Abstract
Human immunodeficiency virus (HIV) is transmitted primarily sexually across mucosal surfaces. After infection, HIV propagates initially in the lamina propria below the polarized epithelium and causes extensive destruction of mucosal T cells. Immunoglobulin A (IgA) antibodies, produced in the lamina propria and then transcytosed across the mucosal epithelium into the lumen, can be the first line of immune defense against HIV. Here, we used IgA monoclonal antibodies against HIV envelope proteins to investigate the abilities of polarized primate and human epithelial cells to excrete HIV virions from the basolateral to the apical surface via polymeric Ig receptor (pIgR)-mediated binding and the internalization of HIV-IgA immune complexes. African green monkey kidney cells expressing pIgR demonstrated HIV excretion that was dependent on the IgA concentration and the exposure time. Matched IgG antibodies with the same variable regions as the IgA antibodies and IgA antibodies to non-HIV antigens had no HIV excretory function. A mixture of two IgA anti-bodies against gp120 and gp41 showed a synergistic increase in the level of HIV excreted. The capacity for HIV excretion correlated with the ability of IgA antibodies to bind HIV and of the resulting immune complexes to bind pIgR. Consistent with the epithelial transcytosis of HIV-IgA immune complexes, the colocalization of HIV proteins and HIV-specific IgA was detected intracellularly by confocal microscopy. Our results suggest the potential of IgA antibodies to excrete HIV from mucosal lamina propria, thereby decreasing the viral burden, access to susceptible cells, and the chronic activation of the immune system.
Human immunodeficiency virus type 1 (HIV-1) is estimated to have newly infected 4.3 million people worldwide in 2006 (41). The transmission of HIV occurs primarily through contact with rectal, genital, or intestinal mucosal surfaces (69). Once at the mucosal barrier, free virus and virally infected cells can enter the body through gaps in the epithelial lining, but both simian immunodeficiency virus and HIV can also cross without apparent damage to the epithelial layer (47, 71). Other routes allowing HIV access to mucosal lymphoid cells include transcytosis across epithelial tight junctions and directly through epithelial cells via the galactosyl ceramide receptor, as well as transepithelial transport by Langerhans cells, dendritic cells, and M cells (2, 5, 8, 37, 49, 70). Human epithelial cell infection in vitro is enhanced by semen complement (11, 33), and gp340, a protein on genital tract epithelial cells, binds HIV and increases infectivity (71). HIV replication in vitro has been reported to occur in epithelial cells from the colon, uterus, and oral cavity and salivary gland cells, although the presence and role of epithelial cell infection in vivo are debated (23, 24, 26, 29, 62, 63, 65, 75, 76, 84).
Early HIV infection causes significantly more damage to mucosal than to systemic lymphoid tissues, and in both rhesus macaques and humans, mucosal CD4+ T cells are rapidly infected and killed within the first few weeks of infection. This rapid decline of mucosal T cells is irrespective of the route of infection (31, 60, 78). The transmission rate correlates with the viral load and is highest per coital act during the first months of infection (79). Therefore, methods to reduce early viremia have implications for lowering transmission rates.
Understanding the interactions of HIV with the main mucosal antibody class, immunoglobulin A (IgA), may help identify methods to decrease viremia. HIV-specific IgA has been detected previously in genital and intestinal secretions, and the IgA collected has been shown to neutralize HIV in vitro (17, 18, 44, 61, 82). Secretory IgA produced after oral immunization has also been able to neutralize HIV, and lymph node immunization in macaques can generate protective mucosal immunity (13, 50). The presence of IgA antibodies against HIV can correlate with resistance in sex workers and in uninfected sexual partners of infected individuals, and in some instances, the antibodies mediate cross-clade protection (7, 18, 19, 44, 45, 57, 58, 64). In contrast, uninfected HIV-exposed individuals have not been shown to have anti-HIV IgG (32, 52).
To protect from HIV and other microbial pathogens, IgA mediates host defense functions via the polymeric Ig receptor (pIgR) that enables the basolateral endocytosis of IgA and its subsequent transcytosis through the mucosal epithelium. Intracellular neutralization is a protective function whereby antiviral IgA interferes with virus production via an intraepithelial cell action. This IgA function has been shown previously for Sendai virus, measles virus, influenza virus, and rotavirus and for HIV via antibodies against envelope gp160, as well as the internal proteins reverse transcriptase (RT) and Gag (15, 25, 27, 54-56, 68, 81, 83). The ability of basolaterally endocytosed IgA antibody to meet apically endocytosed HIV intracellularly and prevent the virus from reaching the basolateral compartment by recycling it to the apical side has also been termed intracellular neutralization (1, 6, 9, 17, 34, 35, 80), although in our view this phenomenon is more accurately described as a variant of immune excretion in which already formed IgA antibody-antigen complexes are endocytosed basolaterally and transcytosed intact to the apical surface (43, 66, 81, 83).
No previous studies have demonstrated the IgA-mediated excretion of HIV from the basolateral surface across polarized epithelial cells to the apical surface. In the present work, we show that IgA antibodies against HIV envelope proteins were capable of binding intact HIV virions and mediating their transport from the basolateral (serosal) compartments across polarized epithelial cells into the apical (luminal) compartments of Transwell chambers. Both monkey kidney cells transfected to express pIgR and human cells with endogenous pIgR expression were permissive for HIV excretion. Of note, two IgA antibodies against gp120 and gp41 were synergistic.
MATERIALS AND METHODS
Cell culture and viruses.
African green monkey kidney cells, Vero C1008 (ATCC CRL 1587) cells, human endometrial adenocarcinoma HEC-1A (ATCC HTB-112) cells, and human colorectal adenocarcinoma HT-29 (ATCC HTB-38) cells from the American Type Culture Collection (ATCC, Rockville, MD) were used to evaluate virus excretion. The Vero C1008 cells had been transfected and stably expressed human pIgR (38). Both pIgR+ and pIgR− Vero cells were grown in Eagle's minimal essential medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; HyClone, Logan, UT) with 0.2 mg/ml Geneticin (Invitrogen) added for pIgR+ cells. The human cell lines HEC-1A and HT-29, which naturally express pIgR, were grown in McCoy's medium (Invitrogen) containing 10% FBS. Cells of the Vero, HEC-1A, and HT-29 lines polarize easily and transport IgA efficiently. All cells were grown on tissue culture-treated, 0.4-μm-pore-size Transwell polyester membranes (Costar Corp., Cambridge, MA) to allow the formation of polarized monolayers. Polarization was assessed by monitoring electrical resistance between the apical and basal chambers (38). Polarized Vero, HEC-1A, and HT-29 cells had values of 60 to 80, 210 to 270, and 120 to 140 Ω per cm2, respectively. Vero cells were plated at 2 × 105 cells per membrane and used on day 4 to 5. HEC-1A cells plated at 3 × 105 cells per membrane were used between days 5 and 7, and HT-29 cells plated at the same density as HEC-1A cells were used on days 7 to 10.
Human T-cell lines CEM-SS and 174 × CEM were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, and both were cultured in RPMI 1640 medium (Invitrogen) with 5% FBS. Proviral DNA (67) from HIV subtype B, an X4 virus (the pKS242 molecular clone supplied by the AIDS Research and Reference Reagent Program), was used to transfect Vero C1008 cells, and the virus was then propagated in CEM-SS cells. Continuous cultures were maintained, and HIV-containing supernatant was collected fresh for each experiment. 174 × CEM cells, which produced more obvious cytopathic effects (CPE) than CEM-SS cells, were used for determining virus infectivity.
MAbs.
