Abstract
The Bacillus subtilis membrane contains diacylglycerol-based lipids with at least five distinct headgroups that together help to define the physical and chemical properties of the lipid bilayer. Here, we describe the phenotypic characterization of mutant strains lacking one or more of the following lipids: glycolipids (ugtP mutants), phosphatidylethanolamine (pssA and psd mutants), lysylphosphatidylglycerol (mprF), and cardiolipin (ywnE and ywjE). Alterations of membrane lipid headgroup composition are generally well-tolerated by the cell, and even severe alterations lead to only modest effects on growth proficiency. Mutants with decreased levels of positively charged lipids display an increased sensitivity to cationic antimicrobial compounds, and cells lacking glycolipids are more sensitive to the peptide antibiotic sublancin and are defective in swarming motility. A quadruple mutant strain (ugtP pssA mprF ywnE), with a membrane comprised predominantly of phosphatidylglycerol, is viable and grows at near-wild-type rates, although it forms long, coiled filaments. Transcriptome comparisons identified numerous regulons with altered expression in cells of the ugtP mutant, the pssA mprF ywnE triple mutant, and the ugtP pssA mprF ywnE quadruple mutant. These effects included a general decrease in expression of the SigD and FapR regulons and increased expression of cell envelope stress responses mediated by σM and the YvrGHb two-component system.
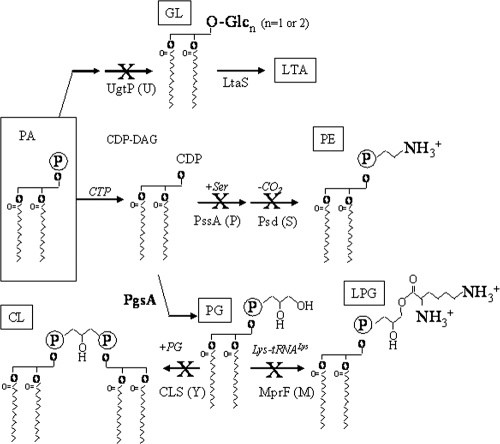
The cytoplasmic membrane forms an essential permeability barrier and is a defining feature of life. In most bacteria, the membrane is a lipid bilayer composed of fatty acid esters with sn-glycerol-3-phosphate (46). These complex lipids vary not only in the length and modifications of the acylated fatty acids but also in the composition of their headgroups, which differ significantly in charge and propensity to form nonbilayer structures. The membrane of the soil bacterium Bacillus subtilis is a complex structure comprised mainly of the anionic phospholipid phosphatidylglycerol (PG) and the zwitterionic phosphatidylethanolamine (PE). Other components include a relatively large amount (∼30%) of neutral glycolipids (GL), a variable amount of positively charged lysylphosphatidylglycerol (LPG), and a small amount of anionic cardiolipin (CL) (Fig. 1).
FIG. 1.
Pathways for membrane lipid synthesis in B. subtilis. Membrane lipid synthesis begins with the common precursor PA and leads to the generation of GL, PG, PE, CL, and LPG. Key enzymes and substrates are indicated, and steps blocked by mutation are denoted with an X. Note that in the absence of UgtP, LTA is still synthesized but the glycerol-phosphate copolymer is linked to the membrane by DAG rather than by diglucosyl-DAG.
The complex composition of the membrane is presumed to be important because the physical properties of membrane lipids influence many cell processes, including division (43), DNA replication (22), and protein transport (13). For example, the anionic lipids PG and CL mediate the cycling of the DNA replication protein DnaA from the ADP-DnaA to ATP-DnaA (active) state and inhibit the binding of DnaA to oriC via sequestration (7). Escherichia coli cells with reduced amounts of anionic phospholipids accumulate outer membrane proteins in the cytoplasm, and some proteins insert incorrectly into the membrane (60). Furthermore, CL and PE facilitate the formation of nonbilayer structures (13) that are important in cell division and sporulation (35). Recent evidence suggests that these various lipid species may assort into spatially distinct lipid microdomains (56), which in turn may affect the localization of membrane proteins (35). In B. subtilis CL-rich and PE-rich domains are localized to the septal regions and the poles (26, 41), corresponding with the FtsZ-dependent subcellular localization of the lipid biosynthesis enzymes PssA, YwnE, and PgsA (phosphatidylglycerophosphate synthase) (41). Moreover, recent results suggest that anionic lipids may assemble into a spiral structure along the long axis of the cell (3).
In B. subtilis, membrane lipids are synthesized from the common precursor phosphatidic acid (PA) (Fig. 1). In the case of PE, PA is converted to CDP-diacylglycerol (CDP-DAG), which is condensed with serine and then rapidly decarboxylated to generate PE. Condensation of CDP-DAG with glycerol-3-phosphate followed by removal of the phosphate leads to PG, the only essential complex lipid in B. subtilis. PG can be further modified to form two minor complex lipids, CL and LPG. CL is formed by the condensation of two PG molecules by cardiolipin synthase (CLS). B. subtilis contains two CLS enzymes: the major form (YwnE; also called ClsA [26]) is expressed during vegetative growth, while the minor (YwjE) is involved in sporulation (26). LPG is formed when MprF transfers a lysyl group from lysyl-tRNALys to PG. Finally, GL are created by dephosphorylation of PA to diacylglycerol, which is then modified by the transfer of one or two glucose molecules from UDP-glucose by UgtP (25). Fatty acid synthesis and desaturation are controlled by the FapR (52) and DesRK (14) regulatory systems, and both chain length and desaturation may be regulated by various stress conditions (31, 32, 38, 49). In contrast, little is known about how membrane headgroup composition is regulated, although the extracytoplasmic function σ factor σX has been shown to contribute to expression of PE biosynthesis genes (11).
Here, we report the characterization of a series of isogenic B. subtilis strains with altered membrane composition. Mutant strains were characterized for growth, antibiotic resistance, morphology, and alterations in global gene expression patterns. Our results suggest that the cell can tolerate even large changes in membrane composition. Remarkably, B. subtilis retains viability and even rapid growth when the membrane is comprised predominantly, if not exclusively, of PG.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All B. subtilis strains used were derivatives of either CU1065 (W168 trpC2 attSPβ) or the related strain NCIB3610 (Table 1). Escherichia coli strain DH5α was used for standard cloning procedures. Bacteria were grown in Luria-Bertani (LB) medium, LB supplemented with 25 mM MgSO4, or modified minimal medium (MM) (9) at 37°C with vigorous shaking. Antibiotics were added to the growth medium when appropriate: 100 μg/ml ampicillin for E. coli; 1 μg/ml erythromycin plus 25 μg/ml of lincomycin (macrolide-lincomycin-streptogramin B [MLS] resistance), 10 μg/ml chloramphenicol, 10 μg/ml kanamycin, 20 μg/ml tetracycline, and 100 μg/ml spectinomycin for B. subtilis. Sensitivity to nisin was measured by growing the different strains in LB and then adding the indicated concentration during early exponential growth (optical density at 600 nm [OD600], ∼0.25). Sensitivity to sublancin was tested via spot-on-lawn assay. Lawns were created by inoculating 100 μl of mid-exponential-phase cultures into 2 ml of 0.7% LB agar. This was poured into one well of an eight-well rectangular multidish (26 mm by 33 mm; Nunc). Once set, the plates were dried for 30 min in a laminar flow hood, and 5-μl aliquots of strains JH642 and HB6164 (JH642 sunA::kan [Table 1]), grown to an OD600 of 0.6, were spotted in the center of the well. Plates were incubated overnight at 37°C in an airtight container with moist paper towels to prevent drying.
TABLE 1.
