Abstract
Human neutrophil peptide α-defensins (HNPs) and human β-defensins (HBDs) are small well-characterized peptides with broad antimicrobial activities and a diversity of innate immune functions. Although the interactions of defensins with bacteria and their membranes have been well characterized, the interactions of defensins with bacterial adhesins have not. Here we determine if HNPs and HBDs bind to the immobilized adhesins of Porphyromonas gingivalis strain 381, recombinant hemagglutinin B (rHagB) and recombinant fimbrillin A (rFimA), by surface plasmon resonance spectroscopy. Association of HNPs and HBDs with rHagB or rFimA was dose dependent and defensin specific. HBD3, HNP-2, and HNP-1 bound more readily to immobilized rHagB than HBD2 and HBD1 did. HNP-2, HNP-1, and HBD3 bound more readily to immobilized rFimA than HBD1 and HBD2 did. Binding of defensins to adhesins may serve to prevent microbial adherence to tissues, attenuate proinflammatory cytokine responses, and facilitate delivery of bound antigen to antigen-presenting cells with defensin receptors.
Human defensins are small, host-derived peptides with a variety of innate and adaptive immune functions (8, 20, 36, 63). They kill gram-negative bacteria, gram-positive bacteria, and fungi and inhibit some viral infections (18, 21, 25, 28, 48); chemoattract monocytes, macrophages, CD45 RA+/CD4+ T lymphocytes, mast cells, and immature dendritic cells (10, 21, 62); enhance antigen-specific immune responses (9, 38); and even possibly regulate pigmentation and body weight via modulation of Mc1r and Mc4r (1). Defensins also bind to bacterial membranes (7, 17) and lipopolysaccharides (17, 39) and neutralize select bacterial toxins (23, 31, 32). Binding of human neutrophil peptide α-defensins (HNPs) and human β-defensins (HBDs) to these materials may facilitate receptor-mediated internalization of microbial antigens to immature dendritic cells (61) or attenuate antigen-induced proinflammatory cytokine responses (44).
Both HNPs and HBDs are detected in oral tissues, salivary glands, salivary secretions, gingival crevicular fluid, and gingival tissue (6, 14, 24, 29, 47, 49). HNP-1 to -4 are present in gingival crevicular fluid (45), and HBD2 (0.0095 μg/ml) (22) and HBD3 (0.1 to 0.89 μg/ml) (15, 22, 52) are present in saliva. Thus, HNPs and HBDs are ideally positioned in the oral cavity to interact with an extensive and diverse group of both commensal and pathogenic microbial antigens. This includes adhesins of Porphyromonas gingivalis, a periodontal pathogen that induces proinflammatory cytokines (11, 51, 64).
Porphyromonas gingivalis is one of the dominant etiologic agents of periodontal disease (26, 35, 43). Attachment and invasion of the organism are promoted by adhesins, including fimbriae (16, 59) and hemagglutinins (27, 30, 37, 40). Local tissue damage and evasion of host defense mechanisms are facilitated by capsular polysaccharides (34, 55), lipopolysaccharide (2, 3, 58), and proteolytic enzymes (4, 5, 13, 46, 53, 54). Previously, we reported that HBD3 binds to immobilized recombinant hemagglutinin B (rHagB) of P. gingivalis strain 381 and produces a significantly higher resonance unit (RU) signal in surface plasmon resonance (SPR) spectroscopic analysis than do HBD2 and HBD1 used as control defensins (44). This led us to ask whether other HNPs and HBDs, which differ in compositions and cationic charges, would bind preferentially to P. gingivalis rHagB or recombinant fimbrillin A (rFimA), nonfimbrial and fimbrial adhesins of different compositions, respectively. We hypothesized that HNPs and HBDs would bind differentially to rHagB and rFimA.
MATERIALS AND METHODS
P. gingivalis adhesin ligands.
HagB is a major virulence factor and is among the more closely studied hemagglutinins of P. gingivalis. rHagB was prepared as previously described (33, 50). Briefly, rHagB (1.4 kb) was cloned into the vector pQE31 (Qiagen Inc., Valencia, CA), designated pQE31-TX1, and expressed in Escherichia coli M15(pREP4)pQE31-TX1. The histidine-tagged rHagB was purified on a nickel-nitrilotriacetic acid affinity column (Bio-Rad Laboratories, Hercules, CA) and dialyzed against 500 mM sodium chloride and 10 mM Tris, pH 7.4. The composition and purity of rHagB were verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, mass spectrometry, and amino acid analysis (High Resolution Mass Spectrometry Facility, University of Iowa, Iowa City, IA). Lipopolysaccharide content was 1.9 ng per 1.0 μg rHagB (QCL-1000 chromogenic Limulus amebocyte lysate assay; Cambrex, Walkersville, MD). The observed mass of rHagB was 49,560.56 Da. For SPR spectroscopy, rHagB was adjusted to contain 2.0 μM in 10 mM sodium acetate, pH 5.0 (immobilization buffer).
rFimA was produced by PCR amplification of the fimA coding sequences from P. gingivalis chromosomal DNA as described before (60). rFimA was expressed in E. coli by using a pET protein expression system (Novagen, Wisconsin). The PCR products were cloned into pCRII-TOPO (Invitrogen, Carlsbad, CA). The fimA fragment was then subcloned into pET-30b downstream of a histidine tag. The rFimA was expressed in E. coli BL21(DE3) cells carrying the pET-30b/fimA plasmid in the presence of IPTG (isopropyl-β-d-thiogalactopyranoside) and kanamycin. His-tagged rFimA was purified with Ni2+-charged His-bind resin (Novagen, Wisconsin). The His tag on the recombinant protein was cleaved with enterokinase and removed by His-bind resin. Enterokinase was then removed by using Ekapture agarose. The purity of the rFimA preparation was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, mass spectrometry, and amino acid analysis (High Resolution Mass Spectrometry Facility, University of Iowa, Iowa City, IA). rFimA has a theoretical pI of 6.99, a predicted monoisotopic mass of 45,342.08 Da, and an observed matrix-assisted laser desorption ionization mass of 45,359.2 Da. Lipopolysaccharide content was 60 pg per 1.0 μg rFimA (QCL-1000 chromogenic Limulus amebocyte lysate assay; Cambrex, Walkersville, MD). For SPR spectroscopy, rFimA was adjusted to contain 2.2 μM in immobilization buffer.
