Abstract
Neisseria gonorrhoeae is a gram-negative diplococcus that in human beings produces gonorrhea. Much clinical evidence has led to the conclusion that gonococcus has important mechanisms to evade host immune functions; however, these mechanisms are only now beginning to be elucidated. In this study, we determined that the BALB/c mouse is a good animal model to study gonococcus infection and examined the immune response against the bacteria. We determined that after intravaginal inoculation of mice with Neisseria gonorrhoeae, the bacteria reached and invaded the upper female reproductive tissues and elicited a T-cell-specific immune response associated with a very weak humoral response, altogether resembling gonococcus infection and disease in women. Remarkably, in the draining lymph nodes of the genital tracts of infected mice, we found an increase of regulatory T lymphocytes, namely, transforming growth factor β1-positive CD4+ T cells and CD4+ CD25+ Foxp3+ T cells. Altogether, results indicate that N. gonorrhoeae induces regulatory T cells, which might be related to the local survival of the pathogen and the establishment of a chronic asymptomatic infection.
Gonorrhea is a sexually transmitted disease produced by the gram-negative diplococcus Neisseria gonorrhoeae. In women, infection affects the cervix and may spread to the uterus and oviduct, inducing endometritis and pelvic inflammatory disease. Strikingly, about 50% of the cases proceed without symptoms, inducing damage mainly in the fallopian tube, while in men infection occurs with distinctive clinical symptoms (11, 21). The hallmark of humoral immune response against N. gonorrhoeae is the extremely low levels of antigonococcal antibodies found in serum and secretions of the human (male and female) during infection (19, 20). Antibodies are directed against several major membrane molecules, such as the pilus- and opacity-associated outer membrane proteins (Pil and Opa, respectively), the porin protein (Por), and the lipooligosaccharide (5, 19, 20, 38, 39, 55). Although some of these have bactericidal activity, they are not protective and seem to be blocked by outer membrane protein 3 (RmP)-specific immunoglobulin Gs (IgGs) (38).
The highly asymptomatic infection in women and the poor immune response related to gonococcus prime and multiple challenges might actually be related to mechanisms of immune evasion acquired by the bacteria to constrain the immune response. Moreover, the presence of a significant, although weak, humoral immune response during gonococcus infection (19) suggests that the bacteria might stimulate a regulatory type of immune response. One of these mechanisms might be the induction of noninflammatory responses dominated by Th2-type cytokines and the activation of regulatory T cells (Tregs), which in turn would contribute to the suppression of most of the mechanisms of protection against intracellular pathogens (2, 40). Tregs are CD4+ T lymphocytes involved in the induction of suppressor responses; it has been determined experimentally that they show several phenotypes. One of the major characteristics is related to the presence of transforming growth factor β1 (TGF-β1) (52), and subgroups can be distinguished because of the expression of CD25 and the Foxp3 transcription factor. CD4+ CD25+ T cells correspond to a subgroup expressing Foxp3, which originate in the thymus and are called natural Tregs, while the CD4+ CD25− T cells are induced at the periphery after antigenic stimulation in the presence of a distinct cytokine environment (28, 31, 50, 52). TGF-β1 blocks T-cell proliferation, inhibits Th1, Th2, and CTL differentiation, and moreover induces Foxp3 expression in Tregs (27).
Overall, primary cell and organ culture systems have been successfully developed to examine the initial phase of gonococcal pathogenesis (9, 10, 13, 29, 48) where several gonococcus membrane components, such as Pil and Opa, are highly relevant (30). However, the mechanisms explaining why female infection occurs in a high number of cases without inflammatory signs, with low levels of antibody induction, and with no disease resolution responses are not understood. Understanding pathogenesis at this level has been greatly hindered due to the ethical considerations associated with human research work and the lack of an animal model of experimental infection (9). Only recently, a murine model introduced by Ann Jerse has allowed studies of protection against gonococcus infection (23, 37).
In this study, we have further investigated the mouse experimental model of N. gonorrhoeae infection and demonstrated that the bacteria reach and invade the upper female reproductive tissues (uterus and oviduct), resembling gonococcus infection and disease in women. The murine model allowed us to determine that infection elicits a T-cell-specific immune response associated with a weak humoral response. In addition, the local response includes the induction of regulatory TGF-β1+ T cells which, acting to suppress the activation of the immune system, would support the occurrence of infection. Altogether, results indicate that N. gonorrhoeae induces regulatory mechanisms of immunity which, in turn, might explain the local survival of the pathogen and the establishment of a chronic asymptomatic infection in women.
MATERIALS AND METHODS
Bacteria and culture conditions.
The following three different variants of N. gonorrhoeae strain P9, kindly provided by Myron Christodoulides (University of Southampton, United Kingdom), were used in this study: P9-13 (Pil− Opaa+), P9-16 (Pil− Opab+), and P9-17 (Pil+ Opab+) (8). Bacteria were routinely grown on GC agar (Becton Dickinson, Maryland) supplemented with 1% IsoVitaleX (Becton Dickinson) for 18 h at 37°C in 5% CO2. Gonococcal variants containing a red shift mutant green fluorescent protein (GFP) plasmid were grown in GC agar containing ampicillin (5 μg/ml). Analysis of colony morphology under a stereomicroscope and Western blotting for the detection of Pil and Opa were routinely performed to discard phenotypic variability. Monoclonal antibodies against Pil and Opa were kindly provided by Mumtaz Virji (University of Bristol, United Kingdom) and Mark Achtman (Max Planck Institute, Berlin, Germany), respectively.
Animals.
BALB/c mice were obtained from the Institute of Public Health (Santiago, Chile), housed under regulated light and temperature conditions, and sacrificed by cervical dislocation. Research was conducted in accordance with institutional guidelines and with the International Guiding Principles for Biomedical Research Involving Animals of the Society for the Study of Reproduction.
Mouse uterine cell cultures.
