Abstract
Vaccine and therapeutic strategies that prevent infections with Yersinia pestis have been sought for over a century. Immunization with live attenuated (nonpigmented) strains and immunization with subunit vaccines containing recombinant low-calcium-response V antigen (rLcrV) and recombinant F1 (rF1) antigens are considered effective in animal models. Current antiplague subunit vaccines in development for utilization in humans contain both antigens, either as equal concentrations of the two components (rF1 plus rLcrV) or as a fusion protein (rF1-rLcrV). Here, we show that immunization with either purified rLcrV (a protein at the tip of type III needles) or a variant of this protein, recombinant V10 (rV10) (lacking amino acid residues 271 to 300), alone or in combination with rF1, prevented pneumonic lesions and disease pathogenesis. In addition, passive immunization studies showed that specific antibodies of macaques immunized with rLcrV, rV10, or rF1, either alone or in combination, conferred protection against bubonic plague challenge in mice. Finally, we found that when we compared the reactivities of anti-rLcrV and anti-rV10 immune sera from cynomolgus macaques, BALB/c mice, and brown Norway rats with LcrV-derived peptides, rV10, but not rLcrV immune sera, lacked antibodies recognizing linear LcrV oligopeptides.
Human infections with the bacterium Yersinia pestis are often secondary sequelae to expansion phases of sylvatic rodent plague foci (17, 24). Expansion events occur in response to conditions that facilitate the dispersal of the infectious agent (32) or to the population dynamics of animal reservoirs (72), the flea vector (5), and human hosts (8, 52). Recent epidemiologic surveys have indicated that plague is widespread throughout the wild rodent populations in the southwestern United States, Southeast Asia, Eastern Europe, central and southern Africa, as well as South America, where human populations are highly susceptible (12, 16, 19). Public health officials must also contend with the emergence of multi-antibiotic-resistant Y. pestis strains (26). Thus, there is a potential for large-scale human epidemics, and this necessitates development of a plague vaccine (57).
Over the last century, vaccine preparations have included numerous formulations, including aliquots of bacterial broth cultures (28), live attenuated strains (27), formalin-killed whole-cell extracts (38, 53), and individual purified bacterial subunits (6, 10). Current efforts to generate subunit vaccines for human use are focusing on preparations containing recombinant F1 (rF1) plus low-calcium-response V antigen (LcrV) (68) and rF1-LcrV fusion protein (29) based on the concept that a combination of two protective antigens can result in higher levels of vaccine success than the individual components alone (3, 63). However, to date, it has not been demonstrated that the presence of rF1 in these subunit vaccines bolsters protective immunity in nonhuman primates, the critical animal model for pneumonic plague, or, even more importantly, in humans (57, 69). We contend that naturally occurring or genetically engineered virulent F1 mutant plague strains cannot be neutralized by F1-specific antibody in vivo, bringing into question the overall advantage of including F1 in a subunit vaccine (11, 18, 51, 70, 71). Further, F1-specific humoral immune responses are a sensitive indicator of infection, and immunization with F1 would interfere with the serological diagnosis of plague (4, 40, 62).
Immunization of mice with purified recombinant LcrV (rLcrV) alone elicits protective immunity (2, 35, 41, 42, 64); however, LcrV-associated suppression of host defense mechanisms may preclude the use of full-length LcrV as a human plague vaccine (9, 45). For example, LcrV has been reported to trigger the release of interleukin-10 by host immune cells and also to suppress the release of proinflammatory cytokines, such as tumor necrosis factor alpha and gamma interferon (44, 45, 55, 56). LcrV forms a multimer at the tip of type III needles (43) and plays an essential role in bacterial secretion of Yop effectors into host phagocytes (15, 47, 48). Goguen and colleagues showed that multimerized LcrV, but not monomeric subunits, can stimulate immune cells in a Toll-like receptor 2-dependent manner, suggesting that LcrV subunit preparations, which are largely monomeric, may not have significant immune suppressive properties in animals (50). Previous work showed that an LcrV variant lacking amino acid residues 271 to 300 (rV10) elicited immune responses that protected mice against a lethal challenge with the fully virulent Y. pestis strain CO92 (20, 46), an isolate from a fatal case of human pneumonic plague (22). Compared to rLcrV immunization, rV10 immunization provided equal levels of vaccine protection to mice (20). rV10 displayed a reduced ability to release interleukin-10 or prevent the release of tumor necrosis factor alpha from lipopolysaccharide-stimulated primary macrophages (46).
In this study, we further pursued analysis of rV10 as a human plague vaccine candidate using aerosol infections of cynomolgus macaques (Macaca fascicularis) as a model for pneumonic plague infection. In a direct comparison with vaccines containing rLcrV or rLcrV plus rF1, rV10 proved to be equally immunogenic and protective. When we compared the reactivities of rLcrV and rV10 immune sera from cynomolgus macaques, BALB/c mice, and brown Norway rats with LcrV-derived peptides, we noted that rV10 immune sera, but not rLcrV immune sera, lacked antibodies that recognize short linear LcrV peptides.
MATERIALS AND METHODS
Purification of rLcrV, rV10, and rF1.
The pET16b (Novagen) expression vectors (61) for rLcrV and rV10 have been described previously (46). The Y. pestis strain KIM coding sequence of caf1 (21) was PCR amplified with primers specifying abutting NdeI and BamHI restriction sites. Amplified DNA fragments were subcloned into the expression vector pET15b (Novagen) and digested with NdeI and BamHI, yielding prF1. Escherichia coli BL21(DE3) carrying the expression vectors was grown overnight at 37°C in Luria-Bertani medium with 100 μg/ml ampicillin. Bacteria were diluted in fresh medium and grown to an optical density at 600 nm of 0.5. T7 polymerase was induced with 1 mM isopropyl-1-thiol-d-galactopyranoside, and the bacteria were grown for an additional 3 h at 37°C. Bacteria were sedimented by centrifugation at 10,000 × g for 15 min, and E. coli cells from a 500-ml culture were disrupted twice with a French pressure cell at 14,000 lb/in2 in 20 ml of 50 mM Tris-HCl (pH 7.5)-150 mM NaCl (column buffer). The lysates were applied to a nickel-nitrilotriacetic acid column (bed volume, 1 ml) preequilibrated with 20 ml column buffer. The column was washed with 20 volumes of the same buffer and then with 20 volumes of column buffer containing 20 mM imidazole. Protein was eluted in 50 mM Tris-HCl (pH 7.5)-150 mM NaCl with 250 mM imidazole. Proteins were extracted with 1% Triton X-114 (Sigma) to remove endotoxin, and the detergent was removed by chromatography with a HiTrap desalting column (GE); proteins were eluted in phosphate-buffered saline. Lipopolysaccharide contamination of vaccine antigens was assayed with Limulus amebocyte lysate (QCL-1000; Cambrex, New Jersey) (1). Protein concentrations were determined by the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL) or by measuring absorption at 280 nm. Proteins were flash frozen with dry ice and ethanol and stored at −80°C until they were used.
Immunization of animals.
