Abstract
Bacillus anthracis spores, the infectious agents of anthrax, are notoriously difficult to remove from contaminated areas because they are resistant to many eradication methods. These resistance properties are due to the spore's dehydration and dormancy and to the multiple protective layers surrounding the spore core, one of which is the cortex. In order for B. anthracis spores to germinate and resume growth, the cortex peptidoglycan must be depolymerized. This study reports on analyses of sleL (yaaH), which encodes a cortex-lytic enzyme. The inactivation of sleL does not affect vegetative growth, spore viability, or the initial stages of germination, including dipicolinic acid release. However, mutant spores exhibit a slight delay in the loss of optical density compared to that of wild-type spores. Mutants also retain more diaminopimelic acid and N-acetylmuramic acid during germination than wild-type spores, suggesting that the cortex peptidoglycan is not being hydrolyzed as rapidly. This finding is supported by high-pressure liquid chromatography analysis of the peptidoglycan structure used to confirm that SleL acts as an N-acetylglucosaminidase. When sleL is inactivated, the cortex peptidoglycan is not depolymerized into small muropeptides but instead is retained within the spore as large fragments. In the absence of the sleL-encoded N-acetylglucosaminidase, other cortex-lytic enzymes break down the cortex peptidoglycan sufficiently to allow rapid germination and outgrowth.
The gram-positive rod Bacillus anthracis transitions between two cellular morphologies, the spore and the vegetative cell, during its intricate life cycle. The spore is the infectious agent that causes all three types of anthrax: cutaneous, gastrointestinal, and inhalational (29). Unlike vegetative cells, spores are resistant to many environmental insults, including extreme pH, high temperatures, chemical treatment, radiation, desiccation, and starvation (34, 43). These characteristics allow the metabolically inactive, dormant morphotype to survive outside a host for centuries (13) and hinder cleanup efforts in contaminated areas. Environmental conditions inside the B. anthracis host support the resumption of metabolic activity, triggering the spores to germinate into vegetative cells. It is the vegetative cells that are responsible for the fatal symptoms associated with certain types of anthrax because they produce a protective capsule and deadly toxins.
Germination is the transition of a spore to a vegetative cell. It is stimulated when the spore encounters nutrient or nonnutrient germinants in its environment (44). Nutrient germinants interact with germinant receptors within the inner membrane of the spore. This initiates a cascade of events, including the release of cations and dipicolinic acid (DPA) from the spore core, partial core rehydration, hydrolysis of the cortex peptidoglycan (PG), and further hydration, causing swelling of the spore and loss of dormancy (30, 36, 42, 44).
Bacillus spores contain two types of PG, the germ cell wall and the cortex PG. The germ cell wall is adjacent to the inner forespore membrane, and this layer may act as a template for vegetative cell wall synthesis after germination (2, 10). The cortex PG is assembled between the germ cell wall and the outer forespore membrane (28). The cortex PG is composed of glycan strands made of alternating N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM), the latter of which may be modified to muramic δ-lactam or whose side chain may be cleaved to a single l-alanine (2, 39, 46). These distinct modifications result in a loosely cross-linked cortex that helps maintain dehydration, promoting spore heat resistance and dormancy (22, 38, 45).
As spores germinate, the cortex PG is hydrolyzed by germination-specific cortex-lytic enzymes (GSLEs). GSLEs can differentiate between cortex and germ cell wall PG by identifying the unique muramic δ-lactam residues of the cortex (2, 8, 25, 40). These types of enzymes have been subclassified as either spore cortex-lytic enzymes (SCLE) or cortical fragment-lytic enzymes (CFLE), which recognize intact or partially hydrolyzed fragments of cortex PG, respectively (25). Studies have identified SCLEs and CFLEs in several Bacillus species. SleB is an SCLE with orthologs in B. thuringiensis (15), B. cereus (24, 33), B. subtilis (32), and B. anthracis (23). The sleB gene is expressed in the forespore under the control of σG (6, 31), and the protein is then translocated to the cortex or the inner membrane in its mature form (9, 27, 31). After recognizing PG-containing muramic δ-lactam, SleB is thought to act as either a lytic transglycosylase or an amidase (6, 31, 33). CwlJ has sequence similarity to one domain of SleB, but this enzyme is apparently associated with the proteinaceous coats of the spore (4, 16). The enzymatic activity of CwlJ has yet to be conclusively determined, but it has been speculated to be an amidase (16). In B. subtilis, SleB and CwlJ are redundant in an essential role during germination. In the absence of both enzymes, spores cannot hydrolyze the cortex PG and complete germination (9).
The product of the B. cereus sleL gene functions as a CFLE (8). Like cwlJ, sleL is expressed in the mother cell under the regulation of σE (19). SleL localizes to the periphery of the cortex or the cortex/coat interface (7, 8, 20). The enzymatic function of SleL has been suggested to be either an N-acetylglucosaminidase (8) or an epimerase (3, 9). We have characterized the role of sleL in B. anthracis spore germination. Analyses of spore viability, germination, and cortex PG hydrolysis indicate that SleL functions as a CFLE and is an N-acetylglucosaminidase.