Bacterial strains and primers used in this study
| Strain or primer | Genotype or sequencea | Source or reference |
|---|---|---|
| Bacterial strains | ||
| CU1065 | W168 trpC2 attSPβ | Lab strain |
| NCIB3610 | Wild-type isolate | BGSC 3A1 |
| JH642 | pheA1 trpC2 | BGSC 1A96 |
| HB6164 | JH642 sunA::kan | 10 |
| HB5337 | CU1065 mprF::kan | LFH-PCR → CU1065 |
| HB5343 | CU1065 psd::MLS | LFH-PCR → CU1065 |
| HB5346 | CU1065 ugtP::MLS | LFH-PCR → CU1065 |
| HB5347 | CU1065 ywnE::tet | LFH-PCR → CU1065 |
| HB5348 | CU1065 ywjE::kan | LFH-PCR → CU1065 |
| HB5361 | CU1065 pssA::spc | LFH-PCR → CU1065 |
| HB5362 | CU1065 ywnE::cat | LFH-PCR → CU1065 |
| HB5445 | CU1065 ywjE::cat | LFH-PCR → CU1065 |
| HB5344 | CU1065 psd::MLS mprF::kan | HB5337 chr DNA → HB5343 |
| HB5349 | CU1065 ywjE::kan ugtP::MLS | LFH-PCR → HB5348 |
| HB5350 | CU1065 mprF::kan ugtP::MLS | LFH-PCR → HB5337 |
| HB5387 | CU1065 ywnE::cat mprF::kan | LFH-PCR → HB5362 |
| HB5388 | CU1065 mprF::kan pssA::spc | LFH-PCR → HB5337 |
| HB5438 | CU1065 ugtP::MLS pssA::spc | LFH-PCR → HB5346 |
| HB5439 | CU1065 ugtP::MLS ywnE::cat | LFH-PCR → HB5346 |
| HB5440 | CU1065 pssA::spc ywnE::cat | LFH-PCR → HB5361 |
| HB5441 | CU1065 ywnE::cat ywjE::kan | HB5348 chr DNA → HB5362 |
| HB5442 | CU1065 ywnE::cat psd::MLS | HB5343 chr DNA → HB5362 |
| HB5443 | CU1065 pssA::spc ywjE::kan | HB5348 chr DNA → HB5361 |
| HB5389 | CU1065 ugtP::MLS mprF::kan pssA::spc | LFH-PCR → HB5350 |
| HB5390 | CU1065 ugtP::MLS mprF::kan ywnE::cat | LFH-PCR → HB5350 |
| HB5436 | CU1065 ugtP::MLS pssA::spc ywnE::cat | LFH-PCR → HB5438 |
| HB5437 | CU1065 mprF::kan pssA::spc ywnE::cat | LFH-PCR → HB5388 |
| HB5391 | CU1065 ugtP::MLS pssA::spc ywnE::cat mprF::kan | LFH-PCR → HB5436 |
| HB5481 | CU1065 ugtP::MLS pssA::spc mprF::kan ywnE::tet | LFH-PCR → HB5389 |
| HB5482 | CU1065 ugtP::MLS pssA::spc mprF::kan ywnE::tet ywjE::cat | HB55445 DNA → HB5481 |
| HB5406 | CU1065 pssA::spc-PxylA | LFH-PCR → CU1065 |
| HB5407 | CU1065 ybfM::spc-PxylA | LFH-PCR → CU1065 |
| HB5461 | NCIB3610 pssA::spc | Transduction → NCIB3610 |
| HB5462 | NCIB3610 ugtP::MLS | Transduction → NCIB3610 |
| HB5463 | NCIB3610 mprF::kan | Transduction → NCIB3610 |
| HB5464 | NCIB3610 ywnE::cat | Transduction → NCIB3610 |
| HB5465 | NCIB3610 ywjE::cat | Transduction → NCIB3610 |
| HB5466 | NCIB3610 ugtP::MLS pssA::spc | Transduction → HB5462 |
| HB5467 | NCIB3610 ugtP::MLS mprF::kan | Transduction → HB5462 |
| HB5468 | NCIB3610 ywnE::cat ugtP::MLS | Transduction → HB5464 |
| HB5469 | NCIB3610 ugtP::MLS ywjE::cat | Transduction → HB5462 |
| HB5470 | NCIB3610 pssA::spc mprF::kan | Transduction → HB5461 |
| HB5471 | NCIB3610 ywnE::cat pssA::spc | Transduction → HB5464 |
| HB5472 | NCIB3610 pssA::spc ywjE::cat | Transduction → HB5461 |
| HB5473 | NCIB3610 ywnE::cat mprF::kan | Transduction → HB5464 |
| HB5474 | NCIB3610 mprF::kan ywjE::cat | Transduction → HB5463 |
| HB5475 | NCIB3610 ugtP::MLS mprF::kan pssA::spc | Transduction → HB5467 |
| HB5476 | NCIB3610 ugtP::MLS pssA::spc ywnE::cat | Transduction → HB5466 |
| HB5477 | NCIB3610 ugtP::MLS mprF::kan ywnE::cat | Transduction → HB5467 |
| HB5478 | NCIB3610 mprF::kan pssA::spc ywnE::cat | Transduction → HB5470 |
| HB5479 | NCIB3610 ugtP::MLS mprF::kan pssA::spc ywnE::cat | Transduction → HB5475 |
| HB5480 | NCIB3610 ugtP::MLS mprF::kan ywnE::cat pssA::spc | Transduction → HB5476 |
| HB5423 | CU1065 amyE::PsigM-lacZ | pLS30 → CU1065 |
| HB5426 | CU1065 ugtP::MLS amyE::PsigM-lacZ | pLS30 → HB5346 |
| Oligonucleotide primer no., name | ||
| 1761, yfiW up fwd | tccaagcgcctgaatcagcc | |
| 1762, yfiW up rev (kan) | CCTATCACCTCAAATGGTTCGCTGtgatgaccatgagcgtacg | |
| 1763, yfiX do fwd (kan) | CGAGCGCCTACGAGGAATTTGTATCGccatgttcctcgttacacg | |
| 1764, yfiX do rev | gagccgtaaagtcatggaagg | |
| 1913, psd up fwd | ccgatccttctggcaatcgg | |
| 1914, psd up rev (MLS) | GAGGGTTGCCAGAGTTAAAGGATCgacagacgatgattcgtcagc | |
| 1915, psd do fwd (MLS) | CGATTATGTCTTTTGCGCAGTCGGCcaagtcggagaactgatagg | |
| 1916, psd do rev | gacatcttctgtgtgccatacagcg | |
| 2092, ugtP up fwd | gacgagctatactacgtgcc | |
| 2093, ugtP up rev (MLS) | GAGGGTTGCCAGAGTTAAAGGATCttggctacctgcacatgtcc | |
| 2094, ugtP do fwd (MLS) | CGATTATGTCTTTTGCGCAGTCGGCggaatcagaaatgatgaccg | |
| 2095, ugtP do rev | tgccgcttatccgtaaagcc | |
| 2100, ywnE up fwd | gctcttcctttatttgtcgtgcg | |
| 2101, ywnE up rev (tet) | GAGAACAACCTGCACCATTGCAAGAccaagatgcactggcatcgc | |
| 2102, ywnE do fwd (tet) | GGGATCAACTTTGGGAGAGAGTTCctatgaggagtatctgcagc | |
| 2103, ywnE do rev | tttgtacagtgacgcagacc | |
| 2367, ywnE up rev (cat) | CTTGATAATAAGGGTAACTATTGCCccaagatgcactggcatcgc | |
| 2368, ywnE do fwd (cat) | GGGTAACTAGCCTCGCCGGTCCACGctatgaggagtatctgcagc | |
| 2104, ywjE up fwd | agaaagcccatgctcatgcc | |
| 2105, ywjE up rev (kan) | CCTATCACCTCAAATGGTTCGCTGgatatcaagcagaatcaaggc | |
| 2106, ywjE do fwd (kan) | CGAGCGCCTACGAGGAATTTGTATCGtggaggatttcagcaagcg | |
| 2107, ywjE do rev | Agacaaacggaacatcctcc | |
| 3880, ywjE up rev (cat) | CTTGATAATAAGGGTAACTATTGCCgatatcaagcagaatcaaggc | |
| 3881, ywjE do fwd (cat) | GGGTAACTAGCCTCGCCGGTCCACGtggaggatttcagcaagcg | |
| 2369, pssA up fwd | cttgagacggcacatctggc | |
| 2370, pssA up rev (spc) | CGTTACGTTATTAGCGAGCCAGTCcctatcgtaatcatacaggg | |
| 2371, pssA do fwd (spc) | CAATAAACCCTTGCCCTCGCTACGcagaaaacctggagtctggg | |
| 2372, pssA do rev | gaaccacaccgtcaacaggg | |
| 2449, pssA up rev (spc xylA) | CAATAAACCCTTGCCCTCGCTACGcctatcgtaatcatacaggg | |
| 2450, pssA do fwd (spc xylA SD) | CCAACCAGATAAGTGCGAGCCAGTCAAGAGGATCTGCatggaattagttcagcagc | |
| 2451, ybfM up rev (spc xylA) | CAATAAACCCTTGCCCTCGCTACGcgtaatccgctatgagctgc | |
| 2866, PsigM fwd (H) | ccgtaagctttgggtactatatagtaatggtg | |
| 436, SigM-r3 | cgggatcccagtaagtcttcagcaagatg |
Uppercase letters indicate sequence complementary to antibiotic resistance genes. Underlined sequences indicate restriction sites.
