Abstract
Strains of Salmonella enterica lacking YggX and the cellular reductant glutathione exhibit defects similar to those resulting from iron deficiency and oxidative stress. Mutant strains are sensitive to hydrogen peroxide and superoxide, deregulate the expression of the Fur-regulated gene entB, and fail to grow on succinate medium. Suppression of some yggX gshA mutant phenotypes by the cell-permeable iron chelator deferoxamine allowed the conclusion that increased levels of cellular Fenton chemistry played a role in the growth defects. The data presented are consistent with a scenario in which glutathione acts as a physiological chelator of the labile iron pool and in which YggX acts upstream of the labile iron pool by preventing superoxide toxicity.
Iron is an essential mineral that is needed for the function of proteins involved in diverse cellular processes. Most cellular iron is tightly bound by metalloenzymes and iron storage proteins (9). However, there is a small pool of iron loosely chelated by cellular metabolites, such as ATP, citric acid, and free thiols, that has been termed the labile iron pool (32). Quantification of this iron pool has been difficult, and the predominant methods to do so rely on an iron chelator and the subsequent detection of the chelator-iron complex (32). Estimates of the labile iron pool are in the low micromolar range, with one estimate placing it at 10 μM in Escherichia coli (24).
Homeostasis of the labile iron pool is closely linked to oxidative stress. Both hydrogen peroxide (H2O2) and superoxide (O2−·) stress oxidize Fe-S cluster proteins of the dehydratase family, releasing iron into the cytoplasm and increasing the labile iron pool (13, 22). This increase in labile iron further increases oxidative stress since iron participates in Fenton chemistry (Fe2+ + H2O2→Fe3+ + OH− + ·OH), generating reactive hydroxyl radicals (·OH). Chelation of labile iron with the cell-permeable chelator deferoxamine protects cells from Fenton chemistry (21).
The need for iron can be increased by oxidative stress. Iron is required to repair oxidized Fe-S clusters in proteins, and the supplementation of iron into the growth medium improves the growth of strains lacking cytoplasmic superoxide dismutase (3). Since oxidative stress can increase both the toxicity of and the need for iron, the cell regulates mechanisms to deal with iron during times of oxidative stress.
One mechanism to minimize the toxic effects of labile iron is to regulate iron uptake. In Salmonella enterica and other microorganisms, the ferric uptake regulator (Fur) is a transcriptional repressor of genes involved in iron uptake and metabolism. Fur is active (i.e., represses) when bound to Fe2+ (reviewed in references 8 and 19). Recently, H2O2 and superoxide stress have been shown to induce the Fur regulon (5, 41). It has been proposed that the Fur-Fe complex can serve as a substrate for the Fenton reaction, resulting in inactivation of Fur upon oxidation of the iron atom (41).
Glutathione is the main cellular reductant in microorganisms and is present in cells at millimolar levels (23). Glutathione is able to chelate metals via a free thiol (25, 31) and has been shown to reduce Fe3+, although at a lower rate than other cellular thiols, such as cysteine (30). Elevated cysteine levels result in rapid reduction of Fe3+, thereby increasing the rate of Fenton chemistry in the cell (30). The lower rate of metal reduction suggests that in addition to its role as a cellular reductant, glutathione would be an ideal thiol buffer for iron in the cell. The involvement of glutathione in processes involving iron is supported by studies showing that the repair rate of the Fe-S cluster enzyme aconitase after H2O2 damage is lower in cells lacking glutathione (14) and by studies with eukaryotic systems that suggest that glutathione is involved in Fe-S cluster synthesis (35).
YggX is a 10-kDa protein found in S. enterica whose loss results in phenotypes indicative of increased oxidative stress, defects in Fe-S cluster metabolism, and defects in iron metabolism (16, 18, 37, 42). Cells lacking YggX have increased sensitivity to superoxide and an increased mutagenesis rate specific to the types of lesions caused by hydroxyl radicals (16, 18). Mutations in yggX exacerbated the defects caused by lesions in other genes involved with Fe-S cluster metabolism (16, 18, 37, 42). Additional studies indicated that the Fur regulon is induced in strains lacking YggX (18) and that YggX is able to bind Fe2+ with a KD (equilibrium dissociation constant) of 20 μM (11). Mutants lacking yggX suffer chronic oxidative stress, and YggX has been proposed to protect Fe-S cluster proteins from oxidative damage, though no enzymatic mechanism to support this has been identified.