MATERIALS AND METHODS
Strains and spore preparation.
B. anthracis strains and plasmids used in this study are described in Table 1. Escherichia coli strains used to propagate plasmids were grown in LB broth or agar medium with the appropriate antibiotics at 37°C. B. anthracis Sterne strain 34F2 (pXO1+, pXO2−) was grown on brain heart infusion (BHI, Difco) broth with antibiotics when indicated. The following concentrations of antibiotics were added to either LB or BHI broth to select resistance markers when necessary: 10 μg ml−1 tetracycline (Jersey Lab Supply), 50 μg ml−1 kanamycin sulfate (Jersey Lab Supply), and 500 μg ml−1 (E. coli) or 5 μg ml−1 (B. anthracis) erythromycin (Fisher). B. anthracis endospores were prepared by growth in modified G broth (18) at the appropriate temperatures for 3 to 4 days. The spores were harvested by centrifugation and washed repeatedly in deionized water. Any remaining vegetative cells were heat killed at 65°C for 20 min. The spores were further purified with a 50% sodium diatrizoate (Sigma-Aldrich) gradient as previously described (35). Spores were stored in deionized water at 4°C until analysis.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotypea | Source or reference |
|---|---|---|
| B. anthracis strains | ||
| Sterne 34F2 | pX01+ pX02− | P. Hanna |
| DPBa27 | sleL-lacZ::pDPV350 Kanr | This study |
| DPBa35 | ΔsleL | This study |
| DPBa36 | ΔsleL, pBKJ236 Err | This study |
| DPBa37 | ΔsleL, pDPV352 Err | This study |
| Plasmids | ||
| pDONRtet | Tetr | 12 |
| pNFd13 | Kanr, pE194 orits, Pspac, lacZ | 12 |
| pBKJ236 | Err orits | 17 |
| pBKJ223 | Tetr, Pamy-I-SceI | 17 |
| pDPV350 | pNFd13::sleL′ | This study |
| pDPV351 | pBKJ236::ΔsleL | This study |
| pDPV352 | pBKJ236::sleL | This study |
Kanr, kanamycin resistance; Err, erythromycin resistance; Tetr, tetracycline resistance; orits, temperature-sensitive origin of replication.
Mutant construction.
An sleL-lacZ transcriptional fusion was created as described previously (12) with slight modifications. Briefly, primers SleLgatFor and SleLgatRev (sequences available upon request) were used to PCR amplify a 442-bp region of the B. anthracis Sterne genome, including the Shine-Dalgarno sequence and the first 142 codons of sleL. The sleL truncation was introduced into the entry vector pDONRtet and then into the destination vector pNFd13 (12) using the Invitrogen Gateway cloning system, and the resulting plasmid was named pDPV350. This plasmid contains the temperature-sensitive pE194 origin of replication and the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible Pspac promoter upstream and the lacZ gene downstream of the sleL insert. Plasmid constructs were verified after each step by restriction-enzyme digestion and sequencing. pDPV350 was prepared from E. coli INV110 (dam dcm) (Invitrogen) and was electroporated into B. anthracis Sterne as described previously (41). To select for the integration of the plasmid into the B. anthracis chromosome, a broth culture of the resulting strain was shifted to 39°C. The correct chromosomal construct of the strain denoted as DPBa27 was verified by Southern blotting and by PCR using primers homologous to regions upstream and downstream of sleL (SleL1 and SleL4, respectively), within lacZ, and upstream of the Pspac promoter. Southern blots were performed using the Gene Images random prime labeling kit and Gene Images ECL detection kit (GE Healthcare) following the manufacturer's protocols.
The sleL gene was deleted by markerless gene replacement as described previously (17) with the following modifications. PCR was used to amplify a 2,377-bp fragment, including sleL and its flanking regions. Primers SleL1 and SleL4 were designed to add a NotI restriction site upstream and a BamHI site downstream of sleL, respectively. The PCR product and pBKJ236 (17) were digested with NotI and BamHI, and the products were ligated, resulting in pDPV352. The inverse PCR of the plasmid using primers SleL2 and SleL3 resulted in a linear 7,373-bp product with BglII sites at both ends. The linear product was digested with BglII and ligated to produce pDPV351. The plasmid was transformed into INV110 and then introduced into B. anthracis via conjugation as described previously (17). Selection at 37°C resulted in the insertion of the plasmid via a single crossover at the sleL locus. pBKJ223 (17) isolated from E. coli INV110 was electroporated into the resulting strain to allow the expression of I-SceI, and strains in which pDPV351 was deleted from the chromosome by a second recombination event were identified by screening for antibiotic sensitivity. A strain carrying the in-frame sleL deletion mutation, denoted DPBa35, was verified by PCR using primers SleL1 and SleL4 and by sequencing of the resulting PCR product. Complementation studies involved the introduction of pBKJ236 (empty vector) or pDPV352 (complementing plasmid) into DPBa35 to produce DPBa36 and DPBa37, respectively. These plasmids are derived from a vector with a copy number of approximately 5 in B. subtilis (21, 37). Plasmids were maintained in the replicative form by conducting complementation studies at 27°C.
Phenotypic analyses.
