Abstract
Mounting evidence suggests the involvement of caspases in the disease process associated with Alzheimer’s disease (AD). The activation of caspases may be responsible for the neurodegeneration associated with AD and several recent studies have suggested that caspases may also play a role in promoting pathogenic mechanisms underlying this disease. Thus, caspase activation and cleavage of the amyloid precursor protein (APP) and tau may facilitate both the production of beta-amyloid (Aβ) as well as the formation of neurofibrillary tangles (NFTs). Because the activation of caspases in AD may be a proximal event that is not just associated with neurodegeneration, caspases are potential therapeutic targets for the treatment of this disorder. In this review, studies documenting the role of caspases in the AD brain will be discussed. In this context, a discussion of the therapeutic value of targeting caspase inhibition in the treatment of AD will be evaluated including drug targets, delivery and selectivity.
Keywords: Apoptosis, caspase, Alzheimer’s disease, Tau, beta-amyloid, amyloid precursor protein, neurofibrillary tangle
Introduction
Alzheimer’s disease (AD) is a neuro-degenerative disorder characterized by extensive neuronal loss leading to cognitive impairment and dementia. AD is diagnosed based upon the extent of senile plaques composed of beta-amyloid (Aβ) and neurofibrillary tangles (NFTs) containing abnormally phosphorylated tau [1]. Currently, there is strong support for the amyloid cascade hypothesis, and many of the current therapeutic strategies poised for clinical trials involve some aspect of modifying Aβ production or clearance [2]. Despite these recent advances, there is no cure for AD and current regimens are only palliative in nature for treating the symptoms of the disease. It is important, therefore, to identify new potential drug targets for the treatment of AD. Also, given the complex nature of the disease and the multiple pathological molecular cascades that are engaged, combination treatments may be more efficacious and target different neurodegenerative pathways. Numerous studies have implicated the activation of caspases as well as the cleavage of critical proteins associated with neuropathology in AD. In this review, we will highlight some of the more relevant studies demonstrating the involvement of caspases in AD and discuss the need for additional studies utilizing caspase inhibitors in animal models of AD. Such studies could provide critical evidence that caspases are a viable target for pharmaceutical development and positive results may stimulate the development and testing of caspase inhibitors for their potential in treating this disease. An examination of potential drug targets including caspase-3 and Bcl-2, as well as potential problems with drug selectivity will also be discussed.
Apoptosis as a Cell-Death Mechanism in AD
The involvement of caspases in AD began with studies examining the mechanism responsible for the neuronal cell death associated with this disease. Many of these early studies consisted of in situ detection of fragmented DNA utilizing terminal deoxyuridine triphosphate nick end-labeling (TUNEL) techniques [3, 4]. The presumption being that during apoptosis, DNA is cleaved into a characteristic profile of fragments [5], which can be detected using TUNEL techniques. However, other studies using the same technique were negative in nature and did not support apoptosis as the major pathway of cell death in the AD brain [6, 7]. Despite whether these early studies supported or refuted a role of apoptosis in the cell death associated with AD, they were important as they stimulated further investigation employing more specific techniques that identified the actual players involved in apoptosis including caspases and their target proteins.
Caspase Activation and Cleavage of Target Proteins in AD
Because early studies suggested that the TUNEL technique may not be specific for apoptosis, newer methodologies were developed to examine the role of apoptosis in AD. One approach to detect apoptosis is to follow the activation caspases or their protein fragments following caspase cleavage. Caspases are indispensable for the execution of apoptosis, being responsible for the phenotypic characteristics of apoptosis following the cleavage of critical cellular proteins [8]. Because caspases are specific in that they cleave only after aspartic residues [9], antibodies can be designed that are based upon consensus caspase-cleavage sites and therefore are highly specific for either the targeted caspase or a specific protein substrate. Much credit for the design of such cleavage site-directed antibodies goes to Greg Cole and colleagues who were the first to utilize this technique and provide evidence for the caspase cleavage of actin in the AD brain [10]. Shortly after the publication of this study, it was demonstrated that the amyloid precursor protein (APP) is a substrate for caspase-3-mediated cleavage, which may contribute to Aβ formation, synaptic loss, and the behavioral changes associated with AD [11]. The principle data supporting this study was obtained using a site-directed caspase-cleavage antibody to APP. Other studies quickly followed employing the same technique and were able to demonstrate the caspase cleavage of fodrin in the AD brain [12] as well as the activation of specific initiator and executioner caspases including caspase-3, -6, -8, and -9 [13–17]. Collectively, these studies firmly established the activation of apoptotic pathways during the course of the disease progression in AD. Moreover, data from these studies suggested that caspases may be playing a proximal role in the disease mechanisms underlying AD including promoting Aβ formation as well as linking plaques to NFTs [18].
