Abstract
In the Saimiri sciureus monkey, erythrocytes infected with the varO antigenic variant of the Plasmodium falciparum Palo Alto 89F5 clone bind uninfected red blood cells (rosetting), form autoagglutinates, and have a high multiplication rate, three phenotypic characteristics that are associated with severe malaria in human patients. We report here that varO parasites express a var gene having the characteristics of group A var genes, and we show that the varO Duffy binding-like 1α1 (DBL1α1) domain is implicated in the rosetting of both S. sciureus and human erythrocytes. The soluble varO N-terminal sequence (NTS)-DBL1α1 recombinant domain, produced in a baculovirus-insect cell system, induced high titers of antibodies that reacted with varO-infected red blood cells and disrupted varO rosettes. varO parasites were culture adapted in vitro using human erythrocytes. They formed rosettes and autoagglutinates, and they had the same surface serotype and expressed the same varO gene as the monkey-propagated parasites. To develop an in vitro model with highly homogeneous varO parasites, rosette purification was combined with positive selection by panning with a varO NTS-DBL1α1-specific mouse monoclonal antibody. The single-variant, clonal parasites were used to analyze seroprevalence for varO at the village level in a setting where malaria is holoendemic (Dielmo, Senegal). We found 93.6% (95% confidence interval, 89.7 to 96.4%) seroprevalence for varO surface-reacting antibodies and 86.7% (95% confidence interval, 82.8 to 91.6%) seroprevalence for the recombinant NTS-DBL1α1 domain, and virtually all permanent residents had seroconverted by the age of 5 years. These data imply that the varO model is a relevant in vivo and in vitro model for rosetting and autoagglutination that can be used for rational development of vaccine candidates and therapeutic strategies aimed at preventing malaria pathology.
Plasmodium falciparum malaria is a major public health burden in intertropical areas, with up to 600 million cases and more than 2 million deaths each year, mainly African children (8). A pathological hallmark of P. falciparum infections is sequestration of mature intraerythrocytic parasite stages in the microvasculature of vital organs. Sequestration results from cytoadherence of P. falciparum-infected red blood cells (iRBC) to microvascular endothelial cells or to circulating blood cells. Rosetting (i.e., the capacity of iRBC to bind uninfected red blood cells [RBC]) has been consistently associated with severe malaria in African children (12, 23, 30, 59, 67, 85). Platelet-mediated clumping of P. falciparum iRBC has been associated with severe malaria in many studies (20, 60, 64, 88) but not in all studies (2, 3). Importantly, children with severe malaria do not have rosette-disrupting antibodies (12). The mechanism by which rosetting contributes to the severity of infection may result from occlusion of the microvasculature (36, 54) and/or from a particularly high parasite multiplication rate, which may be favored by efficient invasion of the uninfected RBC in the rosettes by bursting merozoites (47).
Analysis of the molecular basis of cytoadherence has highlighted the key role played by the variant P. falciparum erythrocyte membrane protein 1 (PfEMP1) encoded by the var multigene family (for a review, see reference 39). PfEMP1 adhesins are high-molecular-mass proteins with a large extracellular region consisting of Duffy binding-like (DBL), constant (C2), and cysteine-rich interdomain region (CIDR) modules. Specific sequence signatures permit grouping of DBL domains into seven distinct classes (DBLα, DBLα1, DBLβ, DBLγ, DBLδ, DBLɛ, and DBLX) and CIDR domains into four classes (CIDRα, CIDRα1, CIDRβ, and CIDRγ) (39, 40, 65, 78). Based on 5′ and 3′ noncoding sequences, domain combinations, chromosomal location, and gene orientation, var genes were classified into three major groups, groups A, B, and C, and two intermediate groups, groups B/A and B/C (39, 40, 65, 78).
Based on the limited number of genes associated thus far with rosetting, it appears that this phenomenon is mediated by a small subset of PfEMP1 variants (9), each of which is involved in a specific interaction(s) with host molecules (for reviews, see references 27 and 50), including RBC surface receptors (29, 70, 86) and serum components (21, 26, 30, 49, 50, 71, 79). To date, two in vitro rosette-forming variants have been studied in detail. The first variant, designated R29, expresses a group A var gene that codes for a PfEMP1 adhesin that binds to complement receptor 1 (CR1)/CD35 (68). The second variant, designated FCR3S1.2, forms giant rosettes and expresses a PfEMP1 molecule that binds to diverse host receptors, including heparan sulfate, blood group A, immunoglobulin M (IgM), PECAM-1/CD31, and CD36 (14, 15, 75). In contrast to that of R29, the FCR3S1.2 var gene does not belong to group A (38). Expression of individual modules from both variants has shown that the N-terminal DBL1α domain of each variant mediates rosetting (15, 68). R29 and FCR3S1.2 are antigenic variants of the FCR3/IT4 line, which is poorly infectious for nonhuman primates (25), which hampers in vivo experimental studies with rosette-forming parasites.
We developed an in vivo experimental model of rosetting in the Saimiri sciureus monkey using the varO antigenic variant of the Palo Alto 89F5 clone, which forms rosettes and autoagglutinates that circulate in the peripheral blood of splenectomized animals (22, 24, 46). This allowed us to show that varO parasites have a higher in vivo multiplication rate than the isogenic, nonrosetting, antigenic variant varR parasites (47). We report here that the varO gene expressed by 89F5 varO parasites has the main features of the genes belonging to the group A/UpsA subset, which is frequently associated with severe malaria in African children (35, 41). Due to high costs associated with S. sciureus studies and the paucity of specific reagents available for this animal species, we developed an in vitro varO model using human RBC culture. We show here that human RBC culture-adapted 89F5 varO parasites express the same varO gene, have the same rosetting and autoagglutination phenotypes, and have the same surface serotype as Saimiri-propagated parasites. To establish a continuous in vitro culture of parasites uniformly expressing the varO gene, rosette enrichment was combined with positive selection by panning with a mouse monoclonal antibody (MAb) raised to a soluble varO N-terminal sequence (NTS)-DBL1α1 recombinant domain. The single-variant preparations obtained were used to investigate the seroprevalence of antibodies against varO parasites in Dielmo, a Senegalese village located in a region where malaria is holoendemic. Very high levels of seroprevalence were observed for varO-infected RBC (varO-iRBC) and for the recombinant rosetting domain. The varO model thus is a pertinent rosetting-autoagglutination model and, indeed, the only system for which both a nonhuman primate host and an in vitro model are available to guide the design of rational intervention strategies and vaccine candidates.
MATERIALS AND METHODS
Parasites.
The 89F5 parasite clone was obtained by cloning by limiting dilution (in human RBC cultures) the uncloned varO antigenic variant of the Saimiri-adapted Palo Atlo strain of P. falciparum (also designated FUP/SP) (24, 47). The 89F5 clone was then inoculated into a naive splenectomized animal and further propagated in splenectomized Saimiri monkeys by serial blood passage (44). RBC infected with the uncloned varO line or with the 89F5 varO clone form rosettes and spontaneously autoagglutinate in the absence of immune serum (24, 46). The nonrosetting varR clone is an isogenic antigenic variant of the Palo Alto FUP/SP line obtained using a varO-infected monkey under immune pressure (24, 45, 46).
Saimiri monkey infection and blood sampling.
Male adult Saimiri monkeys were used in this study. The procedures used for housing, animal handling, and blood sampling have been described previously (24, 45-47). Nonimmune and immune (anti-varO and anti-varR) Saimiri sera were obtained as described previously (47). For RNA and DNA preparation, peripheral blood was collected by venipuncture from anesthetized animals when parasitemia was ≥20%.
Cloning and sequencing of the var gene expressed by 89F5 varO parasites.
In order to obtain RNA preparations devoid of the nonspecific abortive var transcripts present in early stages (74) and with host RNA depleted, infected blood samples collected from infected Saimiri monkeys were treated as follows. After the white blood cells were removed with a CF11 column (31), the mature stages present in the blood samples were lysed with 5% sorbitol, while the young stages were allowed to mature in complete culture medium for 24 h before they were harvested by centrifugation. RNA was extracted from the mature parasite stages as described previously (19). DNase I-treated RNA preparations were used for reverse transcriptase PCRs (RT-PCRs). Comparative RT-PCRs with varO and varR parasites were used to ascertain the variant-specific expression profile. The first varO-specific fragment cloned was derived from the DBL3γ domain, which was cloned from RT-PCR-generated fragments using the UNIEBP5′ and UNIEBP3′ primers described elsewhere (45, 62). This generated the nucleotide sequence from position 3799 to position 4266 of the varO gene. The varO DBL1α fragment was then amplified by RT-PCR using the universal α-AF and α-BR primers (81) and cloned using a TOPO TA cloning kit (Invitrogen). The DBL1α fragment included nucleotide positions 577 to 959 of the varO sequence. Specific primers based on the DBL1α and DBL3γ fragments were then used to clone and sequence the entire varO gene using (i) chromosome walking, which produced flanking fragments for both domains, and (ii) RT-PCR performed with specific and conserved primers, which generated DBL1-DBL3 and DBL3-exon II fragments. Chromosome walking was performed with a Universal genome walker kit (Clontech) using varO DBL1α- or varO DBL3γ-specific primers (Table 1). RT-PCR was used to amplify a 3,071-bp fragment from varO RNA with the DBL1-specific primer DBL1O-fo851 and the varO DBL3-specific primer DBL3O-rev3894 and to amplify a 3,249-bp fragment with the varO DBL3-specific primer DBL3O-fo4129 and exon II conserved primer L5 (14). The PCR fragments were directly sequenced to avoid possible cloning artifacts in the sequence. The cDNA sequence confirmed the sequence data obtained by chromosome walking. The available varO cDNA sequence codes for an 7,378-bp open reading frame starting with the initiator codon ATG. The exon II sequence is a partial sequence.
TABLE 1.
Primer sequences used for varO gene sequencing and for detection of varO expression in 89F5 varO parasite cultures
| Primera | Sequence (5′-3′) | Primer positions (bp) |
|---|---|---|
| Primary PCR | ||
| DBL1O-rev639 | CTTGTAGTCCTTTTTGTACTTTATCATCC | 639-667 |
| DBL1O-rev853 | TCCGTGACTGCTAAAATGTAGAGTACC | 853-879 |
| DBL1O-fo851 | ATGGTACTCTACATTTTAGCAGTCACGG | 851-878 |
| DBL3O-rev3894 | ATATTTATACCAAGCAAAATGTGTTTCTATA | 3894-3924 |
| DBL3O-rev4095 | GAAGAAAGAAAGTAAGGAAAGAAAAAATCC | 4095-4124 |
| Nested PCR | ||
| DBL1O-rev624 | GTACTTTATCATCCTTATTAGGTAAAAAC | 624-652 |
| DBL1O-rev844 | CTGCTAAAATGTAGAGTACCATCTGAACC | 844-872 |
| DBL1O-fo864 | TTTTAGCAGTCACGGAAAGTGCGGCC | 864-889 |
| DBL3O-rev3846 | CTCTGTTCTTATATCTTCTTTTTCTTTTATA | 3846-3876 |
| DBL3O-fo4129 | GAATGGTGGAATGAACATGGAAAGGAG | 4129-4155 |
| L5 (exonII) | CCATCTTCATATTCACTTTCTGA | |
| varO gene expression | ||
| 5ID3Bfo | GCCCAATTGGATCCACAAAACAAAGCATATAAAG | 5764-5797 |
| 5DBL5Bfo | TGTAAGAAGTAGGATCCAGGTAGTTGTCCAGAA | 6085-6117 |
| VarO3exon2Brev | ATATCTATTTGATGATTTCGGGGTAGG | 7216-7242 |
rev, reverse primer; fo, forward primer.
PfEMP1varO sequence analysis and homology search with the databases.
