Abstract
Vaccine reactogenicity has complicated the development of safe and effective live, oral cholera vaccines. Δctx Vibrio cholerae mutants have been shown to induce inflammatory diarrhea in volunteers and interleukin-8 (IL-8) production in cultured intestinal epithelial cells. Bacterial flagellins are known to induce IL-8 production through Toll-like receptor 5 (TLR5). Since the V. cholerae genome encodes five distinct flagellin proteins, FlaA to FlaE, with homology to conserved TLR5 recognition regions of Salmonella FliC, we hypothesized that V. cholerae flagellins may contribute to IL-8 induction through TLR5 and mitogen-activated protein kinase (MAPK) signaling. Each purified recombinant V. cholerae flagellin induced IL-8 production in T84 intestinal epithelial cells and also induced nuclear factor kappa B (NF-κB) activation in HEK293T/TLR5 transfectants, which was blocked by cotransfection with a TLR5 dominant-negative construct, demonstrating TLR5 specificity. Supernatants derived from ΔflaAC and ΔflaEDB mutants induced IL-8 production in HT-29 intestinal epithelial cells and in HEK293T cells overexpressing TLR5, whereas ΔflaABCDE supernatants induced significantly less IL-8 production, demonstrating the contribution of multiple flagellins in IL-8 induction. NF-κB activation by ΔflaABCDE supernatants was partially restored by flaA or flaAC complementation. Western analysis confirmed the presence of V. cholerae flagellins in culture supernatants. Purified recombinant V. cholerae FlaA activated the MAPKs p38, c-jun N-terminal kinase (JNK), and extracellular regulated kinase (ERK) in T84 cells. FlaA-induced IL-8 production in T84 cells was inhibited by the p38 inhibitor in combination with either the JNK or ERK inhibitors. Collectively, these data suggest that V. cholerae flagellins are present in culture supernatants and can induce TLR5- and MAPK-dependent IL-8 secretion in host cells.
The gram-negative bacterium Vibrio cholerae causes cholera, an acute diarrheal disease characterized by voluminous rice water stools and rapid dehydration. V. cholerae produces an ADP-ribosylating enterotoxin called cholera toxin (CT), encoded by ctxA and ctxB, that activates the host cell enzyme adenylate cyclase, resulting in profuse diarrhea in humans (15). The preeminence of CT as a major virulence factor of cholera was confirmed in volunteer studies, making this toxin a prime target in the development of cholera vaccine strains (21, 23). While the first generation of ΔctxA and ΔctxAB V. cholerae vaccine strains did not cause cholera, they nevertheless caused significant reactogenicity in individuals in the form of mild to moderate diarrhea (23). Although the reason for the reactogenicity of the Δctx vaccine strains is unknown, two hypotheses were introduced as possible explanations (22). First, V. cholerae may produce additional unidentified enterotoxins that were previously undetectable because of the dominant effects of CT. Second, adherence of V. cholerae during colonization of the proximal small intestine may cause alterations in small intestine function, resulting in diarrhea. Although further efforts to improve live oral cholera vaccines have focused on deleting newly characterized toxins, the reactogenicity in these strains was not markedly diminished (9, 49, 52). This observation supports the latter hypothesis.
While cholera is not generally considered an inflammatory disease, there is evidence of inflammation in cholera patients and from human and animal vaccine studies. For instance, immune cell infiltration and activation have been observed in patients with cholera (10, 24, 33, 34). More recently, Qadri et al. reported neutrophil infiltration into the lamina propria along with an increase in inflammatory mediators, such as tumor necrosis factor alpha, in adults and children during the acute stage of V. cholerae O1 and O139 serogroup infections (35, 36). In human volunteer studies, the fecal lactoferrin levels induced by the reactogenic Δctx vaccine strain CVD110 were markedly higher than those induced by the wild-type CT-expressing El Tor strain (44). The lactoferrin levels induced by CVD110 were comparable to elevated levels found in volunteers who ingested Shigella, the prototypic inflammatory enteric pathogen (30). Lactoferrin is a major component of polymorphonuclear leukocytes and is secreted by most mucosal membranes during inflammatory responses. Its presence in feces is indicative of leukocyte infiltration and intestinal inflammation. In a rabbit model, elevated levels of interleukin-1β (IL-1β) and IL-8 were observed following infection with two different reactogenic Δctx V. cholerae vaccine strains (E. C. Boedecker and J. B. Kaper, unpublished observations). IL-8 production has also been reported to contribute to the recruitment of neutrophils following infection with the enteric pathogens Salmonella spp. and Escherichia coli (4, 25, 42). Reactogenic V. cholerae vaccine strains were reported previously to induce higher levels of IL-8 production than nonreactogenic strains, and this was attributed to the presence of hap, which encodes a hemagglutinin protease (39). However, experiments with whole cultures and filtered supernatants of the Δctx V. cholerae strain CVD115, which is also hap negative, still resulted in induction of IL-8 production in the intestinal epithelial cell line T84 (57). Treatment of the CVD115 supernatants with proteinase K or trypsin decreased IL-8 production, suggesting that the factor(s) that induces the proinflammatory response is proteinaceous rather than lipopolysaccharide (LPS). These indicators of IL-8 involvement in the response to V. cholerae led us to focus on identifying V. cholerae factors that initiate an IL-8 response.
Bacterial flagellin proteins are known activators of innate immunity (47). Flagellin monomers are recognized by host cells through a direct interaction with Toll-like receptor 5 (TLR5), which mediates a proinflammatory cytokine response, including IL-8 induction. IL-8 expression involves activation of the mitogen-activated protein kinases (MAPKs) p38 kinase, c-jun N-terminal kinase (JNK), and extracellular-regulated kinase (ERK), as well as the transcription factors nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1) (14, 14, 31). Activation of NF-κB and its subsequent translocation into the nucleus occur following phosphorylation and degradation of the inhibitor protein IκBα (51). AP-1 is usually constitutively bound to DNA, and its activation is dependent on its phosphorylation, its abundance, and its binding to protein kinases (14).
Purified native flagellins of V. cholerae (FlaA, FlaC, and FlaD), Salmonella enterica serotype Typhimurium (FliC), and enteroaggregative E. coli (FliC) have been shown to induce IL-8 production in intestinal epithelial cells (11, 48, 53). Flagellins of Salmonella species, E. coli, Legionella pneumophila, Vibrio vulnificus, and V. cholerae (FlaA, FlaC, and FlaD) activate NF-κB through TLR5 (2, 7, 11, 12, 19, 32, 43, 54), and Salmonella, E. coli, and L. pneumophila flagellins have been shown to activate the MAPKs (3, 16, 43, 50, 55, 58). V. cholerae FlaA has also been shown to activate p38 and ERK in the small intestine epithelial cell line Int407 (2). While FlaA is the major V. cholerae flagellin required for flagellar synthesis and motility, V. cholerae expresses five flagellins, FlaA to FlaE (17). Conserved sequences important for TLR5 recognition have been identified within the Salmonella flagellin FliC (32, 46, 47). Collectively, these findings suggest that multiple flagellum components of Δctx V. cholerae vaccine strains may be inducers of IL-8 production. Therefore, we sought to determine if the V. cholerae flagellins are present in culture supernatants and if the flagellins can induce IL-8 production and NF-κB activation through TLR5 and by signaling through MAPKs.
In this report, we demonstrate that purified recombinant V. cholerae flagellins are capable of inducing IL-8 production through TLR5-dependent NF-κB activation and through MAPK signaling. We also show that V. cholerae flagellins are present in culture supernatants, where they can also induce IL-8 production and activate NF-κB. Deletion of all five V. cholerae flagellin genes significantly reduces the ability of culture supernatants to induce IL-8 production or activate NF-κB. Also, complementing five flagellin mutants with the flaAC operon restores motility, the ability to induce IL-8 production, and the ability to activate NF-κB. These findings provide further insight into the interaction of reactogenic V. cholerae vaccine candidates with host cells. Understanding the mechanisms involved in the interaction between V. cholerae and host cells should facilitate the development of improved attenuated V. cholerae vaccines.
MATERIALS AND METHODS
Cells and reagents.
HEK293T cells (ATCC, Rockville, MD) were cultured in Dulbecco modified Eagle medium (DMEM) (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The HT-29 intestinal epithelial cell line (ATCC, Rockville, MD) was cultured in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 2.5 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The T84 intestinal epithelial cell line (ATCC) was cultured in DMEM/F12 (Invitrogen) supplemented with 10% FBS, 2.5 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. SuperFect transfection reagent was purchased from Qiagen (Valencia, CA). All expression plasmid constructs were prepared using an EndoFree plasmid maxi kit (Qiagen). The antibodies used to detect the activated forms of p38 kinase (phospho-p38 MAPK [Thr180/Tyr182] mouse monoclonal antibody [MAb]), JNK1/2 (phospho-SAPK/JNK [Thr183/Tyr185] mouse MAb), and ERK1/2 (phospho-p44/42 MAPK [Thr202/Tyr204] mouse MAb) were purchased from Cell Signaling Technology (Danvers, MA). The antibodies used to detect the nonactivated forms of p38 kinase (sc-535 rabbit polyclonal antibody), JNK1 (sc-571 rabbit polyclonal antibody), and ERK1 (sc-93 rabbit polyclonal antibody) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody used to detect IκBα (IκBα [L35A5] mouse MAb) was purchased from Cell Signaling Technology, and the antibody used to detect β-actin (anti-β-actin MAb) was purchased from Sigma (St. Louis, MO). Anti-GroEL mouse MAb was purchased from Calbiochem (San Diego, CA). The antibody used to detect all five V. cholerae flagellins (FlaA rabbit polyclonal antibody) was generated by Lampire Biological Laboratories (Pipersville, PA). The secondary antibodies used were Alexa Fluor 680-conjugated goat anti-rabbit antibody and Alexa Fluor 750-conjugated goat anti-mouse antibody (Invitrogen). The V. cholerae classical Inaba 569B strain LPS was obtained from Sigma. Protein-free, phenol-water-extracted E. coli K235 LPS was prepared as described elsewhere (26). The inhibitors of p38 (SB202190), JNK (SP600125), and ERK (PD98059), purchased from Calbiochem, were each resuspended in dimethyl sulfoxide (DMSO) at a stock concentration of 10 mM.
