Abstract
The actinomycete Streptomyces scabies 87-22 is the causal agent of common scab, an economically important disease of potato and taproot crops. Sequencing of the S. scabies 87-22 genome revealed the presence of a gene with high homology to the gene encoding the α-tomatine-detoxifying enzyme tomatinase found in fungal tomato pathogens. The tomA gene from S. scabies 87-22 was cotranscribed with a putative family 1 glycosyl hydrolase gene, and purified TomA protein was active only on α-tomatine and not potato glycoalkaloids or xylans. Tomatinase-null mutants were more sensitive to α-tomatine than the wild-type strain in a disk diffusion assay. Interestingly, tomatine affected only aerial mycelium and not vegetative mycelium, suggesting that the target(s) of α-tomatine is not present during vegetative growth. Severities of disease for tomato seedlings affected by S. scabies 87-22 wild-type and ΔtomA1 strains were indistinguishable, suggesting that tomatinase is not important in pathogenicity on tomato plants. However, conservation of tomA on a pathogenicity island in S. acidiscabies and S. turgidiscabies suggests a role in plant-microbe interaction.
Streptomycetes are mainly benign saprophytic soil bacteria that produce nearly two-thirds of the world's naturally occurring antibiotics (5). However, in the genus Streptomyces, there are a few pathogens, some of which cause common scab of potatoes and other taproot crops (38). These pathogens are general necrotic pathogens and aggressively colonize root structures and possess multiple virulence factors (36, 37). Production of the nonribosomally synthesized dipeptide phytotoxin thaxtomin is required for pathogenicity (20, 33). Thaxtomin inhibits cellulose synthesis on actively growing plant tissue (49). The biosynthesis of thaxtomin has been characterized (19, 20, 29), and transcription of the biosynthetic genes is activated by the AraC family regulator TxtR, which binds cellobiose to facilitate transcription (25). Scab-causing streptomycetes also contain a novel necrogenic protein, Nec1, which is important in pathogenesis and may be involved in the suppression of plant defenses (10, 24). Recently, sequencing of a self-mobilizable pathogenicity island in S. turgidiscabies revealed that a putative tomatinase gene was conserved among three phylogenetically distinct scab-causing streptomycetes, S. turgidiscabies Car8, S. acidiscabies, and S. scabies (also known as S. scabiei) 87-22 (28).
Genes encoding tomatinase enzymes are typically found in fungal pathogens of tomato plants, such as Septoria lycopersici and Fusarium oxysporum f. sp. lycopersici, which cause disease on tomato plants. Tomatinase belongs to a class of enzymes called saponinases; such enzymes function as glycosyl hydrolases that detoxify phytoanticipins, which are preformed antimicrobial molecules produced by plants (52). Tomatinase detoxifies the steroidal tomato phytoanticipin α-tomatine by hydrolysis of one or more sugar residues (43) that are essential for α-tomatine activity (30). The toxicity of α-tomatine is typically mediated through complexing with sterols in eukaryotic membranes to ultimately cause membrane pores and cell lysis (30, 31). In addition to nullification of an antimicrobial compound, tomatinases are able to indirectly suppress the induced plant defense response; this presumably results from plant recognition of by-products from α-tomatine hydrolysis (8, 22). Despite the obvious advantage for a tomato pathogen to have a tomatinase in its arsenal, tomatinase-null mutants are not impaired in their ability to cause disease on tomato plants (42, 46). However, a tomatinase enzyme in Septoria lycopersici was shown to be important for infection of tobacco (8), and the avenacin-detoxifying enzyme from Gaeumannomyces graminis is required for pathogenicity in oats and contributes to host range (9).
Recently, a bacterial tomatinase gene in the tomato wilt pathogen Clavibacter michiganensis subsp. michiganensis was characterized; however, tomatinase mutants were not compromised in virulence (27). Interestingly, the closely related subspecies C. michiganensis subsp. sepedonicus, a potato pathogen, lacks a tomatinase gene (4). The presence of a saponinase in the actinomycetes C. michiganensis subsp. michiganensis, S. scabies 87-22, S. acidiscabies, and S. turgidiscabies but not in other bacterial plant pathogens is curious. The location of the tomatinase gene on pathogenicity islands suggests a role in pathogenicity (17, 28). The objectives of this study were to (i) characterize the substrate specificity of the saponinase from the broad-host-range pathogen S. scabies 87-22, (ii) describe streptomycete growth in the presence of saponins and assess whether the saponinase mitigated any negative effects, and (iii) explore the role of the saponinase in virulence of S. scabies 87-22.
MATERIALS AND METHODS
Bacterial strains and culturing conditions.
Escherichia coli strains were cultured as previously described (32, 47). Streptomyces strains were cultured at 28°C using International Streptomyces Project 4 agar media, mannitol-soya flour agar, or tryptic soy broth (TSB) medium. All liquid cultures were shaken at ∼250 rpm. Media were supplemented with antibiotics at the following concentrations: 100 μg/ml apramycin, 50 μg/ml kanamycin, 100 μg/ml ampicillin, 50 μg/ml streptomycin, 25 μg/ml nalidixic acid, and 10 μg/ml thiostrepton. All Streptomyces strains were generated by cross-genus conjugation from the nonmethylating E. coli strain ET12567/pUZ8002 as previously described (32). Strains and plasmids are described in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or commentsa | Source or reference |
|---|---|---|
| S. scabies strains | ||
| 87-22 | Wild type | This study |
| ΔtomA1 | 87-22 tomA-null mutant (isolate 1) | This study |
| ΔtomA2 | 87-22 tomA-null mutant (isolate 2) | This study |
| ΔtomA1 attB ΦC31::pAU34-5 | tomA1-null mutant bearing empty complementation vector | This study |
| ΔtomA1 attB ΦC31::pRFSRL20 | Complemented tomA1-null mutant | This study |
| E. coli strains | ||
| BW25113 | Host for Redirect PCR targeting system | 18 |
| ET12567 | Nonmethylating host for transfer of DNA into Streptomyces spp. (dam, dcm, and hsdS) | 41 |
| DH5α | F− general cloning host | Gibco-BRL |
| TOP10 | Host for pCR2.1TOPO cloning system | Invitrogen |
| Plasmids | This study | |
| pBACher | BAC backbone for S. scabies 87-22 BACs; Camr | Invitrogen |
| 14h01 | pBACher derivative containing tomA locus; Camr | This study |
| pCR2.1TOPO | Cloning vector for PCR products; Kanr Ampr | Invitrogen |
| pET30a | Protein expression vector; IPTG-inducible T7 promoter system; Kanr | Novagen |
| pIJ773 | PCR template for aac3(IV) plus oriT cassette used in Redirect PCR targeting system | 18 |
| pIJ779 | PCR template for aadA cassette used in Redirect PCR targeting system | 18 |
| pUZ8002 | Encodes conjugation machinery for mobilization of plasmids from E. coli to Streptomyces; Kanr | 41 |
| pKD46 | Encodes lambda Red recombination machinery induced by arabinose; Ampr | 12 |
| pAU34-5 | pSET152 derivative, integrates into the ΦC31 attB site in Streptomyces; Aprr Tspr | 7 |
| pHZ1272 | PCR template for pIJ101 origin of replication used to construct pRFSRL36 | Zixin Deng |
| pRFSRL13 | pET30a derivative with ∼1.6-kb tomA gene cloned into NdeI-HindIII sites; Kanr | This study |
| pRFSRL20 | pAU3-45 derivative with a ∼3.4-kb fragment containing the tomA locus cloned into the XbaI site | This study |
| pRFSRL36 | pRFSRL20 derivative with a ∼2.2-kb fragment containing the pIJ101 origin of replication cloned into the SphI-HindIII sites | This study |
Amp, ampicillin; Apr, apramycin; Cam, chloramphenicol; Kan, kanamycin; Tsp, thiostrepton.
