Abstract
Activation of the late prespore-specific RNA polymerase sigma factor σG during Bacillus subtilis sporulation coincides with completion of the engulfment process, when the prespore becomes a protoplast fully surrounded by the mother cell cytoplasm and separated from it by a double membrane system. Activation of σG also requires expression of spoIIIJ, coding for a membrane protein translocase of the YidC/Oxa1p/Alb3 family, and of the mother cell-specific spoIIIA operon. Here we present genetic and biochemical evidence indicating that SpoIIIAE, the product of one of the spoIIIA cistrons, and SpoIIIJ interact in the membrane, thereby linking the function of the spoIIIJ and spoIIIA loci in the activation of σG. We also show that SpoIIIAE has a functional Sec-type signal peptide, which is cleaved during sporulation. Furthermore, mutations that reduce or eliminate processing of the SpoIIIAE signal peptide arrest sporulation following engulfment completion and prevent activation of σG. SpoIIIJ-type proteins can function in cooperation with or independently of the Sec system. In one model, SpoIIIJ interacts with SpoIIIAE in the context of the Sec translocon to promote its correct localization and/or topology in the membrane, so that it can signal the activation of σG following engulfment completion.
Two important issues in developmental biology are how adjacent cells communicate so that coordinated responses emerge and how these signal transduction complexes are assembled at appropriate, discrete sites within the cell. The process of spore differentiation by the gram-positive bacterium Bacillus subtilis provides several examples of cell-cell communication pathways that initiate in one cell and influence the behavior of an adjacent sister cell (11, 15, 39). Sporulation involves the cooperation between two cells formed at the onset of the process by asymmetric division of a sporangial cell. This polar division produces a polar prespore, the future spore, and an adjacent larger mother cell, a terminal cell line, which contributes to spore morphogenesis but that lyses at its completion (11, 15, 39). The programs of gene expression in the prespore and the mother cell are controlled by the successive activation of cell-type-specific RNA polymerase sigma factors. The activation of three of these development-specific sigma factors requires signals conveyed by the adjacent cell. In this way, close coordination between the programs of gene expression in the two cells is achieved. The activity of σF in the prespore is required for activation of σE in the mother cell. Because activation of σF is coupled to the completion of septation, activation of σE is also coupled to this morphological landmark (11, 15, 39).
Following activation of σE, the mother cell initiates a phagocytic-like process whereby it engulfs the prespore, eventually encapsulating it in a double membrane system formed by the inner and outer prespore membranes. Upon engulfment completion, σF is replaced by σG, itself produced under the control of σF, which then controls late stages in development in the prespore (11, 15, 39). σG in turn triggers a signaling pathway that leads to the activation of the late mother cell-specific regulator σK, which administers the final stages of development, including lysis of the mother cell to discharge the mature spore into the environment (11, 15, 39). Activation of σG does not follows its synthesis under σF control but depends upon and coincides with completion of the engulfment process (reviewed in references 11, 15, and 39). However, the mechanism by which σG is kept inactive prior to engulfment completion remains elusive (5, 6, 7, 13, 33, 41). Two anti-sigma factors are known that can bind to and antagonize σG, SpoIIAB (6, 13, 22, 23, 41) and the product of the σF-controlled csfB gene (also called gin) (5, 8, 9, 21). However, making σG refractory to the action of SpoIIAB, or deletion of csfB/gin, even in a strain where σG is insensitive to SpoIIAB, does not cause its massive premature activity prior to engulfment completion (5, 8, 13, 41). The process by which σG is activated following conclusion of the engulfment process is also unknown. It is clear, however, that activation of σG in the engulfed prespore requires a signaling pathway emanating from the mother cell and involving the activity of σE (5, 6, 7, 8, 21, 22, 33, 41, 42, 45).
σE directs the expression of the spoIIIA locus, which encodes eight proteins predicted to be membrane associated and all of which are required for the activation of σG (5, 16, 22, 45). The SpoIIIA-encoded proteins (SpoIIIAA to SpoIIIAH) may assemble into a complex that links engulfment completion to the activation of σG (2, 3, 5, 10, 19, 22, 45). Consistent with this idea, SpoIIIAH localizes to the outer prespore membrane through a direct interaction of its C-terminal extracellular domain with the extracellular domain of the inner prespore membrane protein SpoIIQ (2, 3, 5, 10, 19, 28), which has been implicated in the synthesis and activation of σG (5, 19, 26, 37, 47). The two proteins colocalize and form discrete foci along the spore membranes (2, 3, 10, 37). Recent results indicate that SpoIIIAH and SpoIIQ form a channel during the engulfment process that is required to activate σG following engulfment completion (5, 28). Although evidence suggests that the remaining spoIIIA-encoded proteins form part of a larger complex with SpoIIIAH-SpoIIQ, nothing is known about their localization and assembly. It is also unknown whether the proper assembly of the putative SpoIIIA complex is engulfment dependent and itself the signal for σG activation or whether the complex promotes the transport of a signal between the two chambers of the developing cell. Regardless of the mode of action of the spoIIIA-encoded proteins, it is likely that their correct insertion and assembly into the membranes that surround the spore are prerequisites for function.
In bacterial cells, most membrane proteins are targeted to and inserted into the membrane cotranslationally using the signal recognition particle and the Sec pathway (24, 27, 48, 51, 52). Others depend on the evolutionarily conserved YidC/Oxa1p/Alb3 family of proteins, which may function independently or in concert with the Sec translocon (24, 27, 48, 51, 52). B. subtilis codes for two members of the YidC/Oxa1p/Alb3 family of membrane protein translocases, SpoIIIJ and YqjG. During vegetative growth, SpoIIIJ and YqjG are involved in membrane protein biogenesis and protein secretion, and at least one is required for viability (12, 31, 49). However, deletion of spoIIIJ blocks sporulation following engulfment completion and prevents activation of σG (12, 31, 42). SpoIIIJ is so far the only component of the secretion or membrane protein biogenesis machinery that has been specifically implicated in the engulfment checkpoint (38). Presumably, SpoIIIJ but not YqjG interacts with a sporulation-specific substrate with an essential role in the signaling pathway leading to the activation of σG.
Recently, Camp and Losick used genetic suppression to show that SpoIIIJ and SpoIIIAE are likely to interact (5). Here, in confirmation and extension of those results, we also document a genetic interaction between spoIIIAE and spoIIIJ and show that SpoIIIJ interacts with SpoIIIAE in the membrane, thus linking the function of the two loci in the σG checkpoint. We further show that SpoIIIAE has a functional Sec-type signal sequence which is essential for sporulation. While the signal sequence of SpoIIIAE can be replaced by a different cleavable sequence, mutations that interfered with processing of the native signal sequence resulted in accumulation of an unprocessed form of SpoIIIAE, a block in sporulation following engulfment completion, and inactivity of σG. Altogether, the results suggest a link of SpoIIIAE to both the Sec translocon and to SpoIIIJ. One possibility is that SpoIIIJ interacts with SpoIIIAE in the context of the Sec translocon and directs the final stages in its insertion and/or folding in the membrane.
MATERIALS AND METHODS
Strains and general methods.
The B. subtilis strains used in this work (Table 1) are congenic derivatives of the Spo+ strain MB24 (trpC2 metC3). LB medium was used for growth or maintenance of Escherichia coli and B. subtilis, and sporulation was induced in Difco sporulation medium (DSM) (36, 41). All PCR fragments used for plasmid construction were confirmed by sequencing. Wherever appropriate, the B. subtilis strains were checked by PCR.
TABLE 1.