Bacterial strains and growth conditions.
All bacterial strains (Table 1) were maintained on Luria-Bertani medium. Antibiotics were used at the following concentrations unless otherwise indicated: ampicillin, 100 μg/ml; carbenicillin, 50 μg/ml; chloramphenicol, 20 μg/ml; kanamycin, 50 μg/ml; and polymyxin B, 50 U/ml. Modified L agar with sucrose contained 10 g tryptone per liter, 5 g yeast extract per liter, 1.5% agar, and 10% sucrose.
TABLE 1.
Strains and plasmids used in this studya
| Strain or plasmid | Biotype (serotype) | Relevant characteristic(s) | Reference or source |
|---|---|---|---|
| V. cholerae strains | |||
| CVD110 | El Tor (Ogawa) | ΔctxA Δzot Δace hlyA::mer ΔctxB of E7946 | 29 |
| CVD115 | El Tor (Ogawa) | Δhap ΔrtxA mutant of CVD110 | 57 |
| CVD119 | El Tor (Ogawa) | ΔflaA::cat mutant of CVD115 | This study |
| CVD121 | El Tor (Ogawa) | ΔflaAC mutant of CVD115 | This study |
| CVD122 | El Tor (Ogawa) | ΔflaEDB mutant of CVD115 | This study |
| CVD120 | El Tor (Ogawa) | ΔflaEDB mutant of CVD121 | This study |
| E7946 | El Tor (Ogawa) | Wild type | 20 |
| N16961 | El Tor (Inaba) | Wild type | 13 |
| CVD115/pBAD | El Tor (Ogawa) | CVD115 complemented with pBAD/Myc-HisA | This study |
| CVD115/pACYC177 | El Tor (Ogawa) | CVD115 complemented with pACYC177 | This study |
| CVD119/pBAD | El Tor (Ogawa) | CVD119 complemented with pBAD/Myc-HisA | This study |
| CVD119 /pFlaA | El Tor (Ogawa) | CVD119 complemented with pFlaA | This study |
| CVD121/pACYC177 | El Tor (Ogawa) | CVD121 complemented with pACYC177 | This study |
| CVD121/pFlaAC | El Tor (Ogawa) | CVD121 complemented with pFlaAC | This study |
| CVD120/pBAD | El Tor (Ogawa) | CVD120 complemented with pBAD/Myc-HisA | This study |
| CVD120/pACYC177 | El Tor (Ogawa) | CVD120 complemented with pACYC177 | This study |
| CVD120/pFlaA | El Tor (Ogawa) | CVD120 complemented with pFlaA | This study |
| CVD120/pFlaAC | El Tor (Ogawa) | CVD120 complemented with pFlaAC | This study |
| E. coli strains | |||
| BL21(DE3) | Invitrogen | ||
| DH5α | Invitrogen | ||
| DH5αλpir | 28 | ||
| Mach1T1 | Invitrogen | ||
| SM10λpir | 45 | ||
| TOP10 | Invitrogen | ||
| Plasmids | |||
| pACYC177 | Cloning vector, Kmr Apr | Invitrogen | |
| pBAD102/D-TOPO | Expression vector | Invitrogen | |
| pBAD/Myc-HisA (pBAD) | Expression vector | Invitrogen | |
| pCVD442 | Suicide vector | 8 | |
| pENTR/SD/D-TOPO | Cloning vector | Invitrogen | |
| pGEM-T | Cloning vector | Promega | |
| pJMM68 | pBAD102/D-TOPO::flaC | This study | |
| pJMM69 | pBAD102/D-TOPO::flaB | This study | |
| pJMM73 | pBAD102/D-TOPO::flaD | This study | |
| pJMM96 | pGEM-T::flaAC | This study | |
| pJMM97 | pJMM96::ΔflaAC::Kmr | This study | |
| pJMM98 | pCVD442::ΔflaAC::Kmr | This study | |
| pJMM99 | pENTR/SD/D-TOPO::flaEDB | This study | |
| pJMM102 | pJMM99::ΔflaEDB | This study | |
| pJMM104 | pJMM112::flaEDB | This study | |
| pJMM112 | Suicide vector | Unpublished data | |
| pLHH2144 | pBAD/Myc-HisA::flaE (six-His) | This study | |
| pSSH2188 | pBAD/Myc-HisA::flaA (six-His) | This study | |
| pFlaA | pBAD/Myc-HisA::flaA | This study | |
| pFlaAC | pBAD/Myc-HisA::flaAC | This study |
All V. cholerae strains are serotype O1 strains.
DNA manipulation and plasmid construction.
All DNA manipulations were performed using standard protocols (40). Oligonucleotides used in this study are listed in Table 2. His-tagged flagellin expression plasmids pJMM69 (flaB), pJMM68 (flaC), and pJMM73 (flaD) were generated using genomic DNA (gDNA) from V. cholerae N16961 as the template to amplify flaB, flaC, and flaD with primer sets K4106/K4107, K4104/K4105, and K4115/K4116, respectively, and were directionally cloned into pBAD102/D-TOPO (Invitrogen) according to the manufacturer's instructions. To obtain His-tagged FlaA, the flaA gene was amplified from V. cholerae E7946 gDNA using primer set K4814/K4815. The resulting PCR product was digested with BspHI and SalI and ligated with NcoI-SalI-digested pBAD/myc-HisA (Invitrogen) to generate pSSH2188. To obtain His-tagged FlaE, the flaE gene was amplified from V. cholerae CVD115 gDNA using primer set K5017/K5018, and the resulting PCR product and pBAD/myc-HisA were both digested with NcoI and SalI and ligated to generate pLHH2144. To obtain the FlaA complementation plasmid, the flaA gene was amplified from E7946 gDNA using primer set K4814/K4819. The PCR product was digested with BspHI and PmeI and ligated with NcoI-PmeI-digested pBAD/myc-HisA to generate pFlaA. To obtain the FlaAC complementation plasmid, the flaAC operon, including its native promoter, was amplified from CVD110 gDNA using primer set K5535/K5536 and ligated into pCR-Blunt II-TOPO (Invitrogen) to generate pLH020508. The flaAC operon was then moved as an XhoI-BamHI fragment into XhoI-BamHI-digested pACYC177 to generate pFlaAC. The eukaryotic expression vectors pEF6/V5-His-TLR5 [designated TLR5(WT)] and pEF6/V5-His-DN TLR5 (designated DN-TLR5), which encode V5-tagged wild-type TLR5 and dominant-negative TLR5, respectively, were kindly provided by Andrew Gewirtz (Emory University, Atlanta, GA) and have been described elsewhere (11, 55). The reporter plasmids pELAM-luc (NF-κB reporter) and pCMV1-β-galactosidase were kindly provided by D. Golenbock (University of Massachusetts Medical School) and have been described previously (5). All constructs were confirmed by DNA sequencing analysis using an ABI 3100 gene analyzer (Applied Biosystems, Foster City, CA).
TABLE 2.
Primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| K3632 | TGAGCTTGCGAACTCGATAGGA |
| K3633 | TGGTGTTTACATTCACCGCCAT |
| K4104 | GCCTAGAAGAGCCAGTGCTG |
| K4105 | CACCATGGCGGTGAATGTAAACAC |
| K4106 | TCCCAATAAGCTCAGAGCTGC |
| K4107 | CACCATGGCAATTAATGTAAACACGAAC |
| K4115 | CACCATGGCAGTGAATGTAAATACCAAC |
| K4116 | ACCCAACAGGCTGAGTGCGG |
| K4117 | CACCATGGCCATGACGGTAAATACC |
| K4367 | TTTTGGATCCACTTCAGGTCACGAGCGGTA |
| K4398 | CACCTTTGCCATCAGTAAAA |
| K4414 | AAGGATCCGTTGGTATTTACGTTAATGGTCAT |
| K4422 | AAAGGATCCAGCGTAATGCTCTGCCAGTG |
| K4423 | AAAGGATCCTCTCTGATGTTACATTGCACAAG |
| K4432 | TCTAGATACGGCCAAACTGCATCAGT |
| K4433 | TCTAGACTAGCCTAGAAGAGCCAGTG |
| K4481 | CACCGATATGGCTATGGCTAGGATG |
| K4482 | ACTCGTTCGCGGTCATATTC |
| K4814 | TATATCATGACCATTAACGTAAATACCAAC |
| K4815 | GATAGTCGACCTGCAATAACGAGATTGCAGAG |
| K4919 | ATAGTTTAAACCTACTGCAATAACGAGATTGCAG |
| K5017 | TATACCATGGCCATGACGGTAAATACC |
| K5018 | GATAGTCGACATTACGCAGCAAAAACAGCAC |
| K5535 | GGATCCCTAACCTTCAATGCCTTATGCG |
| K5536 | CTCGAGCTAGCCTAGAAGAGCCAGTGC |
Flagellin gene deletions.
The suicide plasmid pKEK93 (ΔflaA1::cat [17]) was used to generate a flaA mutant. E. coli SM10λpir containing pKEK93 was mated with CVD115 and plated on L agar containing ampicillin and polymyxin B overnight at 37°C. Colonies were picked and grown overnight in L broth (LB) at 37°C to allow double-crossover events. Cultures were then plated and incubated overnight at 37°C on modified L agar containing 10% sucrose. Sucrose-resistant isolates were screened for the flaA deletion by PCR using primers K3632 and K3633, and one confirmed deletion strain was retained and designated CVD119.