PfEMP1varO protein domain sequence boundaries were defined by using previously described criteria (65, 78, 80). Type-specific consensus motifs were used to classify individual varO domains. For each domain, sequence homology searches using P. falciparum isolate 3D7, IT4/25/5, HB3, Dd2, and Ghana and chimpanzee malaria parasite Plasmodium reichenowi partial genome and protein sequence databases were performed using the BLAST tools available and specialized Plasmodium databases. Sequences with high levels of similarity were extracted. Translated sequences were aligned using the ClustalW program (DNASTAR Lasergene software, V7.1.0). The cysteine/position of limited variation (PoLV) classification was used to assign the varO DBL1α domain (9, 10).
In vitro culture of 89F5 varO parasites in human RBC.
Cryopreserved blood of Saimiri monkeys infected with 89F5 varO parasites was thawed and used to establish an in vitro culture with human RBC. The parasites were cultivated and maintained in continuous culture in human O+ RBC (blood bank of Etablissement Français du Sang [EFS], Rungis, France), adjusted to 5% hematocrit (82), in RPMI 1640 medium (Invitrogen) supplemented with 10% human AB+ serum using an atmosphere containing 5% O2, 5% CO2, and 90% N2. Routine screening of cultures for Mycoplasma contamination by PCR using a VenorGeM mycoplasma detection kit (Biovalley) was negative. Rosetting parasites were enriched once a week by centrifugation (30 s, 1,000 × g) on ice-cold Ficoll (1.077 g/ml; Lymphoprep; Abcys) as previously described (48). Rosette formation was monitored after iRBC nuclei were stained with Hoechst dye (Hoechst 33342; Molecular Probes). The rosetting rate was calculated by determining the percentage of rosette-forming iRBC present in the mature parasite population. A weekly enrichment procedure kept the rosetting rate above 90%.
Selection of varO-iRBC by panning with a varO-specific mouse MAb.
To select for the varO phenotype in a heterogeneous population of rosette-forming parasites, varO-iRBC were positively selected by panning with D15-50, a mouse MAb raised to the rNTS-DBL1α1 domain of varO (M. Guillotte, I. Vigan-Womas, A. Juillerat, S. Igonet, F. Marchand, F. Nato, G. Bentley, and O. Mercereau-Puijalon, unpublished data). Briefly, rosette-forming iRBC were first treated with 10 μg/ml dextran sulfate (molecular weight, >500,000; Sigma) to dissociate rosettes. iRBC were then magnetically selected on a MACS column (Miltenyi BioTec) (61), eluted, resuspended in phosphate-buffered saline (PBS) with 2% human AB+ serum, and placed in a culture flask (75 cm2; Corning) that had been coated overnight at 4°C with MAb D15-50 (10 ml of a 100-μg/ml solution in PBS) and saturated for 2 h at room temperature with PBS containing 2% bovine serum albumin. Binding was performed at 37°C for 2 h with gentle rocking every 30 min. Unbound iRBC were removed by washing the preparation with the same medium, and binding of varO-iRBC was checked using an inverted microscope. After addition of fresh RBC along with complete culture medium, the parasites were allowed to reinvade overnight. The culture was then transferred to new flasks and incubated under standard conditions. The percentage of varO-positive iRBC in the culture was routinely assessed by performing surface immunofluorescence assays (S-IFA) with anti-rNTS-DBL1α1 mouse serum or MAb D15-50.
varO gene expression in cultured 89F5 varO parasites.
RT-PCR was performed using total RNA extracted with the Trizol reagent (Invitrogen) from synchronized 89F5 varO parasite cultures at the trophozoite and early-schizont stages. The 5DBL5Bfo-VarO3exon2Brev and 5ID4Bfo-VarO3exon2Brev specific primer pairs (Table 1) were used. One-half of the RNA preparations were treated with RNase-free DNase I (Invitrogen) before RT-PCR amplification. RT-PCRs were performed using the Access RT-PCR introductory system (Promega) according to the manufacturer's instructions. PCR products were analyzed by agarose gel electrophoresis.
Plasmid constructs and production of recombinant baculovirus.
To overcome expression problems caused by the codon usage bias of P. falciparum genes, codon-optimized versions of domain-encoding sequences were synthesized. Recodoned DBL1α1 optimized for Homo sapiens codon usage (positions 96 to 398 of the deduced varO protein sequence), designated rhDBL1α, has been described elsewhere (72). Insect cell-optimized coding sequences of the varO CIDRγ, DBL2βC2, and DBL5β domains (positions 399 to 835, 821 to 1241, and 2031 to 2264 of the deduced varO protein sequence, respectively) were custom-made (Biomethodes, Evry, France). Insect cell-optimized NTS-DBL1α1 (positions 1 to 487 of the deduced varO protein sequence) was synthesized by GeneCust (Evry, France). For each domain, all potential N-glycosylation sites were mutated (NXS/T to NXA), as were restriction sites that could hinder cloning manipulations. The constructs also contained a coding sequence for a six-histidine tag 3′, upstream from, and in frame with a stop codon. The synthesized recombinant genes, designated rhDBL1α, rNTS-DBL1α1, rCIDRγ, rDBL2βC2, and rDBL5β, were cloned in the pMelBacA plasmid (Invitrogen) downstream from and in phase with the honeybee mellitin signal sequence. Each sequence was confirmed base by base for both strands.
Spodoptera frugiperda (Sf9; Invitrogen) and Trichoplusia ni (High-5; Invitrogen) insect cells were grown in monolayer or suspension cultures at 27°C, in SF-900-II serum-free medium (Gibco-BRL) supplemented with 4 mM glutamine (Gibco-BRL), 5% fetal bovine serum, and 50 μg/ml gentamicin. For each domain, recombinant baculovirus was generated using the Bac-N-Blue transfection and expression system (Invitrogen) according to the manufacturer's protocol. Viral stocks were produced in Sf9 insect cells and stored at 4°C. The recombinant viral clones were confirmed by PCR and sequencing.
Cell surface expression of varO recombinant domains and erythrocyte binding assay.
Briefly, 5 × 106 High-5 cells grown in a monolayer were infected with recombinant baculovirus. After 90 min of incubation at 27°C, the infection medium was removed, and infected cells were cultured in complete culture medium for 3 days. Infected cells expressing rhDBL1α, rCIDRγ, rDBL2βC2, or rDBL5β were harvested and analyzed in order to determine surface expression of the recombinant domain by S-IFA using a mouse anti-His tag MAb (Novagen), followed by Alexa Fluor 488-conjugated goat anti-mouse IgGF(ab′)2 (Molecular Probes). Staining was analyzed by fluorescence microscopy (Leica DM4500B) or by flow cytometry (FACS LSR1; Becton Dickinson). Erythrocyte binding on insect cells expressing varO recombinant domains was assessed using 1 × 106 infected High-5 cells washed twice with RPMI 1640 and incubated with human O+ or A+ RBC in RPMI 1640 supplemented with 10% human serum for 3 to 5 h at 27°C. An aliquot of a cell suspension was mounted on a glass slide, and rosette formation was monitored by microscopy.
Production of soluble recombinant domains and mouse immunization.
To produce a soluble rNTS-DBL1α1 domain, suspension cultures of Sf9 cells were infected with recombinant baculovirus harboring the rNTS-DBL1α1 construct. After 3 days of incubation, the soluble secreted protein was harvested from culture supernatants and purified as described previously (7). The sequence of the recombinant protein was verified by mass spectroscopy, and its purity was assessed by Coomassie blue staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and immunoblotting. The N-terminal sequence of the recombinant protein was determined and was shown to match the predicted sequence after cleavage of the mellitin signal sequence. The DBL3γ732 recombinant protein was obtained as described previously (17).
Outbred (OF1) female mice (6 to 8 weeks old; Charles River, France) were immunized by subcutaneous injection at 3-week intervals of 10 μg of soluble rNTS-DBL1α1 or DBL3γ732 protein in the presence of Freund's adjuvant (complete Freund's adjuvant for the first immunization and incomplete Freund's adjuvant for the booster immunizations). Sera were collected 10 days after the third injection, pooled, and analyzed by enzyme-linked immunosorbent assay (ELISA), immunoblotting, S-IFA, and rosette dissociation assay. Mouse MAb D15-50 was obtained from a mouse immunized with rNTS-DBL1α1 as described elsewhere (55).
Collection of human serum from malaria-exposed and non-malaria-exposed individuals.
A longitudinal study was carried out in Dielmo (Senegal), a village located in an area where malaria is holoendemic (the study design has been described previously [58, 66, 84]). For this substudy, we used serum samples collected from 235 (ages, 1 to 85 years; mean age, 23 years) of the 247 villagers living in Dielmo from July to September 1992. During the 1992 rainy season, the entomological inoculation rate was 26 ± 17 infective bites/person/month. Serum samples were stored at −20°C until they were used at the Institut Pasteur in Paris, France. This longitudinal study is part of a malaria immunity program and has been approved by National Council on Health Research of Senegal (reference no. 1971 MSPM/DS/DER; August 2006); it is being conducted in accordance with the Declaration of Helsinki. Informed written consent is obtained when an individual is included in the study and is orally confirmed each year by the volunteers studied. For the varO substudy, the protocol was approved by the institutional review boards of the Institut Pasteur of Paris (reference no. RBm/2006.032; September 2007) and the National Council on Health Research of Senegal (reference no. 05 MSP/DS/CNRS; February 2008). We also used nonimmune human plasma obtained from healthy non-malaria-exposed adults (blood bank of EFS, Rungis, France).
S-IFA.
After rosette enrichment, an aliquot of 89F5 varO rosetting parasites (rosetting rate, >85%) was incubated for 30 min at 37°C with serial dilutions of mouse sera or with human plasma or Saimiri sera (1:20 dilution). After two washes with PBS with 2% fetal calf serum, the binding of IgG or IgM was detected with the appropriate secondary antibody diluted 1:1,000 in PBS with 2% fetal calf serum. Surface-reactive human or Saimiri antibodies were detected with Alexa Fluor 488-conjugated goat anti-human IgG or IgM (Molecular Probes). For mice, Alexa Fluor 488-conjugated goat anti-mouse IgG F(ab′)2 (Molecular Probes) was used. Each secondary antibody was mixed with Hoechst dye (1:1,000 dilution; Hoechst 33342; Molecular Probes) for iRBC nucleus staining. After 30 min of incubation at 37°C and two washes, parasites were fixed with 0.37% formaldehyde (Sigma), and immunofluorescence was analyzed by using fluorescence microscopy or flow cytometry. Pools of sera from nonimmune mice, humans not exposed to malaria, or naïve Saimiri monkeys were used as negative controls (NC).
For the seroprevalence study, a pool of 30 adult sera from Dielmo was used as a positive control (PC). Five thousand events were recorded for Hoechst dye-gated iRBC, and the data were processed with the CellQuest software (Becton Dickinson). For each sample, the percentage of Alexa Fluor-positive iRBC (%iRBC+) and the mean fluorescence intensity (MFI) were calculated. The IgG surface reactivity was expressed in arbitrary units (AU), determined as follows: [(%iRBC+sample − %iRBC+NC)/(%iRBC+PC − %iRBC+NC)] × 100. A reactivity index that took into account the MFI was calculated as follows: [(MFIsample × %iRBC+sample)/(MFIPC × %iRBC+PC)] × 100. The mean surface reactivity plus 3 standard deviations observed with plasma from 20 European donors not exposed to malaria (EFS, Rungis, France) was used as a negative cutoff value. A cutoff value of 20 AU was obtained, and individuals having surface-reactive antibody values above this value were considered positive.
ELISA using varO rNTS-DBL1α1.