Bacillus subtilis strains
| Strain | Relevant genotype | Origin or reference |
|---|---|---|
| MB24 | trpC2 metC3 | Laboratory stock |
| AH2357 | trpC2 metC3 spoIIAC::erm | Laboratory stock |
| AH3562 | trpC2 ΔspoVE85 ΔamyE::PxylA-spoVE-yfp | 35 |
| AH1802 | trpC2 metC3 tasA::Pspac-tasA | 43 |
| JOB9 | trpC2 metC3 ΔyqjG::sp | 42 |
| JOB20 | trpC2 metC3 ΔspoIIIJ::sp | 42 |
| AH3795 | trpC2 ΔspoIIIG | 41 |
| RL2040 | trpC2 pheA1 ΔspoIIIAE | R. Losick |
| RL2041 | trpC2 pheA1 ΔspoIIIAF | R. Losick |
| RL2042 | trpC2 pheA1 ΔspoIIIAA | R. Losick |
| RL2043 | trpC2 pheA1 ΔspoIIIAB | R. Losick |
| RL2044 | trpC2 pheA1 ΔspoIIIACD | R. Losick |
| RL2045 | trpC2 pheA1 ΔspoIIIAG | R. Losick |
| RL2046 | trpC2 pheA1 ΔspoIIIAH | R. Losick |
| BTD | yycR::PsspE-cfp | D. Rudner |
| AH2468 | trpC2 ΔspoIIIAE | This work |
| AH2469 | trpC2 ΔspoIIIAF | This work |
| AH2470 | trpC2 ΔspoIIIAA | This work |
| AH2471 | trpC2 ΔspoIIIAB | This work |
| AH2472 | trpC2 ΔspoIIIACD | This work |
| AH2473 | trpC2 ΔspoIIIAG | This work |
| AH2474 | trpC2 ΔspoIIIAH | This work |
| AH2482 | trpC2 ΔspoIIIAE ΔamyE::PxylA-spoIIIAE | This work |
| AH2485 | trpC2 ΔspoIIIAE ΔyqjG::sp ΔamyE::PxylA-spoIIIAE | This work |
| AH2486 | trpC2 ΔspoIIIAE ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAE | This work |
| AH6566 | trpC2 ΔspoIIIG yycR::PsspE-cfp | This work |
| AH9200 | trpC2 ΔspoIIIAF ΔamyE::PxylA-spoIIIAF | This work |
| AH9201 | trpC2 ΔspoIIIAF ΔyqjG::sp ΔamyE::PxylA-spoIIIAF | This work |
| AH9202 | trpC2 ΔspoIIIAF ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAF | This work |
| AH9212 | trpC2 ΔspoIIIAH ΔamyE::PxylA-spoIIIAH | This work |
| AH9214 | trpC2 ΔspoIIIAH ΔyqjG::sp ΔamyE::PxylA-spoIIIAH | This work |
| AH9215 | trpC2 ΔspoIIIAH ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAH | This work |
| AH9247 | trpC2 ΔspoIIIAE ΔamyE::PxylA-spoIIIAE spoIIIJhis | This work |
| AH9250 | trpC2 ΔspoIIIAE ΔamyE::PspoIIIA-ΔSPspoIIIAE | This work |
| AH9256 | trpC2 ΔspoIIIAE spoIIIJhis | This work |
| AH9257 | trpC2 ΔspoIIIAE ΔamyE::PspoIIIA-spoIIIAE | This work |
| AH9262 | trpC2 ΔspoIIIAE ΔamyE::PspoIIIA-SPsleB-ΔSPspoIIIAE | This work |
| AH9267 | trpC2 ΔspoIIIAE ΔamyE::PspoIIIA-TMspoIFB-ΔSPspoIIIAE | This work |
| AH9270 | trpC2 ΔspoIIIAE ΔamyE::PxylA-spoIIIAE ΔspoIIIJ::sp yqjGhis | This work |
| AH9272 | trpC2 ΔspoIIIAE ΔamyE::PspoIIIA-spoIIIAEA24K | This work |
| AH9274 | trpC2 metC3 tasA::Pspac-SPspoIIIAE--ΔSPtasA | This work |
| AH9279 | trpC2 metC3 tasA::Pspac-SPspoIIIAE(A24K)-ΔSPtasA | This work |
| AH9275 | trpC2 ΔspoVE85 ΔspoIIIJ::sp ΔamyE::PxylA-spoVE-yfp | This work |
| AH9311 | trpC2 ΔspoIIIAA ΔamyE::PxylA-spoIIIAA | This work |
| AH9312 | trpC2 ΔspoIIIAB ΔamyE::PxylA-spoIIIAB | This work |
| AH9313 | trpC2 ΔspoIIIACD ΔamyE::PxylA-spoIIIACD | This work |
| AH9314 | trpC2 ΔspoIIIAG ΔamyE::PxylA-spoIIIAG | This work |
| AH9315 | trpC2 ΔspoIIIAA ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAA | This work |
| AH9316 | trpC2 ΔspoIIIAB ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAB | This work |
| AH9317 | trpC2 ΔspoIIIACD ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIACD | This work |
| AH9318 | trpC2 ΔspoIIIAG ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAG | This work |
| AH9320 | trpC2 ΔspoIIIAB ΔyqjG::sp ΔamyE::PxylA-spoIIIAB | This work |
| AH9321 | trpC2 ΔspoIIIACD ΔyqjG::sp ΔamyE::PxylA-spoIIIACD | This work |
| AH9322 | trpC2 ΔspoIIIAG ΔyqjG::sp ΔamyE::PxylA-spoIIIAG | This work |
| AH9323 | trpC2 ΔspoIIIAA ΔyqjG::sp ΔamyE::PxylA-spoIIIAA | This work |
| AH9335 | trpC2 metC3 spoIIAC::erm yycR::PsspE-cfp | This work |
| AH9336 | trpC2 ΔspoIIIAE yycR::PsspE-cfp | This work |
| AH9337 | trpC2 ΔspoIIIAE ΔamyE::PspoIIIA-spoIIIAE yycR::PsspE-cfp | This work |
| AH9338 | trpC2 ΔspoIIIAE ΔamyE::PspoIIIA-spoIIIAEA24K yycR::PsspE-cfp | This work |
In-frame deletion of the spoIIIA cistrons.
Strains carrying in-frame deletions of each of the spoIIIA cistrons (RL2040 to RL2046; Table 1), originally constructed by P. Stragier (45), were kindly provided by Richard Losick (Harvard University). The in-frame deletions were introduced into MB24 by transformation with chromosomal DNA with selection to methionine prototrophy followed by screening for Spo− congressants. This yielded strains AH2468 through AH2474 (Table 1).
PxylA promoter fusions.
The spoIIIAA (984 bp), spoIIIAB (597 bp), spoIIIACD (694 bp; because of their reduced size, the spoIIIAC and spoIIIAD cistrons were amplified and cloned as a single unit), spoIIIAE (1,373 bp), spoIIIAF (709 bp), spoIIIAG (765 bp), and spoIIIAH (1,133 bp) genes were PCR amplified from DNA of MB24, digested, and cloned into pGR40 (36) downstream of the PxylA promoter. (Note: the sequences of all primers used in this study as well as details of plasmid and strain constructions will be provided upon request.) Strains AH2482, AH9200, AH9311 to AH9314, and AH9212 were obtained by transformation of AH2468 through AH2474 with the various PxylA fusions (Table 1). Derivatives of strains AH2482, AH9200, AH9311, AH9312, AH9313, AH9314, and AH9212 bearing ΔyqjG::sp or ΔspoIIIJ::sp alleles were then obtained by transformation with DNA from JOB9 or JOB20 (Table 1).
Strain AH3562 (spoVE85 ΔamyE::PxylA-spoVE-yfp) (35) was transformed with chromosomal DNA from JOB20 (ΔspoIIIJ::sp), yielding AH9275 (spoVE85 ΔspoIIIJ::sp ΔamyE::PxylA-spoVE-yfp) (Table 1).
Fusions of spoIIIJ and yqjG to a His tag.
The 3′ regions of spoIIIJ (532 bp) and yqjG (445 bp) were PCR amplified from MB24 DNA and introduced into pET30a (Novagen, Darmstadt, Germany) to produce pFV2 and pFV1, respectively. In this way, a six-His tag was fused in frame to the 3′ ends of spoIIIJ and yqjG. Spectinomycin and chloramphenicol resistance cassettes were then isolated from pAH250 or pMS38 (41) and introduced into pFV2 (to give pFV4) or into pFV1 (to produce pMS254), respectively. Strains AH2468 (ΔspoIIIAE) and AH2482 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE) were transformed with pFV4, yielding AH9256 (ΔspoIIIAE spoIIIJhis) and AH9247 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE spoIIIJhis), respectively (Table 1). Strain AH2486 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE ΔspoIIIJ::Sp) was transformed with pMS254 to give AH9270 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE ΔspoIIIJ::sp yqjGhis) (Table 1).
Production of SpoIIIAE, SpoIIIJ-6xHis, and YqjG-6xHis in E. coli.
spoIIIAE (1,257 bp), spoIIIJ (906 bp), and yqjG (828 bp) were first PCR amplified from MB24 DNA. The spoIIIJ and yqjG fragments were then introduced into pETDuet-1 (Novagen, Darmstadt, Germany) to yield pMS266 and pMS273, for overproduction of SpoIIIJ-6xHis or YqjG-6xHis, respectively. The spoIIIAE fragment was cloned into pACYDuet-1 to yield pMS267. Plasmids pMS266, pMS267, and pMS273 were introduced into C43(DE3) cells, and protein production was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) as described previously (29). During the course of this work, we found two disagreements between the spoIIIAE sequences of strains MB24 and 168 (http://genolist.pasteur.fr/SubtiList/). In MB24, an A instead of a G occupies position 86 of the coding sequence (creating a glutamic acid codon), and the absence of a G at position 1100 results in a sequence 4 residues shorter that aligns well with SpoIIIAE orthologues. Camp and Losick have reported identical discrepancies for strain PY79 (5).
Fusion of tasA to the spoIIIAE signal sequence.
We first used PCR to fuse the spoIIIAE signal sequence to the tasA gene. Then, the SPspoIIIAE-ΔSPtasA fragment was cloned downstream of the IPTG-inducible Pspac promoter in pDH88 (41) to yield pMS304. Transformation of MB24 with pMS304 produced the Cmr strain AH9274 (tasA::Pspac-SPspoIIIAE-ΔSPtasA) (Table 1).