Cloning and plasmid construction.
Standard molecular biology procedures were used for all DNA manipulations and plasmid construction in this study (32, 47). Commercial enzymes were purchased from New England Biolabs (Beverly, MA). Unless otherwise noted, insert DNA for cloning was generated by PCR using Phusion Taq polymerase. Integrated DNA Technologies (Coralville, IA) synthesized all oligonucleotides used in PCRs. PCR products were cloned into pCR2.1TOPO (Invitrogen) and sequenced (Biotechnology Resource Center, Cornell University). Vector and pCR2.1TOPO insert-containing plasmids were digested with an appropriate restriction enzyme. Gel-purified insert and vector fragments were ligated together using T4 DNA ligase and transformed into E. coli DH5α cells. Clones were verified by restriction digestion.
Overexpression and purification of TomA-His6.
The tomA gene without its putative secretion signal (15) from S. scabies 87-22 was PCR amplified and cloned into pET30a (Novagen) to produce pRFSRL13 and moved into the E. coli expression strain BL21(λDE3). An overnight culture of BL21(λDE3)/pRFSRL13 (5 ml) was used for inoculation of 500 ml of LB containing kanamycin. Cells were grown to an optical density at 600 nm (OD600) of ∼0.600, at which point TomA-His6 protein production was induced with 1 mM IPTG (isopropyl-d-thiogalactopyranoside). After 3 h of induction, cells were harvested by centrifugation. The cell pellet was resuspended in 50 ml 10 mM Tris-Cl (pH 8.5) and mechanically disrupted by sonication (10-min duration, 20-s pulse, and 20-s rest on ice). TomA-His6 was purified from lysate by Ni-nitrilotriacetic acid (Qiagen) affinity chromatography according to the manufacturer's instructions with a 40 mM imidazole wash step. Eluted fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). TomA-His6 was purified to >90% purity and quantified using the Bradford method with bovine serum albumin as a standard. Prior to activity assays, a buffer exchange using Microcon YM-30 columns (Millipore) was performed with 10 mM Tris-Cl (pH 8.5) to ensure that protein function would not be inhibited by the high concentration of imidazole present in the elution buffer.
TLC analysis.
TomA-His6 reaction products were examined using the method used in reference 46 with the following modifications. TomA-His6 protein (6 μg) or crude lysate from BL21(λDE3)/pET30a (100 μg) was incubated with 1 mM α-tomatine and incubated at 42°C for 2 h. Following NH4OH precipitation of reaction products, pellets were dissolved in 15 μl of 100% methanol and 10 μl of thin-layer chromatography (TLC) solvent and spotted onto a silica 60 TLC plate (Whatman). Metabolites were identified by cochromatography of standards. The chemicals α-tomatine and tomatidine were purchased from LTD chemicals and MP Biomedicals, respectively.
Enzyme activity assays.
Enzyme activity was measured with PAHBAH (p-hydroxybenzoic acid hydrazide) to determine the amount of reducing sugar present (35). Reaction mixtures containing 0.5 mM to 4.0 mM α-tomatine, 1 mM α-solanine or α-chaconine, or 0.5% xylans from birchwood or oat spelt (Sigma) were prepared essentially as described for the TLC analysis; however, 5 μg of TomA-His6 was used instead of 6 μg. One-hundred micrograms of crude lysate from BL21(λDE3)/pET30a served as a negative control. Reactions were stopped by the addition of 800 μl of PAHBAH reagent and processed as described previously (35). The kinetic constant (Km) was estimated by the Lineweaver-Burk method, using glucose as a standard.
Generation of mutant strains.
S. scabies ΔtomA strains were created using the PCR-targeting Redirect technology (18). A disruption cassette consisting of an oriT and the aac(3)IV apramycin resistance gene from pIJ773 was generated by PCR amplification with primers that contained 39 nucleotides (nt) of homology that included the start or stop codons of tomA and 36 nt downstream or upstream of the open reading frame. The resulting PCR product was gel purified and electroporated into E. coli BW25113 containing the λ Red gene-carrying plasmid, pKD46 and tomA bacterial artificial chromosome (BAC) 14h02. Transformants were screened for the presence of mutagenized BAC by colony PCR. Because of antibiotic incompatibilities in downstream procedures, the chloramphenicol resistance gene present on the BAC backbone was replaced with the streptomycin resistance gene aadA from pIJ779 by using λ Red recombination in a manner similar to that described above. Mutagenized BAC DNA was transferred to S. scabies via conjugation. Transconjugants were selected for apramycin resistance and streptomycin sensitivity. Deletion of tomA was confirmed by colony PCR and Southern blot hybridization.
Complementation of the tomA1-null mutant.
To complement the ΔtomA1 mutant, a 3.4-kb fragment containing the tomA gene and ∼1,800 bp of upstream DNA was PCR amplified and cloned into pAU34-5 at the XbaI site. The resulting plasmid, pRFSRL20, was moved to the ΔtomA1 strain by conjugation. Transconjugants were selected for thiostrepton resistance.
α-Tomatine bioassay.
A disk diffusion assay with α-tomatine was used to assess saponin sensitivity. Mannitol-soya flour agar plates (pH 6.8) were seeded with a lawn of bacteria by streaking 100 μl of spores. Sterilized paper disks with a diameter of 6 mm were securely placed on top of the agar. α-Tomatine (0.3 μmol) dissolved in 50 mM Na citrate (pH 4.0) was applied to the center of three disks. Sodium citrate buffer without α-tomatine was used as a negative control. Plates were incubated at 28°C for approximately 3 days before measuring zones of inhibition. A zone of inhibition was defined as the diameter over which aerial mycelium was absent.
To assess α-tomatine sensitivity in liquid culture, we generated a growth curve in the presence or absence of α-tomatine. Five-hundred microliters of overnight culture of wild-type or Δtom1A S. scabies was used for inoculation of 25 ml of TSB (pH 7.0) in a 250-ml flask. Cultures were supplemented with α-tomatine to a final concentration of 0.1 mM or an equivalent volume of Na citrate buffer during early exponential growth phase. One milliliter of culture was harvested every hour, and a spectrophotometer was used to read the absorbance at 600 nm of three biological replicates for each treatment.
Southern blot hybridization.