Defensin analytes.
Stock solutions (200 μg/ml) of HNP-1 and HNP-2 (American Peptide Company, Sunnyvale, CA) and HBD1, HBD2, and HBD3 (PeproTech, Inc., Rocky Hill, NJ) were prepared in 10 mM HEPES, pH 7.4, containing 150 mM NaCl, 3 mM EDTA, and 0.005% surfactant P20 (HBS-EP buffer). The purity, mass, and composition of these peptides were confirmed by matrix-assisted laser desorption ionization-time of flight. Lipopolysaccharide content was 0.1 to 4.5 pg per 1.0 μg HNP-1, HNP-2, HBD1, HBD2, or HBD3 (QCL-1000 chromogenic Limulus amebocyte lysate assay; Cambrex, Walkersville, MD). Content and composition of each defensin were determined by amino acid analysis (High Resolution Mass Spectrometry Facility, University of Iowa, Iowa City, IA).
SPR spectroscopy.
SPR spectroscopy (Biacore 3000; Biacore, Inc., Piscataway, NJ) was performed in CM5 sensor chips. For this, 0.4 M 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride and 0.1 M N-hydroxysuccinimide were passed through the flow cell, adding succinimide esters to the carboxyl groups on the carboxymethyl dextran surface. Then 2.0 μM rHagB or 2.2 μM rFimA in immobilization buffer was passed through the flow cell. Primary amine groups of these ligands react spontaneously with the succinimide esters covalently linking them directly to the carboxymethyl dextran surface. The immobilization levels of rHagB and rFimA were 5,437 and 14,454 RU, respectively. These responses were similar to those of proteins immobilized in other studies (41, 56). Finally, 1.0 M ethanolamine-HCl (pH 8.5) was passed through the flow cell to deactivate any free succinimide esters remaining on the carboxymethyl dextran surface.
Mobile defensin analytes and HBS-EP were passed through the test and control CM5 flow cells at 20 μl/minute for ∼2 to 4 min at 25°C. Dissociation was analyzed for 3 min by passing HBS-EP through the flow cell and monitoring the surface plasmon RU signal responses. After each analysis, the sensor chip surfaces were regenerated with 2.5 M glycine and equilibrated with HBS-EP before the next analysis. The association of each concentration of defensin with rHagB was assessed in three replications. The kinetic association (Ka) and dissociation (Kd) rate constants of the binding curves in the SPR sensorgrams were calculated using BIAevaluation, version 4.1 (Biacore, Inc., Piscataway, NJ). The equilibrium dissociation constant KD was calculated as Kd/Ka. Higher Ka values imply faster binding recognition of the mobile analytes for the immobilized ligands, lower Kd values imply more-stable binding to the ligands, and lower KD values imply higher affinities for the ligands.
Enzyme-linked immunosorbent assay (ELISA).
Solutions of rHagB and rFimA (0.02 μM) were prepared in 0.01 M phosphate-buffered saline, pH 7.2, and 100 μl of these suspensions was put into Immulon 1 microtiter wells and held overnight at 26°C. Unattached ligand and phosphate-buffered saline were aspirated from the wells, and 0.01 M Tris buffer containing 0.145 M NaCl, 1.0% fish gelatin, and 0.05% blocking buffer was added and held for 30 min at 26°C. This step was repeated. Solutions of HBD1, HBD2, and HBD3 (2.0 μM) were prepared in blocking buffer, diluted twofold, and added to the plates containing rHagB and rFimA. Rabbit antiserum prepared to P. gingivalis whole cells was also diluted twofold and added to the plates containing rHagB and rFimA. Blocking buffer was added to the control wells of the plate. After incubation for 1 hour at 26°C, all wells were washed three times with blocking buffer and 100 μl of rabbit anti-HBD1, anti-HBD2, and anti-HBD3 (1 μg/ml; PeproTech, Inc., Rocky Hill, NJ) was added to the respective rows in each plate. After incubation for 1 hour at 26°C, all wells were washed three times with blocking buffer and 100 μl of peroxidase-labeled goat anti-rabbit immunoglobulin G antibody (1 μg/ml; Kirkegaard & Perry Laboratories [KPL], Inc., Gaithersburg, MD) was added. After incubation for 1 hour at 26°C, all wells were washed three times with blocking buffer. Then 100 μl of peroxidase developing reagent (TMB Microwell peroxidase substrate, one component, catalog no. 53-00-02; KPL, Inc., Gaithersburg, MD) was added per well and 100 μl stop reagent (TMB stop, one component, KPL catalog no. 50-85-05) was added per well. The optical densities of the wells in the plate were determined in the spectrophotometer (450 nm; PowerWavex; BioTek Instruments, Inc., Winooski, VT) and corrected by subtracting the optical densities of the blank wells.
Statistics.
The nonparametric Kruskal-Wallis test was used to compare differences among the RU signal responses of the four concentrations of each defensin and to compare differences among the RU signal responses of five defensins at each concentration. Adjustment for multiple pairwise comparisons was accomplished using the adaptation of the Tukey method recommended by Conover (12) in conjunction with an overall 0.05 level of significance. The Spearman rank correlation was used to assess the possibility of an increasing or decreasing dose-response relationship between concentrations and RU signal responses for each defensin using a nominal 0.05 level of significance.
