Abstract
The Frankia genome contains two truncated hemoglobin genes (hboN and hboO) whose functions remain to be determined. Nitric oxide (NO) generated by the addition of 400 μM SNAP (S-nitroso-N-acetylpenicillamine) caused a 10-fold increase in hboN gene expression but had no effect on hboO expression. The addition of the NO scavenger, carboxy-PT10, reduced the effect of SNAP. hboO gene expression increased under low-oxygen conditions, while hboN expression was unaffected. These results suggest that HboN may function in protection from nitrosative stress and that HboO may act as an oxygen transport molecule for increased respiration in hypoxic environments.
Truncated hemoglobins (trHbs), the newest branch of the hemoglobin superfamily, are typically 20 to 40 amino acids shorter than traditional hemoglobin but retain the classically conserved globin fold (1, 21, 28). Three distinct groups of trHb proteins (trHbN, trHbO, and trHbP) are found distributed among eubacteria, protozoans, and plants, and their functions are currently being elucidated. For example, Mycobacterium tuberculosis, which produces two trHbs (trHbN and trHbO), has been hypothesized to use trHbN (glbN) to detoxify nitric oxide produced by macrophages in tubercles (17). M. tuberculosis trHbO (glbO) has been proposed to act as an oxygen delivery protein for terminal oxidases to aid in the stationary survival of this organism within hypoxic tubercles (10, 18).
Frankia is a nitrogen-fixing actinobacterium (gram-positive filamentous bacterium) that forms a symbiotic association with over 200 different species of plants belonging to eight different plant families, which are only distantly related to each other (for reviews, see references 4 and 27). Hemoglobin production seems to be widespread among all of the Frankia isolates (3, 26), but its specific function(s) is unknown. Biochemical studies have shown that the presence or absence of a combined nitrogen source does not affect total hemoglobin production (3), but total hemoglobin levels are greater when cells are grown with 2% oxygen than when they are grown with 20% oxygen.
Analysis of the Frankia genome elucidated the presence of two trHb genes (hboN and hboO) (16), and phylogenetic analysis grouped them closest to their respective Mycobacterium orthologs (15), suggesting potential analogous functions for the two Frankia hemoglobins. Since both microbes are capable of intracellular growth during their life cycle, this hypothesis is not unreasonable. The goal of this work was to evaluate the relative expression levels of the trHbN and trHbO genes in Frankia strain CcI3 under various environmental conditions.
Frankia strain CcI3 was grown and maintained in propionate basal medium with NH4Cl as a nitrogen source as described previously (25). Total RNA was isolated from Frankia strain CcI3 as described by Sung et al. (23). DNA was removed from RNA samples with a DNase treatment using DNase I (NEB) according to the manufacturer's recommendations. Reverse transcriptase PCR (RT-PCR) was performed using a Titan One Tube RT-PCR system (Roche) according to the manufacturer's recommendations. The following primer sets were used: HbNcci92 (5′-CACCCCTCTTTGCCAACC-3′) and HbNcci300 (5′-GGTGGTTTCCGTCGGGAC-3′) for hboN, HbOcci299 (5′-GGGACGCCTGGCTGAAGA-3′) and HbOcci375 (5′-CCAGAGCTGCCTGTCGAGATC-3′) for hboO, and DB41 (5′-TTCTTCATCCACGACCCG-3′) and DB44 (5′-GGCTTCGGCATGAAG GT-3′) for glnA (5). The thermocycling parameters were as follows: (i) reverse transcription at 55°C for 30 min; (ii) initial denaturation at 94°C for 2 min; (iii) 9 cycles of denaturation at 94°C for 20 s, primer annealing at 55°C for 30 s, and primer extension at 68°C for 45 s; (iv) 24 cycles of denaturation at 94°C for 20 s, primer annealing at 55°C for 30 s, and primer extension at 68°C for 50 s, with an additional 5 s added to each progressive cycle; and (v) a final extension step at 68°C for 4 min. Amplicons were resolved by gel electrophoresis, and the respective band intensities were quantified using Quantity One software (Bio-Rad).
