Abstract
African green monkeys (AGM) do not develop overt signs of disease following simian immunodeficiency virus (SIV) infection. While it is still unknown how natural hosts like AGM can cope with this lentivirus infection, a large number of investigations have shown that CD8+ T-cell responses are critical for the containment of AIDS viruses in humans and Asian nonhuman primates. Here we have compared the phenotypes of T-cell subsets and magnitudes of SIV-specific CD8+ T-cell responses in vervet AGM chronically infected with SIVagm and rhesus monkeys (RM) infected with SIVmac. In comparison to RM, vervet AGM exhibited weaker signs of immune activation and associated proliferation of CD8+ T cells as detected by granzyme B, Ki-67, and programmed death 1 staining. By gamma interferon enzyme-linked immunospot assay and intracellular cytokine staining, SIV Gag- and Env-specific immune responses were detectable at variable but lower levels in vervet AGM than in RM. These observations demonstrate that natural hosts like SIV-infected vervet AGM develop SIV-specific T-cell responses, but the disease-free course of infection does not depend on the generation of robust CD8+ T-cell responses.
Humans infected with the human immunodeficiency virus (HIV) and Asian nonhuman primates infected with the simian immunodeficiency virus (SIV) generally experience a progressive loss of CD4+ T cells and eventually develop AIDS. In contrast, African nonhuman primates do not typically develop an AIDS-like disease following infection with their respective SIV strains (25, 73). Among African nonhuman primates, African green monkeys (AGM) belong to those species of natural hosts that have been studied in greater detail (25, 68). AGM are classified into four subspecies: grivet, vervet, sabaeus, and tantalus monkeys (Chlorocebus aethiops, Chlorocebus pygerythrus, Chlorocebus sabaeus, and Chlorocebus tantalus, respectively), each of which are infected in the wild by distinct species-specific SIVagm virus subtypes (2, 25). After reaching maturity, feral populations of AGM have a high seroprevalence for SIV and plasma viral loads that can reach levels similar to those observed in AIDS virus-infected humans or Asian nonhuman primates (7, 19).
Experimental infection of AGM with SIV results initially in high levels of plasma viremia followed by partial viral containment. A transient loss of CD4+ T cells during primary viremia is followed by rapid recovery to preinfection levels within a few weeks after challenge (12, 18, 27, 32, 52, 54, 55). Very infrequently, after a prolonged period of SIV infection, a significant decline of CD4+ T cells has been observed in vervet AGM without causing disease (46). Despite the lack of pathogenicity in vervet AGM, SIV infection of pig-tailed macaques shows that at least one SIVagm strain can lead to CD4+ T-cell loss and development of an AIDS-like disease (20, 26). Thus, SIVagm is not inherently nonpathogenic in nonhuman primates.
In humans and Asian nonhuman primates, the containment of HIV and SIV viremia is likely multifactorial, including innate and adaptive immune factors (1, 37, 57, 61). Adaptive immune responses are usually detectable within a few weeks or months following infection. The relative contribution of humoral immune responses is still a matter of debate. Neutralizing antibodies exert selective immune pressure on the virus, resulting in extensive variation in the envelope gene and immune escape (63). However, most neutralizing antibodies are type specific, and broadly neutralizing antibodies are rarely seen (8, 23). In contrast, cellular immune responses play a central role in partial containment of primary and chronic AIDS virus infections in humans and Asian nonhuman primates (6, 34, 35, 66).
As the reason for the nonpathogenic course of infection in natural hosts of AIDS viruses remains elusive, a number of correlative observations have been made that can explain the differential outcome of infections in natural hosts compared to nonnatural hosts. The most profound observations associated with nonpathogenic SIV infection are the transient immune activation following SIV infection, the strong induction of anti-inflammatory responses, and the relative paucity of CCR5+ CD4+ T-cell target cells, particularly in gastrointestinal tissues (32, 53, 68). It is questionable whether adaptive immune responses contribute to partial viral control and a nonpathogenic course of infection. Since SIV-specific humoral immune responses in chronic infection consist mainly of nonneutralizing envelope-specific antibodies and relatively low titers of Gag-specific antibodies in comparison to AIDS virus-infected humans and Asian nonhuman primates, it is unlikely that humoral immune responses play a major role in the nonpathogenic course of infection (17, 25, 50). Cellular immune responses have been detected in sooty mangabeys (30). However, CD8+ lymphocyte depletion experiments in SIV-infected sooty mangabeys have questioned the relevance of SIV-specific CD8+ T-cell responses in viral containment and a disease-free course of infection (4). CD8+ T cells from SIV-infected vervet AGM can secrete molecules that may interfere with SIV replication in in vitro cultures (15), similar to observations made for humans and Asian nonhuman primates. Only a small subgroup of vaccinated or SIV-infected AGM showed T-cell proliferation upon stimulation with whole inactivated virus (5). However, a detailed investigation of CD8+ T-cell responses in AGM has not been performed so far.
In the present study, we have utilized complex polychromatic flow cytometry to detect T-cell subset changes and performed gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays and intracytoplasmic cytokine staining to determine SIV peptide pool-specific functional CD8+ T-cell responses in SIV-negative and SIV-infected vervet AGM and rhesus monkeys (RM). In comparison to SIV-infected RM, vervet AGM had relatively weak signs of immune activation and proliferation of CD8+ T cells and relatively low levels of SIV-specific CD8+ T-cell responses.
MATERIALS AND METHODS
Animals and viruses.
A total of 33 vervet AGM were recruited for these studies. Twenty-two were imported from Tanzania and included four SIV-negative animals, 11 naturally infected animals, and seven experimentally infected animals. Eleven uninfected vervet AGM that originated from Kenya were imported from Germany. The experimentally infected vervet AGM were inoculated either with the well-characterized SIVagmVer90 isolate (26) or with 2 ml of SIV-infected blood from one of the naturally infected vervet AGM (Ver1) as previously described (18) and were evaluated at 3.5 years following infection. For comparative purposes, a total of 63 SIV-negative RM and 21 RM chronically infected with SIV, all of Indian origin (Macaca mulatta), were recruited. The numbers of RM used for the individual assays are indicated in Results. All SIV-infected RM were negative for the major histocompatibility complex class I allele Mamu-A*01. The RM were challenged either with uncloned SIVmac251 or with SIVsmE660, and blood samples were taken from these animals at 4 to 6 months (n = 16) or 2 years (n = 5) postchallenge. All animals were maintained in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals under an NIAID-approved animal study protocol (48), and all studies and procedures were reviewed and approved by the Institutional Animal Care and Use Committees of the NIH and Harvard University.
Plasma viral load assay.
Plasma levels of viral RNA in RM were measured by an ultrasensitive branched DNA amplification assay with a detection limit of 125 copies per ml (Bayer Diagnostics, Berkeley, CA). A quantitative real-time reverse transcription-PCR (RT-PCR) assay for quantitation of viral RNA in AGM plasma was performed as previously described (19), using methodology based on the 7700 sequence detection system (Applied Biosystems, Foster City, CA) used for SIVsm/mac-specific real-time RT-PCR (71). Briefly, forward and reverse primers to amplify a 122-bp fragment and an internal fluorogenic probe were generated based on the SIVagm155 sequence (GenBank accession no. M29975) as follows: AgmF, 5′-GTC CAG TCT CAG CAT TTA CTT G-3′ (nucleotide 7981); AgmR, 5′-CGG GCA TTG AGG TTT TTC AC-3′ (nucleotide 8090); and probe, 5′-R-CAG ATG TTG AAG CTG ACC ATT TGG GQ-3′ (nucleotide 8041), where R indicates a 6-carboxyfluorescein group and Q indicates a 6-carboxytetramethylrhodamine group conjugated through a linker arm nucleotide linkage. Previous studies have shown that this primer-probe set amplifies divergent SIVagmVer isolates (19).
STLV and SIV serology.