RESULTS
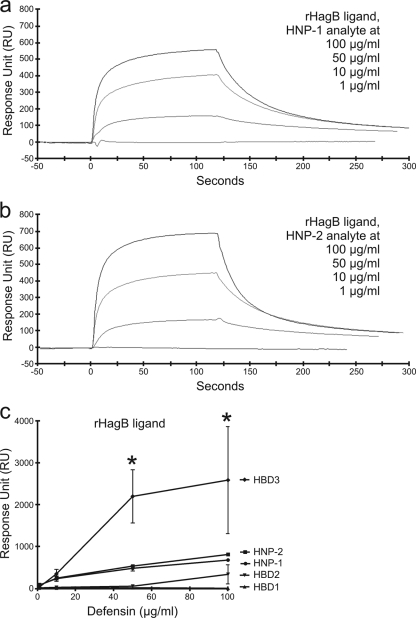
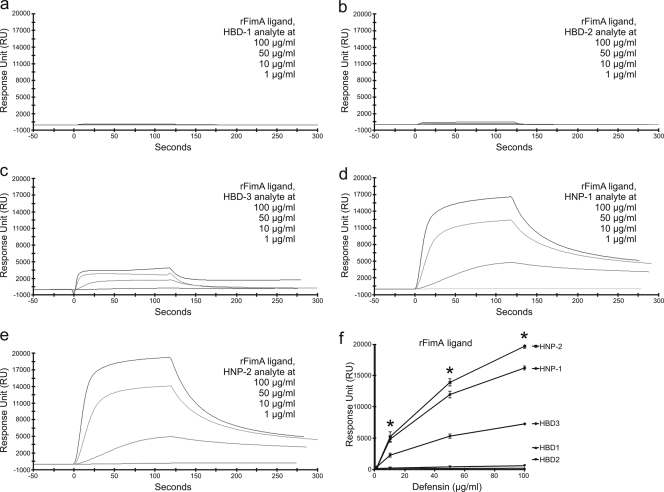
The mean kinetic dissociation constants (KD) of HBD1, HBD2, HBD3, HNP-1, and HNP-2, for rHagB and rFimA, ranged from 2.27 × 10−7 to 4.08 × 10−10 (n = 3) (Table 1), which are all in the range of those reported for the affinities of cell adhesion molecules, receptor ligands, and monoclonal antibodies. Single representative SPR sensorgrams for these interactions are shown in Fig. 1 and 2, and the mean RU signal responses (n = 3) are included in Table 1 and compared in Fig. 1c and 2f.
TABLE 1.
Binding of defensins to adhesins of Porphyromonas gingivalisa
| Ligand and analyte | Constantd
|
Relative response (RU) at concn:
|
|||||
|---|---|---|---|---|---|---|---|
| Ka | Kd | KD | 1 μg | 10 μg | 50 μg | 100 μg | |
| rHagBb | |||||||
| HBD1 | 2.14E+03 (1.14E+03) | 1.37E−05 (2.67E−06) | 8.76E−09 (2.30E−09) | 0.0 (0.0) | 0.0 (0.0) | 13.3 (13.3) | 0.0 (0.0) |
| HBD2 | 1.76E+05 (1.14E+05) | 9.74E−03 (4.86E−03) | 5.00E−08 (2.95E−08) | 9.3 (4.0) | 21.8 (6.1) | 49.5 (20.6) | 333.2 (227.9) |
| HBD3 | 1.83E+04 (1.73E+04) | 6.00E−04 (5.90E−04) | 1.79E−08 (7.83E−09) | 28.7 (18.4) | 345.7 (107.8) | 2,197.0 (635.8) | 2,586.5 (1,278.0) |
| HNP-1 | 1.64E+06 (5.63E+05) | 1.28E−02 (1.11E−03) | 1.08E−08 (4.41E−09) | 77.5 (41.7) | 230.8 (64.2) | 478.2 (65.0) | 675.1 (22.3) |
| HNP-2 | 1.26E+07 (6.15E+06) | 1.38E−02 (8.88E−04) | 1.66E−09 (5.94E−10) | 74.3 (37.1) | 242.6 (42.9) | 531.8 (30.4) | 809.2 (35.9) |
| rFimAc | |||||||
| HBD1 | 4.16E+06 (2.23E+06) | 1.96E−03 (1.17E−03) | 3.70E−09 (3.25E−09) | 192.0 (192.0) | 218.4 (218.4) | 201.0 (154.6) | 237.5 (132.9) |
| HBD2 | 1.16E+10 (1.14E+10) | 6.53E−02 (3.20E−02) | 4.08E−10 (3.01E−10) | 194.3 (188.6) | 260.6 (205.1) | 453.0 (139.3) | 635.8 (103.3) |
| HBD3 | 2.78E+04 (8.24E+03) | 1.14E−02 (4.51E−04) | 4.88E−07 (1.32E−07) | 424.2 (153.5) | 2,324.0 (314.9) | 5,361.9 (342.8) | 7,316.3 (130.7) |
| HNP-1 | 1.57E+04 (2.72E+03) | 6.83E−03 (2.02E−04) | 4.60E−07 (7.47E−08) | 232.2 (184.2) | 4,878.8 (486.9) | 12,012.0 (537.1) | 16,259.0 (323.0) |
| HNP-2 | 1.32E+05 (8.52E+04) | 8.17E−03 (4.15E−04) | 2.27E−07 (1.69E−07) | 332.7 (182.7) | 5,269.9 (791.5) | 13,948.3 (574.5) | 19,730.8 (352.3) |
Values are shown as means (standard errors of the means).
Recombinant hemagglutinin B (rHagB) adhesin of P. gingivalis strain 381.
Recombinant fimbrillin A (rFimA) adhesin of P. gingivalis strain 381.
FIG. 1.
SPR spectroscopy sensorgrams showing concentration-dependent binding of HNP-1 and HNP-2 to immobilized rHagB from P. gingivalis. Sensorgrams (a and b) show the overlay of four binding isotherms generated from decreasing concentrations (top line to bottom line, respectively) of HNP-1 and HNP-2. The binding affinity of each defensin for immobilized rHagB was determined by kinetic analysis calculated from the association (Ka) and dissociation (Kd) rates of the four binding isotherms together in each graph using BIAevaluation software version 4.1 (Pharmacia Biosensor AB). HNP-2 and HNP-1 had high affinities characterized by high association and low dissociation rates for immobilized rHagB. The RU signal responses of HNP-2 and HNP-1 in the graph (c) were higher than those previously reported for HBD2 and HBD1 and yet lower than that previously reported for HBD3 (44). *, P < 0.05.