For quantitative PCR (qPCR), cDNA synthesis was performed using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's directions. qPCR was performed using Power Sybr green PCR master mix (Applied Biosystems) according to the manufacturer's recommendations. The above-described primer sets were used for real-time PCR, except that for HboN expression, primer HbNcci219 (5′-CCTCACCGACGCCCACTT-3′) was used instead of HbNcci92. Amplification was carried out using an ABI GeneAmp 5700 sequence detection system adapted on a 96-well GeneAmp 9600 PCR system (Applied Biosystems). The thermocycling parameters were as follows: activation of the enzyme at 95°C for 10 min, followed by 40 cycles of a two-step denaturation at 95°C for 15 s and primer annealing/extension at 63°C for 1 min. Melting analysis was performed at the end of each PCR to determine the homogeneity of the PCR products. Cycle threshold values were determined and used to calculate the number of RNA copies per microgram of total RNA using a standard curve for a known amount of DNA.
Effect of nitrogen status on gene expression.
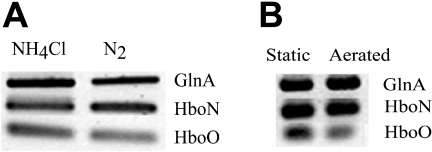
One hypothesis is that trHb functions as an oxygen scavenger, protecting the oxygen-labile nitrogenase complex during aerobic nitrogen fixation. Cyanoglobin (GlbN), a trHb present in Nostoc spp., is synthesized in the absence of combined nitrogen, and the gene locus for glbN resides between two nif operons (8). Frankia strain CcI3 was grown for 6 days in medium with or without NH4Cl to test the effect of nitrogen limitation on trHb expression. Under these conditions, the relative expression levels of the three genes were similar (Fig. 1A). Analysis of the intensities of the bands for the three genes confirmed that the expression levels of both trHb genes relative to glnA expression were similar (data not shown). These results indicate that hemoglobin expression was not regulated by cellular nitrogen status.
FIG. 1.
Nitrogen status does not alter hemoglobin expression, while hypoxic conditions increase the expression of hboO. Transcriptional analysis of hboN, hboO, and glnA was determined by RT-PCR as described in Materials and Methods. (A) Frankia strain CcI3 was incubated in growth medium with and without NH4Cl as a combined nitrogen source. After 6 days, total RNA was isolated as described in Materials and Methods and used as the template in an RT reaction. (B) Frankia strain CcI3 cultures were grown in basal medium supplemented with NH4Cl under hypoxic and oxic conditions by incubating the cultures either statically or with aeration. After 7 days, total RNA was isolated as described in Materials and Methods and used as the template in an RT reaction.
Effect of oxygen conditions on gene expression.
Schwintzer et al. (22) observed an increase in total hemoglobin levels in Frankia strain ArI3 cultures grown under a 1% oxygen atmosphere compared to those in cultures grown under 20% and 40% oxygen atmospheres. We hypothesized that Frankia trHbO may deliver oxygen to terminal oxidases to stimulate their rates of respiration under hypoxic conditions. Consistent with this model, Tjepkema et al. (26) observed rapid oxygen kinetics (oxygen association and oxygen disassociation rates, k′on and koff) for Frankia hemoglobin, suggesting the role of oxygen transport over short distances. In M. tuberculosis, trHbO has been shown to function as an oxygen shuttle to respiratory enzymes (10, 12, 18).
Frankia strain CcI3 cultures were grown for 7 days under oxic conditions (cultures aerated with atmospheric oxygen) and hypoxic conditions (static cultures). The relative expression of trHbO was greater in static cultures than in aerated cultures, while trHbN expression levels were similar under both conditions (Fig. 1B). These results suggest that Frankia TrHbO may function under hypoxic conditions to shuttle oxygen to the respiratory chain, similar to mycobacteria.
Effect of NO stress on gene expression.
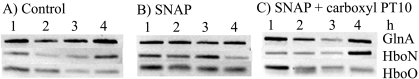
During intracellular pathogenesis, mycobacteria are bombarded with toxic NO species generated by macrophages in tuberculosis granulomas. The trHbN protein functions as a protective molecule, with NO catalytically reacting with oxygen to generate a harmless nitrate molecule (6, 11, 13, 17, 19). Since plants also use NO as a defense mechanism against pathogens (14), we predicted a similar protective function in Frankia. To test our hypothesis, the spontaneous NO donor S-nitroso-N-acetylpenicillamine (SNAP) was added to Frankia strain CcI3 cultures and the relative expression levels were evaluated. RNA samples were taken every hour, and Fig. 2 shows the RT-PCR results from these experiments. The relative expression level of hboO did not change under any of the test conditions. However, the relative expression level of hboN increased through the first 3 h of NO exposure (with SNAP), and the signal decreased after 4 h. The addition of carboxy-PT10 (cPT10), an NO scavenger, to cultures growing in the presence of SNAP decreased the level of hboN expression. Control cultures without SNAP showed no change in their relative expression levels over the same time period. These results indicate that Frankia's hboN gene expression was stimulated by NO and suggests that Frankia trHbN may be involved in nitric oxide detoxification.
