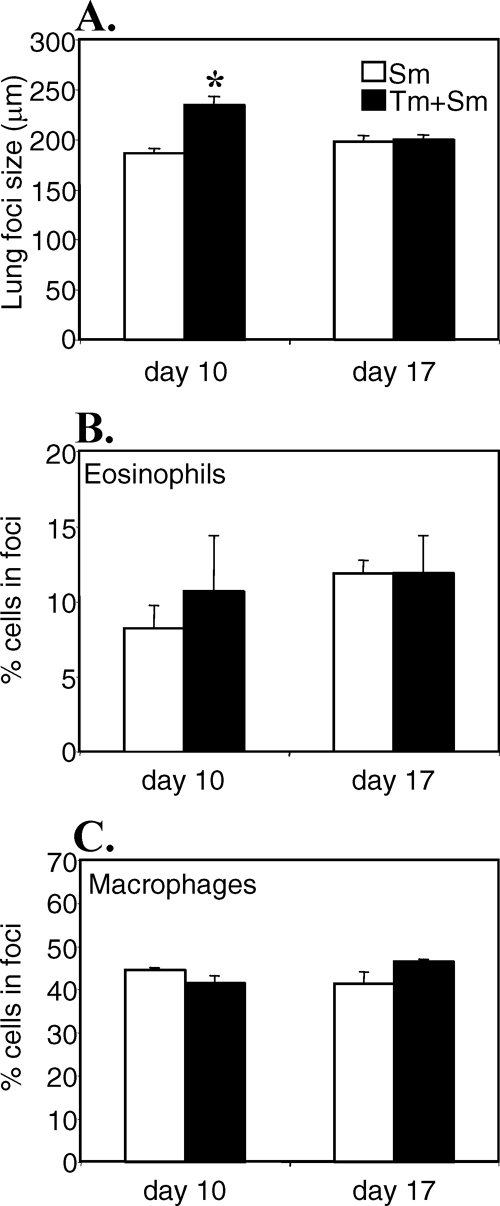
Inflammatory foci develop as the schistosomula migrate through the lungs, possibly as a result of tissue damage caused by the migrating larvae (13). We evaluated the size and cellular composition of the inflammatory reactions in singly infected and coinfected mice on days 10 and 17 post-S. mansoni infection. The data in Fig. 6 show that the mean diameters of the pulmonary reactions containing schistosomula were significantly larger in coinfected than in singly infected mice on day 10 post-S. mansoni infection (216.83 ± 13.07 μm versus 173.35 ± 2.64 μm, P < 0.05). On day 17 post-S. mansoni infection, there was a trend toward the opposite, with the inflammatory foci in mice with only S. mansoni infection becoming larger, while the foci from coinfected mice were shrinking. However, the size difference on day 17 was not statistically significant. There was no difference in the cellular compositions of the inflammatory foci, with similar percentages of infiltrating eosinophils and macrophages in the two groups (Fig. 6B and C). No inflammatory responses were observed in the lungs of mice with only T. muris infection (data not shown).
FIG. 6.
Size and composition of inflammatory foci in the lungs during the migration phase of S. mansoni (Sm). AKR mice were infected orally with 150 T. muris (Tm) eggs, percutaneously with 200 S. mansoni cercariae, or both. S. mansoni infection was started 40 days after T. muris infection. At days 10 and 17 post-S. mansoni infection, lungs were fixed and embedded and sections were cut and stained with hematoxylin and eosin according to routine methods. The mean diameters of inflammatory foci (A) containing schistosomula and the percentage of eosinophils (B) and macrophages (C) in inflammatory foci were determined for 20 to 40 foci per mouse. Means of four to six mice per group and standard errors of the mean are shown. *, statistically significant difference between groups of mice (P < 0.05).
Altered cytokine responses in the lungs of coinfected animals.