ELISA plates (Maxisorp 439454; Nunc, France) were coated with 0.2 μg (in 100 μl) of rNTS-DBL1α1 protein or nonfat milk protein in PBS. After overnight incubation at 4°C, the plates were blocked with 200 μl PBS-5% nonfat milk for 1 h at 37°C. Human or Saimiri sera (1:100 dilution) or anti-rNTS-DBL1α1 mouse sera (twofold serial dilutions) were diluted in PBS-2.5% nonfat milk-0.05% Tween 20 and incubated for 1 h at 37°C. Immunoglobulin binding was detected using horseradish peroxidase-conjugated goat anti-human IgG F(ab′)2 (Cappel, France), anti-mouse IgG (Promega, France), and (for Saimiri sera) anti-human IgG (Promega, France). Enzymatic reactions were developed for 10 min at room temperature after addition of the trimethylbenzene-H2O2 substrate (KPL). Optical densities (OD) at 450 and 655 nm were determined with a multiscan plate reader (Bio-Rad). Each serum was tested in duplicate.
To study the seroprevalence of varO rNTS-DBL1α1, antibody responses were expressed in AU to account for day-to-day variation and were calculated as follows: [(ODsample − ODbackground)/(ODPC − ODbackground)] × 100. A pool of plasma from adults from Dielmo reacting strongly with the surface of varO-iRBC was used as a positive control. The mean OD plus 2 standard deviations obtained with plasma from 20 healthy European donors (EFS, Rungis, France) who had not been exposed to malaria was used as a negative cutoff value. A cutoff value of 7.76 AU was obtained for rNTS-DBL1α1.
Rosette dissociation assay.
An aliquot of rosette-enriched 89F5 varO parasites (20 μl in complete RPMI culture medium; rosetting rate, >85%; 5% parasitemia) was incubated for 30 min at 37°C with twofold serial dilutions of mouse serum pools (range, 1/5 to 1/1,000). The rosetting rate was evaluated by light microscopy. Each serum was tested in duplicate.
Scanning electron microscopy.
varO rosetting parasites were enriched using ice-cold Ficoll. A pellet containing rosetting parasites was washed in PBS, fixed for at least 1 h with 2.5% glutaraldehyde in PBS and 0.1 M cacodylate buffer, washed, and postfixed in 1% osmium tetroxide. After dehydration using a graded ethanol series, samples were critical point dried (Balzers Union CPD30) and coated with gold-palladium (Gatan 681 ion beam coater). Samples were visualized with a JEOL JSM 6700F scanning electron microscope.
Statistical analysis.
Results obtained for surface-reactive and rNTS-DBL1α1-reactive antibodies were log transformed to obtain a normal distribution, and Student's t test was used to compare the groups studied. When the log-transformed data were not normally distributed, Wilcoxon's nonparametric test was used. Comparisons of varO surface-reactive antibodies and rNTS-DBL1α1-reactive antibodies were performed by using the Spearman rank correlation test. Data were analyzed with STATA software (version 9; STATA Corporation, College Station, TX), and a P value of <0.05 was considered statistically significant.
Nucleotide sequence accession number.
The sequence reported in this paper has been deposited in the GenBank database under Palo_Alto_varO GenBank accession number EU9082205.
RESULTS
Characterization of the varO gene expressed by mature varO parasites.
To isolate the var gene specifically expressed by varO parasites in S. sciureus, RT-PCR was carried out with mRNA extracted from mature trophozoites using the degenerate α-AF and α-BR primers. Of the 49 cDNA clones obtained, 28 (57%) had the same DBL1α sequence. This consensus sequence was completely absent from 20 and 23 cDNA clones generated from two sibling variants of the Palo Alto line (varR and varS+, respectively) (22, 24) and hence was considered specific for the varO trophozoite mRNA preparation. RT-PCR using the UNIEBP primers amplified a 450-bp product derived from a DBL3γ domain that was also enriched in varO parasite RNA preparations. We concluded that the two predominant sequences were varO specific and originated from the major var gene expressed by the varO parasites; accordingly, we designated this gene varO. Specific primers derived from these two domains were used to amplify the entire varO exon I sequence and part of varO exon II by combining RT-PCR and chromosome walking (Table 1; also see Materials and Methods). They were also used to confirm expression specificity in independent RNA preparations from four varO- and four varR-infected animals (data not shown). Furthermore, we showed that the same varO gene was expressed in the uncloned Palo Alto FUP/SP varO line and in the 89F5 parasite clone derived from this uncloned population.
Analysis of the varO cDNA sequence obtained yielded an assembled 7,378-bp open reading frame (Palo_Alto_varO GenBank accession no. EU9082205). Domain identification using protein signatures defined previously (78) showed that PfEMP1varO exon I codes for five DBL domains (one DBL1α1 domain, three DBLβ domains, and one DBLγ domain), one CIDRγ domain, one C2 domain, and three unclassified interdomain regions. The PfEMP1varO modular structure organization and amino acid sequence are shown in Fig. 1. varO has a typical group A (also called UpsA) signature, in particular, multiple domains and an NTS-DBL1α1-CIDRγ head structure that is found only in group A var genes (28). Moreover, the PoLV sequence signatures of the varO DBL1α1 domain as defined by Bull et al. (9, 10) place varO DBL1α1 in group 1 (2Cys) (Fig. 2), which is found exclusively in group A var genes.
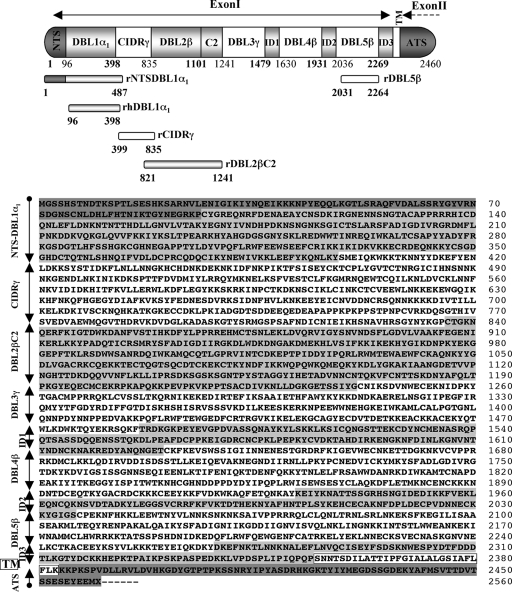
FIG. 1.
Schematic diagram of the domain organization and deduced amino acid sequence for the varO gene expressed by Palo Alto varO parasites. The varO gene encoding the PfEMP1varO adhesin was cloned and sequenced (see Materials and Methods). The varO cDNA sequence codes for a 7,378-bp open reading frame (2,460 amino acids) (Palo_Alto_varO GenBank accession no. EU9082205). varO exon I codes for the extracellular region of the protein. The varO exon II sequence encoding the acidic terminal segment (ATS) is a partial sequence. The amino acid sequence boundaries of each domain, defined using previously described criteria (78, 80), are indicated.
FIG. 2.
PoLV classification of the varO DBL1α1 domain. The cysteine-PoLV classification approach was used to assign the varO DBL1α1 domain to a specific group (9, 10). The DBL1α domains encoded by var genes in the IT4/FCR3 genome with documented involvement in rosetting, namely the varO nearest ortholog IT4/R29 (also designated IT4var9) (68) and FCR3S1.2 (also designated IT4var21) (14, 15) were included, as were the nearest orthologs of the varO DBL1α1 and IT4/R29 DBL1α1 domains in 3D7 (PF13_0003). The PoLV sequence features and the cysteine residues are indicated by a gray background. The conserved amino acids are indicated by a black background. The derived sequence signature and the assigned PoLV group for each DBL1α domain are indicated in the lower panel. nd, not defined.
varO orthologs.
Nucleotide alignment and deduced amino acid sequence alignment did not identify any var gene with a similar domain arrangement or a closely related sequence throughout the entire cDNA varO sequence in any complete genome or previously described sequence. Thus, no varO sensu stricto ortholog was found in any of the genomes investigated, reflecting the internal mosaic structure of the var genes (28, 38, 43). A search for individual domain orthologs in the 3D7 var gene repertoire (28, 38) identified DBL1α1 and DBL2β orthologous domains in the PF13_0003 var gene, which, however, has a markedly different CIDR1γ domain (30.3% sequence identity) (Table 2). The 3D7 orthologs of varO DBL4β and varO DBL5β were from the PF08_0141 var gene. The varO CIDRγ sequence had limited homology with the 3D7 var gene repertoire, and the highest level of identity was 47.2% identity with the PFD0995c CIDR2γ sequence. The varO exon II sequence was a partial sequence, but it had specific signatures. The best match for this sequence in the 3D7 genome was PF11_0521. Interestingly, the 3D7 orthologs of varO DBL1α1, DBL2β, DBL4β, and DBL5β and the probable exon II ortholog are group A var genes (43).
TABLE 2.
varO domain orthologs and protein sequence identity in P. falciparum and P. reichenowi isolatesa
| Parasite | DBL1α1
|
CIDRγ
|
DBL2β
|
DBL3γ
|
DBL4β
|
DBL5β
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| var gene or contig | % Identity | var gene or contig | % Identity | var gene or contig | % Identity | var gene or contig | % Identity | var gene or contig | % Identity | var gene or contig | % Identity | |
| 3D7 | PF13_0003b | 65.4 | PFD0995c | 47.2 | PF13_0003b | 56.2 | PFF0010w | 54.5 | PF08_0141b | 66.4 | PF08_0141b | 81.4 |
| IT4 | IT4var9/R29varb | 67.1 | IT4var8b | 51.8 | IT4var2b | 56.3 | IT4var64b | 47.3 | IT4var13 | 48.6 | IT4var8b | 41.6 |
| HB3 | HB3var5b | 66.7 | HB3var23 | 48.4 | HB3var16 | 54.7 | HB3var48c | 55.1 | HB3var8 | 67.6 | HB3var8 | 50.6 |
| Dd2 | Contig_1.57 | 67.3 | Contig_1.924 | 45.8 | Contig_1.227 | 55.4 | Contig_1.9 | 54.5 | Contig_1.181 | 50.3 | Contig_1.24 | 37.8 |
| Ghana | Contig_20793 | 64.8 | Contig_18537 | 50.9 | Contig_20179 | 58.8 | Contig_21033 | 45.9 | Contig_21299 | 68.9 | Contig_20860 | 50.4 |
| P. reichenowi | Contig_000023499 | 55.3 | Contig_000023127 | 40.3 | Contig_000023807 | 54.9 | Contig_000023387 | 54.1 | Contig_000023779 | 68.5 | Contig_000022699 | 64.7 |
For each domain, sequence homology searches were performed using the genome sequences of the P. falciparum 3D7, IT4/25/5, HB3, Dd2, and Ghana isolates and P. reichenowi, the chimpanzee malaria parasite. The values are the levels of sequence identity. For each varO domain, the var gene with the highest sequence identity is indicated by bold type.
Group A/UpsA var gene.
Pseudogene.
Each varO domain had an ortholog with high sequence identity in the genomes of the IT4, HB3, Dd2, and Ghana isolates (32, 38, 87), but for each individual domain the identity scores were different for the different isolates (Table 2). Interestingly, each domain also had an ortholog in the P. reichenowi genome (32), and some of the identity scores were remarkably high (Table 2). The varO DBL1α1 domain had an ortholog with >60% identity in each P. falciparum genome investigated and thus seems to be the most conserved domain in the isolates. Interestingly, the highest sequence identity to varO DBL1α1 was found with the DBL1α1 domain of varR29 (67.1% identity) (annotated IT4var9 in the IT4 var gene repertoire [38]), which is also implicated in rosetting (68). The DBL1α domain of the FCR3S1.2 rosetting clone (14) (annotated IT4var21 [38]) had a lower level of sequence identity (41.3%). The varO DBL5β domain exhibited 81.4% sequence identity with the PF08_0141 DBL5 locus in the 3D7 genome, which is the highest level of identity found for the varO exon I domains. However, the nearest orthologs of varO DBL5β in the other P. falciparum clones or isolates exhibited lower levels of sequence identity (Table 2). The least conserved domain was varO CIDR1γ, for which the highest level of sequence identity was 51.8% (IT4var8 CIDR1γ). In the P. reichenowi genome, the levels of identity varied with the individual domains, and the highest level was observed for varO DBL4β (68.5%).