This study was initiated to dissect the phenotypic similarities and differences between strains lacking YggX and/or glutathione and to gain insight into the role each played in cellular metabolism. While the hypothesis that YggX and glutathione contribute by distinct yet integrated mechanisms to maintain labile iron homeostasis in S. enterica is not the only possibility, the studies reported are consistent with it.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
All strains used in this study are derived from S. enterica LT2 and are listed with their respective genotypes in Table 1. The NCE medium described by Berkowitz et al. and others (4, 12) (K2HPO4·3H2O, 30 mM; KH2PO4, 30 mM; NaNH4PO4·4H2O, 20 mM) supplemented with 1 mM MgSO4 was used as minimal medium. Glucose (11 mM), gluconate (11 mM), or succinate (16.5 mM) was provided as the sole carbon source. Thiamine was used at a concentration of 100 nM. In an effort to control metal concentrations, all minimal medium was made using Milli-Q filtered water and culture tubes were used a single time. Luria broth (LB) and Difco nutrient broth (NB) (8 g/liter) with NaCl (5 g/liter) were used as rich media, with Difco BiTek agar added to a final concentration of 1.5% for solid media. When indicated, the medium was treated with Chelex resin as described previously (39). The FeCl3 was added to growth medium after sterilization from a 0.1 M stock in 0.1 N HCl. The final concentrations of antibiotics were as follows: tetracycline, 20 μg/ml; kanamycin, 50 μg/ml; gentamicin, 6 μg/liter; and chloramphenicol, 20 μg/ml. All other chemicals were purchased from Sigma Chemical Co., St. Louis, MO.
TABLE 1.
Bacterial strainsa
| Strain | Genotype |
|---|---|
| DM10000 | Wild type |
| DM7622 | yggX::Gm fpr::MudJbzxx-8077::Tn10dc(Cm) |
| DM7623 | fpr::MudJ zxx-8077::Tn10d(Cm) |
| DM7878 | yggX::Gm entB103::MudJ zxx-8077::Tn10d(Cm) |
| DM7879 | entB103::MudJ zxx-8077::Tn10d(Cm) |
| DM7880 | yggX::Gm ghsA101::Tn10d(Tc) entB103::MudJ zxx-8077::Tn10d(Cm) |
| DM7881 | ghsA101::Tn10d(Tc) entB103::MudJ zxx-8077::Tn10d(Cm) |
| DM7885 | entB103::MudJ fur-1 zbj-5123::Tn10(Tc)d |
| DM8759 | fur-1 zbj-5123::Tn10(Tc) |
| DM8792 | ryhB1::Cm fur-1 zbj-5123::Tn10(Tc) |
| DM9673 | yggX::Gm |
| DM9680 | ghsA101::Tn10d(Tc) |
| DM9720 | yggX::Gm ghsA101::Tn10d(Tc) |
| DM10052 | ΔryhB1 acnA::Kn |
| DM10056 | yggX::Gm ΔryhB1 acnA::Kn |
| DM10057 | ghsA101::Tn10d(Tc) ΔryhB1 acnA::Kn |
| DM10063 | yggX::Gm ghsA101::Tn10d(Tc) ΔryhB1 acnA::Kn |
Growth analysis.
Growth in liquid medium was quantified by monitoring three independent cultures for each strain in each condition. Strains were grown overnight at 37°C in NB medium, harvested, and resuspended in an equal volume of saline, and 100 μl was inoculated into 5 ml of the appropriate medium in 18- by 150-mm culture tubes. Cultures were placed in an air shaker at 37°C, and growth was monitored by measuring the optical density at 650 nm (OD650) with a Bausch & Lomb Spectronic 20. The starting OD650 was routinely between 0.02 and 0.07 for growth curves.
Strain construction.
Standard genetic techniques were used to construct and verify multiple mutant strains.
Sensitivity to H2O2.
Strains were grown in triplicate overnight at 37°C in NB medium, harvested, and resuspended in an equal volume of saline, and 100 μl was inoculated into 5 ml of minimal glucose medium supplemented with 100 nM thiamine and 0.2% Casamino Acids in 18- by 150-mm culture tubes. Cells were grown to an OD650 of 0.3 and were then placed on ice until all cultures were ready, after which cultures were placed at room temperature without shaking. Before (time zero) and also 1.5 and 3 h after the addition of 8 mM H2O2, 100 μl of cells from each culture were diluted 1:10 into NCE medium containing 1,300 U/ml catalase. Cells were diluted prior to plating on LB medium and incubated overnight at 37°C. The CFU/ml were recorded for each strain at each time point of H2O2 exposure.
Enzyme assays.