Hybridomas secreting previously characterized anti-HIV IgG antibodies against the gp120 V3 loop (D19 and D47), the CD4 binding site region (D25), the gp41 cluster 1 region (D61), and unknown conformational epitopes (D10 and T33) were generously provided by Patricia Earl (21, 22, 72). Matching IgA monoclonal antibodies (MAbs) were obtained by repetitive cycles of limiting dilution and spontaneous isotype switching of the IgG hybridomas (10, 40). Control IgA was a MAb against measles virus fusion protein (83). The MAb specificities were confirmed by Western blotting. The production and purification of MAbs and the determination of the percentages of IgA oligomers in MAbs were performed as described previously (83).
MAb transcytosis through polarized epithelial cells.
Mouse IgA is known to be efficiently transported by human pIgR (74), and the extent of transport was assessed for both free MAbs and antibody-virus immune complexes. Purified IgA or IgG (12 μg in 120 μl of medium [100 μg/ml]) with or without HIV was incubated at 4°C for 3 h. The free MAbs or immune complexes were then added to the basolateral chambers below polarized pIgR+ or pIgR− Vero C1008, HEC-1A, and HT-29 cells for incubation at 37°C. Apical supernatants were collected after 8 h, and the Ig content was analyzed via enzyme-linked immunosorbent assay (ELISA) (39).
Excretion of HIV.
When cell membranes were polarized (as assessed by monitoring electrical resistance), fresh HIV supernatant from CEM-SS cells, with an average copy number of 4 × 109 virions per ml, was prepared. Equal volumes of virus and IgA or IgG MAb in a total volume of 120 μl were mixed. Between 1 and 12 μg of purified IgA or IgG was used, for a concentration range from 8.3 to 100 μg/ml. Controls employed IgG antibodies with the same variable (V) regions as the IgA, as well as IgA antibodies to non-HIV antigens. HIV-antibody mixtures were added to the basolateral chamber below polarized epithelial cells, with 200 μl of medium in the apical chamber. After 2, 4, 8, or 12 h of incubation at either 4 or 37°C, apical and basolateral samples were collected and viral RNA was extracted from 100 μl with a viral RNA kit by following the instructions of the manufacturer (ZYMO, Orange, CA). RT-PCR with a SuperScript One-Step RT-PCR system (Invitrogen) was performed using primers for the V3 region of the HIV envelope protein gene (59), and the 320-bp fragment was detected by ethidium bromide staining after gel electrophoresis. In some cases, polarized cell monolayers had either free virus or immune complexes added apically rather than basolaterally prior to the 8-h incubation. For those membranes, the basolateral medium was sampled and tested for HIV.
To determine the sensitivity of the assay to detect HIV, the same HIV used to form immune complexes was serially diluted and RNA was extracted for RT-PCR. The positive controls for each experiment were made from a 10−6 dilution of the HIV used to form the immune complexes, and the controls were run concurrently with the apical samples.
Evaluation of binding of IgA to HIV and of immune complexes to pIgR.
The relative affinities of the MAbs for HIV were examined. ELISA plates (96 well; Becton Dickinson) were coated overnight at 4°C with HIV supernatant in a 50% mixture with 50 mM carbonate buffer (pH 9.6). The plates were blocked with 1% bovine serum albumin-phosphate-buffered saline at room temperature for 5 h and then incubated overnight at 4°C with three dilutions of the MAbs. Alkaline phosphatase-labeled goat anti-mouse IgA (Southern Biotech, Birmingham, AL) was added at room temperature for 5 h before detection with a 4-nitrophenyl phosphate disodium salt hexahydrate substrate (Sigma), and the optical densities at 405 nm (OD405) were read with a microplate spectrophotometer. The level of binding of irrelevant IgA was subtracted to eliminate background effects.
The ability of HIV-IgA immune complexes to bind to recombinant human pIgR (R&D Systems, Minneapolis, MN) was assessed via ELISA. The pIgR was bound to ELISA plates at 4 μg/ml (50 μl per well) overnight at 4°C before the washing of the plates and the addition of immune complexes overnight at 4°C. A polyclonal mixture of mouse anti-HIV IgG was added for 5 h at room temperature before detection with alkaline phosphatase-labeled goat anti-mouse IgG (Southern Biotech). After OD determination, any level of binding of HIV individually was subtracted to eliminate background effects.
Infectivity of excreted HIV.
An antibody capable of conventionally neutralizing pKS242 HIV (D47A) at 0.075 μg/ml and one unable to neutralize at 50 μg/ml (D10A) (39) were used for excretion. To determine whether the excreted HIV was still infectious, 2 × 105 174 × CEM T cells were added to the apical compartment 1 h after HIV-IgA immune complexes were added basolaterally to pIgR+ Vero membranes. The 174 × CEM cells were centrifuged for 30 min at 1,500 × g prior to apical addition to improve infection. Excretion was allowed to occur for 8 h at 37°C. The 174 × CEM cells were collected and incubated for another 5 h before being washed three times with Hanks' balanced salt solution (HBSS; HyClone) to remove unabsorbed virus and p24. The T cells were then further incubated at 37°C and monitored for CPE. Supernatants were sampled at days 3, 5, and 7 for viral p24 analysis using an HIV-1 p24 antigen kit (Zeptrometrix, Buffalo, NY).
Epithelial cell monolayers monitored for infection after excretion.
Each epithelial cell line was examined for possible productive HIV infection. After an excretion experiment, the epithelial cells were washed apically and basally three times with HBSS before being washed with 0.25% trypsin-EDTA (Invitrogen) for 1 min and then three more times with HBSS. The usual growth medium was added, cell monolayers were incubated at 37°C, and apical and basal media were sampled at days 3, 7, and 10. The samples were analyzed for viral RNA by RT-PCR and gel electrophoresis for amplicon detection by ethidium bromide staining.
Determination of excreted virus copy numbers.
Apical and basal media were collected after 8 h of excretion through pIgR+ Vero cells. The copy number of excreted virus was determined with an Amplicor Monitor HIV-1 test, version 1.5, per the instructions of the manufacturer (Roche, Switzerland).
Statistical determinations.
The means ± standard deviations (SD) were used to analyze the results of binding and viral copy number analyses. Significance was determined with unpaired two-tailed t tests by using GraphPad Prism (GraphPad Software, San Diego, CA).
Colocalization of IgA and HIV in complexes within polarized epithelial cells.
After 8 h of excretion with pIgR+ Vero, HEC-1A, and HT-29 cell monolayers and a mixture of HIV and 100 μg/ml D10A/D47A or irrelevant IgA MAb in the basolateral chambers, the membranes were washed, fixed in 2% paraformaldehyde, and permeabilized with 0.1% Triton X-100 (39). The HIV proteins were stained with mouse IgG MAbs against gp160 epitopes different from those epitopes bound by D10 or D47. Rhodamine-labeled goat anti-mouse IgG was used to detect HIV proteins (red fluorescence) and fluorescein isothiocyanate-labeled goat anti-mouse IgA (39) was used to stain transcytosing IgA (green fluorescence). Two-color immunofluorescence confocal microscopy (performed at the Case Western Reserve University/Ireland Comprehensive Cancer Center confocal microscopy facility) was used to visualize HIV and IgA antibody within the epithelial cells. A model 510 laser scanning confocal microscope with a 100× alpha Plan-Fluar oil immersion objective lens (Zeiss, Thornwood, NY) was used to detect fluorescence with excitation at 488 nm from an argon laser and at 543 nm from a He/Ne laser.
RESULTS
MAbs.
The anti-gp120 and -gp41 IgG hybridomas were isotype switched to IgA with the same V regions to allow comparison between the matched IgA and IgG antibodies. Table 1 lists the MAbs and the percentages of oligomers (only the oligomers bind pIgR and are able to be transcytosed). The MAbs were more than 90% pure as determined by densitometry after sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
TABLE 1.