FIG. 2.
SPR spectroscopy sensorgrams showing concentration-dependent binding of HNP and HBD defensins to immobilized rFimA from P. gingivalis. Sensorgrams (a to e) show the overlay of four binding isotherms generated from decreasing concentrations (top line to bottom line, respectively) of defensins. The binding affinity of each defensin for immobilized rFimA was determined by kinetic analysis calculated from the association (Ka) and dissociation (Kd) rates of the four binding isotherms together in each graph using BIAevaluation software version 4.1 (Pharmacia Biosensor AB). HBD1 (a) and HBD2 (b) did not appear to bind to rFimA. HNP-2, HNP-1, and HBD3 had higher affinities characterized by high association and low dissociation rates for immobilized rFimA. The RU signal responses of HNP-2, HNP-1, and HBD3 in the graph (f) were higher than those for HBD1 and HBD2. *, P < 0.05.
Defensin binding to rHagB ligand.
Previously, we observed that HBD3 binds to immobilized rHagB using SPR spectroscopy (44). Binding with HBD3 was slow (Ka = 1.83 × 104) and more stable (Kd = 6.00 × 10−4) with lower affinity (KD = 1.79 × 10−8). In comparison, binding with HBD1 was slower (Ka = 2.14 × 103) and more stable (Kd = 1.37 × 10−5) with higher affinity (KD = 8.76 × 10−9) and binding with HBD2 was faster (Ka = 1.76 × 105) and yet stable (Kd = 9.74 × 10−3) with lower affinity (KD = 5.00 × 10−8). HBD3 also produced a significantly higher RU signal response than did both HBD1 and HBD2 (Table 1) (44).
In comparison to the results reported with the β-defensins (44), HNP-1 and HNP-2 had high association rates and lower dissociation rates for immobilized rHagB. Binding of HNP-1 to rHagB was fast (Ka = 1.64 × 106) and less stable (Kd = 1.28 × 10−2) with high affinity (KD = 1.08 × 10−8) (Table 1). Similarly, the binding of HNP-2 was fast (Ka = 1.26 × 107) and less stable (Kd = 1.38 × 10−2) with higher affinity (KD = 1.66 × 10−9) (Table 1).
When the RU signal responses of HNP-1 and HNP-2 to immobilized rHagB were compared with the responses of HBD1, -2, and -3 to immobilized rHagB (44), the response of HBD3 was much higher than (in descending order) HNP-2, HNP-1, HBD2, and HBD1 at all concentrations (Fig. 1c). The RU signal responses of HBD3 (100.0 μg/ml) were significantly higher than those of HBD1, HBD2, and HNP-1; the RU signal responses of HNP-2 were significantly higher than those of HBD1 and HBD2; and the RU signal responses of HNP-1 were significantly higher than that of HBD1. Finally, at 100.0 μg/ml, the RU signal responses for HBD3 and HNP-2, while not significantly different from each other, were significantly higher than those for all other defensins.
To confirm the stronger binding association between HBD3 and rHagB and to rule out nonspecific association of HBD3 with rHagB, HBD1, HBD2, or HBD3 was immobilized to the carboxymethylated dextran via amine coupling and 0.1, 1.0, 10.0, or 100.0 μg/ml rHagB in HBS-EP was then passed over the immobilized HBD surfaces. Similarly, rHagB bound more readily to HBD3 than to (in descending order) HBD2 and HBD1 (data not shown).
Defensin binding to rFimA ligand.
The SPR sensorgrams showed that the binding patterns of HNPs and HBDs to immobilized rFimA were vastly different (Fig. 2a to f). HBD1 and HBD2 did not readily bind to immobilized rFimA (Fig. 2). They had lower RU signal responses than HNP-2, HNP-1, and HBD3 and yet bound with higher affinities (Table 1). In contrast, HNP-2, HNP-1, and HBD3 more readily bound to immobilized rFimA (Fig. 2). They had higher RU signal responses than did HBD1 and HBD2 but had lower affinities (Table 1).
Binding of HBD1 to rFimA was fast (Ka = 4.16 × 106) and more stable (Kd = 1.96 × 10−3) with higher affinity (KD = 3.70 × 10−9) (Table 1). Similarly, the binding of HBD2 was fast (Ka = 1.16 × 1010) and less stable (Kd = 6.53 × 10−2) with higher affinity (KD = 4.08 × 10−10) (Table 1). Binding of HBD3 to rFimA was slow (Ka = 2.78 × 104) and less stable (Kd = 1.14 × 10−2) with lower affinity (KD = 4.88 × 10−7) (Table 1).
Binding of HNP-1 to rFimA was slower (Ka = 1.57 × 104) and more stable (Kd = 6.83 × 10−3) with lower affinity (KD = 4.60 × 10−7) (Table 1). Binding of HNP-2 to rFimA was fast (Ka = 1.32 × 105) and stable (Kd = 8.17 × 10−3) with higher affinity (KD = 2.27 × 10−7) (Table 1).
When the RU signal responses of HNPs and HBDs to immobilized rFimA were compared, the RU signal responses of HNP-2 were much higher than (in descending order) HNP-1, HBD3, HBD1, and HBD2 at all concentrations (Fig. 2f).
ELISA.