All enzyme assays were performed using three independent cultures for each strain. Protein concentrations were determined using the Bradford assay (7), and cell extracts routinely contained ∼500 μg/ml protein.
(i) β-Galactosidase assays.
Overnight NB cultures grown at 37°C were subcultured 50 μl into 5 ml of Chelex resin-treated LB with added MgSO4 (1 mM), trace minerals (1×) lacking iron (2), and FeCl3 (10 μM). Cultures were placed in an air shaker at 37°C and grown to an OD650 of 0.4 before being harvested and resuspended in 14.6 mM NaCl. Cells were assayed for β-galactosidase activity according to the method described by Miller (29).
(ii) Aconitase assays.
Overnight NB cultures grown at 37°C were inoculated 150 μl into 5 ml minimal NCE gluconate medium supplemented with 0.2% Casamino Acids, 100 nM thiamine, and 50 μM FeCl3. Cell extracts were generated, and assays were performed as previously described (36). Values are reported as specific activity (ΔA240/min/mg protein) and also relative activity compared to a parent strain grown under defined conditions.
(iii) SDH assays.
Succinate dehydrogenase (SDH) activity was assayed with the extracts generated for the aconitase assays described above. Assays were performed as described previously (36). Values are reported as specific activity (ΔA600/min/mg protein) and also relative activity compared to a parent strain grown under defined conditions.
RESULTS
Strains lacking YggX and glutathione have increased available iron.
The inhibitory effects of the antibiotic streptonigrin are correlated with iron availability. In the presence of iron, streptonigrin binds DNA and causes damage to the chromosome, resulting in death (6). Growth of S. enterica is slowed in the presence of streptonigrin (data not shown), and a yggX gshA double mutant strain showed enhanced sensitivity to this antibiotic. After 24 h, the wild-type strain grown in NB with or without 0.5 μg/ml streptonigrin reached an OD650 of 0.91 ± 0.01 or 1.14 ± 0.01, respectively. In the same conditions, the double mutant (yggX gshA) reached an OD650 of 0.47 ± 0.13 or 1.16 ± 0.04, respectively. Growth of the yggX and gshA single mutant strains in the presence of streptonigrin was indistinguishable from that of the wild type.
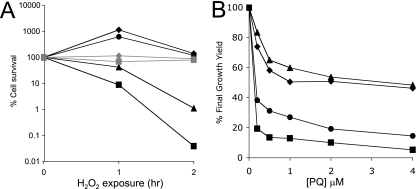
The toxicity of H2O2 is mediated in part through iron participating in the Fenton reaction (22). When challenged with 8 mM H2O2 for 2 h, the yggX gshA double mutant strain showed ∼0.05% survival compared to 100% for the wild type (Fig. 1A). When deferoxamine (2 mM), a cell-permeable iron chelator, was present during the H2O2 challenge, the yggX gshA strain showed ∼80% survival (Fig. 1A). These data supported the conclusion that a larger pool of iron was available for Fenton chemistry in the yggX gshA mutant strain than in the wild type.
FIG. 1.
Strains lacking YggX and GshA display distinct sensitivity to H2O2 and superoxide. Strains DM10000 (wild type; diamonds), DM9673 (yggX; circles), DM9680 (gshA; triangles), and DM9720 (yggX gshA; squares) were tested for sensitivity toward H2O2 and superoxide. In each case, the data points represent the average results from triplicate cultures. (A) Cells were grown at 37°C with shaking to an OD650 of 0.3 in minimal glucose medium containing 100 nM thiamine and 0.2% Casamino Acids with (gray) or without (black) 2 mM deferoxamine. At the indicated times after exposure to 8 mM H2O2, cells were diluted 1:10 into minimal NCE medium containing 1,300 U/ml catalase and then dilution plated onto LB medium. (B) Cells were grown at 37°C with shaking in minimal glucose medium containing 100 nM thiamine and indicated concentrations of Paraquat (PQ). The OD650 after 24 h of growth was recorded and standardized to the growth achieved with no Paraquat to give a percent final growth yield.
Lack of glutathione and a yggX mutation contribute to an elevated available iron pool by different mechanisms.
Two reactive oxygen species (superoxide and H2O2) that impact the labile iron pool were considered in this study. While the yggX gshA double mutant was more sensitive to both reactive species, the single mutants had distinct sensitivity profiles (Fig. 1). The gshA mutant strain was not sensitive to Paraquat, while the yggX mutant was almost as sensitive to this superoxide-generating compound as the double mutant (Fig. 1B) (16). In contrast, the gshA mutant was sensitive to H2O2, while the yggX mutant was unaffected (Fig. 1A).