Mutagenesis of the spoIIIAE signal peptide.
We used PCR to fuse spoIIIAE without its putative signal sequence to a spoIIIA promoter fragment (position −229 to +29 relative to the transcriptional start site). The PspoIIIA-ΔSPspoIIIAE (1,532-bp) fragment was introduced into pDG364 (42) to yield pMS247. Then, strain AH2468 (ΔspoIIIAE) was transformed with pMS247, with selection to Cmr, yielding AH9250 (ΔspoIIIAE ΔamyE::PspoIIIA-ΔSPspoIIIAE; Table 1). A fusion of the complete spoIIIAE gene to the spoIIIA promoter was also constructed as a control. First, the spoIIIA promoter fragment was introduced into pMLK83 (42) to give pMS216. Second, a spoIIIAE PCR fragment (1,360 bp) was cloned downstream of the spoIIIA promoter in pMS216 to generate pMS217. Finally, strain AH2468 (ΔspoIIIAE) was transformed with pMS217, yielding AH9257 (ΔspoIIIAE ΔamyE::PspoIIIA-spoIIIAE).
PCR was also used to produce an allele of spoIIIAE in which its signal peptide sequence was replaced with that of the sleB gene (30) under the control of the spoIIIA promoter. The PspoIIIA-SPsleB-ΔSPspoIIIAE fragment was introduced into pDG364 to yield pMS252. Strain AH2482 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE; see above) was transformed with pMS252 digested with BamHI to create AH9262 (ΔspoIIIAE ΔamyE::PspoIIIA-SPsleB-ΔSPspoIIIAE) (Table 1).
The signal peptide of spoIIIAE was also replaced by the first transmembrane segment of SpoIVFB (14) and the fusion expressed under the control of the spoIIIA promoter. The PspoIIIA-TMspoIVFB-ΔSPspoIIIAE fragment was introduced into pDG364 to yield pMS287. Strain AH2482 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE; see above) was transformed with BamHI-digested pMS287, with selection to Cmr Nmr, to generate AH9267 (ΔspoIIIAE ΔamyE::PspoIIIA- TMspoIVFB-ΔSPspoIIIAE) (Table 1).
Lastly, the alanine codon at position 24 (from the N terminus) of SpoIIIAE was replaced with a lysine codon (AAA) by using the QuikChange site-directed mutagenesis system (Stratagene) in the spoIIIAE and tasA alleles present in pMS217 and pMS304 (see above). This resulted in plasmids pMS303 and pMS310. Strain AH2468 (ΔspoIIIAE) was transformed with pMS303 to create the Nmr strain AH9272 (ΔspoIIIAE ΔamyE::PspoIIIA-spoIIIAEA24K), whereas the Cmr strain AH9279 [tasA::Pspac-SPspoIIIAE(A24K)-ΔSPtasA] resulted from introducing pMS310 into MB24.
An sspE-cfp fusion.
Strains AH2357 (spoIIAC::erm), AH3795 (ΔspoIIIG), AH2468 (ΔspoIIIAE), AH9257 (ΔspoIIIAE ΔamyE::PspoIIIA-spoIIIAE), and AH9272 (ΔspoIIIAE ΔamyE::PspoIIIA-spoIIIAEA24K) were transformed to Cmr with DNA from BTD2633 (ΔyycR::PsspE-cfp) (kindly provided by D. Rudner), yielding AH9335, AH6566, AH9336, AH9337, and AH9338, respectively (Table 1).
Sedimentation analysis.
Cells (100 ml) grown in DSM were collected by centrifugation (6,000 × g for 10 min) 3 h after the onset of sporulation, fractionated into a soluble and a membrane fraction, and analyzed as described by Scott et al. (40).
In vivo protein cross-linking and purification of His-tagged protein complexes.
E. coli C43(DE3) cells expressing spoIII-hisJ, yqjGhis, or spoIIIAE alone or in combination were grown in LB to an A600 of 0.7 and induced with 1 mM IPTG for 3 h. B. subtilis strains were grown in LB in the presence of 1% xylose for 4 h. Following induction, E. coli or B. subtilis cells (10 ml) were centrifuged and washed with 150 mM NaCl, 20 mM NaPO4, pH 7.2. The cells were then concentrated 10-fold in the same buffer before addition of dithiobis(succinimidylpropionate) (DSP), which has a fixed spacer arm of 12 Å and a cleavable disulfide bond (Pierce, Rockford, IL), to 0.2 mM. Cross-linking was carried out for 30 min at 37°C and was quenched with 20 mM Tris-HCl, pH 7.5. Cells were harvested, resuspended in 10 mM Tris-HCl, pH 7.4, 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride, and the suspension was passed twice through a French pressure cell at 19,000 lb/in2. Membranes, isolated by a 60-min centrifugation at 100,000 × g, were solubilized in 8 M urea, 10 mM Tris-HCl, 100 mM NaH2PO4, 1% Triton X-100, 0.2% sarcosyl, adjusted to pH 8.0, mixed with 200 μl 50% Ni-nitrilotriacetic acid resin (Qiagen, Hilden, Germany) for each 10 ml of suspension, and incubated for 1 h with mixing. The resin was washed three times for 10 min with 0.5 M NaCl, 20 mM Tris, 5 mM imidazole, 0.1% sodium dodecyl sulfate (SDS), pH 8.0. Bound proteins were eluted with 8 M urea, 50 mM Tris, 2% SDS, 0.4 M imidazole, pH 6.8. The cross-linker was reduced with 5% β-mercaptoethanol in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (2% SDS, 6.25 mM Tris-HCl, pH 6.8, 5% glycerol, 0.025% bromophenol blue) at 100°C for 5 min.
Immunoblotting.
Cultures of the Pspac-tasA strains were grown in LB to an A600 of 0.6 and induced with 5 mM IPTG for 30 min. Samples of 15 ml were harvested by centrifugation. The cell pellet was resuspended in 1 ml of lysis buffer and the suspension passed twice through a French press (43). Immunoblot analysis of the cell pellets and culture supernatants was as described previously (43) with a mouse anti-His tag antibody (Novagen, Darmstadt, Germany) or with rabbit antibodies raised against peptides derived from SpoIIIAE or SpoIIIJ (residues 66 to 80 and 247 to 261, respectively; Eurogentec, Seraing, Belgium) at a dilution of 1:1,000. Anti-σA and anti-TasA antibodies were used as described previously (36, 43).
Fluorescence microscopy.
Samples (0.6 ml) of DSM cultures were collected about 4 and 6 h after the initiation of sporulation and resuspended in 0.2 ml of phosphate-buffered saline, and the membrane dye FM4-64 (Molecular Probes) was added at a final concentration of 10 μg ml−1 for visualization of membranes. Microscopy was carried out as described previously (41).
RESULTS
A functional interaction between spoIIIAE and spoIIIJ.
SpoIIIAE is coded by the fifth cistron in the operon and has the most complex predicted membrane topology of all the spoIIIA-encoded products, with eight predicted membrane-spanning domains (Fig. 1A) (1, 20). This suggested to us that SpoIIIJ is involved in facilitating its insertion into the prespore membranes, and this motivated the test of a functional connection between spoIIIAE and spoIIIJ. Since either SpoIIIJ or YqjG is required for cell viability (31, 49), we reasoned that in cells lacking SpoIIIJ, overexpression of a gene coding for a SpoIIIJ development-specific substrate could cause a cell growth defect if the overexpressed protein were to block YqjG by means of a nonfunctional interaction or otherwise by titrating YqjG (49). This type of effect would then be reminiscent of the Slo (synthethic lethality by overexpression) phenotype described in other systems (25). We tested if the overexpression of the spoIIIAE gene would cause a synthetic lethal phenotype in growing cells lacking spoIIIJ. We also overexpressed the remaining spoIIIA cistrons (Fig. 1A) from the PxylA promoter in the same background. We first fused the coding region of the spoIIIA cistrons to the xylose-inducible and glucose-repressible PxylA promoter (36). Then, the promoter fusions were introduced at the nonessential amyE locus in strains carrying an in-frame deletion of the corresponding spoIIIA cistron (Table 1). In all strains, induction with 0.5% xylose restored sporulation to wild-type levels, confirming the synthesis of functional spoIIIA-encoded products from the PxylA promoter (data not shown). We next introduced ΔspoIIIJ::sp, and as a control a ΔyqjG::Sp allele, in all the strains carrying the spoIIIA cistrons under the control of the PxylA promoter (Table 1). The various strains were grown in LB medium under conditions of repression (with 0.5% of glucose) or basal expression (no glucose or xylose added) of the PxylA promoter (36). The results in Fig. 1 show that expression of spoIIIAE in a spoIIIJ mutant background results in a growth defect on plates and in a reduced capacity to support exponential growth in liquid medium (Fig. 1B and C). A lag of about 2 h is seen following inoculation of strain AH2486 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE ΔspoIIIJ::sp) before an effect on growth is seen (Fig. 1C). This may be explained by the slow accumulation of SpoIIIAE during growth, because the effect on growth coincides with the time at which SpoIIIAE is first detected by immunoblotting (data not shown). These effects are specific for the spoIIIJ allele, as expression of spoIIIAE in cells lacking yqjG has no influence on growth on plates or liquid medium (Fig. 1B and D). Moreover, expression of the other spoIIIA cistrons in a spoIIIJ or yqjG mutant background did not impair growth on plates (as exemplified by spoIIIAF and spoIIIAH in Fig. 1B) or on liquid medium (data not shown). We noted that in the presence of xylose the growth defect caused by expression of spoIIIAE in the absence of spoIIIJ was somewhat masked by a longer growth period. This was due to utilization of the xylose, as deletion of the xylose utilization genes restored the growth defect (data not shown).