Serology for antibodies to SIVagm was performed by Western blot analysis, as described previously (18). Briefly, virus was pelleted from cell-free supernatant of CEMss cells infected with SIVagm90. Virus particles were disrupted in Laemmli sample buffer, and viral proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. Individual strips containing SIV proteins were reacted with diluted vervet AGM plasma and washed to remove unbound material. The bound SIV-specific antibodies were visualized by subsequent reaction with ImmunoPure A/G protein conjugated with alkaline phosphatase (Pierce Biotechnology, Rockford, IL), followed by the nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Serology for antibodies to simian T-lymphotropic virus type 1 (STLV-1) was performed by enzyme-linked immunosorbent assay (BioReliance, Rockville, MD). To confirm SIV infection of naturally infected vervet AGM, virus isolation was attempted by coculture of phytohemagglutinin- and interleukin-2 (IL-2)-stimulated peripheral blood mononuclear cells (PBMC) with CEMss cells as previously described (19). Cultures were monitored weekly for supernatant reverse transcriptase activity for up to 6 weeks of culture. Virus was isolated from all of the SIV-seropositive vervet AGM and none of the seronegative vervet AGM.
Monoclonal antibodies (MAbs) and immunophenotyping of lymphocytes.
The antibodies used in this study were purchased from BD Biosciences (San Jose, CA) or Beckman Coulter (Miami, FL). The anti-programmed death 1 (PD-1) antibody (EH12) was recently described and is available from Biolegend (13). The antibodies used in this study were anti-tumor necrosis factor alpha (TNF-α)-fluorescein isothiocyanate (MAb11; BD Biosciences), anti-CD95-phycoerythrin (DX2; BD Biosciences), anti-IFN-γ-phycoerythrin-Cy7 (B27; BD Biosciences), anti-CD28-peridinin chlorophyll protein-Cy5.5 (L293; BD Biosciences), anti-IL-2-allophycocyanin (MQ1-17H12; BD Biosciences), anti-CD4-AmCyan (L200; BD Biosciences), anti-CD3-Pacific blue (SP34-2; BD Biosciences), anti-CD8α-allophycocyanin-Cy7 (SK1; BD Biosciences), anti-CD8αβ-energy-coupled dye (2ST8.5H7; Beckman Coulter), anti-PD-1-phycoerythrin (EH12-2H7), anti-Ki-67-fluorescein isothiocyanate (B56; BD Biosciences), and anti-granzyme B-Alexa Fluor 700 (GB11; BD Biosciences).
Whole-blood samples were stained for 15 min with antisurface antibodies (CD3, CD4, CD8α, CD8αβ, CD28, CD95, and PD-1). Red blood cells were lysed by a TQ-Prep instrument (Beckman Coulter), and the cells were washed with phosphate-buffered saline (PBS). Cells were then fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences) and stained with antibodies specific for Ki-67 and granzyme B. Labeled cells were fixed in 1.5% formaldehyde-PBS. Samples were collected on an LSR II instrument (BD Biosciences) and analyzed using FlowJo software (TreeStar Inc., Ashland, OR).
The cross-reactivity of the anti-PD-1 antibody against vervet AGM PD-1 was tested by staining of stimulated vervet AGM PBMC. Purified PBMC (2 × 106) were stimulated for 2 days in RPMI (Invitrogen) supplemented with 10% fetal calf serum (FCS), IL-2 (20 U/ml), and concanavalin A (5 μg/ml).
IFN-γ ELISPOT assays.
For ELISPOT assays, 96-well Multiscreen HA plates (Millipore, Bedford, MA) were coated by overnight incubation (100 μl/well) at 4°C with mouse anti-human IFN-γ MAb (B27; BD Biosciences) at 5 μg/ml in PBS. Plates were washed with PBS and blocked for 2 h at 37°C with 100 μl/well of RPMI 1640 medium containing 10% FCS. Purified PBMC were plated in triplicate at 2 × 105/well in a 100-μl final volume with medium alone or a peptide pool of 2 μg/ml SIVagmVer Gag peptide or 2 μg/ml of SIVagmVer9063 Env peptide. The peptide pools for stimulation of vervet AGM-derived PBMC consisted of overlapping 15-mer peptides spanning the SIVagmVer Env protein or the Gag protein (Mimotopes, Clayton, Australia, and NIH/NIAID Reagent Resource Support Program for AIDS Vaccine Development, Quality Biological, Inc. [R. L. Brown, principal investigator]). The peptide pools for stimulation of RM-derived PBMC consisted of overlapping 15-mer peptides spanning the SIVmac251 Env protein or Gag protein (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Germantown, MD). After 18 h of incubation at 37°C, the plates were washed free of cells with PBS-0.05% Tween 20 and double-distilled water and incubated for 2 h at room temperature with 100 μl of biotinylated rabbit polyclonal anti-human IFN-γ MAb (BD Biosciences) per well at 2 μg/ml. Plates were washed, and 100 μl of streptavidin-alkaline phosphatase (Southern Biotechnology Associates, Birmingham, AL) was added at a 1/500 dilution. After a 2-h incubation, plates were washed and developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate chromogen (Pierce Biotechnology, Rockford, IL). Plates were read on an ImmunoSpot analyzer (Cellular Technology, Cleveland, OH) using Cellular Technologies Ltd. (CTL) ImmunoSpot image processing software. The median number of spots from triplicate wells was calculated for each responder animal and adjusted to represent the median number of spots per 106 cells. Data are presented as the median number of spots per 106 PBMC. All data are reported after background correction. Only wells with spot numbers higher than twice the standard deviation of spots in the medium controls were counted as positive.
PBMC stimulation and ICS.
For intracellular cytokine staining (ICS) assays, purified PBMC were isolated from heparin-anticoagulated blood by Ficoll gradient separation. PBMC were then incubated at 37°C for 6 h in the presence of RPMI 1640-10% FCS alone (unstimulated), pools of 15-mer Gag or Env peptides (5 μg/ml [each peptide]), or phorbol 12-myristate 13-acetate (10 ng/ml; Sigma-Aldrich) and ionomycin (1 μg/ml; Sigma-Aldrich) as a positive control. All cultures contained monensin (GolgiStop; BD Biosciences) as well as 1 μg/ml anti-CD49d (BD Biosciences) and 1 μg/ml anti-CD28 (BD Biosciences). The cultured cells were stained with MAbs (as described above) specific for cell surface molecules (CD3, CD4, CD8α, and CD8αβ). Cells were then fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences) and stained with antibodies specific for IFN-γ, TNF-α, and IL-2. Labeled cells were fixed in 1.5% formaldehyde-PBS. Samples were collected on an LSR II instrument (BD Biosciences) and analyzed using FlowJo software (TreeStar Inc.). At least 200,000 events were collected per sample. The background level of cytokine staining varied between different samples and different cytokine patterns but was <0.15% of the CD8+ T cells (median, 0.05%). All data are reported after background correction. The only samples considered positive were those in which the percentage of cytokine-staining cells was at least twice the standard deviation of the background.
Statistical analyses.
Statistical analyses and graphical presentations were computed with GraphPad Prism (GraphPad Prism Software, La Jolla, CA). P values of <0.05 were considered significant. Mann-Whitney tests were applied for comparison of two groups. Multigroup comparisons were performed with the Kruskal-Wallis test. If the Kruskal-Wallis test showed a significant difference, a Dunn's posttest was applied to determine the level of significance between two individual groups. A Spearman correlation test was performed to analyze the association between plasma viral RNA loads and various parameters (including absolute CD4+ T-cell counts and ratio of naive/memory CD4+ T cells).
RESULTS
Characterization of the vervet AGM study group.
The 33 vervet AGM that were studied are listed in Table 1. All 15 seronegative vervet AGM were also negative by virus isolation from PBMC and plasma viral RNA. SIV-infected vervet AGM included 11 naturally infected animals and seven infected with either SIVagmVer90 or blood from a naturally infected vervet AGM, Ver1, as previously described (18). All infected monkeys were positive by SIV serology, virus isolation from PBMC, and plasma viral RNA assay with a median level of plasma viral RNA of 56,750 copies/ml (range, 2,600 to 838,379) (Table 1). No significant difference (P = 0.77; Mann-Whitney test) was detected in absolute CD4+ T-cell counts between SIV-infected (median, 234 cells/ml; range, 38 to 869) and SIV-negative (median, 287; range, 109 to 1,399) vervet AGM (Table 1).
TABLE 1.