For mice, groups of 6- to 8-week-old female BALB/c mice (Charles River Labs, Massachusetts) were immunized twice by intramuscular injection into the hind leg of 0.1-ml aliquots containing 50 μg of rLcrV, rV10, rF1, rLcrV plus rF1, or rV10 plus rF1 (50 μg of each subunit) in 25% Alhydrogel (Brenntag Biosector, Frederikssund, Denmark) on days 0 and day 21. Blood was drawn on day 42 to determine the serum antibody titers prior to plague challenge. For rats, 12-week-old female brown Norway rats (Charles River Labs, Massachusetts) were immunized twice by intramuscular injection into the hind leg of 0.1-ml aliquots containing 50 μg of rLcrV or rV10 in 25% Alhydrogel (Brenntag Biosector, Frederikssund, Denmark) on days 0 and 21. Blood was drawn on day 35 to determine the serum antibody titers. For nonhuman primates, adult male and female cynomolgus macaques weighing 2.4 to 3.5 kg (Scientific Resources International Ltd., The Netherlands) were immunized by intramuscular injection three times at 3-week intervals using the protein and vaccine formulation (50 μg of each antigen) described above for the mouse experiments. The animals were randomly assigned to six groups. Group 1 animals (n = 4) received 25% adjuvant in PBS; group 2 animals (n = 4) received rLcrV; group 3 animals (n = 4) received rV10; group 4 animals (n = 4) received rF1; group 5 animals (n = 4) received rLcrV plus rF1; and group 6 animals (n = 4) received rV10 plus rF1. Blood samples were collected over the course of immunization on days 21, 42, 49, and 85 following the first injection. For antibody detection, the levels of serum immunoglobulin G (IgG) reactive with specific antigens were determined by a custom enzyme-linked immunosorbent assay (ELISA) designed by the GLRCE Immunology Core at the University of Chicago (20). Briefly, pooled serum samples representative of the immunization group were aliquoted on microtiter plates precoated with either histidine-tagged rF1, rLcrV, or rV10 antigens (1 μg/ml). Binding of serum antibody was detected with secondary antibodies against mouse or monkey immunoglobulins, such as goat anti-monkey IgG, IgM, and IgA (Fitzgerald) and mouse anti-human IgG, IgG1, IgG2, IgG3, and IgG4 (Cell Science). Additional experiments were performed to examine the contribution of anti-histidine antibodies to mean IgG titers. The results revealed that use of histidine-tagged antigen as a coating substrate for our ELISAs and use of nontagged antigen as a coating substrate for our ELISAs yielded similar mean IgG titers for both mouse and nonhuman primate antisera (data not shown). Statistical analysis of antibody levels using a pairwise comparison was performed with the Student t test. Immunoglobulin isotypes of antibodies reactive with vaccine antigens were also determined by ELISA.
Plague challenge of immunized animals.
For mice, two models were used to recapitulate the pathogenesis of bubonic plague (subcutaneous injection) and pneumonic plague (intranasal instillation). For the pneumonic plague model, mice were anesthetized with 17 mg/ml ketamine (Ketsed; Vedco) and 0.7 mg/ml of xylazine (Sigma) injected into the peritoneal cavity and then challenged by intranasal inoculation of 20 μl of a bacterial suspension in PBS which was equivalent to 100 mean lethal doses (MLD) of Y. pestis CO92 (4 × 104 CFU) (20). For this experiment, Y. pestis CO92 was grown in heart infusion broth supplemented with 2.5 mM calcium at 37°C overnight. Bacteria were washed and diluted in sterile PBS to obtain the required concentration. For passive transfer experiments and bubonic plague challenge, mice were inoculated with 200 μl of mouse serum or 500 μl of nonhuman primate serum 1 h prior to challenge by subcutaneous injection of a 0.1-ml suspension containing 20 MLD Y. pestis CO92. For this experiment, plague bacteria were grown in heart infusion broth at 26°C overnight, washed, and diluted in sterile PBS to obtain the required concentration. Immunized mice that had been challenged with Y. pestis CO92 were monitored for morbidity and mortality for 14 days. All mouse experiments were performed in accordance with institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at the University of Chicago. Log rank tests were used to compare the statistical significance of mortality for different vaccine groups as indicated below.
Using head-only inhalation, cynomolgus macaques were challenged with a target dose containing 200 ± 25 MLD of Y. pestis CO92, as previously described (65). The challenges were performed with two groups, containing 11 and 12 animals, 24 h apart. Each group contained two or three members of each immunization group plus one cynomolgus macaque that received adjuvant in PBS. The order of challenge was randomized. Procedurally, male and female cynomolgus macaques were fasted for 12 to 18 h (overnight) prior to exposure to minimize the likelihood of vomiting in response to anesthesia and the possible sequelae of aspiration pneumonia during the procedure. Animals were anesthetized with 2 to 6 mg/kg Telazol (Fort Dodge) approximately 15 min prior to aerosol exposure. For this experiment, Y. pestis CO92 was grown for 72 h at 26°C, washed, suspended in sterile 1% peptone, diluted in brain heart infusion broth, and aerosolized with a Collison nebulizer (MRE-3 jet; BGI, Inc., Waltham, MA). The aerosol was sampled directly with an all-glass impinger (AGI; Ace Glass, Inc., Vineland, NJ), using samples drawn from the head-only exposure chamber, downstream from the macaque's nostrils. The bacterial concentrations and the purity of the challenge preparation were determined by quantitative bacterial culture of aerosol samples and by assessing colony morphology on tryptic soy and Congo red agar. The target particle size was 1 to 3 μm, as determined with a Aerodynamic particle size spectrometer (model 3321; TSI, Inc., Shoreview, MN). Inhaled aerosol doses were determined using Buxco plethysmography. Animal respiratory frequency, tidal volume, and minute volume were recorded during exposure, and the targeted inhaled volume for each exposure was 3.5 liters. The concentrations of Y. pestis in nebulizer suspensions were established prior to aerosol delivery so that a known number of bacteria per minute could be delivered. The nonhuman primate protocol and any amendment(s) or procedures involving the care and use of animals in this study were reviewed and approved by Lovelace Respiratory Research Institute's Institutional Animal Care and Use Committee before the study was conducted. During the study, the care and use of animals was in accordance with the guidelines of the U.S. National Research Council. Log rank tests were used to compare the statistical significance of mortality for different vaccine groups as indicated below.
Histopathology.
Animal tissues obtained during necropsy were fixed in 10% neutral buffered formalin and embedded in paraffin. Blocks were cut to obtain 5-μm sections, which were stained with hematoxylin and eosin prior to microscopy and image analysis.
RESULTS
Humoral immune response to plague subunit vaccines in mice.