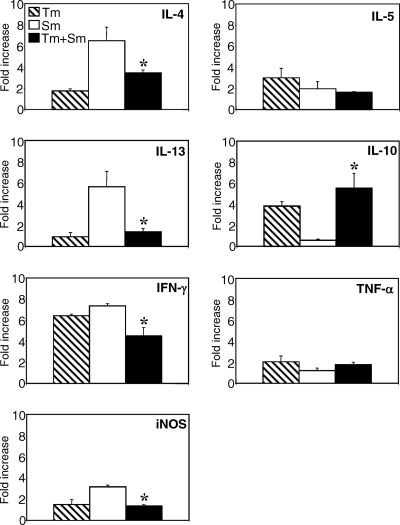
Percutaneous infection with S. mansoni in normal mice typically results in the failure of approximately 50% of the worms to reach the portal system. Most of this natural elimination occurs during the lung phase (15, 36). Studies using the irradiated S. mansoni vaccine model have demonstrated that attrition of schistosomula in the lungs of vaccinated animals is dependent on IFN-γ-producing CD4+ T cells (44, 48) and correlates with macrophage activation (33) and nitric oxide production (32, 52). In order to investigate the influence of T. muris infection on the cytokine environment in the lungs during early S. mansoni larval migration, we analyzed cytokine mRNA expression in lungs on day 7 post-S. mansoni infection by real-time PCR. This time point was chosen since it represents the peak for larval passage through the lungs (16, 50). The data in Fig. 7 show that the migration of S. mansoni larvae is associated with an upregulation of IFN-γ, iNOS, IL-13, and IL-4 mRNAs in the lungs of singly infected mice. Interestingly, mice infected with only chronic T. muris infection had increased expression of IFN-γ and IL-10 in lung tissue compared to uninfected mice, demonstrating that although this parasite is living only in the intestinal mucosa, increased cytokine responses can be detected at other mucosal surfaces such as the lungs. Coinfected mice, however, expressed significantly lower levels of IFN-γ, iNOS, IL-13, and IL-4 and significantly higher levels of IL-10, compared to mice with only S. mansoni infection. The high level of IL-10 in the coinfected group was comparable to that seen in the T. muris-infected group. There was little or no increase in IL-5 or TNF-α expression in any of the groups. Thus, these data show that a chronic T. muris infection alters the cytokine environment in the lungs, resulting in an IL-10-dominated response which appears to inhibit innate antilarval responses during S. mansoni lung-stage migration.
FIG. 7.
Lung cytokine mRNA expression during the migration phase of S. mansoni (Sm). AKR mice were infected orally with 150 T. muris (Tm) eggs, percutaneously with 200 S. mansoni cercariae, or both. S. mansoni infection was started 40 days after T. muris infection. RNA was prepared from freshly isolated lung tissue 7 days post-S. mansoni infection and reverse transcribed, and cytokine mRNA expression was analyzed by real-time PCR. Means of four to six mice per group and standard errors are shown. *, statistically significant difference between S. mansoni singly infected and coinfected groups (P < 0.05).
DISCUSSION
Helminth infections are the most common parasitic infections in the world, and epidemiological studies show that individuals infected with multiple species of helminths often have higher-intensity infections than individuals with single-species infections (3, 5, 45). The associations observed between soil-transmitted nematode species may largely be due to the similarity in transmission of these nematodes, which is closely related to poor hygiene and the lack of adequate sanitation (20). Thus, it is difficult to evaluate the relative contributions of environmental hygiene, socioeconomic, genetic, and pathogen-driven factors in determining rates and prevalence of polyparasitism in humans and it is likely that some or all of these are contributing to polyparasitism to some degree. However, associations between helminths transmitted by different routes (soil versus nonsoil) are more difficult to explain purely based on environmental, geographical, or socioeconomic factors. Nonetheless, increased intensity of infections has been reported in S. mansoni and hookworm coinfection (6, 23, 34) as well as in Schistosoma japonicum- and T. trichiuria-coinfected individuals (20). Interestingly, lower infection intensities have also been reported in S. mansoni and Ascaris lumbricoides coinfections (23). Such lower infection intensities may be explained by reciprocally enhanced Th2-mediated resistance mechanisms, as lower Ascaris worm burdens correlate with increased Th2 responses in humans (46). This is supported by experimental evidence, as mice harboring an established S. mansoni infection are more resistant to concurrent Strongyloides venezuelensis (54) and T. muris (14) infections.