Construction of null mutants.
Chromosomal deletions were created in the CU1065 background by using long-flanking homology PCR (LFH-PCR) as described previously (34) with the following changes: flanking fragments were amplified using Pfu DNA polymerase (Stratagene), and the flanking fragments and antibiotic resistance marker were joined using the Expand Long Template PCR system (Roche). The xylose-inducible mutants were created by LFH using a spectinomycin resistance gene fused with the PxylA region amplified from pTn7SX (8). A detailed protocol is available at http://www.micro.cornell.edu/faculty/helmann/supplemental%20index.htm.
Primer sequences used for mutant constructions are detailed in Table 1.
The null mutants in the NCIB3610 background were created by SPP1-mediated transduction from the CU1065 strains harboring the mutation of interest as previously described (28). Transductants were tested for their ability to grow on the appropriate antibiotic and in MM without the addition of tryptophan.
Lipid extraction and thin-layer chromatography.
Ten-ml aliquots of mid-exponential-phase cultures were centrifuged for 10 min at 4,500 × g, and lipids were extracted from the pellet via a modified Bligh-Dyer method (29). Briefly, the cell pellet was resuspended in 100 μl distilled deionized H2O with the addition of perchloric acid to a final concentration of 1 M. The cell suspension was then incubated at 0°C for 30 min, after which lipids were extracted by the addition of 1 ml methanol-chloroform-water (12:6:2 [vol/vol]) followed by incubation for 50 min on ice. Phase separation was achieved by the sequential addition of 0.3 ml water and 0.3 ml chloroform, after which suspensions were incubated overnight at −20°C and then centrifuged for 5 min at 720 × g at 4°C. The organic phase was then removed and dried under nitrogen. The lipids were resuspended in 20 μl of chloroform-methanol (2:1 [vol/vol]), spotted to silica gel 60 plates (VWR), and separated using the solvent mixture chloroform-methanol-water (65:25:4 [vol/vol]). Phospholipids were detected using molybdenum blue spray reagent (Sigma-Aldrich). PG and PE standards were obtained from Sigma-Aldrich. For two-dimensional thin-layer chromatography (TLC) assays, cells were grown in LB for 4 to 5 h and membrane lipids were labeled with 0.05 μCi of [2-14C]acetic acid per ml. Lipids were extracted and spotted to silica plates as described above. Lipids were first separated (x dimension) using the solvent mixture chloroform-methanol-water (65:25:4 [vol/vol]) and then (y dimension) using the solvent mixture chloroform-acetic acid-methanol-water (80:15:12:4 [vol/vol]). Spots for 14C-labeled lipids were visualized using a Storm 840 PhosphorImager scanner (Molecular Dynamics) after exposure of a PhosphorImager screen.
Microscopy.
Phase-contrast and fluorescence microscopy were performed using an Olympus BX61 epifluorescence microscope, with 40× UPlanFl (numerical aperture, 0.75) and 100× UPlanApo (numerical aperture, 1.35) objectives. The microscope was equipped with filter cubes for viewing fluorescein, 7-aminoactinomycin D/Mitotracker red, and 4′,6-diamidino-2-phenylindole/Hoechst fluorescence. Images were acquired using Cooke SensiCam and Slidebook software (Intelligent Imaging Inc.). Figures were assembled using Adobe Photoshop.
Swarming motility assay.
LB plates containing 0.7% agar were dried in a laminar flow hood for 30 min and then spotted in the center with 5 μl of mid-exponential-phase cultures grown in LB medium. The plates were then dried for another 15 min and incubated overnight at 37°C.
Construction and analysis of PsigM transcriptional fusion.
The DNA fragment of the sigM regulatory region was PCR amplified using primers 2866 and 436. The fragment was digested with HindIII and BamHI and cloned into the vector pDG1661, which contains a promoterless lacZ gene (21), resulting in plasmid pLS30. The sequences of the insert were verified by DNA sequencing (Cornell DNA sequencing facility). B. subtilis CU1065 and HB5346 (ugtP::MLS) were transformed to Cmr with the ScaI-linearized plasmid, which integrated into the amyE locus, creating strains HB5423 and HB5426, respectively. For quantitative measurements of β-galactosidase activity, strains HB5423 and HB5426 were grown in LB at 37°C with vigorous shaking, and samples were collected at different growth stages as determined by the OD600. To test promoter induction, strains HB5423 and HB5426 were grown in LB until the OD600 reached ∼0.3 and then the cultures were split into aliquots, which were either unchallenged or challenged with vancomycin (2 μg/ml) for 30 min. β-Galactosidase activity was measured according to the method of Miller (37), except that cells were lysed by the addition of lysozyme to a final concentration of 20 μg/ml followed by a 30-min incubation at 37°C.
RNA isolation and microarray analysis.
Strains CU1065 (wild type [WT]), HB5346 (ugtP, abbreviated as U), HB5437 (mprF pssA ywnE triple mutant, abbreviated as T), and HB5391 (ugtP mprF pssA ywnE quadruple mutant, abbreviated as Q) were inoculated into LB and grown at 37°C with vigorous shaking until an OD600 of ∼0.4, and RNA isolation was performed using the RNeasy mini kit (Qiagen). RNA was subsequently DNase treated with Turbo DNA-free (Ambion) and precipitated overnight. The RNA was dissolved in RNase-free water and quantified using a NanoDrop spectrophotometer (Nanodrop Tech. Inc., Wilmington, DE). RNA was isolated from three biological replicates.
cDNA synthesis was performed using the SuperScript Plus indirect cDNA labeling system (Invitrogen) as per the manufacturer's instructions with 20 μg of total RNA and then purified using the Qiagen MinElute kit (Qiagen, Maryland) and quantified with NanoDrop. Total cDNA was labeled overnight with Alexa Fluor 555 or Alexa Fluor 647 (Invitrogen) and then purified using the Qiagen MinElute kit (Qiagen, Maryland) and quantified with NanoDrop. Equal amounts (100 to 150 pmol) of labeled cDNA (WT/U, WT/T, and WT/Q) were combined to a final volume of 15 μl, and 1 μl salmon sperm DNA (10 mg/ml; Invitrogen) plus 16 μl 2× hybridization buffer (50% formamide, 10× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% sodium dodecyl sulfate [SDS]) were added. cDNA mix was denatured at 95°C and hybridized for 16 to 18 h at 42°C to DNA microarray slides which had been prehybridized for at least 30 min at 42°C in 1% bovine serum albumin, 5× SSC, 0.1% SDS, washed in water, and dried. Following hybridization the slides were washed sequentially in 2× SSC plus 0.1% SDS for 5 min at 42°C, 2× SSC plus 0.1% SDS for 5 min at room temperature, 2× SSC for 5 min at room temperature, and 0.2× SSC for 5 min at room temperature and finally dipped in water and spun until dry. Arrays were scanned using a GenePix 4000B array scanner (Axon Instruments, Inc.). Our arrays are based on a B. subtilis oligonucleotide library manufactured by Sigma-Genosys consisting of 4,128 oligonucleotides (65-mers) representing 4,106 B. subtilis genes, 10 control oligonucleotides (from E. coli and Brome mosaic virus), and 12 random oligonucleotides. A single oligonucleotide was designed to represent each of the B. subtilis genes as annotated in the genome data, release R16.1 (26 April 2001), at the SubtiList website (http://genolist.pasteur.fr/SubtiList/). The arrays were printed onto poly-l-lysine-coated Corning CMT-Gap slides at the W.M. Keck Foundation Biotechnology Resource Laboratory, Yale University. Each array contains 8,447 features corresponding to duplicates of each open reading frame-specific oligonucleotide, additional oligonucleotides of control genes, and 50% dimethyl sulfoxide blank controls.
Raw data files were produced from the scanned images using the GenePix Pro 4.0 software package (GPR files), and the red/green fluorescence intensity values were normalized such that the ratio of medians of all features was equal to 1. The normalized data were exported to Excel for analysis. The data sets were filtered to remove those genes that were not expressed at levels significantly above background under either condition (sum of mean fluorescence intensity, <30). For analysis, we filtered to identify those genes that were altered at least 1.5-fold in signal intensity in at least two of the three biological replicates (and excluding those genes with an opposing change in the third replicate).