Genomic DNA was isolated from overnight TSB-grown cultures by using the MasterPure kit for gram-positive bacteria (Epicentre) according to the manufacturer's instructions. Five micrograms of genomic DNA was digested with KpnI and run on a 0.8% agarose gel. The DNA was denatured with 0.5 M NaOH and 1.5 M NaCl and transferred to a nylon membrane (Whatman) by capillary transfer with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). DNA was UV cross-linked to the membrane by applying 120,000 μJ/cm2 for 4 min. The membrane was probed with a gene-specific probe for tomA or aac(3)IV that was labeled with dioxigenin-11-dUTP (Roche). Hybridization was performed in a rotary oven at 42°C. Stripping conditions were as follows: 2× SSC at room temperature for 10 min and 0.1× SSC at room temperature for 15 min. Processing of the membrane from this point forward was performed according to the manufacturer's instructions (Roche).
Cotranscription and 5′ transcript mapping.
For cotranscription and transcript mapping experiments, wild-type S. scabies was grown overnight in TSB liquid medium. RNA was extracted as previous described (25). Five micrograms of DNase-treated RNA was reverse transcribed using the Superscript III first-strand synthesis system and 250 ng of random hexamer primers (Invitrogen). Control reactions (non-RT) in which enzyme was omitted were also performed. Cotranscription analysis was performed by PCR using cDNA as the template and four primers (P1 [CACCACCTCAACCTTTC], P2 [CGGTCAAGAGCCCTATGG], P3 [CAGCCCATCGACCTGCT], and P4 [TGCGTCTGCTGATCCA]). PCR products were then gel purified and sequenced to confirm their identities.
To map the transcription start site, we used a 5′ rapid amplification of cDNA ends (5′ RACE) system (version 2.0; Invitrogen). Due to the apparent low abundance of SCAB77311-tomA transcript present in TSB-grown cultures, we cloned the multicopy origin of replication (pIJ101) from pHZ1272 (32) into pRFSRL20 to increase the number of SCAB77311-tomA transcripts and to facilitate mapping of the transcriptional start site. This plasmid pRFSRL36 was introduced into S. scabies by conjugation, and RNA was isolated from the resulting strain and was processed exactly as described by the manufacturer for 5′ RACE of high-G+C-content DNA. Sequences of gene-specific primers were as follows: for GSP1, CTCCGCGTACTTCTCGAA; for GSP2, GGTGGAGCGAGGCCATCAG; and for GSP3, TGAACGTGTCCCAGAT. The final PCR product was gel purified, cloned into pCR2.1TOPO, and sequenced using M13f and M13r primers (Invitrogen). The transcriptional start site was determined to be the nucleotide immediately adjacent to the string of poly(A)s that resulted from deoxyribosyladenine tailing of cDNA.
Quantitative RT-PCR.
S. scabies wild-type and ΔtomA1 strains were grown overnight in TSB liquid medium, and then 200 μl of each was subcultured into 10 ml of fresh TSB medium. Cultures were grown to an OD600 of ∼0.700, at which point either 0.1 mM α-tomatine or an equivalent amount of empty buffer (50 mM Na citrate, pH 4.0) was added. Culture samples were harvested in 20-min intervals until 80 min was reached. RNA was extracted and quantitative reverse transcription-PCR (RT-PCR) was performed exactly as previously described (25). Induction was defined as a >2-fold change in transcript level.
In planta bioassays.
Excised potato tuber (cv. Russet Burbank) assays were performed as described previously (39). Virulence assays with tomato (cv. Pto) were performed essentially as described previously (25), in Magenta boxes on Murahige and Skoog agar medium with 2% sucrose. Overnight cultures of S. scabies wild-type and ΔtomA1 strains were grown in TSB liquid medium. One milliliter of culture was pelleted in a microcentrifuge, washed with 1 ml of sterile water, and resuspended in 1 ml of water. The resuspended cells were then used for inoculation of an entire Magenta box (containing ∼4 plants) by swirling the liquid across the agar surface. Inoculations were performed ∼2 weeks after seed germination, and infection was allowed to proceed for ∼4 weeks. Infection of hydroponically grown radish seedlings was performed with 10 × 104 spores as described previously (24). Plants were inoculated 5 days after germination and inspected for disease 3 weeks after inoculation.
RESULTS
The deduced amino acid sequence of the tomA gene product is homologous with known α-tomatine-detoxifying enzymes.
The tomA gene product encodes a 545-amino-acid (aa) protein (55 kDa) that contains a predicted secretion signal at its N terminus (aa 1 to 27) (15). The TomA protein has two domains, an N-terminal catalytic domain (aa 28 to 356) that belongs to glycosyl hydrolase family 10 and a C-terminal domain (aa 357 to 507) with weak similarity to the E-set superfamily that has been found in other bacterial hydrolases but does not have an ascribed function.
A CLUSTAL W alignment of the predicted amino acid sequence from the S. scabies TomA protein and from closely related proteins showed that TomA is homologous to known α-tomatine-detoxifying enzymes as well as bacterial xylanases (Fig. 1). It is most closely related to the well-characterized tomatinase from the tomato fungal pathogen F. oxysporum f. sp. lycopersici (60% identical) (46) and to the tomatinase from the bacterial wilt pathogen C. michiganensis subsp. michiganensis (58% identical) (27). The TomA protein also contains Glu residues at positions 158 and 266, which align with the acid/base catalysis and nucleophile residues, respectively, from the family 10 glycosyl hydrolase Cex of Cellulomonas fimi (40). TomA protein catalyzes hydrolysis of an O-glycosyl compound, a unique reaction among family 10 glycosyl hydrolases, which characteristically possess either xylanase (EC 3.2.1.8) or endo-1,3-β-xylanase (EC 3.2.1.32) activity (21). The range of catalytic activity observed in glycosyl hydrolase family 10 is in agreement with the low conservation among substrate binding sites in this family (11).
FIG. 1.
Comparison of the amino acids of the predicted catalytic domain from the S. scabies 87-22 TomA protein with closely related proteins. Ssc, tomatinase from S. scabies 87-22 (coordinates 8542762 to 8544363 at http://www.sanger.ac.uk/Projects/S_scabies/); Cmm, tomatinase from C. michiganensis subsp. michiganensis (GenBank accession no. AAP57293); Fol, tomatinase from F. oxysporum f. sp. lycopersici (CAA10112); Sco, xylanase from S. coelicolor A(3)2 (CAB61191); Tfu, xylanase from Thermobifida fusca YX (YP_290847); Tma, xylanase from Thermotoga maritima (AAP97078); Kra, xylanase from Kineococcus radiotolerans (EAM76898); Cfi, xylanase from Cellulomonas fimi (Q59277). Residues identical for all proteins are boxed in black, and residues identical in four or more proteins are boxed in gray. Asterisks mark catalytic residues Glu 158 and Glu 266.
Heterologous overexpression and purification of TomA in E. coli.