The varO gene codes for a PfEMP1 adhesin mediating rosetting.
To ascertain whether the dominant cDNA isolated from varO parasites did indeed encode the PfEMP1 adhesin mediating varO rosetting, individual domains were expressed on the surface of Cos7-L cells as described previously (37) and tested for binding to uninfected Saimiri RBC. Surface expression of DBL1α1 was associated with binding to uninfected RBC from Saimiri monkeys (data not shown), but the expression levels and the numbers of transfected cells were very low (1 to 3%). However, transfection of Cos7-L cells with a recodoned version of the DBL1α1 domain (rhDBL1α) improved the surface expression and subsequent binding to human RBC (72).
To further analyze the binding capacity of varO domains, three other domains were recodoned. varO rhDBL1α, rCIDRγ, rDBL2βC2, and rDBL5β were then cloned in baculovirus and expressed in High-5 insect cells. We chose the baculovirus-insect cell expression system because it has proved to be effective for the production of correctly folded cysteine-rich domains, including DBL domains (5, 17, 33, 63, 73). Surface exposure was monitored by S-IFA using an anti-His tag MAb, and the level of cells expressing each construct was determined by flow cytometry. For each domain, the expression level and the number of insect cells expressing the recombinant protein were very high (Fig. 3A). Binding to O+ and A+ human RBC was readily seen on the surface of varO rhDBL1α-expressing cells; the right panels of Fig. 3B1 show the results of two representative experiments with O+ RBC. There was no binding by surface-expressed recombinant varO rCIDRγ, rDBL2βC2, or rDBL5β, and Fig. 3B2 shows representative examples. The involvement of only DBL1α1 in erythrocyte binding was further confirmed by analysis of binding inhibition using varO DBL1α1-specific antisera (see below).
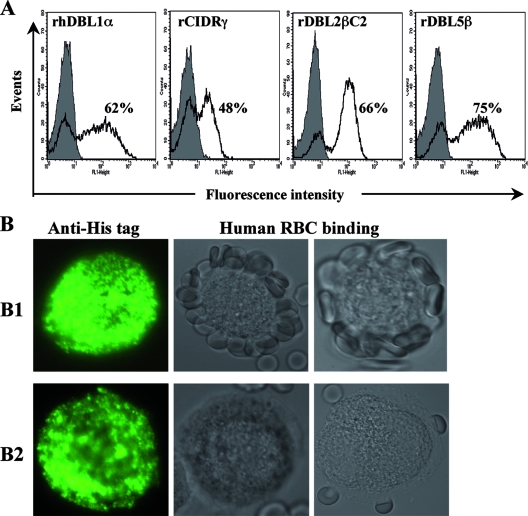
FIG. 3.
Human RBC binding capacity of varO domains expressed on the surface of insect cells infected with recombinant baculovirus. The C-terminal His6-tagged recodoned rhDBL1α, rCIDRγ, rDBL2βC2, and rDBL5β domains of varO were cloned into baculovirus, which was used to infect High-5 insect cells. The sequence boundaries of each construct are shown in Fig. 1. (A) Surface expression of the varO domains on High-5 infected cells was monitored by S-IFA using an anti-His tag MAb and a goat anti-mouse IgG Alexa Fluor 488-conjugated antibody. The level of expression of each construct was analyzed by flow cytometry. The shaded histograms show labeling in the absence of the anti-His tag MAb (incubation with only the conjugate). The percentage of High-5 cells expressing each construct is indicated. (B) Representative results for human RBC binding to the surface of High-5 cells expressing rhDBL1α (panels B1) or rCIDRγ (panels B2). The absence of human RBC binding to the surface of High-5 cells expressing rDBL2βC2 and rDBL5β is not shown. The left panels show surface expression of varO domain constructs by High-5 cells as determined by a fluorescence microscopy analysis of S-IFA generated using anti-His tag MAb. The right panels show the human RBC binding capacity of varO domains expressed on parallel cultures of High-5 infected cells. Representative results of two independent binding experiments performed with human O+ RBC are shown. Only the varO rhDBL1α domain displayed RBC binding capacity.
In vitro varO model of rosetting and autoagglutination.
The 89F5 varO clone was established in continuous in vitro culture in human O+ RBC from an infected Saimiri monkey (see Materials and Methods). The human RBC infected with 89F5 varO parasites had knobs and retained the surface cytoadherence phenotype (formation of rosettes and autoagglutinates) (Fig. 4A). Unlike rosettes, which were observed even at low levels of parasitemia, autoagglutinates were observed only in cultures with ≥8% parasitemia. These two cytoadherence phenotypes were not platelet dependent since all cultures were grown with platelet-poor sera. This suggests that rosette formation and autoagglutinate formation are two distinct manifestations of the same cytoadherence phenotype that depend on the abundance of the respective binding partners. In this regard, varO autoagglutinates may be similar to the “giant rosettes” described by Heddini et al. (30). In addition, in line with previous findings with other rosetting clones and lines (14, 21, 49, 68), varO rosettes and autoagglutinates in human RBC were human serum dependent, suggesting that serum factors may be involved in these cytoadherence phenotypes.
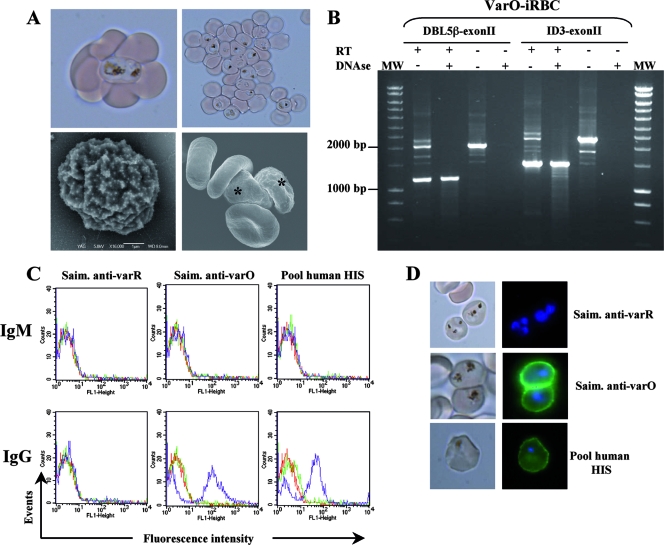
FIG. 4.
Phenotype and serotype of the 89F5 varO rosettes cultivated in vitro in human RBC. (A) 89F5 varO parasites cultivated in human RBC at trophozoite and early-schizont stages are Knob+ parasites that are able to form rosettes and autoagglutinates. The upper panels show rosettes and autoagglutinates of varO-iRBC as viewed by light microscopy, and the lower panels show the results of an analysis of varO-iRBC by scanning electron microscopy. Scale bar = 1 μm. The asterisk indicates varO-iRBC. (B) varO gene expression in 89F5 varO cultures: RT-PCR amplification patterns for varO mRNA extracted from mature stages of rosetting varO parasites using the 5DBL5Bfo-VarO3exon2Brev primer pair (predicted size of genomic DNA, 2,050 bp; predicted size of mRNA, 1,158 bp) or the 5ID3Bfo-VarO3exon2Brev primer pair (predicted size of genomic DNA, 2,400 bp; predicted size of mRNA, 1,479 bp). Control reactions were carried out using samples treated with DNase or without reverse RT as indicated above the lanes. PCR products were analyzed by agarose gel electrophoresis. Representative results of three independent experiments are shown. Lanes MW contained molecular size markers. (C and D) S-IFA analysis of IgM or IgG binding to the surface of human RBC infected by mature-stage 89F5 varO parasites. Binding of nonimmune (green lines) or immune (purple lines) Saimiri (Saim.) or human antibodies reacting with varO surface-exposed antigens was monitored using a goat anti-human IgM or IgG Alexa Fluor 488-conjugated antibody. A varO-iRBC preparation incubated with the conjugate alone was included in each experiment (red lines). varO-iRBC were stained with Hoechst dye (nuclei stained blue). S-IFA results were analyzed by flow cytometry (C) and fluorescence microscopy (D). The results are representative of at least three independent experiments. HIS, hyperimmune sera.
Importantly, as observed with Saimiri RBC, in human RBC the 89F5 varO parasites expressed the varO gene. RT-PCR across the intron (encompassing DBL5β-exon II or ID3-exon II) generated a band that was the size predicted for a correctly spliced product (Fig. 4B). The fragment observed was derived from RNA, since it was not observed when RT was omitted. The varO surface serotype, as defined with sera collected from 89F5 varO-infected S. sciureus, was displayed by the parasites cultured using human RBC. Surface reactivity, as analyzed by S-IFA and flow cytometry (Fig. 4C) and by epifluorescence surface microscopy (Fig. 4D), clearly showed that human-adapted varO parasites were specifically recognized by sera from Saimiri monkeys infected with varO parasites but not by sera from animals infected with the sibling varR variant. Like 89F5 varO-infected Saimiri RBC (47), 89F5 varO-infected human RBC did not bind nonimmune Saimiri IgG or IgM (Fig. 4C). Previous work showed that uncloned varO-infected Saimiri RBC reacted with hyperimmune human sera (24). The 89F5 varO-infected human RBC exhibited this surface reactivity with a pool of hyperimmune human sera from Senegal (Fig. 4C and 4D) and did not react with nonimmune human sera. Here the surface reactivity was also due to IgG and not IgM.
Antisera raised to the rNTS-DBL1α1 domain react with the surface of 89F5 varO-iRBC and disrupt rosettes.
To confirm that the varO gene was responsible for the surface phenotype and serotype assigned to varO parasites, we produced a soluble varO DBL1α1 recombinant protein to raise specific antisera. The rhDBL1α1 expression product used in the High-5 surface-binding assay described above proved to be insoluble. Accordingly, we redesigned the construct to contain the segment from Met-1 to Ser-487 (which includes the 95-residue N-terminal NTS region); thus, it contained 20 cysteine residues instead of the 14 cysteine residues in the first construct (Fig. 1). The new construct had much improved solubility and expression levels. The recodoned varO rNTS-DBL1α1 gene sequence was optimized for the codon usage of the baculovirus-insect cell expression system (see Materials and Methods). Using this new construct, we obtained a 58-kDa soluble, secreted recombinant protein, varO rNTS-DBL1α1, that was purified to homogeneity as judged by SDS-PAGE analysis under reducing conditions (Fig. 5A). N-terminal sequencing indicated that there was a single N terminus corresponding to cleavage at the predicted site after the mellitin signal sequence (data not shown). The protein reacted on an immunoblot with an anti-His tag MAb (data not shown). Thus, the full-length recombinant protein was expressed.