For flaAC operon deletion, a 3,510-bp fragment containing the flaA and flaC genes was PCR amplified from CVD110 using primer set K4432/K4433 and cloned into the pGEM-T vector (Invitrogen) according to the manufacturer's instructions. The resulting clone, pJMM96, was subjected to reverse PCR using primer set K4414/K4367, digested with BamHI, self-ligated, and transformed into E. coli TOP10, resulting in pJMM96ΔflaAC. An 815-bp kanamycin gene was PCR amplified from pACYC177 using primer set K4422/K4423, trimmed with BamHI, and ligated into the BamHI site of pJMM96ΔflaAC, yielding pJMM97. A 2,080-bp XbaI fragment containing the ΔflaAC::kan fragment was isolated from pJMM97 and ligated into the XbaI site of pCVD442, resulting in suicide plasmid pJMM98. pJMM98 was electroporated into E. coli SM10λpir and introduced into V. cholerae CVD115 by conjugal mating. Kanamycin-resistant and ampicillin-sensitive mutants were screened for the flaAC deletion by PCR analysis using primer set K4432/K4433, and a deletion isolate was designated CVD121.
To construct the flaEDB operon deletion, a new suicide vector, pJMM112 (J. Michalski and J. B. Kaper, unpublished data), which utilizes the Gateway system (Invitrogen), was employed. Briefly, primer set K4481/K4482 and Pfu Ultra II Fusion HS polymerase (Stratagene, La Jolla, CA) were used to amplify a 7,450-bp product containing flaEDB with flanking regions 1.6 kb upstream and 2 kb downstream from CVD110, which was directionally cloned into pENTR/SD/D-TOPO and transformed into E. coli Mach1T1 (Invitrogen), yielding pJMM99. Plasmid pJMM99 was digested with PstI, and a 6.6-kb fragment was gel purified, self-ligated, and transformed into E. coli DH5α, yielding pJMM102. Plasmid pJMM102 and suicide vector pJMM112 were incubated together in the presence of the LR Clonase II enzyme mixture (Invitrogen) according to the manufacturer's instructions and transformed into E. coli DH5αλpir. An Apr Cms plasmid, pJMM104, harboring the flaEDB deletion recombined into suicide vector pJMM112, was isolated and transformed into E. coli SM10λpir. E. coli SM10λpir(pJMM104) was conjugally mated with either V. cholerae CVD115 or V. cholerae CVD121, plated on L agar containing ampicillin and polymyxin B, and incubated at 37°C overnight. Approximately 20 colonies were inoculated into 3 ml of LB, incubated for 8 h at room temperature, and then plated on modified L agar containing 10% sucrose. The plates were incubated at room temperature for 24 to 30 h, and sucrose-resistant isolates were screened for the flaEDB deletion by PCR. Isolates exhibiting a 710-bp PCR product with primer set K4117/K4398 were identified as deletion mutants. V. cholerae CVD122 and V. cholerae CVD120 are isogenic ΔflaEDB derivatives of CVD115 and CVD121, respectively.
Flagellin complementation.
V. cholerae CVD115 and ΔflaA, ΔflaAC, and ΔflaABCDE mutants of this strain were electroporated either with empty vector pBAD/myc-HisA or pACYC177 or with the pFlaA or pFlaAC expression construct as previously described (29), except that we used 137 mM sucrose instead of buffer H and all centrifugations were carried out at room temperature.
Bacterial supernatant and WCL preparation.
Freshly plated bacteria were inoculated into 5 ml LB and incubated overnight at 37°C. The overnight cultures were then subcultured (1:33) in LB and incubated at 30°C for 3.5 h. The optical densities at 600 nm of the cultures were adjusted to ∼1.5, and the cultures were centrifuged for 15 min at 2,600 × g to pellet the bacteria. The supernatants were filtered using a 0.22-μm Millex GP syringe-driven filter unit (Millipore, Billerica, MA). One hundred microliters of filtered supernatant was added to each well of a 24-well plate for IL-8 assays (HT-29 or HEK293T transfectants), and 200 μl of filtered supernatant was added to each well of a 12-well plate for NF-κB assays (HEK293T transfectants). The concentrated bacterial supernatants used for polyacrylamide gel electrophoresis (PAGE) were prepared using trichloroacetic acid precipitation. Briefly, 5 μl of 5% deoxycholic acid was added to 1 ml of each filtered supernatant and vortexed. Next, 100 μl trichloroacetic acid was added to each sample and placed on ice 15 to 30 min. Samples were centrifuged at 13,000 rpm for 10 min at 4°C. The supernatants were discarded, and the pellets were resuspended in 15 μl of 2 M Tris base. For sodium dodecyl sulfate (SDS)-PAGE, 15 μl of 2× SDS-PAGE buffer was added, and 6 μl of a total sample was added to each well to correlate with the 200 μl of supernatant used for HEK293T transfectant experiments. Bio-Rad (Hercules, CA) Criterion precast gels (4 to 20% Tris-HCl) were used for SDS-PAGE, and separated proteins were transferred onto Immobilon-FL polyvinylidene difluoride membranes (Millipore) for Western blot analysis to detect V. cholerae flagellins and GroEL. The Western blot analysis using the Odyssey two-color detection system is described below. To obtain whole-cell lysates (WCLs), 100-μl portions of CVD115 and ΔflaABCDE subcultures were pelleted at 13,000 rpm for 1 min to remove LB. The pellets were resuspended in 50 μl of 2× SDS-PAGE sample buffer, and 5 μl of each sample was loaded into a well of the SDS-PAGE gel.
Purification of V. cholerae flagella.
Flagella were purified using the modified methods of Richardson and Parker (38) and Xicohtencatl-Cortés et al. (53). Brucella agar plates were inoculated with V. cholerae N16961 and incubated at 37°C overnight. The plates were scraped, and bacteria were resuspended in 50 ml phosphate-buffered saline (PBS) (pH 7.4). Flagella were sheared off by using a Waring blender for 90 s on setting 5. The bacteria were pelleted by centrifugation at 15,600 × g for 10 min, and the supernatant was centrifuged for 2 h in an SW28 rotor at 39,000 × g. The pelleted flagella were then subjected to differential centrifugation. First, the pellet was resuspended in 11 ml fresh PBS (pH 7.4) and centrifuged at 15,600 × g for 10 min. The supernatant was removed and centrifuged for 2 h at 30,000 × g in an SW41 rotor. These steps were repeated a total of three times. The final pellet was resuspended in 10 ml Tris-EDTA (pH 8.0) containing 0.5% Triton X-100. CsCl was added (density of 1.3; approximately 0.4 g/ml), and samples were centrifuged in an SW41 rotor at 100,000 × g for 48 h. A white band containing the flagella was extracted and dialyzed against three changes of Tris-EDTA buffer (pH 8.0) buffer.
Expression and purification of flagellins.
For expression of FlaA, FlaB, FlaC, FlaD, and FlaE, cultures of E. coli TOP10 containing pSSH2188, pJMM69, pJMM68, pJMM73, and pLHH2144, respectively, were grown in 100 ml LB containing 100 μg/ml ampicillin to mid-log phase and induced with 0.02% arabinose (FlaB, FlaC, and FlaD) or 0.002% arabinose (FlaA and FlaE). The bacterial cells were pelleted and resuspended in PBS (pH 7.4). His-tagged flagellins were purified using a Magna-His purification kit (Promega, Madison, WI) according to the manufacturer's instructions. Samples were concentrated using Amicon/Centricon YM-10 columns (Millipore), which was followed by two washes with 1.5 ml of 100 mM HEPES (pH 7.5). Coomassie blue staining of the gels revealed bands corresponding to the purified His-tagged flagellins (FlaA to FlaE) that were consistent with their sizes (∼42 kDa) as determined using Western blots probed with polyclonal anti-FlaA antibody (data not shown).
Reporter assay.
HEK293T cells were seeded and transfected as previously described (37). Optimized amounts of the TLR5(WT) construct (5 ng/ml) and/or the DN-TLR5 construct (25 ng/ml) were used along with reporter constructs for transfection. Cells were allowed to recover for at least 18 h in fresh medium and were stimulated with 200 μl V. cholerae supernatant (prepared as described above), various concentrations of purified native V. cholerae flagella or boiled (5 min) V. cholerae flagella, or 1 μg/ml purified recombinant V. cholerae FlaA, FlaB, FlaC, FlaD, FlaE, S. enterica serotype Typhimurium FliC, or enteropathogenic E. coli (EPEC) FliC.
IL-8 assay.
HT-29 cells at ∼80% confluence or HEK293T cells (plated and transfected as described above, without the reporter constructs) were treated with V. cholerae supernatants as described above for 18 h or 24 h, respectively, and T84 cells were treated with recombinant flagellins as described above for 18 h. Cell culture supernatants were collected and stored at −80°C. IL-8 production by T84 cells and IL-8 production by HEK293T cells were determined by sandwich enzyme-linked immunosorbent assays (ELISAs) performed at the UMB Cytokine Core Facility, and IL-8 production by HT-29 cells was determined by using an R&D Systems (Minneapolis, MN) human CXCL8/IL-8 Quantikine ELISA kit or at the UMB Cytokine Core Facility.
MAPK activation and Western blot analysis.
