Abstract
Coxsackievirus is the most important cause of meningitis and encephalitis in infants; an infection is sometimes fatal or may lead to neurodevelopmental defects. Here, we show that coxsackievirus B4 (CVB4) induces an autophagy pathway for replication in rat primary neurons. Notably, calpain inhibitors reduce autophagosome formation. Conversely, the inhibition of the autophagy pathway with 3-methyladenine inhibits calpain activation. This work reveals, for the first time, that calpain is essential for the autophagy pathway and viral replication in CVB4-infected neurons.
Coxsackieviruses belong to the enterovirus genus and the picornavirus family and are divided into serogroups A and B, including six types in the B serogroup. The coxsackieviruses can induce aseptic meningitis and encephalitis, especially in infants and children, which can sometimes be fatal and result in sequelae such as neurodevelopmental defects (4, 5).
Autophagy is a process of sequestering of aberrant organelles or protein aggregates into double-membrane vesicles for lysosomal breakdown. Autophagy has been thought to be a protective mechanism directed against intracellular bacteria and viruses (3). However, it has recently been revealed that some viruses rather induce autophagy to assist in their replication. Such viruses include poliovirus, a member of the picornavirus group (6). In contrast, human rhinovirus type 2, another picornavirus, does not use autophagy during replication (1). Thus, determining whether a particular virus, such as coxsackievirus, uses autophagy during replication is very important for understanding viral tropism and developing therapeutic strategies.
Coxsackievirus B4 (CVB4) was propagated and maintained as described previously (7). Primary rat cortical neuron cultures were prepared, CVB4 was inoculated at 1 PFU per cell at in vitro day 4, and cells were washed with fresh medium after 1 h as described previously (7). Drugs were administered 1 h after virus infection at final concentrations of 10 mM for 3-methyladenine (3-MA), 20 nM for rapamycin, and 100 nM for calpain inhibitors. Western blotting and transmission electron microscopy were performed as described previously (12). Antibodies used in this study were directed against LC3 (1:1,000; MBL), VP1 (1:100; Novocastra), spectrin (1:1,000; BioMol), and β-actin (1:5,000; Sigma).
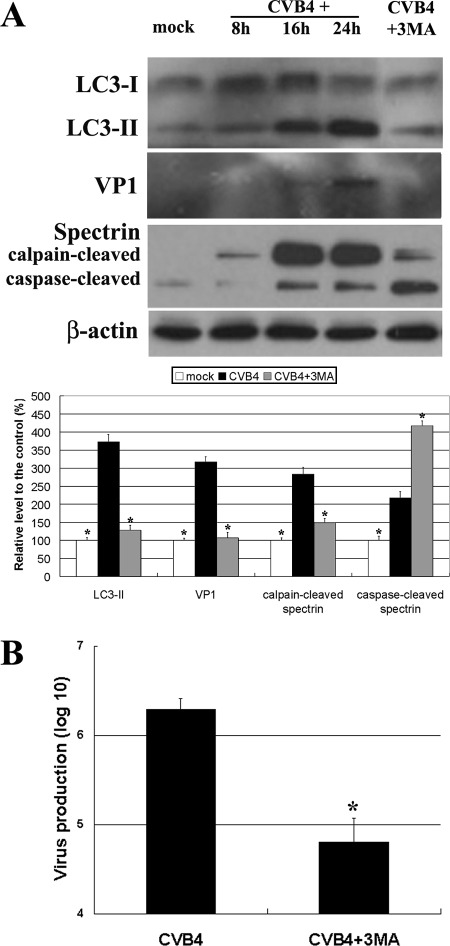
CVB4 increased LC3-II, the autophagosome marker (9), in a time-dependent manner (Fig. 1A), indicating an increase in autophagosomes in primary rat neurons postinfection. With this increase in LC3-II, levels of VP1 (a virus capsid protein) also rose (Fig. 1A). Treatment with 3-MA, an inhibitor of autophagy induction (10), prevented the increases in both LC3-II and VP1 (Fig. 1A) otherwise seen at 24 h postinfection, showing that the autophagy pathway was involved in CVB4 replication in primary neurons. This finding was confirmed by assaying progeny virus production. Whereas control virus levels reached a maximum of 6.4 × 106 ± 0.12 × 106 PFU/ml, 3-MA decreased this figure to 4.8 × 106 ± 0.27 × 106 PFU/ml (Fig. 1B). Rapamycin, an inducer of autophagy, enhanced the increases in LC3-II and VP1 caused by CVB4 at 48 h postinfection (data not shown), further confirming the involvement of autophagy in CVB4 replication in primary neurons.
FIG. 1.