Cohort of vervet AGMa
| Animal | SIVagmVer strain | No. of yrs p.i. | Plasma SIVagmVer RNA (no. of copies/ml) | SIVagmVer serology | SIVagm RT activity | STLV serology | CD4+ T cells (no. of cells/μl) |
|---|---|---|---|---|---|---|---|
| Ver1 | Natural | UK | 161,000 | + | + | − | 206 |
| Ver5 | Natural | UK | 32,700 | + | + | − | 245 |
| Ver10 | Natural | UK | 36,700 | + | + | − | 147 |
| Ver11 | Natural | UK | 2,600 | + | + | − | 116 |
| Ver18 | Natural | UK | 43,800 | + | + | − | 504 |
| Ver3 | Natural | UK | 69,700 | + | + | + | 296 |
| Ver4 | Natural | UK | 11,800 | + | + | + | 477 |
| Ver6 | Natural | UK | 75,400 | + | + | + | 155 |
| Ver12 | Natural | UK | 21,400 | + | + | + | 38 |
| Ver16 | Natural | UK | 13,200 | + | + | + | 228 |
| Ver17 | Natural | UK | 76,800 | + | + | + | 228 |
| Ver20 | SIVagm90 | 3.5 | 31,559 | + | + | − | 239 |
| Ver22 | SIVagm90 | 3.5 | 838,379 | + | + | − | 361 |
| Ver24 | SIVagm90 | 3.5 | 126,595 | + | + | − | 267 |
| Ver2 | SIVagm1 | 3.5 | 166,278 | + | + | − | 174 |
| Ver8 | SIVagm1 | 3.5 | 3,366 | + | + | − | 212 |
| Ver14 | SIVagm1 | 3.5 | 84,472 | + | + | − | 239 |
| Ver19 | SIVagm1 | 3.5 | 147,445 | + | + | − | 869 |
| Ver7 | Naive | NA | <100 | − | − | − | 491 |
| Ver9 | Naive | NA | <100 | − | − | − | 109 |
| Ver13 | Naive | NA | <100 | − | − | − | 194 |
| Ver23 | Naive | NA | <100 | − | − | − | 1,399 |
| Ver302 | Naive | NA | <100 | − | − | − | 263 |
| Ver346 | Naive | NA | <100 | − | − | − | 544 |
| Ver731 | Naive | NA | <100 | − | − | − | 173 |
| Ver5339 | Naive | NA | <100 | − | − | − | 226 |
| Ver5417 | Naive | NA | <100 | − | − | − | 206 |
| Ver5419 | Naive | NA | <100 | − | − | − | 488 |
| Ver5441 | Naive | NA | <100 | − | − | − | 430 |
| Ver5506 | Naive | NA | <100 | − | − | − | 295 |
| Ver5387 | Naive | NA | <100 | − | − | + | 142 |
| Ver5431 | Naive | NA | <100 | − | − | + | 324 |
| Ver5504 | Naive | NA | <100 | − | − | + | 287 |
Abbreviations: UK, unknown; NA, not applicable; p.i., postinfection; RT, reverse transcriptase.
T-cell subsets in vervet AGM and RM.
As previously reported by others, almost all T cells in vervet AGM express the CD8α molecule at various levels (45). To characterize the different T-cell subsets in vervet AGM, we first gated on CD4+ CD8α dimly positive T cells (referred to as CD4+ T cells) or CD4− CD8α intermediately or brightly positive T cells. The CD4− CD8α+ T cells were then further subdivided into CD8αα homodimer-expressing cells and CD8αβ heterodimer-expressing cells (data not shown). The CD8+ T-cell subsets, CD8αα+ and CD8αβ+ T cells, were then further subdivided into naive (CD28+ CD95−), central memory (CM; CD28+ CD95+), and effector memory (EM; CD28− CD95+) T cells according to their expression of CD28 and CD95 (62).
We compared the distribution of maturation-associated T-cell subsets in SIV-negative (n = 12) and SIV-infected (n = 18) vervet AGM to that in SIV-negative (n = 63) and SIVmac251-infected Mamu-A*01-negative (n = 20) RM. We chose to use Mamu-A*01-negative RM for comparison to exclude those RM that may show particularly strong SIV-specific CD8+ T-cell responses that could potentially bias our comparative investigations (43, 44).
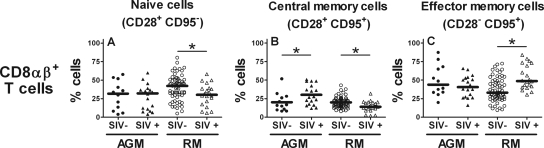
In RM, the percentage of naive and CM CD8αβ+ T cells was lower in SIV-infected than in noninfected animals (naive, P = 0.03; CM, P = 0.006; Mann-Whitney test) (Fig. 1A and B), whereas EM CD8αβ+ T cells were significantly more abundant in SIV-infected animals than in noninfected animals (P = 0.01; Mann-Whitney test) (Fig. 1C). In contrast, SIV-infected vervet AGM showed a significantly higher percentage of CM CD8αβ+ T cells in SIV-infected than in noninfected animals (P = 0.04; Mann-Whitney test) but no difference in the percentage of naive or EM CD8αβ+ T cells between SIV-infected and noninfected animals. In addition, we did not observe a significant difference in the percentages of CD8αα+ T-cell subsets between noninfected and SIV-infected vervet AGM (data not shown).
FIG. 1.
Maturation-associated CD8αβ+ T-cell subsets in uninfected vervet AGM and RM and vervet AGM and RM chronically infected with SIV. Whole-blood samples from vervet AGM and RM were stained with anti-CD3, anti-CD4, anti-CD8α, anti-CD8αβ, anti-CD28, and anti-CD95 MAbs and analyzed by multicolor flow cytometry. The stars indicate a statistically significant difference (P < 0.05; Mann-Whitney test) between uninfected and SIV-infected monkeys (investigated in each of the two monkey species separately).
SIV-specific T-cell responses in vervet AGM.
To assess the quality and quantity of cellular immune responses in SIV-infected vervet AGM, we examined cytokine responses following stimulation with SIV Gag and Env peptide pools. To maximize the sensitivity of IFN-γ detection following SIV antigen-specific stimulation, we used an ELISPOT assay. In addition, we performed an ICS assay following SIV-specific antigen stimulation to simultaneously detect IFN-γ, IL-2, and TNF-α production by different T-cell subsets.
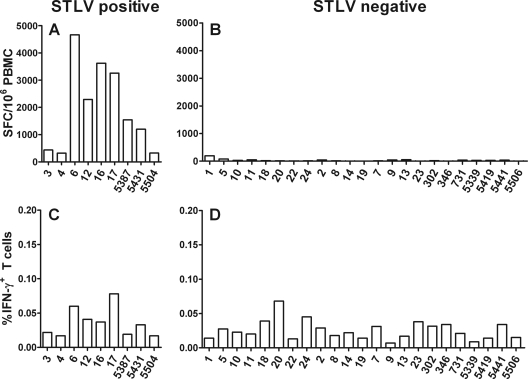
The medium control in the ELISPOT assay from some vervet AGM resulted in a high number of spot-forming cells (SFC) (Fig. 2A and B). For humans, high backgrounds in cytokine detection assays were observed as a result of nonspecific T-cell activation following human T-lymphotropic virus (HTLV) infection (21, 47, 49). Therefore, we tested the animals for antibodies against STLV. The animals with a high IFN-γ response in the medium control wells in the ELISPOT assay (320 to 4,668 SFC/106 cells) were indeed all positive for anti-STLV antibodies (Fig. 2A). The STLV-negative animals showed only a weak background production of IFN-γ in the control wells (0 to 195 SFC/106 cells). HTLV-infected CD4+ T cells spontaneously produce IFN-γ when the T cells are cultured for more than 6 h (21, 47, 49). Therefore, we believe that the high background in the ELISPOT assay was probably due to spontaneous IFN-γ production by CD4+ T cells during the 18-h incubation period. The high background IFN-γ production was not observed in the ICS assay, probably due to the shorter incubation period of 6 h (Fig. 2C and D). Therefore, all STLV-coinfected vervet AGM were excluded from further examination by the IFN-γ ELISPOT assay but not from the ICS assay.
FIG. 2.