As previously reported, yggX mutant strains have increased expression of the SoxR reporter fpr, which is exacerbated by a gshA mutation (37). Significantly, the constitutive expression of the fpr reporter in the yggX and yggX gshA mutants (136 ± 30 and 300 ± 45 Miller units, respectively, compared to the wild type at 51 ± 2) was not affected by the addition of deferoxamine (100 ± 5, 249 ± 30, and 50 ± 3, respectively). These data indicated that the induction of the SoxR regulon in a yggX mutant strain was not due to the hydroxyl radical resulting from iron-mediated Fenton chemistry and showed that not all phenotypes of a yggX mutant are the consequence of increased labile iron.
The status of labile iron can be monitored via expression of the Fur-regulated gene entB.
The ferric uptake regulator, Fur, monitors the status of the labile iron pool and acts as a transcriptional repressor when bound to Fe2+ (reviewed in reference 19). One member of the Fur regulon is entB, encoding a protein involved in the synthesis of the siderophore enterobactin. Therefore, measuring the expression of entB provides an indirect measure of the labile iron pool status via Fur activity. Increased entB expression reflects decreased Fur activity (i.e., repression) (40; reviewed in reference 34).
The expression of entB in cells grown under several conditions was measured, and the results are shown in Table 2. The first column of data gives the basal level of expression for strains grown in medium with low iron. Each of the mutant strains showed significant derepression of the entB gene, indicating that less iron-bound Fur was present than in the wild-type strain under the same conditions. The pattern of expression caused by the addition of 40 μM Paraquat most closely paralleled that found in the yggX mutant, consistent with previous reports indicating that the yggX mutant experiences chronic oxidative stress (37). The addition of FeCl3 to the medium repressed expression in all strains and conditions, indicating that the Fur protein was not damaged; rather, the increased expression reflected a decreased amount of Fe2+ bound to the existing Fur. The effect of a number of additions to the medium on entB expression was measured. The resulting data in Table 2, taken together, made several points that can be summarized as follows.
TABLE 2.
Expression of entB from mutant strainsa
| Strain and condition | β-Galactosidase activity
|
|||||
|---|---|---|---|---|---|---|
| Chelex resin-treated LB | Standing culture | FeCl3 | Na2S | Cysteine | Glutathione | |
| Wild type | 75 ± 10 | 20 ± 5 | 5 ± 1 | 22 ± 6 | 24 ± 1 | 18 ± 3 |
| yggX | 149 ± 22 | 17 ± 4 | 4 ± 2 | 37 ± 9 | 70 ± 15 | 21 ± 8 |
| gshA | 264 ± 52 | 28 ± 6 | 6 ± 2 | 236 ± 46 | 216 ± 45 | 34 ± 12 |
| yggX gshA | 396 ± 43 | 25 ± 5 | 14 ± 2 | 238 ± 76 | 329 ± 12 | 98 ± 25 |
| Wild type + 40 μM Paraquat | 154 ± 23 | 26 ± 6 | 6 ± 3 | 35 ± 3 | 29 ± 2 | 29 ± 4 |
| Wild type + 100 μM EDTA | 440 ± 31 | 357 ± 9 | 5 ± 1 | 277 ± 42 | 340 ± 39 | 354 ± 29 |
Values are averages of β-galactosidase activity in Miller units (± standard deviations) from three independent cultures. When added, FeCl3, Na2S, cysteine, and glutathione were all at a concentration of 0.3 mM.
First, no additions to the media (except iron) eliminated the derepression caused by EDTA. Thus, the iron starvation caused by the presence of EDTA in the medium was intrinsically different than the situation generated in the mutant backgrounds or by Paraquat-induced oxidative stress. One interpretation of this result is that in the former case, there was little iron present to bind Fur, and in the latter, iron was present but was not available to Fur.
Second, limiting available oxygen decreased the expression of entB in all situations (except that with EDTA). These data suggested that a primary contributor to the inactivation of Fur was oxidative damage.
Third, in all situations, entB expression was decreased by the addition of glutathione. This result indicated that in the presence of glutathione, more iron-bound Fur was present. Sulfide and cysteine were able to lower entB expression only in strains that had an intact gshA locus, indicating that their effect was due to conversion into glutathione. These data were consistent with a specific role for glutathione in facilitating the binding of iron by Fur or in maintaining an environment in which iron remains bound to Fur.
Chelation of endogenous iron impacts Fur activity.