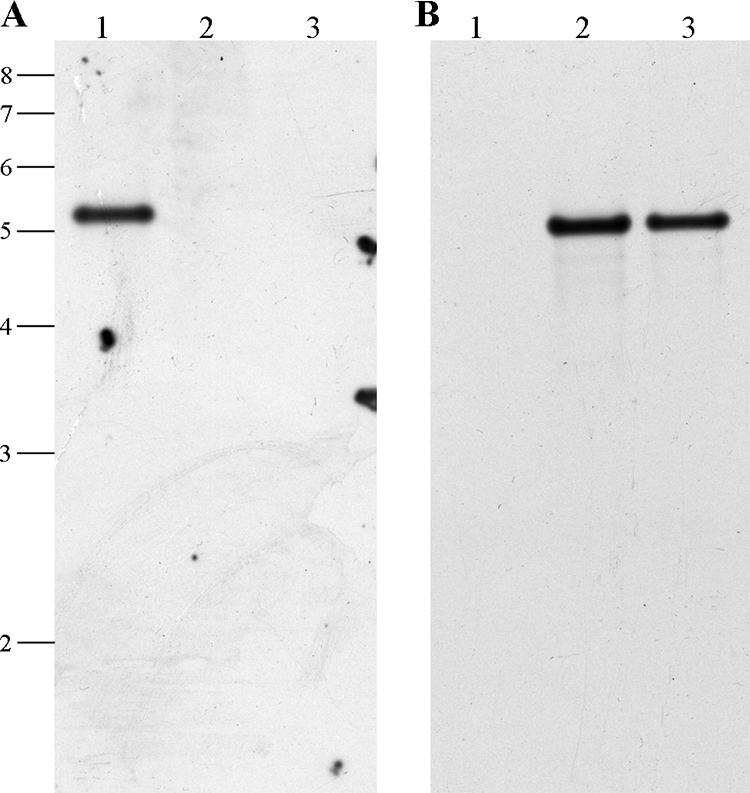
Our initial attempts to purify the TomA protein were made in its natural producer, S. scabies, using pRFSRL12 with tomA transcription driven by ermEp* (6). Despite the use of this strong promoter, we were able to obtain only a small amount of purified protein from culture supernatants. We therefore selected an E. coli expression system for protein expression. A polyhistidine tag (His6) was attached to the C terminus of the TomA protein that lacked the secretion signal. TomA-His6 was purified from E. coli extracts by Ni-nitrilotriacetic acid affinity chromatography. With this purification technique, we were able to achieve >90% purification of TomA-His6 as analyzed by 12% SDS-PAGE (Fig. 2A).
FIG. 2.
Purification of S. scabies 87-22 TomA-His6 protein and TLC analysis of α-tomatine reaction products. (A) Protein extracts were analyzed by 12% SDS-PAGE and were stained with SimplyBlue SafeStain. Lanes: 1, total soluble protein from pET30a vector control; 2, total soluble protein from induced TomA-His6 cultures; 3, TomA-His6 protein purified by nickel affinity chromatography. (B) TLC of TomA-His6 reaction products. Lanes: 1, TomA-His6 (6 μg) plus α-tomatine (0.2 μmol); 2, vector control lysate (100 μg) plus α-tomatine (0.2 μmol); 3, tomatidine standard (0.2 μmol); 4, α-tomatine standard (0.2 μmol). (C) Structure of α-tomatine from tomato. The arrow indicates the point of hydrolysis of lycotetraose by tomatinase enzymes from S. scabies 87-22, C. michiganensis subsp. michiganensis, and F. oxysporum f. sp. lycopersici. (Adapted from reference 46.)
TLC of reactions containing TomA-His6 and α-tomatine showed that the recombinant enzyme was able to catalyze the conversion of α-tomatine to tomatidine, releasing lycotetraose, whereas vector control lysate could not (Fig. 2B and C). The tomatinase enzymes from F. oxysporum f. sp. lycopersici and C. michiganensis subsp. michiganensis also catalyze this reaction (16, 27, 34); however, the tomatinase from Septoria lycopersici hydrolysizes only the terminal β-1,2-linked d-glucose (1, 14). Since TomA-His6 catalyzes the release of the sugar moiety from tomatine, we were able to use a reducing-sugar assay in order to measure enzyme activity (35). We determined a Km of 650 μM for TomA-His6, which is comparable to the Km of 1.1 mM reported for TomA of F. oxysporum f. sp. lycopersici (34), considering the error associated with the Lineweaver-Burk parameter estimation technique (13). Since S. scabies is a general necrotic pathogen with a broad host range, we also analyzed whether TomA-His6 had activity against the potato glycoalkaloids α-chaconine and α-solanine. Incubation of TomA-His6 with these substrates did not yield significant activity (data not shown), suggesting that the tomatinase from S. scabies may not be important in pathogenesis in potatoes. In addition, because TomA displayed very high homologies to xylanases, we assayed TomA-His6 for xylanase activity on xylans from oats or birchwood. Significant xylanase activity was not detected with either xylan (data not shown).
Analysis of the genomic region surrounding tomA in S. scabies revealed the presence of a gene, SCAB77311, encoding a putative family 1 glycosyl hydrolase. This gene is located upstream of tomA and is predicted to be transcribed in the same direction as tomA, which led us to investigate whether the two genes are cotranscribed in an operon. We isolated RNA from log-phase cells grown in TSB and performed RT-PCR with two pairs of oligonucleotide primers that spanned the intergenic region (Fig. 3A). PCR products were obtained for both sets of primers and were sequenced to verify their identities (Fig. 3B); NRT controls confirmed that the PCR products were generated from cDNA and not from genomic DNA, which can contaminate RNA preparations. These data suggest that tomA and SCAB77311 are cotranscribed, despite the relatively large (164-nt) intergenic region.
FIG. 3.
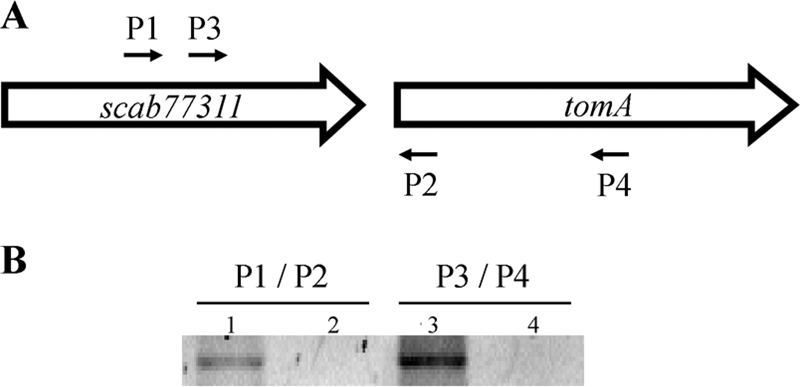
The tomA gene is cotranscribed with SCAB77311, a putative family 1 glycosyl hydrolase. (A) Schematic illustrating the S. scabies 87-22 SCAB77311-tomA locus. P1, P2, P3, and P4 refer to oligonucleotide primers used to demonstrate cotranscription. (B) Results of RT-PCR with oligonucleotide primers P1/P2 and P3/P4. Lanes: 1, RT+; 2, non-RT control; 3, RT+; 4, non-RT control. PCR products were sequenced to confirm their identities.