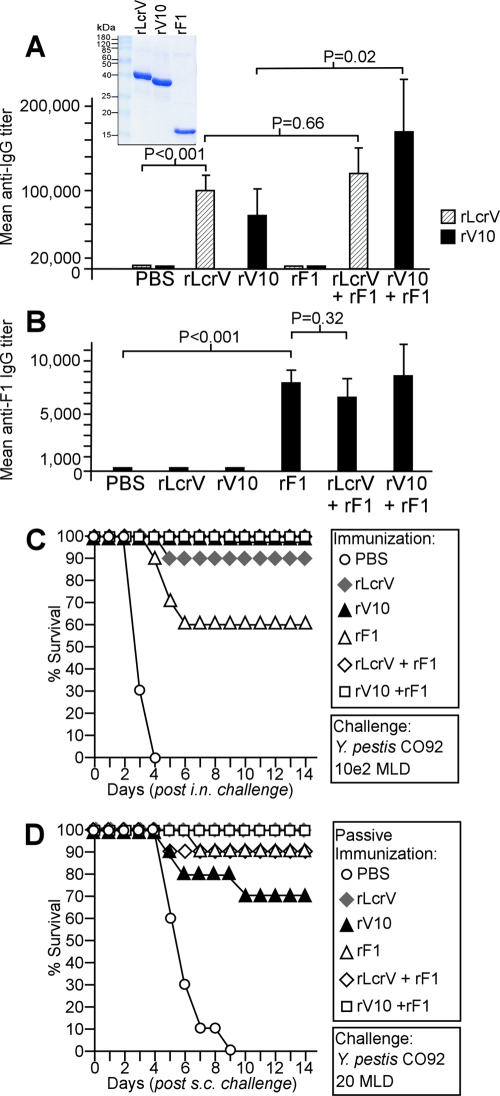
Our goal was to compare the efficacies of subunit vaccines containing rLcrV, rV10, rF1, rLcrV plus rF1, and rV10 plus rF1 in mice and nonhuman primates using the same batches of recombinant proteins that were purified from lysates of E. coli BL21(DE3) strains harboring various expression plasmids (pLcrV, pV10, or pF1). The purity of vaccine subunit preparations was judged to be greater than 90% (Fig. 1A). BALB/c mice (n = 5) were immunized twice by intramuscular injection of purified protein (50 μg) emulsified in 25% (vol/vol) aluminum hydroxide gel with 21 days between the treatments. Forty-two days after the first injection, blood samples were taken and mouse serum was analyzed to determine specific antibody levels using ELISA (Fig. 1A). Mice that had been immunized with rLcrV and mice that had been immunized with rV10 had average rLcrV-specific antibody titers at dilutions of 1:100,000 and 1:75,000, respectively (Fig. 1A). Control animals (which received adjuvant in PBS) or rF1-immunized animals did not raise rLcrV-specific antibodies (for PBS versus rLcrV, P < 0.001). Mice immunized with combinations of subunits (rLcrV plus rF1 or rV10 plus rF1) had average rLcrV-specific antibodies at dilutions of 1:115,000 and 1:170,000, respectively. There was no significant difference between the antibody titers of animals in the rLcrV-immunized group and the antibody titers of animals in the group immunized with rLcrV plus rF1 (P = 0.66). Sera of mice that had been immunized with rF1, rLcrV plus rF1, or rV10 plus rF1 were also analyzed by ELISA, and the sera had rF1-specific antibodies at a 1:7,000 dilution, whereas animals that received adjuvant in PBS did not raise F1 antibodies (for PBS versus rF1, P < 0.001) (Fig. 1B). There was not a significant difference between the F1 antibody level in mice vaccinated with rF1 alone and the F1 antibody level in animals vaccinated with rLcrV plus rF1 or with rV10 plus rF1 (for rF1 versus rLcrV plus rF1, P = 0.32). Isotyping of serum antibodies specific for subunit vaccine antigens indicated that the principal immunoglobulin subclasses were IgG1 and, to a lesser extent, IgG2a/b. IgM type antibodies were not detected (Table 1).
FIG. 1.
Immune responses to plague subunit vaccines in mice. (A) (Inset) Histidine-affinity tagged rLcrV, rV10, and rF1 were purified from lysates of recombinant E. coli, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and stained with Coomassie brilliant blue. Purified rLcrV, rV10, and rF1 (50 μg each) were emulsified with Alhydrogel and injected intramuscularly into 6- to 8-week-old BALB/c mice (n = 5). (A and B) Following two immunizations separated by 21 days, animals were examined to determine their humoral immune responses to rLcrV, rV10, and rF1 by ELISA. (C) Twenty-one days following a second booster immunization, experimental animals were challenged by intranasal (i.n.) inoculation of 100 MLD of Y. pestis wild-type strain CO92. (D) Naïve 6- to 8-week-old BALB/c mice (n = 10) were passively immunized by intraperitoneal injection of 200 μl of mouse immune sera 1 h prior to challenge with 20 MLD of Y. pestis CO92 injected subcutaneously (s.c.).
TABLE 1.
Immunoglobulin isotypes of mouse antibodies directed against plague vaccine antigens
| Group | Antigena | Log10 titers for antigen-specific immunoglobulin isotypes (mean ± SD)
|
|||
|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG2b | IgG3 | ||
| 1 | PBS | NDb | ND | ND | ND |
| 2 | rLcrV | 5.20 ± 3.30 | 2.96 ± 1.80 | 2.90 ± 1.38 | ND |
| 3 | rV10 | 5.20 ± 3.60 | 2.85 ± 1.90 | 2.69 ± 0.90 | ND |
| 4 | rF1 | 4.30 ± 2.60 | 2.54 ± 0.69 | 2.60 ± 0.90 | 2.50 ± 0.69 |
| 5 | rLcrV + (rF1) | 5.34 ± 3.11 | 3.04 ± 1.25 | 2.79 ± 1.00 | ND |
| 5 | (rLcrV) + rF1 | 4.25 ± 2.60 | 2.60 ± 0.69 | 2.60 ± 1.00 | 2.39 ± 1.81 |
| 6 | rV10 + (rF1) | 5.54 ± 3.60 | 3.23 ± 2.47 | 3.00 ± 1.30 | 2.00 ± 1.47 |
| 6 | (rV10) + rF1 | 4.20 ± 3.47 | 2.50 ± 1.30 | 2.47 ± 0.47 | 2.54 ± 2.39 |
BALB/c mice (n = 5) were immunized by intramuscular injection of 50 μg of antigen adsorbed to aluminum hydroxide adjuvant (Alhydrogel) on days 0 and 21 and bled on day 42 postimmunization. Antigen-specific IgG1, IgG2a, IgG2b, and IgG3 in each mouse immune serum were detected by ELISA. Immune sera from animals that received a single antigen displayed specificity for that antigen (data not shown). Immune sera from animals that received combination vaccines (rLcrV plus F1 or rV10 plus F1) were analyzed for each antigen separately, and parentheses indicate the antigen that was not immobilized on microtiter dishes.
ND, rLcrV or rF1 antigen-specific immunoglobulin was not detected.
Comparison of efficacies of subunit vaccines for protecting mice against plague challenge.
BALB/c mice (n = 10) were immunized twice with rLcrV, rV10, rF1, rLcrV plus rF1, or rV10 plus rF1 and infected by intranasal inoculation of 4 × 104 CFU (100 MLD) of Y. pestis CO92, and survival after pneumonic plague challenge was recorded for 14 days (Fig. 1C). Mice immunized with the PBS-adjuvant control died of pneumonic plague within 4 days (Fig. 1C). Animals immunized with rLcrV plus rF1 or rV10 survived the infection (for PBS versus rLcrV plus rF1, P < 0.0001). Statistical analysis of survival after pneumonic plague challenge with log rank tests indicated that immunization with rV10 provided a level of protection similar to that provided by immunization with rV10 plus rF1, rLcrV, or rLcrV plus rF1 (for rV10 versus rLcrV, P = 0.232).
Serum of mice (200 μl) that had been immunized with rLcrV, rV10, rF1, rLcrV plus rF1, or rV10 plus rF1 was injected intraperitoneally into naïve BALB/c mice (n = 10). One hour later, passively immunized animals were subjected to bubonic plague challenge by subcutaneous injection of 20 CFU (20 MLD) Y. pestis CO92, and survival was recorded for 14 days (Fig. 1D) All mice that received serum from animals immunized with adjuvant in PBS succumbed to infection within 9 days (Fig. 1D). In contrast, 9 of 10 mice that had been passively immunized with serum from animals immunized with rLcrV plus rF1 survived the infection (P = 0.0001). Statistical analysis of survival after plague challenge with log rank tests indicated that passive transfer with serum from animals that had been immunized with rV10 also provided protection (70%), which was not significantly different from the protection provided by passive immunization with rLcrV immune serum (for rV10 versus rLcrV, P = 0.06) or rLcrV plus rF1 (Fig. 1D).