MAb specificities and percentages of oligomeric IgA
| Hybridoma | Antigen specificity | % Oligomeric IgAa |
|---|---|---|
| D10A | gp41 | 70 |
| D10G | gp41 | |
| D19A | gp120 | 43 |
| D19G | gp120 | |
| D25A | gp120 | 65 |
| D25G | gp120 | |
| T33A | gp41 | 65 |
| T33G | gp41 | |
| D47A | gp120 | 64 |
| D47G | gp120 | |
| D61A | gp41 | 47 |
| D61G | gp41 | |
| Irrelevant IgA | Measles virus (F protein) | 62 |
| Irrelevant IgG | Measles virus (F protein) |
The percentage of oligomeric IgA was assessed by size exclusion liquid chromatography.
MAb transcytosis through polarized epithelial cells.
MAb transcytosis was assessed at 8 h for each polarized cell line with each MAb singly or combined to yield a final MAb concentration of 100 μg/ml in the basolateral chamber with or without HIV (Table 2). The three pIgR+ cell lines (Vero, HEC-1A, and HT-29) were all capable of transporting IgA but not IgG. The pIgR− Vero cells did not transport IgA: only 22 ± 3 ng/ml of D10/47A was detected after 8 h (data not shown). HEC-1A and HT-29 cells had similar extents of IgA transcytosis, which were lower than that for Vero cells. The presence of HIV, leading to the formation of immune complexes, reduced anti-HIV IgA transcytosis modestly (by 12 to 34%); the transcytosis of irrelevant IgA was unaffected by virus.
TABLE 2.
MAbs transported across polarized Vero, HEC-1, and HT-29 cells
| Cell line | Condition | Amt (ng/ml)a in apical supernatant of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D10A | D61A | D19A | D10/47A | D47A | D25A | T33A | Irrelevant IgA | IgG | ||
| Vero | Without HIV | 711 ± 42 | 631 ± 9 | 569 ± 10 | 911 ± 22 | 787 ± 12 | 698 ± 21 | 588 ± 16 | 559 ± 10 | 26 ± 8 |
| With HIV | 564 ± 37 | 496 ± 9 | 376 ± 12 | 801 ± 25 | 606 ± 31 | 521 ± 20 | 400 ± 14 | 566 ± 5 | 31 ± 9 | |
| HEC-1A | Without HIV | 435 ± 19 | 585 ± 16 | 461 ± 33 | 411 ± 12 | 62 ± 4 | ||||
| With HIV | 328 ± 10 | 515 ± 27 | 389 ± 26 | 396 ± 8 | 58 ± 8 | |||||
| HT-29 | Without HIV | 410 ± 22 | 565 ± 11 | 420 ± 10 | 356 ± 6 | 50 ± 6 | ||||
| With HIV | 352 ± 10 | 464 ± 6 | 367 ± 8 | 349 ± 7 | 51 ± 4 | |||||
Ig levels in apical supernatant were detected by ELISA after 8 h of transcytosis and are expressed as means ± SD.
Detection of HIV by RT-PCR.
To measure HIV, serial dilutions of the virus prior to the addition of MAb were processed by extracting viral RNA for RT-PCR and electrophoresing the products on an agarose gel to visualize a 320-bp amplicon from the gp120 gene (Fig. 1A). HIV was detected to the 10−8 dilution. The positive control for each experiment was generated from a 10−6 dilution of the HIV used to make immune complexes.
FIG. 1.
Transepithelial excretion of HIV through polarized pIgR+ Vero cells mediated by different IgA antibodies. (A) Detection of HIV after fivefold dilutions (10−5 to 10−8) in lanes 2 to 8. Lanes 1 and 9 are DNA ladders (M, molecular size) identifying the 320-bp amplicon location. (B) HIV excretion ability of each IgA at 100 μg/ml. RT-PCR products from apical samples collected after 8 h are shown in lanes 2 to 13. Irrelevant IgA (IgA control [IgA con.]) is in lanes 12 and 13. A positive control (PC; 10−6 dilution of virus) is shown in lane 14 and in lane 8 in panel C. (C) HIV excretion by IgA MAbs at 300 μg/ml.
Abilities of different IgA antibodies against gp120 and gp41 to excrete HIV.
To determine the excretion abilities of different IgA antibodies (Table 1), each MAb (100 or 300 μg/ml) was mixed with HIV for 3 h at 4°C to allow the formation of immune complexes. The immune complexes were then added to the basolateral compartments of Transwell chambers below polarized pIgR+ Vero cells. After 8 h of incubation at 37°C, apical medium was collected and processed to detect viral RNA. At 100 μg/ml, anti-gp41 MAb D10A and anti-gp120 MAb D47A showed robust excretion abilities (Fig. 1B). D25A, also active against gp120, excreted HIV at a lower level. The three anti-HIV IgA MAbs that did not excrete HIV at 100 μg/ml (results for D61A are not shown in Fig. 1B) were tested at 300 μg/ml (Fig. 1C). T33A and D61A still did not excrete HIV, but D19A did. Irrelevant IgA at 300 μg/ml did not excrete HIV (data not shown).
IgA binding to HIV.
Because of differences in the levels of HIV excretion among the IgA antibodies, the ability of each MAb to bind HIV was assessed via ELISA. Three antibody concentrations (100, 50, and 10 μg/ml) were analyzed and yielded identical relative binding patterns. The results with 100 μg/ml are shown for illustration (Fig. 2A). Increased binding to HIV corresponded with more efficient excretion ability, i.e., D10A and D47A yielded the most robust excretion (Fig. 1B) and bound best to HIV (Fig. 2A). Interestingly, when D10A and D47A were combined, IgA binding to HIV was significantly increased over that of D10A (P ≤ 0.0006) or D47A (P ≤ 0.001) individually. D19A and D25A had intermediate binding to HIV and were capable of virus excretion (Fig. 1), though at lower levels than D10A and D47A. The MAbs with the least HIV binding abilities, T33A and D61A, were incapable of excretion even at 300 μg/ml.
FIG. 2.
Relative abilities of IgA MAbs to bind to HIV and of HIV-IgA immune complexes to bind to pIgR. Results are expressed as means ± SD. (A) Binding of MAbs (100 μg/ml) to HIV (n = 3). (B) Binding of immune complexes (100 μg/ml MAb) to pIgR (n = 3).
Immune complex binding to pIgR.
Immune complexes of HIV and IgA antibody (at a 100-μg/ml final MAb concentration) were tested for the ability to bind to pIgR. After immune complex binding to pIgR, the associated HIV was detected by ELISA. D10A and D47A complexes bound to the greatest extent (Fig. 2B). When D10A and D47A were combined to yield mixed immune complexes, there was significantly increased binding compared to that of D10A (P ≤ 0.00001) or D47A (P ≤ 0.0003) alone. D19A and D25A, which were capable of weak excretion (Fig. 1), yielded similar, moderate levels of binding to pIgR. T33A and D61A, which bound weakly to HIV but did not excrete HIV, produced immune complexes that failed to associate with pIgR.
Synergy in HIV excretion.