Using the mouse models of T. muris and S. mansoni, we have investigated the impact an established chronic T. muris infection has on resistance to S. mansoni infection. We observed significant increases in schistosome worm burden in mice previously infected with T. muris (ranging between 64 and 81% of the cercariae applied). Following primary exposure of normal mice to S. mansoni infection, typically only 30 to 50% of the cercariae survive to adulthood. The migration from the skin to the portal system involves a complex series of events in which the parasites spend an average of 2 to 5 days penetrating the skin before entering the blood vessels, where they are transported through the lungs before arriving in the hepatoportal system. Approximately 50 to 70% of cercariae never reach the hepatoportal system and are cleared in the lungs (15, 16, 49). While in the lung, the parasite is of such a size that it may completely occlude the vessels and will have to squeeze its way through the capillaries, distending them, and in some cases causing endothelial damage (13), and it is likely that the elasticity of the capillaries may be of importance in permitting the migration of schistosomula through the lungs. Studies using the irradiated cercarial vaccine have shown that following challenge infection in vaccinated animals, focal aggregates of inflammatory cells quickly develop in the lungs and are believed to be a crucial feature of the resistance mechanism (12). Such inflammatory foci also develop in normal unvaccinated mice after infection, although they are less pronounced and slower to develop. We found that the foci were significantly larger in the lungs of coinfected animals on day 10 post-S. mansoni infection, but there was no difference in the cellular composition of foci between the groups. It is possible that the larger and more diffuse foci observed in the lungs of the coinfected mice may be less efficient in trapping the parasites, and although the significance of this finding is yet to be established, it bears some resemblance to previous studies in anti-IFN-γ-treated vaccinated animals. These animals have reduced protection against S. mansoni (i.e., higher worm recoveries than vaccinated controls) but larger and more diffuse pulmonary foci (44), suggesting an important role for IFN-γ in effector focus formation. In agreement with this, we found that coinfected mice expressed significantly lower IFN-γ and iNOS mRNA levels in the lungs than mice with only S. mansoni infection. The reduction in IFN-γ corresponded to an increase in IL-10 expression, which was also evident in mice infected with only T. muris. Thus, it is clear that although the T. muris worms are entirely restricted to the intestine, their effects on immunoreactivity also extend to other mucosal sites. Lung tissue from animals with S. mansoni infection alone expressed increased levels of IL-4, IL-13, iNOS, and IFN-γ, in agreement with previous studies (51, 52).
Our data suggest that the preexisting T. muris infection is dominating the cytokine response in the lung during coinfection, and it is tempting to speculate that the high levels of T. muris-induced IL-10 are responsible for the suppression of IL-4, IL-13, iNOS, and IFN-γ which is normally observed in the lungs during S. mansoni migration. Attempts to block IL-10 in coinfected mice in order to confirm the function of IL-10 during the lung-stage migration of S. mansoni were unsuccessful, however. Blockade of IL-10 during the chronic stage of T. muris infection rapidly leads to severe intestinal pathology and mortality, preventing us from testing this hypothesis experimentally (data not shown). These findings confirm the requirement for host-protective IL-10 during T. muris infection (43). As such, it is currently not possible to test this hypothesis experimentally without affecting the disease progression dramatically in singly infected animals. It is worth noticing, however, that studies using the irradiated schistosome vaccine have shown that IL-10-deficient mice are more resistant and have increased in vitro schistosomulicidal capacity (30), indicating that high levels of IL-10 are unfavorable in the resistance against migrating lung-stage schistosomula.