Humoral immune response to plague subunit vaccines in nonhuman primates.
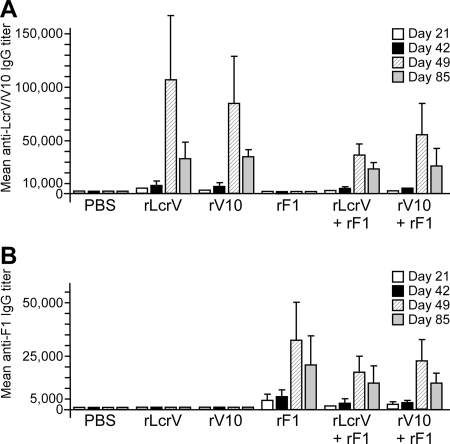
Cynomolgus macaques were immunized by intramuscular injection three times at 3-week intervals of rLcrV, rV10, rF1, rLcrV plus rF1, or rV10 plus rF1. Blood samples were drawn on days 0, 21, 42, 49, and 85 after the first injection. Nonhuman primate serum was analyzed to determine the specific antibody level by ELISA (Fig. 2). Antibodies specific for rLcrV and antibodies specific for rV10 were detected at day 21 with dilutions of 1:5,000 and 1:2,500, respectively (Fig. 2A). For animals immunized with rLcrV plus rF1 or with rV10 plus rF1, antigen-specific antibodies were detected at a dilution of 1:1,800 at day 21. Seven days after the third vaccine injection (on day 49), rLcrV- and rV10-specific antibodies were detected at dilutions of 1:100,000 and 1:85,000, respectively (Fig. 2A). In groups immunized with rV10 plus rF1 and with rLcrV plus rF1, rLcrV- and rV10-specific antibodies were detected at dilutions of 1:38,000 and 1:56,000, respectively. Two weeks prior to challenge (day 85), the dilutions for rLcrV- and rV10-specific antibodies had dropped to 1:30,000 for the groups immunized with the proteins alone or in combination with rF1 (Fig. 2A). The isotypes of antibodies specific for rLcrV, rV10, and rF1 were characterized at day 49 (Table 2). The predominant subclasses consisted of IgG1 and IgM isotypes for all animals immunized with plague subunit vaccines (Table 2).
FIG. 2.
Adult cynomolgus macaques (weight, 2.4 to 3.5 kg) were immunized by intramuscular injection of 50 μg of antigen adsorbed to Alhydrogel on days 0, 21, and 42. The macaques were bled on days 21, 42, 49, and 85 postimmunization. Antibody titers specific either for purified rLcrV or rV10 (A) or for rF1 (B) were detected by ELISA using diluted serum.
TABLE 2.
Nonhuman primate immunoglobulin directed against plague vaccine antigens
| Group | Antigena | Log10 titers for antigen-specific immunoglobulin isotypes (mean ± SD)
|
|
|---|---|---|---|
| IgG1 | IgM | ||
| 1 | PBS | NDb | ND |
| 2 | rLcrV | 3.04 ± 2.71 | 3.29 ± 2.99 |
| 3 | rV10 | 2.98 ± 2.73 | 3.32 ± 2.86 |
| 4 | rF1 | 2.97 ± 2.60 | 3.41 ± 3.27 |
| 5 | rLcrV + (rF1) | 2.65 ± 2.38 | 3.27 ± 2.06 |
| 5 | (rLcrV) + rF1 | 2.79 ± 2.61 | 3.20 ± 2.23 |
| 6 | rV10 + (rF1) | 2.88 ± 2.30 | 3.24 ± 2.37 |
| 6 | (rV10) + rF1 | 2.79 ± 2.10 | 3.17 ± 2.78 |
Cynomolgus macaques (see Table 3 for animal group assignments) were immunized by intramuscular injection of 50 μg antigen adsorbed to aluminum hydroxide adjuvant (Alhydrogel) on days 0, 21, and 42. The monkeys were bled at day 49 postimmunization. Specific IgG1 and IgM were detected by ELISA in serum for individual monkeys.
ND, rLcrV or rF1 antigen-specific immunoglobulin was not detected.
Nonhuman primate antibodies against subunit vaccines protect mice against plague challenge.
To assay the protective attributes of specific antibodies raised in the different groups of immunized macaques, serum from each macaque (500 μl recovered on day 49) was injected (day 52) into two mice 1 h prior to challenge with 20 MLD Y. pestis CO92. The titers of macaque serum antibodies to relevant vaccine antigens, the mean time to death, and survival data for mice passively immunized with nonhuman primate serum are shown in Table 3. As expected, mice that received serum from macaques immunized with PBS-adjuvant succumbed to infection on day 4 or 9 postchallenge. Mice passively immunized with macaque rLcrV or rV10 immune serum were protected against plague challenge; however, 25% of the mice passively immunized with macaque rF1 immune serum did not survive the challenge (Table 3). The serum from macaque 4877, which had a very low F1 antibody titer (1:7,500), did not protect mice, as the mice succumbed to infection on days 10 and 13 postchallenge. Nevertheless, macaque 4877 survived plague aerosol challenge (see below). Two additional mice succumbed to plague infection when they were passively immunized with serum from macaques that had been immunized with rLcrV plus rF1 or rV10 plus rF1. However, no correlation between death and the level of specific antibodies was evident for these groups. This observation is consistent with the results of previous studies indicating that anti-LcrV ELISA titers are not enough to predict plague mortality in mice (7, 69). Altogether, as shown previously, the results of passive immunization studies suggest that specific antibodies of macaques that were immunized with rLcrV, rV10, and rF1, either alone or in combination, confer protection against bubonic plague challenge in mice (69).
TABLE 3.
Vaccine protection against pneumonic plague in cynomolgus macaques
| Vaccine group | Nonhuman primate | rLcrV titera | rV10 titera | rF1 titera | Mouse exptb
|
Nonhuman primate exptc
|
|||
|---|---|---|---|---|---|---|---|---|---|
| No. of survivors/no. challenged | Time of death (days) | Aerosol challenge dose (CFU) | Status 14 days postchallenge | Time of death (days) | |||||
| 1 (PBS/adjuvant) | 4833 | 0 | 0 | 0 | 0/2 | 7-8 | 1.27 × 104 | D | 4 |
| 4885 | 0 | 0 | 0 | 0/2 | 5-9 | 1.45 × 104 | A | NAd | |
| 4846 | 0 | 0 | 0 | 0/1 | 5 | 2.30 × 104 | D | 5 | |
| 13437 | 0 | 0 | 0 | 0/2 | 4-5 | 2.29 × 104 | D | 4 | |
| 2 (rLcrV) | 4817 | 50,000 | NDe | ND | 2/2 | NA | 1.08 × 104 | A | NA |
| 4825 | 175,000 | ND | ND | 2/2 | NA | 1.96 × 104 | A | NA | |
| 13440 | 100,000 | ND | ND | 2/2 | NA | 2.45 × 104 | A | NA | |
| 3 (rV10) | 4875 | ND | 90,000 | ND | 2/2 | NA | 1.27 × 104 | A | NA |
| 4843 | ND | 32,000 | ND | 2/2 | NA | 1.08 × 104 | A | NA | |
| 42147 | ND | 80,000 | ND | 2/2 | NA | 2.38 × 104 | A | NA | |
| 4855 | ND | 140,000 | ND | 2/2 | NA | 2.27 × 104 | A | NA | |
| 4 (rF1) | 24162 | ND | ND | 45,000 | 2/2 | NA | 1.18 × 104 | D | 7 |
| 4877 | ND | ND | 7,500 | 0/2 | 10-13 | 5.45 × 103 | A | NA | |
| 4852 | ND | ND | 20,000 | 2/2 | NA | 2.21 × 104 | A | NA | |
| 24217 | ND | ND | 52,000 | 2/2 | NA | 2.14 × 104 | A | NA | |
| 5 (rLcrV + rF1) | 4864 | 31,000 | ND | 10,000 | 2/2 | NA | 1.07 × 104 | A | NA |
| 4838 | 26,000 | ND | 26,000 | 1/2 | 13 | 1.65 × 104 | A | NA | |
| 4878 | 53,000 | ND | 19,000 | 2/2 | NA | 2.69 × 104 | A | NA | |
| 4809 | 40,000 | ND | 14,000 | 2/2 | NA | 2.76 × 104 | A | NA | |
| 6 (rV10 + rF1) | 42149 | ND | 42,000 | 20,000 | 2/2 | NA | 1.07 × 104 | A | NA |
| 4812 | ND | 23,000 | 10,000 | 2/2 | NA | 8.83 × 103 | A | NA | |
| 4873 | ND | 65,000 | 22,000 | 2/2 | NA | 2.26 × 104 | A | NA | |
| 4836 | ND | 95,000 | 35,000 | 1/2 | 7 | 2.21 × 104 | A | NA | |
Serum antibody titers against specific antigens in nonhuman primates were determined by ELISA.
