Abstract
Herpes simplex virus type 1 (HSV-1) mutants impaired in the activities of the structural protein VP16 and the immediate-early (IE) proteins ICP0 and ICP4 establish a quiescent infection in human fibroblasts, with most cells retaining an inactive, repressed viral genome for sustained periods in culture. To date, the quiescent state has been considered stable, since it has been reversed only by provision of herpesviral proteins, such as ICP0, not by alteration of the cell physiological state. We report that the interaction of HSV-1 with human fibroblasts can be altered significantly by transient treatment of cultures with sodium arsenite, an inducer of heat shock and oxidative stress, or gramicidin D, a toxin that selectively permeabilizes cell membranes, prior to infection. These regimens stimulated gene expression from IE-deficient HSV-1 mutants in a promoter sequence-independent manner and also overcame the replication defect of ICP0-null mutants. Reactivation of gene expression from quiescent HSV-1 genomes and the resumption of virus replication were observed following addition of arsenite or gramicidin D to cultures. Both agents induced reorganization of nuclear domain 10 structures, the sites of quiescent genomes, but appeared to do so through different mechanisms. The results demonstrate that the physiological state of the cell is important in determining the outcome of infection with IE-deficient HSV-1 and show novel methods for reactivating quiescent HSV-1 in fibroblasts with a high efficiency.
Transcription of the herpes simplex virus type 1 (HSV-1) genome at early stages of infection is controlled by the activities of three viral proteins, VP16, ICP4, and ICP0. VP16 is a virion component that stimulates immediate-early (IE) transcription through interaction with the cellular proteins Oct-1 and HCF-1 (51, 77). ICP4 is an IE protein that is essential for early and late gene expression, acting on basal cellular transcription factors (10, 64, 80). The IE protein ICP0 exerts a general stimulatory effect on HSV-1 transcription and is important for initiation of the viral gene expression program (17, 19, 31, 61, 68).
The VP16 and ICP4 proteins can be viewed as conventional transcription activators since they interact with cellular factors that localize to promoter regions of the viral genome. In contrast, the effects of ICP0 are not promoter specific or even restricted to the HSV-1 genome, since the protein stimulates gene expression from a variety of plasmids and viruses (3, 18, 30, 31, 48, 52). One of the most striking features of ICP0 is its effect on cellular intranuclear structures known as promyelocytic leukemia (PML) bodies or nuclear domain 10 (ND10). Early in infection, ICP0 localizes to ND10 and directs the disruption of these bodies (21, 22). Protein components of ND10, including the PML protein itself, are targeted for degradation due to a ubiquitin E3 ligase activity that requires the RING domain of ICP0 (6, 21, 25). HSV-1 mutants that lack the ICP0 coding region exhibit a multiplicity- and cell type-dependent defect in the initiation of productive infection (16, 61, 68, 71). The magnitude of this effect ranges from severe impairment, as observed after infection of human fibroblasts, to an almost complete lack of requirement of ICP0, exemplified by the human osteosarcoma line U2-OS (33, 78). In addition, the cellular metabolic state affects the ability of ICP0-null mutants to initiate replication. Initial studies showed that Vero cells, which normally have intermediate permissiveness, exhibited greater plating efficiencies with ICP0-null mutants at specific stages of the cell cycle, but more recent experiments have demonstrated that the methodology used for cell synchronization also caused cellular stress (7, 8). Heat shock or UV treatment of cell cultures prior to infection enabled ICP0-null mutants to replicate more efficiently, confirming that uncharacterized properties of the host cell have significant effects on the requirement for ICP0 (7).
Infection of human fibroblasts with ICP0-null mutants of HSV-1 results in rapid repression of viral gene expression and subsequent retention of the viral genome in cultures for many days (67). The resulting interaction, in which the viral genome is transcriptionally silent, is referred to as quiescence. The quiescent state is observed most clearly during infection with mutants that are additionally impaired for expression of functional VP16 and/or ICP4. Our laboratory has constructed the mutant in1312, which carries an insertion that inactivates the transcriptional function of VP16, a deletion of the RING domain of ICP0, and a temperature-sensitive mutation in ICP4 (58). The combination of the three mutations reduces cytotoxicity and enables infection of human fibroblasts to be carried out at relatively high multiplicities of infection (MOIs), resulting in the establishment of cultures in which most cells contain a quiescent HSV-1 genome (55). Heterologous promoters, such as the human cytomegalovirus (HCMV) major IE promoter (MIEP), cloned into the in1312 genome, are also rapidly silenced (55). Analogous results have been reported for investigations of similarly impaired mutants by other groups (47, 49, 63). Studies of hybrids between human fibroblasts and U2-OS cells demonstrated that the nonpermissive phenotype is dominant, demonstrating the existence of cellular factors that actively repress the HSV-1 genome in the absence of ICP0 (33).