FIG. 2.
NO release stimulates hboN expression. Transcriptional analysis of hboN, hboO, and glnA was determined by RT-PCR as described in Materials and Methods. Cultures were incubated in growth medium in the presence of a spontaneous NO donor, SNAP. Total RNA was isolated every hour and used as the template in an RT reaction as described in Materials and Methods. (A) Control (no addition); (B) 400 μM SNAP; (C) 400 μM SNAP plus 400 μM cPT10 (an NO scavenger).
The above-mentioned experiments were also performed with Frankia strain EAN1pec, and similar results were obtained for hboN and hboO gene expression levels in response to nitrogen status, oxygen content, and NO stress (data not shown). These results indicate that these responses were not specific to Frankia strain CcI3.
qPCR results.
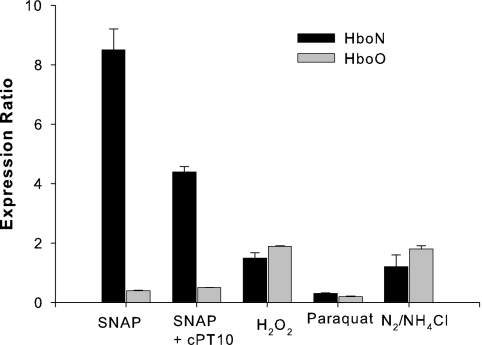
To support the results from the above-described expression studies, we utilized quantitative RT-PCR (qRT-PCR) to measure gene expression in Frankia strain CcI3. In addition to repeating our previous experiments, H2O2 and paraquat were also added to Frankia cultures to evaluate gene expression under oxidative stress conditions. Figure 3 shows the results of these experiments. For each experimental condition, gene expression levels are shown as a ratio to their expression levels in the untreated control cultures.
FIG. 3.
Transcriptional analysis of hboN and hboO gene expression in Frankia strain CcI3 using qRT-PCR. The expression ratio reflects the expression of each gene under the environmental stimulus relative to the expression under control conditions (with no stimulus). The error bars show the standard deviations.
The semi-qRT-PCR data for Frankia strain CcI3 cultured under nitrogen-limiting conditions supported the RT-PCR results described above. There was no substantial difference in gene expression level for either hboO or hboN under nitrogen-sufficient and -limiting conditions (Fig. 3). hboN expression levels were similar under both conditions, while the hboO expression level showed a small increase under growth with NH4Cl. These data, in addition to the previous RT-PCR results, suggest that these two hemoglobin genes were not upregulated under nitrogen fixation conditions.
The oxidative burst of reactive oxygen species by plants is another common defense mechanism against invading pathogens (24). One problem that microbial symbionts face is that they may not be initially distinguished by the host as a friend and not a foe. Thus, a defense mechanism must be utilized to help establish this early stage of symbiosis. Both paraquat and H2O2 were used to test the effects of oxidative stress on gene expression. The addition of 1.0 mM H2O2 caused a small increase in hboN (<1.5-fold) or hboO (<2-fold) gene expression compared to that in the control culture (Fig. 3). Interestingly, the addition of 0.1 mM paraquat, which is metabolized to produce endogenous H2O2, decreased the expression levels of both hboN and hboO nearly 10-fold. It is unclear whether or not these decreases in expression were due to the specific downregulation of these genes or possibly to the cytotoxic effects of intracellular concentrations of H2O2.
To quantify the effect of nitrosative stress on gene expression, Frankia strain CcI3 cultures were exposed to SNAP and/or cPT10 for 3 h before RNA samples were extracted. This time point yielded the strongest band intensity in the previous RT-PCR experiments for hboN (Fig. 2). The expression of hboN increased nearly 10-fold in cells exposed to 400 μM SNAP compared to that in the control (untreated) cells (Fig. 3). The addition of 400 μM cPT10 reduced the effect of 400 μM SNAP and resulted in only a fourfold increase in hboN expression. This result indicates that cPT10 was unable to scavenge all of the NO generated by SNAP under these conditions. hboO expression was reduced 0.5-fold under these conditions. Since cPT10 was unable to relieve this effect, this result would suggest that this reduction was not an NO-specific effect.