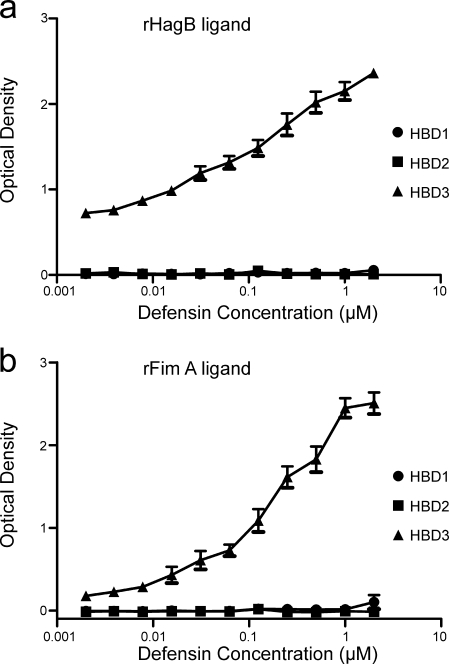
An additional assay was needed to assess and confirm the differential binding patterns of defensins for rHagB and rFimA. Since good antibodies exist for HBD1, HBD2, and HBD3, an ELISA was developed to detect HBD binding to immobilized rHagB and rFimA (Fig. 3). Similar to the SPR spectroscopy results, HBD3, but not HBD1 or HBD2, bound to both rHagB and rFimA immobilized on Immulon 1 plates.
FIG. 3.
Graphs showing strong HBD3 binding to both 0.02 μM rHagB (a) and 0.02 μM rFimA (b) immobilized on Immulon 1 plates and detected with affinity-purified rabbit anti-HBD antibody and peroxidase-labeled goat anti-rabbit antibody. Plotted is the optical density versus the log10 of the dilution of HBD3 starting at 2.0 μM. In each case, binding with HBD3 was dose dependent. In comparison, there was minimal binding of HBD1 and HBD2 to rHagB (a) and rFimA (b).
DISCUSSION
Recently, we reported that HBD3 binds to immobilized rHagB and produces significantly higher RU signal responses in SPR spectroscopic analysis than do HBD2 and HBD1 (44). This led us to ask whether other HNPs and HBDs would bind to rHagB or rFimA, nonfimbrial and fimbrial adhesins of different compositions, respectively. When the results for each ligand were compared, we found that HBD3, HNP-2, and HNP-1, in decreasing order, bind to immobilized rHagB and HNP-2, HNP-1, and HBD3, in decreasing order, bind to immobilized rFimA. Interestingly, HBD1 and HBD2 did not bind particularly well to either rHagB or rFimA. HBD1 and HBD2 generally had lower RU signal responses than did HNP-2, HNP-1, and HBD3 and yet bound with higher affinities. HBD3, HNP-1, and HNP-2 did bind well to rHagB and rFimA. They had significantly higher RU signal responses than did HBD1 and HBD2 and yet bound with lower affinities. Taken together, these results suggest that HNPs and HBDs have the capacity to bind to different P. gingivalis adhesins. These results also suggest that HNPs and HBDs have different binding recognition rates, different dissociation rates, and different affinities for these bacterial adhesins.
The results in this study were consistent with other data in the literature suggesting that HBD3, HNP-1, and HNP-2 generally bind to microbial products and viruses but that HBD1 and HBD2 generally do not. Defensins are known to bind to a variety of bacterial products, often with attenuation of their toxic or inflammation-inducing capacities. For example, the β-defensin DEFB123 prevents lipopolysaccharide-induced production of tumor necrosis factor alpha secretion in murine monocyte RAW 264.7 cells, abolishes lipopolysaccharide-mediated mitogen-activated protein kinase induction in RAW 264.7 cells, protects mice against lipopolysaccharide-mediated acute sepsis, and prevents lipopolysaccharide-induced mortality in C57BL/6 mice (39). HNP-1 binds to Bacillus anthracis lethal factor, causes a conformational change that prevents enzymatic conversion, and protects mice from B. anthracis lethal factor intoxication and death (31). Rhesus θ-defensins 1 to 3, retrocyclins 1 to 3, and several analogues of retrocyclin 1 also bind to B. anthracis lethal factor (57). HNP-1, HNP-3, and HD5 but not HBD1 bind with high affinity to Clostridium difficile toxin B and inhibit toxin B-catalyzed in vitro glucosylation of Rho GTPases (23).
Defensins are also known to bind to a variety of viruses and viral products. HNP1 to HNP4, HD5, HD6, and HBD3 inhibit herpes simplex virus infection, but HBD1 and HBD2 do not (25). HNP-4 and HD6 prevent virus attachment and entry into cells, whereas HNP-1, HNP-2, HNP-3, and HD5 inhibit only postentry events (25). SPR spectroscopy was used to show that HNP-1, HNP-2, HNP-3, and HD5 associated with herpes simplex virus glycoprotein B with high affinity but associated with heparan sulfate, the receptor for attachment, with low affinity (25). HNP-4 and HD6 associated with heparan sulfate but not glycoprotein B. HBD3 associated with both glycoprotein B and heparan sulfate, but HBD1 and HBD2 did not. Retrocyclin 1 bound to CD4 (the primary host receptor for human immunodeficiency virus type 1 [HIV-1]), to galactosylceramide (an alternative cell surface receptor for HIV-1), to gp120 (the envelope glycoprotein of HIV-1) (41, 42, 56), and to the ectodomain of gp41 (HIV-1) (19).
The results in this study also support the findings of our recent work in which we hypothesized that HBD3 directly binds to rHagB (44). The binding between HBD3 and rHagB attenuated the proinflammatory cytokine responses induced by rHagB in human myeloid dendritic cell culture supernatants and significantly attenuated the extracellular signal-regulated kinase 1/2 response in human myeloid dendritic cell lysates. The results suggested that HBD3 serves as an upstream suppressor of cytokine signaling that has the potential to regulate and attenuate proinflammatory cytokine responses in mucosal secretions and tissues to microbial antigens. The results in this study also show that other HNPs and HBDs may have the same capacity. Whether other HNPs and HBDs can attenuate the proinflammatory cytokine responses induced by rHagB or rFimA in human myeloid dendritic cell culture supernatants or phosphorylated mitogen-activated protein kinase proteins in human myeloid dendritic cell lysates is not yet known.