Since tomatinase gene expression is induced by α-tomatine in F. oxysporum f. sp. lycopersici and Septoria lycopersici, we analyzed whether or not the same was true for S. scabies. We performed quantitative RT-PCR of RNA isolated from exponentially growing cells in TSB liquid medium. Cells were supplemented with α-tomatine and transcript abundance was assessed in 20-min intervals until 80 min after supplementation. The addition of α-tomatine did not increase transcript levels of the SCAB77311-tomA locus (data not shown), suggesting that transcription of tomA is not induced by its substrate.
Mapping of the tomA transcriptional start site by 5′ RACE.
Our initial attempts at mapping the SCAB77311-tomA transcriptional start site failed because of the presence of several nonspecific PCR products, presumably resulting from low transcript abundance for the SCAB77311-tomA locus. Indeed, expression analysis using quantitative RT-PCR suggested that the tomA gene is transcribed from a weak promoter (data not shown). In order to facilitate mapping of transcriptional start site by 5′ RACE, we cloned the high-copy-number origin of replication from pIJ101 into pRFSRL20, and the resulting plasmid, pRFSRL36, was conjugated into wild-type S. scabies. RNA was isolated from log-phase cells grown in TSB and was subjected to 5′ RACE analysis. Using this technique, we were able to successfully map the transcriptional start site for the SCAB77311-tomA operon (Fig. 4). Transcription is initiated from a cytosine nucleotide located 23 bp upstream of the putative ATG start codon for SCAB77311. Sequence analysis identified −10 and −35 promoter regions that were identical to Streptomyces promoters that do not display typical characteristics of promoters recognized by E. coli σ70-like sigma factors (51). The −10 region was identical to the −10 region of the hydroxystreptomycin phosphotransferase gene, sphP, from S. glaucescens (51, 53) and the −35 region was identical to that of the bialaphos resistance gene, bar, from S. coelicolor A3(2) (2, 51). The distance between −10 and −35 elements is 20 nt, longer than the typical 17- or 18-nt spacer of streptomycete promoters.
FIG. 4.

Mapping of the SCAB77311-tomA transcriptional start site by 5′ RACE. (A) A 2% agarose gel showing the PCR product obtained by 5′ RACE. (B) Nucleotide schematic showing the transcriptional start site determined by sequencing of the PCR product shown in panel A. A double underline indicates the −35 sequence, and a single underline indicates the −10 sequence; the bold nucleotide with an arrow indicates the initiating nucleotide and direction of transcription, and the bold “atg” indicates the putative start codon of the SCAB77311 gene.
α-Tomatine inhibits aerial but not vegetative growth of S. scabies.
A tomatinase-null mutant strain was created using the λ Red-based Redirect system for gene deletions in Streptomyces spp. (18). Two mutant strains (ΔtomA1 and ΔtomA2) were isolated and subsequently verified by Southern blot hybridization (Fig. 5). S. scabies wild-type and ΔtomA strains were analyzed for sensitivity to α-tomatine in a disk diffusion assay. The ΔtomA1 and ΔtomA2 mutant strains behaved similarly in this assay; therefore we present data for only the ΔtomA1 strain.
FIG. 5.
Deletion of the S. scabies 87-22 tomA gene. (A) Southern blot hybridization using a PCR-generated dioxigenin-11-dUTP-labeled fragment internal to tomA. The positions of the DNA marker are indicated at the left in kb. Lanes: 1, S. scabies wild type; 2, ΔtomA1 mutant; 3, ΔtomA2 mutant. (B) The membrane from panel A was stripped and probed with a dioxigenin-11-dUTP-labeled internal fragment of the apramycin resistance gene aac(3)IV.
The growths of S. scabies wild-type and ΔtomA1 strains were unaffected by the addition of buffer alone. However, the addition of 0.3 μmol of α-tomatine inhibited the formation of the fluffy white aerial mycelium that is characteristic of streptomycetes (Fig. 6A and B). The size of the zone of inhibition increased in a dose-dependent manner (data not shown); however, for experimental ease, we chose to work with 0.3 μmol of α-tomatine only, which is within the physiologically relevant range for the level of α-tomatine that is produced by tomato plants (3). Inhibition of aerial mycelium formation was more severe in the ΔtomA1 strain, with nearly a 50% increase in the diameter of inhibited growth (Table 2), suggesting that TomA detoxifies α-tomatine in vivo. To ensure that the observed phenotype was due solely to the deletion of tomA, we cloned a ∼3.4-kb fragment containing the tomA gene into the integrative vector pAU3-45 (7) and moved it to the ΔtomA1 strain by conjugation. As expected, the complemented strain returned to wild-type levels of inhibited aerial growth, whereas the presence of the vector alone had no effect (Fig. 6C and D and Table 2).
FIG. 6.
TomA mitigates the effect of α-tomatine on aerial growth of S. scabies 87-22. (A) S. scabies wild type. (B) ΔtomA1 mutant. (C) ΔtomA1 attB ΦC31::pAU34-5. (D) ΔtomA1 attB ΦC31::pRFSRL20. One spot of 50 mM Na citrate control buffer (upper left corner) and three replicate spots of 0.3 μmol of α-tomatine were spotted on each plate.
TABLE 2.
Inhibition of aerial growth of S. scabies by α-tomatinea
| S. scabies strain | Diam of inhibition (mm)b | Inhibition increase over wild-type value (%)c |
|---|---|---|
| Wild-type 87-22 | 11.7 ± 1.2 | |
| ΔtomA1 | 17.2 ± 0.3 | 47.0 |
| ΔtomA1attB ΦC31::pAU34-5 | 21.0 ± 1.0 | 80.0 |
| ΔtomA1 attB ΦC31::pRFSRL20 | 11.3 ± 0.6 | −3.4 |
A total of 0.3 μmol α-tomatine was used in this assay.
Zone of inhibition ± standard deviation for three replicates.
Increase of inhibition is expressed as a percentage of the wild-type value.
Next, we analyzed vegetatively growing cells of S. scabies wild-type and ΔtomA1 strains in liquid TSB medium for inhibition by α-tomatine. Cultures were grown to an OD600 of ∼0.200, and then either buffer alone or α-tomatine (final concentration, 0.1 mM) was added. The addition of 0.1 mM α-tomatine is equivalent to the addition of 0.3 μmol of α-tomatine in a disk diffusion assay. Samples were harvested at 1-h intervals and were analyzed spectrophotometrically. There was no observable change in growth of S. scabies wild-type or ΔtomA1 strains after the addition of α-tomatine compared to that of cells treated with buffer alone (Fig. 7).
FIG. 7.

The growth of S. scabies 87-22 vegetative mycelium is not affected by α-tomatine. Vegetatively growing cells were supplemented at ∼3.5 h (arrow) with 0.1 mM α-tomatine or 50 mM Na citrate buffer and analyzed spectrophotometrically. Diamonds, wild type plus Na citrate; squares, wild type plus 0.1 mM α-tomatine; triangles, ΔtomA1 plus Na citrate; asterisks, ΔtomA1 plus 0.1 mM α-tomatine.
tomA is not required for virulence on excised potato tissue or tomato seedlings.