This NO-specific induction of hboN is physiologically relevant for Frankia. During initiation of nodule formation, the nitrogen-fixing microsymbiont is initially recognized as an intruder and subjected to host defenses (20). Microsymbionts have developed mechanisms to adapt and regulate these host responses (for a review, see reference 24). NO, the key regulatory molecule of plant pathogen responses (7, 9), has been shown to accumulate in functional legume nodules (2) and also functions as a defense mechanism. Thus, Frankia HboN could function to detoxify NO during the initiation stages of the host plant infection process to establish symbiosis or to maintain a functional nodule structure.
Acknowledgments
This investigation was supported in part by Hatch grant 486, by USDA NRI grant 2003-35319-13787, by NSF grant EF-0333177, and by the College of Life Sciences and Agriculture, University of New Hampshire, Durham, NH.
We thank Karen Charlton for her advice and assistance with the real-time PCR experiments.
Footnotes
Published ahead of print on 26 September 2008.
Scientific contribution 2367 from the New Hampshire Agricultural Experiment Station.
REFERENCES
- 1.Ascenzi, P., M. Bolognesi, M. Milani, M. Guertin, and P. Visca. 2007. Mycobacterial truncated hemoglobins: from genes to functions. Gene 39842-51. [DOI] [PubMed] [Google Scholar]
- 2.Baudouin, E., L. Pieuchot, G. Engler, N. Pauly, and A. Puppo. 2006. Nitric oxide is formed in Medicago truncatula-Sinorhizobium meliloti functional nodules. Mol. Plant-Microbe Interact. 19970-975. [DOI] [PubMed] [Google Scholar]
- 3.Beckwith, J., J. D. Tjepkema, R. E. Cashon, C. R. Schwintzer, and L. S. Tisa. 2002. Hemoglobin in five genetically diverse Frankia strains. Can. J. Microbiol. 481048-1055. [DOI] [PubMed] [Google Scholar]
- 4.Benson, D. R., and W. B. Silvester. 1993. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 57293-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clawson, M. L., A. Bourret, and D. R. Benson. 2004. Assessing the phylogeny of Frankia-actinorhizal plant nitrogen-fixing root nodule symbioses with Frankia 16S rRNA and glutamine synthetase gene sequences. Mol. Phylogenet. Evol. 31131-138. [DOI] [PubMed] [Google Scholar]
- 6.Couture, M., S. R. Yeh, B. A. Wittenberg, J. B. Wittenberg, Y. Oullet, D. L. Rousseau, and M. Guertin. 1999. A cooperative oxygen-binding hemoglobin from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 9611223-11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grun, S., C. Lindermayr, S. Sell, and J. Durner. 2006. Nitric oxide and gene regulation in plants. J. Exp. Bot. 57507-516. [DOI] [PubMed] [Google Scholar]
- 8.Hill, D. R., T. J. Belbin, M. V. Thorsteinsson, D. Bassam, S. Brass, A. Ernst, P. Böger, H. Paerl, M. E. Mulligan, and M. Potts. 1996. GlbN (cyanoglobin) is a peripheral membrane protein that is restricted to certain Nostoc spp. J. Bacteriol. 1786587-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klessig, D. F., J. Durner, R. Noad, D. A. Navarre, D. Wendehenne, D. Kumar, J. M. Zhou, J. Shah, S. Zhang, P. Kachroo, Y. Trifa, D. Pontier, E. Lam, and H. Silva. 2000. Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. USA 978849-8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, C., Y. He, and Z. Chang. 2004. Truncated hemoglobin o of Mycobacterium tuberculosis: oligomeric state change and the interaction with membrane components. Biochem. Biophys. Res. Commun. 3161163-1172. [DOI] [PubMed] [Google Scholar]
- 11.Milani, M., A. Pesce, Y. Ouellet, P. Ascenzi, M. Guertin, and M. Bolognesi. 2001. Mycobacterium tuberculosis hemoglobin N displays a protein tunnel suited for O2 diffusion to heme. EMBO J. 203902-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milani, M., P. Y. Savard, H. Ouellet, P. Ascenzi, M. Guertin, and M. Bolognesi. 2003. A TyrCD1/TrpG8 hydrogen bond network and a TyrB10-TyrCD1 covalent link shape the distal heme distal site of Mycobacterium tuberculosis hemoglobin O. Proc. Natl. Acad. Sci. USA 1005766-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milani, M., A. Pesce, Y. Ouellet, S. Dewilde, J. Friedman, P. Ascenzi, M. Guertin, and M. Bolognesi. 2004. Heme-ligand tunneling in group I truncated hemoglobins. J. Biol. Chem. 27921520-21525. [DOI] [PubMed] [Google Scholar]