Background of IFN-γ ELISPOT assay and ICS in PBMC from vervet AGM. PBMC were isolated by Ficoll gradient centrifugation and either used in an IFN-γ ELISPOT assay or stained for intracellular IFN-γ cytokine production and analyzed by flow cytometry. Cells were cultured in RPMI supplemented with 10% FCS for 18 h at 37°C for the ELISPOT assay and for 6 h at 37°C for ICS without additional peptide or mitogen stimulation. (A and B) Numbers of SFC in 106 cells detected by ELISPOT assay in either STLV-positive (A) or STLV-negative (B) vervet AGM regardless of their SIV status. (C and D) Percentages of intracellular IFN-γ+ T cells detected by flow cytometry in either STLV-positive (C) or STLV-negative (D) vervet AGM regardless of their SIV status.
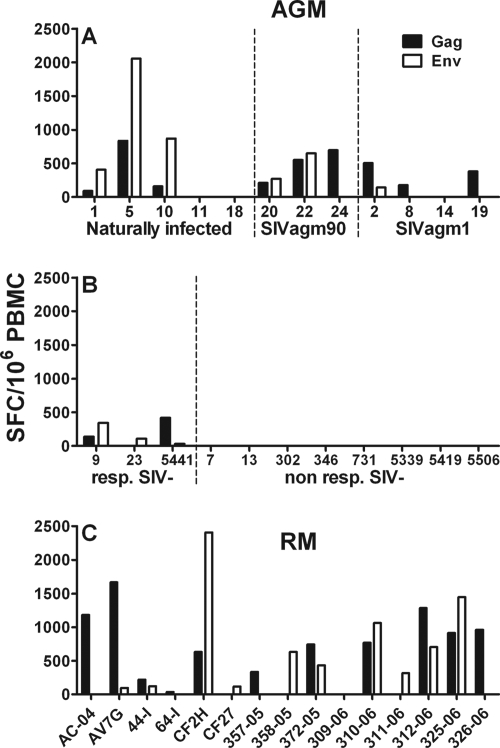
To analyze SIV-specific immune responses in lymphocytes obtained from STLV-negative vervet AGM by the ELISPOT assay, we stimulated the cells with SIVagmVer Gag and Env peptide pools. Three of the 12 SIV-infected vervet AGM did not show any responses to either Gag or Env peptide pools (Ver11, Ver14, and Ver18) (Fig. 3A). The remaining nine animals all responded to stimulation with the Gag peptide pool, but only six animals showed responses to the Env peptide pools. All responses, except for the Env responses of Ver5, were below 1,000 SFC/106 PBMC. No differences in the magnitude and breadth of SIV Gag and Env responses were observed between the naturally infected and experimentally infected vervet AGM. Interestingly, 3 of the 11 noninfected vervet AGM showed IFN-γ responses following stimulation with SIV peptides (Fig. 3B). The responses were low but above the threshold of twice the standard deviation of the mean of the medium control. To ensure that this observation was not due to an artifact in the peptide preparation, we performed additional IFN-γ ELISPOT assays on all vervet AGM with a Gag peptide pool that was obtained from another manufacturer, and we observed comparable results (data not shown).
FIG. 3.
SIV-specific IFN-γ cytokine release in PBMC obtained from SIV-negative and SIV-infected vervet AGM and SIVmac-infected RM. (A and B) PBMC from 12 SIV-infected (A) and 11 SIV-negative (B) vervet AGM were stimulated with SIVagm-specific Gag and Env peptide pools for 18 h at 37°C. (A) Animals were divided into three groups, according to infection history and different strains of virus used for infection. (B) Animals were separated into two groups: SIV-negative animals that showed responses (resp. SIV-) and animals that showed no responses (non resp. SIV-). (C) PBMC from 15 SIVmac-infected RM were stimulated with SIVmac-specific Gag and Env peptide pools for 18 h at 37°C. The bars represent the numbers of SFC in 106 PBMC in response to the Gag (black) and Env (white) peptide pools. Responses were considered positive if their value was greater than twice the standard deviation of the mean of the medium control wells. All values were background corrected using the medium controls. Responses and background were determined separately for cells obtained from vervet AGM and RM.
The RM showed more consistent immune responses in the IFN-γ ELISPOT assays (Fig. 3C). The median IFN-γ responses in RM were slightly higher than those in vervet AGM without, however, reaching statistical significance (SIV Gag peptide pool in RM, 630 SFC/106 cells [range, 0 to 1,665]; SIV Gag peptide pool in vervet AGM, 94 SFC/106 cells [range, 0 to 837] [Mann-Whitney test, P = 0.11]; SIV Env peptide pool in RM, 120 SFC/106 cells [range, 0 to 2,405]; SIV Env peptide pool in vervet AGM, 73 SFC/106 cells [range, 0 to 2,058] [Mann-Whitney test, P = 0.18]).
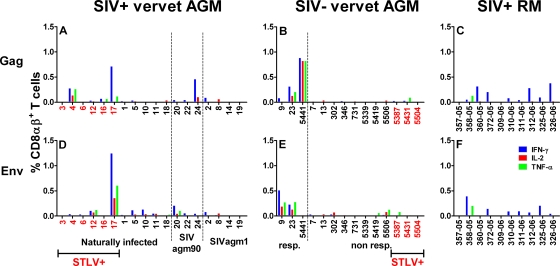
To further elucidate the SIV-specific immune responses in vervet AGM, we stained SIV peptide-stimulated T cells intracellularly with antibodies against IL-2, IFN-γ, and TNF-α. The cells were first gated on CD3+ and CD8αβ+ T cells. CD8αβ+ T-cell responses against Gag and Env peptide pools were relatively weak in SIV-infected vervet AGM (Fig. 4A and D). CD8αα+ T cells from SIV-infected and noninfected vervet AGM had cytokine responses comparable to, but generally lower than, those of CD8αβ+ T cells (data not shown). The highest responses for all three cytokines were observed in Ver17, which was coinfected with STLV. Another STLV-infected AGM, Ver4, also showed above-average responses for IFN-γ, IL-2, and TNF-α. Surprisingly, three of the noninfected vervet AGM had high cytokine responses when stimulated with Gag and/or Env. These animals also produced IFN-γ in the ELISPOT assay after SIV peptide stimulation. Nevertheless, all methods used to detect SIV or STLV in these animals were negative. Since we could not completely rule out the possibility that these three vervet AGM were infected with SIV, we excluded these animals from all phenotyping experiments.
FIG. 4.
Intracellular expression of IFN-γ, IL-2, and TNF-α in SIV Gag and Env peptide-stimulated CD8αβ+ T cells. Freshly isolated PBMC were stimulated for 6 h at 37°C in the presence of either SIVagmVer- or SIVmac-specific peptide pools. The intracellular staining in CD8αβ+ T cells stimulated with Gag or Env peptide pools is shown for SIV-infected vervet AGM (A and D), SIV-negative vervet AGM (B and E), and SIVmac-infected RM (C and F). (A and D) Animals were divided into three groups according to infection histories and the different strains used for infection. (B and E) Animals were separated into two groups: SIV-negative animals that showed responses (resp.) and animals that showed no responses (non resp.). In panels A, B, D, and E, vervet AGM positive for STLV are marked in red. The blue bars represent the percentages of IFN-γ+ CD8αβ+ T cells, the red bars indicate the percentages of cells staining positive for IL-2, and the green bars represent the percentages of cells staining positive for TNF-α. Responses were considered positive if their value was greater than twice the standard deviation of the mean of the medium control wells. All values were background corrected using the medium controls. Responses and background were determined separately for cells obtained from vervet AGM and RM.
Eight of 10 SIV-infected RM were positive for intracellular IFN-γ production after stimulation with Gag or Env peptide pools. Only one animal also produced TNF-α after stimulation with both peptide pools. The median IFN-γ responses following stimulation with SIV Gag and Env peptide pools in SIV-infected RM (SIV Gag, 0.09% [range, 0% to 0.38%]; SIV Env, 0.08% [range: 0% to 0.39%]) were again slightly higher than those of STLV-negative SIV-infected vervet AGM (SIV Gag, 0.01% [range, 0% to 0.46%]; SIV Env, 0.05% [range, 0% to 0.20%]). The comparison of the SIV Gag responses approached an almost significant difference between SIV-infected vervet AGM and RM (P = 0.08, Mann-Whitney test). No statistically significant differences were detected in the comparison of SIV Env responses (P = 0.51, Mann-Whitney test).
In summary, although it did not reach a statistically significant level, a trend of lower immune responses against SIV Gag and Env was detected in vervet AGM chronically infected with SIV compared to RM chronically infected with SIV.