Naïve BALB/c mice were passively immunized by intraperitoneal injection of 500 μl of nonhuman primate serum 60 min prior to plague challenge via subcutaneous injection of 20 MLD of Y. pestis CO92.
Nonhuman primates were challenged with Y. pestis CO92 using an aerosol. A, alive; D, dead.
NA, not applicable.
ND, not determined.
Repertoires of antibodies against rLcrV and rV10 antigens in different animal species.
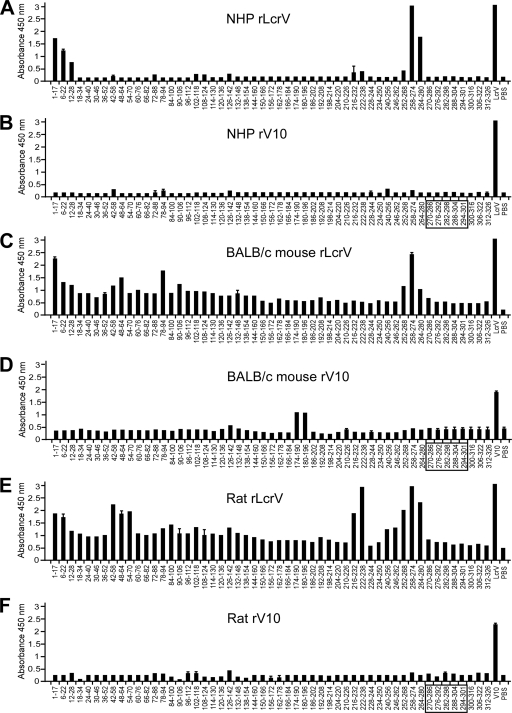
To define epitopes within the LcrV antigen that are recognized by antibodies from cynomolgus macaques immunized with either rLcrV or rV10, we utilized an array of 53 peptides ranging from 15 to 17 residues long and with 11- or 12-amino-acid overlaps (Fig. 3A and B). Pooled serum from macaques immunized with rLcrV recognized four peptides, including two pairs of peptides with an overlapping peptide sequence (amino acid residues 1 to 17 and 6 to 22 and amino acid residues 258 to 274 and 264 to 280). On the other hand, serum from macaques that had been immunized with rV10 did not react with any of the oligopeptides on the array. We wondered whether similar profiles could be observed with both rLcrV- and rV10-derived immune serum from BALB/c mice or brown Norway rats. Interestingly, we observed some similarity in binding specificity between the nonhuman primates and the rodent species for rLcrV antisera; mice and rats raised antibodies that recognized amino acid residues 1 to 17 and 258 to 274, and additional unique epitopes were recognized at amino acid residues 48 to 64 for mice and rats and at amino acid residues 222 to 238 for only rats (Fig. 3C to F).
FIG. 3.
Comparison of specific antibody responses in animals immunized with rLcrV and rV10. rLcrV or rV10 immune sera (diluted 1:510) from cynomolgus macaques (A and B), BALB/c mice (C and D), and brown Norway rats (E and F) were analyzed to determine immune reactivity to overlapping 15- to 17-mer peptides spanning the full LcrV antigen with an ELISA. The box indicates amino acid residues that were deleted during construction of rV10.
Comparison of subunit vaccine efficacies for protecting nonhuman primates against plague challenge.
Cynomolgus macaques were challenged by aerosol with about 200 MLD (1 × 104 CFU) Y. pestis CO92 on day 105; the calculated inhaled dose for each macaque is shown in Table 3. Following exposure, animals were monitored for 14 days for signs of pneumonic plague. Three of the four macaques immunized with PBS-adjuvant developed pneumonic plague and were euthanized or succumbed to infection on days 4 and 5 postinfection. Macaques immunized with rLcrV or rV10 alone or in combination with rF1 did not display clinical symptoms of pneumonic plague and appeared to be healthy for the duration of the experiment. Rather unexpectedly, macaque 24162 in the rF1 immunization group succumbed to pneumonic plague. The survival data for animals in the vaccine groups were compared using log rank tests. The comparisons of survival for the group immunized with PBS-adjuvant and the groups immunized with rV10, rV10 plus F1, and rLcrV plus F1 revealed a significant difference (P < 0.0388). Because the group immunized with rLcrV was smaller, no significance could be determined for the comparison of this group and the group immunized with PBS-adjuvant (P = 0.0703); during vaccination, one of the rLcrV-immunized nonhuman primates developed a fatal, unrelated bacterial infection that could not be controlled by antibiotic treatment. A comparison of the survival data for the groups immunized with PBS-adjuvant and with rF1 using a log rank test did not detect significant differences (P = 0.3865).
Pathological evidence for pneumonic plague in cynomolgus macaques.
To discern whether pulmonary infection with Y. pestis CO92 in the macaque primate model caused pathological lesions similar to those observed in human patients (13, 37), we examined the lungs of infected animals for both gross changes and histopathologic changes associated with infection. Macaques that succumbed to infection or that were humanely euthanized at the end of the study were necropsied, and their lungs were exteriorized and processed for histopathology. Gross examination of the lungs revealed clear differences between the sham (PBS-adjuvant)-immunized controls and macaques that were vaccinated with rLcrV, rV10, or rF1 and persisted during the 14-day observation period, which were euthanized on the final day (Fig. 4). The lungs of sham-vaccinated macaques and one rF1-vaccinated macaque, all of which had succumbed to infection, contained multiple areas of severe congestion and hemorrhage (Fig. 4B to E). Microscopic examination corroborated these findings (Fig. 4G to J). Significant pulmonary lesions were observed in all four macaques (macaques 4833,4846,13437, and 24162) that succumbed to plague infection. Tissue sections from macaques revealed moderate to severe, multifocal, acute bronchopneumonia. In several fields, portions of alveolar lumina were plugged with edematous fluid, fibrin filaments, numerous neutrophils, monocytes, and macrophages. Alveolar macrophages occasionally contained necrotic cellular debris and, to a lesser extent, bacteria. Large aggregates of bacteria were distributed in interstitial spaces and within alveoli (Fig. 4). Within the lung parenchyma there were multifocal, coalescing areas of necrosis that obliterated the normal architecture and that, in some areas, represented large abscesses. Abscesses were characterized by central foci of lytic necrosis surrounded by degenerate neutrophils interspersed with numerous bacteria entangled in the fibrin and erythrocytes deposited. The macaques belonging to group 2 (rLcrV), group 3 (rV10), group 5 (rLcrV plus rF1), and group 6 (rV10 plus rF1) and the three survivors belonging to group 4 (rF1) did not have any appreciable gross or histopathological changes in their lung tissues (Fig. 4A and F show representative samples).