- 14.Neill, S. J., R. Desikan, and J. T. Hancock. 2003. Tansley review: nitric oxide signaling in plants. New Phytol. 15911-35. [DOI] [PubMed] [Google Scholar]
- 15.Niemann, J. M., J. D. Tjepkema, and L. S. Tisa. 2005. Identification of the truncated hemoglobin gene in Frankia. Symbiosis 3991-95. [Google Scholar]
- 16.Normand, P., P. Lapierre, L. S. Tisa, N Alloisio, E. Bagnarol, A. M. Berry, B. Cournoyer, C. Lavire, J. Marechal, P. Pujic, J. P. Gogarten, Y. Huang, J. E. Mastronunzio, D. M. Bickhard, C. A. Bassi, T. Rawnsley, J. Niemann, M. P. Francino, A. Lapidus, M. Martinez, E. Goltsman, C. Medigue, N. Choisne, A. Couloux, S. Cruveillier, V. Daubin, L. Labarre, Z. Rouy, D. Vallenet, N. Demange, B. C. Mullin, O. R. Kopp, Y. Wang, J. P. Tomkins, A. Sellstedt, C. Schenowitz, F. Tavares, C. Valverde, L. G. Wall, and D. R. Benson. 2007. Genome characteristics of facultatively symbiotic Frankia sp. strains reflect host range and host plant biogeography. Genome Res. 177-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ouellet, H., Y. Ouellet, C. Richard, M. Labarre, B. Wittenberg, J. Wittenberg, and M. Guertin. 2002. Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc. Natl. Acad. Sci. USA 995902-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathania, R., N. K. Navani, G. Rajamohan, and K. L. Dikshit. 2002. Mycobacterium tuberculosis hemoglobin HbO associates with membranes and stimulates cellular respiration of recombinant Escherichia coli. J. Biol. Chem. 27715293-15302. [DOI] [PubMed] [Google Scholar]
- 19.Pathania, R., N. K. Navani, A. M. Gardner, P. R. Gardner, and K. L. Dikshit. 2002. Nitric oxide scavenging and detoxification by Mycobacterium tuberculious haemoglobin, HbN in Escherichia coli. Mol. Microbiol. 451303-1314. [DOI] [PubMed] [Google Scholar]
- 20.Santos, R., D. Hérouart, S. Sigaud, D. Touati, and A. Puppo. 2001. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol. Plant-Microbe Interact. 1486-89. [DOI] [PubMed] [Google Scholar]
- 21.Sarma, H. K., B. K. Sharma, S. C. Tiwari, and A. K. Mishra. 2005. Truncated hemoglobins: a single structural motif with versatile functions in bacteria, plants and unicellular eukaryotes. Symbiosis 39151-158. [Google Scholar]
- 22.Schwintzer, C. R., and J. D. Tjepkema. 2005. Effect of oxygen concentration on growth and hemoglobin production in Frankia. Symbiosis 3977-82. [Google Scholar]
- 23.Sung, K., S. A. Khan, M. S. Nawaz, and A. A. Khan. 2003. A simple and efficient Triton X-100 boiling and chloroform extraction method of RNA isolation from gram-positive and gram-negative bacteria. FEMS Microbiol. Lett. 22997-101. [DOI] [PubMed] [Google Scholar]
- 24.Tavares, F., C. L. Santos, and A. Sellstedt. 2007. Reactive oxygen species in legume and actinorhizal nitrogen-fixing symbioses: the microsymbiont's responses to an unfriendly reception. Physiol. Plant. 130344-356. [Google Scholar]
- 25.Tisa, L. S., M. S. Chval, G. D. Krumholz, and J. Richards. 1999. Antibiotic resistance patterns of Frankia strains. Can. J. Bot. 771257-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjepkema, J. D., R. E. Cashon, J. Beckwith, and C. R. Schwintzer. 2002. Hemoglobin in Frankia, a nitrogen-fixing actinomycete. Appl. Environ. Microbiol. 682629-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wall, L. G. 2000. The actinorhizal symbiosis. J. Plant Growth Regul. 19167-182. [DOI] [PubMed] [Google Scholar]
- 28.Wittenberg, J. B., M. Bolognesi, B. A. Wittenberg, and M. Guertin. 2002. Truncated hemoglobins: a new family of hemoglobins widely distributed in bacteria, unicellular eukaryotes, and plants. J. Biol. Chem. 277871-874. [DOI] [PubMed] [Google Scholar]