FIG. 1.
A functional linkage between spoIIIAE and spoIIIJ. (A) Deduced membrane topology of the spoIIIA-encoded proteins (AA to AH; not to scale) (1, 20) in the prespore outer membrane (OPM). Except for SpoIIIAA, the predicted localization of the N and C termini of the various proteins is represented. Note that SpoIIIAE is represented with its putative signal peptide and that the topology of SpoIIIAH has been established experimentally (2, 3, 28). MC, mother cell; IMS, intermembrane space. (B) Strains AH2486 (1; ΔspoIIIAE ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAE), AH2485 (2; ΔspoIIIAE ΔyqjG::sp ΔamyE::PxylA-spoIIIAE), AH9201 (3; ΔspoIIIAF ΔyqjG::sp ΔamyE::PxylA-spoIIIAF), AH9214 (4; ΔspoIIIAH ΔyqjG::sp ΔamyE::PxylA-spoIIIAH), AH9215 (5; ΔspoIIIAH ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAH), and AH9202 (6; ΔspoIIIAF ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAF), grown on LB plates with or without 0.5% glucose, for 12 h at 37°C. (C and D) Strains AH2486 (ΔspoIIIAE ΔspoIIIJ::sp ΔamyE::PxylA-spoIIIAE) (B) and AH2485 (ΔspoIIIAE ΔyqjG::sp ΔamyE::PxylA-spoIIIAE) (C) were grown in LB with 0.5% glucose until the optical density at 600 nm reached approximately 1.0 and then diluted 100-fold (T0) into fresh LB medium with (squares) or without (circles) 0.5% glucose.
As a further test for the specificity of the synthetic lethal effect caused by expression of spoIIIAE in a spoIIIJ mutant, we tested overexpression of a functional spoVE-yfp fusion, which codes for another sporulation-specific polytopic membrane protein (35) (Table 1). We found that induction of spoVE-yfp expression upon xylose addition did not influence growth of a spoIIIJ mutant (data not shown). Together, these results suggest that spoIIIJ and spoIIIAE are functionally linked.
SpoIIIAE and SpoIIIJ form membrane-bound complexes in B. subtilis.
The functional linkage between SpoIIIAE and SpoIIIJ prompted us to test whether the two proteins were present in complexes. We made use of a His-tagged allele of spoIIIJ to test whether a SpoIIIJ-SpoIIIAE complex could be isolated by in vivo cross-linking followed by affinity chromatography. Introduction of the spoIIIJhis fusion into an otherwise-wild-type strain (MB24) did not impair sporulation (data no shown) and, thus, the activity of SpoIIIJ does not seem to be inhibited by its fusion to the His tag. We first attempted to detect an interaction between SpoIIIJ and SpoIIIAE in sporulating cells. However, under our conditions SpoIIIAE was barely detectable during sporulation (not shown), and no interaction could be observed. We then tested for an interaction between SpoIIIJ and SpoIIIAE using strain AH9247 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE spoIIIJhis; see above), which bears a xylose-inducible spoIIIAE allele. The cross-linking agent DSP was added to cultures of AH9247 at the end of the logarithmic phase of growth in LB containing xylose (Fig. 1), membrane extracts were prepared, and the proteins were resolved by SDS-PAGE and identified by immunoblotting with antibodies against the His tag (Fig. 2A). In addition to a species of 28 kDa corresponding to the SpoIIIJ monomer, a higher-molecular-mass species of approximately 50 kDa was detected (Fig. 2A, lane 1). Another member of the SpoIIIJ family, YidC from E. coli, also accumulates as a mixture of monomers and dimers (51), and we presume that the 50-kDa species corresponds to a SpoIIIJ dimer.
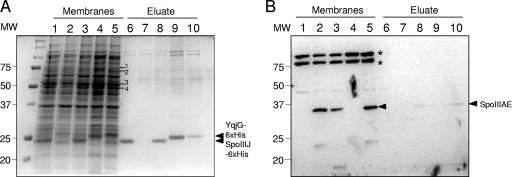
FIG. 2.
Interaction between SpoIIIJ and SpoIIIAE in B. subtilis. (A) Whole-cell extracts of B. subtilis cultures expressing SpoIIIJ-6xHis (AH 9247; ΔspoIIIAE ΔamyE::PxylA-spoIIIAE ΔspoIIIJhis; lane 1) or YqjG-6xHis (AH9270; ΔspoIIIAE ΔamyE::PxylA-spoIIIAE ΔspoIIIJ::sp yqjGhis; lane 2) were analyzed by Western blotting using antibodies against the His tag. The position of molecular mass markers (in kDa) is also shown. (B and C) B. subtilis strains AH2482 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE; lane 1), AH9256 (ΔspoIIIAE spoIIIJhis; lane 2), AH9247 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE spoIIIJhis; lane 3), and AH9270 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE spoIIIJ::sp ΔyqjGhis; lane 4), expressing the indicated proteins (+ signs), were grown in LB in the presence of 0.5% xylose and subjected to in vivo cross-linking with DSP at the end of exponential growth. Membranes were isolated, and proteins were resolved by SDS-PAGE under reducing conditions (to cleave the cross-linker) and identified by Western blotting using antibodies against the His tag or SpoIIIAE, as indicated, before (B) or after (C) purification of SpoIIIJ-6xHis or YqjG-6xHis-containing complexes by Ni2+ affinity chromatography. The band marked with an asterisk in panel B represents a nonspecific cross-reactive species.
Complexes containing SpoIIIJ-6xHis were then purified by Ni2+ affinity chromatography and treated with dithiothreitol to cleave the cross-linker. The protein components of the complex were resolved by SDS-PAGE and identified by immunoblotting with antibodies against the His tag or SpoIIIAE (Fig. 2C). SpoIIIJ-6xHis was affinity purified from the extracts of the strains containing the spoIIIJhis fusion, AH9256 (ΔspoIIIAE spoIIIJhis) and AH9247 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE spoIIIJhis) (Fig. 2B and C, lanes 2 and 3). SpoIIIAE copurified with SpoIIIJ-6xHis from extracts of AH9247, expressing both the spoIIIJhis fusion and spoIIIAE (Fig. 2B and C, lane 3), but did not purify in a spoIIIJhis-independent manner from extracts of AH2482 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE) (Fig. 2B and C, lane 1). In addition, SpoIIIAE did not copurify with the membrane protein SpoVE-6xHis, produced from PxylA at the end of the logarithmic phase of growth in LB containing xylose (data not shown). These results show that SpoIIIAE and SpoIIIJ are present in the same membrane-bound complex or complexes in B. subtilis and suggest that SpoIIIAE is a development-specific substrate for SpoIIIJ.
SpoIIIAE also interacts with YqjG.
We also used a YqjG-6xHis fusion to test for its ability to interact with SpoIIIAE. We inferred that the His-tagged allele of yqjG was functional because strain AH9270 (ΔspoIIIAE ΔamyE::PxylA-spoIIIAE spoIIIJ::sp yqjGhis) was viable. As for SpoIIIJ-6xHis, YqjG-6xHis also accumulates in membranes prepared from this strain as a mixture of monomers and (presumably) dimers (Fig. 2A, lane 2). The DSP cross-linker was then added to cultures of AH9270 at the end of the logarithmic phase of growth in LB in the presence of xylose. YqjG-6xHis-containing complexes were purified by Ni2+ chromatography from membranes of strain AH9270 and treated with dithiothreitol to cleave the cross-linker, and the protein components were resolved by SDS-PAGE and immunoblotted with anti-His tag or anti-SpoIIIAE antibodies (Fig. 2B and C). We found that SpoIIIAE also copurified with YqjG-6xHis (Fig. 2B and C, lane 4). Because YqjG is not able to support normal growth in the absence of SpoIIIJ when SpoIIIAE is overexpressed, we presume that the interaction of SpoIIIAE with YqjG blocks the latter, resulting in impaired growth of strains AH2486 and AH9270 (see above).
SpoIIIAE and SpoIIIJ form membrane-bound complexes in Escherichia coli.