T84 cells at ∼80% confluence were treated with 100 mM HEPES (pH 7.5) for 30 min or with 1 μg/ml EPEC FliC (58) or V. cholerae FlaA for 15, 30, and 60 min. Following treatment, the cells were washed twice with cold 1× Tris-buffered saline (TBS), removed using 1 ml 1× TBS, and pelleted by centrifugation at 1,000 rpm for 5 min. The pellets were then lysed in modified RIPA buffer (1.0% Nonidet P-40, 1.0% sodium deoxycholate, 150 mM NaCl, 10 mM Tris-HCl [pH 7.5], 5.0 mM sodium pyrophosphate, 1.0 mM NaVO4, 5.0 mM NaF, 1.0 μg/ml aprotinin, 1.0 μg/ml leupeptin, 0.1 mM phenylmethylsulfonyl fluoride) for 15 min at 4°C. The lysates were passed through 23- and 27-gauge needles to shear the DNA and then centrifuged at 13,000 rpm for 15 min to clear the lysates. The cleared lysates were kept at −80°C until they were used. The protein contents of cell lysates were determined using a DC protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of total protein (50 μg protein per gel lane) were separated by SDS-PAGE on 12.5% gels and transferred to Immobilon-FL polyvinylidene difluoride membranes (Millipore). The membranes were blocked in Odyssey blocking solution (a 1:1 mixture of 1× TBS and Odyssey blocking buffer [Li-Cor Biosciences, Lincoln, NE]) for 1 h at room temperature. The blots were then double probed with antibodies against the activated (mouse MAb at a 1:2,000 dilution) and nonactivated (rabbit polyclonal antibody at a 1:1,000 dilution) forms of p38 kinase, JNK1/2, or ERK1/2. Briefly, the blots were incubated with antibodies in Odyssey blocking solution containing 0.1% Tween overnight at 4°C. The blots were then washed four times with 1× TBS-0.1% Tween (1× TBS/T) for 5 min and incubated with the secondary antibodies (Alexa Fluor 680-conjugated goat anti-rabbit and Alexa Fluor 750-conjugated goat anti-mouse) at 1:10,000 dilutions for two-color detection in Odyssey blocking solution containing 0.1% Tween for 30 min at room temperature. The blots were washed four times with 1× TBS/T for 5 min and once with 1× TBS for at least 5 min before they were scanned and quantified using the Odyssey infrared detection system (Li-Cor). The blots were stripped by rinsing them with 1× TBS and incubating them with 20 ml Restore Western stripping buffer (Pierce) at 42°C for 45 min. They were then rinsed three times with 1× TBS/T and subsequently washed three times with 1× TBS/T for 5 min. The blots were then ready for the next set of primary antibodies mentioned above using the same protocol. The same blots were also probed for IκBα degradation and β-actin using the protocol described above, except that only one of the secondary antibodies (anti-mouse antibody) was used for single-color detection.
Inhibitor studies.
In six-well tissue culture plates, T84 cells were grown to approximately 80% confluence and pretreated for 1 h in DMEM/F12 with each inhibitor at a concentration of 10 μM or with the following combinations of inhibitors (10 μM each): SB202190 and SP600125; SB202190 and PD98059; SP600125 and PD98059; and SB202190, SP600125, and PD98509. As a control, T84 cells were also pretreated in DMEM/F12 containing DMSO at a final concentration of 0.3%, which was the maximum concentration of DMSO in the three-inhibitor preparation. The medium was then removed and replaced by fresh medium containing the same pretreatment preparations in the presence of 1 μg/ml V. cholerae FlaA, and the cultures were incubated for 18 h. Cell culture supernatants were collected, and the IL-8 levels were determined by an ELISA for each treatment. As determined by microscopic observation, DMSO did not appear to be cytotoxic since the T84 cell confluence continued to increase during the incubation period.
Statistical analyses.
Quantitative data for NF-κB activation in transfected HEK293T cells, IL-8 production in HT-29 cells, HEK293T transfectants, and T84 cells, and MAPK phosphorylation in T84 cells were expressed as means ± standard errors. All of the data were analyzed using a one-way analysis of variance with repeated measures, followed by post hoc comparisons using Tukey's multiple paired comparison test (GraphPad PRISM 4 program for Windows).
RESULTS
Purified V. cholerae flagellins activate NF-κB and induce IL-8 production through TLR5.
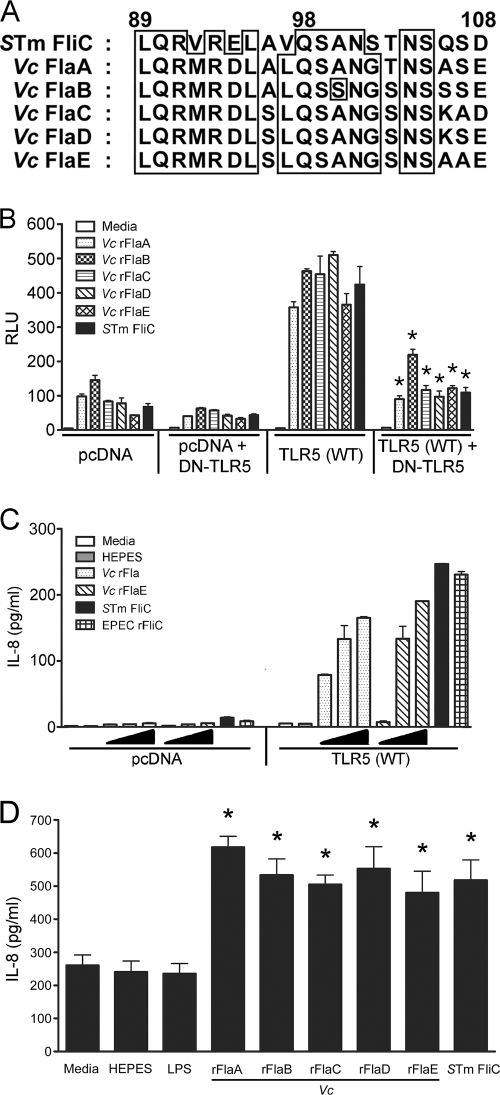
The eukaryotic extracellular receptor TLR5 recognizes bacterial flagellin and activates the transcription factor NF-κB, leading to induction of proinflammatory cytokine production in mammalian cells (12). A conserved TLR5 recognition site has been identified in Salmonella FliC (32, 46). Alignment of the protein sequence of the conserved TLR5 recognition region of S. enterica serovar Typhimurium FliC with the five V. cholerae flagellin sequences revealed that the sequences contain homologous TLR5 recognition sequences (Fig. 1A). The levels of amino acid homology for the TLR5 recognition regions of V. cholerae flagellins and S. enterica serovar Typhimurium FliC are 70% for FlaA, 60% for FlaB, FlaC, and FlaD, and 55% for FlaE. Therefore, it seemed reasonable to hypothesize that all five V. cholerae flagellins may be able to interact with TLR5.
FIG. 1.
(A) Sequence alignment of the conserved TLR5 recognition domain of S. enterica serovar Typhimurium FliC with corresponding sequences of V. cholerae flagellins: ClustalW alignment of the TLR5 recognition domain of S. enterica serovar Typhimurium (STm) FliC with the homologous sequences of V. cholerae (Vc) FlaA, FlaB, FlaC, FlaD, and FlaE. The levels of amino acid homology in this region for the different V. cholerae flagellins and S. enterica serovar Typhimurium FliC are 55 to 70%. (B) NF-κB activation in HEK293T/TLR5 transfectants and TLR5(WT)/DN-TLR5 cotransfectants in response to purified V. cholerae flagellins. A total of 2 × 105 cells/well transfected with pcDNA3.1 or TLR5(WT), with or without DN-TLR5, together with NF-κB and β-galactosidase reporter constructs were treated for 6 h with 1 μg/ml V. cholerae recombinant FlaA (rFlaA), rFlaB, rFlaC, rFlaD, rFlaE, or S. enterica serovar Typhimurium FliC. NF-κB and β-galactosidase activities were determined using cell lysates (n = 3). NF-κB activation was expressed in relative luciferase units (RLU) by normalizing NF-κB activity with constitutive β-galactosidase activity. *, P < 0.001 for a comparison of TLR5(WT) and TLR5 (WT)/DN-TLR5 cotransfectants. (C) Dose-dependent IL-8 production in HEK293T/TLR5 transfectants in response to purified V. cholerae flagellins. A total of 1 × 105 cells/well were transfected with pcDNA3.1 or TLR5(WT) without reporter constructs and then treated for 24 h with purified V. cholerae rFlaA or rFlaE at concentrations of 0.01, 0.1, and 1 μg/ml, S. enterica serovar Typhimurium FliC (1 μg/ml), or EPEC rFliC (1 μg/ml). The IL-8 levels in the cell culture supernatants were determined by ELISA (n = 3). (D) IL-8 production in T84 cells in response to purified V. cholerae flagellins. T84 cells at approximately 80% confluence were treated with 100 ng/ml of purified V. cholerae LPS (569B) or 1 μg/ml of purified V. cholerae rFlaA, rFlaB, rFlaC, rFlaD, rFlaE, or S. enterica serovar Typhimurium FliC for 18 h. The levels of IL-8 present in the cell culture supernatants were determined by ELISA (n = 3). *, P < 0.001 for a comparison of the 100 mM HEPES and flagellin treatments.