Evidence that Caspases Contribute to Pathology Associated with AD
The main purpose of this review is to discuss the relevance that caspases are viable therapeutic targets for the treatment of AD. An effective strategy can only be idealized if it is demonstrated that the activation of caspases do not simply represent end-stage events associated with AD. Based on earlier studies, a hypothesis was formulated suggesting that caspases may be acting further upstream in the sequence of events associated with AD than originally realized. In this manner, caspase activation and cleavage of tau may link Aβ to the formation of NFTs (for a review on this hypothesis, see [18]). Since first publishing the hypothesis that caspase cleavage of tau may be a proximal event in NFT formation, several reports have now supported this view [19–22]. Further support for caspase activation as an early event in progression of AD come from Gastard et al, who demonstrated the activation of caspase-3 within immature tangles in subjects with mild AD [23]. In addition, a study by Guillozet Bongaarts et al, demonstrated that the truncation of tau by caspases may occur relatively early in the disease state prior to the key folding steps leading to the formation of PHFs [24]. Other studies have shown that tau truncated at Asp421 aggregates more rapidly and to a greater degree than full-length tau [20, 21, 25]. This suggests that caspase cleavage of tau may facilitate filament formation, a key event in the evolution of NFTs associated with AD. Collectively, these studies support a role for caspase cleavage of tau as an early event that may link Aβ and NFTs in AD [26]. Thus, therapeutics aimed at inhibiting the caspase-cleavage of tau may prove beneficial in not only preventing NFT formation, but also slowing cognitive decline.
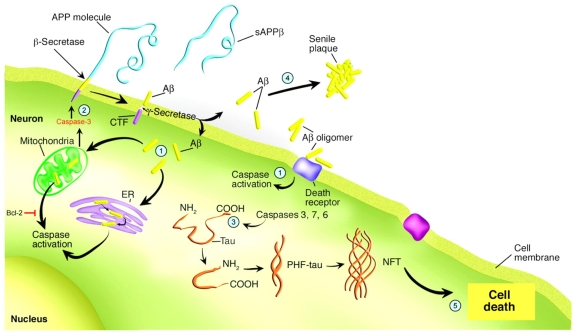
In addition to the caspase-cleavage of tau, evidence suggests APP is a substrate for caspase-mediated cleavage and that this is an important early step in the disease process that may contribute to Aβ formation, synaptic loss, and the behavioral changes associated with AD [11, 28–30]. Figure 1 summarizes the proximal role of caspases in promoting the pathology associated with AD.
Figure 1.
The role of caspases in promoting the pathology associated with AD. This figure summarizes the contribution of caspases to the Aβ cascade hypothesis. Step 1: activation of caspases occurs through stimulation of either the receptor-mediated pathway of apoptosis involving cross-linking of death receptors by Aβ (i.e., Fas), through the mitochondrial-mediated Aβ-induced oxidative stress, or finally by ER stress. Step 2: Following the activation of executioner caspases, particularly caspase-3, c-terminal cleavage of APP may facilitate the production of Aβ. This in turn can create a vicious feed-forward cycle of Aβ production and caspase-3 activation. Bcl-2 is an anti-apoptotic protein expressed in mitochondria, which prevents the release of cytochrome c and other pro-apoptotic factors, thus preventing caspase-9 and caspase-3 activation through an interaction with Apaf-1. Step 3: Conversely, caspase-3 cleavage of tau may promote tau aggregation and PHF formation thereby linking Aβ to NFTs. Step 4: Promotion of pathology by the activation of caspases occurs not only through the production of NFTs, but also by leading to Aβ deposition and plaque formation. Step 5: Ultimately, the activation of caspases and cleavage of critical cellular proteins may disrupt axonal and dendritic transport processes leading to cell death and neurodegeneration. Adapted and reproduced by permission from Dickson [27].