Because the interaction of SpoIIIJ-6xHis or YqjG-6xHis with SpoIIIAE was difficult to detect in B. subtilis (Fig. 2), we used in vivo cross-linking and affinity chromatography to probe for interactions in E. coli cells, under conditions where the two proteins being tested were coproduced (Fig. 3). Cells of the various E. coli strains were treated with DSP and lysed, the membrane proteins were solubilized, and the His tag-containing complexes were affinity purified from the extracts. Proteins in the total membrane preparations and the isolated complexes were first resolved by SDS-PAGE and the gels stained with Coomassie blue. Several proteins known to associate with the inner membrane in E. coli could be identified by mass spectrometry in the preparation of total membranes, including FtsH (70.7 kDa; labeled as 1 in Fig. 3A, lane 5), succinate dehydrogenase (64.4 kDa; number 2), and the α-subunit (55 kDa) and β-subunit (50 kDa) of the F0F1-ATPase (numbers 3 and 4, respectively). In addition, note that SpoIIIJ-6xHis (lanes 1 and 3) and YqjG-6xHis (lanes 4 and 5) were also visible in the Coomassie-stained gel of the total membrane protein, while SpoIIIAE (lanes 2, 3, and 5) could not be detected by this method. SpoIIIJ-6xHis (lane 7) and YqjG-6xHis (lane 9) were readily pulled out by Ni2+-affinity chromatography in a single step, but note that none of the abundant E. coli membrane proteins seemed to copurify to detectable levels. Affinity-purified complexes were then analyzed by immunoblotting with anti-SpoIIIAE-specific antibodies (Fig. 3B). SpoIIIAE copurified with SpoIIIJ-6xHis (Fig. 3B, lanes 3 and 8) but did not purify in a SpoIIIJ-6xHis-independent manner (Fig. 3B, lanes 2 and 7). SpoIIIAE also copurified with YqjG-6xHis (Fig. 3, lanes 5 and 10). Unless a highly conserved protein is involved, these results suggest that SpoIIIAE and SpoIIIJ or YqjG interact directly.
FIG. 3.
Interaction between SpoIIIJ and SpoIIIAE in E. coli. E. coli strains were grown in LB medium and induced with IPTG to produce SpoIIIJ-6xHis (lanes 1 and 6), SpoIIIAE (lanes 2 and 7), both SpoIIIJ-6xHis and SpoIIIAE (lanes 3 and 8), YqjG-6xHis (lanes 4 and 9), or both YqjG-6xHis and SpoIIIAE (lanes 5 and 10). Following in vivo cross-linking with DSP, complexes containing SpoIIIJ-6xHis or YqjG-6xHis complexes were purified from membrane preparations by Ni2+ affinity chromatography. Cross-linked proteins were electrophoretically resolved under reducing conditions to cleave the cross-linker. Total protein levels in lysates and eluates were detected by Coomassie staining (A), and SpoIIIAE protein levels were determined by immunoblot analysis with antibodies raised against a peptide derived from SpoIIIAE (B). The position of size markers (in kDa) is indicated. The positions of SpoIIIAE, SpoIIIJ-6xHis, YqjG-6xHis, and cross-reactive species (asterisks) are indicated. In panel A, the positions of the following proteins identified by mass spectrometry are also indicated in lane 5: band 1, FtsH (70.7 kDa); band 2, succinate dehydrogenase (64.4 kDa); bands 3 and 4, the α-subunit (55 kDa) and β-subunit (50 kDa) of the F0F1-ATPase.
SpoIIIJ is not essential for the association of SpoIIIAE with the membrane.
Members of the SpoIIIJ family promote the insertion of proteins in the membrane or assist in their correct folding or assembly following membrane insertion (24, 27, 50, 51, 52). To determine whether the association of SpoIIIAE with the membrane requires SpoIIIJ, we used anti-SpoIIIAE antibodies to monitor the distribution of SpoIIIAE in extracts of wild-type and spoIIIJ cells. Three hours after the onset of sporulation, extracts from the wild type (MB24) and the spoIIIAE (AH2468) and spoIIIJ (JOB20) mutants were prepared and fractionated into a soluble and a membrane fraction (see Materials and Methods). The membranes were then resuspended in buffer containing Triton X-100 (0.5%) and analyzed by SDS-PAGE and immunoblotting in parallel with the soluble fraction (Fig. 4). SpoIIIAE was detected in the membrane fraction prepared from the wild type (Fig. 4, lane 4) but not from a spoIIIAE deletion mutant (Fig. 4, lane 5). Furthermore SpoIIIAE accumulated and associated with the membrane in cells of a spoIIIJ mutant (Fig. 4, lane 6). SpoIIIAE was not detected in the soluble fraction of any of the cultures (Fig. 4, lanes 1 to 3). Hence, SpoIIIAE behaves like a membrane protein independently of the presence of SpoIIIJ. As controls, SpoIIIJ was found in the membrane fraction (Fig. 4, lanes 4 and 5), whereas RNA polymerase sigma factor σA, a cytoplasmic protein, was found in the soluble fraction (Fig. 4, lanes 1 to 3). This analysis indicates that SpoIIIAE can be inserted in the membrane in a spoIIIJ-independent manner and suggests that SpoIIIJ may function in the correct folding or assembly of SpoIIIAE past a membrane insertion step.
FIG. 4.
SpoIIIAE associates with the membrane independently of SpoIIIJ. Strains MB24 (wild type; lanes 1 and 4), AH2468 (ΔspoIIIAE; lanes 2 and 5), and JOB20 (ΔspoIIIJ::sp; lanes 3 and 6) were grown in DSM and samples were collected 3 h after the onset of sporulation. The cells were lysed and fractionated into a soluble and a membrane fraction, and samples were electrophoretically resolved and analyzed by Western blotting with antibodies against SpoIIIAE, SpoIIIJ, or σA, as indicated. The bands marked with asterisks represent nonspecific cross-reactive species.
SpoIIIAE has a functional Sec-type signal peptide.
SpoIIIAE has eight to nine predicted transmembrane segments, the first of which resembles a Sec-type signal peptide with a potential cleavage site between two adjacent alanine residues (positions 24 and 25) (Fig. 5A) (1). Interestingly this putative Sec-type signal peptide is conserved in SpoIIIAE proteins of other spore-formers, including at least some clostridial species (Fig. 5A). The Sec system is involved in translocation across the cytoplasmic membrane of proteins with an N-terminal cleavable signal sequence, and its components were shown to interact with members of the YidC/Oxa1p/Alb3 family during the Sec-dependent insertion of certain membrane proteins (24, 27, 51, 52). Furthermore, a link between SpoIIIJ and YqjG and the Sec system has been previously suggested (48, 49). To examine the functionality of the signal peptide of SpoIIIAE, we replaced the signal peptide of the secreted protein TasA (43, 44) with the first 24 amino acids of SpoIIIAE. The fusion SPspoIIIAE-ΔSPtasA (deletion of the TasA signal peptide and replacement by that of SpoIIIAE) was introduced at the tasA locus under the control of the IPTG-inducible Pspac promoter (AH9274). Accumulation of mature TasA in cell extracts and supernatants was monitored by SDS-PAGE followed by Western blot analysis using anti-TasA antibodies and anti-σA antibodies to control for contamination by cytoplasmic proteins (Fig. 5B). We were able to detect the mature form of TasA 30 min after induction in the supernatants of cultures of strains expressing either wild-type tasA (AH1802; Fig. 5B, lane 2) or the tasA allele in which the putative SpoIIIAE signal peptide replaced the original signal peptide (AH9274; Fig. 5B, lane 4). We conclude that the first 24 amino acids of SpoIIIAE are able to target TasA for secretion and therefore to function as a signal peptide. Furthermore, we constructed a mutant with a single amino acid substitution of the alanine at position −1, relative to the putative cleavage site of the SpoIIIAE signal sequence (residue 24 from the N terminus), for a lysine (AH9279). Sec-type signal peptides in B. subtilis show a highly conserved alanine at position −1 (48). This position can also tolerate small or uncharged residues, but the alanine-to-lysine substitution is predicted to severely affect the function of the SpoIIIAE signal peptide (47). Indeed, the A24K mutation was sufficient to prevent secretion of TasA (Fig. 5B, lanes 6 and 6*).
FIG. 5.
SpoIIIAE has a functional signal peptide. (A) Alignment of the N-terminal region of the SpoIIIAE protein from selected spore-forming species. Positively charged residues at the N terminus of the depicted region are highlighted in blue. The two residues at positions −1 and +1 relative to the predicted cleavage site are highlighted in yellow, and the predicted cleavage site is indicated by the red arrow. The black arrowhead shows the position of a single amino acid substitution of the alanine (A) at position 24 of B. subtilis SpoIIIAE by a lysine (K). (B) The putative SpoIIIAE signal peptide is able to substitute for an already characterized signal peptide of a secreted protein (TasA). The inducer IPTG was added to LB cultures of Pspac-tasA (lanes 1 and 2), Pspac-SPspoIIIAE-tasA (lanes 3 and 4), and Pspac-SPspoIIIAE(A24K)-tasA (lanes 5 and 7), and samples of cells and supernatants (Sup) collected at the time of addition (lanes 1, 3, and 5) and 30 min after (lanes 2, 4, and 6) were subjected to immunoblot analysis. Anti-TasA and anti-σA antibodies (as controls for cells and supernatants) were used. For the cells (two upper panels) or supernatants (two bottom panels) analyzed, the corresponding cellular or supernatant fractions were also probed with TasA or σA antibodies (lane 7). Note that the region corresponding to lanes 5, 6, and 7 in the upper panel was digitally enhanced and is shown on the side (lanes labeled 5*, 6*, and 7*) to make clearly visible the precursor form of TasA.