Cultures were prepared from mouse uteri as previously described (17). Briefly, uterine horns were washed in RPMI 1640 medium (GIBCO Invitrogen Co., Carlsbad, CA) containing 100 U/ml penicillin and 100 μg/ml streptomycin (GIBCO Invitrogen Co.). Organs were cut into small pieces and then transferred to a plate containing 0.25% trypsin and 2.5% pancreatin (Life Technologies, Grand Island, NY) and incubated for 60 min at 4°C followed by 60 min at 20°C. Organs were transferred to a sterile tube containing 15 ml of cold Hanks' balanced salt solution (GIBCO Invitrogen Co.). Digested uteri were then vortexed and the released epithelial cells were recovered and transferred to a clean tube. The procedure was repeated three times and cells in suspension were collected, centrifuged for 8 min at 180 × g, and resuspended in RPMI 1640 supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc., Logan, UT), 1 mM l-glutamine, 100 μg/ml streptomycin, and 100 U/ml penicillin. Uterine cells were cultured at 37°C in 5% CO2 until they reached 90% confluence. Positive immunostaining with a mouse anticytokeratin monoclonal antibody (Chemicon International, Temecula, CA) and negative staining with antivimentin antibody (Chemicon International) confirmed the epithelial origin of these cells. Typically, cultures had more than 95% epithelial cells.
Infection of primary uterine cell cultures.
Gonococcal isolates were taken from frozen stocks and cultured on GC agar plates at 37°C in a 5% CO2-air atmosphere. Bacteria were then scraped from confluent culture plates and resuspended in 1 ml of Dulbecco's modified Eagle's medium without phenol red (GIBCO Invitrogen Co.). The concentration was estimated by comparison as 1 optical density unit at 600 nm, corresponding to 3.2 ×109 CFU/ml. Cells were infected with N. gonorrhoeae fluorescence variants at a multiplicity of infection of 10 and cultured at 37°C in 5% CO2 until they reached 90% confluence. To determine the uptake of bacteria, epithelial cells were treated with 100 μg/ml of gentamicin for 60 min at 37°C to kill extracellular bacteria. Cells were then diluted in phosphate-buffered saline (PBS) containing 1% saponin and incubated for 15 min at 37°C. A tenfold dilution series was prepared and 100-μl portions were spread on GC plates. The internalization of bacteria in epithelial cells was evident by the recovery of colonies composed of gram-negative, oxidase-positive diplococci after 18 h of growth at 37°C.
Mouse experimental infection.
Experimental infection of BALB/c mice was performed as previously described (23) with some modifications. Briefly, 7 days before bacterium inoculation, four groups of five female BALB/c mice (6 to 8 weeks old) were daily injected with 3 μg of cetrorelix acetate (subcutaneous administration). On day 4, half of the groups were additionally inoculated daily with 300 ng of estradiol [estra-1,3,5(10)-triene-3,17-beta-diol; Sigma] until the end of the experiment. On day 7, one estradiol-treated group and one control group were intravaginally inoculated with 108 CFU N. gonorrhoeae fluorescence variants suspended in Dulbecco's modified Eagle's medium without phenol red (GIBCO Invitrogen Co.). The other two groups were treated with medium. The procedure was performed using a sterile syringe connected to flexible tubing which allowed instillation. Tubing was inserted into the vagina, first dorsally and then cranially, until the cervix was reached. Fifty-microliter portions of bacterial suspension were inoculated in the mice. At 1, 3, and 5 days postinoculation, the genital tracts of infected and control mice were removed. Uteri were weighed to assess the effectiveness of estradiol treatment.
Microscopy.
For microscopic analysis, cells were cultured on coverslips and infected as described above. After the indicated times, cells were fixed with 1% paraformaldehyde in PBS. In the cases of vaginas, uteri, and oviducts, tissues were fixed in 4% paraformaldehyde in PBS for 1 h before sequential transfer to 10% sucrose in PBS for 1 h, 20% sucrose in PBS for 1 h, and 30% sucrose in PBS overnight. Organs were mounted in embedding compound (Cryo-M-Bed; Bright Instrument Co. Ltd., Huntingdon, United Kingdom) and frozen at −20°C. Slices of 5 to 10 μm were cut using a Bright Starlet cryostat at −20°C. Fixed cultured cells and tissue sections were counterstained using a solution of 1 μg/ml propidium iodide in PBS and mounted in a solution of PBS containing 10% (vol/vol) 1,4-diazobicyclo[2.2.2] octane (Dabco; Sigma) and 90% (vol/vol) glycerol. Analysis of interactions of N. gonorrhoeae with uterine cells was performed by confocal microscopy (Zeiss LSM510). Briefly, confocal z-plane slices were obtained and orthogonal views and three-dimensional images were generated from isolated cells and tissues. For transmission electron microscopy analysis, cells were fixed in 1% glutaraldehyde in PBS (pH 7.4) and dehydrated for embedding in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate. Control and infected samples were viewed with a Philips EM-200 transmission electron microscope.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was performed for serum titration. Briefly, whole-protein extracts were prepared by freeze-thawing N. gonorrhoeae strain P9-17 in water, and protein levels were determined by the Bradford assay. Extracts prepared from Escherichia coli JM109 were used as a negative control. Microtiter plates (Falcon; Becton Dickinson Co., Santiago, Chile) were activated with 10 μg of protein per well and incubated overnight at 4°C for 24 h. After the washing, sera collected from infected (n = 4) and control (n = 4) mice were added to plates (at 1:20, 1:50, 1:100, 1:200, and 1:400 dilutions). Specific antibodies to gonococcus were detected with goat anti-mouse IgG-alkaline phosphatase conjugate (Sigma) and p-nitrophenyl phosphate (Sigma). Each mouse serum sample was assayed in duplicate.
T-cell antigen proliferation assays.