Conversion to the quiescent state renders the viral genome insensitive to treatments, such as provision of VP16, that are able to activate gene expression if provided at the time of infection, indicating that cellular silencing mechanisms result in extensive repression of the viral genome (33, 55, 56). The only known methods of reversing repression, thereby provoking the resumption of viral gene expression, require the activities of viral gene products. Provision of ICP0 is particularly effective, although the HCMV protein pp71 or ICP4 supplied by superinfection with ICP0-null HSV-1 is also effective, albeit in only a proportion of quiescently infected cells (35, 54, 55, 63, 71). Currently, there are no known alternative treatments that influence expression from the quiescent genome in human fibroblasts, a fact that has hindered a detailed analysis of the cellular mechanisms that mediate repression and activity of the HSV-1 genome.
The study of quiescent infection in human fibroblasts is important for understanding early virus-cell interactions and for investigations of HSV-1 latency in neurons. During latency, lytic gene expression is repressed and only one transcription unit, specifying latency-associated transcripts, is active (14, 38, 73). Reactivation from latency can occur spontaneously or in response to stimuli that are collectively recognized as causing stress. The stressors may act on the neuron, at the body surface, or systemically, but at present there are few clues, and no details, regarding the cellular factors or pathways that result in resumption of viral gene expression. This situation hampers attempts to understand reactivation and to develop agents that interfere with it. The study presented here demonstrates that stress-inducing agents can prevent the attainment of the quiescent state and can even reverse it, resulting in the resumption of viral gene expression and replication. Two agents, sodium arsenite and gramicidin D, were particularly effective, and their activities form the basis of this report.
Sodium arsenite is a toxin that inhibits many enzymes, mainly through its affinity for sulfhydryl groups. Metabolites of arsenite also have multiple targets within cells. Chronic exposure to arsenite, through contamination of drinking water, is associated with increased incidences of human diseases such as atherosclerosis, diabetes, and cancer (41). Unsurprisingly, in view of its multiple targets, arsenite has significant effects on cellular gene expression and protein content, with the exact response dependent on the magnitude and time of exposure to the toxin (42, 60, 79). It is well established, however, that arsenite is a strong inducer of heat shock and oxidative stress (40-42, 79).
Gramicidin D is a collective name for a mixture of three linear pentadecapeptides, gramicidins A, B, and C, produced by the soil bacterium Bacillus brevis. The three species differ at only two residues and are regarded as having equivalent properties. Gramicidins A, B, and C have alternating D and L residues, and their most relevant property is the ability to form helical dimers that penetrate cell membranes to form an ion channel, rendering the membrane permeable to protons and the monovalent cations Na+ and K+ (74). Gramicidin D has been used extensively for structural studies of ion channels in model membranes and has been shown to cause Na+ influx and K+ efflux in mammalian cells (28, 39, 44, 74). The selective permeabilization of membranes presumably accounts for the neutralization of human immunodeficiency virus and HSV by gramicidin D (5).
MATERIALS AND METHODS
Viruses.
Mutant in1312, derived from HSV-1 strain 17, is defective for VP16, ICP0, and ICP4 function (58). Derivatives of this mutant containing the Escherichia coli lacZ coding sequences controlled by the HCMV MIEP (in1374 and in1382) or the simian CMV MIEP (in1357) have been described previously (36, 43). Mutant in1359 has lacZ controlled by the murine CMV MIEP (527 bp, from positions −491 to +36), derived from plasmid pMH4 (1), and in0131 has the ICP0 promoter (856 bp, from positions −807 to +49) controlling lacZ inserted at the UL43 locus, together with rescue of the VP16 mutation. Mutant in0131 is therefore impaired only for ICP0 and ICP4 function. Mutants tsK, with a temperature-sensitive mutation in ICP4, dl1403Y, deleted for ICP0, and its rescued counterpart, dl1403YR, have been described previously (13, 53, 54, 68). Viruses were titrated on U2-OS cells at 32°C, with 3 mM hexamethylene bisacetamide added for mutants with nonfunctional VP16 (45).
Cells.