The exact mechanism for the binding of HNPs or HBDs to these adhesins is not readily known but is likely related to the unique properties of the defensins and the differing compositions of P. gingivalis adhesins. It could be a nonspecific binding based on electrostatic interactions between the defensin analyte and the immobilized adhesin ligand. If so, we would expect to see binding recognition rates, different dissociation rates, and different affinities align with the cationic charge of the defensin analyte. Primary sequence algorithms show that HBD1 has a net +3 charge, HBD2 has a net +7 charge, HBD3 has a net +11 charge, and HNP-1 and HNP-2 have a net +3 charge. These predicted values do not accurately reflect the molecular dynamics required for the specific analyte-ligand interactions shown in Table 1 and Fig. 1 and 2. Rather, it is more likely that there may be defensin-specific binding domains on these adhesins that favor higher-affinity binding of one defensin over another defensin. It is too soon to speculate that this is a rudimentary recognition and signaling mechanism of the innate immune system.
In summary, HNPs and HBDs, which differ in compositions and cationic charges, bind preferentially to P. gingivalis rHagB or rFimA, nonfimbrial and fimbrial adhesins of different compositions, respectively. It may be possible that binding of defensins to these adhesins may prevent their adherence to tissues, attenuate adhesin-induced proinflammatory cytokine responses, and facilitate delivery of bound antigen to antigen-presenting cells with defensin receptors.
Acknowledgments
We are grateful to James D. Herd for preparation of the figures and to Elizabeth A. Schmitt, Cairn Communications, Mahtomedi, MN, for critically reading the manuscript.
This work was supported by funds from by NIH/NIDCR T32 DE014678 and R01 DE014390.
Editor: F. C. Fang
Footnotes
Published ahead of print on 13 October 2008.
REFERENCES
- 1.Abdel-Malek, Z. A., and D. Supp. 2008. Beta-defensin 3: a novel and unexpected key that unlocks the melanocortin 1 receptor. Pigment Cell Melanoma Res. 217-8. [DOI] [PubMed] [Google Scholar]
- 2.Bainbridge, B. W., and R. P. Darveau. 1999. Lipopolysaccharide from oral bacteria: role in innate host defense and chronic inflammatory disease, p. 899-913. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker, Inc., New York, NY.
- 3.Bainbridge, B. W., and R. P. Darveau. 2001. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol. Scand. 59131-138. [DOI] [PubMed] [Google Scholar]
- 4.Banbula, A., M. Bugno, J. Goldstein, J. Yen, D. Nelson, J. Travis, and J. Potempa. 2000. Emerging family of proline-specific peptidases of Porphyromonas gingivalis: purification and characterization of serine dipeptidyl peptidase, a structural and functional homologue of mammalian prolyl dipeptidyl peptidase IV. Infect. Immun. 681176-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banbula, A., P. Mak, M. Bugno, J. Silberring, A. Dubin, D. Nelson, J. Travis, and J. Potempa. 1999. Prolyl tripeptidyl peptidase from Porphyromonas gingivalis. A novel enzyme with possible pathological implications for the development of periodontitis. J. Biol. Chem. 2749246-9252. [DOI] [PubMed] [Google Scholar]
- 6.Bissell, J., S. Joly, G. K. Johnson, C. C. Organ, D. Dawson, P. B. McCray, and J. M. Guthmiller. 2004. Expression of beta-defensins in gingival health and in periodontal disease. J. Oral Pathol Med. 33278-285. [DOI] [PubMed] [Google Scholar]
- 7.Bohling, A., S. O. Hagge, S. Roes, R. Podschun, H. Sahly, J. Harder, J. M. Schroder, J. Grotzinger, U. Seydel, and T. Gutsmann. 2006. Lipid-specific membrane activity of human beta-defensin-3. Biochemistry 455663-5670. [DOI] [PubMed] [Google Scholar]
- 8.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3238-250. [DOI] [PubMed] [Google Scholar]
- 9.Brogden, K. A., M. Heidari, R. E. Sacco, D. Palmquist, J. M. Guthmiller, G. K. Johnson, H. P. Jia, B. F. Tack, and P. B. McCray. 2003. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol. Immunol. 1895-99. [DOI] [PubMed] [Google Scholar]
- 10.Chen, X., F. Niyonsaba, H. Ushio, M. Hara, H. Yokoi, K. Matsumoto, H. Saito, I. Nagaoka, S. Ikeda, K. Okumura, and H. Ogawa. 2007. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur. J. Immunol. 37434-444. [DOI] [PubMed] [Google Scholar]
- 11.Chou, H. H., H. Yumoto, M. Davey, Y. Takahashi, T. Miyamoto, F. C. Gibson III, and C. A. Genco. 2005. Porphyromonas gingivalis fimbria-dependent activation of inflammatory genes in human aortic endothelial cells. Infect. Immun. 735367-5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conover, W. 1999. Practical nonparametric statistics, 3rd ed. Wiley, New York, NY.