Cleavage of the signal peptide is required for SpoIIIAE function.
To examine the importance of the N-terminal region for the function of SpoIIIAE during sporulation, its signal peptide (residues 1 to 24) was deleted (strain AH9250) or replaced by either the SleB signal peptide (residues 1 to 29; SPsleB-ΔSP-SpoIIIAE; AH9262) or the first transmembrane segment of the SpoIVFB protein (residues 1 to 31; TMspoIVB-ΔSP-SpoIIIAE; AH9267). SleB is a spore cortex hydrolase with a Sec-type signal peptide that is translocated to the cortex region across the prespore inner membrane (29), and SpoIVFB is a membrane metalloprotease involved in the activation of σK (14). The spoIIIAE variants were introduced at amyE under the control of the spoIIIA promoter in strains carrying an in-frame deletion of the spoIIIAE gene (Table 1). As shown in Table 2, the strain carrying a spoIIIAE allele with a deletion of the putative signal peptide (AH9250) was severely impaired in sporulation, whereas the spoIIIAE mutant in which the SleB signal sequence replaced that of SpoIIIAE (AH9262) sporulated with wild-type efficiency (AH9257) (Table 2). This indicates that the signal sequence of SpoIIIAE is functionally similar to that of SleB and essential for the function of the protein. Accordingly, strains producing the TMspoIVB-ΔSpoIIIAE form of SpoIIIAE (AH9267) or the spoIIIAEA24K allele (AH9272) were also impaired in sporulation, although not to the level of the strain deleted for the entire SpoIIIAE signal peptide (Table 2). To determine whether the A24K substitution interfered with the activation of σG, we monitored expression of a fusion of the σG-controlled sspE promoter to cfp in both the wild-type (AH9337) and spoIIIAEA24K (AH9338) strains. The resulting strains were induced to sporulate and the CFP patterns analyzed at hour 4 of sporulation (T4), at which time the engulfment process is completed in most of the population, and 2 h thereafter. Under the conditions used, about 32% of the wild-type cells examined at T4 showed a weak fluorescent signal (Fig. 6A and B, class a cells), whereas 57% of the cells showed stronger fluorescence (Fig. 6, class b cells). The representation of class a cells dropped to about 5% for the wild type at T6, whereas class b cells increased to 87%. In contrast, in the spoIIIAEA24K mutant (AH9338), the proportion of class b cells did not increase significantly from T4 to T6, and the fraction of class a cells remained high (Fig. 6B). Class b corresponds to cells in which the engulfment process has been completed and σG is activated (Fig. 6A). Very few class b cells were found for a ΔspoIIIAE mutant (AH9336) (Fig. 6B), and only class a cells were found in a ΔsigG (AH6566) mutant at either T4 or T6 (Fig. 6B). We did not observe expression of PsspE-cfp in a ΔsigF mutant (AH9335) (not shown). Therefore, we presume that the low level of fluorescence associated with class a cells in the wild type or in cells unable to activate σG results mainly from utilization of the sspE promoter by σF (46). We conclude that the cells bearing the A24K substitution fail to efficiently activate σG.
TABLE 2.
Efficiency of sporulationa of strains bearing various spoIIIAE alleles
| Strain | Relevant genotype | Viable cell count | Heat-resistant cell count | Sporulation (%) |
|---|---|---|---|---|
| AH2468 | spoIIIAE | 1.0 × 108 | 1.8 × 104 | 0.02 |
| AH9257 | spoIIIAE PspoIIIA-spoIIIAE | 4.0 × 108 | 3.3 × 108 | 82.5 |
| AH9250 | spoIIIAE PspoIIIA-ΔSPspoIIIAE | 1.2 × 108 | 5.2 × 105 | 0.4 |
| AH9262 | spoIIIAE PspoIIIA-SPsleB-ΔSPspoIIIAE | 2.9 × 108 | 1.5 × 108 | 51.7 |
| AH9267 | spoIIIAE PspoIIIA-TMspoIFB-ΔSPspoIIIAE | 1.0 × 108 | 1.1 × 107 | 11 |
| AH9272 | spoIIIAE PspoIIIA-spoIIIAEA24K | 1.4 × 108 | 1.1 × 107 | 7.9 |
The titers of viable cells and heat-resistant spores were measured 24 h after the onset of sporulation in DSM (see Materials and Methods).
FIG. 6.
SpoIIIAE processing is required for σG activation. (A) Expression patterns of a PsspE-cfp fusion in DSM cultures of strains expressing wild-type spoIIIAE (AH9337) or the spoIIIAEA24K allele (AH9338) from the spoIIIA promoter. Samples were collected at the indicated times (in hours) after the onset of sporulation, stained with the membrane dye FM4-64, and observed by fluorescence microscopy. Lowercase letters indicate fluorescence intensity patterns (low [a] or high [b]). (B) Quantitative analysis of the PsspE-cfp expression patterns observed at hours 4 and 6 of sporulation for strains AH9337 (black) and AH9338 (dark gray) and in mutants with deletion of the spoIIIAE (AH9336; white) or spoIIIG (AH6566; light gray) allele. Note that the last two strains are not shown in panel A, for simplicity, and that no fluorescence was detected for the ΔsigF mutant AH9335 (not shown). At least 200 cells were scored for the fluorescence patterns designated by low intensity (class a; pixel intensity of <300; see Material and Methods) or high intensity (class b; pixel intensity of >300). (C) An anti-SpoIIIAE immunoblot of a membrane fraction prepared from cells of MB24 (wild type; lane 1), AH2468 (ΔspoIIIAE; lane 2), AH9257 (PspoIIIA-spoIIIAE; lane 3), AH9272 (PspoIIIA-spoIIIAEA24K; lane 4), and AH9262 (PspoIIIA-SPsleB-ΔSPspoIIIAE; lane 5), at hour 3 of sporulation in DSM. The black and white arrowheads show the positions of the precursor and mature forms of SpoIIIAE, respectively. Bar, 1 μm.
Lastly, we examined whether during sporulation the signal peptide of SpoIIIAE was cleaved off and, if so, whether the A24K substitution interfered with processing. We examined the accumulation of the various forms of SpoIIIAE (see above) by immunoblot analysis using SpoIIIAE antibodies (Fig. 6A). We found that SpoIIIAEA24K exhibits a slower electrophoretic migration (Fig. 5C, lanes 4) compared to the wild type (lanes 1 and 3) or to the SPsleB-ΔSpoIIIAE variant (lane 5) and compatible with the expected mass increase of 2.7 kDa corresponding to the uncleaved signal peptide. Together, these results underscore the importance of the Sec-type signal sequence in SpoIIIAE during sporulation and suggest a functional linkage to the Sec system.
DISCUSSION
The results reported herein link the function of SpoIIIJ to that of SpoIIIAE, both of which are required for the activation of σG following engulfment completion. We found that expression of spoIIIAE during growth in the absence of spoIIIJ but not in the absence of its paralogue yqjG causes cell lysis (Fig. 1), a synthetic lethal effect that functionally associates the spoIIIAE and spoIIIJ loci. Presumably, in the absence of SpoIIIJ the accumulation of improperly folded SpoIIIAE in the membrane is lethal or, alternatively, the interaction of SpoIIIAE with YqjG somehow blocks its function, which is essential in a spoIIIJ background. The synthetic lethal effect was specific for the spoIIIAE cistron of the spoIIIA operon, but a functional association of other spoIIIA cistrons with spoIIIJ cannot be excluded.
Furthermore, we found that SpoIIIAE and SpoIIIJ could be cross-linked in membrane preparations of B. subtilis and E. coli, suggesting that the two proteins directly interact (Fig. 2 and 3). That YqjG may also interact with SpoIIIAE supports the idea that the synthetic lethal effect caused by overexpression of spoIIIAE in a spoIIIJ background develops because the interaction of SpoIIIAE with YqjG interferes with its function. These results are in consonance with those of Camp and Losick (5), who have found that mutations able to improve the sporulation efficiency of a spoIIIJ mutant map to either spoIIIAE or to yqjG. Presumably, the spoIIIAE alleles may improve the autonomous insertion of SpoIIIAE into the membrane or may convert SpoIIIAE into a better substrate for YqjG (5). Conversely, the yqjG alleles found by Camp and Losick (5) may render more productive the interaction of SpoIIIAE with YqjG, which then partially replaces SpoIIIJ. An alternative view is that YqjG has the intrinsic ability to interact with SpoIIIAE but that the wild-type protein, and to a lesser extent the mutant forms found by Camp and Losick (5), is either modified or proteolytically inactivated during sporulation. The basis for the specific requirement for SpoIIIJ for sporulation is not yet understood, as proteins of the YidC family are highly permissive to mutagenesis and several examples of cross-species and even cross-kingdom heterologous complementation of mutants have been described (17, 18).