Spore viability was analyzed by conducting colony formation assays. Equivalent amounts of spores of each strain were heat activated at 70°C for 30 min to synchronize germination. After activation, the initial optical density at 600 nm (OD600) of the spore solutions was measured. The suspensions were then serially diluted, and the spores were plated on BHI broth without antibiotics. The plates were incubated overnight at 39°C and were used to calculate the CFU per OD600.
Starter cultures for growth and sporulation assays were cultivated on BHI plates with the appropriate antibiotics. Isolated colonies were inoculated into modified G broth without antibiotics and incubated at 39°C, unless otherwise noted, until the OD600 was ∼0.5. The cultures were back-diluted 1:25, and OD readings were recorded over 8 h. To determine the activity of the wild-type sleL promoter, 1-ml culture samples were collected, and the cells were pelleted and stored at −80°C. β-Galactosidase activity was assayed as previously described (35).
For the germination and outgrowth studies, spores were heat activated in water at 70°C for 30 min and then briefly cooled on ice. Germination was initiated by diluting spores to an OD600 of 0.2 in BHI broth and shaking at 39°C. Germination and outgrowth were monitored as changes in the OD600 over time and are graphically depicted as the initial OD percentage relative to incubation time.
Assays of biochemical changes during germination.
One milliliter of purified spores at an OD600 of 50 were heat activated at 70°C for 30 min. NaPO4 (pH 7.0) was added to achieve a final concentration of 40 mM, and the mixture was incubated at 37°C for 5 min. Germination was initiated by the addition of l-alanine (Fisher) to 10 mM and inosine (Sigma) to 1 mM. The spores were incubated at 37°C until the OD600 decreased at least 40% (approximately 4 min), at which time samples were collected for muropeptide, NAM, diaminopimelic acid (Dpm), and DPA analyses. The germinating spore pellets and exudates were separated by centrifugation, and PG was prepared and analyzed as previously described (11). Both spore exudates and pellet samples were assayed for DPA content as described previously (35). Briefly, spore pellets were disrupted by boiling the samples resuspended in 10 mM Tris HCl (pH 8.0) for 20 min. After cooling on ice, both the pellet and exudate samples were centrifuged to remove any insoluble material. The supernatants were combined with DPA assay reagent, and the absorbance at 440 nm was measured. The amount of DPA in the pellets or exudates was compared to a standard curve produced using purified DPA (Sigma). Muramic acid and amino acid analyses of the pellet and exudate samples collected during germination were done as previously described (28).
RESULTS
Identification and expression of sleL.
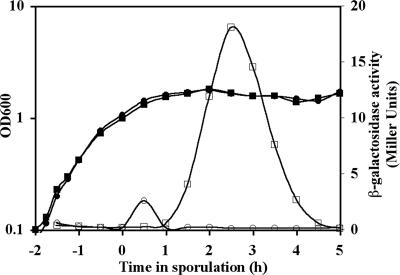
Database searches revealed only a single strong homolog of the B. subtilis (49% amino acid identity) and B. cereus SleL (YaaH) (98% amino acid identity) proteins encoded in each of the available B. anthracis genome sequences. Locus BAS3402 was identified as the B. anthracis Sterne ortholog of sleL. The genes flanking BAS3402 are oriented in the opposite direction, indicating that, as in B. subtilis (19) and B. cereus (8), B. anthracis sleL is a monocistronic locus. By creating a transcriptional fusion between the native sleL promoter and lacZ, we determined the activity of the promoter. While wild-type B. anthracis had only background levels of β-galactosidase activity, the native sleL promoter in DPBa27 was inactive during growth, became active at the first hour of sporulation (t1), was most active at t2.5, and became inactive by t5 (Fig. 1).
FIG. 1.
Sporulation-specific expression of sleL. Strains were incubated in modified G medium at 39°C for 7 h, during which samples were collected for β-galactosidase assays. Growth (filled symbols) and β-galactosidase activity (open symbols) were assayed for wild-type (circles) and DPBa27 (sleL-lacZ::pDPV350) (squares) B. anthracis. The graph is representative of three independent experiments.
Construction and characterization of the sleL mutant strains.
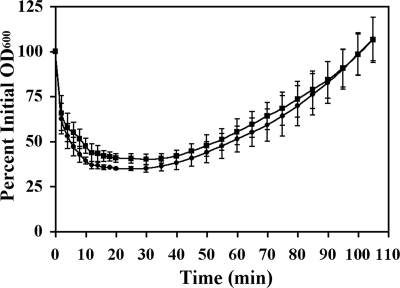
Vegetative growth assays (Fig. 1) indicated that the wild-type and both the sleL plasmid insertion (DPBa27) and in-frame ΔsleL (DPBa35) mutant strains had similar doubling rates in modified G broth. Both mutants also produced equivalent amounts of viable, heat-resistant spores compared to the wild-type (data not shown). Purified spores were analyzed to determine the role of sleL in germination and outgrowth. When spores were germinated in BHI broth, both the wild-type and mutant strains reached 70% of their maximum drop in OD600 within 4 min after the addition of germinants (Fig. 2). However, the wild-type spores reached their maximum drop in OD600 20 min after germination initiation, at which point they lost an average of 65% ± 1% of their initial OD. The plasmid insertion sleL mutant spores, however, reached their maximum OD decrease slightly later, 30 min after induction, and lost only 60% ± 3% of their initial OD600. Repeated measures two-way analysis of variance indicates that there is a significant difference (P ≤ 0.0098) between the two strains from 10 to 35 min after the initiation of germination. Each strain then progressed through outgrowth at approximately the same rate (Fig. 2), reaching the point of the first cell division at ∼110 min after germination initiation (data not shown). Similar results were obtained with the in-frame ΔsleL mutant strain (data not shown).