Human fetal foreskin fibroblasts (HFFF2) were obtained from the European Collection of Cell Cultures and propagated in Dulbecco's modified Eagle medium supplemented with 5% fetal calf serum, 5% newborn calf serum, 1 mM glutamine, nonessential amino acids, 100 units of penicillin per ml, and 100 μg of streptomycin per ml. For the establishment of quiescent infection, monolayers of 8 × 105 HFFF2 cells were infected with 3 × 106 PFU of in1374 and incubated at 38.5°C in Dulbecco's modified Eagle medium containing 2% fetal calf serum and the additives listed above, with medium changes every 2 to 3 days. For reactivation studies, cultures were trypsinized after 8 days at 38.5°C and dispensed into 24-well dishes.
Inhibitors.
Sodium arsenite, gramicidin D, diethyldithiocarbamic acid (DDTC), sodium pyrrolidinedithiocarbamate (PDTC), and cycloheximide were purchased from Sigma-Aldrich. Gramicidin A, Z-VAD-FMK, and staurosporine were purchased from Calbiochem.
Antibodies.
The sources of antibodies used were as follows: mouse anti-poly(ADP-ribose) polymerase (anti-PARP) and mouse anti-heme oxygenase 1 (anti-HO-1) were obtained from BD Bioscience, rabbit anti-Daxx and mouse anti-Fas clone C11 were obtained from Upstate, mouse anti-PML was obtained from Santa Cruz, mouse anti-HSP70 was obtained from Stressgen, and mouse anti-actin was obtained from Sigma-Aldrich. Mouse monoclonal antibodies directed against VP5 and UL42 were described previously (19).
Immunofluorescence.
Monolayers were fixed and analyzed by confocal microscopy as described previously (20). For analysis of ND10 numbers, 10 randomly selected fields from each of two coverslips stained for PML were captured and processed using Adobe Photoshop to produce grayscale images of the fields. Numbers of ND10 per cell were scored manually for 300 nuclei, and Microsoft Excel was used to calculate averages, standard deviations (SD), and significance (by two-tailed t test).
Protein immunoblotting.
Cell lysates were analyzed by protein immunoblotting as described previously (25).
Radiolabeling.
Monolayers of 105 HFFF2 cells were treated with 100 μM sodium arsenite or 12 μg/ml gramicidin D for 2 h. The inhibitors were then removed, the monolayers were washed, and fresh medium was added. At various times, cells were radiolabeled by incubation with [35S]methionine-cysteine (Easy Tag Express protein labeling mix; Perkin-Elmer) (50 μCi/ml) or [5,6-3H]uridine (Perkin-Elmer) (15 μCi/ml) for 1 h at 37°C. Trichloroacetic acid-insoluble incorporation was determined and expressed as a percentage of the value for untreated cultures.
LDH release.
Necrosis was analyzed by release of lactate dehydrogenase (LDH) from monolayers, using a CytoTox kit (Promega). Cells were lysed by the addition of 0.5% (vol/vol) Nonidet P-40 to provide a measure of total cellular LDH.
Histochemical detection of β-galactosidase.
Monolayers were fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) substrate as described previously (37).
RESULTS
Stimulation of HCMV IE promoter activity by stress treatment of cells.
The HSV-1 mutant in1382 is impaired in the transcriptional activities of VP16, ICP0, and ICP4 at 38.5°C and, additionally, contains an insertion of E. coli lacZ, controlled by the HCMV MIEP, at the TK locus. Upon infection of HFFF2 cells, expression from the in1382 genome is severely restricted such that at an MOI of 0.15 (3 × 104 PFU applied to 2 × 105 cells), few cells (<5) detectably produce β-galactosidase. Coinfection with the tsK mutant, which is defective only for ICP4 at 38.5°C, reverses the repression of the in1382 genome due to the production of ICP0, and the number of β-galactosidase-positive cells approximates the number of PFU initially added. The differential between the “on” and “off” states is therefore a factor of approximately 104, emphasizing that HFFF2 fibroblasts are severely restricted for gene expression in the absence of ICP0.
Initial investigations revealed that pretreatment of HFFF2 cultures with agents known to induce a stress response, particularly sodium arsenite, considerably increased the numbers of β-galactosidase-positive cells after infection with in1382. As shown in Table 1 and Fig. 1, the numbers of β-galactosidase-positive cells were increased by treatment of cells with sodium arsenite for 2 h prior to infection. At the maximum dose of arsenite tested (150 μM), the number of β-galactosidase-positive cells was 49% of that obtained by coinfection with tsK, a stimulation of 5,000-fold over the value for untreated cultures after taking account of the 2-fold reduction in the value with tsK. At the higher arsenite concentrations, cytopathology occurred, as reflected in a reduction in expression upon coinfection with tsK. Different regimens of arsenite addition were tested, but although stimulation was observed when the compound was added after infection, pretreatment was most effective (results not shown).