Members of the YidC/Oxa1p/Alb3 family of proteins function in both Sec-dependent and Sec-independent modes in protein export or membrane protein assembly (24, 27, 51, 52). Since the function of the Sec proteins is conserved between B. subtilis and E. coli (48), it seems plausible that SpoIIIJ and YqjG may also operate in both a Sec-dependent and a Sec-independent mode. Our results show that SpoIIIAE has a Sec-type signal peptide, which was also noticed by Camp and Losick (5), and which is required for its function (Fig. 5 and 6 and Table 2). We base this assertion on the following lines of evidence. First, we have shown that the signal peptide of SpoIIIAE can direct secretion of the extracellular protein TasA to the culture medium. Moreover, secretion of TasA was severely curtailed by the presence of a lysine residue at the +1 position of the SpoIIIAE signal peptide, an alteration predicted to interfere strongly with processing by a type I signal peptidase (48). In addition, the signal peptide of SpoIIIAE could neither be deleted nor replaced by a noncleavable transmembrane domain. It could, however, be replaced by an unrelated, cleavable signal peptide. Lastly, and importantly, the lysine substitution at position +1 caused accumulation of a mature form of SpoIIIAE and blocked the activation of σG. Hence, the A24K substitution mimicked all the effects of other known point or deletion mutations in the spoIIIA locus, including a complete deletion of the operon. We suggest that the signal peptide is required to route the polytopic SpoIIIAE protein to the spore membranes via signal peptide-dependent recognition of the Sec translocon and that SpoIIIJ interacts with SpoIIIAE only in the context of the Sec translocon. Membrane proteins that undergo signal peptide cleavage are rare in prokaryotes (4), but in one example, the membrane insertion of FliP (a component of the flagellar export apparatus) is dependent both on the Sec translocon, involving cleavage of a signal peptide, and on YidC (34). One problem with the proposal that both the Sec translocon and SpoIIIJ contribute to proper insertion of SpoIIIAE is that most of the components of the secretion machinery that have been examined, do not seem to be specifically enriched in the spore membranes during sporulation. In fact, only FtsY appears to be enriched, although transiently, in the septal membranes soon after asymmetric division (38). It is tempting to speculate that FtsY may present nascent SpoIIIAE to the Sec translocon, but it is presently not known whether targeting of SpoIIIAE to the membrane requires FtsY. In any event, proper functioning of SpoIIIAE and SpoIIIJ (in cooperation with the Sec translocon) does not seem to require enrichment of Sec components at the spore membranes.
It is presently unclear at what level SpoIIIJ acts on SpoIIIAE. Presumably, SpoIIIJ could assist in the insertion of SpoIIIAE into the lipid bilayer, but deletion of spoIIIJ did not affect detectably the level of SpoIIIAE associated with the membrane. However, the kinetics of insertion of SpoIIIAE in the membrane was not investigated in this study and may be significantly different, and even not sufficient to support efficient sporulation, in the absence of SpoIIIJ (49). The presence of SpoIIIAE in the membrane in a spoIIIJ mutant may also indicate that SpoIIIJ is involved in a step after the membrane insertion of SpoIIIAE, possibly in its folding or assembly. In E. coli, for instance, YidC is required for the proper folding of the LacY and MalF permeases and for the assembly of the latter into a maltose-transporting complex (32, 50), as well as other multimeric membrane protein complexes (52).
Our results, along with those from the genetic suppression studies of Camp and Losick (5), strongly argue that SpoIIIAE is a development-specific substrate for SpoIIIJ. We showed previously that production of SpoIIIJ from a prespore-specific promoter but not from a mother cell-specific promoter was sufficient for σG activation and sporulation (42). This previous report seems contradictory to our present findings, because SpoIIIAE is a mother cell protein. One possibility is that the promoter (the spoIIQ promoter) used to express spoIIIJ in the prespore allows for sufficient expression of the gene during vegetative growth and/or in the mother cell, as also proposed by Camp and Losick (5). It is nevertheless possible that SpoIIIJ has a prespore-specific substrate, because expression of spoIIIJ from a mother cell-specific promoter in single copy or from a replicative plasmid did not support efficient sporulation (42). This putative substrate may not be related to the activation of σG (5) (unpublished results). In any event, the interaction of SpoIIIJ with SpoIIIAE is sufficient to explain the involvement of SpoIIIJ in the activation of σG upon engulfment completion. The activation of σG may be achieved under certain conditions by a minimal pathway formed by the SpoIIIAH-SpoIIQ channel, because a mutation that partially bypasses the need for SpoIIIJ for σG activation also partially suppresses the requirement for spoIIIAA through spoIIIAG, but not spoIIIAH and spoIIQ (5). Nevertheless, the presence of the spoIIIA operon in all endospore-forming species (28), as well as the suggestion that some mechanistic details involved in its function, such as processing of SpoIIIAE (Fig. 6A) are conserved, emphasizes the importance of all the spoIIIA-encoded proteins for sporulation in wild-type cells. The elucidation of the structural organization of the spoIIIA-encoded complex, or subcomplexes, and the mechanism by which it signals the activation of σG are major goals for future work.
Acknowledgments
We thank Amy Camp, Rich Losick, David Rudner, John Walker, and Joen Luirink for strains and antibodies and Patrick Piggot, Jeff Meisner, David Rudner, Joen Luirink, and Jan Marteen van Dijl for helpful discussions and comments on the manuscript. We also thank Jon Pohl (Emory Microchemical Facility) for the mass spectrometry analysis.
This work was supported by grants POCI/BIA-BCM/60855/2004 and PRAXIS/BIO/35109/99 from the FCT to A.O.H. and GM54395 from the National Institutes of Health to C.P. Moran, Jr. M.S. was the recipient of a postdoctoral fellowship (SFRH/BPD/14966/2004) from the FCT.
Footnotes
Published ahead of print on 26 September 2008.
REFERENCES
- 1.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340783-795. [DOI] [PubMed] [Google Scholar]
- 2.Blaylock, B., X. Jiang, A. Rubio, C. P. Moran, Jr., and K. Pogliano. 2004. Zipper-like interaction between proteins in adjacent daughter cells mediates protein localization. Genes Dev. 182916-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broder, D. H., and K. Pogliano. 2006. Forespore engulfment mediated by a ratchet-like mechanism. Cell 126917-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broome-Smith, J. K., S. Gnaneshan, L. A. Hunt, F. Mehraein-Ghomi, L. Hashemzadeh-Bonehi, M. Tadayyon, and E. S. Hennessey. 1994. Cleavable signal peptides are rarely found in bacterial cytoplasmic membrane proteins. Mol. Membr. Biol. 113-8. [DOI] [PubMed] [Google Scholar]
- 5.Camp, A. H., and R. Losick. 2008. A novel pathway of intercellular signaling in Bacillus subtilis involves a protein with similarity to a component of type III secretion channels. Mol. Microbiol. 69402-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chary, V. K., M. Meloni, D. W. Hilbert, and P. J. Piggot. 2005. Control of the expression and compartmentalization of σG activity during sporulation of Bacillus subtilis by regulators of σF and σE. J. Bacteriol. 1876832-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chary, V. K., P. Xenopoulos, and P. J. Piggot. 2006. Blocking chromosome translocation during sporulation of Bacillus subtilis can result in prespore-specific activation of σG that is independent of σE and of engulfment. J. Bacteriol. 1887267-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chary, V. K., P. Xenopoulos, and P. J. Piggot. 2007. Expression of the σF-directed csfB locus prevents premature appearance of σG activity during sporulation of Bacillus subtilis. J. Bacteriol. 1898754-8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decatur, A., and R. Losick. 1996. Identification of additional genes under the control of the transcription factor σF of Bacillus subtilis. J. Bacteriol. 1785039-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doan, T., K. A. Marquis, and D. Z. Rudner. 2005. Subcellular localization of a sporulation membrane protein is achieved through a network of interactions along and across the septum. Mol. Microbiol. 551767-1781. [DOI] [PubMed] [Google Scholar]
- 11.Errington, J. 2003. Regulation of endospore formation in Bacillus subtilis. Nat. Rev. Microbiol. 1117-126. [DOI] [PubMed] [Google Scholar]
- 12.Errington, J., L. Appleby, R. A. Daniel, H. Goodfellow, S. R. Partridge, and M. D. Yudkin. 1992. Structure and function of the spoIIIJ gene of Bacillus subtilis: a vegetative expressed gene that is essential for sigma G activity at an intermediate stage of sporulation. J. Gen. Microbiol. 1382609-2618. [DOI] [PubMed] [Google Scholar]
