Abstract
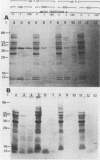
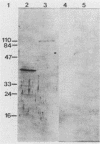
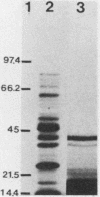
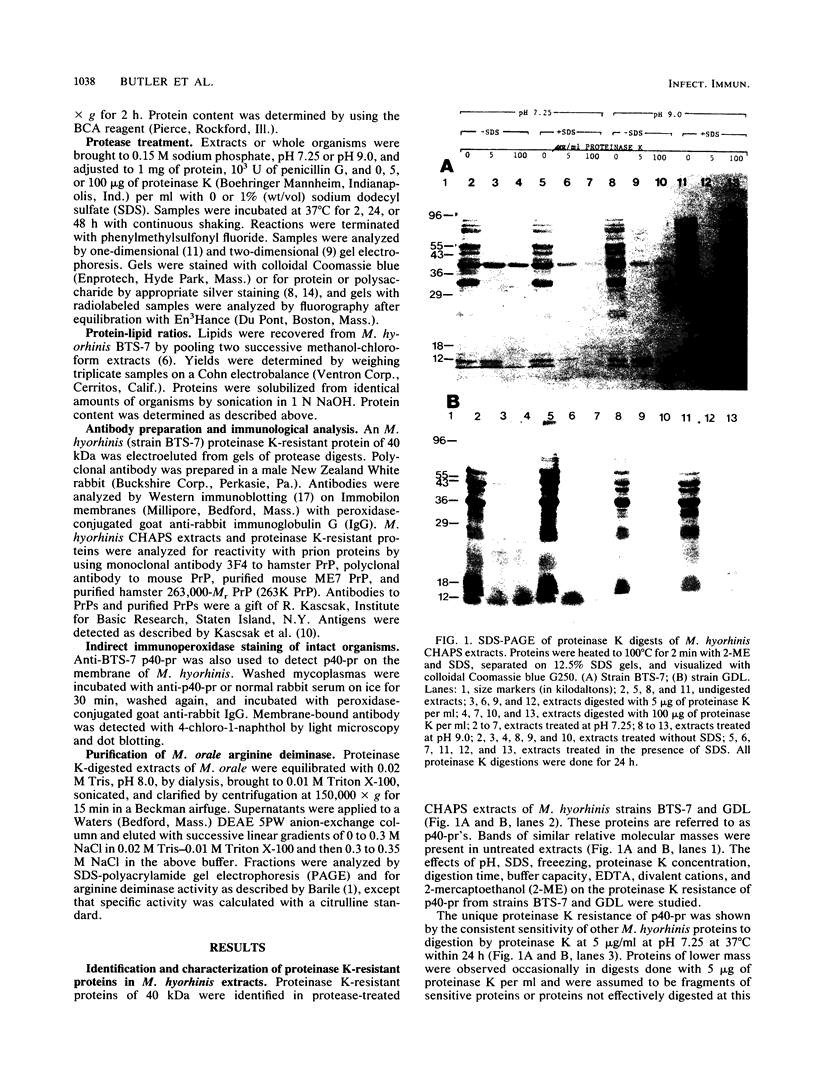
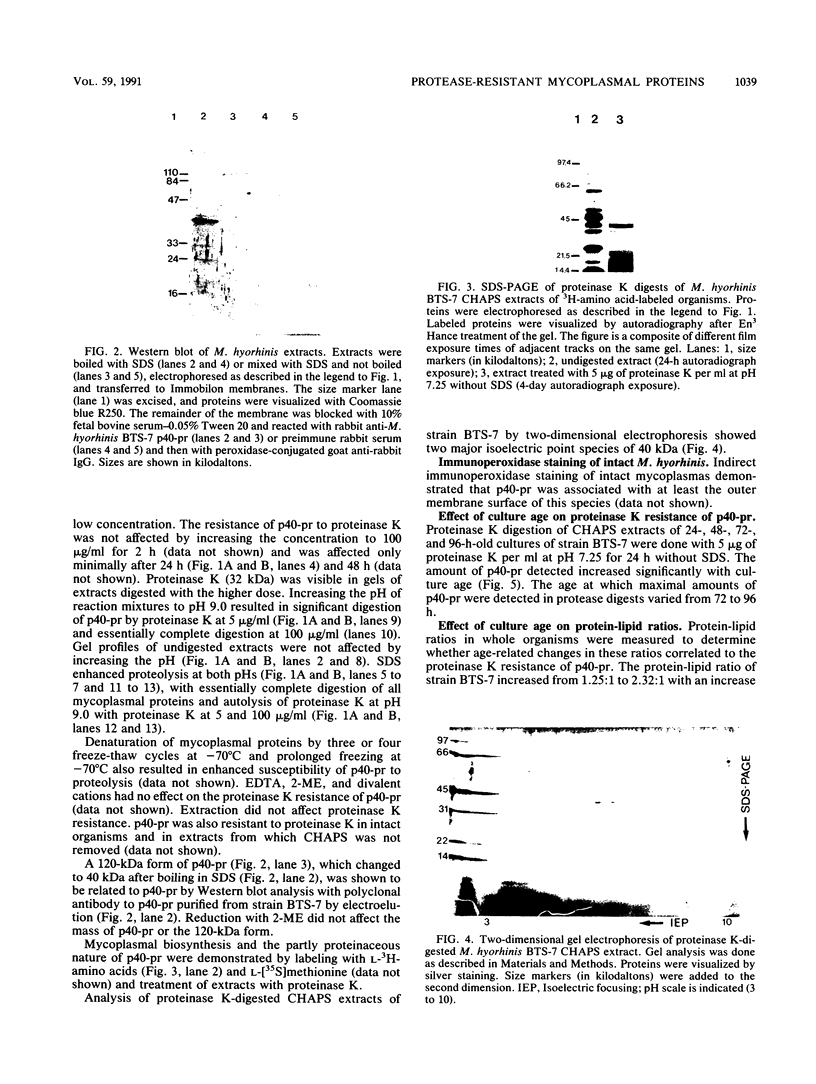
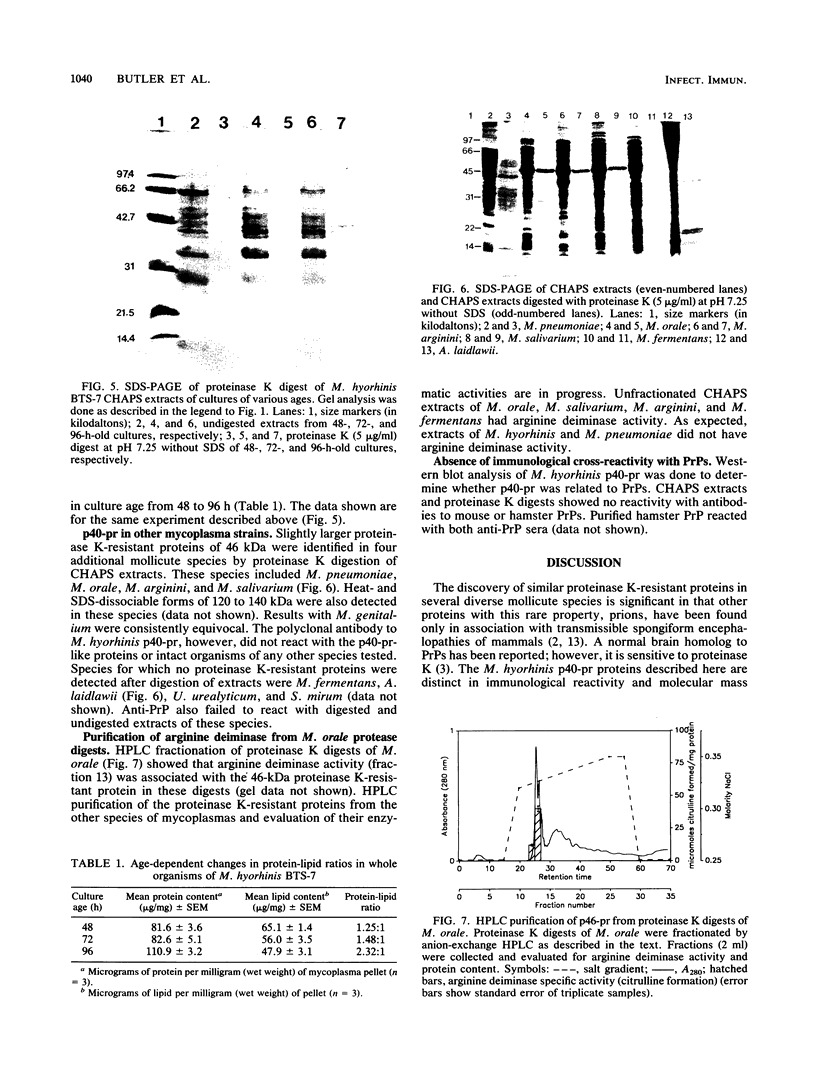
Proteins resistant to proteinase K are rare because of the potency, wide pH optimum, and low peptide bond specificity of this enzyme. Previously, only the prion proteins associated with transmissible spongiform encephalopathies, possibly related proteins in the mollicute Spiroplasma mirum, and proteinase K itself have been reported. We identified a new proteinase K-resistant protein, p40-pr, in two strains of Mycoplasma hyorhinis and in extracts of these organisms. p40-pr's are similar to prion proteins in their resistance to high doses of proteinase K and in the reversal of this resistance by strong denaturing conditions. However, p40-pr's were distinct immunologically, in relative molecular mass, and in their method of extraction. Two immunologically related forms of p40-pr were identified on sodium dodecyl sulfate (SDS) gels and Western immunoblots, a 40-kDa species in boiled samples and a 120-kDa species dissociable by boiling in SDS. Reduction with 2-mercaptoethanol did not affect the mass of p40-pr's or the 120-kDa forms. The development of proteinase K resistance of p40-pr correlated to age-dependent increases