TABLE 1.
Stimulation of in1382 gene expressiona
| Treatment | % of β-galactosidase-positive cells
|
|
|---|---|---|
| −tsK | +tsK | |
| None | 0.01 | 100 |
| 50 μM arsenite | 9 | 85 |
| 75 μM arsenite | 22 | 76 |
| 100 μM arsenite | 26 | 67 |
| 150 μM arsenite | 26 | 53 |
| 6 μg/ml gramicidin D | 4.5 | 56 |
| 9 μg/ml gramicidin D | 11 | 54 |
| 12 μg/ml gramicidin D | 61 | 63 |
| 15 μg/ml gramicidin D | 45 | 56 |
| 12 μg/ml gramicidin A | 46 | 68 |
| 50 μM DDTC plus medium change | 15 | 62 |
| 50 μM PDTC plus medium change | 13 | 45 |
| 50 μM DDTC | 0.2 | 100 |
| 50 μM PDTC | 4.6 | 89 |
| 50 μM DDTC plus 10 μM CuSO4 | 24 | ND |
| 50 μM DDTC plus 7.5 μM ZnCl2 | 18 | ND |
Monolayers of HFFF2 cells were pretreated with various agents for 2 h at 37°C. The medium was then removed, and monolayers were infected with in1382 (amounts ranging from 30 to 3 × 104 PFU per monolayer of 105 cells), with or without coinfection of 2 × 105 PFU of tsK. After a 1-h adsorption period, fresh medium was added and monolayers were incubated overnight at 38.5°C. Cells were histochemically stained for the presence of β-galactosidase. The number obtained for untreated cells coinfected with tsK was taken as 100%. The means of at least three determinations are shown.
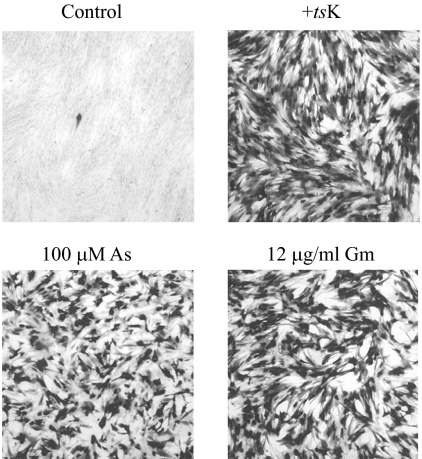
FIG. 1.
Stimulation of gene expression by arsenite and gramicidin. HFFF2 monolayers were left untreated or pretreated with 100 μM sodium arsenite (As) or 12 μg/ml gramicidin D (Gm) for 2 h, washed, and infected with 105 PFU of in1382, with or without coinfection of 2 × 105 PFU of tsK. After incubation overnight at 38.5°C, monolayers were stained for the presence of β-galactosidase.
Other compounds that activate stress responses were tested. Following the observations with arsenite, dithiocarbamates were added to cells for 2 h prior to infection with in1382 (Table 1). The compounds tested, DDTC and PDTC, gave detectable stimulation when added directly to the medium of cultures that had been maintained overnight at 37°C after being seeded but were more effective if they were added in fresh culture medium. Dithiocarbamates have many reported effects on cells, one of which is the ability to act as ionophores for heavy metal ions (15, 29). When CuSO4 or ZnSO4 was added at low concentrations (5 to 10 μM) together with 50 μM DDTC without a medium change, a large increase in the number of β-galactosidase-positive cells was observed. It should be noted that CuSO4 and ZnSO4, even at 50 μM, had no detectable effects in the absence of DDTC (results not shown). It appears, therefore, that heavy metal toxicity is another stress that affects gene expression and that the greater effect of dithiocarbamates in fresh medium was due to the replenishment of heavy metal salts.