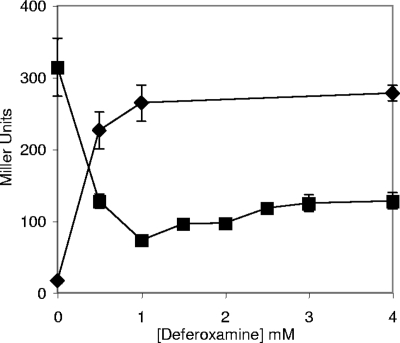
The expression of entB in a wild-type strain and a yggX gshA strain was measured in the presence of increasing amounts of the cell-permeable iron chelator deferoxamine. In the wild-type strain, entB expression increased with deferoxamine concentration as expected (Fig. 2). In contrast, entB expression in the yggX gshA strain was decreased by deferoxamine, indicating the presence of more iron-bound Fur. These data are consistent with a scenario in which the increased entB expression in the yggX gshA strain (Table 2) was due partially to increased Fenton chemistry. In this scenario, deferoxamine chelated iron and removed it from the pool that participated in Fenton chemistry. After the addition of 1 mM deferoxamine, entB transcription leveled off significantly above the value obtained when Fur was completely occupied. These results suggested that equilibrium was achieved between damage generated by iron-mediated Fenton chemistry and iron available to Fur. In the absence of glutathione, the level of entB transcription in the presence of 2 mM deferoxamine is significantly lower than in the strains able to synthesize glutathione (Fig. 2 and data not shown). One explanation could be that the combined chelation power of the millimolar levels of glutathione and deferoxamine limit the availability of iron to Fur more than that limited by the presence of deferoxamine alone.
FIG. 2.
The expression of entB is affected by the presence of deferoxamine differently in strains lacking YggX and GshA. Cells containing an entB::lacZ gene fusion, DM7879 (wild type; diamonds) and DM7880 (yggX and gshA mutant; squares), were grown in Chelex resin-treated LB growth medium with added trace minerals, 10 μM FeCl3, and the indicated concentration of deferoxamine to an OD650 of 0.4 before being harvested and assayed for β-galactosidase activity. Error bars show variations in results from triplicate cultures.
The labile iron pool impacts the activity of Fe-S cluster enzymes.
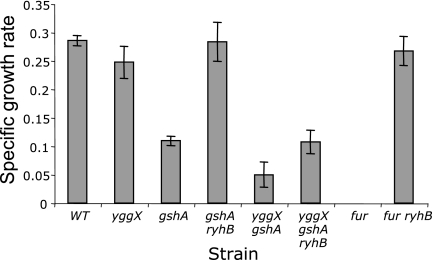
A fur mutant is unable to grow on succinate as a sole carbon source due to a lack of positive regulation of the genes encoding the Fe-S cluster containing the enzymes SDH, fumarase A, and aconitase B (AcnB) of the tricarboxylic acid (TCA) cycle by Fur, which is mediated by the small RNA RyhB (26, 27). A yggX gshA strain was similarly defective in growth on succinate medium (Fig. 3). This growth defect could be caused by RyhB via Fur and/or decreased cluster occupancy of the relevant enzymes in the TCA cycle. A mutation in ryhB restored growth of both a fur mutant and a gshA strain (Fig. 3), indicating that in these strains, the growth defect was due primarily to regulatory effects through Fur. However, mutating ryhB failed to restore growth of the yggX gshA strain in the same medium, suggesting that the defect in the double mutant went beyond the regulatory effect mediated via Fur.
FIG. 3.
Cells lacking YggX and GshA are unable to grow in minimal succinate medium. The indicated strains were grown at 37°C with shaking in minimal succinate medium containing 100 nM thiamine with the addition of 50 μM FeCl3. Growth is reported as the specific growth rate (μ) and was calculated by the equation ln(X/X0)/T, where X is A650, X0 is A650 at time zero, and T is time (in hours). Bars represent errors from triplicate cultures. No growth was seen for the fur mutant strain.
The activities of two relevant enzymes, SDH and AcnB, were monitored to address the hypothesis that the growth defect in the yggX gshA mutant strain on succinate was due to the status of Fe-S cluster metabolism in the strain. SDH and AcnB activities were assayed by using a ryhB acnA background to negate regulatory effects and contributions from the oxygen-stable aconitase A protein. Western blot analysis indicated that similar levels of AcnB protein were present in all strains tested for activity. Both enzyme activities were significantly decreased in the yggX gshA double mutant strain (Table 3). These data support the hypothesis that low activity of multiple TCA cycle enzymes prevented growth of the double mutant (yggX gshA) on succinate medium.