- 13.Curtis, M. A., J. Aduse-Opoku, and M. Rangarajan. 2001. Cysteine proteases of Porphyromonas gingivalis. Crit. Rev. Oral Biol. Med. 12192-216. [DOI] [PubMed] [Google Scholar]
- 14.Dale, B. A., J. R. Kimball, S. Krisanaprakornkit, F. Roberts, M. Robinovitch, R. O'Neal, E. V. Valore, T. Ganz, G. M. Anderson, and A. Weinberg. 2001. Localized antimicrobial peptide expression in human gingiva. J. Periodontal Res. 36285-294. [DOI] [PubMed] [Google Scholar]
- 15.Dale, B. A., R. Tao, J. R. Kimball, and R. J. Jurevic. 2006. Oral antimicrobial peptides and biological control of caries. BMC Oral Health 6(Suppl. 1)S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dashper, S. G., N. M. O'Brien-Simpson, P. S. Bhogal, A. D. Franzmann, and E. C. Reynolds. 1998. Purification and characterization of a putative fimbrial protein/receptor of Porphyromonas gingivalis. Aust. Dent. J. 4399-104. [PubMed] [Google Scholar]
- 17.Dhople, V., A. Krukemeyer, and A. Ramamoorthy. 2006. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim. Biophys. Acta 17581499-1512. [DOI] [PubMed] [Google Scholar]
- 18.Feng, Z., G. R. Dubyak, M. M. Lederman, and A. Weinberg. 2006. Cutting edge: human beta defensin 3—a novel antagonist of the HIV-1 coreceptor CXCR4. J. Immunol. 177782-786. [DOI] [PubMed] [Google Scholar]
- 19.Gallo, S. A., W. Wang, S. S. Rawat, G. Jung, A. J. Waring, A. M. Cole, H. Lu, X. Yan, N. L. Daly, D. J. Craik, S. Jiang, R. I. Lehrer, and R. Blumenthal. 2006. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 28118787-18792. [DOI] [PubMed] [Google Scholar]
- 20.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3710-720. [DOI] [PubMed] [Google Scholar]
- 21.Garcia, J. R., F. Jaumann, S. Schulz, A. Krause, J. Rodriguez-Jimenez, U. Forssmann, K. Adermann, E. Kluver, C. Vogelmeier, D. Becker, R. Hedrich, W. G. Forssmann, and R. Bals. 2001. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 306257-264. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh, S. K., T. A. Gerken, K. M. Schneider, Z. Feng, T. S. McCormick, and A. Weinberg. 2007. Quantification of human beta-defensin-2 and -3 in body fluids: application for studies of innate immunity. Clin. Chem. 53757-765. [DOI] [PubMed] [Google Scholar]
- 23.Giesemann, T., G. Guttenberg, and K. Aktories. 2008. Human alpha-defensins inhibit Clostridium difficile toxin B. Gastroenterology 1342049-2058. [DOI] [PubMed] [Google Scholar]
- 24.Harder, J., J. Bartels, E. Christophers, and J. M. Schroder. 2001. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 2765707-5713. [DOI] [PubMed] [Google Scholar]
- 25.Hazrati, E., B. Galen, W. Lu, W. Wang, Y. Ouyang, M. J. Keller, R. I. Lehrer, and B. C. Herold. 2006. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. J. Immunol. 1778658-8666. [DOI] [PubMed] [Google Scholar]
- 26.Hutter, G., U. Schlagenhauf, G. Valenza, M. Horn, S. Burgemeister, H. Claus, and U. Vogel. 2003. Molecular analysis of bacteria in periodontitis: evaluation of clone libraries, novel phylotypes and putative pathogens. Microbiology 14967-75. [DOI] [PubMed] [Google Scholar]
- 27.Inoshita, E., A. Amano, T. Hanioka, H. Tamagawa, S. Shizukuishi, and A. Tsunemitsu. 1986. Isolation and some properties of exohemagglutinin from the culture medium of Bacteroides gingivalis 381. Infect. Immun. 52421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joly, S., C. Maze, P. B. McCray, Jr., and J. M. Guthmiller. 2004. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J. Clin. Microbiol. 421024-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joly, S., C. C. Organ, G. K. Johnson, P. B. McCray, Jr., and J. M. Guthmiller. 2005. Correlation between beta-defensin expression and induction profiles in gingival keratinocytes. Mol. Immunol. 421073-1084. [DOI] [PubMed] [Google Scholar]
- 30.Katz, J., K. P. Black, and S. M. Michalek. 1999. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect. Immun. 674352-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, C., N. Gajendran, H. W. Mittrucker, M. Weiwad, Y. H. Song, R. Hurwitz, M. Wilmanns, G. Fischer, and S. H. Kaufmann. 2005. Human alpha-defensins neutralize anthrax lethal toxin and protect against its fatal consequences. Proc. Natl. Acad. Sci. USA 1024830-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, C., Z. Slavinskaya, A. R. Merrill, and S. H. Kaufmann. 2006. Human alpha-defensins neutralize toxins of the mono-ADP-ribosyltransferase family. Biochem. J. 399225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohler, J. J., L. Pathangey, A. Hasona, A. Progulske-Fox, and T. A. Brown. 2000. Long-term immunological memory induced by recombinant oral Salmonella vaccine vectors. Infect. Immun. 684370-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laine, M. L., and A. J. van Winkelhoff. 1998. Virulence of six capsular serotypes of Porphyromonas gingivalis in a mouse model. Oral Microbiol. Immunol. 13322-325. [DOI] [PubMed] [Google Scholar]
- 35.Ledder, R. G., P. Gilbert, S. A. Huws, L. Aarons, M. P. Ashley, P. S. Hull, and A. J. McBain. 2007. Molecular analysis of the subgingival microbiota in health and disease. Appl. Environ. Microbiol. 73516-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehrer, R. I. 2004. Primate defensins. Nat. Rev. Microbiol. 2727-738. [DOI] [PubMed] [Google Scholar]
- 37.Lepine, G., and A. Progulske-Fox. 1996. Duplication and differential expression of hemagglutinin genes in Porphyromonas gingivalis. Oral Microbiol. Immunol. 1165-78. [DOI] [PubMed] [Google Scholar]
- 38.Lillard, J. W., Jr., P. N. Boyaka, O. Chertov, J. J. Oppenheim, and J. R. McGhee. 1999. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl. Acad. Sci. USA 96651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motzkus, D., S. Schulz-Maronde, A. Heitland, A. Schulz, W. G. Forssmann, M. Jubner, and E. Maronde. 2006. The novel beta-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J. 201701-1702. [DOI] [PubMed] [Google Scholar]