Agents that alter intracellular ion balance also stimulated viral gene expression in cells infected with in1382. Initial experiments determined that pretreatment of human fetal lung fibroblasts with 0.3 μM ouabain gave a significant effect, with the number of β-galactosidase-positive cells reaching 10% of the value upon coinfection with tsK (results not shown). The compound was also effective in HFFF2 fibroblasts, but to a lesser extent than in lung fibroblasts. Ouabain inhibits the membrane Na+-K+ ATPase, resulting in a net outflow of K+ ions and an inflow of Na+ ions. Gramicidin D is another compound that affects the intracellular ion balance, by inserting into the cell membrane and acting as a channel for small cations, particularly Na+. Pretreatment of cells with gramicidin D was a very effective way of increasing the number of β-galactosidase-positive cells, with the value after addition of the compound at 12 μg/ml reaching almost 100% of that in untreated, tsK-coinfected cells. Pure gramicidin A was similarly effective in stimulating gene expression (Fig. 1 and Table 1).
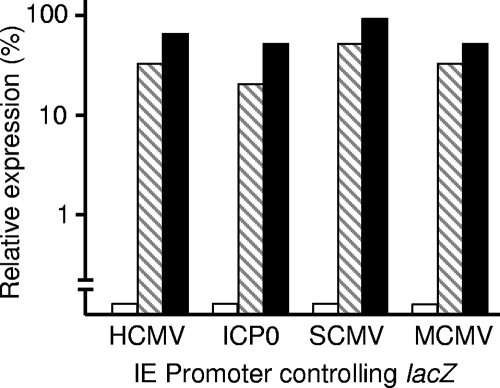
An increase in the number of β-galactosidase-positive cells could result from failure of the HSV-1 genome to be repressed or to a direct stimulatory action on the HCMV IE promoter. The latter mechanism is unlikely, since agents known to activate the promoter, such as phorbol esters or compounds that raise cyclic AMP (cAMP) levels, gave relatively small increases (<10-fold) in the number of positive cells (results not shown). To further investigate the specificity of the effects, the responses of other promoters controlling β-galactosidase production were tested (Fig. 2). Stimulation equivalent to that observed with in1382 was observed with mutants in1357 (containing the simian CMV IE promoter controlling lacZ), in1359 (containing the murine CMV IE promoter), and in0131 (containing the HSV-1 ICP0 promoter) in cultures pretreated with arsenite or gramicidin D. The response to the stressors was therefore not restricted to the HCMV IE promoter. The absence of any detectable promoter specificity suggests that the stressors exert a general effect on the viral genome by overcoming cellular repression mechanisms.
FIG. 2.
Arsenite and gramicidin D stimulate expression from various promoters. HFFF2 monolayers were left untreated (white bars) or pretreated with 100 μM sodium arsenite (hatched bars) or 12 μg/ml gramicidin D (black bars) for 2 h, washed, and infected with HSV-1 mutants. The numbers of β-galactosidase-positive cells are presented as percentages of the value for cultures coinfected with tsK. SCMV, simian CMV; MCMV, murine CMV.
Stressors compensate for the absence of ICP0.
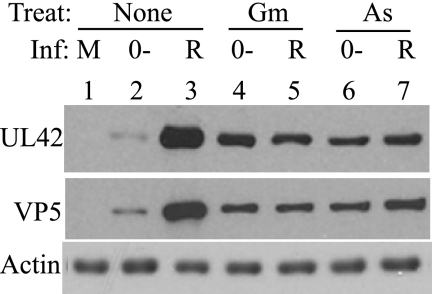
The conclusion that arsenite and gramicidin D act in a promoter-independent manner suggests that an ICP0-negative HSV-1 mutant should be competent for replication in inhibitor-treated cultures. This prediction was tested by infection of HFFF2 cells at an MOI of 1 with the ICP0-null mutant dl1403Y or the rescued mutant dl1403YR and analysis of viral gene expression by monitoring the production of the early protein UL42 or the late protein VP5. The results (Fig. 3) show that expression of UL42 and VP5 was increased in arsenite- or gramicidin D-pretreated cells compared with that observed in untreated cells. As anticipated, pretreatment with the toxic agents resulted in reduced UL42 and VP5 expression in cells infected with dl1403YR, and when this overall effect was taken into account, the mutant and rescued mutant exhibited equivalent levels of gene expression (Fig. 3, lanes 4 to 7). Pretreatment of cells with arsenite or gramicidin therefore overcomes the requirement for ICP0 in launching the gene expression program.
FIG. 3.
Arsenite and gramicidin D overcome the requirement for ICP0. HFFF2 monolayers were treated with 100 μM sodium arsenite (As) or 12 μg/ml gramicidin D (Gm) for 2 h, washed, and infected with 1 PFU of the ICP0-null mutant dl1403Y (0−) or rescued mutant dl1403YR (R) per cell. At 7 h postinfection, cells were harvested and analyzed for UL42, VP5, and actin protein levels.