To analyze the importance of TomA in plant pathogenicity, we first inoculated excised potato tuber disks with plugs of agar containing mycelia of wild-type or ΔtomA1 S. scabies. Potato tuber slices were heavily necrotized by both wild-type and ΔtomA1 strains 7 days postinoculation, suggesting that TomA is not important in pathogenicity on potatoes.
Next, we infected tomato seedlings in vitro. Plants were grown in Magenta boxes on Murahige and Skoog agar containing 2% sucrose. Seedlings were inoculated 1 week after germination with mycelia grown overnight in TSB. Necrosis on primary and lateral roots was apparent on S. scabies wild-type strain- and ΔtomA1 strain-inoculated plants as early as 1 week postinoculation; roots of mock-inoculated seedlings were not necrotic. Four weeks postinoculation, roots of wild-type strain- and ΔtomA1 strain-inoculated plants were severely stunted, and lateral root tips were necrotized and swollen compared to those of control plants (data not shown). These data suggest that the tomatinase protein in S. scabies is not important for pathogenicity on tomato plants. We also inoculated hydroponically grown radish (cv. Burpee White) seedlings and did not observe a difference in pathogenicity for S. scabies wild-type and ΔtomA1 strains.
DISCUSSION
We have shown that the saponin-detoxifying enzyme present in S. scabies is a tomatinase that hydrolyzes α-tomatine, releasing lycotetraose. We determined a Km of 650 μM for this enzyme, which is comparable to the Km of 1.1 mM reported for the tomatinase enzyme from F. oxysporum f. sp. lycopersici (34) when the error associated with the Lineweaver-Burk parameter estimation technique is considered (13). Our substrate utilization analysis showed that TomA from S. scabies does not have xylanase activity, nor is it active on α-chaconine or α-solanine. Taken together, these data suggest that the tomatinase from S. scabies has a high substrate specificity, which is in agreement with the case for tomatinases from other phytopathogens (34, 42).
Our genetic analysis showed that the tomA gene is cotranscribed with SCAB77311, which encodes a putative family 1 glycosyl hydrolase. The closest homolog of this protein is BlgA (73% identity) from C. michiganensis subsp. michiganensis; the blgA gene is located just downstream of the tomA homologue in this organism (27). InteroPro scanning of the deduced amino acid sequence for the SCAB77311 protein showed high homology with family 1 glycosyl hydrolase proteins (InterPro identification no. IPR001360). These proteins hydrolyze the glycosidic bond between two carbohydrates or between a carbohydrate and a noncarbohydrate moiety. The presence of a family 1 glycosyl hydrolase near the tomA gene in both S. scabies and C. michiganensis subsp. michiganensis suggests that the SCAB77311 protein and BlgA might perform the same function and might possibly be involved in hydrolyzing the lycotetraose released during α-tomatine hydrolysis. It is attractive to speculate that the family 1 glycosyl hydrolase encoded by SCAB77311 acts either to hydrolyze lycotetraose into four monosaccharides or two disaccharides, the latter of which would require another protein for hydrolysis to four monosaccharides before entry into glycolysis.
Mapping the transcriptional start site by 5′ RACE identified a promoter sequence that does not display characteristics typical of promoters recognized by E. coli σ70-like sigma factors but is identical to streptomycete promoters involved in antibiotic resistance (2, 51, 53). Our quantitative RT-PCR experiments analyzing α-tomatine-induced transcription of tomA suggest that this gene is not induced by the presence of its substrate. This contrasts with tomatinase transcription in Septoria lycopersici and F. oxysporum f. sp. lycopersici, as well as C. michiganensis subsp. michiganensis, in which tomA gene expression is induced by the presence of α-tomatine (27, 46, 48). The mechanism by which α-tomatine induces transcription in unknown.
Our α-tomatine bioassays showed that S. scabies is sensitive to α-tomatine but that only aerial growth was inhibited, while vegetative mycelium was unaffected. This was an unexpected result, because in both Septoria lycopersici and F. oxysporum f. sp. lycopersici as well as in C. michiganensis subsp. michiganensis, total inhibition of growth was observed upon treatment with α-tomatine (27, 46, 48). It is curious why S. scabies and C. michiganensis subsp. michiganensis are sensitive to α-tomatine in the first place, because α-tomatine complexes with sterols to form membrane pores (30, 31) and analysis of the genomic sequences of both of these organisms did not reveal the presence of a sterol biosynthetic pathway (http://www.sanger.ac.uk/Projects/S_scabies/ and http://gib.genes.nig.ac.jp/single/index.php?spid=Cmic_NCPPB382). However, growth is clearly adversely affected in both S. scabies and C. michiganensis subsp. michiganensis, so α-tomatine must interact with a target that is critical for normal growth by these bacteria. Interestingly, analysis of the S. scabies genome sequence revealed the presence of a putative hopanoid biosynthetic gene cluster. Hopanoids are functionally analogous to sterols and are structurally similar (26) and could potentially serve as a target for α-tomatine in S. scabies. However, analysis of the C. michiganensis subsp. michiganensis genome sequence did not reveal an obvious hopanoid biosynthetic gene cluster. It is interesting that in F. oxysporum f. sp. lycopersici, the observed sensitivity to α-tomatine is a result of α-tomatine-induced programmed cell death in the fungus rather than an effect on membranes (23). Furthermore, the α-tomatine aglycone tomatidine inhibits sterol biosynthesis in Saccharomyces cerevisiae (50), indicating that the biological properties of saponins and their degradation products are complex.
Uncompromised virulence of the ΔtomA1 mutant in a potato tuber disk assay is consistent with our in vitro data showing that TomA from S. scabies does not act on the important glycoalkaloids in potatoes. Infection of tomato plants with tomatinase-null mutants from C. michiganensis subsp. michiganensis, F. oxysporum f. sp. lycopersici, and Septoria lycopersici did not yield observable differences in virulence (27, 42, 46). However, recent studies claim that the F. oxysporum f. sp. lycopersici tomatinase is important during the early stages of tomato infection (44), and curiously, tomatinase was a required virulence factor in Septoria lycopersici for infection of Nicotiana benthamiana, a species that presumably does not produce α-tomatine (8). Since tomatinase is not required for full virulence of C. michiganensis subsp. michiganensis, F. oxysporum f. sp. lycopersici, and Septoria lycopersici on tomato plants, it is not surprising that we did not observe a decrease in virulence on potato tuber tissue or tomato plants inoculated with the S. scabies ΔtomA1 mutant. The inoculation of roots rather than leaves is consistent with the manner in which S. scabies infects plants in the field. However, since tomato roots likely contain lower levels of α-tomatine than leaves (43, 45), it is possible that the inoculation of leaves could have revealed a virulence phenotype for ΔtomA1. It may be that the TomA protein facilitates reproduction on α-tomatine-containing tissue, since α-tomatine inhibits aerial growth and thereby sporulation of S. scabies. Data presented here do not rule out the possibility that tomatinase has a role in suppression of induced plant defenses in some hosts, just as it does in F. oxysporum f. sp. lycopersici and Septoria lycopersici (8, 22). Expression analysis of genes involved in induced defense responses of wild-type strain- and ΔtomA1 strain-inoculated plants will evaluate this hypothesis.