With respect to protective antischistosomula mechanisms, in vitro studies have shown that IFN-γ-activated macrophages or endothelial cells can kill larval schistosomes through an arginine-dependent mechanism involving production of reactive nitrogen oxide (NO) (32, 39). Furthermore, it is possible that occlusion of pulmonary vessels by the activation of endothelial cells might impede the migration of larvae in the lungs or force them into long-term contact with activated endothelial cells producing toxic mediators such as NO. Studies using the irradiated schistosome vaccine have shown that peak IFN-γ and iNOS responses in the lungs occur at the time when challenge parasites are believed to be eliminated, and iNOS can be identified in the pulmonary inflammatory foci around the migrating larvae (52). Interestingly, previous studies have shown that S. mansoni infection in nonvaccinated mice given hemiguanidine sulfate to block production of NO (52) or infection of iNOS-deficient mice (11) results in significantly increased S. mansoni worm burdens compared to those in normal mice. This may be due to cytotoxic effects of NO, or it could simply reflect the function of NO as a vascular relaxing factor (37), affecting smooth muscle tone and vessel diameter and thus affecting the parasites' migration through the capillaries. Significantly, IL-10 is a major negative regulator of NO production (25). Thus, our findings of increased IL-10 and reduced IFN-γ and iNOS levels in the lungs of T. muris-coinfected mice may, at least in part, provide an explanation for the enhanced survival rate of S. mansoni observed in T. muris-coinfected animals. In this context, it is also worth emphasizing that both Th1 and Th2 cytokines are known to play different roles during different stages of schistosome infection and that an excessive IFN-γ-dominated response during the egg-laying stage leads to severe host pathology (29). Thus, different cytokines play different roles during different stages of the infection. Other factors than IFN-γ and iNOS may also be involved in regulating the migration success for S. mansoni larvae. Studies in rats have demonstrated that sublethal irradiation of normal rats before infection resulted in higher worm burdens (24). This was also the case for complement (47) and mast cell depletion (24), indicating that a number of factors may be contributing to innate resistance. Of relevance is also the fact that immunodeficient rats or mice do not develop higher worm burdens, showing that the mechanisms regulating resistance to pulmonary migration are indeed part of the innate system (1, 24).
Analysis of the cytokine response to SEA in the spleen 7 weeks post-S. mansoni infection revealed no differences between singly infected and coinfected animals. In the MLN, however, SEA-specific levels of IL-4 and IL-13 production were significantly reduced, although this was not accompanied by a shift toward a Th1 response. Conversely, the T. muris-specific cytokine response in the MLN was intact in coinfected animals, while the antigen-specific IFN-γ and IL-10 responses were significantly reduced in the spleen. Again, there was no shift in T helper cytokine profile. Thus, it appears that the two infections are dominating the immune response in separate anatomical and lymphoid compartments and suppressing antigen-specific responses without skewing the cytokine profiles. Interestingly, we also found that the T. muris worm burdens were unaffected by the S. mansoni infection. This is in contrast to results from a previous study where S. mansoni infection preceded T. muris infection (14). As such, it is clear that the timing and sequence of infections are of vital importance for the outcome of coinfections.
The most striking finding from our present study is the demonstration that a preestablished chronic T. muris infection resulted in significantly increased S. mansoni worm burden. Crucially, this increase in adult worm burden also resulted in significantly increased schistosome egg burden in the liver as well as increased levels of the profibrotic cytokine IL-13. Since there was no corresponding increase in the antifibrotic cytokine IFN-γ (29), this raises the possibility that Trichuris coinfection may increase the risk or level of liver fibrosis during chronic schistosomiasis. Although several human studies have reported increased intensity of infection in schistosome-infected individuals during intestinal nematode coinfection (6, 20, 23, 34), we have not been able to find any studies reporting on parameters of clinical morbidity, such as periportal fibrosis. Further studies are needed to confirm whether such an increase in schistosome infection intensity is of clinical importance in human schistosome-associated morbidity.
In conclusion, we have provided the first demonstration that a preexisting infection with a gastrointestinal nematode can promote the infectivity and establishment of the trematode helminth S. mansoni, resulting in higher worm burdens and increased egg-induced pathology. The underlying immunological mechanisms appear to involve nematode-induced alterations in lung cytokine responses and reduction in innate resistance mechanisms. We believe this study may represent the first experimental insight into the aggregation of multiple helminth infections seen in nature, providing a foundation for future investigations into the immunology of helminth-helminth coinfections.
Acknowledgments
We thank Eleanor Riley for critical reading of the manuscript.
This study was funded by Wellcome Trust grant GR067320.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 29 September 2008.