Reactivation of quiescent virus.
The in1374 mutant is identical to in1382 except that the HCMV IE promoter-lacZ insertion is at UL43 instead of TK. Cultures of HFFF2 cells infected with in1374 (or in1382) at an MOI of 3 retain the viral genome in a quiescent state for at least 2 weeks (24, 55). We investigated whether treatment with arsenite or gramicidin D reactivated expression from quiescent viral genomes.
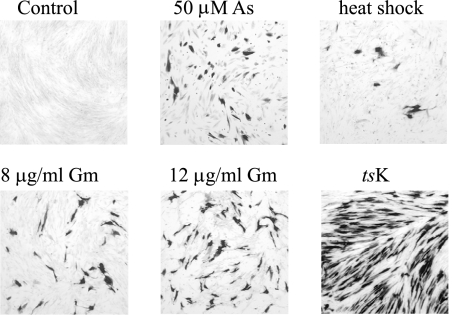
Cultures of HFFF2 cells containing quiescent in1374 were treated with arsenite or gramicidin D for 2 to 6 h, and medium was then replaced and incubation continued at 38.5°C for 20 h. In both cases, β-galactosidase expression resumed in a proportion of cells, but it was found that the cultures were significantly more resistant to the effects of the inducing agents after incubation at 38.5°C for 8 days and that longer exposure times were necessary to achieve the maximum effect. Incubation with 50 μM sodium arsenite for 16 h, followed by further incubation without the agent for 24 h, resulted in a large proportion of cells expressing β-galactosidase (Fig. 4), demonstrating that the treatment reversed the repression of the HCMV MIEP in the quiescent genome. Heat shock of cultures at 44°C for 45 min also induced β-galactosidase expression (Fig. 4), but the balance between activation and cytotoxicity after heat shock was found to be too variable for routine use of this procedure. Treatment with gramicidin D for 16 h also resulted in reactivation of expression from the HCMV MIEP, with an efficiency approaching but not equaling that achieved by infection with tsK (Fig. 4).
FIG. 4.
Reactivation of expression at 38.5°C. HFFF2 monolayers containing quiescent in1374 were left untreated (control), incubated with 50 μM sodium arsenite (As) for 16 h at 38.5°C, incubated at 44°C for 45 min (heat shock), incubated with 8 μg/ml or 12 μg/ml gramicidin D (Gm) for 16 h at 38.5°C, or infected with 3 PFU of tsK per cell. After treatment, cells were incubated at 38.5°C for 20 h and stained for the presence of β-galactosidase.
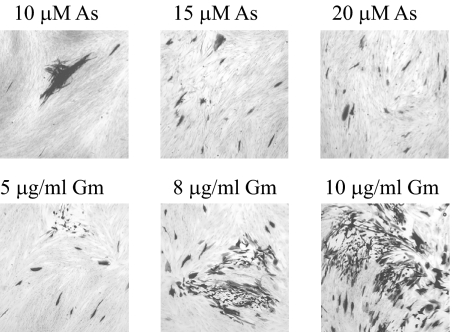
To determine whether arsenite and gramicidin D could induce replication of quiescent virus in addition to gene expression, monolayers containing quiescent in1374 were treated with the stress-inducing agents and incubated at 32°C, a temperature permissive for replication of in1374. Concentrations of arsenite and, to a lesser extent, gramicidin D used for short-term experiments were too toxic over the period of 5 days that was necessary for plaque formation at 32°C, even though the agents were applied only for 16 h, and therefore it was necessary to modify the doses used. The addition of 10 μM arsenite for 16 h induced plaque formation after 5 days at 32°C, as shown in Fig. 5, and increasing the arsenite concentration to 15 and 20 μM gave significantly greater numbers of small plaques (Fig. 5). Gramicidin D was less toxic over periods of days at 32°C, and plaque formation was observed when cultures were treated with this compound, especially at a concentration of 10 μg/ml for 16 h. In addition to plaques, many β-galactosidase-expressing single cells were observed after treatment with arsenite or gramicidin D. To confirm that infectious virus was produced, cultures were analyzed by infectious center assay of U2-OS cells, and in addition, total virus produced after incubation without human serum at 32°C for 5 days was quantified. In six independent determinations, treatment with 20 μM arsenite for 16 h yielded 40 to 59 infectious centers and 2.5 × 103 to 3 × 104 PFU of virus, and treatment with 10 μg/ml gramicidin D gave 421 to 603 infectious centers and 2 × 104 to 8 × 105 PFU of virus. The majority (80%) of untreated cultures did not yield infectious centers or produce virus, with the remainder exhibiting one or two infectious centers.