Acknowledgments
We thank D. R. D. Bignell for performing quantitative RT-PCR experiments and for providing suggestions to improve the manuscript.
This project was supported by the National Research Initiative of the United States Department of Agriculture (USDA) Cooperative State Research, Education, and Extension Service, grant no. 2005-35319-15289. Financial support was also provided to R.F.S. through the USDA Multidisciplinary Graduate Education Traineeship Program.
Footnotes
Published ahead of print on 3 October 2008.
REFERENCES
- 1.Arneson, P. A., and R. D. Durbin. 1967. Hydrolysis of tomatine by Septoria lycopersici: a detoxification mechanism. Phytopathology 571358-1360. [Google Scholar]
- 2.Bedford, D. J., C. G. Lewis, and M. J. Buttner. 1991. Characterization of a gene conferring bialaphos resistance in Streptomyces coelicolor A3(2). Gene 10439-45. [DOI] [PubMed] [Google Scholar]
- 3.Beimen, A. A. B., D. Meletzus, R. Eichenlaub, and W. Barz. 1992. Accumulation of phenolic compounds in leaves of tomato plants after infection with Clavibacter michiganense subsp. michiganense. Z. Naturforsch. C 47898-909. (In German.) [Google Scholar]
- 4.Bentley, S. D., C. Corton, S. E. Brown, A. Barron, L. Clark, J. Doggett, B. Harris, D. Ormond, M. A. Quail, G. May, D. Francis, D. Knudson, J. Parkhill, and C. A. Ishimaru. 2008. Genome of the actinomycete plant pathogen Clavibacter michiganensis subsp. sepedonicus suggests recent niche adaptation. J. Bacteriol. 1902150-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentley, S. D., K. F. Chater, A.-M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C.-H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M.-A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417141-147. [DOI] [PubMed] [Google Scholar]
- 6.Bibb, M. J., J. White, J. M. Ward, and G. R. Janssen. 1994. The mRNA for the 23S rRNA methylase encoded by the ermE gene of Saccharopolyspora erythraea is translated in the absence of a conventional ribosome-binding site. Mol. Microbiol. 14533-545. [DOI] [PubMed] [Google Scholar]
- 7.Bignell, D. R. D., K. Tahlan, K. R. Colvin, S. E. Jensen, and B. K. Leskiw. 2005. Expression of ccaR, encoding the positive activator of cephamycin C and clavulanic acid production in Streptomyces clavuligerus, is dependent on bldG. Antimicrob. Agents Chemother. 491529-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouarab, K., R. Peart, D. Baulcombe, and A. E. Osbourn. 2002. A saponin-detoxifying enzyme mediates suppression of plant defences. Nature 418889-892. [DOI] [PubMed] [Google Scholar]
- 9.Bowyer, P., B. R. Clarke, P. Lunness, M. J. Daniels, and A. E. Osbourn. 1995. Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science 267371-374. [DOI] [PubMed] [Google Scholar]
- 10.Bukhalid, R. B., and R. Loria. 1997. Cloning and expression of a gene from Streptomyces scabies encoding a putative pathogenicity factor. J. Bacteriol. 1797776-7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charnock, S. J., T. D. Spurway, H. Xie, M.-H. Beylot, R. Virden, R. A. J. Warren, G. P. Hazlewood, and H. J. Gilbert. 1998. The topology of the substrate binding clefts of glycosyl hydrolase family 10 xylanases are not conserved. J. Biol. Chem. 27332187-32199. [DOI] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowd, J. E., and D. S. Riggs. 1965. A comparison of estimates of Michaelis-Menten kinetic constants from various linear transformations. J. Biol. Chem. 240863-869. [PubMed] [Google Scholar]
- 14.Durbin, R. D., and T. F. Uchytil. 1969. Purification and properties of a fungal β-glucosidase acting on α-tomatine. Biochim. Biophys. Acta 191176-178. [DOI] [PubMed] [Google Scholar]
- 15.Emanuelsson, O., S. Brunak, G. von Heijne, and H. Nielson. 2007. Locating proteins in the cell using TargetP, SignalP, and related tools. Nat. Protoc. 2953-971. [DOI] [PubMed] [Google Scholar]
- 16.Ford, J. E., D. J. McCance, and R. B. Drysdale. 1977. The detoxification of α-tomatine by Fusarium oxysporum f.sp. lycopersici. Phytochemistry 16545-546. [Google Scholar]
- 17.Gartemann, K.-H., B. Abt, T. Bekel, A. Burger, J. Engemann, M. Flugel, L. Gaigalat, A. Goesmann, I. Grafen, J. Kalinowski, O. Kaup, O. Kirchner, L. Krause, B. Linke, A. McHardy, F. Meyer, S. Pohle, C. Ruckert, S. Schneiker, E.-M. Zellermann, A. Puhler, R. Eichenlaub, O. Kaiser, and D. Bartels. 2008. The genome sequence of the tomato-pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis reveals a large island involved in pathogenicity. J. Bacteriol. 1902138-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gust, B., G. L. Challis, K. Fowler, T. Kieser, and K. F. Chater. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 1001541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy, F. G., S. B. Krasnoff, M. Wach, D. M. Gibson, and R. Loria. 2002. Involvement of a cytochrome P450 monooxygenase in thaxtomin A biosynthesis by Streptomyces acidiscabies. J. Bacteriol. 1842019-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healy, F. G., S. B. Krasnoff, M. Wach, D. M. Gibson, and R. Loria. 2000. The txtAB genes of the plant pathogen Streptomyces acidiscabies encode a peptide synthetase required for phytotoxin thaxtomin A production and pathogenicity. Mol. Microbiol. 38794-804. [DOI] [PubMed] [Google Scholar]
- 21.Henrissat, B., and G. Davies. 1997. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 7637-644. [DOI] [PubMed] [Google Scholar]
- 22.Ito, S.-I., T. Eto, S. Tanaka, N. Yamauchi, H. Takahara, and T. Ikeda. 2004. Tomatidine and lycotetraose, hydrolysis products of α-tomatine by Fusarium oxysporum tomatinase, suppress induced defense responses in tomato cells. FEBS Lett. 57131-34. [DOI] [PubMed] [Google Scholar]
- 23.Ito, S.-I., T. Ihara, H. Tamura, S. Tanaka, T. Ikeda, H. Kajihara, C. Dissanayake, F. F. Abdel-Motaal, and M. A. El-Sayed. 2007. α-Tomatine, the major saponin in tomato, induces programmed cell death mediated by reactive oxygen species in the fungal plant pathogen Fusarium oxysporum. FEBS Lett. 5813217-3222. [DOI] [PubMed] [Google Scholar]
- 24.Joshi, M., X. Rong, S. Moll, J. Kers, C. Franco, and R. Loria. 2007. Streptomyces turgidiscabies secretes a novel virulence protein, Nec1, which facilitates infection. Mol. Plant-Microbe Interact. 20599-608. [DOI] [PubMed] [Google Scholar]