FIG. 2.
Germination of sleL spores is slightly delayed. Heat-activated spores were germinated in BHI medium at 39°C. Germination and outgrowth of wild-type (•) and DPBa27 (sleL-lacZ::pDPV350) (▪) B. anthracis spores were followed as changes in OD600 value over time. Error bars represent 1 standard deviation of the mean of three independent assays. The difference in the loss of OD600 between the two strains is significant (P ≤ 0.0098) from 10 to 35 min after germination initiation.
DPA, NAM, and Dpm release during germination.
Heat-activated B. anthracis spores were induced to germinate with l-alanine and inosine in buffered solution as described in Materials and Methods. Germination was allowed to proceed until the spores lost 40% of their initial OD600 value, which occurred within 5 min. Samples were collected and analyzed for the release of DPA, NAM, and Dpm from the spores. The amount of DPA released from all strains studied was similar (Fig. 3). Nearly all of the DPA contained within the spore core was released within 10 min of germination initiation.
FIG. 3.
Release of DPA and NAM during germination. Heat-activated spores were germinated with l-alanine and inosine in buffer at 37°C for 10 min, and samples were removed and centrifuged for assay of exudate and spore pellet contents. The percentages of DPA and NAM released from B. anthracis spores of the wild type (black bars), the ΔsleL mutant (DPBa35; white bars), the ΔsleL mutant with pBKJ236 vector control (DPBa36; light gray bars), and the ΔsleL mutant with pDPV352 complementing vector (DPBa37; dark gray bars) are shown. Error bars represent 1 standard deviation of the mean of three independent experiments. Asterisks indicate a statistically significant difference (P ≤ 0.0001) compared to wild-type B. anthracis. A cross indicates a statistically significant difference (P ≤ 0.007) compared to either the wild-type or DPBa35.
However, the amount of NAM released differed significantly (Tukey's adjusted P value of ≤0.0001 as determined using analysis of variance with transformed data) between wild-type and ΔsleL mutant spores (Fig. 3). Wild-type spores that had germinated for approximately 10 min released 73% ± 10% of their NAM, whereas the ΔsleL mutant spores released 7% ± 5%, which is likely the result of SleB and CwlJ lytic activity. When the ΔsleL mutant was complemented by the addition of a vector containing the full-length gene under the control of the native promoter (DPBa37), the amount of hexosamine released from the spore increased significantly (Tukey's adjusted P value of ≤0.007) to 33% ± 6%. Partial complementation was likely the result of sporulating DPBa37 in modified G broth without antibiotics, so there was no selective pressure for maintaining the complementation vector. The screening of cells for the plasmid antibiotic resistance revealed that only ∼20% of the cells entering sporulation (at t0) still carried pDPV352. Given that other lytic enzymes produce the 7% NAM release observed for the ΔsleL mutant spores, the 26% increase we observed for the complemented strain is consistent with the low level of plasmid maintenance. Sporulation in the absence of antibiotics was done due to concerns about changes in sporulation efficiency and spore physiology in the presence of varied media components for different strains. Subsequent sporulation in the presence of erythromycin to select for the complementing plasmid resulted in no significant change in the number of heat-resistant spores produced per unit of culture. Germination studies of these spores revealed stronger complementation, leading to the release of 90% of the NAM observed for the wild type during early germination (data not shown). The same pattern of diminished release in the ΔsleL mutant and partial complementation was seen for Dpm, another component of the cortex PG (data not shown).
Muropeptide structural dynamics.
In order to analyze the muropeptide composition of the wild-type, ΔsleL mutant, and complemented spores, heat-activated spores were germinated in buffer with l-alanine and inosine. Germination proceeded for 5 min before spore pellets and exudate samples were separated by centrifugation. To inactivate lytic enzymes, the spore pellets were then disrupted by boiling in a solution containing detergent and dithiothreitol, while exudate samples were simply boiled. The pellet samples and half of the exudate sample volumes were digested with mutanolysin, which cleaves the PG between NAM and NAG, leaving NAM as the terminal reducing sugar. Finally, all samples were reduced and muropeptides were separated using high-pressure liquid chromatography (HPLC) (Fig. 4). The identities of the muropeptides represented by labeled peaks were previously determined by amino acid analysis and mass spectrometry (11) and are listed in Table 2.
FIG. 4.