To determine specific T-cell responses, mice were intravaginally infected as described above and then boosted intraperitoneally on day 15. Seven days after the boost (day 22), mice were sacrificed and the spleens and lymph nodes (renal, iliac, and caudal) were removed (34). CD4+ T cells were isolated by negative selection using antibody-coated magnetic beads (Dynal; Invitrogen). Isolated T cells (1 × 105 cells/well) from treated and control mice were cultured with mitomycin-treated autologous splenocytes (2 × 104 cells/well) previously pulsed with 1 or 10 μg of whole-protein extract of N. gonorrhoeae. Experiments were carried out in 96-well plates in triplicate. As a positive control, T cells were stimulated with phytohemagglutinin (PHA) (10 μl/ml; GIBCO Invitrogen Co.). Plates were incubated at 37°C and 5% CO2 for 72 h and then pulsed with 1 μCi methyl-[3H]thymidine (Amersham Biosciences, Buckinghamshire, United Kingdom) for the final 18 h before harvesting. The amount of incorporated radioactivity was measured using a liquid scintillation counter (Tri-Carb 2100 TR; Packard). Data were expressed as experimental minus control counts per minute.
Leukocyte isolation.
A previously described protocol was followed (24). Briefly, uteri dissected from mice (four animals/experiment) were placed in sterile ice-cold Hanks' balanced salt solution (GIBCO, Grand Island, NY), weighed, and then transferred to a mixture of pancreatin (GIBCO), trypsin (Sigma, St. Louis, MO), and DNase (Worthington, Lakewood, NJ). Under sterile conditions, uterine tissues in the enzyme mixture were cut into small pieces, transferred to six-well culture plates, and incubated first for 1 h at 4°C and then for an additional hour at room temperature with gentle shaking. Cells were recovered from the supernatant and were pooled for each experiment.
Immunofluorescence staining and analysis.
For TGF-β1 detection, leukocytes (5 × 105 cells/ml) were incubated with anti-TGF-β1 antibody (1:100; Santa Cruz Biotechnology) for 1 h at 4°C. After being washed with PBS, cells were incubated with a fluorescein isothiocyanate-conjugated polyclonal anti-rabbit IgG (1:200; Santa Cruz Biotechnology) for 1 h at 4°C. For CD11b detection, cells were incubated for 30 min on ice with 2 ml of RPMI-10% fetal bovine serum with 10% heat-inactivated normal mouse serum to reduce the nonspecific binding of antibodies. After incubation, 106 cells/ml were labeled with phycoerythrin-conjugated anti-mouse CD11b (Santa Cruz Biotechnology, Inc.) for 1 h at 4°C. For analysis of CD4+ CD25+ Foxp3+ T lymphocytes, 1 × 106 cells were labeled using PE-Cy5.5-conjugated anti-CD4 antibody (clone GK1.5; eBioscience, San Diego, CA) and fluorescein isothiocyanate-conjugated anti-CD25 antibody (clone PC61.5; eBioscience). After being washed, cells were resuspended in 200 μl of Fix/Perm buffer (eBioscience) and left at 4°C for 30 min. After being washed, cells were incubated with phycoerythrin-conjugated anti-mouse Foxp3 (clone FJK-16S; eBioscience, San Diego, CA) for 30 min. Isotypic controls were routinely included in all experiments. Labeled cells were analyzed on a FACScan flow cytometer (Becton Dickinson) with CellQuest software.
Mixed lymphocyte reaction (MLR).
Fresh T cells isolated from the lymph nodes and spleens of control and infected BALB/c mice (H-2d) were first labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE). T-cell responders (105) were incubated with spleen cells (5 × 105) isolated from C57BL/6 (H-2b) mice in 200 μl of RPMI at 37°C. After 5 days of culture in 96-well round-bottom plates, cells were washed and fixed in 1% paraformaldehyde, and CFSE dilution was assessed by flow cytometry.
RESULTS
N. gonorrhoeae infects isolated murine uterine cells.
Previously, it had been extensively described that N. gonorrhoeae is a strictly human pathogen; nevertheless, recent reports indicated that the bacteria are also able to colonize the mouse genital female tract. Before initiating studies of infection in vivo, our first aim was to demonstrate that the bacteria attach, bind, and invade murine genital tract cells. Isolated mouse uterine epithelial cells were chosen as targets for in vitro studies. Cells were infected with N. gonorrhoeae fluorescent (GFP) strain P9, variant P9-17 (Pil+ Opab+), at a multiplicity of infection of 10 bacteria per cell. A simultaneous detection of GFP-expressing gonococci and the red-stained nucleus on 0.4-μm-thickness cross-sectional images by confocal microscopy showed that, after 24 h of incubation, gonococci were found associated with the epithelial cells, with a significant number inside the cells surrounding the nucleus (Fig. 1A). Similar results were obtained for variants P9-16 and P9-13. The intracellular localization of the bacteria was confirmed by transmission electron microscopy, as micrographs exhibited N. gonorrhoeae P9-16 enclosed in membrane-bound vesicles of the mouse uterine cells (Fig. 1C and D). Similar structures were not found in control cells (not shown). In addition, an average of 3.5 × 104 intracellular gonococci per well (24-well plate) was recovered from infected uterine epithelial cells after selective antibiotic killing of extracellular bacteria. Colonies had the expected morphology, i.e., variants exhibited small opaque colonies with sharp edges, which correspond to Pil+ Opa+ gonococci. Much higher numbers of bacteria were observed under fluorescent microscopy, indicating that the major portion of gonococci appear attached to the uterine cell, exactly as previously described for the infection of human endometrial and oviductal cells (8). Results demonstrated that N. gonorrhoeae is able to attach and invade the uterine epithelial, thus unequivocally identifying murine cells as a target of gonococcus infection.
FIG. 1.
(A) Confocal photomicrograph of serial sections (0.4 μm) shows the presence of N. gonorrhoeae (P9-17) around the nuclei of uterine epithelial cells. Bacteria appear green, and the cell nuclei are red. (B) Uterine cells; control cultures stained with propidium iodide. (C and D) Transmission electron microscopy images of isolated uterine epithelial cells infected with N. gonorrhoeae (variant P9-16) show multiple cytoplasmic bacteria enclosed in membrane-bound vesicles (arrowheads). Bars, 20 μm (B); 1 μm (C); 0.1 μm (D).