- 25.Joshi, M. V., D. R. D. Bignell, E. G. Johnson, J. P. Sparks, D. M. Gibson, and R. Loria. 2007. The AraC/XylS regulator TxtR modulates thaxtomin biosynthesis and virulence in Streptomyces scabies. Mol. Microbiol. 66633-642. [DOI] [PubMed] [Google Scholar]
- 26.Kannenberg, E. L., and K. Poralla. 1999. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften 86168-176. [Google Scholar]
- 27.Kaup, O., G. Ines, E.-M. Zellerman, R. Eichenlaub, and K.-H. Gartemann. 2005. Identification of a tomatinase in the tomato-pathogenic actinomycete Clavibacter michiganensis subsp. michiganensis NCPPB382. Mol. Plant-Microbe Interact. 181090-1098. [DOI] [PubMed] [Google Scholar]
- 28.Kers, J. A., K. D. Cameron, M. V. Joshi, R. A. Bukhalid, J. E. Morello, M. J. Wach, D. M. Gibson, and R. Loria. 2005. A large, mobile pathogenicity island confers plant pathogenicity on Streptomyces species. Mol. Microbiol. 551025-1033. [DOI] [PubMed] [Google Scholar]
- 29.Kers, J. A., M. J. Wach, S. B. Krasnoff, J. Widom, K. D. Cameron, R. A. Bukhalid, D. M. Gibson, B. R. Crane, and R. Loria. 2004. Nitration of a peptide phytotoxin by bacterial nitric oxide synthase. Nature 42979-82. [DOI] [PubMed] [Google Scholar]
- 30.Keukens, E. A. J., T. de Vrije, C. van den Boom, P. de Waard, H. H. Plasman, F. Thiel, V. Chupin, W. M. F. Jongen, and B. de Kruijff. 1995. Molecular basis of glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta 1240216-228. [DOI] [PubMed] [Google Scholar]
- 31.Keukens, E. A. J., T. de Vrije, L. A. M. Jansen, H. de Boer, M. Janssen, A. I. P. M. de Kroon, W. M. F. Jongen, and B. de Kruijff. 1996. Glycoalkaloids selectively permeabilize cholesterol containing biomembranes. Biochim. Biophys. Acta 1279243-250. [DOI] [PubMed] [Google Scholar]
- 32.Kieser, T., M. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 33.King, R. R., C. H. Lawrence, M. C. Clark, and L. A. Calhoun. 1989. Isolation and characterization of phytotoxins associated with Streptomyces scabies. J. Chem. Soc. Chem. Commun. 13849-850. [Google Scholar]
- 34.Lairini, K., A. Perez-Espinosa, M. Pineda, and M. Ruiz-Rubio. 1996. Purification and characterization of tomatinse from Fusarium oxysporum f. sp. lycopersici. Appl. Environ. Microbiol. 621604-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lever, M. 1972. A new reaction for colorimetric determination of carbohydrates. Anal. Biochem. 47273-279. [DOI] [PubMed] [Google Scholar]
- 36.Loria, R., D. R. D. Bignell, S. Moll, J. C. Huguet-Tapia, M. V. Joshi, E. G. Johnson, R. F. Seipke, and D. M. Gibson. 2008. Thaxtomin biosynthesis: the path to plant pathogenicity in the genus Streptomyces. Antonie van Leeuwenhoek 943-10. [DOI] [PubMed] [Google Scholar]
- 37.Loria, R., J. Coombs, M. Yoshida, J. A. Kers, and R. A. Bukhalid. 2003. A paucity of bacterial roots diseases: Streptomyces succeeds where others fail. Physiol. Mol. Plant Pathol. 6265-72. [Google Scholar]
- 38.Loria, R., J. Kers, and M. Joshi. 2006. Evolution of plant pathogenicity in Streptomyces. Annu. Rev. Phytopathol. 4416.1-16.19. [DOI] [PubMed] [Google Scholar]
- 39.Loria, R., R. A. Bukhalid, R. A. Creath, R. H. Leiner, and M. Olivier. 1995. Differential production of thaxtomins by pathogenic Streptomyces species in vitro. Phytopathology 85537-541. [Google Scholar]
- 40.MacLeod, A. M., T. Lindorst, S. G. Withers, and R. A. J. Warren. 1994. The acid/base catalyst in the exoglucanase/xylanase from Cellulomonas fimi glutamic acid 127: evidence from detailed kinetic studies of mutants. Biochemistry 336371-6376. [DOI] [PubMed] [Google Scholar]
- 41.MacNeil, D. J., K. M. Gewain, C. L. Ruby, G. Dezeny, P. H. Gibbons, and T. MacNeil. 1992. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 11161-68. [DOI] [PubMed] [Google Scholar]
- 42.Martin-Hernandez, A. M., M. Dufresne, V. Hugouvieux, R. Melton, and A. E. Osbourn. 2000. Effects of targeted replacement of the tomatinase gene on the interaction of Septoria lycopersici with tomato plants. Mol. Plant-Microbe Interact. 131301-1311. [DOI] [PubMed] [Google Scholar]
- 43.Morrissey, J. P., and A. E. Osbourn. 1999. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 63708-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pareja-Jamie, Y., M. I. G. Roncero, and M. C. Ruiz-Roldan. 2008. Tomatinase from Fusarium oxysporum f. sp. lycopersici is required for full virulence on tomato plants. Mol. Plant-Microbe Interact. 21728-736. [DOI] [PubMed] [Google Scholar]
- 45.Roddick, J. G. 1974. The steroidal glycoalkaloid α-tomatine. Phytochemistry 139-25. [Google Scholar]
- 46.Roldan-Arjona, T., A. Perez-Espinosa, and M. Ruiz-Rubio. 1999. Tomatinase from Fusarium oxysporum f. sp. lycopersici defines a new class of saponinases. Mol. Plant-Microbe Interact. 12852-861. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Manniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 48.Sandrock, R. W., D. DellaPenna, and H. D. VanEtten. 1995. Purification and characterization of β2-tomatinase, an enzyme involved in the degradation of α-tomatine and isolation of the gene encoding β2-tomatinase from Septoria lycopersici. Mol. Plant-Microbe Interact. 8960-970. [DOI] [PubMed] [Google Scholar]
- 49.Scheible, W.-R., B. Fry, A. Kochevenko, D. Schindelasch, L. Zimmerli, S. Somerville, R. Loria, and C. R. Somerville. 2003. An Arabidopsis mutant resistant to thaxtomin A, a cellulose synthase inhibitor from Streptomyces species. Plant Cell 151781-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simons, V., J. P. Morrissey, M. Latijnhouwers, M. Csukai, A. Cleaver, C. Yarrow, and A. Osbourn. 2006. Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 502732-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strohl, W. R. 1992. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 20961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanEtten, H. D., J. W. Mansfield, J. A. Bailey, and E. E. Farmer. 1994. Two classes of plant antibiotics: phytoalexins versus “phytoanticipins”. Plant Cell 61191-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vögtli, M., and R. Hutter. 1987. Characterization of the hydroxystreptomycin phosphotransferase gene (sph) of Streptomyces glaucescens: nucleotide sequence and promoter analysis. Mol. Gen. Genet. 208195-203. [DOI] [PubMed] [Google Scholar]