in organism protein-lipid ratios. p40-pr-like proteinase K-resistant proteins of 46 to 50 kDa were identified in four of eight additional species of the class Mollicutes but not in S. mirum. However, these mycoplasmal proteins did not react with antibody to the denatured 40-kDa form of M. hyorhinis p40-pr purified by electroelution. The chromatographically purified 46-kDa proteinase K-resistant protein of Mycoplasma orale was an arginine deiminase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian F. O., Jennings R. A., Gardner W. A. Antiserum to scrapie-associated fibril protein cross-reacts with Spiroplasma mirum fibril proteins. J Clin Microbiol. 1987 Dec;25(12):2430–2431. doi: 10.1128/jcm.25.12.2430-2431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G. H., Göbel U., Stanbridge E. J. Blast transformation of mouse splenic B-lymphocytes with extracts of Mycoplasma hyorhinis. Isr J Med Sci. 1984 Sep;20(9):891–894. [PubMed] [Google Scholar]
- Ebeling W., Hennrich N., Klockow M., Metz H., Orth H. D., Lang H. Proteinase K from Tritirachium album Limber. Eur J Biochem. 1974 Aug 15;47(1):91–97. doi: 10.1111/j.1432-1033.1974.tb03671.x. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser D. F., Harrington M. G., Hochstrasser A. C., Miller M. J., Merril C. R. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988 Sep;173(2):424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Carp R. I., Robakis N. K., Wisniewski H. M., Diringer H. Immunological comparison of scrapie-associated fibrils isolated from animals infected with four different scrapie strains. J Virol. 1986 Sep;59(3):676–683. doi: 10.1128/jvi.59.3.676-683.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liberski P. P. The nature of spiroplasma-like inclusions in experimental scrapie. Neuropatol Pol. 1987;25(1):53–57. [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Nielsen B. L., Brown L. R. The basis for colored silver-protein complex formation in stained polyacrylamide gels. Anal Biochem. 1984 Sep;141(2):311–315. doi: 10.1016/0003-2697(84)90047-2. [DOI] [PubMed] [Google Scholar]
- SCHIMKE R. T., BARILE M. F. ARGININE METABOLISM IN PLEUROPNEUMONIA-LIKE ORGANISMS ISOLATED FROM MAMMALIAN CELL CULTURE. J Bacteriol. 1963 Aug;86:195–206. doi: 10.1128/jb.86.2.195-206.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickmann J. L., Fahrney D. E. Arginine deiminase from Mycoplasma arthritidis. Evidence for multiple forms. J Biol Chem. 1977 Apr 25;252(8):2615–2620. [PubMed] [Google Scholar]
- Weickmann J. L., Himmel M. E., Squire P. G., Fahrney D. E. Arginine deiminase from Mycoplasma arthritidis. Properties of the enzyme from log phase cultures. J Biol Chem. 1978 Sep 10;253(17):6010–6015. [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]