TABLE 3.
Lack of YggX and GshA decrease enzyme activities
| Strain | Relevant genotype | Sp act (relative activity)a
|
|
|---|---|---|---|
| AcnB | SDH | ||
| DM10052 | ryhB acnA | 3,954 ± 331 (1.00) | 2,854 ± 254 (1.00) |
| DM10056 | yggX ryhB acnA | 2,777 ± 241 (0.70) | 2,148 ± 148 (0.75) |
| DM10057 | gshA ryhB acnA | 3,024 ± 158 (0.76) | 1,618 ± 421 (0.57) |
| DM10063 | yggX gshA ryhB acnA | 1,507 ± 111 (0.38) | 546 ± 154 (0.19) |
The specific activity (ΔA/min/mg protein [average ± standard deviation]) for three independent cultures is given. The relative activity, obtained by dividing the activity of the relevant strain by the activity of the ryhB acnA parent, is shown in parentheses.
DISCUSSION
The integration of iron homeostasis and reactive oxygen species complicates efforts to dissect the role of cellular components that balance the beneficial and harmful aspects of the iron pool. The data presented herein confirmed that the small protein YggX and the cellular reductant glutathione are players in iron homeostasis. Specifically, the data showed that a yggX gshA mutant strain had increased levels of Fenton chemistry, the Fur iron complex is a target of hydroxyl radical stress, and the action of YggX in preventing Fenton chemistry is most significant for superoxide stress and upstream of labile iron effects. A working model incorporates these data with previously observed interactions between various forms of endogenously generated oxidative stress and the labile iron pool. Superoxide damages labile Fe-S clusters, resulting in the release of iron (13). This iron can react with H2O2 in the Fenton reaction, creating the reactive hydroxyl radical (24). Our current model suggests that YggX functions to prevent the action of superoxide on oxygen-labile Fe-S clusters, possibly by preventing the endogenous formation of superoxide. In this scenario, glutathione acts as a physiological chelator of the labile iron pool that prevents the Fenton reaction.
The increased Fenton chemistry in strains lacking YggX and glutathione has multiple consequences. The yggX gshA mutant strain exhibits behavior consistent with increased internal levels of Fenton chemistry affecting multiple processes (1, 18, 28, 42). Here, we describe two additional phenotypes of the yggX gshA mutant strain (increased sensitivity to H2O2 and increased expression of the Fur regulon, specifically entB) that are corrected by deferoxamine and thus likely caused by Fenton chemistry (24). Consistent with a role for Fenton chemistry in generating the yggX gshA mutant phenotypes, phenotypes described here and previously are corrected by growth under anoxic or oxygen-limiting conditions (17, 37). Deregulation of the Fur regulon was recognized for E. coli cells exposed to superoxide and H2O2 stress (5, 41), both of which can increase cellular Fenton chemistry (24, 30). Correction of the Fur regulatory defect in the yggX gshA strain by deferoxamine is consistent with Fur being a target sensitive to the hydroxyl radical. In our model, the lack of glutathione results in increased iron available to Fenton chemistry, resulting in increased hydroxyl radical stress, which can damage Fur. The damage to Fur by the hydroxyl radical could occur at sites other than the iron binding site, in which case deferoxamine could prevent Fur damage by chelating labile iron without necessarily chelating iron away from the Fur protein.
Based on previous literature and results presented herein, we propose that glutathione is a physiologically relevant chelating agent for the labile iron pool. Glutathione is able to chelate iron as well as other metals (20, 25, 31, 38) and is found at millimolar levels in the cytoplasm (20, 23). In the absence of glutathione and YggX, labile iron is more available to bind to another chelator, streptonigrin. The conclusion that more iron is available to participate in Fenton chemistry in strains lacking glutathione can be drawn from the finding that the intracellular iron chelator deferoxamine reversed the defects of a gshA mutant, such as H2O2 sensitivity and increased entB transcription.
While it is not the only possibility, our preferred model suggests that glutathione plays an essential role that allows the cell to maintain a labile iron pool while minimizing Fenton chemistry. This is accomplished by preventing redox cycling of iron while ensuring that iron is available to cellular processes. Compounds such as cysteine and flavins contribute to iron redox cycling by reducing Fe3+, resulting in increased Fenton chemistry (30, 44). When tested both in vivo and in vitro, glutathione reduced Fe3+ at a significantly lower rate and did not increase levels of Fenton chemistry (30, 44). Deferoxamine traps iron in the 3+ oxidation state with a binding constant of 1031. Although this effectively prevents Fenton chemistry, cells exposed to deferoxamine are starved for iron. The cellular abundance and moderate chelation ability of glutathione suggest it could be a component in maintaining homeostasis of the labile iron pool.