Changes related to autophagy in CVB4-infected neurons. (A) Western blotting shows that levels of LC3-II, VP1, and the calpain-cleaved spectrin fragment increase in CVB4-infected neurons but decrease in 3-MA-treated neurons. *, P < 0.01 versus the result for CVB4-infected untreated neurons. (B) The assay of progeny virus production and a sequential plaque assay showed that 3-MA decreases virus replication. *, P < 0.01.
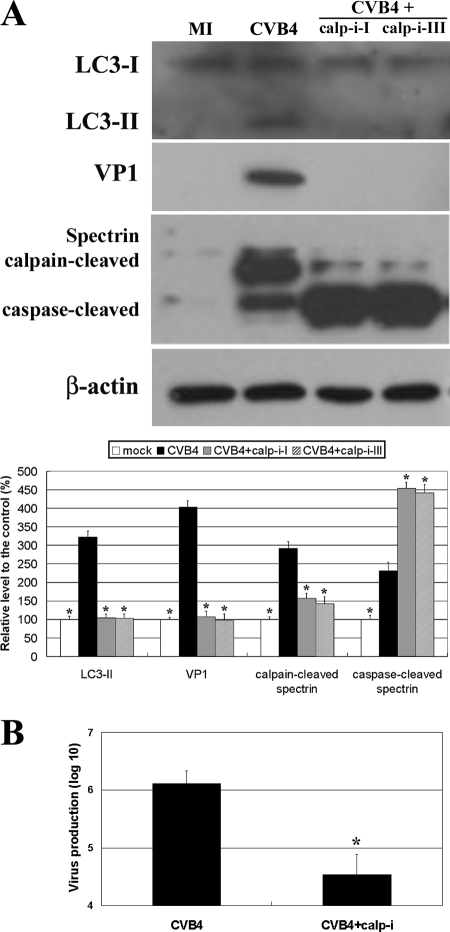
We further examined whether calpain may be involved in the CVB4-induced autophagy pathway because the involvement of calpain in autophagy remains controversial (2, 13). Interestingly, CVB4 increased and 3-MA decreased the level of the calpain-cleaved spectrin fragment (Fig. 1A), indicating that calpain was activated in CVB4-infected primary neurons in which autophagy was occurring. Moreover, the increase in LC3-II was eliminated in neurons treated with calpain inhibitors (Fig. 2), showing that autophagosome formation in CVB4-infected neurons was calpain dependent. Calpain inhibitors also reduced VP1 levels, suggesting that viral replication was weakened by the decrease in autophagosomal formation arising from calpain inhibition. This result was further confirmed by assaying progeny virus production, which showed that virus reached a maximal level of 6.11 × 106 ± 0.23 × 106 PFU/ml in control cells but that calpain inhibitor I decreased virus levels to 4.5 × 106 ± 0.35 × 106 PFU/ml (Fig. 2).
FIG. 2.
Effects of calpain inhibitors on autophagy and virus production. (A) Western blotting shows that calpain inhibitors prevent increases in LC3-II and VP1 in CVB4-infected neurons. MI, mock infection; calp-i-I and calp-i-III, calpain inhibitors I and III. *, P < 0.01 versus the result for CVB4-infected neurons in the absence of inhibitors. (B) The assay of progeny virus production and a sequential plaque assay showed that calpain inhibitor I decreases virus replication. *, P < 0.01.
CVB4 induces apoptosis in primary neurons (7). Interestingly, 3-MA and calpain inhibitors increased the levels of the caspase-cleaved spectrin fragment in CVB4-infected neurons (Fig. 1A and 2A). This finding suggests that drug-mediated autophagy inhibition triggered apoptosis, reflecting cross talk between autophagy and apoptosis (8).
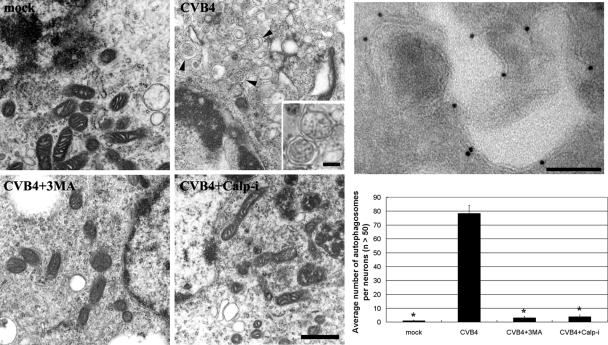
In accordance with the Western blot analysis of LC3-II levels (Fig. 1 and 2), transmission electron microscopy showed that CVB4 infection resulted in increases in autophagosomal structures surrounded by double membranes (Fig. 3). However, both 3-MA and calpain inhibitor I caused significant decreases in autophagosomal structures (Fig. 3), further confirming the Western blot results.
FIG. 3.