HPLC analysis of wild-type and ΔsleL mutant B. anthracis muropeptides. Heat-activated spores were germinated for 5 min with l-alanine and inosine in buffer at 37°C. Samples were removed and centrifuged for analysis of PG in the exudate and spore pellet. PG was collected, digested, and reduced, and muropeptides were separated using a methanol gradient as previously described (11). Muropeptides were derived from dormant spores (A), from the exudates of germinating spores (B to E), from the exudates of germinating spores digested with mutanolysin (F and G), and from germinating spore pellets (H and I). Samples were from spores of the wild type (A, B, F, and H), the ΔsleL mutant (DPBa35) (C, G, and I), the ΔsleL mutant with pBKJ236 vector control (DPBa36) (D), and the ΔsleL mutant with pDPV352 complementing vector (DPBa37) (E). Peaks G3, G10u, and G12 are clearly visible upon the magnification of panel E but are not marked for clarity. Peaks are labeled as in reference 11 and Table 2, except the first “a” in the germination-specific peak names is omitted for space considerations. The identities of peaks aG7a and aG7b are unpublished data. Ino indicates the added germinant inosine. Large unlabeled peaks eluting between 10 and 30 min for exudate samples are other small molecules released from germinating spores that were analyzed and found not to contain peptidoglycan components. Peaks labeled b are buffer components.
TABLE 2.
Muropeptide peak identification
| Muropeptide | Structurea |
|---|---|
| Produced from dormant spore peptidoglycan | |
| A | DS-TriP |
| B | DS-Ac-TriP |
| C | DS-TriP+Am |
| D | DS-Ac-TriP+Am |
| E | DS-Ala |
| F | DS-TP |
| G | DS-Ac-TP |
| H | TS-TP open lactam |
| I | TS Red-TP |
| J | TS Red-Ala |
| K | DS-Ac-TP × TriP+Am-DS-Ac |
| L | TS-TP-Ac |
| M | DS-Ac-TP+Am × TriP+Am-DS-Ac |
| N | TS-TP |
| O | TS-TP × TP |
| P | DS-TP × TP-TS Red |
| Q | TS-Ala |
| R | HSRed-Ac-TP (right lactam reduced) |
| S | HSRed-Ac-TP (left lactam reduced) |
| T | DS-TP × TP-TS |
| U | HS-TP-Ac |
| V | TS-TP × TP-TS |
| W | HS-Ala-Ac |
| X | HS-Ala-Ac |
| Y | HS-TP |
| Z | HS-Ala |
| AA | TS-TP × TP-HS |
| Produced from germinated spore exudate following mutanolysin digestion | |
| aG1 | TriS-TP Red |
| aG4 | TriS-TP |
| aG5 | TriS-Ala |
| aG8 | PS-TP-Ac |
| Produced from germinated spore exudate with no mutanolysin digestion | |
| aG2 | TS-TP NAGr Red |
| aG3 | TS-Ala NAGr Red |
| aG6 | TS-TP NAGr |
| aG7 | TS-Ala NAGr |
| aG7a | TS-TP anhydro |
| aG7b | TS-Ala anhydro |
| aG10u | HS-TP-Ac NAGr |
| aG12 | HS-Ala-Ac NAGr |
DS, disaccharide (NAG-NAM); TS, tetrasaccharide (NAG-lactam-NAG-NAM); HS, hexasaccharide (NAG-lactam-NAG-lactam-NAG-NAM); TriS, trisaccharide (lactam-NAG-NAM); PS, pentasaccharide (lactam-NAG-lactam-NAG-NAM); TriP, tripeptide (Ala-Glu-Dpm); TP, tetrapeptide (Ala-Glu-Dpm-Ala); -Ac, deacetylated glucosamine; +Am, amidated Dpm; Red, reduced lactam (an artifact of sample preparation [47]); NAGr, NAG at the reducing end; ×, cross-link between two peptides; anhydro, anhydro form of NAM at the reducing end.
Wild-type exudate-associated muropeptide profiles differed from ΔsleL mutant profiles in that the former contained peaks G2, G3, G6, G7, G10u, and G12 representing tetrasaccharide (TS) or hexasaccharide (HS) muropeptides with NAG as the terminal reducing sugar (N-acetylglucosaminitol when reduced) (Fig. 4B). The loss of sleL resulted in the disappearance of these products (Fig. 4C). Therefore, the majority of the germination-associated muropeptides released from the ΔsleL mutant spores were aG7a and aG7b, which appear to be the result of SleB-lytic transglycosylase activity (unpublished data). When the ΔsleL mutant was complemented with pDPV352, the exudate muropeptide profile was more similar to that of the wild type (Fig. 4E). aG7a and aG7b were still released, but like the wild type, considerable amounts of the G2, G6, and G7 muropeptides were released from the spore, as well as small amounts of G3, G10u, and G12. This muropeptide profile is consistent with the enzymatic digestion pattern of a glucosaminidase (11). The profile changes were not the result of the pBKJ236 vector because only aG7a and aG7b germination-associated muropeptides were present in the chromatogram of DPBa36 containing the empty vector (Fig. 4D).