- 13.Evans, L., J. Clarkson, M. D. Yudkin, J. Errington, and A. Feucht. 2003. Analysis of the interaction between the transcription factor σG and the anti-sigma factor SpoIIAB of Bacillus subtilis. J. Bacteriol. 1854615-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, D. H., and S. M. Cutting. 2000. Membrane topology of the Bacillus subtilis pro-σK processing complex. J. Bacteriol. 182278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilbert, D. W., and P. J. Piggot. 2004. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol. Mol. Biol. Rev. 68234-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Illing, N., and J. Errington. 1991. The spoIIIA operon in Bacillus subtilis defines a new temporal class of mother-cell-specific sporulation genes under the control of the sigma E form of RNA polymerase. Mol. Microbiol. 51927-1940. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, F., L. Yi, M. Moore, M. Chen, T. Rohl, K. J. Van Wijk, J. W. De Gier, R. Henry, and R. E. Dalbey. 2002. Chloroplast YidC homolog Albino3 can functionally complement the bacterial YidC depletion strain and promote membrane insertion of both bacterial and chloroplast thylakoid proteins. J. Biol. Chem. 3119281-19288. [DOI] [PubMed] [Google Scholar]
- 18.Jiang, F., M. Chen, L. Yi, J. W. de Gier, A. Kuhn, and R. E. Dalbey. 2003. Defining the regions of Escherichia coli YidC that contribute to activity. J. Biol. Chem. 27848965-48972. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, X., A. Rubio, S. Chiba, and K. Pogliano. 2005. Engulfment-regulated proteolysis of SpoIIQ: evidence that dual checkpoints control sigma activity. Mol. Microbiol. 58102-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, D. T., W. R. Taylor, and J. M. Thornton. 1994. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry 333038-3049. [DOI] [PubMed] [Google Scholar]
- 21.Karmazyn-Campelli, C., L. Rhayat, R. Carballido-López, S. Duperrier, N. Frandsen, and P. Stragier. 2008. How the early sporulation sigma factor σF delays the switch to late development in Bacillus subtilis. Mol. Microbiol. 671169-1180. [DOI] [PubMed] [Google Scholar]
- 22.Kellner, E. M., A. Decatur, and C. P. Moran, Jr. 1996. Two-stage regulation of an anti-sigma factor determines developmental fate during bacterial endospore formation. Mol. Microbiol. 21913-924. [DOI] [PubMed] [Google Scholar]
- 23.Kirchman, P. A., H. DeGrazia, E. M. Kellner, and C. P. Moran, Jr. 1993. Forespore-specific disappearance of the sigma-factor antagonist SpoIIAB: implications for its role in determination of cell fate in Bacillus subtilis. Mol. Microbiol. 8663-671. [DOI] [PubMed] [Google Scholar]
- 24.Kol, S., N. Nouwen, and A. J. Driessen. 25 July 2008. Mechanisms of YidC-mediated insertion and assembly of multimeric membrane protein complexes. J. Biol. Chem. [Epub ahead of print.] [DOI] [PubMed]
- 25.Kroll, E. S., K. M. Hyland, P. Hieter, and J. J. Li. 1996. Establishing genetic interactions by a synthetic dosage lethality phenotype. Genetics 14395-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Londoño-Vallejo, J.-A., C. Fréhel, and P. Stragier. 1997. spoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol. Microbiol. 2429-39. [DOI] [PubMed] [Google Scholar]
- 27.Luirink, J., G. von Heijne, E. Houben, and J. W. de Gier. 2005. Biogenesis of inner membrane proteins in Escherichia coli. Annu. Rev. Microbiol. 59329-355. [DOI] [PubMed] [Google Scholar]
- 28.Meisner, J., X. Wang, M. Serrano, A. O. Henriques, and C. P. Moran, Jr. 2008. A channel connecting the mother cell and forespore during bacterial endospore formation. Proc. Natl. Acad. Sci. USA 10515100-15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miroux, B., and J. E. Walker. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260289-298. [DOI] [PubMed] [Google Scholar]
- 30.Moriyama, R., H. Fukuoka, S. Miyata, S. Kudoh, A. Hattori, S. Kozuka, Y. Yasuda, K. Tochikubo, and S. Makino. 1999. Expression of a germination-specific amidase, SleB, of bacilli in the forespore compartment of sporulating cells and its localization on the exterior side of the cortex in dormant spores. J. Bacteriol. 1812373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami, T., K. Haga, M. Takeuchi, and T. Sato. 2002. Analysis of the Bacillus subtilis spoIIIJ gene and its paralogue gene, yqjG. J. Bacteriol. 1841998-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagamori, S., I. N. Smirnova, and H. R. Kaback. 2004. Role of YidC in folding of polytopic membrane proteins. J. Cell Biol. 16553-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partridge, S., and J. Errington. 1993. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol. Microbiol. 8945-955. [DOI] [PubMed] [Google Scholar]
- 34.Pradel, N., A. Decorps, C. Ye, C. L. Santini, and L. F. Wu. 2005. YidC-dependent translocation of green fluorescence protein fused to the FliP cleavable signal peptide. Biochimie 87191-196. [DOI] [PubMed] [Google Scholar]
- 35.Real, G., A. Fay, A. Eldar, S. M. Pinto, A. O. Henriques, and J. Dworkin. 2008. Determinants for the subcellular localization and function of a nonessential SEDS protein. J. Bacteriol. 190363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Real, G., and A. O. Henriques. 2006. Localization of the Bacillus subtilis murB gene within the dcw cluster is important for growth and sporulation. J. Bacteriol. 1881721-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubio, A., and K. Pogliano. 2004. Septal localization of forespore membrane proteins during engulfment in Bacillus subtilis. EMBO J. 231636-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubio, A., X. Jiang, and K. Pogliano. 2005. Localization of translocation complex components in Bacillus subtilis: enrichment of the signal recognition particle receptor at early sporulation septa. J. Bacteriol. 1875000-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudner, D. Z., and R. Losick. 2001. Morphological coupling in development: lessons from prokaryotes. Dev. Cell 1733-742. [DOI] [PubMed] [Google Scholar]
- 40.Scott, J. M., J. Ju, T. Mitchell, and W. G. Haldenwang. 2000. The Bacillus subtilis GTP binding protein Obg and regulators of the σB stress response transcription factor cofractionate with ribosomes. J. Bacteriol. 1822771-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serrano, M., A. Neves, C. M. Soares, C. P. Moran, Jr., and A. O. Henriques. 2004. Role of the anti-sigma SpoIIAB in regulation of σG during Bacillus subtilis sporulation. J. Bacteriol. 1864000-4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano, M., L. Côrte, J. Opdyke, C. P. Moran, Jr., and A. O. Henriques. 2003. Expression of spoIIIJ in the prespore is sufficient for the activation of σG and for sporulation in Bacillus subtilis. J. Bacteriol. 1853905-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serrano, M., R. Zilhão, A. J. Ozin, E. Ricca, C. P. Moran, Jr., and A. O. Henriques. 1999. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J. Bacteriol. 1813632-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stöver, A. G., and A. Driks. 1999. Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein. J. Bacteriol. 1811664-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stragier, P., and R. Losick. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30297-341. [DOI] [PubMed] [Google Scholar]
- 46.Sun, D., P. Fajardo-Cavazos, M. D. Sussman, F. Tovar-Rojo, R. M. Cabrera-Martinez, and P. Setlow. 1991. Effect of chromosome location of Bacillus subtilis forespore genes on their spo gene dependence and transcription by σF: identification of features of good σF-dependent promoters. J. Bacteriol. 1737867-7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun, Y.-L., M. D. Sharp, and K. Pogliano. 2000. A dispensable role for the forespore-specific gene expression in engulfment of the forespore during sporulation of Bacillus subtilis. J. Bacteriol. 1822919-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjalsma, H., H. Antelmann, J. D. H. Jongbloed, P. G. Braun, E. Darmon, R. Dorenbos, J. Y. F. Dubois, H. Westers, G. Zanen, W. J. Quax, O. P. Kuipers, S. Bron, M. Hecker, and J. M. van Dijl. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tjalsma, H., S. Bron, and J. M. van Dijl. 2003. Complementary impact of paralogous Oxa1-like proteins of Bacillus subtilis on post-translocational stages in protein secretion. J. Biol. Chem. 27815622-15632. [DOI] [PubMed] [Google Scholar]
- 50.Wagner, S., O. Pop, G. J. Haan, L. Baars, G. Koningstein, M. M. Klepsch, P. Genevaux, J. Luirink, and J. W. de Gier. 2008. Biogenesis of MalF and the MalFGK(2) maltose transport complex in Escherichia coli requires YidC. J. Biol. Chem. 28317881-17890. [DOI] [PubMed] [Google Scholar]
- 51.Xie, K., and R. E. Dalbey. 2008. Inserting proteins into the bacterial cytoplasmic membrane using the Sec and YidC translocases. Nat. Rev. Microbiol. 6234-244. [DOI] [PubMed] [Google Scholar]
- 52.Yi, L., and R. E. Dalbey. 2005. Oxa1/Alb3/YidC system for insertion of membrane proteins in mitochondria, chloroplasts and bacteria. Mol. Membr. Biol. 22101-111. [DOI] [PubMed] [Google Scholar]