N. gonorrhoeae invades the upper female reproductive tract of the mouse.
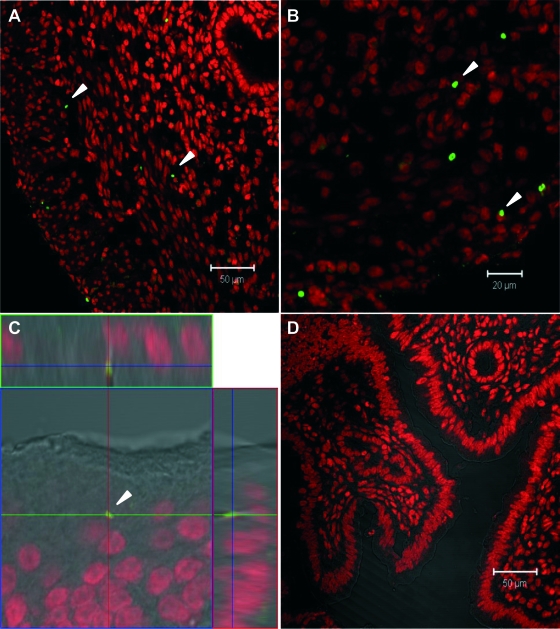
Once it was determined that N. gonorrhoeae is able to colonize isolated uterine epithelial cells, the next step was to corroborate that the bacteria colonize the upper reproductive tract in mice, as seen for women. Because estradiol seems to be crucial for gonococcus infection in mice (23), we inoculated bacterial strain P9-17 expressing GFP (108 CFU) into the vaginas of estradiol-treated BALB/c mice. Uteri were removed after several days postinoculation, processed as described in Materials and Methods, and analyzed by confocal microscopy. Fluorescent bacteria were found in the uteri of all inoculated mice (n = 25), particularly in the epithelial and subepithelial tissues of the organs. On day 1 after inoculation, gonococci were mostly observed in the epithelium and occasionally in the stroma, while on day 3, most of the bacteria were present in the stroma (Fig. 2A). On day 5, the bacteria were distributed throughout the stroma but also in the most external tissues of the uterus (Fig. 2B). An orthogonal view of the infected uterus (midplane z section; height, 1.3 μm,) confirmed the bacterial localization within the depths of the tissue (Fig. 2C). As the bacteria were also found in the epithelial and subepithelial tissues of the vaginas and the oviducts, overall these results indicate that intravaginal inoculation of N. gonorrhoeae allows the bacteria to reach, attach to, and invade the mucosal tissues of the lower and upper organs of the mouse genital tract. Further experiments were performed to evaluate the contribution of estradiol in allowing infection. The experiments were repeated several times with different mice at random stages of the reproductive cycle, and surprisingly, infection was also observed for all mice tested. Moreover, infection seemed to be persistent, because the bacteria were detected as late as 22 days postinoculation; however, as is the case for women, no clinical signs of the disease were apparent in the mice.
FIG. 2.
Confocal images of uterine sections of mice intravaginally infected with GFP-expressing N. gonorrhoeae (variant P9-17). The cell nuclei are stained with propidium iodide. (A) Three days after inoculation. Bar, 50 μm. (B) Five days after inoculation. Bar, 20 μm. (C) Orthogonal views of a midplane z section; height, 1.3 μm. (D) Negative control. Arrows denote fluorescent bacteria.
Immune response.
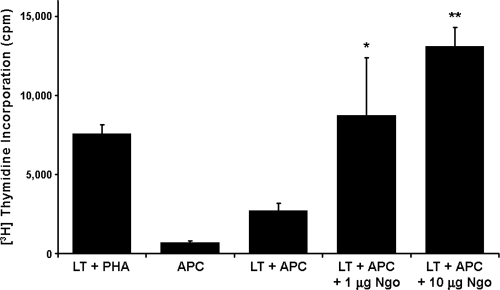
After corroborating that gonococcus infects the mouse genital tract, as it does in humans, we wanted to establish whether the bacteria also induce an antigen-specific response in mice. To evaluate the humoral immune response, sera from infected and control animals obtained at day 22 after treatment were tested using ELISA against a whole-protein extract of the P9-17 bacterial variant. Very low antibody titers in response to N. gonorrhoeae were detected for two out of the four infected mice (Fig. 3), while as expected, no detectable antibody was observed in serum samples of control mice. To examine the T-cell response, CD4+ T lymphocytes isolated from uterus-draining lymph nodes from infected and control mice were examined for the ability to proliferate in response to a whole-protein extract of the bacteria (variant P9-17). The results show that T cells from infected BALB/c mice exhibited a dose-dependent T-cell-proliferative response to gonococcus protein extract (Fig. 4). These findings indicate that N. gonorrhoeae induces a local antigen-specific T-cell response associated with a low- or no-antibody response during experimental infection in the mouse.
FIG. 3.
Analysis of serum response to N. gonorrhoeae by ELISA. Mouse sera were assayed on a whole-protein extract of the P9-17 bacterial variant in a reciprocal dilution series starting at 1:50, and binding was detected with an alkaline phosphatase anti-mouse conjugate. Black symbols represent infected mice (n = 4) and open symbols control mice (n = 4).
FIG. 4.
Proliferative response of isolated CD4+ T cells (LT) from infected mice to a protein extract of N. gonorrhoeae. T cells were stimulated with PHA or added to BALB/c splenocytes (antigen-presenting cells [APC]) pretreated with 1 or 10 μg of whole-protein extract of N. gonorrhoeae P9-17 (Ngo). Proliferation was measured by [3H]thymidine incorporation. Each bar represents the mean ± standard error of the mean (SEM) from triplicates. Data were expressed as experimental minus control counts per minute. Two independent experiments were done with similar results. Asterisks and double asterisks indicate P values of <0.05 and <0.0001, respectively, versus values for LT plus APC only by analysis of variance.
Tregs increase in infected animals.