Animal Models Supporting a Proximal Role for Caspases in AD
Although there is considerable evidence that correlates the activation of caspases as a potential link between Aβ and NFT formation, direct evidence for this hypothesis is lacking. Our lab recently addressed this question utilizing an animal model of AD developed by LaFerla and colleagues [31]. These mice, termed 3×Tg-AD mice progressively develop both plaques and tangles with plaques occurring prior to tangle formation, consistent with the amyloid cascade hypothesis [31]. To test the involvement of caspases in AD disease progression, we generated 3×Tg-AD mice that overexpress the antiapoptotic protein, Bcl-2. Following successful generation of such mice, which we term 3×Tg-AD/Bcl-2 (overexpressors, OE), we examined brain regions for extracellular Aβ deposition and NFT formation. Overexpression of Bcl-2 in the neurons of 3×Tg-AD mice blocked caspase activation (caspase-9 and caspase-3) and the cleavage of tau leading to its accumulation within neurons [32]. Interestingly, despite the high protein levels of tau, there was little evidence for fibrillary tangle formation in 3×TgAD mice overexpressing Bcl-2, suggesting that the caspase-cleavage of tau is a critical step leading to NFT formation (Figure 2). What was even more surprising about our results was the apparent role that caspases had in processing APP. We found that in 3×Tg-AD mice overexpressing Bcl-2, higher levels of intracellular APP was observed in cortical neurons compared to 3×Tg-AD mice alone [32]. In 3×Tg-AD/Bcl-2 OE mice, it appeared as though APP was not being turned over properly and accumulated in the apical dendritic compartments of cortical neurons. The results from this study suggested a novel mechanism by which caspase activation contributes to the processing of APP and tau and suggests that caspases play a pivotal role in the initiation and progression of the pathology associated with AD.
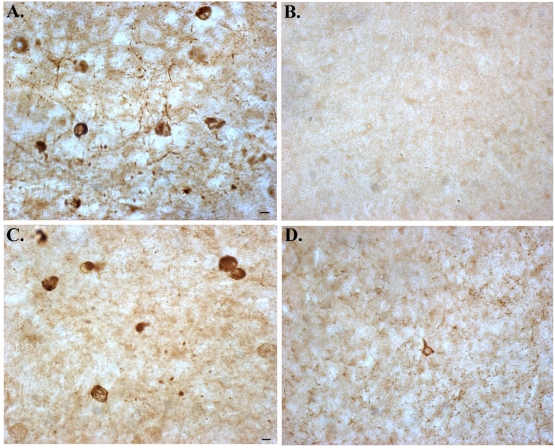
Figure 2.
Overexpression of Bcl-2 in 3×Tg-AD mice prevents caspase-cleavage and hyperphosphorylation of tau in the amygdala. Panels show representative staining from the amygdala of either 18 month-old 3×Tg-AD mice alone (A and C), or in 3×Tg-AD/Bcl-2 OE mice (B and D). (A and B): representative staining using the tau caspase-cleavage product antibody, an antibody that specifically detects caspase-cleaved tau [25] revealed the presence of caspase-cleaved tau within neurons in 3×Tg-AD mice that was prevented following overexpression of Bcl-2. (C and D): identical to Panels A and B with the exception that an early tangle marker, AT8 was employed. In this case, overexpression of Bcl-2 largely prevented the hyperphosphorylation of tau (D). All scale bars represent 10 μm.
Two other studies utilizing animal models have provided support for a role of caspases in the progression of AD. In a seminal study by Spires-Jones et al, the authors employed the use of in vivo imaging to examine caspase activation, the cleavage of tau, and NFT formation in an animal model of tauopathy [33]. Recently, the same group has utilized this technique consisting of in vivo multiphoton microscopy to demonstrate that extracellular Aβ plaques develop rapidly and expand to their full size in a matter of days [34]. In the study by Spires-Jones et al, caspase activation as well as the caspase-cleavage of tau was evident in neurons that morphologically were consistent with NFTs [33]. An interesting outcome of this study was caspase activation was found within neurons that did not display typical features of apoptosis including nuclear condensation and fragmentation, suggesting that the activation of caspases and completion of the apoptotic program may be largely separated in time. Overall, their in vivo findings strongly support a relationship between caspase activation and NFTs.
Finally, Galvan et al reported on PDAPP AD mice in which the C-terminal caspase-3 cleavage site within APP was deleted and compared the phenotype of these mice with mice that contained the functional Aspartic 664 caspase cleavage site [30]. Examination of mice indicated that deletion of this cleavage consensus site did prevent caspase-cleavage of APP yet had no effect on Aβ production and deposition in vivo [30]. Surprisingly, despite the apparent lack of effect on Aβ production and deposition, the D664A substitution did prevent synaptic loss and behavioral deficits associated with these animals. However, the mechanism by which this protection occurs is as yet unknown.