FIG. 5.
Reactivation of virus replication at 32°C. HFFF2 monolayers containing quiescent in1374 were treated with sodium arsenite (As) or gramicidin D (Gm) for 16 h and incubated at 32°C for 5 days, with 2% human serum added to the culture medium. Monolayers were stained for the presence of β-galactosidase.
Distinct modes of action of stressors.
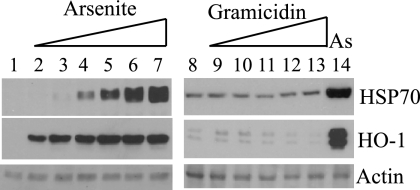
Toxic agents such as arsenite, heavy metals, and gramicidin D affect many cellular processes, and few studies on this subject have used human fibroblasts. We sought to identify a common mode of action for the various compounds that compensate for the absence of ICP0, focusing on the most effective, arsenite and gramicidin D. Arsenite is known to be a strong inducer of heat shock and oxidative stress, and therefore the synthesis of HSP70 and HO-1, a protein induced by both stresses, was examined after exposure to the agents. As expected, cultures exposed to arsenite showed strong activation of HSP70 and HO-1 expression (Fig. 6, lanes 2 to 7 and 14). Gramicidin D, however, did not alter endogenous levels of these proteins, indicating that the agent did not impose heat or oxidative stress (Fig. 6, lanes 9 to 13). In addition, pretreatment with 10 mM N-acetyl cysteine (NAC), a potent antioxidant, did not antagonize the effects of gramicidin D on HSV-1 gene expression (results not shown).
FIG. 6.
Induction of heat shock and oxidative stress by arsenite. HFFF2 monolayers were treated with sodium arsenite or gramicidin D for 2 h at 38.5°C, washed, and harvested after a further 4 h at 38.5°C. Cells were treated with no additions (lanes 1 and 8), sodium arsenite at 5 μM (lane 2), 10 μM (lane 3), 15 μM (lane 4), 20 μM (lane 5), 30 μM (lane 6), or 50 μM (lanes 7 and 14), or gramicidin D at 5 μg/ml (lane 9), 7.5 μg/ml (lane 10), 10 μg/ml (lane 11), 12.5 μg/ml (lane 12), or 15 μg/ml (lane 13).
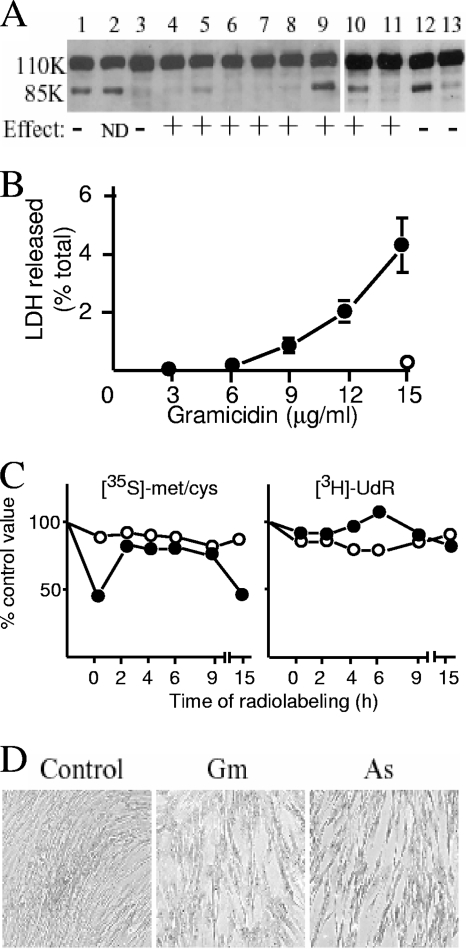
The possible role of apoptosis or necrosis in affecting HSV-1 gene expression was investigated in view of the known toxicity of arsenite and gramicidin D. Neither compound induced the cleavage of PARP, a characteristic of apoptosis (Fig. 7A, lanes 4 to 8), but treatment with 50 μM DDTC in fresh medium (Fig. 7A, lanes 9 and 10) was almost as effective as the addition of the known apoptosis inducers staurosporine (Fig. 7A, lanes 1 and 12) and anti-Fas plus cycloheximide (Fig. 7A, lane 2). However, the addition of the apoptosis inhibitor Z-VAD-FMK did not affect the activation of gene expression by arsenite, gramicidin D, or DDTC (Fig. 7A and results not shown), even though the compound effectively inhibited cleavage of PARP after treatment of cells with staurosporine or DDTC (Fig. 7A, lanes 11 and 13). Necrosis was assessed by the release of LDH 16 h after treatment (Fig. 7B). Arsenite did not significantly induce LDH release at concentrations of up to 150 μM, whereas pretreatment of cells with high levels of gramicidin D (12 and 15 μg/ml) resulted in release of 2% and 4.5%, respectively, of total cellular LDH into the culture medium. Therefore, gramicidin D caused membrane alterations that indicated a limited degree of necrosis, whereas this effect was not detected after the addition of arsenite.