REFERENCES
- 1.Amiri, P., R. M. Locksley, T. G. Parslow, M. Sadick, E. Rector, D. Ritter, and J. H. McKerrow. 1992. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature 16604-617. [DOI] [PubMed] [Google Scholar]
- 2.Bethony, J., S. Brooker, M. Albonico, S. M. Geiger, A. Loukas, D. Diemert, and P. J. Hotez. 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 3671521-1532. [DOI] [PubMed] [Google Scholar]
- 3.Booth, M., D. A. Bundy, M. Albonico, H. M. Chwaya, K. S. Alawi, and L. Savioli. 1998. Associations among multiple geohelminth species infections in schoolchildren from Pemba Island. Parasitology 11685-93. [DOI] [PubMed] [Google Scholar]
- 4.Borkow, G., and Z. Bentwich. 2006. HIV and helminth co-infection: is deworming necessary? Parasite Immunol. 28605-612. [DOI] [PubMed] [Google Scholar]
- 5.Brooker, S., E. A. Miguel, S. Moulin, A. I. Luoba, D. A. Bundy, and M. Kremer. 2000. Epidemiology of single and multiple species of helminth infections among school children in Busia District, Kenya. East Afr. Med. J. 77157-161. [DOI] [PubMed] [Google Scholar]
- 6.Chamone, M., C. A. Marques, G. S. Atuncar, A. L. Pereira, and L. H. Pereira. 1990. Are there interactions between schistosomes and intestinal nematodes? Trans. R. Soc. Trop. Med. Hyg. 84557-558. [DOI] [PubMed] [Google Scholar]
- 7.Chan, M.-S. 1997. The global burden of intestinal nematode infections—50 years on. Parasitol. Today 13438-443. [DOI] [PubMed] [Google Scholar]
- 8.Cliffe, L. J., and R. K. Grencis. 2004. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 57255-307. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, P. J., M. E. Chico, C. Sandoval, I. Espinel, A. Guevara, M. M. Levine, G. E. Griffin, and T. B. Nutman. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 691574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, P. J., I. Espinel, W. Paredes, R. H. Guderian, and T. B. Nutman. 1998. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for IL-10. J. Infect. Dis. 1781133-1138. [DOI] [PubMed] [Google Scholar]
- 11.Coulson, P. S., L. E. Smythies, C. J. Betts, N. A. Mabbott, J. M. Sternberg, X. G. Wei, F. Y. Liew, and R. A. Wilson. 1998. Nitric oxide produced in the lungs of mice immunized with the radiation-attenuated schistosome vaccine is not the major agent causing challenge parasite elimination. Immunology 9355-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulson, P. S., and R. A. Wilson. 1988. Examination of the mechanisms of pulmonary phase resistance to Schistosoma mansoni in vaccinated mice. Am. J. Trop. Med. Hyg. 38529-539. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree, J. E., and R. A. Wilson. 1986. Schistosoma mansoni: an ultrastructural examination of pulmonary migration. Parasitology 92343-354. [DOI] [PubMed] [Google Scholar]
- 14.Curry, A. J., K. J. Else, F. Jones, A. Bancroft, R. K. Grencis, and D. W. Dunne. 1995. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J. Exp. Med. 181769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean, D. A., and B. L. Mangold. 1992. Evidence that both normal and immune elimination of Schistosoma mansoni take place at the lung stage of migration prior to parasite death. Am. J. Trop. Med. Hyg. 47238-248. [DOI] [PubMed] [Google Scholar]
- 16.Dean, D. A., B. L. Mangold, J. R. Georgi, and R. H. Jacobson. 1984. Comparison of Schistosoma mansoni migration patterns in normal and irradiated cercaria-immunized mice by means of autoradiographic analysis. Evidence that worm elimination occurs after the skin phase in immunized mice. Am. J. Trop. Med. Hyg. 3389-96. [DOI] [PubMed] [Google Scholar]
- 17.Doenhoff, M., Q. Bickle, E. Long, J. Bain, and A. McGregor. 1978. Factors affecting the acquisition of resistance against Schistosoma mansoni in the mouse. I. Demonstration of resistance to reinfection using a model system that involves perfusion of mice within three weeks of challenge. J. Helminthol. 52173-186. [DOI] [PubMed] [Google Scholar]