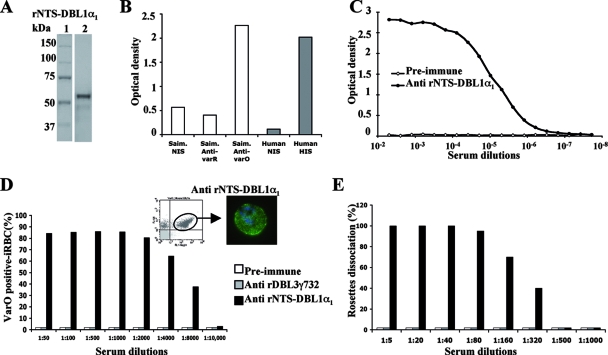
FIG. 5.
varO rNTSDBL1α1 recombinant protein elicits surface-reacting and rosette-disrupting antibodies. (A) The soluble rNTS-DBL1α1 recombinant protein (3.5 μg, 58 kDa) was loaded on a 10% SDS-PAGE gel under reducing conditions and stained with Coomassie blue. Lane 1, molecular mass standards; lane 2, rNTS-DBL1α1 recombinant protein. (B) The reactivity of Saimiri sera (open bars), including Saimiri nonimmune sera (Saim. NIS) and immune sera (Saim. Anti-varO and Saim. Anti-varR), and human sera (filled bars), including pools of human hyperimmune sera from adults living in Dielmo (Human HIS) or non-malaria-immune European blood donors (Human NIS), was tested by using ELISA and the soluble varO rNTS-DBL1α1 domain. The optical densities are indicated. The results are representative of three independent experiments. (C to E) Soluble rNTS-DBL1α1 and rDBL3γ732 domains were used to immunize groups of five outbred (OF1) mice. The pools of preimmune, anti-rNTS-DBL1α1, and/or anti-rDBL3γ732 mouse sera were then analyzed using ELISA (C), S-IFA and flow cytometry (D), and the rosette dissociation assay (E). (C) Titration of anti-rNTS-DBL1α1 sera on the rNTS-DBL1α1 recombinant protein by ELISA. Twofold serial dilutions were prepared, ranging from 5 × 10−3 (1:200 dilution) to 2 × 10−8 (1:52,428,800 dilution). The pool of preimmune sera, which was used as a control, was negative. (D) Representative results obtained for titration of preimmune, anti-rDBL3γ732, and anti-rNTS-DBL1α1 antibodies with live varO-iRBC by S-IFA and flow cytometry. For each dilution tested, the percentage of surface-positive varO-iRBC was calculated. The left inset shows a representative histogram obtained by flow cytometry (FL1, anti-human IgG Alexa Fluor 488 staining; FL4, Hoechst dye staining of varO parasite culture); varO NTS-DBL1α1-positive cells are indicated. The right inset shows S-IFA staining of varO-iRBC visualized by epifluorescence microscopy. (E) Rosette dissociation assay results obtained after incubation of varO rosettes (>90% rosette-forming parasites) with serial dilutions of preimmune, anti-rNTS-DBL1α1, and anti-rDBL3γ732 mouse sera. The percentage of rosetting parasites in the presence of preimmune sera was used as the reference. The rosette dissociation capacity of each antiserum pool is indicated. Each assay was performed in duplicate.
The varO rNTS-DBL1α1 protein exhibited the predicted specificity pattern as determined by ELISA; it was recognized by sera from varO-infected monkeys but not by sera from varR-infected monkeys (Fig. 5B). Interestingly, it reacted with a pool of sera from Senegalese adults living in an area where malaria is endemic. Mice immunized with the soluble varO rNTS-DBL1α1 domain produced high antibody titers in ELISA (end point titer, 1:1,638,400) (Fig. 5C). Anti-varO rNTS-DBL1α1 antisera also reacted with the surface of live 89F5 varO-infected human RBC (Fig. 5D and insets), which they also agglutinated (data not shown). Importantly, these anti-varO rNTS-DBL1α1 mouse antisera disrupted the 89F5 varO rosettes, unlike preimmune sera or sera raised to irrelevant control proteins, such as DBL3γ732 (Fig. 5E). The end point titer for 89F5 varO surface-reactive antibodies was substantially higher (1:8,000) than that for rosette-disrupting antibodies (1:320).
Positive selection of varO parasites by panning.
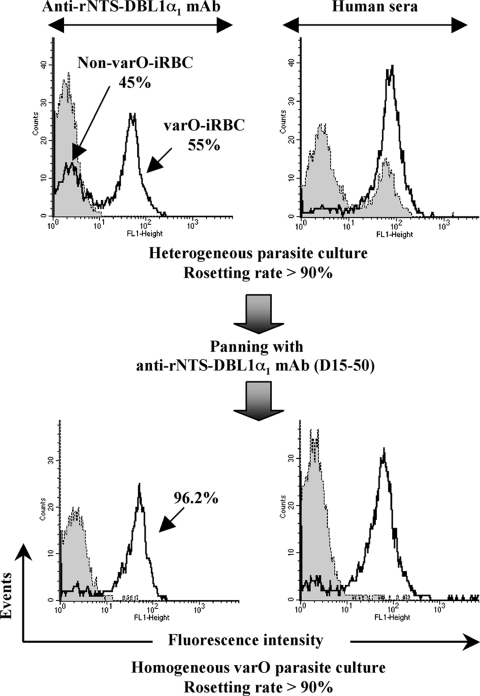
The var gene repertoire of Palo Alto is not known at present; our data indicate that it includes at least two rosetting types designated varO and varS (22, 24, 45), which display a different serotype. However, based on the sequenced genome of IT4/FCR3 (38), we predict that four or five var genes are involved in rosetting in the Palo Alto genome. Weekly enrichment of rosettes by selective centrifugation did allow maintenance of rosette-forming parasites at a high rate but did not ensure long-term maintenance of a pure varO rosetting sero/phenotype in culture. Indeed, the preparation containing rosettes obtained after several months of continuous in vitro culture in human RBC contained additional rosette-forming variants that did not react with varO rNTS-DBL1α1 mouse antisera (Fig. 6, upper panel). Moreover, unlike varO-iRBC, these non-varO rosetting variants were able to bind nonimmune human IgG and were recognized by hyperimmune human sera, which hampered assessment of the varO-specific surface reactivity. To circumvent the progressive loss resulting from antigenic variation, varO-iRBC were positively selected by panning with MAb D15-50, a mouse MAb raised to varO rNTS-DBL1α1 (see Materials and Methods). This panning procedure, which was carried out bimonthly, allowed a pure varO rosetting sero/phenotype to be maintained with >95% of the mature stages of the 89F5 varO-iRBC cultured in human RBC expressing the varO serotype (Fig. 6, lower panel), thus creating a stable in vitro model of varO rosetting. Such homogeneous parasite cultures expressing the PfEMP1varO adhesin were used to analyze seroprevalence in an African setting.
FIG. 6.
Specific selection of varO-iRBC by panning with an anti-rNTS-DBL1α1 MAb. varO parasites were maintained in continuous in vitro culture in human RBC. The surface reactivities obtained after several weeks of culture and several rounds of rosette enrichment by centrifugation on ice-cold Ficoll (upper panels) and after positive selection of iRBC expressing the varO NTS-DBL1α1 domain by panning with MAb D15-50 directed against the varO rNTS-DBL1α1 protein (lower panels) are shown. Mature-stage parasites were stained with a pool of preimmune mouse sera and with MAb D15-50 (left panels) or with pools of nonimmune or hyperimmune (Dielmo) human sera (right panels). The surface reactivity on iRBC was monitored by S-IFA and flow cytometry using a goat anti-mouse or anti-human IgG Alexa Fluor 488-conjugated antibody. The shaded histograms show labeling with nonimmune sera. The S-IFA reactivity obtained with immune sera is indicated by open histograms. As indicated, after panning of a heterogeneous culture, >95% of mature parasites expressed the varO NTS-DBL1α1 domain and had a typical varO serotype (i.e., they did not bind nonimmune human IgG).
Very high seroprevalence for varO at the rural community level in a Senegalese setting where malaria is endemic.
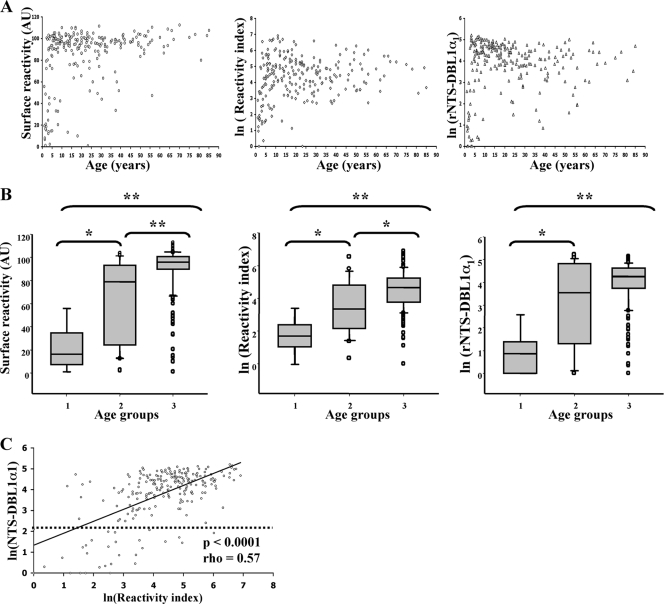
As shown above, 89F5 varO parasites infecting both Saimiri and human RBC were surface positive when they were tested with a pool of hyperimmune sera from Dielmo volunteers. When sera were tested separately, each individual serum from this pool reacted with 89F5 varO parasites, ruling out the possibility of a pool artifact. To investigate the seroprevalence for the culture-adapted 89F5 varO parasites at the village level (i.e., the seroprevalence in volunteers in all age groups living in a defined setting where malaria is endemic), we studied IgG surface reactivity by flow cytometry using an archived collection of sera collected from 235 of the 247 villagers living in Dielmo. A very high proportion (93.6%; 95% confidence interval [CI], 89.7 to 96.4%) of Dielmo villagers had antibodies that reacted with the surface of 89F5 varO-iRBC (Fig. 7A, left panel). There was a marked age dependence (Fig. 7B, left panel), with low seroprevalence in the <2-year-old children (prevalence, 33%; median surface reactivity, 16.48 AU; 25 to 75% interquartile range [IQ25-75], 8.15 to 21.05 AU), higher prevalence in the 2- to <5-year-old age group (prevalence, 78%; median surface reactivity, 79.23 AU; IQ25-75, 24.65 to 93.43 AU), and very high prevalence in the >5-year-old group (prevalence, 98%; median surface reactivity, 96.2 AU; IQ25-75, 90.05 to 101.08 AU). The reactivity index (which takes into account the MFI and the percentage of varO-positive iRBC) showed similar age-associated acquisition of varO surface-reactive antibodies.
FIG. 7.
Seroprevalence for 89F5 varO-iRBC and varO rNTSDBL1α1 recombinant domains at the village level in Dielmo (Senegal). Blood samples were obtained from 235 (ages, 1 to 85 years; mean age, 23 years) of 247 villagers living in Dielmo during the 1992 transmission season. For each villager, antibodies (IgG) reacting with the surface of live varO-iRBC were analyzed by S-IFA and flow cytometry. Antibodies against the varO rNTS-DBL1α1 domain were analyzed by ELISA. The results were expressed in AU (see Materials and Methods) and log transformed (ln) to obtain an approximately normal distribution. (A) Age distribution of varO-iRBC surface-reactive antibodies (expressed as surface reactivity and reactivity index) and rNTS-DBL1α1-reactive antibodies. (B) Acquisition of surface- and rNTS-DBL1α1-reactive antibodies in three age groups, group 1 (<2 years old; n = 9), group 2 (2 to <5 years old; n = 23), and group 3 (>5 years old; n = 203). The boundaries of the boxes indicate the 25th and 75th percentiles, and the line in each box indicates the median. The whiskers above and below the boxes indicate the 90th and 10th percentiles. The outlying dots indicate values exceeding the 90th and 10th percentiles. The results of Wilcoxon's nonparametric test are indicated by asterisks, as follows: *, P < 0.01; **, P < 0.0001. (C) Linear regression of ln(rNTS-DBL1α1) (ordinate) as a function of ln(reactivity index) (abscissa). The estimated regression equation is f(x) = 1.3947 + 0.5550x, and the correlation coefficient (r2) is 0.34. The Spearman rank correlation coefficient (ρ) and the P value are indicated.