FAME analysis.
Two 500-ml biological replicas of mid-exponential-phase cultures of either the WT, ugtP::MLS single mutant, mprF::kan pssA::spc ywnE::cat triple mutant, or ugtP::MLS mprF::kan pssA::spc ywnE::cat quadruple mutant grown in LB were combined, and the frozen pellets were submitted for fatty acid methyl ester (FAME) analysis at Microbial ID, Newark, DE (http://www.microbialid.com/). Results of this analysis are summarized in Table S1 of the supplemental material.
Microarray data accession numbers.
The microarray data sets and related files are available at NCBI GEO under accession numbers GSE13036.
RESULTS AND DISCUSSION
The Bacillus subtilis cytoplasmic membrane can be dramatically simplified.
Bacterial cytoplasmic membranes display a great variety of lipid compositions. PE is a dominant inner membrane component in E. coli (75% of total [13]), whereas Staphylococcus aureus membranes are predominantly formed from PG (∼90% of total [40]). In Mycobacterium tuberculosis, membranes are dominated by phosphatidylinositol mannosides (∼70% [51]). In many other cases, no single lipid type dominates, and in all systems there are at least three or more different types of lipid headgroups represented. These observations are consistent with the hypothesis that membrane function requires lipid components with different physico-chemical properties.
Here, we set out to characterize a set of strains with altered membrane compositions by using B. subtilis as a model system. We inactivated genes involved in the biosynthesis of complex lipids, including genes necessary for the synthesis of PE (pssA; P), GL (ugtP; U), LPG (mprF; M), and CL (ywnE; Y), by allelic replacement with antibiotic resistance cassettes (Table 1). We also constructed mutants of psd (S) and ywjE (J). The psd mutant accumulates phosphatidylserine but is unable to synthesize PE. The ywjE mutant is missing a minor CLS thought to be present during sporulation (26). Allelic replacements were verified by PCR, and the absence of PE and LPG was verified by TLC (Fig. 2 and data not shown, but see also Fig. S1 in the supplemental material). By additional rounds of transformation we created a suite of mutants comprised of various combinations of these mutations with increasingly simplified membrane compositions. Surprisingly, we were able to create a viable quadruple mutant strain (Q; ugtP pssA mprF ywnE) with membranes containing predominantly, if not exclusively, PG (Table 1). The only other DAG-based lipids that might have been present were PA and DAG itself. Previously, DAG was reported to be present in low levels in B. subtilis membranes (5). DAG is formed as a precursor to GL and is also generated as an intermediate during the synthesis of lipoteichoic acids (24).
FIG. 2.
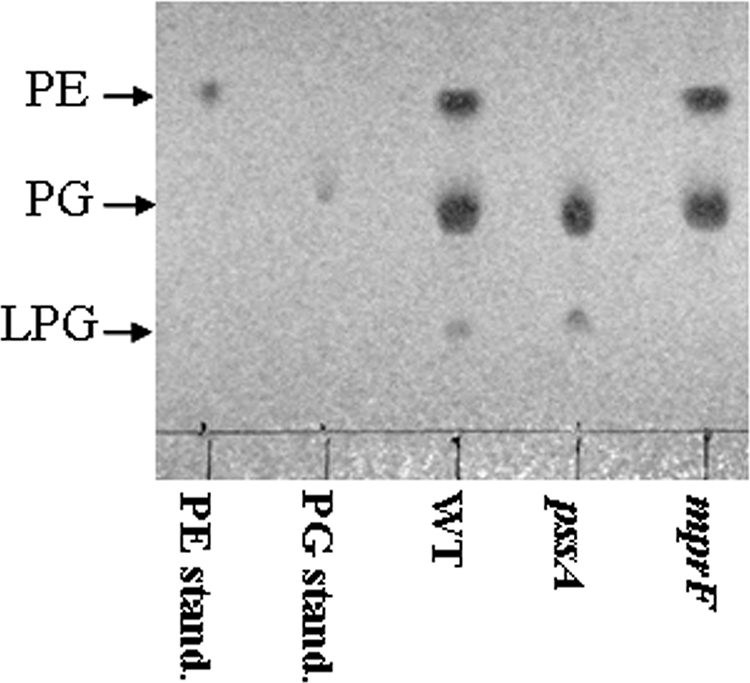
TLC analysis of membrane mutants. Membrane lipids were extracted from exponential cultures of WT or membrane mutants grown in LB using a modified Bligh-Dyer method (6). Extracted lipids were spotted to silica TLC plates, detected with molybdenum blue (Sigma), and compared to standards as indicated.
We hypothesized that alterations to membrane headgroup composition might be compensated, to some extent, by alterations in fatty acid composition. To test this idea, the fatty acid compositions of the wild-type, ugtP single, MPY triple, and the UPMY quadruple mutant strains were determined by FAME analysis (see Table S1 in the supplemental material). While overall composition was quite similar in these strains, the results were consistent with a slight decrease in C15 (15:0 iso) and a corresponding increase in C17 lipids in the ugtP and Q mutant strains (but not the MPY triple mutant). Thus, perturbation of membrane headgroup composition, and in particular a lack of GL, may be compensated in part by alterations of fatty acid synthesis and/or desaturation.
We monitored growth rates for each of our strains by using a BioScreen automated growth curve (optical density) analysis system. All mutant strains were able to grow in a variety of liquid and solid media, including LB, LB plus 2% glucose, Difco sporulation medium (DSM), and minimal competence medium, as well as a defined MM. However, some of the strains had increased doubling and/or lag times. In LB medium, most of the single mutants had doubling times comparable to wild type, with the exception of the mutant strain lacking the neutral lipid GL (ugtP mutant; U) (Table 2). Surprisingly, the quadruple mutant (Q; UPMY) exhibited a doubling time only slightly longer than the wild type in LB, but it was significantly impaired in growth rate in MM (Table 2). In addition, the lag time of the Q strain, as well as all strains lacking ugtP, was considerably longer than that of the WT (data not shown). It is important to note, however, that UgtP plays several roles in the cell. In addition to the synthesis of GL, UgtP is involved in generating anchor lipids for lipoteichoic acids (25) and functions as a sensor to couple cell division to the availability of nutrients (61).
TABLE 2.
Effects of membrane headgroup alteration on cell growth
| Straina | Membrane lipidb (charge)
|
Doubling time (min)c in:
|
|||||
|---|---|---|---|---|---|---|---|
| PG (−1) | CL (−2) | PE (0) | GL (0) | LPG (+1) | LB | MM | |
| WT | + | + | + | + | + | 34 ± 1 | 48 ± 3 |
| P | + | + | − | + | + | 37 ± 2 | 49 ± 3 |
| U | + | + | + | − | + | 50 ± 3 | 51 ± 4 |
| M | + | + | + | + | − | 38 ± 3 | 45 ± 2 |
| Y | + | − | + | + | + | 36 ± 4 | 50 ± 1 |
| S | + | + | − | + | + | 37 ± 2 | 52 ± 2 |
| J | + | +/− | + | + | + | 38 ± 0 | 49 ± 2 |
| UP | + | + | − | − | + | 37 ± 5 | 50 ± 3 |
| UM | + | + | + | − | − | 44 ± 7 | 62 ± 8 |
| UY | + | − | + | − | + | 47 ± 8 | 51 ± 2 |
| UJ | + | +/− | + | − | + | 40 ± 2 | 51 ± 2 |
| PM | + | + | − | + | − | 40 ± 4 | 44 ± 5 |
| PY | + | − | − | + | + | 41 ± 5 | 50 ± 2 |
| PJ | + | +/− | − | + | + | 39 ± 1 | 55 ± 2 |
| MY | + | − | + | + | − | 40 ± 4 | 50 ± 3 |
| MS | + | + | − | + | − | 38 ± 2 | 47 ± 2 |
| YJ | + | − | + | + | + | 41 ± 1 | 49 ± 2 |
| YS | + | − | − | + | + | 39 ± 3 | 52 ± 4 |
| UPM | + | + | − | − | − | 37 ± 2 | 55 ± 6 |
| UPY | + | − | − | − | + | 53 ± 9 | 50 ± 4 |
| UMY | + | − | + | − | − | 42 ± 9 | 49 ± 5 |
| MPY (T) | + | − | − | + | − | 37 ± 3 | 46 ± 7 |
| UPMY (Q) | + | − | − | − | − | 40 ± 7 | 74 ± 9 |
P, pssA::spc; U, ugtP::MLS; M, mprF::kan; Y, ywnE::cat; S, psd::MLS; J, ywjE::kan; T, mprF::kan pssA::spc ywnE::cat triple mutant; Q, ugtP::MLS mprF::kan pssA::spc ywnE::cat quadruple mutant.