- 18.Elias, D., D. Wolday, H. Akuffo, B. Petros, U. Bronner, and S. Britton. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 123219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott, D. E., R. W. Summers, and J. V. Weinstock. 2005. Helminths and the modulation of mucosal inflammation. Curr. Opin. Gastroenterol. 2151-58. [PubMed] [Google Scholar]
- 20.Ellis, M. K., G. Raso, Y. S. Li, Z. Rong, H. G. Chen, and D. P. McManus. 2007. Familial aggregation of human susceptibility to co- and multiple helminth infections in a population from the Poyang Lake region, China. Int. J. Parasitol. 371153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Else, K. J., and R. K. Grencis. 1991. Cellular immune responses to the nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology 72508-513. [PMC free article] [PubMed] [Google Scholar]
- 22.Else, K. J., L. Hultner, and R. K. Grencis. 1992. Cellular immune responses to the nematode parasite Trichuris muris. II. Differential induction of Th cell subsets in resistant versus susceptible mice. Immunology 75232-237. [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming, F. M., S. Brooker, S. M. Geiger, I. R. Caldas, R. Correa-Oliveira, P. J. Hotez, and J. M. Bethony. 2006. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Trop. Med. Int. Health 1156-64. [DOI] [PubMed] [Google Scholar]
- 24.Ford, M. J., Q. D. Bickle, and M. G. Taylor. 1987. Immunity to Schistosoma mansoni in congenitally athymic, irradiated and mast cell-depleted rats. Parasitology 94313-326. [DOI] [PubMed] [Google Scholar]
- 25.Gazzinelli, R. T., I. P. Oswald, S. L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ activated macrophages. J. Immunol. 1481792-1796. [PubMed] [Google Scholar]
- 26.Gryseels, B., K. Polman, J. Clerinx, and L. Kestens. 2006. Human schistosomiasis. Lancet 3681106-1118. [DOI] [PubMed] [Google Scholar]
- 27.Hartgers, F. C., and M. Yazdanbakhsh. 2006. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol. 28497-506. [DOI] [PubMed] [Google Scholar]
- 28.Helmby, H., K. Takeda, S. Akira, and R. K. Grencis. 2001. Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. J. Exp. Med. 194355-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 1646406-6416. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann, K. F., S. L. James, A. W. Cheever, and T. A. Wynn. 1999. Studies with double cytokine-deficient mice reveal that highly polarized Th1- and Th2-type cytokine and antibody responses contribute equally to vaccine-induced immunity to Schistosoma mansoni. J. Immunol. 163927-938. [PubMed] [Google Scholar]
- 31.Howard, S. C., C. A. Donnelly, N. B. Kabatereine, R. C. Ratard, and S. Brooker. 2002. Spatial and intensity-dependent variations in associations between multiple species helminth infections. Acta Trop. 83141-149. [DOI] [PubMed] [Google Scholar]
- 32.James, S. L., and J. Glaven. 1989. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J. Immunol. 1434208-4212. [PubMed] [Google Scholar]
- 33.James, S. L., P. C. Natovitz, W. L. Farrar, and E. J. Leonard. 1984. Macrophages as effector cells of protective immunity in murine schistosomiasis: macrophage activation in mice vaccinated with radiation-attenuated cercariae. Infect. Immun. 44569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keiser, J., E. K. N′Goran, M. Traoré, K. L. Lohourignon, B. H. Singer, C. Lengeler, M. Tanner, and J. Utzinger. 2002. Polyparasitism with Schistosoma mansoni, geohelminths, and intestinal protozoa in rural Côte d'Ivoire. J. Parasitol. 88461-466. [DOI] [PubMed] [Google Scholar]
- 35.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. E. Allen. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 20189-116. [DOI] [PubMed] [Google Scholar]
- 36.Mangold, B. L., D. A. Dean, P. S. Coulson, and R. A. Wilson. 1986. Site requirements and kinetics of immune-dependent elimination of intravascularly administered lung stage schistosomula in mice immunized with highly irradiated cercariae of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 35332-344. [DOI] [PubMed] [Google Scholar]