Lack of increased proliferation and granzyme B expression in CD8+ T cells from SIV-infected vervet AGM.
Chronic immune activation in HIV infection and in SIV infection of Asian nonhuman primates is characterized by elevated T-cell proliferation. The nuclear expression of the Ki-67 molecule is widely accepted as a marker for recent T-cell proliferation in HIV-infected individuals (42). We analyzed Ki-67 expression in CM and EM CD8+ T-cell subsets in vervet AGM and RM by intracellular staining. In general, we found that Ki-67 levels in RM were markedly higher than those in vervet AGM CD8+ T cells, even in noninfected RM (Table 2). CD8αβ+ T cells from SIV-infected RM (n = 15) expressed about twice as much Ki-67 as did those from noninfected RM (n = 28) (Table 2). This was observed in CM as well as in EM CD8αβ+ T-cell subsets (CM, P < 0.0001; EM, P = 0.0012; Mann-Whitney test). In contrast, noninfected and SIV-infected vervet AGM showed only a marginal difference in Ki-67 expression on CD8αβ+ T cells (median Ki-67 expression in CM, 5.4% [SIV negative] and 7.7% [SIV positive]; median Ki-67 expression in EM, 4.0% [SIV negative] and 5.8% [SIV positive]) (Table 2). Similarly, CM and EM CD8αα+ T cells from noninfected and SIV-infected vervet AGM also showed only a marginal difference in Ki-67 expression (median Ki-67 expression in CM, 5.4% [SIV negative] and 5.7% [SIV positive]; median Ki-67 expression in EM, 4.3% [SIV negative] and 6.1% [SIV positive]) (Table 2). Ki-67 expression was not analyzed for the CD8αα+ T-cell subsets in RM since these T-cell subsets were present in only a very small percentage of RM.
TABLE 2.
Ki-67 expression in memory T-cell subsets of vervet AGM and RM
| T-cell subset and memory type | Median percent Ki-67 expression (range)
|
|||
|---|---|---|---|---|
| AGM
|
RM
|
|||
| SIV− | SIV+ | SIV− | SIV+ | |
| CD8αβ+ | ||||
| CM | 5.4 (2.1-12.3) | 7.7 (3.7-10.9) | 17.6 (11.1-35.3) | 30.6 (11.8-42.5) |
| EM | 4.0 (1.1-9.6) | 5.8 (3.1-10.4) | 12.1 (6.6-26.5) | 26.9 (10.7-54.9) |
| CD8αα+ | ||||
| CM | 5.4 (2.2-12.8) | 5.7 (3.7-8.5) | NAa | NA |
| EM | 4.3 (2.4-10.6) | 6.1 (2.4-10.9) | NA | NA |
NA, not applicable.
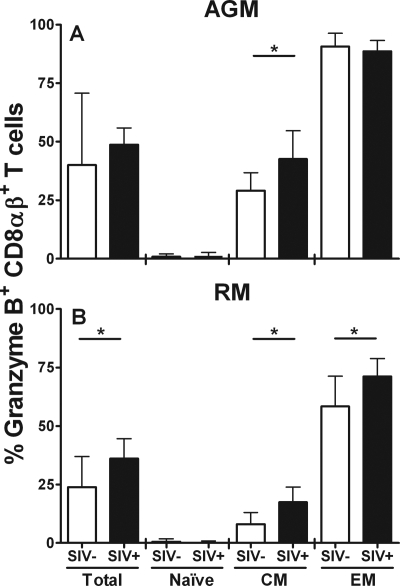
Granzyme B is a serine protease that is produced by CTL. It is stored in a nonactive form in lytic granules of CTL and is released in conjunction with other effector proteins, including perforin, upon activation (39, 75). The granzyme B content of CTL correlates with effector function in vivo (10). We performed an intracellular granzyme B staining of CD8+ T cells from vervet AGM in combination with anti-CD28 and anti-CD95 antibodies to distinguish between naive, CM, and EM cells. There was no significant difference between the expression of granzyme B in total CD8αβ+ T cells from SIV-infected vervet AGM and that in cells from noninfected animals (P = 0.57; Mann-Whitney test) (Fig. 5A). However, SIV-infected vervet AGM had a significantly higher level of granzyme B-expressing CM CD8αβ+ T cells than did noninfected animals (P = 0.03; Mann-Whitney test). Almost all EM CD8αβ+ T cells from vervet AGM expressed granzyme B regardless of whether the animals were SIV infected. As expected, granzyme B expression levels in vervet AGM were highest in EM, intermediate in CM, and almost absent in naive CD8αβ+ T cells. A similar pattern of granzyme B expression was also observed in CD8αα+ T cells from vervet AGM (data not shown).
FIG. 5.
Granzyme B expression in CD8αβ+ T cells obtained from SIV-negative and SIV-infected vervet AGM and RM. Whole-blood samples from 12 SIV-negative vervet AGM, 18 SIV-infected vervet AGM, 18 SIV-negative RM, and 15 SIVmac-infected RM were stained for intracellular expression of granzyme B in maturation-associated CD8αβ+ T-cell subsets. (A) Granzyme B expression in total CD8αβ+ T cells, naive, and CM and EM CD8αβ+ T cells obtained from vervet AGM; (B) results obtained from RM cells. The black bars represent SIV-infected animals, and the white bars represent SIV-negative animals. All bars represent median values, and the error bars show the interquartile ranges. The stars indicate a statistically significant difference between SIV-negative and SIV-infected animals (P < 0.05; Mann-Whitney test).
In RM, total CD8αβ+ T cells and the CM and EM subsets of CD8αβ+ T cells obtained from SIV-infected animals had significantly higher granzyme B expression levels than did cells from noninfected animals (total CD8αβ+ T cells, P = 0.04; CM CD8αβ+ T cells, P = 0.001; EM CD8αβ+ T cells, P = 0.02; Mann-Whitney test) (Fig. 5B). Similar to vervet AGM, the highest expression of granzyme B was observed in EM cells and an intermediate expression was seen in CM cells; meanwhile, naive cells, as expected, were almost completely negative. In general, vervet AGM showed constitutively higher levels of granzyme B expression than did RM. In particular, almost all EM CD8αβ+ T cells from vervet AGM expressed granzyme B. Thus, SIV infection of vervet AGM only marginally influenced the expression pattern of granzyme B on CD8αβ+ T cells (total CD8αβ+ T cells, P = 0.57; CM CD8αβ+ T cells, P = 0.03; EM CD8αβ+ T cells, P = 0.92; Mann-Whitney test).
PD-1 expression pattern in SIV-infected vervet AGM is similar to that of SIV-infected RM.
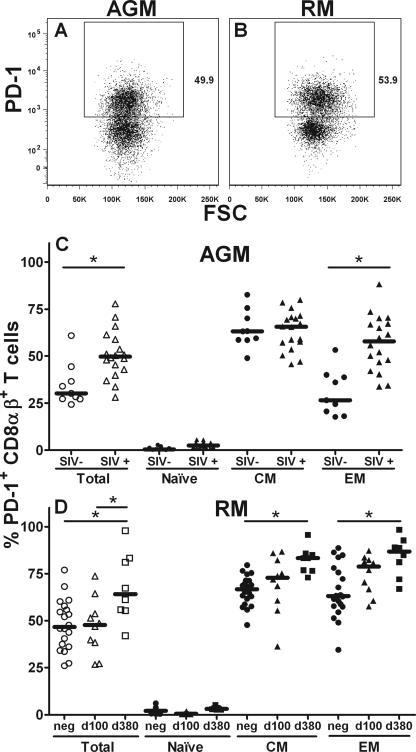
The PD-1 surface molecule is a member of the B7:CD28 family of costimulatory molecules that regulate T-cell activation (22). PD-1 upregulation is associated with exhaustion of HIV-specific T cells and disease progression (11, 72, 76). In vitro blockage of PD-1 interaction with its ligand PD-L1 leads to a partial functional restoration of virus-specific CTL in AIDS virus-infected human and Asian nonhuman primates (16, 60, 72, 74). To compare cross-reactivities of the PD-1 antibody in vervet AGM and RM, we performed staining of freshly isolated CD8αβ+ T cells from both species with PD-1 antibody (Fig. 6A and B). The fluorescent signal intensities of the phycoerythrin-coupled PD-1 antibody on CD8αβ+ T cells from the two species were similar.