Evidence that Caspase Inhibition is Protective in Other Neurodegenerative Disorders
Collectively, the studies discussed suggest a critical involvement of caspases in the etiology associated with AD. Can targeted inhibition of this class of proteases provide an effective means to treat this disease? Before answering this question, it might be worthwhile to discuss studies that have tested the pharmacological inhibition of caspases to treat specific neurodegenerative diseases other than AD. Many of these studies were carried out in the late 1990’s and provided momentum for the development of caspase inhibitors as therapeutic agents. For example, in a study by Li et al, the authors used the pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp fluoromethyl-ketone (Z-VAD-fmk) to treat transgenic mice expressing mutant human SOD1, a model of amyotrophic lateral sclerosis (ALS)[35]. In this case, administration of Z-VAD-fmk delayed disease onset and mortality in ALS mice [35]. Supporting a role for caspases in ALS was a parallel study by which transgenic ALS mice were crossed with Bcl-2 OE mice developed by Martinou et al [36]. These were the same mice we used in our study to breed with 3×Tg-AD mice described above. In this case the authors found that overexpression of Bcl-2 attenuated neurodegeneration, delayed the activation of caspases, and prevented the cleavage of beta-actin in ALS mice [37]. A similar approach by the same authors demonstrated that intracerebroventricular administration of a caspase inhibitor delayed disease progression and death in a mouse model of Huntington’s disease [38]. Other studies have found significant protection and neurological improvement following caspase inhibition in animal models of acute neurologic diseases including ischemia or traumatic injury (for review, see [39]). Finally, caspase inhibition reduced apoptosis, increased the survival of dopaminergic neurons and improved functional recovery in a rat model of Parkinson’s disease [40]. Collectively, it was studies such as the aforementioned that raised expectations that therapeutics would soon follow. Unfortunately, these promising early studies employing caspase inhibitors have not yielded practical therapeutics that inhibits apoptosis in the clinical setting. The reasons for this are unclear but may represent potential toxic side effects caused by the fluoromethyl-ketone residue and poor tissue penetration of Z-VAD-fmk [41]. However, these studies support the idea that administration of caspase inhibitors can be an effective strategy in the treatment of certain neurodegenerative diseases and similar types of experiments should be carried out in animal models of AD. The critical next step is to test directly whether inhibitors of the apoptosis machinery blocks neuronal cell death or other aspects of pathology using animal models of AD.
Potential Drug Targets to Test the Apoptotic Hypothesis in AD
Based on the available data in animal models and studies involving postmortem AD patients several potential candidates of the apoptotic machinery have been uncovered as possible drug targets for the treatment of AD. Clearly, there is abundant evidence supporting a role for executioner caspases (i.e., -3, -6 and -7) in the etiology associated with AD. Initial studies to test the role for caspase-3 should come from using animal models employing peptide-based inhibitors such as Z-VAD-fmk or more specifically, Z-DEVD-fmk. A major obstacle to overcome is the experimental design of drug delivery. Any type of inhibitor must be administered continuously, for an extended time period that may include 3-24 months. Not taking into account that peptide-based inhibitors such as Z-VAD-fmk may prove cost prohibitive, the more daunting task is drug administration. Two options for drug delivery would include direct intracerebroventricular injection of the peptides or the use of miniosmotic pumps, which would require surgery and recovery. It is noteworthy that in the study by Li et al, osmotic pumps were utilized and Z-VAD-fmk was continuously delivered for a time period of 28 days [35]. Considering that in any typical animal model of AD, the minimum time of drug exposure would have to be on the order of 3-6 months then it quickly becomes apparent how difficult it may be to carry out such studies. In lieu of peptide inhibitors of caspase-3, a more effective strategy may be to use non-peptide small molecules that could be given orally and pass through the blood brain barrier. However, in this case drug selectivity becomes a major issue. Apoptosis regulates and controls cell death and tissue homeostasis during development and maturation. It has been estimated that roughly 100,000 cells die by apoptosis every second [42]. Therefore, distinguishing “good” apoptosis from “bad” apoptosis during the course of treatment may contribute to untoward side effects attributable to caspase inhibition.
An appealing alternative to Z-VAD-fmk may be the quinolyl-valyl-O-methylaspartyl-[-2, 6-difluorophenoxy]-methyl ketone (Q-VD-OPh). Q-VD-OPh has been shown to be a potent caspase inhibitor [43]. Improvements over Z-VAD-fmk include potency, stability, and cell permeability [43]. In addition, Q-VD-OPh has demonstrated efficacy in affording neuroprotection in animal models of Parkinson’s, Huntington’s disease and stroke [41, 44]. More importantly, this compound is not toxic to cells even at extremely high concentrations and is systemically active [45]. Therefore, Q-VD-OPh would seem to be an ideal candidate to test whether pharmacological inhibition of caspases in vivo can prevent the pathology associated with AD.