A growing body of data links YggX to a specific role in preventing superoxide toxicity. YggX is a member of the Sox regulon (33), and its absence has been shown to induce the expression of fpr, another member of the Sox regulon (37). A yggX mutant strain is more sensitive to the superoxide-generating compound Paraquat (16), yet the same mutant strain was shown here not to be sensitive to a different reactive oxygen species, H2O2. Although past observations have been interpreted to mean YggX was involved in iron trafficking, data presented here are most consistent with YggX acting upstream of the labile iron pool. The effects of YggX on labile iron homeostasis illustrated here and elsewhere can be explained with superoxide as an intermediate. Specifically, deferoxamine did not decrease the expression of fpr in a yggX mutant strain, indicating that this phenotype was not mediated through the labile iron pool.
The complex interplay between labile iron homeostasis and oxidative stress involves several constituents. The mutants with a loss of yggX and glutathione display additive defects impacting both systems, and the work here was used to begin unraveling the specific roles of these cellular components. These additional insights will facilitate continued efforts to understand the integrated system that functions in vivo.
Acknowledgments
This work was supported by the competitive grant program of the National Science Foundation (MCB-0445654). Funds were also provided from a 21st Century Scientists Scholars Award from the J. M. McDonnell fund to D.M.D. M.P.T. was supported by a Biotechnology Traineeship from the NIH (T32 GM08349) and a Jerome J. Stefaniak Predoctoral Fellowship from the Department of Bacteriology.
Footnotes
Published ahead of print on 3 October 2008.
REFERENCES
- 1.Aruoma, O. I., B. Halliwell, E. Gajewski, and M. Dizdaroglu. 1989. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J. Biol. Chem. 26420509-20512. [PubMed] [Google Scholar]
- 2.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benov, L., and I. Fridovich. 1998. Growth in iron-enriched medium partially compensates Escherichia coli for the lack of manganese and iron superoxide dismutase. J. Biol. Chem. 27310313-10316. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz, D., J. M. Hushon, H. J. Whitfield, J. Roth, and B. N. Ames. 1968. Procedure for identifying nonsense mutations. J. Bacteriol. 96215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard, J. L., W. Y. Wholey, E. M. Conlon, and P. J. Pomposiello. 2007. Rapid changes in gene expression dynamics in response to superoxide reveal SoxRS-dependent and independent transcriptional networks. PLoS ONE 2e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolzan, A. D., and M. S. Bianchi. 2001. Genotoxicity of streptonigrin: a review. Mutat. Res. 48825-37. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 8.Braun, V. 1997. Avoidance of iron toxicity through regulation of bacterial iron transport. Biol. Chem. 378779-786. [PubMed] [Google Scholar]
- 9.Briat, J. F. 1992. Iron assimilation and storage in prokaryotes. J. Gen. Microbiol. 1382475-2483. [DOI] [PubMed] [Google Scholar]
- 10.Castilho, B. A., P. Olfson, and M. J. Casadaban. 1984. Plasmid insertion mutagenesis and lac gene fusion with mini-Mu bacteriophage transposons. J. Bacteriol. 158488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui, Q., M. P. Thorgersen, W. M. Westler, J. L. Markley, and D. M. Downs. 2006. Solution structure of YggX: a prokaryotic protein involved in Fe(II) trafficking. Proteins 62578-586. [DOI] [PubMed] [Google Scholar]
- 12.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 13.Flint, D. H., J. F. Tuminello, and M. H. Emptage. 1993. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 26822369-22376. [PubMed] [Google Scholar]
- 14.Gardner, P. R., and I. Fridovich. 1993. Effect of glutathione on aconitase in Escherichia coli. Arch. Biochem. Biophys. 30198-102. [DOI] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Gralnick, J., and D. Downs. 2001. Protection from superoxide damage associated with an increased level of the YggX protein in Salmonella enterica. Proc. Natl. Acad. Sci. USA 988030-8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gralnick, J., E. Webb, B. Beck, and D. Downs. 2000. Lesions in gshA (encoding γ-l-glutamyl-l-cysteine synthetase) prevent aerobic synthesis of thiamine in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 1825180-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gralnick, J. A., and D. M. Downs. 2003. The YggX protein of Salmonella enterica is involved in Fe(II) trafficking and minimizes the DNA damage caused by hydroxyl radicals: residue CYS-7 is essential for YggX function. J. Biol. Chem. 27820708-20715. [DOI] [PubMed] [Google Scholar]
- 19.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4172-177. [DOI] [PubMed] [Google Scholar]
- 20.Helbig, K., C. Bleuel, G. J. Krauss, and D. H. Nies. 2008. Glutathione and transition-metal homeostasis in Escherichia coli. J. Bacteriol. 1905431-5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imlay, J. A., and S. Linn. 1988. DNA damage and oxygen radical toxicity. Science 2401302-1309. [DOI] [PubMed] [Google Scholar]
- 22.Jang, S., and J. A. Imlay. 2007. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 282929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jocelyn, P. C. 1972. Biochemistry of the sulfhydryl group. Academic Press, New York, NY.