The muropeptide profile of wild-type spore exudate digested with mutanolysin reveals the presence of high levels of G1, G4, G5, and G8 (Fig. 4F). These trisaccharides and pentasaccharides result when NAG is cleaved from the reducing ends of G2, G6, G7, and G10u, respectively. When ΔsleL mutant exudate samples were digested and analyzed, there were no peaks at the positions of G1, G4, G5, or G8 (Fig. 4G). The muropeptides that were released from the mutant spores, L, N, and Q, must be produced by the mutanolysin digestion of larger PG fragments that have been released from the spores but are present in lower amounts than for the wild type, because most of the cortex PG is retained within the mutant spores (Fig. 4I). There was no difference in the release of aG7a or aG7b between the wild-type and ΔsleL mutant spores, however, suggesting that these products of cortex digestion are small enough to be released to the exudate and are unaffected by mutanolysin digestion.
The major differences between the muropeptide profiles of wild-type- and ΔsleL mutant-germinated spore-associated material is that wild-type spores retain very few muropeptide constituents 5 min after germination initiation (Fig. 4H). Notably, essentially undetectable amounts of the predominant cortex-associated muropeptides N and Q were identified in wild-type profiles. ΔsleL mutant spores, however, had much higher amounts of not only N and Q but also other dormant spore-associated muropeptides, including other TS, HS, and cross-linked muropeptides (Fig. 4I). Therefore, more cortex PG was retained in ΔsleL mutant spores as a result of the loss of SleL enzymatic activity.
DISCUSSION
The B. anthracis sleL gene encoding a GSLE was mutated and evaluated for its role in germination. The loss of sleL slightly delays germination because mutant spores take 10 min longer to reach their maximum drop in OD600 value. In refined studies of the progression of germination, our findings indicate that SleL is involved in the depolymerization of cortex PG. Stage I of germination appears to proceed normally in the sleL mutant. Both wild-type and mutant spores release equivalent amounts of DPA, and their drop in OD is equivalent for the first 5 min after germination initiation. However, mutants progress more slowly within stage II of germination, which includes cortex hydrolysis (44). At this point, mutant spores are not hydrolyzing their cortex PG as efficiently. The fact that sleL mutants complete outgrowth as quickly as the wild-type strain suggests that they are not impaired in spore core rehydration and resumption of metabolism.
It was previously proposed that SleL may degrade partially digested cortex PG (8). In the absence of SleL, the cortex continues to be depolymerized by SCLEs, presumably SleB and CwlJ, but the spore retains these large fragments. This would explain why we see ∼65% more NAM and ∼50% more Dpm being retained in germinating sleL mutant spores. The retention of these cortex-associated products indicates that SleL is involved in their release and may also explain why sleL spores continue to be more optically dense than wild-type B. anthracis. Despite these alterations, sleL mutants depolymerize the cortex sufficiently, presumably via the lytic enzymes SleB and CwlJ, so that germination is completed and outgrowth begins.
Chen et al. (8) subjected B. cereus PG fragments to purified SleL and found that the cortex-lytic enzyme was capable of digesting partially disrupted cortex PG and is therefore classified as a CFLE. By using reversed phase HPLC, they also determined that the enzyme acts as an N-acetylglucosaminidase involved in spore cortex depolymerization. Sequence studies reveal that the SleL protein is part of a large family of glycosyl hydrolases that includes chitinases (26), which cleave a polymer of N-acetylglucosamine. Despite the fact that B. cereus and B. anthracis SleL have 48% amino acid identity to B. subtilis SleL, reports of muropeptide structure and composition analyses in the latter species suggested that SleL is a putative muramic δ-lactam epimerase (9). Atrih et al. (3) first proposed the role of an epimerase after concluding that the cortex PG was modified in a way uncharacteristic of an amidase or hydrolytic enzyme. Instead, the enzymatic activity occurred only at muropeptides containing muramic δ-lactam and possibly altered the stereochemistry of muramic acid residues. Chirakkal et al. (9) evaluated a B. subtilis sleL null mutant for cortex PG muropeptide dynamics of dormant and germinated spores. They reported that the sleL strain did not produce epimerase products but continued to release glucosaminidase products to the exudate. We cannot explain the results of Chirakkal et al. (9), but our results are consistent with those of Chen et al. (8) in predicting that SleL is an N-acetylglucosaminidase.
In our analyses, peaks G2, G3, G6, G7, G10u, and G12 were absent from the ΔsleL mutant's germination muropeptide profile. Muropeptides with similar retention times and identical masses were previously identified as epimerase products (1, 3). However, Dowd et al. (11) recently reported that the most evident lytic activity detected during B. anthracis germination is that of a glucosaminidase. When the exudate fractions were digested with mutanolysin and reduced, the original TS and HS were converted to tri- and pentasaccharides, indicating that the native exudate muropeptides had NAG at their reducing termini rather than NAM. Dowd et al. (11) went on to suggest that in B. subtilis, the glucosaminidase products were misidentified as epimerase products. All of our data would indicate that the enzymatic activity of SleL is not an epimerase but actually an N-acetylglucosaminidase.