Another potential therapeutic avenue may be compounds that activate Bcl-2 or increase expression of Bcl-2 in neuronal populations. As discussed above, the overexpression of Bcl-2 prevented pathology associated with 3×Tg-AD mice [32]. Therefore, a drug that would selectively activate Bcl-2 in neurons of the CNS may prove beneficial. Currently, there are no known drugs designed to activate Bcl-2. Indeed, the majority of drugs targeting Bcl-2 are inhibitors, which are being tested for their utility to selectively induce programmed cell death in cancer cells [46]. It seems logical that using the same approach to synthesize small molecule inhibitors to Bcl-2 should be able to generate activators of this anti-apoptotic molecule. Even with the development of a compound capable of specifically activating Bcl-2, serious side effects are foreseeable. In this case, possible side effects resulting from Bcl-2 activation could be the unwanted formation of tumors. We have found for example, that in 3×Tg-AD/Bcl-2 OE mice that although there has been no evidence for tumor formation in the CNS, we have identified tumors in the vaginal area (unpublished observations). In these mice, the overexpression of Bcl-2 is driven by the promoter enolase, which appears also to be expressed in vaginal areas of female mice. In fact, for breeding, only male mice Bcl-2 OE can be used, as the Bcl-2 gene leads to failure of vaginal opening during development [47]. Therefore, as with the caspase inhibitors, any drug targeted to activate Bcl-2 would most likely need to be delivered specifically to the CNS, which presents another problem, namely crossing the blood brain barrier.
Another potential avenue would be the delivery of Bcl-2 utilizing viral vectors. Adenoviral vectors are capable of infecting a variety of types, including neurons, and may provide an efficient way of delivering Bcl-2 genes into mammalian cells. In this technique, neurons infected with a recombinant adenovirus could express the therapeutic gene Bcl-2, but because essential genes for replication are deleted, the vector cannot replicate. The validity of this approach in elevating expression levels of Bcl-2 family members has been reported. For example, adeno-associated virus-mediated delivery of Bcl-w, an anti-apoptotic member of the Bcl-2 family, improved neurological function and reduced infarct size in an experimental model of focal cerebral ischemia [48]. Delivery of Bcl-XL, an additional anti-apoptotic member of the Bcl-2 family, led to a long-term in vivo protection of CNS neurodegeneration when expressed in retinal ganglion cells of the adult rat retina [49]. Finally, Azzouz et al showed intraspinal injection of an adeno-associated virus encoding Bcl-2 in transgenic ALS mice led to sustained Bcl-2 expression in motor neurons and significantly increased the number of surviving neurons at the end-stage of disease. Moreover, functional neurological improvement was demonstrated following forced expression of Bcl-2 [50]. Together, these studies support the rationale of using adeno-associated virus strategies as a delivery mechanism to treat neurodegenerative disorders including AD. A first approach would be to test this type of gene transfer therapy in several transgenic mouse models of AD to determine if similar positive outcomes could be achieved. Table 1 lists the potential advantages and disadvantages of gene transfer of Bcl-2 as a therapeutic tool in treating AD.
Table 1.