+, lipid is present in the membrane; −, lipid is absent; +/−, CL may be partially lacking, at least under some growth conditions, due to loss of the minor CLS encoded by ywjE.
Doubling time calculated for maximum specific growth rate.
In minimal medium, in which cells grow considerably slower, the only strain to demonstrate a significantly increased doubling time was the UPMY quadruple mutant (74 min versus 48 min for WT). Growth rates in DSM were similar to those in LB (data not shown). All strains were capable of sporulating: phase-bright spores were observed after growth in DSM for 24 h, and all strains formed pigmented colonies when streaked on DSM plates.
The viability and robust growth of strains with greatly simplified membrane composition are surprising in light of previous studies of E. coli mutants. For example, an E. coli pssA null mutant had severe growth defects and requires divalent metal ion supplementation for growth (15), and a mutant lacking both PE and CL was nonviable (42). These defects are thought to result from a requirement for the formation of nonbilayer structures within the membrane (facilitated by PE) as well as the proper localization of membrane proteins (2, 36). These differences may result, in part, from the fact that B. subtilis only contains a single membrane, whereas E. coli requires both an inner and outer membrane.
mprF and ugtP mutants exhibit increased sensitivity to cationic antimicrobial peptides.
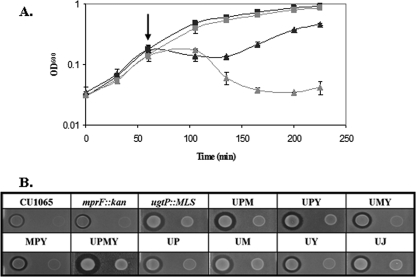
Modification of the membrane has been proposed as a strategy for protection against cationic antimicrobial peptides (57). We tested our collection of strains for their sensitivity to a variety of cell envelope-active compounds, including vancomycin, nisin, sublancin, and duramycin. In the presence of the glycopeptide antibiotic vancomycin, most strains grew to an OD600 comparable to that of WT. However, all ugtP mutant strains were vancomycin sensitive (data not shown). Conversely, in the presence of the cationic lantibiotic nisin, mprF mutants were sensitive (Fig. 3A). LPG can constitute up to 20% of the B. subtilis membrane (16) and has been shown to impart resistance to cationic antimicrobials, including nisin, vancomycin, and human defensins, in S. aureus (50, 57).
FIG. 3.
Variations of cytoplasmic membrane composition result in altered antibiotic sensitivity. A. Deletion of mprF leads to increased sensitivity to nisin. WT (black symbols) and mprF::kan cells (gray symbols) were grown in LB to early log phase and then either left untreated (squares) or treated with 10 μg/ml nisin (triangles). The arrow denotes the time at which nisin was added. B. Strains lacking ugtP demonstrated increased sensitivity to sublancin. Lawns of various strains spotted with the sublancin-producing B. subtilis JH642 (left spot) or a JH642 strain containing a sunA deletion are shown.
Next we tested sensitivity to the bacteriocin sublancin produced by B. subtilis strains harboring the SPβ prophage. Our mutant strains were constructed in B. subtilis CU1065, which lacks the SPβ prophage and is therefore sublancin sensitive. To test sublancin sensitivity, lawns were spotted with B. subtilis JH642 (a sublancin producer) or an isogenic strain unable to produce sublancin (sunA::kan mutant). Among the singly mutant strains, an increased zone of inhibition was only observed for the ugtP mutant (Fig. 3B). Similarly, the Q mutant (UPMY) was significantly more sensitive to sublancin than the MPY triple mutant. These results suggest that the presence of GL in the membrane provides some protection against sublancin.
Duramycin is a lantibiotic produced by Streptoverticillium cinnamoneum. Previous studies have reported that strains selected for increased resistance to duramycin demonstrate decreased levels of PE and increased levels of CL (17), and these studies have predicted a mode of action in which duramycin binds to PE and then inserts into the membrane, creating pores (23). Consistent with this expectation, pssA and psd mutant strains exhibited greatly increased resistance to duramycin relative to the WT strain (data not shown), consistent with previous results (8). No other single mutation conferred this resistance, and double and triple mutants lacking either pssA or psd showed similar resistance levels to that of the single mutants. Unexpectedly, the UPMY quadruple mutant was more sensitive to duramycin than other PE-lacking mutants, but still more resistant than the WT. This suggests that the requirement for PE in the biological action of duramycin may be bypassed in strains with grossly perturbed membrane structure.
In B. subtilis, the PE biosynthetic genes (pssA and psd) are part of a tricistronic operon, pssA ybfM psd. In order to study the possible role of ybfM in PE biosynthesis and duramycin sensitivity, we constructed a strain in which ybfM was replaced by a spectinomycin resistance cassette and psd was placed under the control of a xylose-inducible promoter (ybfM::spc-PxylA). In the presence of xylose, strain ybfM::spc-PxylA was as sensitive to duramycin as WT, whereas in the presence of glucose it was as resistant as a pssA null mutant (data not shown). This suggests that ybfM is not required for the synthesis or localization of PE. In other organisms containing homologs of these proteins, the tricistronic operon structure is not conserved, and often pssA and psd are even situated in separate operons.
Cells containing simplified cytoplasmic membranes exhibit aberrant morphologies.
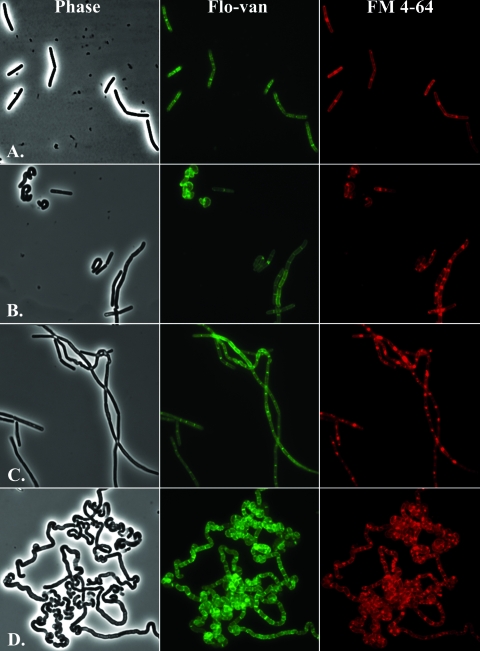
Microscopic observation indicates that mutants with an altered membrane composition have distinctive changes in cell morphology. During mid-exponential growth, the ugtP mutant was the only single mutant to demonstrate a change in cell shape: cells were shorter and tended to curl at the ends (Fig. 4B). Double mutants lacking ugtP appeared mostly as curling short chains of cells or clumps of curled cells, while triple mutants demonstrated lengthened filaments of curled cells (data not shown). The most dramatic effect was observed in the quadruple mutant, in which the filamentation and curling was even more pronounced (Fig. 4D). Double mutants that still contain ugtP resembled WT cells, with a small amount of cells in longer chains (data not shown), while the MPY triple mutant did not curl but was highly filamentous (Fig. 4C). At later growth stages in LB the filamentation phenotype is lost and cells appear as either single cells or short chains (data not shown). When the MPY and UPMY mutants were grown in LB supplemented with Mg2+, which is known to stabilize the effects of PE loss in E. coli (13), the filamentation was reduced. Staining of these filamentous cells with the membrane stain FM 4-64 and fluorescently labeled vancomycin (which targets un-cross-linked peptidoglycan-containing pentapeptide side chains) clearly demonstrated that these are septated filaments (Fig. 4C and D). Fluorescently labeled vancomycin stains the septal regions and also a characteristic helical pattern along the axis of the cell, as also seen for WT cells (59) (Fig. 4 and data not shown), suggesting that peptidoglycan synthesis and incorporation remains mostly unimpaired, even in the quadruple mutant. Staining with FM 4-64 showed a similar accumulation at the septal region, with no obvious aberrant membrane formations, such as involutions or blebs.
FIG. 4.
Alteration of the cytoplasmic membrane leads to aberrant morphology. Exponential-phase cultures grown in LB were treated with both the membrane dye FM 4-64 and fluorescently labeled vancomycin. (A) Wild-type; (B) ugtP::MLS single mutant; (C) mprF::kan pssA::spc ywnE::cat triple mutant; (D) ugtP::MLS mprF::kan pssA::spc ywnE::cat quadruple mutant.