FIG. 6.
PD-1 molecule expression in CD8αβ+ T cells obtained from SIV-negative and SIV-infected vervet AGM and RM. CD8αβ+ T cells from vervet AGM and RM were stained with an anti-human PD-1 antibody conjugated to phycoerythrin. (A and B) Representative examples for vervet AGM (A) and RM (B) are shown. Whole-blood samples obtained from nine SIV-negative vervet AGM, 18 SIV-infected vervet AGM, 20 SIV-negative RM, and 18 SIV-infected RM were stained for PD-1 expression in maturation-associated CD8αβ+ T-cell subsets. (C) Expression of PD-1 in CD8αβ+ T cells obtained from SIV-negative (circles) and SIV-infected (triangles) vervet AGM. (D) Expression of PD-1 in CD8αβ+ T cells obtained from SIV-negative RM (circles) and RM infected with SIV for either 100 days (triangles) or 380 days (squares). The open symbols represent the percentages of PD-1-positive cells in total CD8αβ+ T cells, and the filled symbols represent the percentages of PD-1-positive cells in CD8αβ+ T-cell subsets. The stars indicate a statistically significant difference (Mann-Whitney test [C] or Kruskal-Wallis test and a Dunn's posttest [D]).
Next, we analyzed the PD-1 expression in CD8αβ+ T cells obtained from vervet AGM chronically infected with SIV and noninfected vervet AGM. Total CD8αβ+ T cells obtained from SIV-infected vervet AGM expressed significantly higher levels of PD-1 than did noninfected animals (P = 0.004; Mann-Whitney test) (Fig. 6C). This observation was mainly a result of higher PD-1 expression on EM CD8αβ+ T cells of SIV-infected vervet AGM than on cells of noninfected vervet AGM (P = 0.0006; Mann-Whitney test). CM CD8αβ+ T cells of SIV-infected and noninfected vervet AGM expressed comparable levels of PD-1. As expected, naive CD8αβ+ T cells did not express significant amounts of PD-1 (Fig. 6C). CD8αα+ T cells showed similar patterns of PD-1 expression in SIV-infected and noninfected vervet AGM (data not shown), with high PD-1 expression in both groups.
Interestingly, PD-1 expression on CD8αβ+ T cells from noninfected RM was significantly higher (Fig. 6D) than that on cells from noninfected vervet AGM (P = 0.02; Mann-Whitney test), and the expression observed in RM was of a magnitude comparable to that in a recently published study by Petrovas et al. (60). Total CD8αβ+ T cells from RM that were SIV infected for 100 days did not express higher PD-1 levels than did noninfected animals (Fig. 6D). RM that were SIV infected for 380 days, however, expressed significantly higher levels of PD-1 than did noninfected animals (P = 0.01; Mann-Whitney test). Similar patterns of PD-1 expression were observed in the CM and EM CD8αβ+ T-cell subsets. Expression of PD-1 was significantly higher in RM infected with SIV for 380 days (CM, P ≤ 0.05; EM, P ≤ 0.05; Kruskal-Wallis test) but only slightly higher in RM infected for 100 days (CM, P > 0.05; EM, P > 0.05; Kruskal-Wallis test) than in noninfected animals. Naive CD8αβ+ T cells from all three groups of RM expressed very low levels of PD-1.
In summary, the PD-1 staining experiments showed that chronic SIV infection resulted in upregulation of PD-1 expression on memory CD8+ T cells in both species. However, this upregulation was more consistently observed on CM and EM cells from RM than on cells from vervet AGM, where we found an upregulation of PD-1 only on EM cells.
DISCUSSION
CD8+ T-cell responses play a major role in partial viral containment of pathogenic AIDS virus infections in humans and Asian nonhuman primates (6, 28, 34, 35, 37, 38, 41, 66). However, little is known about CD8+ T-cell responses in SIV-infected natural hosts like AGM. Here, we performed comparative analyses to determine phenotypic subset changes and SIV-specific immune responses in CD8+ T cells from SIV-negative and SIV-infected RM and vervet AGM. We found that SIV infection of vervet AGM was associated with weaker immune activation and proliferation than those for RM as evidenced by phenotypic analysis of granzyme B, Ki-67, and PD-1 expression on T cells. In addition, SIV infection of RM resulted in more significant changes in the relative distribution of naive, CM, and EM CD8+ T-cell subsets. SIV-specific CD8+ T-cell responses were variable between animals and showed a trend toward lower levels in SIV-infected vervet AGM than in SIV-infected Mamu-A*01-negative RM.
The Ki-67 protein, which is tightly associated with somatic cell proliferation (14), is one of the key molecules used to demonstrate increased activation and lymphocyte turnover following AIDS virus infection of nonnatural hosts (31, 64, 65). However, vervet AGM chronically infected with SIV showed only a very small increase in Ki-67 on CM and EM CD8+ T cells, indicating the subdued SIVagm-mediated lymphocyte activation that is characteristic of AIDS virus infections in natural hosts (56, 69, 70).
Granzyme B, a critical mediator of cell-mediated cytotoxicity (58), was significantly upregulated in memory CD8+ T cells from SIV-infected RM in contrast to SIV-negative RM. The much smaller change in granzyme B expression on memory CD8+ T cells between uninfected and SIV-infected vervet AGM was partly due to a much higher level of granzyme B expression in EM CD8+ T cells in vervet AGM (already close to 100% in SIV-negative vervet AGM) than in RM. The increased granzyme B expression in vervet AGM may give this species of natural hosts an advantage of more-efficient killing of infected cells.
While SIV infection in vervet AGM was associated with an increased expression of PD-1 on only EM CD8αβ+ T cells, SIV-infected RM showed an increase of PD-1 expression on both CM and EM CD8αβ+ T cells in animals chronically infected with SIV. Recently, it was shown that the interaction of PD-1 with its ligand PD-L1 greatly reduces the activity of virus-specific CD8+ T cells in HIV and lymphocytic choriomeningitis virus infection (3, 11, 16, 59, 72, 76). The higher PD-1 expression is associated with exhaustion of these cells and an inability to properly respond to stimulation by virus-infected cells, which is thought to lead to a more rapid disease progression. The relatively high PD-1 expression of peripheral blood EM CD8αβ+ T cells in vervet AGM chronically infected with SIV suggests that these cells are also in a functionally impaired state. However, vervet AGM do not progress to AIDS and PD-1 expression might have a regulatory role in vervet AGM preventing prolonged, unnecessarily high CTL responses.
Although the differences in SIV Gag and Env ELISPOT responses between RM and vervet AGM did not reach statistical significance, we observed more consistent responses in the IFN-γ ELISPOT assay performed on RM. We also detected about six-times-higher median levels of SIV Gag responses and about two-times-higher median levels of SIV Env responses in RM than in vervet AGM. A similar pattern emerged when we performed the ICS assay: SIV-specific Gag and Env CD8+ T-cell responses were more readily detectable in RM than in vervet AGM.
Greater magnitudes of AIDS virus-specific CD8+ T-cell responses in nonnatural hosts are correlated with a more-controlled course of infection (24, 43, 51). However, an inverse correlation between these two parameters has also been shown (33). In SIV-challenged RM, pathogenicity studies and vaccine trials have suggested that robust, multifunctional CD8+ T-cell responses are correlated with a less-pathogenic course of infection (36, 67). It is evident that the quantity and the quality of immune responses are critical for more-efficient containment of AIDS virus infections (9, 40, 67). The assays performed in the present study did not detect a qualitative difference in the cytokine pattern of the CD8+ T-cell responses between the RM and vervet AGM chronically infected with SIV but did detect a lower number of responding CD8+ T cells in the vervet AGM. CD8+ T cells in vervet AGM may be less functionally impaired; thus, lower cell numbers are sufficient for partial viral control. It is therefore likely that natural hosts of SIV, including vervet AGM, utilize multiple protection mechanisms, in addition to antiviral CD8+ T-cell responses, that allow a “peaceful” coexistence between the lentivirus and its host despite an ongoing relatively high-level viremia (56, 68).