To determine if each individual V. cholerae flagellin can directly activate NF-κB and IL-8 production through TLR5, we heterologously expressed and purified each recombinant V. cholerae flagellin protein from E. coli. TLR5 was overexpressed in the human kidney epithelial cell line HEK293T along with reporter constructs for ELAM (NF-κB) luciferase and β-galactosidase. As reported previously, HEK293T cells are totally unresponsive to LPS (5, 27) as they do not express any endogenous TLR4, CD14, and MD-2, and this was confirmed by our observation that even 5 ng/ml of E. coli K235 LPS failed to induce any detectable NF-κB luciferase activity (data not shown). However, HEK293T cells are minimally responsive to flagellins since they express low levels of endogenous TLR5 (56), while HEK293T/TLR5 transfectants exhibited a robust response to flagellins. HEK293T/TLR5 transfectants were treated with 1 μg/ml of each V. cholerae flagellin for 6 h, and lysates were analyzed for NF-κB-driven luciferase activity by normalizing the results using constitutive β-galactosidase activity. Figure 1B shows that each of the recombinant V. cholerae flagellins was capable of activating NF-κB in HEK293T/TLR5 transfectants to a level that was significantly greater than the level in the HEK293T/pcDNA control transfectants (Fig. 1B, left panel) and was similar to levels activated by S. enterica serovar Typhimurium FliC. Cotransfection of HEK293T cells with the dominant-negative TLR5 construct (DN-TLR5) along with TLR5(WT) resulted in significantly reduced NF-κB activation upon treatment with individual flagellins (Fig. 1B, right panel). In the negative controls, the low level of endogenous TLR5 activity in HEK293T/pcDNA transfectants treated with V. cholerae flagellins was also somewhat reduced by cotransfection with the DN-TLR5 construct (Fig. 1B, two left panels). FlaA and FlaE, the V. cholerae flagellins with the most and least homology to S. enterica serovar Typhimurium FliC, respectively, were also able to induce IL-8 production in a dose-dependent manner in the HEK293T/TLR5 transfectants (Fig. 1C). The other flagellins were not tested in this assay; however, they were all tested using the intestinal epithelial cell line T84, and they were all able to significantly induce IL-8 production to levels above the levels in medium-, HEPES-, and LPS (100 ng/ml)-treated controls (Fig. 1D). These results demonstrate that each of the V. cholerae flagellins is able to activate NF-κB through TLR5 recognition and to induce IL-8 production in TLR5-expressing cells.
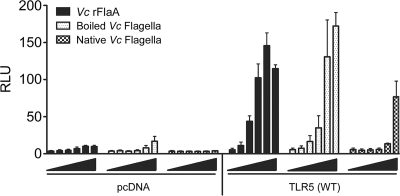
Since the TLR5 recognition site of flagellin monomers is inaccessible in the flagellar filament of S. enterica serovar Typhimurium flagella (46), the TLR5 recognition site of V. cholerae flagellins is also probably unavailable in the flagellar filament. Figure 2 shows the dose response for NF-κB activity in HEK293T/TLR5 transfectants treated with recombinant V. cholerae FlaA, native V. cholerae flagella, or V. cholerae flagella boiled to release the monomeric flagellins. HEK293T cells transfected with pcDNA showed very low endogenous TLR5 activity for all three treatments (Fig. 2, left panel), whereas cells transfected with TLR5 showed a robust, dose-dependent response to recombinant V. cholerae FlaA and boiled flagella (Fig. 2, right panel). Native flagella activated NF-κB only at a concentration of 100 ng/ml, whereas boiled flagella activated NF-κB at a concentration 2.5 ng/ml (Fig. 2, right panel). These data suggest that boiling V. cholerae flagella exposes TLR5 epitopes that are otherwise inaccessible in the native conformation, presumably through the release of monomers.
FIG. 2.
Dose-response comparison of V. cholerae recombinant FlaA, boiled flagella, and native flagella. A total of 2 × 105 cells/well were transfected with either pcDNA3.1 or the TLR5(WT) construct together with NF-κB and β-galactosidase reporter constructs and then treated with 0, 0.1, 1, 2.5, 10, or 100 ng/ml of recombinant V. cholerae (Vc) FlaA, boiled V. cholerae flagella, or native V. cholerae flagella for 6 h. NF-κB and β-galactosidase activities were determined in duplicate using cell lysates, and the averages were calculated (n = 2). NF-κB activation is expressed in relative luciferase units (RLU).
Filtered supernatants from V. cholerae flaA+ strains, but not from ΔflaABCDE strains, activate NF-κB and induce IL-8 production through TLR5.
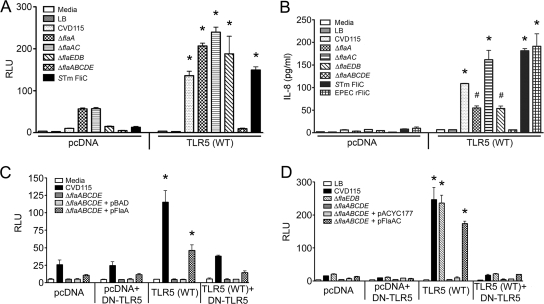
In order to test the effects of flagellin deletions in El Tor strain CVD115 (57), we generated isogenic flagellin deletion mutants, including CVD120 (ΔflaABCDE), CVD119 (ΔflaA), CVD121 (ΔflaAC), and CVD122 (ΔflaEDB). The ΔflaA and ΔflaAC mutants showed little or no motility on motility agar plates, while the ΔflaABCDE mutant was completely nonmotile (data not shown). The ΔflaEDB mutant, which is flaA+, was as motile as the parental strain (data not shown). Since FlaA has previously been shown to be the only flagellin required for flagellar assembly and motility in the classical Ogawa 395 strain (17), we introduced a flaA-overexpressing plasmid (pFlaA) or a flaAC-expressing plasmid (pFlaAC) into the ΔflaABCDE mutant. Complementation with pFlaA alone or with pFlaAC restored motility to the ΔflaABCDE mutant, albeit not at wild-type levels (data not shown).
We next sought to determine whether supernatants derived from our flagellin deletion mutants were able to induce IL-8 production through TLR5 activation in HEK293T cells. HEK293T cells transfected with the control vector pcDNA responded weakly to the supernatants, as expected, due to the presence of low levels of endogenous TLR5 (Fig. 3A, left panel), whereas the response was far greater when the HEK293T cells were exogenously transfected with the TLR5(WT) expression construct (Fig. 3A, right panel). Filtered culture supernatants from CVD115 and isogenic ΔflaA, ΔflaAC, and ΔflaEDB mutant strains activated NF-κB significantly (P < 0.001) in HEK293T/TLR5 transfectants (Fig. 3A), suggesting that the remaining encoded flagellins are expressed and released into culture supernatants, where they can signal through TLR5. Most notably, the ΔflaABCDE strain supernatants completely failed to activate NF-κB, suggesting that the five flagellin proteins are solely responsible for NF-κB activation in this in vitro system. Supernatants from the ΔflaA, ΔflaAC, and ΔflaEDB strains also significantly induced IL-8 production in HEK293T/TLR5 transfectants (P < 0.05 or P < 0.001), while the IL-8 levels in cells treated with the ΔflaABCDE strain supernatants were basal levels (Fig. 3B). Both S. enterica serovar Typhimurium FliC and EPEC FliC significantly induced IL-8 production in HEK293T/TLR5 transfectants compared to HEK293T/pcDNA transfectants (Fig. 3B). The low level of activity observed when IL-8 (Fig. 3B) was measured compared with the NF-κB level (Fig. 3A) was likely due to the greater sensitivity of the NF-κB-driven luciferase readout.
FIG. 3.
NF-κB activation and IL-8 production in HEK293T/TLR5 transfectants in response to V. cholerae supernatants. A total of 2 × 105 cells/well (for the NF-kB assay) or 1 × 105 cells/well (for the IL-8 assay) were transfected with either pcDNA3.1 or TLR5(WT) together with reporter constructs (no reporters were used for the IL-8 assay) and treated with supernatants from CVD115 or isogenic ΔflaA, ΔflaAC, ΔflaEDB, and ΔflaABCDE mutants (A) for 6 h to detect NF-κB activation or (B) for 24 h to detect secreted IL-8. Also, 2 × 105 cells/well were transfected with either pcDNA3.1 or TLR5(WT) in the presence or absence of the DN-TLR5 construct together with reporter constructs and then treated with supernatants from CVD115 or the ΔflaABCDE mutant complemented with either (C) pFlaA or (D) pFlaAC for 6 h to detect NF-κB activation. NF-κB activation is expressed in relative luciferase units (RLU) for cell lysates (n = 3). The levels of IL-8 present in the cell culture supernatants were determined in duplicate by ELISA (n = 3). Representative graphs are shown for each experiment. #, P < 0.05 for a comparison of the pcDNA and TLR5(WT) treatments; *, P < 0.001 for a comparison of the pcDNA and TLR5(WT) treatments. STm FliC, S. enterica serovar Typhimurium FliC.
Complementing the ΔflaABCDE strain with pFlaA partially restored the ability of the supernatant to activate NF-κB in HEK293T/TLR5 transfectants (Fig. 3C), consistent with the partial complementation of motility (data not shown). In addition, when the ΔflaABCDE strain was complemented with pFlaAC, its supernatant yielded an even greater increase in NF-κB activation in HEK293T/TLR5 transfectants than in the HEK293T/pcDNA transfectants, even though the NF-κB activation was slightly less than that obtained with CVD115 supernatants (Fig. 3D). The partial restoration of NF-κB activation through pFlaA (Fig. 3C, right panel) and pFlaAC (Fig. 3D, right panel) complementation was significantly diminished when HEK293T/TLR5 transfectants were cotransfected with DN-TLR5, further demonstrating the specificity of TLR5-induced activation by V. cholerae flagellins.