In general, the most dramatic morphological changes were observed in those cells lacking GL. ugtP codes for a multifunctional UDP-glucose:diacylglycerol glucosyltransferase that is required for (i) formation of GL (25, 48), (ii) synthesis of diglucosyldiacylglycerol, the membrane anchor for lipotechoic acids (25, 30), and (iii) coupling nutrient availability to cell division in B. subtilis (61). When nutrients are available, UgtP is expressed at high levels, evenly distributed in the cytoplasm, and concentrated at the septum, colocalizing and interacting with FtsZ (61). UgtP is proposed to inhibit the assembly of FtsZ rings by sequestering the FtsZ subunits, resulting in larger cells at the time of division (55, 61). Upon carbon depletion, localization of UgtP to the septum is abolished resulting in smaller cells (61). This dual role of UgtP in envelope biosynthesis and regulation of cell size could explain the severity of the defects seen in ugtP null mutants.
Cells containing simplified cytoplasmic membranes are altered for swarming motility.
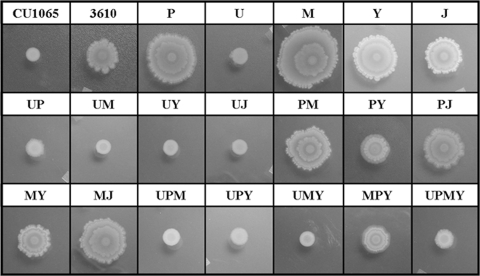
In order to study the effects of alteration of cytoplasmic membrane composition on motility and biofilm formation, we transferred our mutant collection into an NCIB3610 background which, unlike most B. subtilis 168 laboratory strains, exhibits a robust swarming phenotype (28). In this background, the ugtP mutant strains exhibited decreased swarming while, curiously, some of the single mutants displayed enhanced swarming (Fig. 5). This enhanced swarming was reduced only in the PY and MY double mutants, as well as in the MPY triple mutant. This does not seem to be due to a specific lack of CL, since the Y single mutant did not show a similar decrease. Surprisingly, all strains tested were able to form characteristic pellicles on the air-liquid interface of MSgg medium (8a). However, the biofilms of strains lacking ugtP were somewhat flatter and more matte, while that of the MPY triple mutant resembled the WT (data not shown).
FIG. 5.
Deletion of ugtP results in a swarming defect. Membrane mutants in the NCIB3610 background were grown to mid-exponential phase in LB and then spotted to 0.7% LB agar plates. Plates were incubated overnight.
Transcriptome comparisons.
We conducted DNA microarray experiments to compare the transcriptional profiles of the ugtP single mutant, MPY triple mutant, and UPMY quadruple mutant to that of WT cells in LB medium at mid-log stage. These mutations are highly pleiotropic, and we identified a large number of genes that were reproducibly altered in expression levels relative to WT (detailed in Tables S2 to S4 in the supplemental material). These included genes involved in carbon and protein metabolism, cell wall and membrane biosynthesis, DNA metabolism and repair, motility and chemotaxis, and production of secondary metabolites, as well as a large number of uncharacterized membrane proteins. In addition, the expression levels of many known or putative ABC transporter and other transport permeases were affected, including multidrug efflux pumps and antibiotic resistance proteins. Alterations of membrane composition also affected the levels of expression of numerous known and predicted transcriptional regulators. In general, these changes were most dramatic for the ugtP mutant and the UPMY quadruple mutant, with generally more modest changes noted in the MPY triple mutant. These results suggest that cell envelope perturbations caused by the absence of UgtP are potentially the most deleterious to the cell and result in a shift in the transcriptional profile.
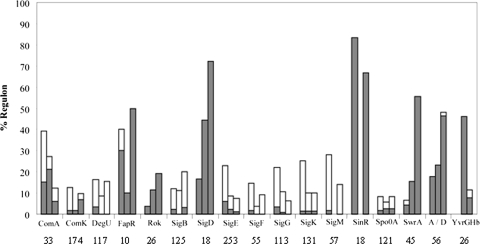
To determine the likely origins of these large shifts in transcriptional patterns, we have linked many of the genes affected (either positively or negatively) by membrane alterations to known regulons. As summarized in Fig. 6, large fractions of several known regulons were altered in their expression in one or more of the tested mutant backgrounds. For example, genes regulated by σD (involved in motility, chemotaxis, and autolysin synthesis [33]) were expressed at lower levels in all three mutant backgrounds. In contrast, numerous members of the large DegU and σB (general stress response) regulons were elevated in expression in these strains. There was also a general increase in the expression of genes under the control of the sporulation-specific sigma factors σE, σF, σG, and σK in all backgrounds (Fig. 6). This suggests that sporulation may have initiated earlier and asynchronously within this growing population, whereas sporulation genes are generally not expressed during logarithmic growth in the WT.
FIG. 6.
Analysis of regulons, demonstrating differential expression patterns. Microarray experiments compared WT with either ugtP, MPY, or UPMY mutant strains. RNA was extracted from mid-log-phase cells grown in LB. Results represent averages of three separate experiments. Each three bars correspond to a specific regulon; the order of experiments is WT versus ugtP, WT versus MPY, and WT versus UPMY. The height of a bar represents the overall percentage of the regulon with altered expression. Gray sections represent the percentage of the regulon that was downregulated at least 1.5-fold, and white sections represent the percentage of the regulon that was upregulated at least 1.5-fold The number of genes in each regulon is beneath the regulon name (adapted from references 1, 4, 12, 18-20, 27, 39, 45, 47, 52 to 54, and 58).
In several cases, the role of UgtP appeared to be most important, since the transcriptome changes were most dramatic for the U and UPMY mutations with relatively little effect noted in the MPY triple mutant. For example, genes involved in biosynthesis of fatty acids and under the negative control of FapR were strongly downregulated in both the U and UPMY backgrounds but only slightly altered in the MPY triple mutant. Genes negatively regulated by SinR, involved in the production of exopolysaccharides and biofilm formation (12), were also downregulated only in a U and UPMY background. In addition, the σM cell envelope stress regulon (18) was induced only in the U and UPMY backgrounds. This induction was verified by β-galactosidase assays, which showed that in a ugtP null background the expression level of the PsigM promoter was higher throughout all growth phases (Fig. 7A), although it could still be additionally induced by treatment with vancomycin (Fig. 7B). Interestingly, the expression of the regulons of extracytoplasmic function sigma factors σW (which provides intrinsic resistance to antimicrobial compounds [10]) and σX (which regulates modification of the cell envelope and resistance to cationic antimicrobial peptides [11]) was unaltered. A contrasting example is provided by analysis of genes assigned to the YvrGHb TCS regulon. YvrGHb is reported to regulate cell surface homeostatic functions (53). This regulon was expressed at a reduced level in the MPY triple mutant but affected to a lesser extent in the quadruple mutant.
FIG. 7.
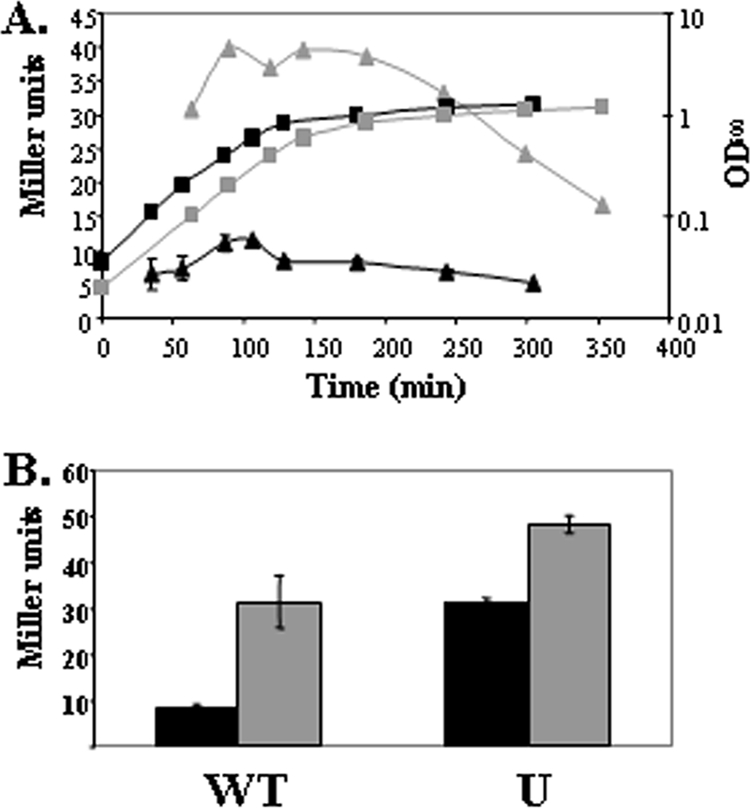
Activity of the σM promoter is increased in a ugtP::MLS background. A. WT (black symbols) and ugtP::MLS (gray symbols) cells containing a PsigM-lacZ promoter fusion were grown in LB. Samples were taken periodically, and the OD600 (squares) and LacZ activity (triangles) were monitored. B. PsigM can still be induced in a ugtP::MLS background. Mid-log-phase cells were either untreated (black) or treated with 2 μg/ml vancomycin for 30 min.