The ability of different IgA antibodies to work synergistically in virus excretion was assessed by using D10A and D47A in combination to form HIV-IgA immune complexes. Immune complexes with D10A (50 μg/ml) plus D47A (50 μg/ml) in the basolateral chamber below pIgR+ Vero cells yielded increased levels of HIV excretion compared to that yielded by each individual IgA at 100 μg/ml. The apical copy number of HIV with the combination of D10A and D47A (50 μg/ml each) was 289,000 (±68,000) virions per ml, whereas that with D10A alone (100 μg/ml) was 96,000 (±17,000) virions per ml and that with D47A alone (100 μg/ml) was 173,000 (±59,000) virions per ml (Fig. 3A). Virus excretion with the combined MAbs was therefore increased 1.7-fold over that with D47A alone (P ≤ 0.042) and 3-fold over that with D10A alone (P ≤ 0.002). Figure 3B shows the viral band intensities after excretion by the individual and combined IgAs. The individual IgAs at 50 μg/ml each yielded the least intense bands, and the bands from 100-μg/ml concentrations of the individual MAbs were less intense than those from the combination of D10A and D47A. Vero cells without the pIgR receptor were unable to transport IgA and did not excrete HIV, even with the combination of D10A and D47A at 100 μg/ml (data not shown). Incubation at 4°C rather than 37°C did not allow excretion to occur (data not shown).
FIG. 3.
Synergy of HIV excretion through pIgR+ Vero cells with a combination of D10A (anti-gp41) and D47A (anti-gp120). (A) Viral copy number in virions per milliliter (mean ± SD; n = 2) detected in the apical compartment after 8 h of excretion with D10A or D47A alone and in combination at a final MAb concentration of 100 μg/ml. (B) RT-PCR products comparing virus excretion levels with D10A or D47A at 50 and 100 μg/ml and the D10A/D47A mixture (50 and 50 μg/ml). Lanes 12 to 15 contain the IgG control (con.), a 100-μg/ml mixture of D10G and D47G, and the irrelevant IgA control. The positive control (PC) was as described in the legend to Fig. 1. M, molecular size markers.
HIV excretion is IgA antibody concentration dependent.
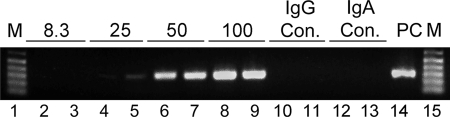
To observe HIV excretion as a function of the IgA antibody concentration, equal volumes of HIV and D10A/D47A at concentrations ranging from 8.3 to 100 μg/ml were mixed and the mixture was placed into the basolateral chambers below polarized pIgR+ Vero cell monolayers. After 8 h of excretion at 37°C, HIV in the apical medium was assessed by the extraction of viral RNA, followed by RT-PCR and gel electrophoresis analysis of the PCR product. At the lowest MAb concentration (8.3 μg/ml), no viral excretion was detected. At 25 μg/ml, apical HIV was detected, and the extent of virus excretion increased further according to the IgA concentration (Fig. 4). The amount of virus detected after the addition of 200 μg/ml of IgA was comparable to that detected after the addition of 100 μg/ml, indicating a plateau (data not shown). Neither V-region-matched IgG nor irrelevant IgA at 100 μg/ml excreted HIV.
FIG. 4.
HIV excretion through pIgR+ Vero cells at different IgA antibody concentrations. Apical supernatants were analyzed after 8 h of excretion of basolateral virus from medium containing equal amounts of D10A and D47A at total concentrations of 8.3 (lanes 2 and 3), 25 (lanes 4 and 5), 50 (lanes 6 and 7), and 100 (lanes 8 and 9) μg/ml. IgG and IgA controls (con.) were as described in the legend to Fig. 3. The positive control (PC) was as described in the legend to Fig. 1. M, molecular size markers.
HIV excretion by IgA antibody is time dependent.
To determine whether IgA-mediated excretion depends on the duration of basolateral exposure to immune complexes, HIV-IgA complexes (containing D10A/D47A at 100 μg/ml) were added to the basolateral chamber below polarized pIgR+ Vero cells and incubated at 37°C. At 2, 4, 8, and 12 h, apical medium was collected and viral RNA was extracted for RT-PCR and detected by gel electrophoresis. Apical HIV was detected first at 4 h, and the amount increased with further incubation (Fig. 5). Irrelevant IgA and V-region-matched IgG were not able to transport HIV through the cell monolayer after 12 h of incubation.
FIG. 5.
HIV excretion at different times. Apical samples from pIgR+ Vero cells were analyzed after 2 (lanes 2 and 3), 4 (lanes 4 and 5), 8 (lanes 6 and 7), and 12 (lanes 8 and 9) h of basolateral exposure to HIV immune complexes with D10A/D47A at 100 μg/ml. IgG and IgA controls (con.) at 12 h (lanes 10 to 13) were as described in the legend to Fig. 3. The positive control (PC) was as described in the legend to Fig. 1. M, molecular size markers.
Infectivity of excreted HIV.
The infectivity of the excreted HIV transported with either conventionally nonneutralizing D10A or neutralizing D47A through pIgR+ Vero cell monolayers was analyzed. 174 × CEM T cells placed in the apical compartment during virus excretion mediated by D10A became infected, as indicated by CPE and p24 production (Fig. 6). T cells incubated with HIV from D47A-mediated excretion produced no visible CPE, and p24 levels were below the level of detection (data not shown).
FIG. 6.
Infectivity of HIV excreted by nonneutralizing D10A through pIgR+ Vero cells. Shown are the levels (means ± SD; n = 2) of p24 produced by target T cells at different days postinfection during 8-h incubations.
HIV excretion through polarized human epithelial cells.
To assess whether human epithelial cell lines that naturally express pIgR can mediate excretion, as observed with pIgR-transfected Vero (primate) cells, HEC-1A, a human uterine endometrial cell line, and HT-29, a human colorectal cell line, were tested for excretion. HIV and IgA, a combination of D10A (50 μg/ml) and D47A (50 μg/ml), were mixed as described above and added to the basolateral compartment under polarized human cell monolayers, and the chamber was incubated at 37°C. The apical medium was sampled at 8 h, and the viral RNA was extracted, amplified by RT-PCR, and analyzed by gel electrophoresis. Both human cell lines, HEC-1A and HT-29, allowed IgA antibody to excrete HIV (Fig. 7). Excretion did not occur with irrelevant IgA or V-region-matched IgG.
FIG. 7.
HIV excretion through polarized pIgR+ cells of human epithelial cell lines. Apical HIV was detected 8 h after the exposure of the monolayers to basolateral HIV mixed with D10A/D47A (100 μg/ml total). Both cell lines, HEC-1A and HT-29, were capable of excreting HIV-IgA immune complexes. Specific IgG and irrelevant IgA controls (con.) were as described in the legend to Fig. 3. The positive control (PC) was as described in the legend to Fig. 1. M, molecular size markers.
Lack of productive infection of cell monolayers during excretion.
Vero, HEC-1A, and HT-29 cell monolayers were washed thoroughly after HIV excretion by either D10A or D47A antibodies. Cells were then further incubated, and apical and basal media were collected on days 3, 7, and 10. All samples were negative for viral RNA (data not shown).
Lack of recycling of HIV to the basolateral chamber.
Reports of the transcytosis of apically added cell-free HIV have been inconsistent and strain dependent (16, 33). The possibility that HIV excreted into the apical chamber may be recycled back to the basolateral chamber was assessed in our system. The addition of free HIV or HIV-IgA immune complexes to the apical compartment yielded no detectable HIV in the basolateral chamber after 8 h of incubation (data not shown).
Colocalization of HIV and IgA antibody during excretion.