Potential compounds targeting apoptotic members for the treatment of AD
| Drug Target | Advantages | Disadvantages |
|---|---|---|
| Z-VAD-fmk, pan caspase inhibitor | Extensively used in vitro with a proven efficacy in preventing apoptosis. Previous studies using Z-VAD-fmk in animal models of diseases have proven to be successful and efficacious | Transport into the CNS, bioavailability, and selectivity for specific caspases could be problematic. Toxicity as a result of the ketone group has been reported. |
| Q-VD-OPh, small molecule inhibitor of caspases | Improvements over Z-VAD-fmk include potency, stability, and cell permeability. Low toxicity and systemically actively as compared to Z-VAD-fmk | Limited number of studies available compared to Z-VAD-fmk. Selection between “good” versus “bad” apoptosis. Cost prohibitive for long-term study (3-24 months) |
| Bcl-2 agonists | Represents a critical convergence point in apoptotic pathway. High-affinity small molecule antagonists have already been synthesized for treatment of cancer | Presently, no selective agonist drugs are available for testing. Potential for widespread tumor formation is possible following inhibition of apoptosis. |
| Adeno-vector virus-mediated delivery of Bcl-2 family members | Efficient way to target neurons and thereby limit potential side effects including tumor formation. Can confer long-term transgene expression potentially decreasing drug dosing | Expression may be transient depending on vector used. Cell-specific targeting difficult to achieve. May be pathogenic and immunogenic. Depending on vector, size of foreign DNA may be limiting. |
| Minocycline | Orally available and demonstrated effectiveness in a number of other model degenerative diseases. Proven track record for safety in human. Potential dual functions as apoptotic inhibitor and anti-inflammatory agent may beneficial in treating AD | Limited testing in animal models of AD. Minocycline is no longer covered by patent and therefore, drug companies may not be interested in pursuing it as a potential remedy. |
Besides approaches to activate or increase the expression of Bcl-2, another potential drug of interest that already has an impressive history is minocycline. Minocycline is a second-generation tetracycline that has been demonstrated to be effective in the treatment of ischemia-induced injury [51, 52] and neuroprotective in mouse models of ALS, Huntington’s disease, Parkinson’s disease as well as in multiple sclerosis [39, 53, 54]. The advantage of minocycline is that it is orally available, crosses the blood brain barrier, and appears to be safe in humans [39]. Minocycline would seem to be an ideal drug to be tested in the treatment of AD based partly on its putative mechanism of action. Neuroprotection by minocycline appears to be afforded by its ability to directly inhibit the release of cytochrome c and prevent the activation of caspase-3 [55, 56]. In addition, minocycline has been shown to prevent reactive microgliosis [57, 58], a key feature that has been associated with AD [59]. Minocycline has been tested in an animal model of AD and was shown to slow neuronal cell death and improve cognitive impairment in Tg2576 mice as well as in Aβ1-42-infused rats [60]. However, whether minocycline prevents Aβ deposition or NFT formation was not addressed in this study. In another study by Seabrook et al, the authors examined the effects of minocycline on Aβ plaque formation and microglial function in J20 APP-Tg mice [61]. Their results indicated that minocycline was able to reduce Aβ stimulated microglial activation; however, an increase in Aβ plaque burden was observed in young mice [61]. These two studies suggest that minocycline may be a good candidate as a therapeutic agent for AD but additional studies are needed. It is interesting to speculate whether minocycline would prevent the pathology and improve cognition in 3×Tg-AD mice, which recapitulate most of the pathological features that occur in AD [31].
Concluding Remarks
Historically, the role of apoptosis in the neurodegeneration associated with AD has been controversial. However, numerous studies now suggest the activation of apoptotic pathways and the participation of caspases as major players underlying the pathological disease mechanisms associated with AD. Thus, along with cellular death, caspase activation and cleavage of APP and tau may contribute to the normal turnover and clearance of these proteins as well as contribute to Aβ and tangle formation. Based on the available evidence supporting the involvement of caspases in AD, it is now time to test drugs in animal models that target either the inhibition of caspases or other components of the apoptotic cascade. It is only after demonstrated efficacy of compounds in animal models of AD that therapeutics aimed at inhibiting apoptosis can move from the bench to the clinic. Although there are numerous disease-modifying candidates now in clinical trials, a practical approach to treating AD will include a multifaceted approach targeting both upstream and downstream molecules. The hope of this present review is that it will stimulate further studies and provide the necessary impetus for the development of pharmaceutics that can modulate apoptosis to be used for the treatment of this devastating disease.
Acknowledgments
Funded by NIH/NCRR grant #P20RR016454 and a grant from the American Health Assistance Foundation (AHAF) to T.T.R. and NIH/NIA UCI ADRC P50 AG16573.