FIG. 4.
(A to E) Gross lung pathology of macaques challenged with Y. pestis aerosol: dorsal view of lungs from cynomolgus macaques that either succumbed to plague during infection (B to E) or were euthanized at the end of the 14-day observation period (A). (F to J) Corresponding hematoxylin- and eosin-stained lung tissue viewed by microscopy at a magnification of ×200, revealing either normal lung tissue (F) or the histopathologic features of pneumonic plague (G to J). See the text for details.
DISCUSSION
Y. pestis, although most often associated with bubonic plague, causes lethal pneumonia in human populations (37, 72). The latter disease can be contracted either secondarily from a bubonic plague infection, directly from contact with reservoirs where plague is endemic, such as burrowing rodents, or, equally devastatingly, from aerosol transmission from another human (37). Although antibiotics have proven to be invaluable in the treatment of bubonic plague, the control of pestilential spread, the nonspecificity of clinical signs, and the rapidity of a typical pneumonic case often do not allow sufficient time for selection or use of these drugs (31). Alarmingly, recent reports have described a growing incidence of multi-drug-resistant plague strains (25). Particularly notorious is the strain Y. pestis 17/95, which was isolated in 1995 in the Ambalavao district of Madagascar and is resistant to antibiotics that are classically recommended for therapy and prophylaxis (26). In light of the emergence of antibiotic-resistant virulent strains, the recrudescence of plague infections, global biodefense initiatives, and, equally importantly, the increased volume of research being conducted by a susceptible population of researchers during this third pandemic of plague, the development of a vaccine and the identification of novel therapeutics are of paramount importance (14).
The first diagnosis of human primary pneumonic plague was reported by Childe in 1896 (13). Many virulence attributes of Y. pestis contribute to the establishment of pneumonic plague; however, the pathogen has been reported to deploy its Pla protease for the pathogenesis of this unique disease manifestation (33, 34, 60). Nevertheless, much additional work is needed to unravel the biology of Y. pestis aerosol transmission and the pathogenesis of pneumonic plague as defined by K. F. Meyer (37). For instance, it has yet to be shown in a natural infection whether aerosol transmission is the result of direct deposition of Y. pestis into the airways or is simply secondary sequelae to infection of tonsillar tissue (Waldeyer's ring) (37). Several studies of primary pneumonic plague acquired either via intranasal installation or aerosolization have been conducted with nonhuman primates, mice, rats, and guinea pigs and have shown that the animal models of disease do in fact show details of both gross and microscopic pathology similar to those of human disease (23, 58, 59). Histopathology results observed here with four cynomolgus macaques that succumbed to pneumonic plague infection reflected the findings of the previous reports (Fig. 4) (58). More importantly, immunization with rV10 subunit antigen alone or in combination with rF1 prevented pneumonic lesions and disease pathogenesis.
Through use of rodent animal model systems, various plague vaccine formulations have been examined, and humoral immunity was identified as an important component of protection against Y. pestis infection (57). Our studies described here corroborated the findings of other workers that LcrV of Y. pestis (41, 64) and its variant, rV10 (20, 46), are able to activate host immunity and that rLcrV- and rV10-specific antibodies can protect against Y. pestis infection via subcutaneous, intranasal, or aerosol challenge. Epitope mapping studies conducted during this study revealed commonalities in the immune reactivity profiles of rLcrV and rV10 polyclonal sera in different species. Of particular interest to us is the region containing amino acid residues 258 to 274, which falls within the region previously described for the murine monoclonal antibody 7.3, which was mapped broadly to amino acids 135 to 275 (30). All animals immunized with rLcrV (mice, rats, and nonhuman primates) generated antibodies against the LcrV residue 258 to 274 epitope, whereas animals immunized with rV10 did not. Clearly, antibodies directed against LcrV residues 258 to 274 cannot be solely responsible for protection against plague, as rV10-derived immune sera provided immunity even without these antibodies (Fig. 3). The near absence of antibodies against linear peptide epitopes in rV10 immune sera suggests two things. First, antibodies directed at conformational epitopes of LcrV may be able to provide protective immunity and may do so by binding LcrV at the tip of type III needles (43). This could explain the molecular attributes of protective LcrV antibodies, which must block type III injection of immune cells (48) and thereby enable phagocytosis and clearance of the invading pathogen by macrophages and polymorphonuclear leukocytes (15). Second, small changes in the LcrV sequence (for example, deletion of residues 270 to 300) could precipitate fundamental changes in the antibody repertoire of immunized animals, likely because the mechanisms by which LcrV is perceived by the host immune system have been altered (54).
Nevertheless, it is certainly also plausible that antigen-antibody interactions may dictate different methods of neutralization. For example, workers may find that when multiple protective monoclonal antibodies are generated, with each antibody recognizing a discrete linear or discontinuous epitope, one antibody may play a role in opsonophagocytic clearance, another may act via steric hindrance to prevent type III injection of target cells, and another may directly inhibit or physically block the secretion of Yop proteins (49). Future studies should explore these possibilities and the role of rV10 in stimulating T-cell-dependent cellular immunity in a nonhuman primate model of pneumonic plague (57).
We sought to investigate the types of immune responses generated by vaccination of cynomolgus macaques with rLcrV and rV10, as well as combination vaccines that include the highly immunogenic and protective capsular antigen fraction 1 (Caf1 or F1) (39), and to compare the protective immunities against lethal pneumonic plague challenge provided by the antigens mentioned above. Previous studies have shown that F1 and LcrV combination vaccines protect macaques against plague infection (7, 36, 66). Here we demonstrate that both rLcrV and rV10 alone are immunogenic and protective in cynomolgus macaques at a clinically relevant dose, 50 μg per subunit, when they are adsorbed to an aluminum hydroxide gel adjuvant. Following immunization with three doses of vaccine, a peak specific IgG titer, primarily an IgG1 isotype titer, developed by day 49 and conferred protection in a murine passive transfer assay. Interestingly, we did not find a significant difference in titer or in protection between macaques immunized with rLcrV and macaques immunized with rLcrV plus rF1, whereas we did observe a difference between the group immunized with rV10 and the group immunized with rV10 plus rF1. Future studies should address the possible benefit of rV10 compared with rLcrV. There does not appear to be an advantage to incorporating rF1 in either rLcrV or rV10 vaccine formulations for nonhuman primates, which differs from the findings for immunological synergism and increased protection of mice obtained when LcrV was combined with F1 (29, 67, 69). In an attempt to gauge the duration of specific long-term antibody production, we examined the titers for each macaque prior to aerosol challenge on day 85 and noted that for most of the groups, the antibody titers seemed to decline between 30 and 50%. More work is needed to understand the kinetics and longevity of the immune response of macaques to rV10 and how it correlates to that of humans immunized with the same antigen. Future work in assessing plague vaccine candidates must consider challenge studies with highly virulent F1 mutant Y. pestis strains to examine each candidate's true spectrum of protection against a plague outbreak (51).