- 40.Okuda, K., A. Yamamoto, Y. Naito, I. Takazoe, J. Slots, and R. J. Genco. 1986. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect. Immun. 54659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owen, S. M., D. Rudolph, W. Wang, A. M. Cole, M. A. Sherman, A. J. Waring, R. I. Lehrer, and R. B. Lal. 2004. A theta-defensin composed exclusively of D-amino acids is active against HIV-1. J. Pept. Res. 63469-476. [DOI] [PubMed] [Google Scholar]
- 42.Owen, S. M., D. L. Rudolph, W. Wang, A. M. Cole, A. J. Waring, R. B. Lal, and R. I. Lehrer. 2004. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 201157-1165. [DOI] [PubMed] [Google Scholar]
- 43.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 1833770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pingel, L. C., K. G. Kohlgraf, C. J. Hansen, C. G. Eastman, D. E. Dietrich, K. K. Burnell, R. N. Srikantha, X. Xiao, M. Bélanger, A. Progulske-Fox, J. E. Cavanaugh, J. M. Guthmiller, G. K. Johnson, S. Joly, Z. B. Kurago, D. V. Dawson, and K. A. Brogden. 2008. August 19. Human b-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol. Cell Biol. [Epub ahead of print.] doi: 10.1038/icb.2008.56. [DOI] [PubMed]
- 45.Pisano, E., T. Cabras, C. Montaldo, V. Piras, R. Inzitari, C. Olmi, M. Castagnola, and I. Messana. 2005. Peptides of human gingival crevicular fluid determined by HPLC-ESI-MS. Eur. J. Oral Sci. 113462-468. [DOI] [PubMed] [Google Scholar]
- 46.Potempa, J., and J. Travis. 1996. Porphyromonas gingivalis proteinases in periodontitis, a review. Acta Biochim. Pol. 43455-465. [PubMed] [Google Scholar]
- 47.Premratanachai, P., S. Joly, G. K. Johnson, P. B. McCray, Jr., H. P. Jia, and J. M. Guthmiller. 2004. Expression and regulation of novel human beta-defensins in gingival keratinocytes. Oral Microbiol. Immunol. 19111-117. [DOI] [PubMed] [Google Scholar]
- 48.Quinones-Mateu, M. E., M. M. Lederman, Z. Feng, B. Chakraborty, J. Weber, H. R. Rangel, M. L. Marotta, M. Mirza, B. Jiang, P. Kiser, K. Medvik, S. F. Sieg, and A. Weinberg. 2003. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 17F39-F48. [DOI] [PubMed] [Google Scholar]
- 49.Saitoh, M., Y. Abiko, S. Shimabukuro, K. Kusano, M. Nishimura, T. Arakawa, K. Nakashima, T. Takuma, T. Kaku, and S. Igarashi. 2004. Correlated expression of human beta defensin-1, -2 and -3 mRNAs in gingival tissues of young children. Arch. Oral Biol. 49799-803. [DOI] [PubMed] [Google Scholar]
- 50.Song, H., M. Belanger, J. Whitlock, E. Kozarov, and A. Progulske-Fox. 2005. Hemagglutinin B is involved in the adherence of Porphyromonas gingivalis to human coronary artery endothelial cells. Infect. Immun. 737267-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi, Y., M. Davey, H. Yumoto, F. C. Gibson III, and C. A. Genco. 2006. Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells. Cell. Microbiol. 8738-757. [DOI] [PubMed] [Google Scholar]
- 52.Tao, R., R. J. Jurevic, K. K. Coulton, M. T. Tsutsui, M. C. Roberts, J. R. Kimball, N. Wells, J. Berndt, and B. A. Dale. 2005. Salivary antimicrobial peptide expression and dental caries experience in children. Antimicrob. Agents Chemother. 493883-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Travis, J., A. Banbula, and J. Potempa. 2000. The role of bacterial and host proteinases in periodontal disease. Adv. Exp. Med. Biol. 477455-465. [DOI] [PubMed] [Google Scholar]
- 54.Travis, J., R. Pike, T. Imamura, and J. Potempa. 1997. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J. Periodontal Res. 32120-125. [DOI] [PubMed] [Google Scholar]
- 55.van Winkelhoff, A. J., B. J. Appelmelk, N. Kippuw, and J. de Graaff. 1993. K-antigens in Porphyromonas gingivalis are associated with virulence. Oral Microbiol. Immunol. 8259-265. [DOI] [PubMed] [Google Scholar]
- 56.Wang, W., A. M. Cole, T. Hong, A. J. Waring, and R. I. Lehrer. 2003. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J. Immunol. 1704708-4716. [DOI] [PubMed] [Google Scholar]
- 57.Wang, W., C. Mulakala, S. C. Ward, G. Jung, H. Luong, D. Pham, A. J. Waring, Y. Kaznessis, W. Lu, K. A. Bradley, and R. I. Lehrer. 2006. Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J. Biol. Chem. 28132755-32764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson, M. 1995. Biological activities of lipopolysaccharides from oral bacteria and their relevance to the pathogenesis of chronic periodontitis. Sci. Prog. 7819-34. [PubMed] [Google Scholar]
- 59.Wu, H., and P. M. Fives-Taylor. 2001. Molecular strategies for fimbrial expression and assembly. Crit. Rev. Oral Biol. Med. 12101-115. [DOI] [PubMed] [Google Scholar]
- 60.Xie, H., W. O. Chung, Y. Park, and R. J. Lamont. 2000. Regulation of the Porphyromonas gingivalis fimA (fimbrillin) gene. Infect. Immun. 686574-6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang, D., A. Biragyn, D. M. Hoover, J. Lubkowski, and J. J. Oppenheim. 2004. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 22181-215. [DOI] [PubMed] [Google Scholar]
- 62.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23291-296. [DOI] [PubMed] [Google Scholar]
- 63.Yang, D., O. Chertov, and J. J. Oppenheim. 2001. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell. Mol. Life Sci. 58978-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, P., M. Martin, S. M. Michalek, and J. Katz. 2005. Role of mitogen-activated protein kinases and NF-κB in the regulation of proinflammatory and anti-inflammatory cytokines by Porphyromonas gingivalis hemagglutinin B. Infect. Immun. 733990-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]