- 24.Keyer, K., and J. A. Imlay. 1996. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA 9313635-13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, N. C., and R. A. Manning. 1955. Some metal complexes of sulfur-containing amino acids. J. Am. Chem. Soc. 775225-5227. [Google Scholar]
- 26.Massé, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. USA 994620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massé, E., C. K. Vanderpool, and S. Gottesman. 2005. Effect of RyhB small RNA on global iron use in Escherichia coli. J. Bacteriol. 1876962-6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride, T. J., B. D. Preston, and L. A. Loeb. 1991. Mutagenic spectrum resulting from DNA damage by oxygen radicals. Biochemistry 30207-213. [DOI] [PubMed] [Google Scholar]
- 29.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 30.Park, S., and J. A. Imlay. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J. Bacteriol. 1851942-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrin, D. D., and A. E. Watt. 1971. Complex formation of zinc and cadmium with glutathione. Biochim. Biophys. Acta 23096-104. [DOI] [PubMed] [Google Scholar]
- 32.Petrat, F., H. de Groot, R. Sustmann, and U. Rauen. 2002. The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 383489-502. [DOI] [PubMed] [Google Scholar]
- 33.Pomposiello, P. J., A. Koutsolioutsou, D. Carrasco, and B. Demple. 2003. SoxRS-regulated expression and genetic analysis of the yggX gene of Escherichia coli. J. Bacteriol. 1856624-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raymond, K. N., E. A. Dertz, and S. S. Kim. 2003. Enterobactin: an archetype for microbial iron transport. Proc. Natl. Acad. Sci. USA 1003584-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sipos, K., H. Lange, Z. Fekete, P. Ullmann, R. Lill, and G. Kispal. 2002. Maturation of cytosolic iron-sulfur proteins requires glutathione. J. Biol. Chem. 27726944-26949. [DOI] [PubMed] [Google Scholar]
- 36.Skovran, E., and D. M. Downs. 2000. Metabolic defects caused by mutations in the isc gene cluster in Salmonella enterica serovar Typhimurium: implications for thiamine synthesis. J. Bacteriol. 1823896-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skovran, E., C. T. Lauhon, and D. M. Downs. 2004. Lack of YggX results in chronic oxidative stress and uncovers subtle defects in Fe-S cluster metabolism in Salmonella enterica. J. Bacteriol. 1867626-7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiura, Y., and H. Tanaka. 1972. Iron-sulfide chelates of some sulfur-containing peptides as model complex of non-heme iron proteins. Biochem. Biophys. Res. Comm. 46335-340. [DOI] [PubMed] [Google Scholar]
- 39.Thorgersen, M. P., and D. M. Downs. 2007. Cobalt targets multiple metabolic processes in Salmonella enterica. J. Bacteriol. 1897774-7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsolis, R. M., A. J. Baumler, I. Stojiljkovic, and F. Heffron. 1995. Fur regulon of Salmonella typhimurium: Identification of new iron-regulated genes. J. Bacteriol. 1774628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varghese, S., A. Wu, S. Park, K. R. Imlay, and J. A. Imlay. 2007. Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol. Microbiol. 64822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vivas, E., E. Skovran, and D. M. Downs. 2006. Salmonella enterica strains lacking the frataxin homolog CyaY show defects in Fe-S cluster metabolism in vivo. J. Bacteriol. 1881175-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Way, J. C., M. A. Davis, D. Morisato, D. E. Roberts, and N. Kleckner. 1984. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene 32369-379. [DOI] [PubMed] [Google Scholar]
- 44.Woodmansee, A. N., and J. A. Imlay. 2002. Reduced flavins promote oxidative DNA damage in non-respiring Escherichia coli by delivering electrons to intracellular free iron. J. Biol. Chem. 27734055-34066. [DOI] [PubMed] [Google Scholar]