Transmission electron microscopy images. Compared to mock-infected neurons, CVB4-treated neurons show extensive accumulations of autophagosomal structures (arrowheads). The inset is a magnified view of an autophagosomal structure surrounded by a double membrane. The autophagosomal structures were greatly decreased in 3-MA- and calpain inhibitor I (Calp-I)-treated neurons. Negative staining of a cryoelectron microscopy image shows the immunogold labeling of LC3-II on autophagosomal structures (right upper panel). Bar in CVB4+Calp-I image, 500 nm; bars in inset and right upper panel, 100 nm. *, P < 0.01 versus the result for CVB4-infected neurons in the absence of 3-MA and calpain inhibitors.
Here, we describe the novel finding that calpain and the autophagy pathway were connected with each other during CVB4 infection in primary neurons. This finding may be interpreted to indicate that (i) the autophagy pathway was directly activated by calpain, (ii) calpain was directly activated by the autophagy pathway, or (iii) both the autophagy pathway and calpain were indirectly activated by the abundance of viral proteins influenced by an effect of 3-MA and calpain inhibitors on viral replication.
The effects of calpain on the autophagy pathway vary with cell type, the degree of cell starvation, the presence of drugs such as rapamycin, and the nature of the infecting virus. CVB4 requires calpain activation for autophagy induction and virus replication (Fig. 2). However, since the calpain inhibitors could inhibit several other proteinases, more exquisite studies using approaches such as small interfering RNA would clarify this issue more clearly in the future. Interestingly, during the preparation of our manuscript, Upla and colleagues reported that calpain is required for CVB3 replication and suggested that the formation of replication complexes is dependent on calpain activity (11). We suggest here that the autophagosome is one of the CVB4 replication complexes dependent on calpain activity.
Acknowledgments
This study was supported by a Korea Research Foundation grant funded by the South Korean government (MOEHRD, Basic Research Promotion Fund; grant no. KRF-2007-412-J00304) and grants from the Korea Science and Engineering Foundation (contract grant no. R13-2008-023) and the Korea Basic Science Institute (grant no. T28032).
Footnotes
Published ahead of print on 17 September 2008.
REFERENCES
- 1.Brabec-Zaruba, M., U. Berka, D. Blaas, and R. Fuchs. 2007. Induction of autophagy does not affect human rhinovirus type 2 production. J. Virol. 8110815-10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demarchi, F., C. Bertoli, T. Copetti, I. Tanida, C. Brancolini, E. L. Eskelinen, and C. Schneider. 2006. Calpain is required for macroautophagy in mammalian cells. J. Cell Biol. 175595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deretic, V. 2006. Autophagy as an immune defense mechanism. Curr. Opin. Immunol. 18375-382. [DOI] [PubMed] [Google Scholar]
- 4.Euscher, E., J. Davis, I. Holzman, and G. J. Nuovo. 2001. Coxsackie virus infection of the placenta associated with neurodevelopmental delays in the newborn. Obstet. Gynecol. 981019-1026. [DOI] [PubMed] [Google Scholar]
- 5.Gear, J. H., and V. Measroch. 1973. Coxsackievirus infections of the newborn. Prog. Med. Virol. 1542-62. [PubMed] [Google Scholar]
- 6.Jackson, W. T., T. H. Giddings, Jr., M. P. Taylor, S. Mulinyawe, M. Rabinovitch, R. R. Kopito, and K. Kirkegaard. 2005. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 3e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo, C. H., Y. K. Kim, H. Lee, H. Hong, S. Y. Yoon, and D. Kim. 2002. Coxsackievirus B4-induced neuronal apoptosis in rat cortical cultures. Neurosci. Lett. 326175-178. [DOI] [PubMed] [Google Scholar]
- 8.Maiuri, M. C., E. Zalckvar, A. Kimchi, and G. Kroemer. 2007. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8741-752. [DOI] [PubMed] [Google Scholar]
- 9.Mizushima, N., A. Yamamoto, M. Matsui, T. Yoshimori, and Y. Ohsumi. 2004. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 151101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petiot, A., E. Ogier-Denis, E. F. Blommaart, A. J. Meijer, and P. Codogno. 2000. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275992-998. [DOI] [PubMed] [Google Scholar]
- 11.Upla, P., V. Marjomaki, L. Nissinen, C. Nylund, M. Waris, T. Hyypia, and J. Heino. 2008. Calpain 1 and 2 are required for RNA replication of echovirus 1. J. Virol. 821581-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon, S. Y., J. E. Choi, J. H. Yoon, J. W. Huh, and D. H. Kim. 2006. BACE inhibitor reduces APP-beta-C-terminal fragment accumulation in axonal swellings of okadaic acid-induced neurodegeneration. Neurobiol. Dis. 22435-444. [DOI] [PubMed] [Google Scholar]
- 13.Yousefi, S., R. Perozzo, I. Schmid, A. Ziemiecki, T. Schaffner, L. Scapozza, T. Brunner, and H. U. Simon. 2006. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 81124-1132. [DOI] [PubMed] [Google Scholar]