Acknowledgments
The following reagent was obtained from the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Peptide Array Y. pestis V antigen NR-2867.
We acknowledge membership in and support received from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH award 1-U54-AI-057153). This work was also supported in part by NIH/NIAID challenge award U01-AI070559.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 15 September 2008.
REFERENCES
- 1.Aida, Y., and M. J. Pabst. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods. 132191-195. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, G. W., Jr., S. E. C. Leary, E. D. Williamson, R. C. Titball, S. C. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 644580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, G. P., D. G. Heath, G. W. Anderson, Jr., S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 642180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbaji, A., S. Kharabsheh, S. Al-Azab, M. Al-Kayed, Z. S. Amr, M. Abu Baker, and M. C. Chu. 2005. A 12-case outbreak of pharyngeal plague following the consumption of camel meat, in north-eastern Jordan. Ann. Trop. Med. Parasitol. 99789-793. [DOI] [PubMed] [Google Scholar]
- 5.Bacot, A. W., and C. J. Martin. 1914. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. 13423-439. [PMC free article] [PubMed] [Google Scholar]
- 6.Baker, E. E., H. Sommer, L. E. Foster, E. Meyer, and K. F. Meyer. 1952. Studies on immunization against plague. I. The isolation and characterization of the soluble antigen of Pasteurella pestis. J. Immunol. 68131-145. [PubMed] [Google Scholar]
- 7.Bashaw, J., S. Norris, S. Weeks, S. Trevino, J. J. Adamovicz, and S. Welkos. 2007. Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin. Vaccine Immunol. 14605-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boisier, P., L. Rahalison, M. Rasolomaharo, M. Ratsitorahina, M. Mahafaly, M. Razafimahefa, J.-M. Duplantier, L. Ratsifasomanana, and S. Chanteau. 2002. Epidemiologic features of four successive annual outbreaks of bubonic plague in Mahajanga, Madagascar. Emerg. Infect. Dis. 8311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brubaker, R. R. 2003. Interleukin-10 and the inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 713673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrows, T. W. 1956. An antigen determining virulence in Pasteurella pestis. Nature 177426-427. [DOI] [PubMed] [Google Scholar]
- 11.Burrows, T. W., and G. A. Bacon. 1958. The effect of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br. J. Exp. Pathol. 39278-291. [PMC free article] [PubMed] [Google Scholar]
- 12.Chanteau, S., M. Ratsitorahina, L. Rahalison, B. Rasoamanana, F. Chan, P. Boisier, D. Rabeson, and J. Roux. 2000. Current epidemiology of human plague in Madagascar. Microbes Infect. 225-31. [DOI] [PubMed] [Google Scholar]
- 13.Childe, L. F. 1898. The pathology of plague. Br. Med. J. 2858-862. [Google Scholar]
- 14.Cornelius, C., L. Quenee, D. Anderson, and O. Schneewind. 2007. Protective immunity against plague. Adv. Exp. Med. Biol. 603415-424. [DOI] [PubMed] [Google Scholar]
- 15.Cowan, C., A. V. Philipovskiy, C. R. Wulff-Strobel, Z. Ye, and S. C. Straley. 2005. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect. Immun. 736127-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craven, R. B., G. O. Maupin, M. L. Beard, T. J. Quan, and A. M. Barnes. 1993. Reported cases of human plague infections in the United States 1970-1991. J. Med. Entomol. 30758-761. [DOI] [PubMed] [Google Scholar]
- 17.Crook, L. D., and B. Tempest. 1992. Plague: a clinical review of 27 cases. Arch. Intern. Med. 1521253-1256. [DOI] [PubMed] [Google Scholar]
- 18.Davis, K. J., D. L. Fritz, M. L. M. Pitt, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopitheus aethiops). Arch. Pathol. Lab. Med. 120156-163. [PubMed] [Google Scholar]
- 19.Davis, S., M. Begon, L. De Bruyn, V. S. Ageyev, N. L. Klasovskiy, S. B. Pole, H. Viljugrein, N. C. Stenseth, and H. Leirs. 2004. Predictive thresholds for plague in Khazakhstan. Science 304736-738. [DOI] [PubMed] [Google Scholar]
- 20.DeBord, K. L., D. M. Anderson, M. M. Marketon, K. A. Overheim, R. W. DePaolo, N. A. Ciletti, B. Jabri, and O. Schneewind. 2006. Immunogenicity and protective immunity against bubonic and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 744910-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng, W., V. Burland, G. R. Plunkett, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 1844601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doll, J. M., P. S. Zeitz, P. Ettestad, A. L. Bucholtz, T. Davis, and K. L. Gage. 1994. Cat-transmitted fatal pneumonic plague in a person who traveled from Colorado to Arizona. Am. J. Trop. Med. Hyg. 51109-114. [DOI] [PubMed] [Google Scholar]
- 23.Finegold, M. J. 1969. Pneumonic plague in monkeys. An electron microscopic study. Am. J. Pathol. 54167-185. [PMC free article] [PubMed] [Google Scholar]
- 24.Gage, K. L., D. T. Dennis, K. A. Orloski, P. Ettestad, T. L. Brown, P. J. Reynolds, W. J. Pape, C. L. Fritz, L. G. Carter, and J. D. Stein. 2000. Cases of cat-associated human plague in the Western US, 1977-1998. Clin. Infect. Dis. 30893-900. [DOI] [PubMed] [Google Scholar]
- 25.Galimand, M., E. Carniel, and P. Courvalin. 2006. Resistance of Yersinia pestis to antimicrobial agents. Antimicrob. Agents Chemother. 503233-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galimand, M., A. Guiyoule, G. Gerbaud, B. Rasoamanana, S. Chanteau, E. Carniel, and P. Courvalin. 1997. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 337677-680. [DOI] [PubMed] [Google Scholar]
- 27.Girard, G., and J. Robic. 1942. L'état actuel de la peste à Madagascar et la prophylaxie vaccinale par le virus-vaccin E. V. Bull. Soc. Pathol. Exot. 3542-49. [Google Scholar]
- 28.Haffkine, W. M. 1897. Remarks on the plague prophylactic fluid. Br. Med. J. 11461-1462. [Google Scholar]
- 29.Heath, D. G., G. W. Anderson, Jr., J. M. Mauro, S. L. Welkos, G. P. Andrews, J. J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 161131-1137. [DOI] [PubMed] [Google Scholar]
- 30.Hill, J., S. E. C. Leary, K. Griffin, E. D. Williamson, and R. W. Titball. 1997. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 654476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inglesby, T. V., D. T. Dennis, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, J. F. Koerner, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, M. Schoch-Spana, and K. Tonat. 2000. Plague as a biological weapon: medical and public health management. JAMA 2832281-2290. [DOI] [PubMed] [Google Scholar]
- 32.Kellogg, W. H. 1920. An epidemic of pneumonic plague. Am. J. Public Health 10599-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lathem, W. W., S. D. Crosby, V. L. Miller, and W. E. Goldman. 2005. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc. Natl. Acad. Sci. USA 10217786-17791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lathem, W. W., P. A. Price, V. L. Miller, and W. E. Goldman. 2007. A plaminogen-activating protease specifically controls the development of primary pneumonic plague. Science 315509-513. [DOI] [PubMed] [Google Scholar]
- 35.Leary, S. E. C., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 632854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mett, V., J. Lyons, K. Musiychuk, J. A. Chichester, T. Brasil, R. Couch, R. Sherwood, G. A. Palmer, S. J. Streatfield, and V. Yusibov. 2007. A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine 253014-3017. [DOI] [PubMed] [Google Scholar]