We next assessed the seroprevalence of the same sera with the varO rNTS-DBL1α1 recombinant protein by performing ELISA. The percentage of antibodies reacting with the rosetting domain of the PfEMP1varO adhesin was very high (87.7%; 95% CI, 82.8 to 91.6%), and it increased with age, from a low value (prevalence, 11.1%; median, 2.38 AU; IQ25-75, 0.89 to 3.42 AU) in the <2-year-old children to an elevated value (prevalence, 65%; median, 35.65 AU; IQ25-75, 3.76 to 123.57 AU) in the 2- to <5-year-old children to a very high value (prevalence, 94%; median, 71.18 AU; IQ25-75, 42.39 to 104.5 AU) in the >5-year-old group (Fig. 7A and 7B, right panels). The varO surface-reactive antibodies were positively correlated with the ELISA reactivity to varO rNTS-DBL1α1 (Fig. 7C), as determined by Spearman's rank correlation test (for surface reactivity versus rNTS-DBL1α1, rho = 0.60 [P < 0.0001]; for reactivity index versus rNTS-DBL1α1, rho = 0.57 [P < 0.0001]).
DISCUSSION
Our findings show that 89F5 varO parasites adapted to culture in human RBC express the same var gene, the same rosetting and autoagglutination cytoadherence phenotype, and the same surface serotype as the original uncloned varO line and the 89F5 varO clone propagated in S. sciureus monkeys. Mature varO parasites expressed a var gene that we designated varO, which possesses group A var gene characteristics and a varO DBL1α1 PoLV group 1 (Cys2) sequence signature, two molecular features associated with rosetting and/or severe malaria in African children (9, 41, 59). Establishment of an in vitro model with a homogeneous parasite population expressing the varO gene and the varO sero/phenotype (i.e., a “single-variant” culture of the 89F5 clone) allowed us to analyze with a high degree of confidence varO field seroprevalence. The results obtained showed that the varO rosetting and autoagglutination serotype was very prevalent in Dielmo, a Senegalese setting where malaria is holoendemic, and moreover that varO seroconversion occurs early in life. It is interesting to place these characteristics in the perspective of the acquired immunity to severe malaria of young African children, which correlates with seroconversion to so-called frequent PfEMP1 types (11, 57) associated with group A genes (33, 34). The age profile for seroconversion for varO parasites in Dielmo and the varO cytoadherence characteristics are consistent with varO classification in the subset of group A genes implicated in malaria pathogenesis.
As found with other var genes expressed by rosette-forming parasites (14, 68), the varO DBL1α1 domain mediated erythrocyte binding on the surface of Cos7-L cells. This was observed both when the wild-type sequence was used and when a recodoned sequence devoid of N-glycosylation sites was used. The same DBL1α1 coding sequence also mediated binding to human RBC when it was expressed on the surface of insect cells. Furthermore, antibodies raised to the soluble recombinant varO NTS-DBL1α1 domain reacted with high titers to the surface of 89F5 varO-iRBC and totally disrupted varO rosettes. This is convincing evidence that the varO rosetting is indeed mediated by the varO NTS-DBL1α1 domain encoded by the varO gene. Using in vitro cultures with >90% of the rosetting parasites stained positively with the anti-NTS-DBL1α1 antibodies (and hence expressing varO), both rosetting or autoagglutinates and giant rosettes were observed, suggesting strongly that PfEMP1varO mediates both rosetting and autoagglutination. It is worth noting that antibodies to varO NTS-DBL1α1 disrupted rosettes but did not dissociate autoagglutinates. Indeed, while serial dilutions showed a marked reduction in the number of rosettes, the number of agglutinates in the sample remained unchanged (except at dilutions inducing antibody-dependent iRBC agglutination, where all iRBC were observed in aggregates). Further work is needed to clarify this point and to identify the domain mediating platelet-independent varO autoagglutination.
Production of recombinant DBL domains with native folding is difficult, possibly because the richness of disulfide bridges constrains the domain structures. We have used the baculovirus-insect cell production system because, for example, it has been used successfully to produce cysteine-rich MSP1-19 EGF domains (18, 63) and has also been used for DBL domains (4, 5). A surface-expressed domain displaying binding activity was produced by using the domain boundaries originally defined by Smith et al. (77). However, this protein was insoluble and not secreted (despite being cloned in the vector used to secrete MSP1-19 [18]). Difficulty generating soluble DBL1α domains is not new and, indeed, was confirmed by computer prediction by Ahuja et al. (1). Nevertheless, a soluble protein could be produced using a redesigned construct with an elongated domain, based on folding of the EBA-175 DBL domains (76). The C-terminal boundaries of PfEMP1 DBL domains are more difficult to identify because of substantial divergence from EBA in this region. However, we obtained an apparently properly folded protein using this approach, because the protein elicited high titers of surface-reacting antibodies and of rosette-disrupting antibodies. It is worth mentioning that mutation of the six N-glyscosylation sites did not result in major alterations in the antigenicity. Another important consequence of using a recodoned version was the sequence stability of the plasmid constructs. The native uncodoned sequence frequently mutated upon propagation in Escherichia coli and recloning in an expression vector. This was not observed once a recodoned version with a less biased G+C content was used.
The varO soluble rNTS-DBL1α1 domain elicited high antibody titers in mice that appeared to be better than those previously reported by other groups for other recombinant DBL domains. In particular, we observed surface reactivity at dilutions that were 10 to 100 times higher than the dilutions at which surface reactivity was observed for recombinant DBL1α domains from other rosette-forming parasites using different expression systems, such as E. coli or Semliki Forest virus (16, 51). An important observation that we made with varO anti-rNTS-DBL1α1 antibodies raised in mice was that the rosette-disrupting titers were substantially lower than the titers for surface-reactive antibodies and rNTS-DBL1α1 ELISA-reactive antibodies. This suggests that only a fraction of surface-reactive antibodies react with the receptor-binding site and/or that only the antibodies with a very high affinity are able to disrupt rosettes. Nonetheless, the varO rNTS-DBL1α1 domain elicited titers of surface-reactive, rNTS-DBL1α1-reactive, and/or cytoadherence-disrupting antibodies that were substantially higher than the titers obtained so far using other antigen production systems (5, 6, 16, 34). We attribute this to the baculovirus-insect cell expression and/or to the fact that the elongated construct enabled production of a recombinant domain with adequate conformational fidelity to the native protein and epitope presentation.
The in vitro varO model with cultivated human RBC is experimentally easier to handle than the in vivo infection model with Saimiri monkeys. To develop a model with all iRBC displaying the same PfEMP1varO adhesin and the varO-iRBC serotype, weekly rosette purification was not sufficient. Positive selection with an anti-rNTS-DBL1α1 antibody was necessary to eliminate the non-varO rosetting sibling variants enriched along with varO by centrifugation on ice-cold Ficoll (48). The varO model is thus the first rosetting model to be developed in this way. We anticipate that such a procedure will be necessary for the other var genes mediating rosetting and, more generally, for every var gene mediating a cytoadherence phenotype shared by multiple members of the var repertoire, such as CD36 binding. Working with a homogeneous parasite population expressing a single var gene and a single serotype is critical for seroprevalence and cross-reactivity studies, as well as for biochemical host receptor interaction studies.
The 89F5 varO parasites were shown here to express an iRBC surface serotype and an NTS-DBL1α1 serotype that have very high seroprevalence in Dielmo, Senegal. Palo Alto parasites originated from Uganda and were collected in 1978. We do not know whether the varO gene or its individual modular domains circulate as such in the west African setting analyzed here more than 14 years after Palo Alto was collected or whether the observed serologic reactions reflect strict varO-specific reactions or cross-reactions with varO-related sequences. To answer this question, analysis of the local var gene repertoire is needed, as is analysis of surface cross-reactivity, which is a vast undertaking in view of the large field diversity of var repertoires. The question of cross-reactivity between closely related variants is still open; there is some evidence of cross-reactivity with animal sera raised to individual domains (51), and there have been reports of limited cross-reactivity in the field with human sera. Mixed agglutination assays have indicated, at best, marginal cross-reactivity (56). The conservative conclusion from our data is that the varO serotype (if not the varO genotype, including mosaic genes with reshuffled individual domains) is indeed frequent in this setting where malaria is endemic.
Using the recombinant NTS-DBL1α1 domain, which appears to be correctly folded since it elicits surface-reacting and rosette-disrupting antibodies, we could investigate seroprevalence for the first varO domain. The seroprevalence was high and correlated with surface reactivity. The two reactivities, however, did not fully overlap, suggesting that antibodies to other varO domains and/or surface antigens might contribute to surface reactivity. Cloning and expression of additional varO domains are under way to explore this hypothesis. This seroepidemiological survey also showed that the prevalence of antibodies reacting with varO parasites and/or varO rNTS-DBL1α1 increased early in life and was very high from the age of 5 years onward (5 years is the age at which the risk of malaria episodes drops substantially in the village studied) (83). In this regard, the seroprevalence for the varO-iRBC surface is much higher than reported for the FCR3S1.2 rosetting clone or field isolates in semi-immune Kenyan children (51). Additional work is in progress (in particular, a longitudinal follow-up study of individual children) to dissect the kinetics of acquisition of antibodies to the varO-iRBC surface and to individual domains and to study the functional role of these antibodies and their possible association with protection.
Analysis of severe malaria in human patients has identified a broad range of rosette, autoagglutinate, or platelet clumping frequencies (12, 13, 23, 30, 64, 67, 69, 85, 88). Only a minority of isolates display high rosette frequencies. Consistent with this, multiple var genes are expressed by the circulating parasite pool (42) and by the sequestered population (52, 53). Such heterogeneity precludes analysis of the contributions of specific parasite features to malaria pathology. We show here that the varO phenotype is related to severe malaria in several respects, including the cytoadherence phenotype, the elevated multiplication rate contributing to high parasite densities, the expression of a var gene having the characteristics of the group A/UpsA var gene subset, and the expression of a serotype with very elevated prevalence in semi-immune children. The Palo Alto varO model is the first in vitro and in vivo model for such combined phenotypes and provides a unique tool for exploring the contribution of rosetting or autoagglutination cytoadherence to the pathogenesis of malaria.
Acknowledgments
The personnel in charge of animal care at the Pasteur Institute of French Guiana are warmly thanked. We are grateful to C. El Euch for her help with sequencing of the varO gene. We express our gratitude to the villagers of Dielmo, the medical staff, and our collaborators at the Institut Pasteur of Dakar and Institut de Recherche et de Developpement of Dakar (in particular, C. Rogier, J. F. Trape, C. Sokhna, A. Touré-Baldé, and J. Faye) for epidemiological data and sample collection. We thank F. Marchand for her help with MAb production, A. Namane and J. D'Alayer for mass spectrometry and protein sequence analysis, and A. Mallet and N. Cayet for their technical assistance with scanning electron microscopy analysis. We thank P. David, N. Mohandas, and T. Fandeur for helpful comments on the manuscript.
This study received financial support from the French National Research Agency (Agence Nationale de la Recherche grant MIME 021 01-02); A.J. was supported by this Agence Nationale de la Recherche grant. This work was part of the activities of the BioMalPar European Network of Excellence supported by European grant LSHP-CT-2004-503578 from Priority 1 “Life Sciences, Genomics and Biotechnology for Health” in the 6th Framework Program.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 22 September 2008.