By creating an sleL-lacZ transcriptional fusion, we were able to show that B. anthracis expresses sleL around t2 or midsporulation. This result is consistent with a microarray analysis of B. anthracis that indicates sleL is transcribed during wave 4 of gene expression, which includes t1.5 to 4 (5, 23). Because both B. anthracis and B. subtilis express sleL around t2, and given that Kodama et al. (19) have shown that B. subtilis sleL is under the control of σE, it is reasonable to predict that B. anthracis sleL is most likely expressed in the mother cell under the regulation of σE. The protein is then apparently localized to the developing forespore, where it is incorporated into the mature spore (8, 19).
The SleL N-acetylglucosaminidase plays a major role in determining the structure of muropeptides released into the medium by germinating B. anthracis. These muropeptides may have a major role in modulating the host immune system during an anthrax infection (14). To fully understand the contributions of GSLEs during B. anthracis germination, it will be imperative to create and analyze combinatorial double and triple mutants. This will allow us to determine if and how these enzymes cooperate to produce particular muropeptide products and to allow for such a fast, efficient morphological transition.
Acknowledgments
This research was supported by public health service grant AI060726 from the National Institute of Allergy and Infectious Disease.
We thank Sunan Zhao and Sara Wang at the VT Laboratory for Interdisciplinary Statistical Analysis for their advice.
Footnotes
Published ahead of print on 3 October 2008.
REFERENCES
- 1.Atrih, A., G. Bacher, R. Korner, G. Allmaier, and S. J. Foster. 1999. Structural analysis of Bacillus megaterium KM spore peptidoglycan and its dynamics during germination. Microbiology 1451033-1041. [DOI] [PubMed] [Google Scholar]
- 2.Atrih, A., P. Zollner, G. Allmaier, and S. J. Foster. 1996. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J. Bacteriol. 1786173-6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atrih, A., P. Zollner, G. Allmaier, M. P. Williamson, and S. J. Foster. 1998. Peptidoglycan structural dynamics during germination of Bacillus subtilis 168 endospores. J. Bacteriol. 1804603-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagyan, I., and P. Setlow. 2002. Localization of the cortex lytic enzyme CwlJ in spores of Bacillus subtilis. J. Bacteriol. 1841219-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman, N. H., E. C. Anderson, E. E. Swenson, M. M. Niemeyer, A. D. Miyoshi, and P. C. Hanna. 2006. Transcriptional profiling of the Bacillus anthracis life cycle in vitro and an implied model for regulation of spore formation. J. Bacteriol. 1886092-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boland, F. M., A. Atrih, H. Chirakkal, S. J. Foster, and A. Moir. 2000. Complete spore-cortex hydrolysis during germination of Bacillus subtilis 168 requires SleB and YpeB. Microbiology 14657-64. [DOI] [PubMed] [Google Scholar]
- 7.Buist, G., A. Steen, J. Kok, and O. P. Kuipers. 2008. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol. Microbiol. 68838-847. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y., S. Fukuoka, and S. Makino. 2000. A novel spore peptidoglycan hydrolase of Bacillus cereus: biochemical characterization and nucleotide sequence of the corresponding gene, sleL. J. Bacteriol. 1821499-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chirakkal, H., M. O'Rourke, A. Atrih, S. J. Foster, and A. Moir. 2002. Analysis of spore cortex lytic enzymes and related proteins in Bacillus subtilis endospore germination. Microbiology 1482383-2392. [DOI] [PubMed] [Google Scholar]
- 10.Cleveland, E. F., and C. Gilvarg. 1975. Selective degradation of peptidoglycan from Bacillus megaterium spores during germination, p. 458-464. In P. Gerhardt, R. N. Costilow, and H. L. Sadoff (ed.), Spores VI. American Society for Microbiology, Washington, DC.
- 11.Dowd, M. M., B. Orsburn, and D. L. Popham. 2008. Cortex peptidoglycan lytic activity in germinating Bacillus anthracis spores. J. Bacteriol. 1904541-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher, N., and P. Hanna. 2005. Characterization of Bacillus anthracis germinant receptors in vitro. J. Bacteriol. 1878055-8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould, G. W. 2006. History of science—spores. J. Appl. Microbiol. 101507-513. [DOI] [PubMed] [Google Scholar]
- 14.Guan, R., and R. A. Mariuzza. 2007. Peptidoglycan recognition proteins of the innate immune system. Trends Microbiol. 15127-134. [DOI] [PubMed] [Google Scholar]
- 15.Hu, K., H. Yang, G. Liu, and H. Tan. 2007. Cloning and identification of a gene encoding spore cortex-lytic enzyme in Bacillus thuringiensis. Curr. Microbiol. 54292-295. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, S., K. Yamane, and J. Sekiguchi. 1998. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 1801375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janes, B. K., and S. Stibitz. 2006. Routine markerless gene replacement in Bacillus anthracis. Infect. Immun. 741949-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, H. U., and J. M. Goepfert. 1974. A sporulation medium for Bacillus anthracis. J. Appl. Bacteriol. 37265-267. [DOI] [PubMed] [Google Scholar]