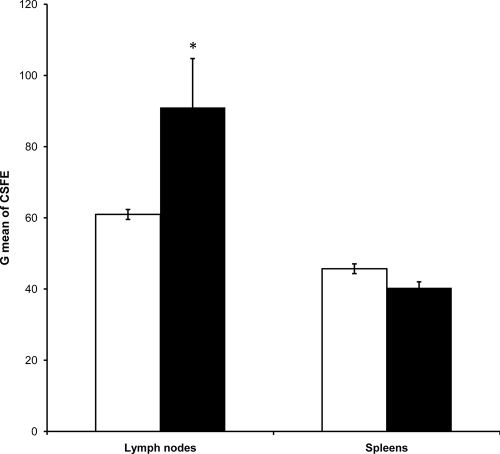
As mentioned previously, specific stimulation of Tregs might explain the weak antigonococcal humoral immune response and the absence of protective immunity. Thus, we evaluated whether the BALB/c T-cell response to gonococcus also involves the stimulation of Tregs. As the major phenotype of Tregs is the synthesis of TGF-β1, we first quantified the percentage of TGF-β1-producing T cells locally induced 22 days after infection. In six independent experiments, the percentages of CD4+ TGF-β1+ T cells isolated from the regional lymph nodes of infected mice (Fig. 5C, top right, and F) were twofold higher than those of cells of the control group of mice (Fig. 5B, top right, and F). No changes in the CD4+ TGF-β1+ T lymphocytes were observed for T cells isolated from spleens of the same animals (Fig. 5D and E, top right, and F). Interestingly, a strong shift in CD4− TGF-β1+ splenocytes from infected (Fig. 5E) versus noninfected (Fig. 5D) mice occurred. This effect was not observed in the regional lymph nodes, and the cells might correspond to a different type of regulatory cells. In addition, we examined the stimulated T cells in four independent experiments to determine the presence of CD25 and Foxp3, additional molecular markers of Tregs. Results revealed that the percentage of CD4+ CD25+ Foxp3+ T cells detected in the local lymph nodes of the group of infected animals was higher than that of the control group (P = 0.005) (Fig. 6A and B, top right, and E). Once again, no statistically significant differences were found when cells in the spleens of the animals were quantified (Fig. 6C and D, top right, and E). Then, we tested the suppressive function of Tregs in an allogeneic MLR. Only cells from the lymph nodes of infected BALB/c mice, where Tregs have been expanded by gonococcus infection, showed suppression in the MLR stimulated by C57BL/6 spleen cells, while cells from the lymph nodes of control mice and from the spleens of infected mice, where no T-regulatory expansion was observed, showed a regular response to allogeneic C57BL/6 stimulation (Fig. 7). Overall, these results indicate that gonococcus infection of the murine genital tract induces a significant stimulation of Tregs as part of the local immune response.
FIG. 5.
Flow cytometric analysis of TGF-β1-producing CD4+ T cells. (A to E) Representative experiment. (A) Control of autofluorescence. (B) Cells isolated from the regional lymph nodes of control mice. (C) Cells isolated from the regional lymph nodes of infected mice. (D) Splenocytes of control mice. (E) Splenocytes of infected mice. The percentage of TGF-β1+ CD4+ T cells is shown in the top right panel of each figure. (F) Percentages of TGF-β1+ CD4+ T cells in lymph nodes and spleens of infected (white bars) and control (black bars) BALB/c mice. Bars represent means ± SEM from six independent experiments. Lymph nodes of infected mice showed a significantly higher percentage of TGF-β1-producing CD4+ T cells than did the lymph nodes of the control group. No differences were found in spleens. *, P value of <0.02 by the Mann-Whitney U test.
FIG. 6.
Flow cytometric detection of regulatory CD4+ T cells. (A to D) Representative experiment; results are expressed as the percentage of CD25+ or CD25− T cells expressing FoxP3 in the gated CD4+ T-cell population of local lymph nodes of control BALB/c mice (A), local lymph nodes of infected BALB/c mice (B), spleens of control mice (C), and spleens of infected mice (D). (E) Percentages of CD4+ CD25+ Foxp3+ T cells in lymph nodes and spleens of infected (white bars) and control (black bars) mice. Bars represent means ± SEM of four independent experiments. Lymph nodes of infected mice showed a significantly higher percentage of CD4+ CD25+ Foxp3+ T cells than did the lymph nodes of the control group. No differences were found in spleens. *, P value of <0.05 by the Mann-Whitney U test.
FIG. 7.
Geometric mean fluorescence of CFSE-labeled T cells. Responder cells were isolated from the lymph nodes and spleens of control (white bars) and infected (black bars) BALB/c mice. For MLR, stimulators were obtained from C57BL/6 spleens. CSFE-labeled responder cells were incubated with stimulator cells (ratio of 1:5) for 5 days and CFSE dilution was assessed by flow cytometry at the end of the experiment. Bars represent geometric means (G mean) ± SEM from nine independent experiments. Only cells from the lymph nodes, where Tregs have been expanded by gonococcus infection, showed suppression to the MLR. *, P value of <0.0001 by the Kruskal-Wallis test.
TGF-β1+ cells infiltrate the uterine tissues.
We further investigated whether a local immune response was also accompanied by a uterine infiltration of TGF-β1+ cells. Because N. gonorrhoeae in vitro induces the expression of TGF-β1 in macrophages (unpublished data), cells isolated from the uteri of infected and control animals were labeled with anti-TGF-β1 and anti-CD11b (macrophage marker) antibodies. Data from a flow cytometric analysis show that infected animals have a moderate but significant increase of CD11b+ TGF-β1+ cells in the uteri (Fig. 8C and D, top right), which indicates that gonococcus induces a mucosal infiltration of macrophages whose phenotype would favor the differentiation of Tregs. Interestingly, in addition to being associated with the infiltration of macrophages, infection is also associated with the infiltration of a great number of CD11b− TGF-β1+ cells (Fig. 8E), which might correspond to Tregs.
FIG. 8.
Flow cytometry analysis of CD11b+ TGF-β1+ cells infiltrating genital tract tissues following infection with N. gonorrhoeae. (A) Autofluorescence control. (B) Secondary antibody control. (C and D) CD11b+ TGF-β1+ cells from control (C) and infected (D) tissues. (E) White bars, TGF-β1+ cells from control groups; black bars, TGF-β1+ cells from infected mice.
DISCUSSION
In this study, we established that N. gonorrhoeae invades the murine mucosa of the upper genital tract, results that confirm and extend the extensive mouse work developed by Ann Jerse and collaborators (23, 37, 43). Moreover, we demonstrated that infection induces a weak humoral immune response associated with a local increase of TGF-β1+ CD4+ Tregs.