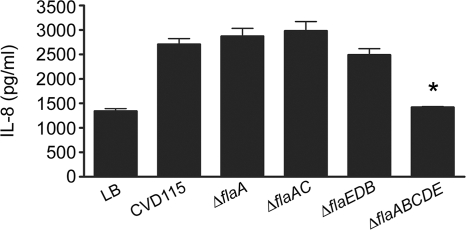
We also tested the effects of the flagellin deletions on IL-8 induction in the intestinal epithelial cell line HT-29. Consistent with the HEK293T/TLR5 data, supernatants of the ΔflaA, ΔflaAC, and ΔflaEDB strains induced IL-8 production in HT-29 cells (Fig. 4). The ΔflaABCDE supernatants yielded basal levels of IL-8 that were comparable to the levels obtained with LB (Fig. 4). Supernatants of the ΔflaABCDE strain complemented with pFlaAC induced production of IL-8 levels similar to the levels obtained with the CVD115 supernatants (data not shown). Collectively, these data suggest that flagellins are present in culture supernatants, where they can activate NF-κB-driven genes and induce IL-8 production specifically through TLR5.
FIG. 4.
Induction of IL-8 production in HT-29 cells in response to V. cholerae supernatants. HT-29 cells at approximately 80% confluence were treated for 18 h with supernatants from CVD115 or the CVD115 ΔflaA, ΔflaAC, ΔflaEDB, and ΔflaABCDE mutants. The levels of IL-8 present in the cell culture supernatants were determined by ELISA, and the averages were calculated (n = 3). *, P < 0.001 for a comparison of the CVD115 and ΔflaABCDE supernatant treatments.
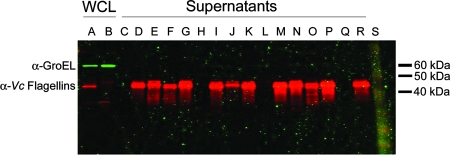
To demonstrate directly the presence of V. cholerae flagellins in filtered supernatants and to test for bacterial lysis, we examined WCLs of the CVD115 and ΔflaABCDE strains and supernatants from the flagellin mutant strains for the cytoplasmic protein GroEL and flagellins using Western blot analysis. The polyclonal antiflagellin antibody recognized all five purified V. cholerae flagellins on Western blots (data not shown). Flagellin proteins were present in all of the supernatants except the supernatants of the ΔflaABCDE strain, the ΔflaABCDE strain with pBAD, and the ΔflaABCDE strain with pACYC177, as expected (Fig. 5, red bands). GroEL was not detected in any of the supernatants but was present in WCLs (Fig. 5, green bands), demonstrating that the flagellins present in supernatants were not due to bacterial cell lysis. Since flagellins were present in the ΔflaA, ΔflaAC, and ΔflaEDB strain supernatants, it appeared that the two operons expressed flagellins independent of each other. Furthermore, these data demonstrated that V. cholerae flagellins are secreted, suggesting that they could contribute to TLR5-mediated IL-8 production in intestinal epithelial cells regardless of whether a functional flagellum is present.
FIG. 5.
Western blot of V. cholerae WCLs and concentrated supernatants. Blots containing (lane A) CVD115 and (lane B) ΔflaABCDE WCLs along with concentrated supernatants from (lane C) LB, (lane D) CVD115, (lane E) ΔflaA, (lane F) ΔflaAC, (lane G) ΔflaEDB, (lane H) ΔflaABCDE, (lane I) CVD115/pBAD, (lane J) ΔflaA/pBAD, (lane K) ΔflaA/pFlaA, (lane L) ΔflaABCDE/pBAD, (lane M) ΔflaABCDE/pFlaA, (lane N) CVD115/pACYC177, (lane O) ΔflaAC/pACYC177, (lane P) ΔflaAC/pFlaAC, (lane Q) ΔflaABCDE/pACYC177, and (lane R) ΔflaABCDE/pFlaAC cultures at amounts equivalent to the doses used to treat HEK293T cells were probed with both mouse anti-GroEL (green bands) and rabbit anti-FlaA (red bands) antibodies for simultaneous two-color detection. Lane S contained a protein ladder. The molecular mass of GroEL is approximately 60 kDa, and the molecular mass of the V. cholerae flagellins is approximately 42 kDa. A representative blot is shown (n = 3). α-GroEL, anti-GroEL antibody; α-Vc Flagellins, anti-V. cholerae flagellin antibodies.
Purified V. cholerae flagellins induce MAPK activation and IκBα degradation in T84 cells.
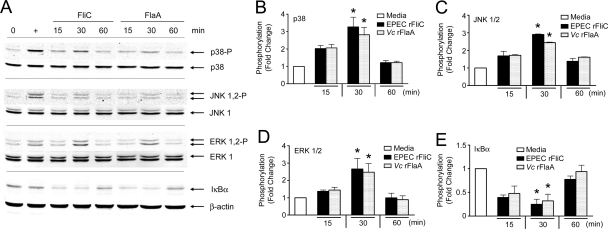
In addition to the NF-κB pathway, signaling through TLR5 can lead to activation of MAPKs, which are involved in the inflammatory cytokine response through activation of transcription factors such as AP-1 (6, 14, 18). Therefore, we sought to determine if V. cholerae flagellins could activate the MAPKs p38, JNK1/2, and ERK1/2 in T84 cells, using recombinant FlaA as the stimulus. Following treatment of T84 cells with 1 μg/ml purified recombinant FlaA for 15, 30, and 60 min, we examined the WCLs by performing Western analysis with antibodies specific for the nonphosphorylated and phosphorylated (activated) forms of p38, JNK1/2, and ERK1/2 (Fig. 6A). Significant but transient increases in phosphorylation were observed for p38 (Fig. 6B), JNK1/2 (Fig. 6C), and ERK1/2 (Fig. 6D) at 30 min in T84 cells treated with FlaA compared to cells treated with medium alone (P < 0.001). The levels of phosphorylated forms were decreased significantly by 1 h. To examine IκBα degradation, the same blots were reprobed with an anti-IκBα antibody. By 30 min, FlaA had caused significant degradation of IκBα in T84 cells (P < 0.001) (Fig. 6E). IκBα degradation leads to NF-κB activation; hence, these data further confirm our finding obtained with HEK293T/TLR5 transfectants that the NF-κB pathway is activated by flagellin proteins. These data demonstrate that V. cholerae flagellins are able to activate MAPK signaling pathways, in addition to the NF-κB pathway, in T84 cells, suggesting that these pathways are involved in the flagellin-induced IL-8 production.
FIG. 6.
MAPK activation and IκBα degradation by V. cholerae FlaA in T84 cells. (A) T84 cells at approximately 80% confluence were treated with 1 μg/ml of V. cholerae recombinant FlaA or EPEC FliC for various times, and cell lysates were analyzed for p38, JNK1/2, and ERK1/2 phosphorylation and for IκBα degradation by Western blotting. The levels of phosphorylation of (B) p38, (C) JNK1/2, and (D) ERK1/2 and the levels of degradation of (E) IκBα were determined using the Odyssey infrared detection system, and the averages were calculated (n = 3). *, P < 0.001 for a comparison of untreated and flagellin-treated cells. Vc rFlaA, V. cholerae recombinant FlaA.
MAPK inhibitors downregulate FlaA-induced IL-8 production in T84 cells.
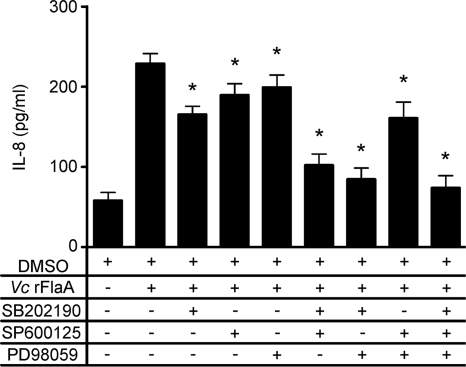
To determine if MAPK phosphorylation in T84 cells is directly involved in the IL-8 production induced by V. cholerae flagellins, T84 cells were treated with 1 μg/ml of recombinant FlaA in the absence or presence of the specific kinase inhibitors SB202190 (p38), SP600125 (JNK1/2), and PD98059 (ERK1/2) solubilized in DMSO. Supernatants were collected after 18 h of treatment and assayed for IL-8. There was no significant difference in IL-8 levels between cells treated with medium only and cells treated with medium containing the vehicle (DMSO) (data not shown). Treatment of cells with FlaA and DMSO resulted in IL-8 production that was significantly greater than the IL-8 production in controls that received only medium (P < 0.001) (Fig. 7). Following the addition of each kinase inhibitor individually, there was a significant decrease in FlaA-induced IL-8 production in T84 cells. A combination of two or three inhibitors further decreased FlaA-induced IL-8 production to basal levels (Fig. 7), especially when SB202190 was included. These data indicate that p38, JNK1/2, and ERK1/2 can each contribute to the FlaA-induced IL-8 production in T84 cells.
FIG. 7.
IL-8 production in T84 cells treated with V. cholerae FlaA in the presence or absence of MAPK inhibitors. T84 cells at approximately 80% confluence were pretreated with medium containing DMSO or various combinations of the p38 (SB202190), JNK (SP600125), and ERK (PD98059) inhibitors (10 μM each) for 1 h and then treated with medium containing 1 μg/ml V. cholerae recombinant FlaA (Vc rFlaA) in the presence of DMSO or the same combination of inhibitors for 18 h. Cell culture supernatants were harvested and assayed for IL-8 by ELISA, and the averages were calculated (n = 3). *, P < 0.001 for a comparison of treatment with FlaA and DMSO and treatment with FlaA in the presence of MAPK inhibitors.