To confirm the continued interaction of IgA and HIV inside epithelial cells during the excretion of immune complexes, two-color immunofluorescence with a confocal laser scanning microscope was used. Polarized cell monolayers were collected after an 8-h excretion experiment with HIV and either a mixture of D10A/D47A or irrelevant IgA (anti-measles virus F protein) at 100 μg/ml. Membranes with pIgR+ Vero or HT-29 cells were processed and stained with rhodamine conjugate (red) for HIV protein and with fluorescein isothiocyanate conjugate (green) for IgA. Figure 8 shows the HIV protein and IgA (D10A/D47A or irrelevant) in apical, middle, and basal cell sections. Both Vero (Fig. 8A) and HT-29 (Fig. 8B) cells showed the colocalization of anti-HIV IgA (green) and HIV protein (red) at all levels (staining in HT-29 cells was more diffuse). Virtually no HIV was observed in the absence of immune complexes (Fig. 8, right panels), i.e., irrelevant IgA showed no colocalization with HIV.
FIG. 8.
Intracellular colocalization of IgA and HIV protein within polarized epithelial cells after the endocytosis of virus-IgA immune complexes observed by confocal immunofluorescence microscopy. Apical, middle, and basal horizontal sections through the cell monolayers after 8 h of excretion are shown for the red channel (HIV protein), the green channel (IgA), and merged red and green channels. A yellow to orange signal in the merged channel indicates colocalization. Vero C1008 pIgR+ cells and HT-29 pIgR+ cells are shown. Cells transcytosing irrelevant IgA showed no HIV, while cells transcytosing immune complexes of HIV and specific IgA antibody showed the colocalization of HIV protein and IgA at all levels. Bars, 10 μm.
DISCUSSION
Mucosal surfaces, where IgA is the main class of antibody, are the primary site of heterosexual, homosexual, and vertical HIV transmission. Anti-HIV IgA in mucosal fluids from infected patients, exposed seronegative partners, and immunized macaques is capable of conventionally neutralizing HIV, preventing viral transcytosis from the apical surface through epithelial cells (14, 19, 45, 46, 50, 57, 61, 82), and has been correlated with protection in exposed seronegative individuals (7, 17, 18, 44, 45, 57, 58).
Another defense function of IgA antibodies, immune excretion, has been demonstrated for viruses by the transport of measles virus from the basolateral compartment through polarized epithelial cells to the apical compartment of Transwell chambers (83) and for inert antigens in a mouse model in which ovalbumin was excreted from the intestinal lamina propria into the lumen (66). The present study is the first to demonstrate IgA-mediated immune excretion of HIV across polarized epithelial cells, indicating the potential for IgA antibodies to remove HIV from the mucosal laminae propriae of infected individuals.
The immune excretion of HIV demonstrated in this study required the presence of metabolically active pIgR on the basolateral surfaces of the epithelial cells since excretion was not observed with either pIgR− Vero cells or pIgR+ Vero cells incubated at 4°C. Excretion was also dependent on immune complexes containing IgA antibody since V-region-matched IgGs, which do not bind pIgR, were unable to excrete HIV. Furthermore, irrelevant IgA that cannot bind HIV but is transcytosed via the pIgR does not excrete virus, indicating that HIV-specific IgA is needed. Thus, HIV excretion requires both pIgR+ epithelial cells and HIV-specific IgA.
Individual IgA antibodies can have different protective abilities, as indicated by a previous study with measles virus, which described an anti-fusion protein IgA that is nonneutralizing conventionally but moderate in intracellular neutralization and robust at virus excretion in comparison to a neutralizing antihemagglutinin IgA which is weak in excretion (83). The antiviral capabilities also varied among the different anti-HIV IgA MAbs used in this study, in which excretion capacities ranged from none to robust. Such variations in activity presumably reflect differences in viral antigen specificity and the affinity of binding.
To help understand the observed excretion differences among the MAbs, they were tested for epithelial cell transcytosis ability in the presence of HIV, binding to HIV, and their binding as immune complexes to pIgR. While the transcytosis of the anti-HIV IgAs was reduced when HIV was present (Table 2), this effect did not fully explain the excretion differences since the MAbs showed similar reductions. A correlation between viral binding and excretion was observed. Among individual MAbs, D10A (anti-gp41) and D47A (anti-gp120) produced the highest levels of excretion (Fig. 1) and also bound best both to virus and as immune complexes to pIgR (Fig. 2). D25A (anti-gp120) and D19A (anti-gp120) both had lower levels of binding to virus and as immune complexes to pIgR than D10A and D47A but were still capable of excreting HIV. T33A and D61A (anti-gp41) had the lowest levels of HIV binding, and their immune complexes showed virtually no binding to pIgR, in keeping with their failure to excrete virus. In summary, the ability of IgA antibodies to mediate excretion appears to be related to their ability to bind to virus, as well as their ability in immune complexes to bind pIgR.
After appropriate immunization, the mucosal lamina propria contains a polyclonal population of antibodies, whose collective properties determine the effectiveness of protection. The results in prior reports have shown the ability of some MAbs to work synergistically to mediate HIV neutralization (3, 48, 51, 53). The potential for antibody synergy in excretion function was tested here with IgAs against gp120 (D47A) and gp41 (D10A), and synergy was indeed observed (Fig. 3). A likely explanation for the increased excretion is the ability of these two MAbs when combined to transcytose at higher rates than the individual MAbs (Table 2), bind HIV more efficiently (Fig. 2A), and bind better to pIgR when in complexes with HIV (Fig. 2B).
HIV excretion was detected at 25 μg/ml IgA antibody and increased as the IgA antibody concentration was raised. The effect plateaued at 100 μg/ml (Fig. 4), which is roughly consistent with the concentration of IgA in human external secretions (40) and dog mesenteric lymph (77). These results suggest that physiological IgA antibody concentrations may mediate excretion in vivo, especially since the heterogeneous polyclonal IgA antibodies produced during actively induced immune responses would include antibodies of greater affinity than the MAbs employed in the present study. Furthermore, the extensive daily production of IgA, greater than that of all the other Ig classes combined (42), allows the mucosae to have newly synthesized IgA continuously available for the binding and transport of antigens. Given the enormous surface area of the body's mucosae, especially in the intestinal tract, the ongoing excretion of virus has the potential to reduce viremia and, in the case of HIV, to limit damage to mucosal T cells whose loss can set the course of future disease (4, 28, 31).
Though IgA-mediated immune excretion removes antigen from the lamia propria, it has not been shown to cause the intracellular degradation of antigen (66). D10A antibody, in contrast to D47A, lacks conventional and intracellular neutralization activities (39) but is capable of binding and excreting HIV, indicating that excretion function is not directly related to neutralization ability. The different neutralizing abilities of D10A and D47A provided an opportunity to study whether excreted virus could remain infectious. Not unexpectedly, neutralizing D47A MAb excreted noninfectious virions while D10A transported infectious virus as indicated by CPE and confirmed by p24 production by infected T cells (Fig. 6). Other studies have shown that the apical-to-basolateral transcytosis of HIV through epithelial cells can be interrupted by IgA or IgM antibody transcytosing from the basolateral compartment and that virus redirected to the apical side remains infectious (8, 35). Additionally, phagocytes can become infected after ingesting antibody-bound HIV via Fc receptor intake (36, 73), and since epithelial cells may be capable of supporting HIV replication, it is possible that virus from antibody-mediated excretion may infect epithelial cells. However, for the Vero, HEC-1A, and HT-29 cells studied here, productive infection resulting from excretion was not detected.
HEC-1A, a uterine endometrial cell line, and HT-29, a colorectal cell line, are both derived from mucosae where IgA transcytosis through the lining epithelium occurs. Both these human cell lines express endogenous pIgR and therefore demonstrate the potential capacity of human mucosal epithelial cells to excrete HIV from the lamina propria, thereby helping to protect mucosal T cells.