REFERENCES
- 1.Ahuja, S., F. Pettersson, K. Moll, C. Jonsson, M. Wahlgren, and Q. Chen. 2006. Induction of cross-reactive immune responses to NTS-DBL-1alpha/x of PfEMP1 and in vivo protection on challenge with Plasmodium falciparum. Vaccine 246140-6154. [DOI] [PubMed] [Google Scholar]
- 2.al-Yaman, F., B. Genton, D. Mokela, A. Raiko, S. Kati, S. Rogerson, J. Reeder, and M. Alpers. 1995. Human cerebral malaria: lack of significant association between erythrocyte rosetting and disease severity. Trans. R. Soc. Trop. Med. Hyg. 8955-58. [DOI] [PubMed] [Google Scholar]
- 3.Arman, M., A. Raza, L. J. Tempest, K. E. Lyke, M. A. Thera, A. Kone, C. V. Plowe, O. K. Doumbo, and J. A. Rowe. 2007. Platelet-mediated clumping of Plasmodium falciparum infected erythrocytes is associated with high parasitemia but not severe clinical manifestations of malaria in African children. Am. J. Trop. Med. Hyg. 77943-946. [PMC free article] [PubMed] [Google Scholar]
- 4.Badaut, C., G. Faure, N. G. Tuikue Ndam, G. Bertin, A. Chaffotte, A. Khattab, M. Q. Klinkert, P. Deloron, and G. A. Bentley. 2007. Receptor-binding studies of the DBLγ domain of Plasmodium falciparum erythrocyte membrane protein 1 from a placental isolate. Mol. Biochem. Parasitol. 15189-99. [DOI] [PubMed] [Google Scholar]
- 5.Barfod, L., M. A. Nielsen, L. Turner, M. Dahlback, A. T. Jensen, L. Hviid, T. G. Theander, and A. Salanti. 2006. Baculovirus-expressed constructs induce immunoglobulin G that recognizes VAR2CSA on Plasmodium falciparum-infected erythrocytes. Infect. Immun. 744357-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bir, N., S. S. Yazdani, M. Avril, C. Layez, J. Gysin, and C. E. Chitnis. 2006. Immunogenicity of Duffy binding-like domains that bind chondroitin sulfate A and protection against pregnancy-associated malaria. Infect. Immun. 745955-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet, S., S. Petres, I. Holm, T. Fontaine, S. Rosario, C. Roth, and S. Longacre. 2006. Soluble and glyco-lipid modified baculovirus Plasmodium falciparum C-terminal merozoite surface protein 1, two forms of a leading malaria vaccine candidate. Vaccine 245997-6008. [DOI] [PubMed] [Google Scholar]
- 8.Breman, J. G., and C. N. Holloway. 2007. Malaria surveillance counts. Am. J. Trop. Med. Hyg. 7736-47. [PubMed] [Google Scholar]
- 9.Bull, P. C., M. Berriman, S. Kyes, M. A. Quail, N. Hall, M. M. Kortok, K. Marsh, and C. I. Newbold. 2005. Plasmodium falciparum variant surface antigen expression patterns during malaria. PLoS Pathog. 1e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bull, P. C., S. Kyes, C. O. Buckee, J. Montgomery, M. M. Kortok, C. I. Newbold, and K. Marsh. 2007. An approach to classifying sequence tags sampled from Plasmodium falciparum var genes. Mol. Biochem. Parasitol. 15498-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bull, P. C., B. S. Lowe, M. Kortok, and K. Marsh. 1999. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect. Immun. 67733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson, J., H. Helmby, A. V. Hill, D. Brewster, B. M. Greenwood, and M. Wahlgren. 1990. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 3361457-1460. [DOI] [PubMed] [Google Scholar]
- 13.Carlson, J., G. B. Nash, V. Gabutti, F. al-Yaman, and M. Wahlgren. 1994. Natural protection against severe Plasmodium falciparum malaria due to impaired rosette formation. Blood 84:3909-3914. [PubMed] [Google Scholar]
- 14.Chen, Q., A. Barragan, V. Fernandez, A. Sundstrom, M. Schlichtherle, A. Sahlen, J. Carlson, S. Datta, and M. Wahlgren. 1998. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J. Exp. Med. 18715-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, Q., A. Heddini, A. Barragan, V. Fernandez, S. F. Pearce, and M. Wahlgren. 2000. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J. Exp. Med. 1921-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, Q., F. Pettersson, A. M. Vogt, B. Schmidt, S. Ahuja, P. Liljestrom, and M. Wahlgren. 2004. Immunization with PfEMP1-DBL1α generates antibodies that disrupt rosettes and protect against the sequestration of Plasmodium falciparum-infected erythrocytes. Vaccine 222701-2712. [DOI] [PubMed] [Google Scholar]
- 17.Chia, Y. S., C. Badaut, N. G. Tuikue Ndam, A. Khattab, S. Igonet, N. Fievet, G. A. Bentley, P. Deloron, and M. Q. Klinkert. 2005. Functional and immunological characterization of a duffy binding-like gamma domain from Plasmodium falciparum erythrocyte membrane protein-1 expressed by a placental isolate. J. Infect. Dis. 1921284-1293. [DOI] [PubMed] [Google Scholar]
- 18.Chitarra, V., I. Holm, G. A. Bentley, S. Petres, and S. Longacre. 1999. The crystal structure of C-terminal merozoite surface protein 1 at 1.8 A resolution, a highly protective malaria vaccine candidate. Mol. Cell 3457-464. [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162156-159. [DOI] [PubMed] [Google Scholar]
- 20.Chotivanich, K., J. Sritabal, R. Udomsangpetch, P. Newton, K. A. Stepniewska, R. Ruangveerayuth, S. Looareesuwan, D. J. Roberts, and N. J. White. 2004. Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J. Infect. Dis. 1891052-1055. [DOI] [PubMed] [Google Scholar]
- 21.Clough, B., F. A. Atilola, J. Black, and G. Pasvol. 1998. Plasmodium falciparum: the importance of IgM in the rosetting of parasite-infected erythrocytes. Exp. Parasitol. 89129-132. [DOI] [PubMed] [Google Scholar]
- 22.Contamin, H., C. Behr, O. Mercereau-Puijalon, and J. Michel. 2000. Plasmodium falciparum in the squirrel monkey (Saimiri sciureus): infection of non-splenectomised animals as a model for exploring clinical manifestations of malaria. Microbes Infect. 2945-954. [DOI] [PubMed] [Google Scholar]
- 23.Deans, A. M., K. E. Lyke, M. A. Thera, C. V. Plowe, A. Kone, O. K. Doumbo, O. Kai, K. Marsh, M. J. Mackinnon, A. Raza, and J. A. Rowe. 2006. Low multiplication rates of African Plasmodium falciparum isolates and lack of association of multiplication rate and red blood cell selectivity with malaria virulence. Am. J. Trop. Med. Hyg. 74554-563. [PMC free article] [PubMed] [Google Scholar]
- 24.Fandeur, T., C. Le Scanf, B. Bonnemains, C. Slomianny, and O. Mercereau-Puijalon. 1995. Immune pressure selects for Plasmodium falciparum parasites presenting distinct red blood cell surface antigens and inducing strain-specific protection in Saimiri sciureus monkeys. J. Exp. Med. 181283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fandeur, T., O. Mercereau-Puijalon, and B. Bonnemains. 1996. Plasmodium falciparum: genetic diversity of several strains infectious for the squirrel monkey (Saimiri sciureus). Exp. Parasitol. 841-15. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez, V., C. J. Treutiger, G. B. Nash, and M. Wahlgren. 1998. Multiple adhesive phenotypes linked to rosetting binding of erythrocytes in Plasmodium falciparum malaria. Infect. Immun. 662969-2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flick, K., and Q. Chen. 2004. var genes, PfEMP1 and the human host. Mol. Biochem. Parasitol. 1343-9. [DOI] [PubMed] [Google Scholar]
- 28.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Handunnetti, S. M., M. R. van Schravendijk, T. Hasler, J. W. Barnwell, D. E. Greenwalt, and R. J. Howard. 1992. Involvement of CD36 on erythrocytes as a rosetting receptor for Plasmodium falciparum-infected erythrocytes. Blood 802097-2104. [PubMed] [Google Scholar]
- 30.Heddini, A., F. Pettersson, O. Kai, J. Shafi, J. Obiero, Q. Chen, A. Barragan, M. Wahlgren, and K. Marsh. 2001. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect. Immun. 695849-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Homewood, C. A., and K. D. Neame. 1976. A comparison of methods used for the removal of white cells from malaria-infected blood. Ann. Trop. Med. Parasitol. 70249-251. [DOI] [PubMed] [Google Scholar]
- 32.Jeffares, D. C., A. Pain, A. Berry, A. V. Cox, J. Stalker, C. E. Ingle, A. Thomas, M. A. Quail, K. Siebenthall, A. C. Uhlemann, S. Kyes, S. Krishna, C. Newbold, E. T. Dermitzakis, and M. Berriman. 2007. Genome variation and evolution of the malaria parasite Plasmodium falciparum. Nat. Genet. 39120-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen, A. T., P. Magistrado, S. Sharp, L. Joergensen, T. Lavstsen, A. Chiucchiuini, A. Salanti, L. S. Vestergaard, J. P. Lusingu, R. Hermsen, R. Sauerwein, J. Christensen, M. A. Nielsen, L. Hviid, C. Sutherland, T. Staalsoe, and T. G. Theander. 2004. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 1991179-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joergensen, L., L. Turner, P. Magistrado, M. A. Dahlback, L. S. Vestergaard, J. P. Lusingu, M. Lemnge, A. Salanti, T. G. Theander, and A. T. Jensen. 2006. Limited cross-reactivity among domains of the Plasmodium falciparum clone 3D7 erythrocyte membrane protein 1 family. Infect. Immun. 746778-6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaestli, M., I. A. Cockburn, A. Cortes, K. Baea, J. A. Rowe, and H. P. Beck. 2006. Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study. J. Infect. Dis. 1931567-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaul, D. K., E. F. Roth, Jr., R. L. Nagel, R. J. Howard, and S. M. Handunnetti. 1991. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood 78812-819. [PubMed] [Google Scholar]
- 37.Khattab, A., J. Kun, P. Deloron, P. G. Kremsner, and M. Q. Klinkert. 2001. Variants of Plasmodium falciparum erythrocyte membrane protein 1 expressed by different placental parasites are closely related and adhere to chondroitin sulfate A. J. Infect. Dis. 1831165-1169. [DOI] [PubMed] [Google Scholar]
- 38.Kraemer, S. M., S. A. Kyes, G. Aggarwal, A. L. Springer, S. O. Nelson, Z. Christodoulou, L. M. Smith, W. Wang, E. Levin, C. I. Newbold, P. J. Myler, and J. D. Smith. 2007. Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: comparisons of geographically diverse isolates. BMC Genomics 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraemer, S. M., and J. D. Smith. 2006. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 9374-380. [DOI] [PubMed] [Google Scholar]
- 40.Kyes, S. A., S. M. Kraemer, and J. D. Smith. 2007. Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. Eukaryot. Cell 61511-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyriacou, H. M., G. N. Stone, R. J. Challis, A. Raza, K. E. Lyke, M. A. Thera, A. K. Kone, O. K. Doumbo, C. V. Plowe, and J. A. Rowe. 2006. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol. Biochem. Parasitol. 150211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavstsen, T., P. Magistrado, C. C. Hermsen, A. Salanti, A. T. Jensen, R. Sauerwein, L. Hviid, T. G. Theander, and T. Staalsoe. 2005. Expression of Plasmodium falciparum erythrocyte membrane protein 1 in experimentally infected humans. Malar. J. 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavstsen, T., A. Salanti, A. T. Jensen, D. E. Arnot, and T. G. Theander. 2003. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar. J. 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Scanf, C., B. Carcy, S. Bonnefoy, T. Fandeur, and O. Mercereau-Puijalon. 1997. A modification in restriction pattern of the Plasmodium falciparum Pf60 multigene family associated with a specific antigenic variation switch in the Palo Alto line. Behring Inst. Mitt. 9916-24. [PubMed] [Google Scholar]
- 45.Le Scanf, C., T. Fandeur, S. Bonnefoy, M. Guillotte, and O. Mercereau-Puijalon. 1999. Novel target antigens of the variant-specific immune response to Plasmodium falciparum identified by differential screening of an expression library. Infect. Immun. 6764-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Scanf, C., T. Fandeur, M. E. Morales-Betoulle, and O. Mercereau-Puijalon. 1997. Plasmodium falciparum: altered expressions of erythrocyte membrane-associated antigens during antigenic variation. Exp. Parasitol. 85135-148. [DOI] [PubMed] [Google Scholar]
- 47.Le Scanf, C., I. Vigan-Womas, H. Contamin, M. Guillotte, E. Bischoff, and O. Mercereau-Puijalon. 2008. Rosetting is associated with increased Plasmodium falciparum in vivo multiplication rate in the Saimiri sciureus monkey. Microbes Infect. 10447-451. [DOI] [PubMed] [Google Scholar]
- 48.Ljungström, O., H. Perlmann, M. Schlichtherle, A. Scherf, and A. Wahlgren. 2004. Methods in malaria research, 4th ed. Malaria Research and Reference Reagent Resource Center (MR4)/ATCC, Manassas, VA.