Until now, it has been widely accepted that N. gonorrhoeae is a strictly human pathogen, which calls the validity of the use of mice as an animal model of experimental infection into question. This is reinforced by the fact that most of human receptors for attachment and invasion do not seem to be expressed in the mouse (30). However, we demonstrated here that N. gonorrhoeae is able to infect murine epithelial cells isolated from the uterus; the uptake of gonococcus by murine uterine epithelial cells was demonstrated by means of three independent assays, a gentamicin resistance test, confocal multiple scan analysis, and transmission electron microscopy. Moreover, in vivo, we showed that GFP-expressing gonococci are able to reach the upper genital organs and to invade uterine tissues in all infected animals. Because in the experiments there were no sources of green fluorescence other than that of the bacteria, the uterus is normally sterile, and colonies of gonococcus were recovered from the uterine cells of infected mice, we are confident that the 1- to 2-μm fluorescent spots seen in tissues by confocal microscopy correspond to bacteria. Therefore, although mice are not natural hosts for N. gonorrhoeae, results confirmed that gonococcus infects the lower genital tract of BALB/c mice (23) and demonstrated for the first time that the bacteria not only reach the upper genital tract but also invade the upper murine mucosae, as occurs in humans.
Analyses performed to characterize the immune response of BALB/c mice showed that once gonococcus colonizes the female genital tract, bacterial antigens are detected by the immune system, as revealed by the ability of CD4+ T lymphocytes to respond to the bacterial extract and by the presence of specific antibodies in serum. However, as has been observed for humans, antibodies that recognize N. gonorrhoeae were detected at low levels in the infected mice. Gonococcal activation of CD4+ T cells has also been described for humans, as gonococcal pilus interaction with CD4+ T cells induces the activation and proliferation of lymphocytes and stimulates the secretion of interleukin-10 (IL-10) (36). In contrast, it has also been shown that N. gonorrhoeae Opa proteins mediate binding to CEACAM-1 expressed by CD4+ T cells and suppress the activation and proliferation of naive lymphocytes (4, 25). Although not fully comparable, this does not seem to occur in mice, since PHA-stimulated naive T cells from control mice showed proliferation (not shown).
The analysis of the nature of the CD4+ T cells stimulated by gonococci during the experimental infection leads us to observe that infection induces TGF-β1+ CD4+ T-cell responders in the mucosal lymph nodes, including a subset of CD25+ Foxp3+ Tregs. In particular, these Tregs showed a small but significant increase in number which is similar to that observed after infection with parasites (15, 47, 53). Interestingly, the induction of this type of immunity did not occur at the systemic level. Even more, the regulatory activity was confirmed in vitro in an allogeneic MLR, indicating that N. gonorrhoeae might induce this type of response to avoid the host mechanisms of protection. The results support the idea that suppression is induced at least in part by TGF-β1, which either in a cell-surface-bound or a secreted form inhibits the immune response at a variety of levels, i.e., inhibits IL-2 production, IL-12-dependent cell activation, and Th1 development, among other responses (27, 46). The source of TGF-β1 was not demonstrated in this study; nevertheless, epithelial and stromal cells of the reproductive organs of the mouse and human, which are targets of gonococcus infection, express high levels of TGF-β1 and other molecules involved in conditioning immune privilege sites (6, 17, 22, 51). Because TGF-β1 also has a role in the induction of Tregs (54) and, moreover, because CD4+ CD25+ peripheral T cells can be converted to Foxp3+ Tregs by stimulation via the T-cell receptor in the presence of TGF-β1 (7, 12), we believe that the cytokine milieu found in the reproductive tract subsidizes the induction of Tregs by N. gonorrhoeae. Moreover, antigen-presenting cells, such as macrophages and dendritic cells, regularly present in the reproductive tissues (16, 44) might also contribute to Treg differentiation, as they may produce TGF-β1 after infection. Actually, we detected an increase of CD11b+ macrophages infiltrating the reproductive mucosae of infected mice, which is consistent with such a role. Moreover, a high number of CD11b− TGF-β1+ cells, which seem to be T cells by morphology criteria, also infiltrated the mucosal reproductive tissues during infection, suggesting that Tregs also play an important role at the mucosal level.
If extrapolated to humans, the results would indicate that gonococcus infection in women might also be related to a similar Treg induction, explaining in part the lack of protective immune response. In fact, there are various examples of evasion through pathogen-induced modulation of the immune response; one of these has been reported for the human filarial infection, where the parasite also induces an imbalance toward the Th2 response, which is at the same time accompanied by a diminished production of inflammatory factors and an increase of anti-inflammatory components, including Tregs (1). In human beings, N. gonorrhoeae can induce other highly efficient ways to overcome immune defense mechanisms, resulting in disease or chronic infection (14). Previous studies have focused attention on antigenic variation (49), epitope mimicry (18, 33), and phagosome subversion (3) as molecular mechanisms of immune evasion. Moreover, recent studies have explored a putative role of T cells as a determinant of successful gonococcus infection. As mentioned above, Opa proteins from gonococci have the ability to inhibit CD4+ T-cell proliferation, which will prevent adaptive immune response. What is more interesting is the gonococcus-dependent induction of IL-10 (36), a cytokine that it is also involved in the differentiation of Tregs (Tr1 type) (26, 41). A study of infected patients will shed light on the mechanism of pathogenesis and the presumed role of Tregs.
Altogether, the results showed that the infection of mice with N. gonorrhoeae induces a tolerant type of response which may correspond to a form of immune evasion that has not been previously studied for gonorrhea. Most studies describing these evasion mechanisms have been reported for parasite infection and chronic diseases caused by viruses (14, 32, 35, 42, 45). In those cases, immune regulation seems to favor the persistence of infection, which becomes evident when Tregs are depleted, and the disease is soon controlled by the immune system and the pathogen cleared. We are currently investigating the effect that the depletion of Tregs may have on the development of gonorrhea; moreover, studies of human subjects are currently in progress to investigate the role of these regulatory cells during infection in women.