- 37.Meyer, K. F. 1961. Pneumonic plague. Bacteriol. Rev. 25249-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer, K. F. 1970. Effectiveness of live or killed plague vaccines in man. Bull. W. H. O. 42653-666. [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer, K. F., J. A. Hightower, and F. R. McCrumb. 1974. Plague immunization. VI. Vaccination with the fraction 1 antigen of Yersinia pestis. J. Infect. Dis. 129S13-S18. [DOI] [PubMed] [Google Scholar]
- 40.Mittal, V., D. Bhattacharya, U. V. Rana, A. Rai, S. T. Pasha, A. Kumar, A. K. Harit, R. L. Ichhpujani, U. K. Baveja, S. Lal, and S. P. Agarwal. 2006. Prompt laboratory diagnosis in timely containment of a plague outbreak in India. J. Commun. Dis. 38317-324. [PubMed] [Google Scholar]
- 41.Motin, V. L., R. Nakajima, G. B. Smirvov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 624192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motin, V. M., Y. A. Nedialkov, and R. R. Brubaker. 1996. V antigen-polyhistidine fusion peptide: binding to LcrH and active immunity against plague. Infect. Immun. 644313-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller, C. A., P. Broz, S. A. Muller, P. Ringler, F. Erne-Brand, I. Sorg, M. Kuhn, A. Engel, and G. R. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310674-676. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 6123-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 633021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Overheim, K. A., R. W. Depaolo, K. L. Debord, E. M. Morrin, D. M. Anderson, N. M. Green, R. R. Brubaker, B. Jabri, and O. Schneewind. 2005. LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 735152-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry, R. D., P. A. Harmon, W. S. Bowmer, and S. C. Straley. 1986. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect. Immun. 54428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettersson, J., A. Holmstrom, J. Hill, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, A. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32961-976. [DOI] [PubMed] [Google Scholar]
- 49.Philipovskiy, A. V., C. Cowan, C. R. Wulff-Strobel, S. H. Burnett, E. J. Kerschen, D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2005. Antibody against V antigen prevents Yop-dependent growth of Yersinia pestis. Infect. Immun. 731532-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pouliot, K., N. Pan, S. Wang, S. Lu, E. Lien, and J. D. Goguen. 2007. Evaluation of the role of LcrV-Toll-like receptor 2-mediated immunomodulation in the virulence of Yersinia pestis. Infect. Immun. 753571-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quenee, L., C. A. Cornelius, N. A. Ciletti, D. Elli, and O. Schneewind. 2008. Yersinia pestis caf1 (F1) variants and the limits of plague vaccine protection. Infect. Immun. 762025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratsitorahina, M., S. Chanteau, L. Rahalison, L. Ratisofasoamanana, and P. Boisier. 2000. Epidemiological and diagnostic aspects of the outbreak of pneumonic plague in Madagascar. Lancet 355111-113. [DOI] [PubMed] [Google Scholar]
- 53.Russell, P., S. M. Eley, S. E. Hibbs, R. J. Manchee, A. J. Stagg, and R. W. Titball. 1995. A comparison of plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine 131551-1556. [DOI] [PubMed] [Google Scholar]
- 54.Shim, H. K., J. A. Musson, H. M. Harper, H. V. McNeill, N. Walker, H. Flick-Smith, A. von Delwig, E. D. Williamson, and J. H. Robinson. 2006. Mechanisms of major histocompatibility complex class II-restricted processing and presentation of the V antigen of Yersinia pestis. Immunology 119385-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sing, A., A. Roggenkamp, A. M. Geiger, and J. Heesemann. 2002. Yersinia enterocolitica evasion of the host immune response by V antigen-induced IL-10 production of macrophages is abrogated in IL-10-deficient mice. J. Immunol. 1681315-1321. [DOI] [PubMed] [Google Scholar]
- 56.Sing, A., D. Rost, N. Tvardovaskaia, A. Roggenkamp, A. Wiedemann, C. Kirschning, J. M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 1961017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smiley, S. T. 2008. Current challenges in the development of vaccines for pneumonic plague. Expert Rev. Vaccines 7209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith, P. N. 1959. Pneumonic plague in mice: gross and histopathology in untreated and passively immunized animals. J. Infect. Dis. 10478-84. [DOI] [PubMed] [Google Scholar]
- 59.Smith, P. N., J. McCamish, J. Seeley, and G. M. Cooke. 1957. The development of pneumonic plague in mice and the effect of paralysis of respiratory cilia upon the course of infection. J. Infect. Dis. 100215-222. [DOI] [PubMed] [Google Scholar]
- 60.Sodeinde, O., Y. Subrahmanyam, K. Stark, T. Quan, Y. Bao, and J. Goguen. 1992. A surface protease and the invasive character of plague. Science 2581004-1007. [DOI] [PubMed] [Google Scholar]
- 61.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 polymerase to direct expression of cloned genes. Methods Enzymol. 18560-89. [DOI] [PubMed] [Google Scholar]
- 62.Thullier, P., V. Guglielmo, M. Rajerison, and S. Chanteau. 2003. Serodiagnosis of plague in humans and rats using a rapid test. Am. J. Trop. Med. Hyg. 69450-451. [PubMed] [Google Scholar]
- 63.Titball, R. W., and E. D. Williamson. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 194175-4184. [DOI] [PubMed] [Google Scholar]
- 64.Une, T., and R. R. Brubaker. 1984. Roles of V antigen in promoting virulence and immunity in yersiniae. J. Immunol. 1332226-2230. [PubMed] [Google Scholar]
- 65.Van Andel, R., R. Sherwood, C. Gennings, C. R. Lyons, J. Hutt, A. Gigliotti, and E. Barr. 2008. Clinical and pathological features of Cynomologus macaques (Macaca fascicularis) infected with aerosolized Yersinia pestis. Comp. Med. 5868-75. [PMC free article] [PubMed] [Google Scholar]
- 66.Welkos, S., S. Norris, and J. Adamovicz. 2008. Modified caspase-3 assay indicates correlation of caspase-3 activity with immunity of nonhuman primates to Yersinia pestis infection. Clin. Vaccine Immunol. 151134-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 2000. A single dose sub-unit vaccine protects against pneumonic plague. Vaccine 19566-571. [DOI] [PubMed] [Google Scholar]
- 68.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 1997. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine 151079-1084. [DOI] [PubMed] [Google Scholar]
- 69.Williamson, E. D., H. C. Flick-Smith, C. LeButt, C. A. Rowland, S. M. Jones, E. L. Waters, R. J. Gwyther, J. Miller, P. J. Packer, and M. Irving. 2005. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 733598-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winter, C. C., W. B. Cherry, and M. D. Moody. 1960. An unusual strain of Pasteurella pestis isolated from a fatal case of human plague. Bull. W. H. O. 23408-409. [PMC free article] [PubMed] [Google Scholar]
- 71.Worsham, P. L., M. P. Stein, and S. L. Welkos. 1995. Construction of defined F1 negative mutants of virulent Yersinia pestis. Contrib. Microbiol. Immunol. 13325-328. [PubMed] [Google Scholar]
- 72.Yersin, A. 1894. La peste bubonique à Hong-Kong. Ann. Inst. Pasteur 2428-430. [Google Scholar]