- 37.Moncada, S., and E. A. Higgs. 2006. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 147S193-S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Needham, C., H. T. Kim, N. V. Hoa, L. D. Cong, E. Michael, L. J. Drake, A. Hall, and D. A. Bundy. 1998. Epidemiology of soil-transmitted nematode infections in Ha Nam Province, Vietnam. Trop. Med. Int. Health 3904-912. [DOI] [PubMed] [Google Scholar]
- 39.Oswald, I. P., I. A. Eltoum, T. A. Wynn, B. Schwartz, P. Caspar, D. Paulin, A. Sher, and S. L. James. 1994. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc. Natl. Acad. Sci. USA 91999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overbergh, L., D. Valckx, M. Waer, and C. Mathieu. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11305-312. [DOI] [PubMed] [Google Scholar]
- 41.Pearce, E. J., and A. S. MacDonald. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2499-511. [DOI] [PubMed] [Google Scholar]
- 42.Sabin, E. A., M. I. Araujo, E. M. Carvalho, and E. J. Pearce. 1996. Impairment of tetanus-specific Th1-like immune response in humans infected with Schistosoma mansoni. J. Infect. Dis. 173269-272. [DOI] [PubMed] [Google Scholar]
- 43.Schopf, L. R., K. F. Hoffmann, A. W. Cheever, J. F. Urban, and T. A. Wynn. 2002. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 1682383-2392. [DOI] [PubMed] [Google Scholar]
- 44.Smythies, L. E., P. S. Coulson, and R. A. Wilson. 1992. Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J. Immunol. 1493654-3658. [PubMed] [Google Scholar]
- 45.Tchuem Tchuenté, L. A., J. M. Behnke, F. S. Gilbert, V. R. Southgate, and J. Vercruysse. 2003. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Trop. Med. Int. Health 8975-986. [DOI] [PubMed] [Google Scholar]
- 46.Turner, J. D., H. Faulkner, J. Kamgno, F. Cormont, J. Van Snick, K. J. Else, R. K. Grencis, J. M. Behnke, M. Boussinesq, and J. E. Bradley. 2003. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J. Infect. Dis. 1881768-1775. [DOI] [PubMed] [Google Scholar]
- 47.Vignali, D. A., Q. D. Bickle, M. G. Taylor, G. Tennent, and M. B. Pepys. 1988. Comparison of the role of complement in immunity to Schistosoma mansoni in rats and mice. Immunology 6355-61. [PMC free article] [PubMed] [Google Scholar]
- 48.Vignali, D. A., P. Crocker, Q. D. Bickle, S. Cobbold, H. Waldmann, and M. G. Taylor. 1989. A role for CD4+ but not CD8+ T cells in immunity to Schistosoma mansoni induced by 20 krad-irradiated and Ro 11-3128-terminated infections. Immunology 67466-472. [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson, R. A., and P. S. Coulson. 1986. Schistosoma mansoni: dynamics of migration through the vascular system of the mouse. Parasitology 9283-100. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, R. A., P. S. Coulson, and B. Dixon. 1986. Migration of the schistosomula of Schistosoma mansoni in mice vaccinated with radiation-attenuated cercariae, and normal mice: an attempt to identify the timing and site of parasite death. Parasitology 92101-116. [DOI] [PubMed] [Google Scholar]
- 51.Wynn, T. A., D. Jankovic, S. Hieny, A. W. Cheever, and A. Sher. 1995. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J. Immunol. 1544701-4709. [PubMed] [Google Scholar]
- 52.Wynn, T. A., I. P. Oswald, I. A. Eltoum, P. Caspar, C. J. Lowenstein, F. A. Lewis, S. L. James, and A. Sher. 1994. Elevated expression of Th1 cytokines and nitric oxide synthase in the lungs of vaccinated mice after challenge infection with Schistosoma mansoni. J. Immunol. 1535200-5209. [PubMed] [Google Scholar]
- 53.Yazdanbakhsh, M., and S. Wahyuni. 2005. The role of helminth infections in protection from atopic disorders. Curr. Opin. Allergy Clin. Immunol. 5386-391. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida, A., H. Maruyama, Y. Yabu, T. Amano, T. Kobayakawa, and N. Ohta. 1999. Immune response against protozoal and nematodal infection in mice with underlying Schistosoma mansoni infection. Parasitol. Int. 4873-79. [DOI] [PubMed] [Google Scholar]