The vesicular transcytosis of polymeric IgA begins with its binding to pIgR, followed by the endocytosis of IgA and pIgR at the basolateral cell membrane before transport through a series of endosomal compartments and release into the apical mucosal secretions (42). Confocal microscopy showed the colocalization of transcytosing IgA antibody and HIV within the basal, middle, and apical portions of polarized epithelial cells, consistent with the established pathway of pIgR-mediated transport for apical release (Fig. 8). Colocalization was observed for both a pIgR-transfected primate cell line and a human cell line with endogenous pIgR expression.
It is apparent that IgA antibodies can manifest a number of anti-HIV properties. IgA has been shown previously to block the apical adherence of HIV to epithelial cells and inhibit the apical-to-basolateral transcytosis of HIV (1, 2, 16). We have previously demonstrated that IgA antibodies against the envelope proteins gp120 and gp41, as well as the internal proteins RT and Gag, can mediate HIV neutralization intracellularly during their epithelial transcytosis (39, 81). In the present work, we provided evidence in vitro for another host defense function in which anti-HIV IgA excretes virus across epithelial cells. Determining the significance of our findings for mucosal defense in vivo must, of course, await future studies, including experiments with R5 viruses, which tend to be associated with initial infection.
Currently, there is considerable interest in the possibility that an increase in microbially derived antigens in the intestinal mucosa leads to a detrimental chronic activation of the immune system during HIV infection (12, 20, 30). Therefore, mucosal IgA antibodies that can excrete intact HIV and its individual components, as well as the products of other microbes, may be significant in attempts to improve the clinical response to HIV exposure. The findings of the present study and others illustrating the multiple protective functions of anti-HIV IgA would seem to support the rationale for mucosal immunization strategies against HIV.
Acknowledgments
We thank the Confocal Microscopy Core in the Comprehensive Cancer Center of Case School of Medicine/University Hospitals of Cleveland (funded by NIH grant no. P30 CA43703-12) for the use of their facility.
This research was supported by grants AI-36359 and CA-43703 from the NIH.
Footnotes
Published ahead of print on 1 October 2008.
REFERENCES
- 1.Alfsen, A., P. Iniguez, E. Bouguyon, and M. Bomsel. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 1666257-6265. [DOI] [PubMed] [Google Scholar]
- 2.Alfsen, A., H. Yu, A. Magerus-Chatinet, A. Schmitt, and M. Bomsel. 2005. HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol. Biol. Cell 164267-4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaway, G. P., A. M. Ryder, G. A. Beaudry, and P. J. Maddon. 1993. Synergistic inhibition of HIV-1 envelope-mediated cell fusion by CD4-based molecules in combination with antibodies to gp120 or gp41. AIDS Res. Hum. Retrovir. 9581-587. [DOI] [PubMed] [Google Scholar]
- 4.Ambrose, Z., K. Larsen, J. Thompson, Y. Stevens, E. Finn, S. L. Hu, and M. L. Bosch. 2001. Evidence for early local viral replication and local production of antiviral immunity upon mucosal simian-human immunodeficiency virus SHIV89.6 infection in Macaca nemestrina. J. Virol. 758589-8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amerongen, H. M., R. Weltzin, C. M. Farnet, P. Michetti, W. A. Haseltine, and M. R. Neutra. 1991. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J. Acquir. Immune Defic. Syndr. 4760-765. [PubMed] [Google Scholar]
- 6.Belec, L., P. D. Ghys, H. Hocini, J. N. Nkengasong, J. Tranchot-Diallo, M. O. Diallo, V. Ettiegne-Traore, C. Maurice, P. Becquart, M. Matta, A. Si- Mohamed, N. Chomont, I. M. Coulibaly, S. Z. Wiktor, and M. D. Kazatchkine. 2001. Cervicovaginal secretory antibodies to human immunodeficiency virus type 1 (HIV-1) that block viral transcytosis through tight epithelial barriers in highly exposed HIV-1-seronegative African women. J. Infect. Dis. 1841412-1422. [DOI] [PubMed] [Google Scholar]
- 7.Beyrer, C., A. W. Artenstein, S. Rugpao, H. Stephens, T. C. VanCott, M. L. Robb, M. Rinkaew, D. L. Birx, C. Khamboonruang, P. A. Zimmerman, K. E. Nelson, C. Natpratan, et al. 1999. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. J. Infect. Dis. 17959-67. [DOI] [PubMed] [Google Scholar]
- 8.Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 342-47. [DOI] [PubMed] [Google Scholar]
- 9.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9277-287. [DOI] [PubMed] [Google Scholar]
- 10.Boot, J. H., M. E. Geerts, E. R. De Groot, and L. A. Aarden. 1988. Murine monoclonal isotype switch variants. Detection with rat monoclonal antibodies in ELISA and isolation by sequential sublining. J. Immunol. Methods 106195-202. [DOI] [PubMed] [Google Scholar]
- 11.Bouhlal, H., N. Chomont, N. Haeffner-Cavaillon, M. D. Kazatchkine, L. Belec, and H. Hocini. 2002. Opsonization of HIV-1 by semen complement enhances infection of human epithelial cells. J. Immunol. 1693301-3306. [DOI] [PubMed] [Google Scholar]
- 12.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 121365-1371. [DOI] [PubMed] [Google Scholar]
- 13.Bukawa, H., K. Sekigawa, K. Hamajima, J. Fukushima, Y. Yamada, H. Kiyono, and K. Okuda. 1995. Neutralization of HIV-1 by secretory IgA induced by oral immunization with a new macromolecular multicomponent peptide vaccine candidate. Nat. Med. 1681-685. [DOI] [PubMed] [Google Scholar]
- 14.Burnett, P. R., T. C. VanCott, V. R. Polonis, R. R. Redfield, and D. L. Birx. 1994. Serum IgA-mediated neutralization of HIV type 1. J. Immunol. 1524642-4648. [PubMed] [Google Scholar]
- 15.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272104-107. [DOI] [PubMed] [Google Scholar]
- 16.Chomont, N., H. Hocini, J. C. Gody, H. Bouhlal, P. Becquart, C. Krief-Bouillet, M. Kazatchkine, and L. Belec. 2008. Neutralizing monoclonal antibodies to human immunodeficiency virus type 1 do not inhibit viral transcytosis through mucosal epithelial cells. Virology 370246-254. [DOI] [PubMed] [Google Scholar]
- 17.Devito, C., K. Broliden, R. Kaul, L. Svensson, K. Johansen, P. Kiama, J. Kimani, L. Lopalco, S. Piconi, J. J. Bwayo, F. Plummer, M. Clerici, and J. Hinkula. 2000. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 1655170-5176. [DOI] [PubMed] [Google Scholar]
- 18.Devito, C., J. Hinkula, R. Kaul, J. Kimani, P. Kiama, L. Lopalco, C. Barass, S. Piconi, D. Trabattoni, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2002. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J. Acquir. Immune Defic. Syndr. 30413-420. [DOI] [PubMed] [Google Scholar]
- 19.Devito, C., J. Hinkula, R. Kaul, L. Lopalco, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2000. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 141917-1920. [DOI] [PubMed] [Google Scholar]
- 20.Douek, D. 2007. HIV disease progression: immune activation, microbes, and a leaky gut. Top. HIV Med. 15114-117. [PubMed] [Google Scholar]