Among the most highly induced genes in the UPMY and MPY mutant backgrounds were the yrkNM operon, encoding a GCN5-related N-acetyltransferase and an unknown protein, respectively, and yrkO, which encodes a putative transporter. yrkN and yrkO are thought to be positively regulated by the divergently encoded response regulator YrkP, which was suggested to regulate membrane function (44). However, the expression of the ykcBC operon, also thought to be regulated by YrkP (44), remained unchanged in all three backgrounds tested.
Concluding remarks.
We report here the construction of a large set of isogenic strains with alterations in membrane lipid composition. Perhaps the most surprising finding from this work is that the membrane of B. subtilis can be greatly simplified by removal of GL, PE, LPG, and CL, and the cells grow robustly. These cells are morphologically abnormal and exhibit a combination of cell separation and cell shape defects: they grow as long and curled filaments. In this initial survey, we have highlighted the effects of grossly perturbing membrane composition on antibiotic sensitivity, swarming motility, and global transcription patterns. In many of our phenotypic assays, the loss of ugtP appears to have a dominant effect, with changes in the ugtP single mutant often more dramatic than in the MPY triple mutant. Additional studies are clearly required to further understand these complex phenotypes and to determine if the ugtP phenotypes are due to the loss of GL or in addition or instead to the important role of this protein as a metabolic sensor.
Supplementary Material
Acknowledgments
We thank Esther Angert, Jenna Mendell, and Rebekah Ward for assistance with fluorescence microscopy and William Ghiorse and Andreas Toba for assistance with electron microscopy. We further thank Bronwyn Butcher and Ahmed Gaballa for invaluable advice and discussion and Sheila Habib for initial experiments and construction of pLS30.
This work was supported by a grant from the National Institutes of Health (GM-047446).
Footnotes
Published ahead of print on 26 September 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Albano, M., W. K. Smits, L. T. Ho, B. Kraigher, I. Mandic-Mulec, O. P. Kuipers, and D. Dubnau. 2005. The Rok protein of Bacillus subtilis represses genes for cell surface and extracellular functions. J. Bacteriol. 1872010-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badyakina, A. O., Y. A. Koryakina, N. E. Suzina, and M. A. Nesmeyanova. 2003. Membrane phospholipid composition of Escherichia coli affects secretion of periplasmic alkaline phosphatase into the medium. Biochemistry (Moscow) 68752-759. [DOI] [PubMed] [Google Scholar]
- 3.Barak, I., K. Muchova, A. J. Wilkinson, P. J. O'Toole, and N. Pavlendova. 2008. Lipid spirals in Bacillus subtilis and their role in cell division. Mol. Microbiol. 681315-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berka, R. M., J. Hahn, M. Albano, I. Draskovic, M. Persuh, X. Cui, A. Sloma, W. Widner, and D. Dubnau. 2002. Microarray analysis of the Bacillus subtilis K-state: genome-wide expression changes dependent on ComK. Mol. Microbiol. 431331-1345. [DOI] [PubMed] [Google Scholar]
- 5.Bishop, D. G., L. Rutberg, and B. Samuelsson. 1967. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur. J. Biochem. 2448-453. [DOI] [PubMed] [Google Scholar]
- 6.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37911-917. [DOI] [PubMed] [Google Scholar]
- 7.Boeneman, K., and E. Crooke. 2005. Chromosomal replication and the cell membrane. Curr. Opin. Microbiol. 8143-148. [DOI] [PubMed] [Google Scholar]
- 8.Bordi, C., B. G. Butcher, Q. Shi, A. B. Hachmann, J. E. Peters, and J. D. Helmann. 2008. In vitro mutagenesis of Bacillus subtilis by using a modified Tn7 transposon with an outward-facing inducible promoter. Appl. Environ. Microbiol. 743419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Branda, S. S., J. E. Gonzalez-Pastor, S. Ben-Yehuda, R. Losick, and R. Kolter. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA 9811621-11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bsat, N., L. Chen, and J. D. Helmann. 1996. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J. Bacteriol. 1786579-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butcher, B. G., and J. D. Helmann. 2006. Identification of Bacillus subtilis sigma-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by bacilli. Mol. Microbiol. 60765-782. [DOI] [PubMed] [Google Scholar]
- 11.Cao, M., and J. D. Helmann. 2004. The Bacillus subtilis extracytoplasmic-function σX factor regulates modification of the cell envelope and resistance to cationic antimicrobial peptides. J. Bacteriol. 1861136-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu, F., D. B. Kearns, S. S. Branda, R. Kolter, and R. Losick. 2006. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 591216-1228. [DOI] [PubMed] [Google Scholar]
- 13.Cronan, J. E. 2003. Bacterial membrane lipids: where do we stand? Annu. Rev. Microbiol. 57203-224. [DOI] [PubMed] [Google Scholar]
- 14.Cybulski, L. E., G. del Solar, P. O. Craig, M. Espinosa, and D. de Mendoza. 2004. Bacillus subtilis DesR functions as a phosphorylation-activated switch to control membrane lipid fluidity. J. Biol. Chem. 27939340-39347. [DOI] [PubMed] [Google Scholar]
- 15.DeChavigny, A., P. N. Heacock, and W. Dowhan. 1991. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J. Biol. Chem. 2665323-5332. [PubMed] [Google Scholar]
- 16.den Kamp, J. A., I. Redai, and L. L. van Deenen. 1969. Phospholipid composition of Bacillus subtilis. J. Bacteriol. 99298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunkley, E. A., Jr., S. Clejan, A. A. Guffanti, and T. A. Krulwich. 1988. Large decreases in membrane phosphatidylethanolamine and diphosphatidylglycerol upon mutation to duramycin resistance do not change the protonophore resistance of Bacillus subtilis. Biochim. Biophys. Acta 94313-18. [DOI] [PubMed] [Google Scholar]
- 18.Eiamphungporn, W., and J. D. Helmann. 2008. The Bacillus subtilis σM regulon and its contribution to cell envelope stress responses. Mol. Microbiol. 67830-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichenberger, P., S. T. Jensen, E. M. Conlon, C. van Ooij, J. Silvaggi, J. E. Gonzalez-Pastor, M. Fujita, S. Ben-Yehuda, P. Stragier, J. S. Liu, and R. Losick. 2003. The σE regulon and the identification of additional sporulation genes in Bacillus subtilis. J. Mol. Biol. 327945-972. [DOI] [PubMed] [Google Scholar]
- 20.Feucht, A., L. Evans, and J. Errington. 2003. Identification of sporulation genes by genome-wide analysis of the σE regulon of Bacillus subtilis. Microbiology 1493023-3034. [DOI] [PubMed] [Google Scholar]
- 21.Guerout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 18057-61. [DOI] [PubMed] [Google Scholar]
- 22.Ichihashi, N., K. Kurokawa, M. Matsuo, C. Kaito, and K. Sekimizu. 2003. Inhibitory effects of basic or neutral phospholipid on acidic phospholipid-mediated dissociation of adenine nucleotide bound to DnaA protein, the initiator of chromosomal DNA replication. J. Biol. Chem. 27828778-28786. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto, K., T. Hayakawa, M. Murate, A. Makino, K. Ito, T. Fujisawa, and T. Kobayashi. 2007. Curvature-dependent recognition of ethanolamine phospholipids by duramycin and cinnamycin. Biophys. J. 931608-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerga, A., Y. J. Lu, G. E. Schujman, D. de Mendoza, and C. O. Rock. 2007. Identification of a soluble diacylglycerol kinase required for lipoteichoic acid production in Bacillus subtilis. J. Biol. Chem. 28221738-21745. [DOI] [PubMed] [Google Scholar]
- 25.Jorasch, P., F. P. Wolter, U. Zahringer, and E. Heinz. 1998. A UDP glucosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1,2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products. Mol. Microbiol. 29419-430. [DOI] [PubMed] [Google Scholar]