References
- 1.Golde TE, Dickson D, Hutton M. Filling the gaps in the abeta cascade hypothesis of Alzheimer's disease. Curr Alzheimer Res. 2006;3:421–430. doi: 10.2174/156720506779025189. [DOI] [PubMed] [Google Scholar]
- 2.Christensen DD. Alzheimer's disease: progress in the development of anti-amyloid disease-modifying therapies. CNS Spectr. 2007;12:113–116. 119–123. doi: 10.1017/s1092852900020629. [DOI] [PubMed] [Google Scholar]
- 3.Su JH, Anderson AJ, Cummings BJ, Cotman CW. Immunohistochemical evidence for DNA fragmentation in neurons in the AD brain. Neuroreport. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Anderson AJ, Su JH, Cotman CW. DNA damage and apoptosis in Alzheimer's disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J Neurosci. 1996;16:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadelmann C, Bruck W, Bancher C, Jellinger K, Lassmann H. Alzheimer disease: DNA fragmentation indicates increased neuronal vulnerability, but not apoptosis. J Neuropathol Exp Neurol. 1998;57:456–464. doi: 10.1097/00005072-199805000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Bancher C, Lassmann H, Breitschopf H, Jellinger KA. Mechanisms of cell death in Alzheimer's disease. J Neural Transm Suppl. 1997;50:141–152. doi: 10.1007/978-3-7091-6842-4_14. [DOI] [PubMed] [Google Scholar]
- 8.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 9.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F, Sun X, Beech W, Teter B, Wu S, Sigel J, Vinters HV, Frautschy SA, Cole GM. Antibody to caspase-cleaved actin detects apoptosis in differentiated neuroblastoma and plaque-associated neurons and microglia in Alzheimer's disease [see comments] Am J Pathol. 1998;152:379–389. [PMC free article] [PubMed] [Google Scholar]
- 11.Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer's amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 12.Rohn TT, Head E, Su JH, Anderson AJ, Bahr BA, Cotman CW, Cribbs DH. Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer's disease. Am J Pathol. 2001;158:189–198. doi: 10.1016/S0002-9440(10)63957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Bruck W, Jellinger K, Lassmann H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer's disease. Evidence for apoptotic cell death. Am J Pathol. 1999;155:1459–1466. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW. Activated caspase-3 expression in Alzheimer's and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001;898:350–357. doi: 10.1016/s0006-8993(01)02018-2. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc A, Liu H, Goodyer C, Bergeron C, Hammond J. Caspase-6 role in apoptosis of human neurons, amyloidogenesis, and Alzheimer's disease. J Biol Chem. 1999;274:23426–23436. doi: 10.1074/jbc.274.33.23426. [DOI] [PubMed] [Google Scholar]
- 16.Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH. Activation of caspase-8 in the Alzheimer's disease brain. Neurobiol Dis. 2001;8:1006–1016. doi: 10.1006/nbdi.2001.0449. [DOI] [PubMed] [Google Scholar]
- 17.Rohn TT, Rissman RA, Davis MC, Kim Y-E, Cotman C, Head E. Caspase-9 Activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis. 2002;11:341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- 18.Rohn TT, Rissman RA, Head E, Cotman CW. Caspase activation in the Alzheimer’s disease brain: tortuous and torturous. Drug News Perspect. 2002;15:549–557. doi: 10.1358/dnp.2002.15.9.740233. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC. Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol. 2004;165:523–531. doi: 10.1016/S0002-9440(10)63317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin H, Kuret J. C-terminal truncation modulates both nucleation and extension phases of tau fibrillization. FEBS Lett. 2006;580:211–215. doi: 10.1016/j.febslet.2005.11.077. [DOI] [PubMed] [Google Scholar]
- 22.Ding H, Matthews TA, Johnson GV. Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation. J Biol Chem. 2006;281:19107–19114. doi: 10.1074/jbc.M511697200. [DOI] [PubMed] [Google Scholar]
- 23.Gastard MC, Troncoso JC, Koliatsos VE. Caspase activation in the limbic cortex of subjects with early Alzheimer’s disease. Ann Neurol. 2003;54:393–398. doi: 10.1002/ana.10680. [DOI] [PubMed] [Google Scholar]
- 24.Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Tau truncation during neurofibrillary tangle evolution in Alzheimer’s disease. Neurobiol Aging. 2005;26:1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotman CW, Poon WW, Rissman RA, Blurton-Jones M. The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2005;64:104–112. doi: 10.1093/jnen/64.2.104. [DOI] [PubMed] [Google Scholar]