- 21.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 712674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earl, P. L., W. Sugiura, D. C. Montefiori, C. C. Broder, S. A. Lee, C. Wild, J. Lifson, and B. Moss. 2001. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J. Virol. 75645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fantini, J., N. Yahi, S. Baghdiguian, and J. C. Chermann. 1992. Human colon epithelial cells productively infected with human immunodeficiency virus show impaired differentiation and altered secretion. J. Virol. 66580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantini, J., N. Yahi, and J. C. Chermann. 1991. Human immunodeficiency virus can infect the apical and basolateral surfaces of human colonic epithelial cells. Proc. Natl. Acad. Sci. USA 889297-9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng, N., J. A. Lawton, J. Gilbert, N. Kuklin, P. Vo, B. V. Prasad, and H. B. Greenberg. 2002. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6-specific IgA mAb. J. Clin. Investig. 1091203-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming, S. C., M. S. Kapembwa, T. T. MacDonald, and G. E. Griffin. 1992. Direct in vitro infection of human intestine with HIV-1. AIDS 61099-1104. [DOI] [PubMed] [Google Scholar]
- 27.Fujioka, H., S. N. Emancipator, M. Aikawa, D. S. Huang, F. Blatnik, T. Karban, K. DeFife, and M. B. Mazanec. 1998. Immunocytochemical colocalization of specific immunoglobulin A with Sendai virus protein in infected polarized epithelium. J. Exp. Med. 1881223-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George, M. D., E. Reay, S. Sankaran, and S. Dandekar. 2005. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J. Virol. 792709-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han, Y., C. L. Ventura, K. P. Black, J. E. Cummins, Jr., S. D. Hall, and S. Jackson. 2000. Productive human immunodeficiency virus-1 infection of epithelial cell lines of salivary gland origin. Oral Microbiol. Immunol. 1582-88. [DOI] [PubMed] [Google Scholar]
- 30.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 171881-1888. [DOI] [PubMed] [Google Scholar]
- 31.Hel, Z., J. R. McGhee, and J. Mestecky. 2006. HIV infection: first battle decides the war. Trends Immunol. 27274-281. [DOI] [PubMed] [Google Scholar]
- 32.Hirbod, T., and K. Broliden. 2007. Mucosal immune responses in the genital tract of HIV-1-exposed uninfected women. J. Intern. Med. 26244-58. [DOI] [PubMed] [Google Scholar]
- 33.Hocini, H., P. Becquart, H. Bouhlal, N. Chomont, P. Ancuta, M. D. Kazatchkine, and L. Belec. 2001. Active and selective transcytosis of cell-free human immunodeficiency virus through a tight polarized monolayer of human endometrial cells. J. Virol. 755370-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hocini, H., L. Belec, S. Iscaki, B. Garin, J. Pillot, P. Becquart, and M. Bomsel. 1997. High-level ability of secretory IgA to block HIV type 1 transcytosis: contrasting secretory IgA and IgG responses to glycoprotein 160. AIDS Res. Hum. Retrovir. 131179-1185. [DOI] [PubMed] [Google Scholar]
- 35.Hocini, H., and M. Bomsel. 1999. Infectious human immunodeficiency virus can rapidly penetrate a tight human epithelial barrier by transcytosis in a process impaired by mucosal immunoglobulins. J. Infect. Dis. 179(Suppl. 3)S448-S453. [DOI] [PubMed] [Google Scholar]
- 36.Homsy, J., M. Meyer, M. Tateno, S. Clarkson, and J. A. Levy. 1989. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science 2441357-1360. [DOI] [PubMed] [Google Scholar]
- 37.Hu, J., B. Murray, M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 746087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang, Y. T., C. J. Miller, V. Wong, H. Fujioka, J. G. Nedrud, and M. E. Lamm. 1999. Replication and budding of simian immunodeficiency virus in polarized epithelial cells. Virology 25724-34. [DOI] [PubMed] [Google Scholar]
- 39.Huang, Y. T., A. Wright, X. Gao, L. Kulick, H. Yan, and M. E. Lamm. 2005. Intraepithelial cell neutralization of HIV-1 replication by IgA. J. Immunol. 1744828-4835. [DOI] [PubMed] [Google Scholar]
- 40.Jackson, S., J. Mestecky, Z. Moldoveanu, and P. Spearman. 1999. Collection and processing of human mucosal secretions, p. 1567-1575. In J. Mestecky, P. L. Ogra, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology, 2nd ed. Academic Press, San Diego, CA.
- 41.Joint United Nations Programme on HIV/AIDS. 30 May 2006, posting date. 2006 report on the global AIDS epidemic: a UNAIDS 10th anniversary special edition, p. 7-50. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2006/default.asp.
- 42.Kaetzel, C. S., and K. Mostov. 2005. Immunoglobulin transport and the polymeric immunoglobulin receptor, p. 211-250. In J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, J. R. McGhee, and L. Mayer (ed.), Mucosal immunology, 3rd ed., vol. 1. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 43.Kaetzel, C. S., J. K. Robinson, K. R. Chintalacharuvu, J. P. Vaerman, and M. E. Lamm. 1991. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc. Natl. Acad. Sci. USA 888796-8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaul, R., F. Plummer, M. Clerici, M. Bomsel, L. Lopalco, and K. Broliden. 2001. Mucosal IgA in exposed, uninfected subjects: evidence for a role in protection against HIV infection. AIDS 15431-432. [DOI] [PubMed] [Google Scholar]
- 45.Kaul, R., D. Trabattoni, J. J. Bwayo, D. Arienti, A. Zagliani, F. M. Mwangi, C. Kariuki, E. N. Ngugi, K. S. MacDonald, T. B. Ball, M. Clerici, and F. A. Plummer. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 1323-29. [DOI] [PubMed] [Google Scholar]
- 46.Kozlowski, P., R. W. Buckheit, Jr., and S. Jackson. 1995. Neutralization of HIV infection by serum IgA antibodies. Adv. Exp. Med. Biol. 371B1027-1030. [PubMed] [Google Scholar]
- 47.Kozlowski, P. A., and M. R. Neutra. 2003. The role of mucosal immunity in prevention of HIV transmission. Curr. Mol. Med. 3217-228. [DOI] [PubMed] [Google Scholar]
- 48.Laal, S., S. Burda, M. K. Gorny, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1994. Synergistic neutralization of human immunodeficiency virus type 1 by combinations of human monoclonal antibodies. J. Virol. 684001-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lagaye, S., M. Derrien, E. Menu, C. Coito, E. Tresoldi, P. Mauclere, G. Scarlatti, G. Chaouat, F. Barre-Sinoussi, and M. Bomsel. 2001. Cell-to-cell contact results in a selective translocation of maternal human immunodeficiency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J. Virol. 754780-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehner, T., Y. Wang, M. Cranage, L. A. Bergmeier, E. Mitchell, L. Tao, G. Hall, M. Dennis, N. Cook, R. Brookes, L. Klavinskis, I. Jones, C. Doyle, and R. Ward. 1996. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat. Med. 2767-775. [DOI] [PubMed] [Google Scholar]
- 51.Li, A., H. Katinger, M. R. Posner, L. Cavacini, S. Zolla-Pazner, M. K. Gorny, J. Sodroski, T. C. Chou, T. W. Baba, and R. M. Ruprecht. 1998. Synergistic neutralization of simian-human immunodeficiency virus SHIV-vpu+ by triple and quadruple combinations of human monoclonal antibodies and high-titer anti-human immunodeficiency virus type 1 immunoglobulins. J. Virol. 723235-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopalco, L., C. Pastori, A. Cosma, S. E. Burastero, B. Capiluppi, E. Boeri, A. Beretta, A. Lazzarin, and A. G. Siccardi. 2000. Anti-cell antibodies in exposed seronegative individuals with HIV type 1-neutralizing activity. AIDS Res. Hum. Retrovir. 16109-115. [DOI] [PubMed] [Google Scholar]