DISCUSSION
Attempts to develop more effective Δctx V. cholerae vaccine strains have been fraught with difficulty due to the reactogenicity associated with an underlying inflammatory response that is probably suppressed by the effects of CT in normal infection. Although cholera is the prototypical noninflammatory disease, volunteers ingesting Δctx vaccine candidates, but not volunteers ingesting wild-type strains, had inflammatory diarrhea with levels of fecal lactoferrin (an indirect measure of neutrophil infiltration) equivalent to the levels seen with Shigella infections (44). In recent years, mounting evidence has shown that CT is a potent immunomodulator with anti-inflammatory effects on immune cells, including inhibition of polymorphonuclear leukocyte migration, tumor necrosis factor alpha and nitric oxide suppression in macrophages, and MAPK signaling downregulation in macrophages (41, 44). Since previous work in our lab had shown that a proteinaceous proinflammatory component(s) in filtered supernatants of the V. cholerae El Tor strain CVD115 induced IL-8 production in intestinal epithelial cells (57) and several reports have indicated that bacterial flagellins are a source of IL-8 induction (1, 11, 19, 32, 48, 53, 58), we sought to test the potential role of all five V. cholerae flagellins in this inflammatory signaling pathway.
All five flagellins of V. cholerae (FlaA to FlaE) contain a consensus amino acid sequence that is homologous to the conserved TLR5 recognition region of Salmonella FliC (Fig. 1A). We showed that each purified His-tagged V. cholerae flagellin is capable of activating NF-κB and inducing IL-8 production in HEK293T/TLR5 transfectants (Fig. 1B and 1C). We further verified our results for V. cholerae flagellin-induced NF-κB activation by analyzing the degradation of IκBα in T84 cells treated with recombinant V. cholerae FlaA or EPEC FliC (Fig. 6A and 6E). Furthermore, each V. cholerae flagellin induced IL-8 production in T84 cells (Fig. 1D). Neither E. coli nor V. cholerae LPS induced IL-8 production in either HT-29 or HEK293T/TLR5 transfectants (data not shown), consistent with our previous report that the stimulatory activity was abolished by treatment with trypsin or proteinase K (57). The specificity of flagellin-induced TLR5 activation was confirmed by use of a DN-TLR5 construct that significantly reduced NF-κB activation in HEK293T/TLR5 transfectants (Fig. 1B, right panel), consistent with the fact that HEK293T/TLR5 cells are known to be insensitive to LPS (5, 27). TLR5 has been shown to recognize purified Salmonella, E. coli, L. pneumophila, and V. vulnificus flagellins and to induce signaling cascades that result in the production of proinflammatory cytokines, such as IL-8 (7, 11, 12, 19, 32). Other reports have touched on the role of V. cholerae flagellins in inflammation (2, 53, 54); however, our results show directly that all five purified V. cholerae flagellins can induce IL-8 production and activate NF-κB specifically through TLR5. Consistent with our findings, Bandyopadhaya et al. recently reported that recombinant V. cholerae FlaA caused NF-κB translocation into the nucleus of Int407 cells, a small intestine epithelial cell line (2), and Yoon and Mekalanos recently demonstrated that recombinant V. cholerae FlaC and FlaD activated NF-κB in the pulmonary epithelial cell line A549 (54). However, until our study, direct TLR5-dependent activation of NF-κB by FlaA, FlaB, or FlaE had not been demonstrated.
Studies with Salmonella flagella have indicated that TLR5 activation by flagellins is mediated by TLR5 recognition of flagellin monomers rather than surfaces of the flagellar filament (46). We show here that monomeric V. cholerae flagellins (recombinant proteins or boiled flagella) activate NF-κB in HEK293T/TLR5 transfectants at lower concentrations than native V. cholerae flagella (Fig. 2), consistent with a previous report that showed that monomers are the activating form (46). However, at the highest concentration tested (100 ng/ml), some NF-κB activation by native V. cholerae flagella was detected. This may have been due to the increased sensitivity of our system since TLR5 was overexpressed, or perhaps there were enough flagellin monomers present in the flagellum preparation to elicit a response at higher concentrations.
Using isogenic flagellin deletion mutants, we found that deletion of all five flagellin genes in CVD115 (ΔflaABCDE) resulted in a strain that was completely nonmotile (data not shown), and the abilities of supernatants of this strain to activate NF-κB in HEK293T/TLR5 transfectants and to induce IL-8 production in HEK293T/TLR5 transfectants and HT-29 cells were significantly reduced (Fig. 3A, 3B, and 4). Supernatants of the ΔflaA, ΔflaAC, and ΔflaEDB strains were able to induce IL-8 production and activate NF-κB, suggesting that the remaining flagellins in each strain were capable of inducing an immunological response, which is supported by our results obtained with purified flagellins. We also demonstrated that supernatants of CVD115 and all of the flagellin mutant strains except the ΔflaABCDE strain contained flagellins (Fig. 5). The presence of the flagellins in supernatants was independent of bacterial lysis since the cytoplasmic protein GroEL was not found in culture supernatants (Fig. 5). V. cholerae strains with a deletion in flaA are not flagellated (17), indicating that the flagellins present in supernatants of ΔflaA strains are not derived from filamentous flagella. Yoon and Mekalanos recently suggested that the V. cholerae flagellar sheath may help V. cholerae flagella elude recognition by the innate immune system by suppressing monomer dissociation from V. cholerae flagella (54). However, our results indicate that the presence of intact sheathed flagella may not entirely determine whether V. cholerae flagellins are detected by the innate immune system since monomeric flagellins capable of inducing NF-κB and IL-8 production are clearly present in culture supernatants of both flaA+ and flaA V. cholerae strains (Fig. 5). The mechanism of release of unassembled V. cholerae flagellins into culture supernatants is not known; whether flagellins are actively exported, passively leaked during flagellum assembly, or released through some other mechanism remains to be determined.
While our HT-29 cell data indicate that supernatants of ΔflaA, ΔflaAC, and ΔflaEDB mutants induce production of IL-8 levels comparable to the level obtained with the parental El Tor strain CVD115 (Fig. 4), Xicohtencatl-Cortés et al. showed that boiled V. cholerae supernatants of strains having similar mutations in the classical Ogawa strain 395 background induced production of significantly lower levels of IL-8 in HT-29 cells than those induced by supernatants of wild-type Ogawa strain 395 (53). The variations may reflect differences in V. cholerae flagellin regulation, possibly due to differences between classical and El Tor biotypes, or differences in culture supernatant preparation.
In addition to NF-κB activation, MAPKs have also been implicated in the induction of IL-8 production (14). E. coli, L. pneumophila, and Salmonella flagellins have been shown to activate MAPKs in tissue culture cells, leading to the production of IL-8 (16, 43, 58). Here, we report that purified V. cholerae FlaA was able to activate all three of the MAPKs (p38, JNK1/2, and ERK1/2) in T84 cells at levels comparable to the levels induced by an equivalent amount of EPEC FliC (Fig. 6A, 6B, 6C, and 6D). By using specific kinase inhibitors, we also demonstrated that IL-8 production in T84 cells was most sensitive to inhibition of p38, yet all three MAPKs seem to contribute to signaling (Fig. 7). In line with our observations, Bandyopadhaya et al. recently reported that recombinant V. cholerae FlaA activates p38 and ERK1/2 in the small intestine epithelial cell line Int407 (2); however, they did not look at JNK1/2 activation. Inhibition of p38 and ERK1/2 was also found to reduce the production of the proinflammatory cytokine IL-1β in Int407 cells (2), mirroring our results for IL-8. Other reports have shown that inhibition of p38 significantly reduced IL-8 production by intestinal epithelial cells treated with E. coli (16, 58) and Salmonella (55) flagellins, which is also in line with our results. Also, Hoffman et al. reported that the ERK pathway may also contribute to, but not have a primary role in, induction of IL-8 production (14), which correlates with our finding that even when there is inhibition of ERK, IL-8 production in T84 cells treated with V. cholerae FlaA can still occur.
In summary, our data support the hypothesis that all five V. cholerae flagellins have a direct role in the induction of IL-8 production through activation of NF-κB and MAPKs. Since IL-8 is the primary neutrophil chemokine in humans, it is likely that stimulation of TLR5 by flagellins contributes to the inflammatory responses seen with vaccine strains. Further experiments are under way to determine whether flagellin-induced IL-8 production contributes to vaccine reactogenicity.
Acknowledgments
We thank Andrew Gewirtz for providing the TLR5 constructs, Douglas Golenbock for providing the ELAM (NF-κB) luciferase and β-galactosidase reporter constructs, and Karl Klose for providing the suicide plasmid pKEK93. We also thank Mary-Jane Lombardo for critical reading of the manuscript, Tamara Hilton for technical assistance, and Alexei Maigkov of NovaScreen (Hanover, MD) for preliminary TLR screening data.
This work was funded by National Institutes of Health grants F32-DK077209 (to L.M.H.), R37-AI18797 (to S.N.V.), and R01-AI19716 (to J.B.K.).