Acknowledgments
This work was supported by grants 1020354 from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), 020743IB and 020540AC from the Dirección de Investigaciones Científicas y Tecnológicas (DICYT), and IPA06 from the Programa Bicentenario en Ciencia y Tecnología (PBCT).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 29 September 2008.
REFERENCES
- 1.Babu, S., C. P. Blauvelt, V. Kumaraswami, and T. B. Nutman. 2006. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 1763248-3256. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid, Y., R. B. Blank, and I. Suffia. 2006. Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol. Rev. 212287-300. [DOI] [PubMed] [Google Scholar]
- 3.Binker, M. G., L. I. Cosen-Binker, M. R. Terebiznik, G. V. Mallo, S. E. McCaw, E. L. Eskelinen, M. Willenborg, J. H. Brumell, P. Saftig, S. Grinstein, and S. D. Gray-Owen. 2007. Arrested maturation of Neisseria-containing phagosomes in the absence of the lysosome-associated membrane proteins, LAMP-1 and LAMP-2. Cell. Microbiol. 92153-2166. [DOI] [PubMed] [Google Scholar]
- 4.Boulton, I. C., and S. D. Gray-Owen. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3229-236. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, G. F., and C. J. Lammel. 1989. Humoral immune response to gonococcal infections. Clin. Microbiol. Rev. 2(Suppl.)S5-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chegini, N., Y. Zhao, R. S. Williams, and K. C. Flanders. 1994. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains [125I]TGF beta 1-binding sites. Endocrinology 135439-449. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., W. Jin, N. Hardegen, K. J. Lei, L. Li, N. Marinos, G. McGrady, and S. M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 1981875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christodoulides, M., J. S. Everson, B. L. Liu, P. R. Lambden, P. J. Watt, E. J. Thomas, and J. E. Heckels. 2000. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol. Microbiol. 3532-43. [DOI] [PubMed] [Google Scholar]
- 9.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 2000. Host cell invasion by pathogenic neisseriae. Subcell. Biochem. 3361-96. [DOI] [PubMed] [Google Scholar]
- 10.Draper, D. L., E. A. Donegan, J. F. James, R. L. Sweet, and G. F. Brooks. 1980. Scanning electron microscopy of attachment of Neisseria gonorrhoeae colony phenotypes to surfaces of human genital epithelia. Am. J. Obstet. Gynecol. 138818-826. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, J. L., and M. A. Apicella. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 17965-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantini, M. C., C. Becker, G. Monteleone, F. Pallone, P. R. Galle, and M. F. Neurath. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 1725149-5153. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez, R., P. Nelson, J. Delgado, J. Aguilera, R. Massai, L. Velasquez, M. Imarai, H. B. Croxatto, and H. Cardenas. 2001. Increased adhesiveness and internalization of Neisseria gonorrhoeae and changes in the expression of epithelial gonococcal receptors in the Fallopian tube of copper T and Norplant users. Hum. Reprod. 16463-468. [DOI] [PubMed] [Google Scholar]
- 14.Finlay, B. B., and G. McFadden. 2006. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124767-782. [DOI] [PubMed] [Google Scholar]
- 15.Finney, C. A., M. D. Taylor, M. S. Wilson, and R. M. Maizels. 2007. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. Eur. J. Immunol. 371874-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Givan, A. L., H. D. White, J. E. Stern, E. Colby, E. J. Gosselin, P. M. Guyre, and C. R. Wira. 1997. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am. J. Reprod. Immunol. 38350-359. [DOI] [PubMed] [Google Scholar]
- 17.Grant, K. S., and C. R. Wira. 2003. Effect of mouse uterine stromal cells on epithelial cell transepithelial resistance (TER) and TNFalpha and TGFbeta release in culture. Biol. Reprod. 691091-1098. [DOI] [PubMed] [Google Scholar]
- 18.Harvey, H. A., W. E. Swords, and M. A. Apicella. 2001. The mimicry of human glycolipids and glycosphingolipids by the lipooligosaccharides of pathogenic Neisseria and Haemophilus. J. Autoimmun. 16257-262. [DOI] [PubMed] [Google Scholar]
- 19.Hedges, S. R., M. S. Mayo, J. Mestecky, E. W. Hook III, and M. W. Russell. 1999. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect. Immun. 673937-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedges, S. R., D. A. Sibley, M. S. Mayo, E. W. Hook III, and M. W. Russell. 1998. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 178742-751. [DOI] [PubMed] [Google Scholar]
- 21.Hook, E. I., and H. H. Handsfield. 1999. Gonococcal infections in the adult, p. 451-466. In K. K. Holmes, P. F. Sparling, P. A. Mårdh, S. M. Lemon, W. A. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill, New York, NY.