- 53.Mascola, J. R., M. K. Louder, T. C. VanCott, C. V. Sapan, J. S. Lambert, L. R. Muenz, B. Bunow, D. L. Birx, and M. L. Robb. 1997. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 717198-7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazanec, M. B., C. L. Coudret, and D. R. Fletcher. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 691339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazanec, M. B., C. S. Kaetzel, M. E. Lamm, D. Fletcher, and J. G. Nedrud. 1992. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 896901-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazanec, M. B., J. G. Nedrud, C. S. Kaetzel, and M. E. Lamm. 1993. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 14430-435. [DOI] [PubMed] [Google Scholar]
- 57.Mazzoli, S., L. Lopalco, A. Salvi, D. Trabattoni, S. Lo Caputo, F. Semplici, M. Biasin, C. Blé, A. Cosma, C. Pastori, F. Meacci, F. Mazzotta, M. L. Villa, A. G. Siccardi, and M. Clerici. 1999. Human immunodeficiency virus (HIV)-specific IgA and HIV neutralizing activity in the serum of exposed seronegative partners of HIV-seropositive persons. J. Infect. Dis. 180871-875. [DOI] [PubMed] [Google Scholar]
- 58.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 31250-1257. [DOI] [PubMed] [Google Scholar]
- 59.Meng, G., X. Wei, X. Wu, M. T. Sellers, J. M. Decker, Z. Moldoveanu, J. M. Orenstein, M. F. Graham, J. C. Kappes, J. Mestecky, G. M. Shaw, and P. D. Smith. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8150-156. [DOI] [PubMed] [Google Scholar]
- 60.Miller, C. J., Q. Li, K. Abel, E. Y. Kim, Z. M. Ma, S. Wietgrefe, L. La Franco-Scheuch, L. Compton, L. Duan, M. D. Shore, M. Zupancic, M. Busch, J. Carlis, S. Wolinsky, and A. T. Haase. 2005. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 799217-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moja, P., C. Tranchat, I. Tchou, B. Pozzetto, F. Lucht, C. Desgranges, and C. Genin. 2000. Neutralization of human immunodeficiency virus type 1 (HIV-1) mediated by parotid IgA of HIV-1-infected patients. J. Infect. Dis. 1811607-1613. [DOI] [PubMed] [Google Scholar]
- 62.Moore, J. S., S. D. Hall, and S. Jackson. 2002. Cell-associated HIV-1 infection of salivary gland epithelial cell lines. Virology 29789-97. [DOI] [PubMed] [Google Scholar]
- 63.Moore, J. S., F. Rahemtulla, L. W. Kent, S. D. Hall, M. R. Ikizler, P. F. Wright, H. H. Nguyen, and S. Jackson. 2003. Oral epithelial cells are susceptible to cell-free and cell-associated HIV-1 infection in vitro. Virology 313343-353. [DOI] [PubMed] [Google Scholar]
- 64.Pastori, C., C. Barassi, S. Piconi, R. Longhi, M. L. Villa, A. G. Siccardi, M. Clerici, and L. Lopalco. 2000. HIV neutralizing IgA in exposed seronegative subjects recognise an epitope within the gp41 coiled-coil pocket. J. Biol. Regul. Homeost. Agents 1415-21. [PubMed] [Google Scholar]
- 65.Qureshi, M. N., C. E. Barr, T. Seshamma, J. Reidy, R. J. Pomerantz, and O. Bagasra. 1995. Infection of oral mucosal cells by human immunodeficiency virus type 1 in seropositive persons. J. Infect. Dis. 171190-193. [DOI] [PubMed] [Google Scholar]
- 66.Robinson, J. K., T. G. Blanchard, A. D. Levine, S. N. Emancipator, and M. E. Lamm. 2001. A mucosal IgA-mediated excretory immune system in vivo. J. Immunol. 1663688-3692. [DOI] [PubMed] [Google Scholar]
- 67.Sakai, K., X. Y. Ma, I. Gordienko, and D. J. Volsky. 1991. Recombinational analysis of a natural noncytopathic human immunodeficiency virus type 1 (HIV-1) isolate: role of the vif gene in HIV-1 infection kinetics and cytopathicity. J. Virol. 655765-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz-Cornil, I., Y. Benureau, H. Greenberg, B. A. Hendrickson, and J. Cohen. 2002. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J. Virol. 768110-8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith, P. D., and S. M. Wahl. 2005. Immunobiology of mucosal HIV-1 infection, p. 1199-1211. In J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, J. R. McGhee, and L. Mayer (ed.), Mucosal immunology, 3rd ed., vol. 1. Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 70.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoddard, E., G. Cannon, H. Ni, K. Kariko, J. Capodici, D. Malamud, and D. Weissman. 2007. gp340 expressed on human genital epithelia binds HIV-1 envelope protein and facilitates viral transmission. J. Immunol. 1793126-3132. [DOI] [PubMed] [Google Scholar]
- 72.Sugiura, W., C. C. Broder, B. Moss, and P. L. Earl. 1999. Characterization of conformation-dependent anti-gp120 murine monoclonal antibodies produced by immunization with monomeric and oligomeric human immunodeficiency virus type 1 envelope proteins. Virology 254257-267. [DOI] [PubMed] [Google Scholar]
- 73.Sullivan, N. J. 2001. Antibody-mediated enhancement of viral disease. Curr. Top. Microbiol. Immunol. 260145-169. [DOI] [PubMed] [Google Scholar]
- 74.Tamer, C. M., M. E. Lamm, J. K. Robinson, J. F. Piskurich, and C. S. Kaetzel. 1995. Comparative studies of transcytosis and assembly of secretory IgA in Madin-Darby canine kidney cells expressing human polymeric Ig receptor. J. Immunol. 155707-714. [PubMed] [Google Scholar]
- 75.Tan, X., R. Pearce-Pratt, and D. M. Phillips. 1993. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J. Virol. 676447-6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan, X., and D. M. Phillips. 1996. Cell-mediated infection of cervix derived epithelial cells with primary isolates of human immunodeficiency virus. Arch. Virol. 1411177-1189. [DOI] [PubMed] [Google Scholar]
- 77.Vaerman, J. P., and J. F. Heremans. 1970. Origin and molecular size of immunoglobulin-A in the mesenteric lymph of the dog. Immunology 1827-38. [PMC free article] [PubMed] [Google Scholar]
- 78.Veazey, R. S., and A. A. Lackner. 2004. Getting to the guts of HIV pathogenesis. J. Exp. Med. 200697-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wawer, M. J., R. H. Gray, N. K. Sewankambo, D. Serwadda, X. Li, O. Laeyendecker, N. Kiwanuka, G. Kigozi, M. Kiddugavu, T. Lutalo, F. Nalugoda, F. Wabwire-Mangen, M. P. Meehan, and T. C. Quinn. 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 1911403-1409. [DOI] [PubMed] [Google Scholar]
- 80.Wolbank, S., R. Kunert, G. Stiegler, and H. Katinger. 2003. Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J. Virol. 774095-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wright, A., H. Yan, M. E. Lamm, and Y. T. Huang. 2006. Immunoglobulin A antibodies against internal HIV-1 proteins neutralize HIV-1 replication inside epithelial cells. Virology 356165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu, X., S. Hall, and S. Jackson. 2003. Tropism-restricted neutralization by secretory IgA from parotid saliva of HIV type 1-infected individuals. AIDS Res. Hum. Retrovir. 19275-281. [DOI] [PubMed] [Google Scholar]
- 83.Yan, H., M. E. Lamm, E. Bjorling, and Y. T. Huang. 2002. Multiple functions of immunoglobulin A in mucosal defense against viruses: an in vitro measles virus model. J. Virol. 7610972-10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeaman, G. R., A. L. Howell, S. Weldon, D. J. Demian, J. E. Collins, D. M. O'Connell, S. N. Asin, C. R. Wira, and M. W. Fanger. 2003. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology 109137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]