- 26.Kawai, F., M. Shoda, R. Harashima, Y. Sadaie, H. Hara, and K. Matsumoto. 2004. Cardiolipin domains in Bacillus subtilis Marburg membranes. J. Bacteriol. 1861475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearns, D. B., and R. Losick. 2005. Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev. 193083-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kearns, D. B., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49581-590. [DOI] [PubMed] [Google Scholar]
- 29.Lacombe, C., and B. Lubochinsky. 1988. Specific extraction of bacterial cardiolipin from sporulating Bacillus subtilis. Biochim. Biophys. Acta 961183-187. [DOI] [PubMed] [Google Scholar]
- 30.Lazarevic, V., B. Soldo, N. Medico, H. Pooley, S. Bron, and D. Karamata. 2005. Bacillus subtilis α-phosphoglucomutase is required for normal cell morphology and biofilm formation. Appl. Environ. Microbiol. 7139-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez, C. S., H. Heras, S. M. Ruzal, C. Sanchez-Rivas, and E. A. Rivas. 1998. Variations of the envelope composition of Bacillus subtilis during growth in hyperosmotic medium. Curr. Microbiol. 3655-61. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Lara, I. M., J. L. Gao, M. J. Soto, A. Solares-Perez, B. Weissenmayer, C. Sohlenkamp, G. P. Verroios, J. Thomas-Oates, and O. Geiger. 2005. Phosphorus-free membrane lipids of Sinorhizobium meliloti are not required for the symbiosis with alfalfa but contribute to increased cell yields under phosphorus-limiting conditions of growth. Mol. Plant-Microbe Interact. 18973-982. [DOI] [PubMed] [Google Scholar]
- 33.Marquez, L. M., J. D. Helmann, E. Ferrari, H. M. Parker, G. W. Ordal, and M. J. Chamberlin. 1990. Studies of σD-dependent functions in Bacillus subtilis. J. Bacteriol. 1723435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascher, T., N. G. Margulis, T. Wang, R. W. Ye, and J. D. Helmann. 2003. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 501591-1604. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto, K., J. Kusaka, A. Nishibori, and H. Hara. 2006. Lipid domains in bacterial membranes. Mol. Microbiol. 611110-1117. [DOI] [PubMed] [Google Scholar]
- 36.Mileykovskaya, E., Q. Sun, W. Margolin, and W. Dowhan. 1998. Localization and function of early cell division proteins in filamentous Escherichia coli cells lacking phosphatidylethanolamine. J. Bacteriol. 1804252-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Minnikin, D. E., and H. Abdolrahimzadeh. 1974. Effect of pH on the proportions of polar lipids, in chemostat cultures of Bacillus subtilis. J. Bacteriol. 120999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molle, V., M. Fujita, S. T. Jensen, P. Eichenberger, J. E. Gonzalez-Pastor, J. S. Liu, and R. Losick. 2003. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 501683-1701. [DOI] [PubMed] [Google Scholar]
- 40.Nishi, H., H. Komatsuzawa, T. Fujiwara, N. McCallum, and M. Sugai. 2004. Reduced content of lysyl-phosphatidylglycerol in the cytoplasmic membrane affects susceptibility to moenomycin, as well as vancomycin, gentamicin, and antimicrobial peptides, in Staphylococcus aureus. Antimicrob. Agents Chemother. 484800-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishibori, A., J. Kusaka, H. Hara, M. Umeda, and K. Matsumoto. 2005. Phosphatidylethanolamine domains and localization of phospholipid synthases in Bacillus subtilis membranes. J. Bacteriol. 1872163-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishijima, S., Y. Asami, N. Uetake, S. Yamagoe, A. Ohta, and I. Shibuya. 1988. Disruption of the Escherichia coli cls gene responsible for cardiolipin synthesis. J. Bacteriol. 170775-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norris, V., and I. Fishov. 2001. Hypothesis: membrane domains and hyperstructures control bacterial division. Biochimie 8391-97. [DOI] [PubMed] [Google Scholar]
- 44.Ogura, M., T. Ohsawa, and T. Tanaka. 2008. Identification of the sequences recognized by the Bacillus subtilis response regulator YrkP. Biosci. Biotechnol. Biochem. 72186-196. [DOI] [PubMed] [Google Scholar]
- 45.Ogura, M., H. Yamaguchi, K. Yoshida, Y. Fujita, and T. Tanaka. 2001. DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res. 293804-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereto, J., P. Lopez-Garcia, and D. Moreira. 2004. Ancestral lipid biosynthesis and early membrane evolution. Trends Biochem. Sci. 29469-477. [DOI] [PubMed] [Google Scholar]
- 47.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Volker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 1835617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price, K. D., S. Roels, and R. Losick. 1997. A Bacillus subtilis gene encoding a protein similar to nucleotide sugar transferases influences cell shape and viability. J. Bacteriol. 1794959-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rigomier, D., J. P. Bohin, and B. Lubochinsky. 1980. Effects of ethanol and methanol on lipid metabolism in Bacillus subtilis. J. Gen. Microbiol. 121139-149. [DOI] [PubMed] [Google Scholar]
- 50.Ruzin, A., A. Severin, S. L. Moghazeh, J. Etienne, P. A. Bradford, S. J. Projan, and D. M. Shlaes. 2003. Inactivation of mprF affects vancomycin susceptibility in Staphylococcus aureus. Biochim. Biophys. Acta 1621117-121. [DOI] [PubMed] [Google Scholar]
- 51.Sareen, M., and G. K. Khuller. 1990. Cell wall and membrane changes associated with ethambutol resistance in Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 341773-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schujman, G. E., L. Paoletti, A. D. Grossman, and D. de Mendoza. 2003. FapR, a bacterial transcription factor involved in global regulation of membrane lipid biosynthesis. Dev. Cell 4663-672. [DOI] [PubMed] [Google Scholar]
- 53.Serizawa, M., K. Kodama, H. Yamamoto, K. Kobayashi, N. Ogasawara, and J. Sekiguchi. 2005. Functional analysis of the YvrGHb two-component system of Bacillus subtilis: identification of the regulated genes by DNA microarray and northern blot analyses. Biosci. Biotechnol. Biochem. 692155-2169. [DOI] [PubMed] [Google Scholar]
- 54.Serizawa, M., H. Yamamoto, H. Yamaguchi, Y. Fujita, K. Kobayashi, N. Ogasawara, and J. Sekiguchi. 2004. Systematic analysis of SigD-regulated genes in Bacillus subtilis by DNA microarray and Northern blotting analyses. Gene 329125-136. [DOI] [PubMed] [Google Scholar]
- 55.Shiomi, D., and W. Margolin. 2007. A sweet sensor for size-conscious bacteria. Cell 130216-218. [DOI] [PubMed] [Google Scholar]
- 56.Somerharju, P., J. A. Virtanen, and K. H. Cheng. 1999. Lateral organisation of membrane lipids. The superlattice view. Biochim. Biophys. Acta 144032-48. [DOI] [PubMed] [Google Scholar]
- 57.Staubitz, P., and A. Peschel. 2002. MprF-mediated lysinylation of phospholipids in Bacillus subtilis—protection against bacteriocins in terrestrial habitats? Microbiology 1483331-3332. [DOI] [PubMed] [Google Scholar]
- 58.Steil, L., M. Serrano, A. O. Henriques, and U. Volker. 2005. Genome-wide analysis of temporally regulated and compartment-specific gene expression in sporulating cells of Bacillus subtilis. Microbiology 151399-420. [DOI] [PubMed] [Google Scholar]
- 59.Tiyanont, K., T. Doan, M. B. Lazarus, X. Fang, D. Z. Rudner, and S. Walker. 2006. Imaging peptidoglycan biosynthesis in Bacillus subtilis with fluorescent antibiotics. Proc. Natl. Acad. Sci. USA 10311033-11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Dalen, A., and B. de Kruijff. 2004. The role of lipids in membrane insertion and translocation of bacterial proteins. Biochim. Biophys. Acta 169497-109. [DOI] [PubMed] [Google Scholar]
- 61.Weart, R. B., A. H. Lee, A. C. Chien, D. P. Haeusser, N. S. Hill, and P. A. Levin. 2007. A metabolic sensor governing cell size in bacteria. Cell 130335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.