- 19.Kodama, T., H. Takamatsu, K. Asai, K. Kobayashi, N. Ogasawara, and K. Watabe. 1999. The Bacillus subtilis yaaH gene is transcribed by SigE RNA polymerase during sporulation, and its product is involved in germination of spores. J. Bacteriol. 1814584-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kodama, T., H. Takamatsu, K. Asai, N. Ogasawara, Y. Sadaie, and K. Watabe. 2000. Synthesis and characterization of the spore proteins of Bacillus subtilis YdhD, YkuD, and YkvP, which carry a motif conserved among cell wall binding proteins. J. Biochem. 128655-663. [DOI] [PubMed] [Google Scholar]
- 21.Kok, J., J. M. van der Vossen, and G. Venema. 1984. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl. Environ. Microbiol. 48726-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis, J. C., N. S. Snell, and H. K. Burr. 1960. Water permeability of bacterial spores and the concept of a contractile cortex. Science 132544-545. [DOI] [PubMed] [Google Scholar]
- 23.Liu, H., N. H. Bergman, B. Thomason, S. Shallom, A. Hazen, J. Crossno, D. A. Rasko, J. Ravel, T. D. Read, S. N. Peterson, J. Yates, III, and P. C. Hanna. 2004. Formation and composition of the Bacillus anthracis endospore. J. Bacteriol. 186164-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makino, S., N. Ito, T. Inoue, S. Miyata, and R. Moriyama. 1994. A spore-lytic enzyme released from Bacillus cereus spores during germination. Microbiology 1401403-1410. [DOI] [PubMed] [Google Scholar]
- 25.Makino, S., and R. Moriyama. 2002. Hydrolysis of cortex peptidoglycan during bacterial spore germination. Med. Sci. Monit. 8RA119-127. [PubMed] [Google Scholar]
- 26.Marchler-Bauer, A., J. B. Anderson, M. K. Derbyshire, C. DeWeese-Scott, N. R. Gonzales, M. Gwadz, L. Hao, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, D. Krylov, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, S. Lu, G. H. Marchler, M. Mullokandov, J. S. Song, N. Thanki, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2007. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 35D237-D240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masayama, A., H. Fukuoka, S. Kato, T. Yoshimura, M. Moriyama, and R. Moriyama. 2006. Subcellular localization of a germination-specific cortex-lytic enzyme, SleB, of Bacilli during sporulation. Genes Genet. Syst. 81163-169. [DOI] [PubMed] [Google Scholar]
- 28.Meador-Parton, J., and D. L. Popham. 2000. Structural analysis of Bacillus subtilis spore peptidoglycan during sporulation. J. Bacteriol. 1824491-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55647-671. [DOI] [PubMed] [Google Scholar]
- 30.Moir, A., and D. A. Smith. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44531-553. [DOI] [PubMed] [Google Scholar]
- 31.Moriyama, R., H. Fukuoka, S. Miyata, S. Kudoh, A. Hattori, S. Kozuka, Y. Yasuda, K. Tochikubo, and S. Makino. 1999. Expression of a germination-specific amidase, SleB, of Bacilli in the forespore compartment of sporulating cells and its localization on the exterior side of the cortex in dormant spores. J. Bacteriol. 1812373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moriyama, R., A. Hattori, S. Miyata, S. Kudoh, and S. Makino. 1996. A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine-mediated germination. J. Bacteriol. 1786059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriyama, R., S. Kudoh, S. Miyata, S. Nonobe, A. Hattori, and S. Makino. 1996. A germination-specific spore cortex-lytic enzyme from Bacillus cereus spores: cloning and sequencing of the gene and molecular characterization of the enzyme. J. Bacteriol. 1785330-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Hartwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons Ltd., Chichester, England.
- 36.Paidhungat, M., B. Setlow, W. B. Daniels, D. Hoover, E. Papafragkou, and P. Setlow. 2002. Mechanisms of induction of germination of Bacillus subtilis spores by high pressure. Appl. Environ. Microbiol. 683172-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez-Casal, J., J. A. Price, E. Maguin, and J. R. Scott. 1993. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol. Microbiol. 8809-819. [DOI] [PubMed] [Google Scholar]
- 38.Popham, D. L., M. E. Gilmore, and P. Setlow. 1999. Roles of low-molecular-weight penicillin-binding proteins in Bacillus subtilis spore peptidoglycan synthesis and spore properties. J. Bacteriol. 181126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J. Bacteriol. 1786451-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. USA 9315405-15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn, C. P., and B. N. Dancer. 1990. Transformation of vegetative cells of Bacillus anthracis with plasmid DNA. J. Gen. Microbiol. 1361211-1215. [DOI] [PubMed] [Google Scholar]
- 42.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage in spore germination. J. Bacteriol. 1834894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setlow, P. 2000. Resistance of bacterial spores, p. 217-230. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, DC.
- 44.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550-556. [DOI] [PubMed] [Google Scholar]
- 45.Warth, A. D. 1985. Mechanisms of heat resistance, p. 209-225. In G. J. Dring, D. J. Ellar, and G. W. Gould (ed.), Fundamental and applied aspects of bacterial spores. Academic Press, London, United Kingdom.
- 46.Warth, A. D., and J. L. Strominger. 1972. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry 111389-1396. [DOI] [PubMed] [Google Scholar]
- 47.Warth, A. D., and J. L. Strominger. 1969. Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc. Natl. Acad. Sci. USA 64528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]