Interestingly, three of the wild-caught SIV-negative vervet AGM exhibited low but consistent levels of immune responses against SIV Gag and Env peptide pools. All assays employed to detect signs of SIV infection including RT-PCR, serology, and SIV reverse transcriptase activity were negative. However, repeated ICS and ELISPOT assays, including the use of an SIVagm Gag peptide pool from a different vendor, resulted in a similar magnitude of responses in these animals. Even combined in vivo depletion of CD8+ and CD20+ cells in two of these animals did not activate SIV replication to a level detectable by our assays (unpublished observation). Thus, these immune responses may be an indication of a previous encounter with SIV that did not result in a productive infection, similar to observations in humans who were exposed to HIV but were not productively infected (29). It is also possible that the animals were previously exposed to an unknown antigen with sequence homology to SIVagm.
In this study, we have demonstrated variable, relatively low-level SIVagm Gag and Env-specific CD8+ T-cell responses in vervet AGM chronically infected with SIV. Since all SIV-infected vervet AGM used in this study experienced an indistinguishable nonpathogenic course of infection regardless of the relative magnitude of SIV-specific CD8+ T-cell responses, the role of immune responses in partial viral containment and lack of development of an AIDS-like disease in natural hosts is difficult to assess. Studies that seek to temporally activate or inhibit innate and/or adaptive immune responses during primary and/or chronic SIVagm infection will aid in determining whether immune responses contribute to viral containment and maintenance of a disease-free course of infection in natural hosts such as vervet AGM.
Acknowledgments
This work was supported by the NIH grants AI065335 (to J.E.S.) and AI56299 (to G.J.F.), NIAID Center for HIV/AIDS Vaccine Immunology (CHAVI) grant AI067854 (to N.L.L.), Harvard Medical School Center for AIDS Research (CFAR) grant AI060354, the Foundation for the NIH through the Grand Challenges in Global Health initiative (G.J.F.), and the Division of Intramural Research, NIAID, NIH (V.M.H., S.G., and C.R.B.).
Footnotes
Published ahead of print on 1 October 2008.
REFERENCES
- 1.Ahmed, R. K., G. Biberfeld, and R. Thorstensson. 2005. Innate immunity in experimental SIV infection and vaccination. Mol. Immunol. 42251-258. [DOI] [PubMed] [Google Scholar]
- 2.Allan, J. S., M. Short, M. E. Taylor, S. Su, V. M. Hirsch, P. R. Johnson, G. M. Shaw, and B. H. Hahn. 1991. Species-specific diversity among simian immunodeficiency viruses from African green monkeys. J. Virol. 652816-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439682-687. [DOI] [PubMed] [Google Scholar]
- 4.Barry, A. P., G. Silvestri, J. T. Safrit, B. Sumpter, N. Kozyr, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2007. Depletion of CD8+ cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species. J. Immunol. 1788002-8012. [DOI] [PubMed] [Google Scholar]
- 5.Beer, B., M. Baier, J. zur Megede, S. Norley, and R. Kurth. 1997. Vaccine effect using a live attenuated nef-deficient simian immunodeficiency virus of African green monkeys in the absence of detectable vaccine virus replication in vivo. Proc. Natl. Acad. Sci. USA 944062-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broussard, S. R., S. I. Staprans, R. White, E. M. Whitehead, M. B. Feinberg, and J. S. Allan. 2001. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 752262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5233-236. [DOI] [PubMed] [Google Scholar]
- 9.Calarota, S. A., A. Foli, R. Maserati, F. Baldanti, S. Paolucci, M. A. Young, C. M. Tsoukas, J. Lisziewicz, and F. Lori. 2008. HIV-1-specific T cell precursors with high proliferative capacity correlate with low viremia and high CD4 counts in untreated individuals. J. Immunol. 1805907-5915. [DOI] [PubMed] [Google Scholar]
- 10.Curtsinger, J. M., D. C. Lins, C. M. Johnson, and M. F. Mescher. 2005. Signal 3 tolerant CD8 T cells degranulate in response to antigen but lack granzyme B to mediate cytolysis. J. Immunol. 1754392-4399. [DOI] [PubMed] [Google Scholar]
- 11.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 12.Diop, O. M., A. Gueye, M. Dias-Tavares, C. Kornfeld, A. Faye, P. Ave, M. Huerre, S. Corbet, F. Barre-Sinoussi, and M. C. Muller-Trutwin. 2000. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J. Virol. 747538-7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorfman, D. M., J. A. Brown, A. Shahsafaei, and G. J. Freeman. 2006. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 30802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endl, E., and J. Gerdes. 2000. The Ki-67 protein: fascinating forms and an unknown function. Exp. Cell Res. 257231-237. [DOI] [PubMed] [Google Scholar]
- 15.Ennen, J., H. Findeklee, M. T. Dittmar, S. Norley, M. Ernst, and R. Kurth. 1994. CD8+ T lymphocytes of African green monkeys secrete an immunodeficiency virus-suppressing lymphokine. Proc. Natl. Acad. Sci. USA 917207-7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman, G. J., E. J. Wherry, R. Ahmed, and A. H. Sharpe. 2006. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 2032223-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gicheru, M. M., M. Otsyula, P. Spearman, B. S. Graham, C. J. Miller, H. L. Robinson, N. L. Haigwood, and D. C. Montefiori. 1999. Neutralizing antibody responses in Africa green monkeys naturally infected with simian immunodeficiency virus (SIVagm). J. Med. Primatol. 2897-104. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein, S., C. R. Brown, I. Ourmanov, I. Pandrea, A. Buckler-White, C. Erb, J. S. Nandi, G. J. Foster, P. Autissier, J. E. Schmitz, and V. M. Hirsch. 2006. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T-cell dynamics in vervet and sabaeus African green monkeys. J. Virol. 804868-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein, S., I. Ourmanov, C. R. Brown, B. E. Beer, W. R. Elkins, R. Plishka, A. Buckler-White, and V. M. Hirsch. 2000. Wide range of viral load in healthy African green monkeys naturally infected with simian immunodeficiency virus. J. Virol. 7411744-11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein, S., I. Ourmanov, C. R. Brown, R. Plishka, A. Buckler-White, R. Byrum, and V. M. Hirsch. 2005. Plateau levels of viremia correlate with the degree of CD4+-T-cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J. Virol. 795153-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goon, P. K. C., E. Hanon, T. Igakura, Y. Tanaka, J. N. Weber, G. P. Taylor, and C. R. M. Bangham. 2002. High frequencies of Th1-type CD4+ T cells specific to HTLV-1 Env and Tax proteins in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Blood 993335-3341. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23515-548. [DOI] [PubMed] [Google Scholar]
- 23.Haynes, B. F., and D. C. Montefiori. 2006. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev. Vaccines 5347-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hel, Z., J. Nacsa, E. Tryniszewska, W. P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 1694778-4787. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch, V. M. 2004. What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS? AIDS Rev. 640-53. [PubMed] [Google Scholar]
- 26.Hirsch, V. M., G. Dapolito, P. R. Johnson, W. R. Elkins, W. T. London, R. J. Montali, S. Goldstein, and C. Brown. 1995. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 69955-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holzammer, S., E. Holznagel, A. Kaul, R. Kurth, and S. Norley. 2001. High virus loads in naturally and experimentally SIVagm-infected African green monkeys. Virology 283324-331. [DOI] [PubMed] [Google Scholar]
- 28.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaul, R., J. Rutherford, S. L. Rowland-Jones, J. Kimani, J. I. Onyango, K. Fowke, K. MacDonald, J. J. Bwayo, A. J. McMichael, and F. A. Plummer. 2004. HIV-1 Env-specific cytotoxic T-lymphocyte responses in exposed, uninfected Kenyan sex workers: a prospective analysis. AIDS 182087-2089. [DOI] [PubMed] [Google Scholar]
- 30.Kaur, A., L. Alexander, S. I. Staprans, L. Denekamp, C. L. Hale, H. M. McClure, M. B. Feinberg, R. C. Desrosiers, and R. P. Johnson. 2001. Emergence of cytotoxic T lymphocyte escape mutations in nonpathogenic simian immunodeficiency virus infection. Eur. J. Immunol. 313207-3217. [DOI] [PubMed] [Google Scholar]