- 22.Imarai, M., L. Varela-Nallar, C. Figueroa-Gaete, P. Gonzalez, D. Valdes, L. Velasquez, H. Cardenas, and K. Maisey. 2005. Fas ligand in the uterus of the non-pregnant mouse induces apoptosis of CD4+ T cells. J. Reprod. Immunol. 6613-32. [DOI] [PubMed] [Google Scholar]
- 23.Jerse, A. E. 1999. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect. Immun. 675699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushic, C., K. Grant, M. Crane, and C. R. Wira. 2000. Infection of polarized primary epithelial cells from rat uterus with Chlamydia trachomatis: cell-cell interaction and cytokine secretion. Am. J. Reprod. Immunol. 4473-79. [DOI] [PubMed] [Google Scholar]
- 25.Lee, H. S., M. A. Ostrowski, and S. D. Gray-Owen. 2008. CEACAM1 dynamics during Neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. J. Immunol. 1806827-6835. [DOI] [PubMed] [Google Scholar]
- 26.Levings, M. K., S. Gregori, E. Tresoldi, S. Cazzaniga, C. Bonini, and M. G. Roncarolo. 2005. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood 1051162-1169. [DOI] [PubMed] [Google Scholar]
- 27.Li, M. O., Y. Y. Wan, S. Sanjabi, A. K. Robertson, and R. A. Flavell. 2006. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2499-146. [DOI] [PubMed] [Google Scholar]
- 28.Lohr, J., B. Knoechel, and A. K. Abbas. 2006. Regulatory T cells in the periphery. Immunol. Rev. 212149-162. [DOI] [PubMed] [Google Scholar]
- 29.Maisey, K., G. Nardocci, M. Imarai, H. Cardenas, M. Rios, H. B. Croxatto, J. E. Heckels, M. Christodoulides, and L. A. Velasquez. 2003. Expression of proinflammatory cytokines and receptors by human fallopian tubes in organ culture following challenge with Neisseria gonorrhoeae. Infect. Immun. 71527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merz, A. J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16423-457. [DOI] [PubMed] [Google Scholar]
- 31.Miyara, M., and S. Sakaguchi. 2007. Natural regulatory T cells: mechanisms of suppression. Trends Mol. Med. 13108-116. [DOI] [PubMed] [Google Scholar]
- 32.Montagnoli, C., S. Bozza, R. Gaziano, T. Zelante, P. Bonifazi, S. Moretti, S. Bellocchio, L. Pitzurra, and L. Romani. 2006. Immunity and tolerance to Aspergillus fumigatus. Novartis Found. Symp. 27966-79, 216-219. [PubMed] [Google Scholar]
- 33.Moran, A. P., M. M. Prendergast, and B. J. Appelmelk. 1996. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol. Med. Microbiol. 16105-115. [DOI] [PubMed] [Google Scholar]
- 34.Parr, M. B., and E. L. Parr. 1990. Antigen recognition in the female reproductive tract. I. Uptake of intraluminal protein tracers in the mouse vagina. J. Reprod. Immunol. 17101-114. [DOI] [PubMed] [Google Scholar]
- 35.Peters, N., and D. Sacks. 2006. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunol. Rev. 213159-179. [DOI] [PubMed] [Google Scholar]
- 36.Plant, L. J., and A. B. Jonsson. 2006. Type IV pili of Neisseria gonorrhoeae influence the activation of human CD4+ T cells. Infect. Immun. 74442-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plante, M., A. Jerse, J. Hamel, F. Couture, C. R. Rioux, B. R. Brodeur, and D. Martin. 2000. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J. Infect. Dis. 182848-855. [DOI] [PubMed] [Google Scholar]
- 38.Plummer, F. A., H. Chubb, J. N. Simonsen, M. Bosire, L. Slaney, I. Maclean, J. O. Ndinya-Achola, P. Waiyaki, and R. C. Brunham. 1993. Antibody to Rmp (outer membrane protein 3) increases susceptibility to gonococcal infection. J. Clin. Investig. 91339-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plummer, F. A., H. Chubb, J. N. Simonsen, M. Bosire, L. Slaney, N. J. Nagelkerke, I. Maclean, J. O. Ndinya-Achola, P. Waiyaki, and R. C. Brunham. 1994. Antibodies to opacity proteins (Opa) correlate with a reduced risk of gonococcal salpingitis. J. Clin. Investig. 931748-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulendran, B. 2004. Modulating TH1/TH2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol. Res. 29187-196. [DOI] [PubMed] [Google Scholar]
- 41.Roncarolo, M. G., S. Gregori, M. Battaglia, R. Bacchetta, K. Fleischhauer, and M. K. Levings. 2006. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 21228-50. [DOI] [PubMed] [Google Scholar]
- 42.Rouse, B. T., P. P. Sarangi, and S. Suvas. 2006. Regulatory T cells in virus infections. Immunol. Rev. 212272-286. [DOI] [PubMed] [Google Scholar]
- 43.Spencer, S. E., I. E. Valentin-Bon, K. Whaley, and A. E. Jerse. 2004. Inhibition of Neisseria gonorrhoeae genital tract infection by leading-candidate topical microbicides in a mouse model. J. Infect. Dis. 189410-419. [DOI] [PubMed] [Google Scholar]
- 44.Stagg, A. J., M. Tuffrey, C. Woods, E. Wunderink, and S. C. Knight. 1998. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue. Infect. Immun. 663535-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suvas, S., and B. T. Rouse. 2006. Treg control of antimicrobial T cell responses. Curr. Opin. Immunol. 18344-348. [DOI] [PubMed] [Google Scholar]
- 46.Taylor, A., J. Verhagen, K. Blaser, M. Akdis, and C. A. Akdis. 2006. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology 117433-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor, M. D., A. Harris, S. A. Babayan, O. Bain, A. Culshaw, J. E. Allen, and R. M. Maizels. 2007. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J. Immunol. 1794626-4634. [DOI] [PubMed] [Google Scholar]
- 48.Tjia, K. F., J. P. van Putten, E. Pels, and H. C. Zanen. 1988. The interaction between Neisseria gonorrhoeae and the human cornea in organ culture. An electron microscopic study. Graefes Arch. Clin. Exp. Ophthalmol. 226341-345. [DOI] [PubMed] [Google Scholar]
- 49.van der Woude, M. W., and A. J. Baumler. 2004. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17581-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Boehmer, H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6338-344. [DOI] [PubMed] [Google Scholar]
- 51.Wada, K., S. Nomura, E. Morii, Y. Kitamura, Y. Nishizawa, A. Miyake, and N. Terada. 1996. Changes in levels of mRNAs of transforming growth factor (TGF)-beta1, -beta2, -beta3, TGF-beta type II receptor and sulfated glycoprotein-2 during apoptosis of mouse uterine epithelium. J. Steroid Biochem. Mol. Biol. 59367-375. [DOI] [PubMed] [Google Scholar]
- 52.Wan, Y. Y., and R. A. Flavell. 2006. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol. Rev. 212114-130. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, M. S., M. D. Taylor, A. Balic, C. A. Finney, J. R. Lamb, and R. M. Maizels. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 2021199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamagiwa, S., J. D. Gray, S. Hashimoto, and D. A. Horwitz. 2001. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 1667282-7289. [DOI] [PubMed] [Google Scholar]
- 55.Zheng, H. 1997. Analysis of the antigen-antibody specificity in the semen of patients with Neisseria gonorrhoeae. Chin. Med. Sci. J. 1247-49. [PubMed] [Google Scholar]