- 49.Luginbuhl, A., M. Nikolic, H. P. Beck, M. Wahlgren, and H. U. Lutz. 2007. Complement factor D, albumin, and immunoglobulin G anti-band 3 protein antibodies mimic serum in promoting rosetting of malaria-infected red blood cells. Infect. Immun. 751771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercereau-Puijalon, O., M. Guillotte, and I. Vigan-Womas. 2008. Rosetting in Plasmodium falciparum: a cytoadherence phenotype with multiple actors. Transfus. Clin. Biol. 1562-71. [DOI] [PubMed] [Google Scholar]
- 51.Moll, K., F. Pettersson, A. M. Vogt, C. Jonsson, N. Rasti, S. Ahuja, M. Spangberg, O. Mercereau-Puijalon, D. E. Arnot, M. Wahlgren, and Q. Chen. 2007. Generation of cross-protective antibodies against Plasmodium falciparum sequestration by immunization with an erythrocyte membrane protein 1-duffy binding-like 1α domain. Infect. Immun. 75211-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montgomery, J., D. A. Milner, Jr., M. T. Tse, A. Njobvu, K. Kayira, C. P. Dzamalala, T. E. Taylor, S. J. Rogerson, A. G. Craig, and M. E. Molyneux. 2006. Genetic analysis of circulating and sequestered populations of Plasmodium falciparum in fatal pediatric malaria. J. Infect. Dis. 194115-122. [DOI] [PubMed] [Google Scholar]
- 53.Montgomery, J., F. A. Mphande, M. Berriman, A. Pain, S. J. Rogerson, T. E. Taylor, M. E. Molyneux, and A. Craig. 2007. Differential var gene expression in the organs of patients dying of falciparum malaria. Mol. Microbiol. 65959-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nash, G. B., B. M. Cooke, J. Carlson, and M. Wahlgren. 1992. Rheological properties of rosettes formed by red blood cells parasitized by Plasmodium falciparum. Br. J. Haematol. 82757-763. [DOI] [PubMed] [Google Scholar]
- 55.Nato, F., K. Reich, S. Lhopital, S. Rouyre, C. Geoffroy, J. C. Mazie, and P. Cossart. 1991. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect. Immun. 594641-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newbold, C. I., R. Pinches, D. J. Roberts, and K. Marsh. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 75281-292. [DOI] [PubMed] [Google Scholar]
- 57.Nielsen, M. A., T. Staalsoe, J. A. Kurtzhals, B. Q. Goka, D. Dodoo, M. Alifrangis, T. G. Theander, B. D. Akanmori, and L. Hviid. 2002. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J. Immunol. 1683444-3450. [DOI] [PubMed] [Google Scholar]
- 58.Noranate, N., R. Durand, A. Tall, L. Marrama, A. Spiegel, C. Sokhna, B. Pradines, S. Cojean, M. Guillotte, E. Bischoff, M. T. Ekala, C. Bouchier, T. Fandeur, F. Ariey, J. Patarapotikul, J. Le Bras, J. F. Trape, C. Rogier, and O. Mercereau-Puijalon. 2007. Rapid dissemination of Plasmodium falciparum drug resistance despite strictly controlled antimalarial use. PLoS ONE 2e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Normark, J., D. Nilsson, U. Ribacke, G. Winter, K. Moll, C. E. Wheelock, J. Bayarugaba, F. Kironde, T. G. Egwang, Q. Chen, B. Andersson, and M. Wahlgren. 2007. PfEMP1-DBL1α amino acid motifs in severe disease states of Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 10415835-15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pain, A., D. J. Ferguson, O. Kai, B. C. Urban, B. Lowe, K. Marsh, and D. J. Roberts. 2001. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc. Natl. Acad. Sci. USA 981805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paul, F., S. Roath, D. Melville, D. C. Warhurst, and J. O. Osisanya. 1981. Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet ii70-71. [DOI] [PubMed] [Google Scholar]
- 62.Peterson, D. S., L. H. Miller, and T. E. Wellems. 1995. Isolation of multiple sequences from the Plasmodium falciparum genome that encode conserved domains homologous to those in erythrocyte-binding proteins. Proc. Natl. Acad. Sci. USA 927100-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pizarro, J. C., V. Chitarra, D. Verger, I. Holm, S. Petres, S. Dartevelle, F. Nato, S. Longacre, and G. A. Bentley. 2003. Crystal structure of a Fab complex formed with PfMSP1-19, the C-terminal fragment of merozoite surface protein 1 from Plasmodium falciparum: a malaria vaccine candidate. J. Mol. Biol. 3281091-1103. [DOI] [PubMed] [Google Scholar]
- 64.Roberts, D. J., A. Pain, O. Kai, M. Kortok, and K. Marsh. 2000. Autoagglutination of malaria-infected red blood cells and malaria severity. Lancet 3551427-1428. [DOI] [PubMed] [Google Scholar]
- 65.Robinson, B. A., T. L. Welch, and J. D. Smith. 2003. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol. Microbiol. 471265-1278. [DOI] [PubMed] [Google Scholar]
- 66.Roussilhon, C., C. Oeuvray, C. Muller-Graf, A. Tall, C. Rogier, J. F. Trape, M. Theisen, A. Balde, J. L. Perignon, and P. Druilhe. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 4e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowe, A., J. Obeiro, C. I. Newbold, and K. Marsh. 1995. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect. Immun. 632323-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowe, J. A., J. M. Moulds, C. I. Newbold, and L. H. Miller. 1997. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388292-295. [DOI] [PubMed] [Google Scholar]
- 69.Rowe, J. A., J. Obiero, K. Marsh, and A. Raza. 2002. Short report: positive correlation between rosetting and parasitemia in Plasmodium falciparum clinical isolates. Am. J. Trop. Med. Hyg. 66458-460. [DOI] [PubMed] [Google Scholar]
- 70.Rowe, J. A., S. J. Rogerson, A. Raza, J. M. Moulds, M. D. Kazatchkine, K. Marsh, C. I. Newbold, J. P. Atkinson, and L. H. Miller. 2000. Mapping of the region of complement receptor (CR) 1 required for Plasmodium falciparum rosetting and demonstration of the importance of CR1 in rosetting in field isolates. J. Immunol. 1656341-6346. [DOI] [PubMed] [Google Scholar]
- 71.Rowe, J. A., J. Shafi, O. K. Kai, K. Marsh, and A. Raza. 2002. Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am. J. Trop. Med. Hyg. 66692-699. [DOI] [PubMed] [Google Scholar]
- 72.Russell, C., O. Mercereau-Puijalon, C. Le Scanf, M. Steward, and D. E. Arnot. 2005. Further definition of PfEMP-1 DBL-1α domains mediating rosetting adhesion of Plasmodium falciparum. Mol. Biochem. Parasitol. 144109-113. [DOI] [PubMed] [Google Scholar]
- 73.Salanti, A., T. Staalsoe, T. Lavstsen, A. T. Jensen, M. P. Sowa, D. E. Arnot, L. Hviid, and T. G. Theander. 2003. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 49179-191. [DOI] [PubMed] [Google Scholar]
- 74.Scherf, A., R. Hernandez-Rivas, P. Buffet, E. Bottius, C. Benatar, B. Pouvelle, J. Gysin, and M. Lanzer. 1998. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 175418-5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scholander, C., C. J. Treutiger, K. Hultenby, and M. Wahlgren. 1996. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat. Med. 2204-208. [DOI] [PubMed] [Google Scholar]
- 76.Singh, S. K., R. Hora, H. Belrhali, C. E. Chitnis, and A. Sharma. 2006. Structural basis for Duffy recognition by the malaria parasite Duffy-binding-like domain. Nature 439741-744. [DOI] [PubMed] [Google Scholar]
- 77.Smith, J. D., A. G. Craig, N. Kriek, D. Hudson-Taylor, S. Kyes, T. Fagan, R. Pinches, D. I. Baruch, C. I. Newbold, and L. H. Miller. 2000. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc. Natl. Acad. Sci. USA 971766-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith, J. D., G. Subramanian, B. Gamain, D. I. Baruch, and L. H. Miller. 2000. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 110293-310. [DOI] [PubMed] [Google Scholar]
- 79.Somner, E. A., J. Black, and G. Pasvol. 2000. Multiple human serum components act as bridging molecules in rosette formation by Plasmodium falciparum-infected erythrocytes. Blood 95674-682. [PubMed] [Google Scholar]
- 80.Su, X. Z., V. M. Heatwole, S. P. Wertheimer, F. Guinet, J. A. Herrfeldt, D. S. Peterson, J. A. Ravetch, and T. E. Wellems. 1995. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 8289-100. [DOI] [PubMed] [Google Scholar]
- 81.Taylor, H. M., S. A. Kyes, D. Harris, N. Kriek, and C. I. Newbold. 2000. A study of var gene transcription in vitro using universal var gene primers. Mol. Biochem. Parasitol. 10513-23. [DOI] [PubMed] [Google Scholar]
- 82.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193673-675. [DOI] [PubMed] [Google Scholar]
- 83.Trape, J. F., and C. Rogier. 1996. Combating malaria morbidity and mortality by reducing transmission. Parasitol. Today 12236-240. [DOI] [PubMed] [Google Scholar]
- 84.Trape, J. F., C. Rogier, L. Konate, N. Diagne, H. Bouganali, B. Canque, F. Legros, A. Badji, G. Ndiaye, P. Ndiaye, et al. 1994. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am. J. Trop. Med. Hyg. 51123-137. [DOI] [PubMed] [Google Scholar]
- 85.Treutiger, C. J., I. Hedlund, H. Helmby, J. Carlson, A. Jepson, P. Twumasi, D. Kwiatkowski, B. M. Greenwood, and M. Wahlgren. 1992. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am. J. Trop. Med. Hyg. 46503-510. [DOI] [PubMed] [Google Scholar]
- 86.Vogt, A. M., G. Winter, M. Wahlgren, and D. Spillmann. 2004. Heparan sulphate identified on human erythrocytes: a Plasmodium falciparum receptor. Biochem. J. 381593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Volkman, S. K., P. C. Sabeti, D. DeCaprio, D. E. Neafsey, S. F. Schaffner, D. A. Milner, Jr., J. P. Daily, O. Sarr, D. Ndiaye, O. Ndir, S. Mboup, M. T. Duraisingh, A. Lukens, A. Derr, N. Stange-Thomann, S. Waggoner, R. Onofrio, L. Ziaugra, E. Mauceli, S. Gnerre, D. B. Jaffe, J. Zainoun, R. C. Wiegand, B. W. Birren, D. L. Hartl, J. E. Galagan, E. S. Lander, and D. F. Wirth. 2007. A genome-wide map of diversity in Plasmodium falciparum. Nat. Genet. 39113-119. [DOI] [PubMed] [Google Scholar]
- 88.Wassmer, S. C., T. Taylor, C. A. Maclennan, M. Kanjala, M. Mukaka, M. E. Molyneux, and G. E. Grau. 2008. Platelet-induced clumping of Plasmodium falciparum-infected erythrocytes from Malawian patients with cerebral malaria—possible modulation in vivo by thrombocytopenia. J. Infect. Dis. 19772-78. [DOI] [PMC free article] [PubMed] [Google Scholar]