Editor: V. J. DiRita
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Bambou, J. C., A. Giraud, S. Menard, B. Begue, S. Rakotobe, M. Heyman, F. Taddei, N. Cerf-Bensussan, and V. Gaboriau-Routhiau. 2004. In vitro and ex vivo activation of the TLR5 signaling pathway in intestinal epithelial cells by a commensal Escherichia coli strain. J. Biol. Chem. 27942984-42992. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhaya, A., M. Sarkar, and K. Chaudhuri. 2008. IL-1beta expression in Int407 is induced by flagellin of Vibrio cholerae through TLR5 mediated pathway. Microb. Pathog. 44524-536. [DOI] [PubMed] [Google Scholar]
- 3.Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell. Microbiol. 4635-648. [DOI] [PubMed] [Google Scholar]
- 4.Betis, F., P. Brest, V. Hofman, J. Guignot, M. F. Bernet-Camard, B. Rossi, A. Servin, and P. Hofman. 2003. The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect. Immun. 711068-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 27410689-10692. [DOI] [PubMed] [Google Scholar]
- 6.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103239-252. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly, M. A., and T. S. Steiner. 2002. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of Toll-like receptor 5. J. Biol. Chem. 27740456-40461. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 594310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasano, A., B. Baudry, D. W. Pumplin, S. S. Wasserman, B. D. Tall, J. M. Ketley, and J. B. Kaper. 1991. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 885242-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangarosa, E. J., W. R. Beisel, C. Benyajati, H. Sprinz, and P. Piyaratn. 1960. The nature of the gastrointestinal lesion in Asiatic cholera and its relation to pathogenesis: a biopsy study. Am. J. Trop. Med. Hyg. 9125-135. [DOI] [PubMed] [Google Scholar]
- 11.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 1671882-1885. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 4101099-1103. [DOI] [PubMed] [Google Scholar]
- 13.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72847-855. [PubMed] [Google Scholar]
- 15.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 848-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan, M. A., J. Kang, and T. S. Steiner. 2004. Enteroaggregative Escherichia coli flagellin-induced interleukin-8 secretion requires Toll-like receptor 5-dependent p38 MAP kinase activation. Immunology 112651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klose, K. E., and J. J. Mekalanos. 1998. Differential regulation of multiple flagellins in Vibrio cholerae. J. Bacteriol. 180303-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan, J., K. Selvarajo, M. Tsuchiya, G. Lee, and S. Choi. 2007. Toll-like receptor signal transduction. Exp. Mol. Med. 39421-438. [DOI] [PubMed] [Google Scholar]
- 19.Lee, S. E., S. Y. Kim, B. C. Jeong, Y. R. Kim, S. J. Bae, O. S. Ahn, J. J. Lee, H. C. Song, J. M. Kim, H. E. Choy, S. S. Chung, M. N. Kweon, and J. H. Rhee. 2006. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect. Immun. 74694-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine, M. M., R. E. Black, M. L. Clements, D. R. Nalin, L. Cisneros, and R. A. Finkelstein. 1981. Volunteer studies in development of vaccines against cholera and enterotoxigenic Escherichia coli: a review, p. 443-459. In T. Holme, J. Holmgren, M. H. Merson, and R. Mollby (ed.), Acute enteric infections in children. New prospects for treatment and prevention. Elsevier, Amsterdam, The Netherlands.
- 21.Levine, M. M., J. B. Kaper, R. E. Black, and M. L. Clements. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol. Rev. 47510-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine, M. M., J. B. Kaper, D. Herrington, J. Ketley, G. Losonsky, C. O. Tacket, B. Tall, and S. Cryz. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. ii467-470. [DOI] [PubMed] [Google Scholar]
- 23.Levine, M. M., J. B. Kaper, D. Herrington, G. Losonsky, J. G. Morris, M. L. Clements, R. E. Black, B. Tall, and R. Hall. 1988. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 56161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathan, M. M., G. Chandy, and V. I. Mathan. 1995. Ultrastructural changes in the upper small intestinal mucosa in patients with cholera. Gastroenterology 109422-430. [DOI] [PubMed] [Google Scholar]
- 25.McCormick, B. A., C. A. Parkos, S. P. Colgan, D. K. Carnes, and J. L. Madara. 1998. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J. Immunol. 160455-466. [PubMed] [Google Scholar]
- 26.McIntire, F. C., H. W. Sievert, G. H. Barlow, R. A. Finley, and A. Y. Lee. 1967. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry 62363-2372. [DOI] [PubMed] [Google Scholar]
- 27.Medvedev, A. E., and S. N. Vogel. 2003. Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J. Endotoxin Res. 960-64. [DOI] [PubMed] [Google Scholar]
- 28.Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 1755899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michalski, J., J. E. Galen, A. Fasano, and J. B. Kaper. 1993. CVD110, an attenuated Vibrio cholerae O1 El Tor live oral vaccine strain. Infect. Immun. 614462-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, J. R., L. J. Barrett, K. Kotloff, and R. L. Guerrant. 1994. A rapid test for infectious and inflammatory enteritis. Arch. Intern. Med. 1542660-2664. [DOI] [PubMed] [Google Scholar]
- 31.Mukaida, N., S. Okamoto, Y. Ishikawa, and K. Matsushima. 1994. Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56554-558. [PubMed] [Google Scholar]
- 32.Murthy, K. G., A. Deb, S. Goonesekera, C. Szabo, and A. L. Salzman. 2004. Identification of conserved domains in Salmonella muenchen flagellin that are essential for its ability to activate TLR5 and to induce an inflammatory response in vitro. J. Biol. Chem. 2795667-5675. [DOI] [PubMed] [Google Scholar]
- 33.Norris, H. T. 1974. The pathology of cholera, p. 169-187. In B. Burrows (ed.), Cholera. Saunders, Philadelphia, PA.
- 34.Pastore, G., G. Schiraldi, G. Fera, E. Sforza, and O. Schiraldi. 1976. A bioptic study of gastrointestinal mucosa in cholera patients during an epidemic in southern Italy. Am. J. Dig. Dis. 21613-617. [DOI] [PubMed] [Google Scholar]
- 35.Qadri, F., T. R. Bhuiyan, K. K. Dutta, R. Raqib, M. S. Alam, N. H. Alam, A. M. Svennerholm, and M. M. Mathan. 2004. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induces increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut 5362-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qadri, F., R. Raqib, F. Ahmed, T. Rahman, C. Wenneras, S. K. Das, N. H. Alam, M. M. Mathan, and A. M. Svennerholm. 2002. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab Immunol. 9221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rallabhandi, P., J. Bell, M. S. Boukhvalova, A. Medvedev, E. Lorenz, M. Arditi, V. G. Hemming, J. C. Blanco, D. M. Segal, and S. N. Vogel. 2006. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J. Immunol. 177322-332. [DOI] [PubMed] [Google Scholar]
- 38.Richardson, K., and C. D. Parker. 1985. Identification and occurrence of Vibrio cholerae flagellar core proteins in isolated outer membrane. Infect. Immun. 47674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez, B. L., A. Rojas, J. Campos, T. Ledon, E. Valle, W. Toledo, and R. Fando. 2001. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect. Immun. 69613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook, J., and M. J. Gething. 1989. Protein structure. Chaperones, paperones. Nature 342224-225. [DOI] [PubMed] [Google Scholar]
- 41.Satchell, K. J. 2003. Activation and suppression of the proinflammatory immune response by Vibrio cholerae toxins. Microbes Infect. 51241-1247. [DOI] [PubMed] [Google Scholar]
- 42.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 644480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmeck, B., P. D. N′Guessan, M. Ollomang, J. Lorenz, J. Zahlten, B. Opitz, A. Flieger, N. Suttorp, and S. Hippenstiel. 2007. Legionella pneumophila-induced NF-κB- and MAPK-dependent cytokine release by lung epithelial cells. Eur. Respir. J. 2925-33. [DOI] [PubMed] [Google Scholar]
- 44.Silva, T. M., M. A. Schleupner, C. O. Tacket, T. S. Steiner, J. B. Kaper, R. Edelman, and R. Guerrant. 1996. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect. Immun. 642362-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simon, R., U. Prefiefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 46.Smith, K. D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M. A. Bergman, S. L. Barrett, B. T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 41247-1253. [DOI] [PubMed] [Google Scholar]
- 47.Steiner, T. S. 2007. How flagellin and Toll-like receptor 5 contribute to enteric infection. Infect. Immun. 75545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 1051769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tacket, C. O., G. Losonsky, J. P. Nataro, S. J. Cryz, R. Edelman, A. Fasano, J. Michalski, J. B. Kaper, and M. M. Levine. 1993. Safety and immunogenicity of live oral cholera vaccine candidate CVD 110, a ΔctxA Δzot Δace derivative of El Tor Ogawa Vibrio cholerae. J. Infect. Dis. 1681536-1540. [DOI] [PubMed] [Google Scholar]
- 50.Tallant, T., A. Deb, N. Kar, J. Lupica, M. J. de Veer, and J. A. DiDonato. 2004. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-κB and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thanos, D., and T. Maniatis. 1995. NF-kappa B: a lesson in family values. Cell 80529-532. [DOI] [PubMed] [Google Scholar]
- 52.Trucksis, M., J. E. Galen, J. Michalski, A. Fasano, and J. B. Kaper. 1993. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc. Natl. Acad. Sci. USA 905267-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xicohtencatl-Cortés, J., S. Lyons, A. P. Chaparro, D. R. Hernandez, Z. Saldana, M. A. Ledesma, M. A. Rendon, A. T. Gewirtz, K. E. Klose, and J. A. Girón. 2006. Identification of proinflammatory flagellin proteins in supernatants of Vibrio cholerae O1 by proteomics analysis. Mol. Cell Proteomics 52374-2383. [DOI] [PubMed] [Google Scholar]
- 54.Yoon, S. S., and J. J. Mekalanos. 2008. Decreased potency of the Vibrio cholerae sheathed flagellum to trigger host innate immunity. Infect. Immun. 761282-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, Y., H. Zeng, S. Lyons, A. Carlson, D. Merlin, A. S. Neish, and A. T. Gewirtz. 2003. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 285G282-G290. [DOI] [PubMed] [Google Scholar]
- 56.Zhao, L., M. J. Kwon, S. Huang, J. Y. Lee, K. Fukase, N. Inohara, and D. H. Hwang. 2007. Differential modulation of Nods signaling pathways by fatty acids in human colonic epithelial HCT116 cells. J. Biol. Chem. 28211618-11628. [DOI] [PubMed] [Google Scholar]
- 57.Zhou, X., D. Q. Gao, J. Michalski, J. A. Benitez, and J. B. Kaper. 2004. Induction of interleukin-8 in T84 cells by Vibrio cholerae. Infect. Immun. 72389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, X., J. A. Girón, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 712120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]