- 27.Dickson DW. Apoptotic mechanisms in Alzheimer neurofibrillary degeneration: cause or effect? J Clin Invest. 2004;114:23–27. doi: 10.1172/JCI22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu DC, Soriano S, Bredesen DE, Koo EH. Caspase cleavage of the amyloid precursor protein modulates amyloid beta-protein toxicity. J Neurochem. 2003;87:733–741. doi: 10.1046/j.1471-4159.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M, Su J, Head E, Cotman CW. Accumulation of caspase cleaved amyloid precursor protein represents an early neurodegenerative event in aging and in Alzheimer’s disease. Neurobiol Dis. 2003;14:391–403. doi: 10.1016/j.nbd.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Galvan V, Gorostiza OF, Banwait S, Ataie M, Logvinova AV, Sitaraman S, Carlson E, Sagi SA, Chevallier N, Jin K, Greenberg DA, Bredesen DE. Reversal of Alzheimer’s-like pathology and behavior in human APP transgenic mice by mutation of Asp664. Proc Natl Acad Sci USA. 2006;103:7130–7135. doi: 10.1073/pnas.0509695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 32.Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci. 2008;28:3051–3059. doi: 10.1523/JNEUROSCI.5620-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spires-Jones TL, de Calignon A, Matsui T, Zehr C, Pitstick R, Wu HY, Osetek JD, Jones PB, Bacskai BJ, Feany MB, Carlson GA, Ashe KH, Lewis J, Hyman BT. In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. J Neurosci. 2008;28:862–867. doi: 10.1523/JNEUROSCI.3072-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model [see comments] Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 36.Martinou J-C, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J. Overexpression of bcl-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 37.Vukosavic S, Stefanis L, Jackson-Lewis V, Guegan C, Romero N, Chen C, Dubois-Dauphin M, Przedborski S. Delaying caspase activation by Bcl-2 A clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2000;20:9119–9125. doi: 10.1523/JNEUROSCI.20-24-09119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, Chung WM, Frey AS, Menon AS, Li XJ, Stieg PE, Yuan J, Penney JB, Young AB, Cha JH, Friedlander RM. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 39.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 40.Schierle GS, Hansson O, Leist M, Nicotera P, Widner H, Brundin P. Caspase inhibition reduces apoptosis and increases survival of nigral transplants. Nat Med. 1999;5:97–100. doi: 10.1038/4785. [DOI] [PubMed] [Google Scholar]
- 41.Braun JS, Prass K, Dirnagl U, Meisel A, Meisel C. Protection from brain damage and bacterial infection in murine stroke by the novel caspase-inhibitor Q-VD-OPH. Exp Neurol. 2007;206:183–191. doi: 10.1016/j.expneurol.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 42.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 43.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Sugama S, Mischak RP, Kiaei M, Bizat N, Brouillet E, Joh TH, Beal MF. A novel systemically active caspase inhibitor attenuates the toxicities of MPTP, malonate, and 3NP in vivo. Neurobiol Dis. 2004;17:250–259. doi: 10.1016/j.nbd.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 45.Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E. Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ. 2007;14:387–391. doi: 10.1038/sj.cdd.4402044. [DOI] [PubMed] [Google Scholar]
- 46.Letai A. BCL-2 found bound and drugged! Trends Mol Med. 2005;11:442–444. doi: 10.1016/j.molmed.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez I, Araki K, Khatib K, Martinou JC, Vassalli P. Mouse vaginal opening is an apoptosis-dependent process which can be prevented by the overexpression of Bcl2. Dev Biol. 1997;184:115–121. doi: 10.1006/dbio.1997.8522. [DOI] [PubMed] [Google Scholar]
- 48.Sun Y, Jin K, Clark KR, Peel A, Mao XO, Chang Q, Simon RP, Greenberg DA. Adeno-associated virus-mediated delivery of BCL-w gene improves outcome after transient focal cerebral ischemia. Gene Ther. 2003;10:115–122. doi: 10.1038/sj.gt.3301868. [DOI] [PubMed] [Google Scholar]
- 49.Malik JM, Shevtsova Z, Bahr M, Kugler S. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol Ther. 2005;11:373–381. doi: 10.1016/j.ymthe.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Azzouz M, Hottinger A, Paterna JC, Zurn AD, Aebischer P, Bueler H. Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum Mol Genet. 2000;9:803–811. doi: 10.1093/hmg/9.5.803. [DOI] [PubMed] [Google Scholar]
- 51.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hersch S, Fink K, Vonsattel JP, Friedlander RM. Minocycline is protective in a mouse model of Huntington’s disease. Ann Neurol. 2003;54:841. doi: 10.1002/ana.21891. author reply 842-843. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Narayanan M, Friedlander RM. Additive neuroprotective effects of minocycline with creatine in a mouse model of ALS. Ann Neurol. 2003;53:267–270. doi: 10.1002/ana.10476. [DOI] [PubMed] [Google Scholar]
- 55.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Li AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci USA. 2003;100:10483–10487. doi: 10.1073/pnas.1832501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-Daspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- 58.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Streit WJ. Microglia and neuroprotection: implications for Alzheimer’s disease. Brain Res Brain Res Rev. 2005;48:234–239. doi: 10.1016/j.brainresrev.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 60.Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Kim HS, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, Kim S, Suh YH. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology. 2007;32:2393–2404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- 61.Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia. 2006;53:776–782. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]