- 31.Kaur, A., C. L. Hale, S. Ramanujan, R. K. Jain, and R. P. Johnson. 2000. Differential dynamics of CD4+ and CD8+ T-lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J. Virol. 748413-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornfeld, C., M. J. Ploquin, I. Pandrea, A. Faye, R. Onanga, C. Apetrei, V. Poaty-Mavoungou, P. Rouquet, J. Estaquier, L. Mortara, J. F. Desoutter, C. Butor, R. Le Grand, P. Roques, F. Simon, F. Barre-Sinoussi, O. M. Diop, and M. C. Muller-Trutwin. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Investig. 1151082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31677-686. [DOI] [PubMed] [Google Scholar]
- 34.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 1625127-5133. [PubMed] [Google Scholar]
- 36.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 3121530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letvin, N. L., and B. D. Walker. 2003. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat. Med. 9861-866. [DOI] [PubMed] [Google Scholar]
- 38.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 7510187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lord, S. J., R. V. Rajotte, G. S. Korbutt, and R. C. Bleackley. 2003. Granzyme B: a natural born killer. Immunol. Rev. 19331-38. [DOI] [PubMed] [Google Scholar]
- 40.Maino, V. C., and H. T. Maecker. 2004. Cytokine flow cytometry: a multiparametric approach for assessing cellular immune responses to viral antigens. Clin. Immunol. 110222-231. [DOI] [PubMed] [Google Scholar]
- 41.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohri, H., A. S. Perelson, K. Tung, R. M. Ribeiro, B. Ramratnam, M. Markowitz, R. Kost, A. Hurley, L. Weinberger, D. Cesar, M. K. Hellerstein, and D. D. Ho. 2001. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 1941277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mothe, B. R., H. Horton, D. K. Carter, T. M. Allen, M. E. Liebl, P. Skinner, T. U. Vogel, S. Fuenger, K. Vielhuber, W. Rehrauer, N. Wilson, G. Franchini, J. D. Altman, A. Haase, L. J. Picker, D. B. Allison, and D. I. Watkins. 2002. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muhl, T., M. Krawczak, P. Ten Haaft, G. Hunsmann, and U. Sauermann. 2002. MHC class I alleles influence set-point viral load and survival time in simian immunodeficiency virus-infected rhesus monkeys. J. Immunol. 1693438-3446. [DOI] [PubMed] [Google Scholar]
- 45.Murayama, Y., A. Amano, R. Mukai, H. Shibata, S. Matsunaga, H. Takahashi, Y. Yoshikawa, M. Hayami, and A. Noguchi. 1997. CD4 and CD8 expressions in African green monkey helper T lymphocytes: implication for resistance to SIV infection. Int. Immunol. 9843-851. [DOI] [PubMed] [Google Scholar]
- 46.Murayama, Y., R. Mukai, M. Inoue-Murayama, and Y. Yoshikawa. 1999. An African green monkey lacking peripheral CD4 lymphocytes that retains helper T cell activity and coexists with SIVagm. Clin. Exp. Immunol. 117504-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura, T., Y. Nishiura, K. Ichinose, S. Shirabe, A. Tsujino, H. Goto, T. Furuya, and S. Nagataki. 1996. Spontaneous proliferation of and cytokine production by T cells adherent to human endothelial cells in patients with human T-lymphotropic virus type I-associated myelopathy. Intern. Med. 35195-199. [DOI] [PubMed] [Google Scholar]
- 48.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 49.Nishiura, Y., T. Nakamura, K. Ichinose, S. Shirabe, A. Tsujino, H. Goto, T. Furuya, and S. Nagataki. 1996. Increased production of inflammatory cytokines in cultured CD4+ cells from patients with HTLV-I-associated myelopathy. Tohoku J. Exp. Med. 179227-233. [DOI] [PubMed] [Google Scholar]
- 50.Norley, S., and R. Kurth. 2004. The role of the immune response during SIVagm infection of the African green monkey natural host. Front. Biosci. 9550-564. [DOI] [PubMed] [Google Scholar]
- 51.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 2792103-2106. [DOI] [PubMed] [Google Scholar]
- 52.Pandrea, I., C. Apetrei, J. Dufour, N. Dillon, J. Barbercheck, M. Metzger, B. Jacquelin, R. Bohm, P. A. Marx, F. Barre-Sinoussi, V. M. Hirsch, M. C. Muller-Trutwin, A. A. Lackner, and R. S. Veazey. 2006. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J. Virol. 804858-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pandrea, I., C. Apetrei, S. Gordon, J. Barbercheck, J. Dufour, R. Bohm, B. Sumpter, P. Roques, P. A. Marx, V. M. Hirsch, A. Kaur, A. A. Lackner, R. S. Veazey, and G. Silvestri. 2007. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood 1091069-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandrea, I., C. Kornfeld, M. J. Ploquin, C. Apetrei, A. Faye, P. Rouquet, P. Roques, F. Simon, F. Barre-Sinoussi, M. C. Muller-Trutwin, and O. M. Diop. 2005. Impact of viral factors on very early in vivo replication profiles in simian immunodeficiency virus SIVagm-infected African green monkeys. J. Virol. 796249-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandrea, I., G. Silvestri, R. Onanga, R. S. Veazey, P. A. Marx, V. Hirsch, and C. Apetrei. 2006. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: common patterns and species-specific differences. J. Med. Primatol. 35194-201. [DOI] [PubMed] [Google Scholar]
- 56.Pandrea, I., D. L. Sodora, G. Silvestri, and C. Apetrei. 2008. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 29419-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pantaleo, G., and R. A. Koup. 2004. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat. Med. 10806-810. [DOI] [PubMed] [Google Scholar]
- 58.Peters, P. J., J. Borst, V. Oorschot, M. Fukuda, O. Krahenbuhl, J. Tschopp, J. W. Slot, and H. J. Geuze. 1991. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 1731099-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2032281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrovas, C., D. A. Price, J. Mattapallil, D. R. Ambrozak, C. Geldmacher, V. Cecchinato, M. Vaccari, E. Tryniszewska, E. Gostick, M. Roederer, D. C. Douek, S. H. Morgan, S. J. Davis, G. Franchini, and R. A. Koup. 2007. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood 110928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Picker, L. J. 2006. Immunopathogenesis of acute AIDS virus infection. Curr. Opin. Immunol. 18399-405. [DOI] [PubMed] [Google Scholar]
- 62.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 16829-43. [DOI] [PubMed] [Google Scholar]
- 63.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 1004144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenzweig, M., M. A. DeMaria, D. M. Harper, S. Friedrich, R. K. Jain, and R. P. Johnson. 1998. Increased rates of CD4(+) and CD8(+) T lymphocyte turnover in simian immunodeficiency virus-infected macaques. Proc. Natl. Acad. Sci. USA 956388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sachsenberg, N., A. S. Perelson, S. Yerly, G. A. Schockmel, D. Leduc, B. Hirschel, and L. Perrin. 1998. Turnover of CD4+ and CD8+ T lymphocytes in HIV-1 infection as measured by Ki-67 antigen. J. Exp. Med. 1871295-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 67.Seder, R. A., P. A. Darrah, and M. Roederer. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8247-258. [DOI] [PubMed] [Google Scholar]
- 68.Silvestri, G., M. Paiardini, I. Pandrea, M. M. Lederman, and D. L. Sodora. 2007. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Investig. 1173148-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18441-452. [DOI] [PubMed] [Google Scholar]
- 70.Sodora, D. L., and G. Silvestri. 2008. Immune activation and AIDS pathogenesis. AIDS 22439-446. [DOI] [PubMed] [Google Scholar]
- 71.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retrovir. 14183-189. [DOI] [PubMed] [Google Scholar]
- 72.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 121198-1202. [DOI] [PubMed] [Google Scholar]
- 73.VandeWoude, S., and C. Apetrei. 2006. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 19728-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Velu, V., S. Kannanganat, C. Ibegbu, L. Chennareddi, F. Villinger, G. J. Freeman, R. Ahmed, and R. R. Amara. 2007. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J. Virol. 815819-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wowk, M. E., and J. A. Trapani. 2004. Cytotoxic activity of the lymphocyte toxin granzyme B. Microbes Infect. 6752-758. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, J. Y., Z. Zhang, X. Wang, J. L. Fu, J. Yao, Y. Jiao, L. Chen, H. Zhang, J. Wei, L. Jin, M. Shi, G. F. Gao, H. Wu, and F. S. Wang. 2007. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood 1094671-4678. [DOI] [PubMed] [Google Scholar]