FIG. 7.
Toxic effects on HFFF2 cells. (A) HFFF2 monolayers were treated in various ways, and extracts were analyzed for cleavage of PARP (revealed by the presence of an 85-kDa band) after incubation overnight at 38.5°C. In parallel, cells were infected with in1382 after treatment, incubated overnight at 38.5°C, stained for β-galactosidase, and scored as + (30 to 100% of the tsK value), − (not increased >5-fold above the untreated value), or not determined (ND). Treatments were as follows: staurosporine (100 nM) for 24 h (lanes 1 and 12), anti-Fas (125 ng/ml) plus cycloheximide (10 μg/ml) for 24 h (lane 2), no treatment (lane 3), sodium arsenite at 50 μM (lane 4) or 100 μM (lane 5) for 2 h, gramicidin D at 10 μg/ml (lane 6), 12.5 μg/ml (lane 7), or 15 μg/ml (lane 8) for 2 h, 50 μM DDTC plus medium change for 2 h (lanes 9 and 10), DDTC plus medium change plus Z-VAD-FMK (50 μM) for 2 h (lane 11), and staurosporine (100 nM) plus Z-VAD-FMK (50 μM) for 24 h (lane 13). (B) HFFF2 monolayers were treated with gramicidin D for 2 h, washed, and incubated at 38.5οC for 16 h. Released LDH in cell-free medium was assayed (filled circles) and expressed as a percentage of the value from detergent-lysed cells. The amount of LDH released after incubation with 150 μM sodium arsenite for 2 h followed by incubation at 38.5°C for 16 h is shown (open circle). (C) Protein and RNA synthesis. Monolayers were treated with 100 μM sodium arsenite (open circles) or 12 μg/ml gramicidin D (filled circles) for 2 h, washed, and radiolabeled at various times to determine rates of protein or RNA synthesis. The zero time point represents radiolabeling immediately after removal of agents. Values were expressed as percentages of those for untreated cultures radiolabeled in parallel. The points represent the means of duplicate samples, which did not vary by more than 10% of the mean value. (D) Photographs of HFFF2 cultures after no treatment (control) or addition of 10 μg/ml gramicidin D (Gm) or 50 μM sodium arsenite (As) for 2 h, followed by incubation at 37°C for 16 h.
The effects of arsenite and gramicidin D on protein and RNA synthesis were examined (Fig. 7C). Pretreatment with gramicidin D resulted in a twofold decrease in the rate of protein synthesis immediately after removal of the compound and also at 15 h. At other time points, rates of protein synthesis were no less than 63% of the control value. Rates of RNA synthesis ranged between 67% and 124% of control values. Therefore, arsenite and gramicidin D did not induce a major impairment of cellular metabolic activity.
None of the most obvious candidate mechanisms of action accounted for the stimulatory effects of both arsenite and gramicidin D, and thus it is likely that they act through different pathways. One point of similarity, however, was in the gross cytological changes observed 16 h after treatment with suboptimal concentrations of the compounds. Cells lost contact with each other, resulting in a typical morphology that was grossly similar in arsenite- and gramicidin D-treated cultures (Fig. 7D).
Arsenite and gramicidin D induce changes in ND10.
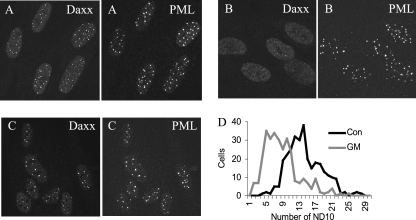
In view of the importance of ND10 as sites of genome repression, the effects of arsenite and gramicidin D on these structures were investigated. After 2 h of treatment with 100 μM arsenite or 12 μg/ml gramicidin D, cells were fixed, permeabilized, and examined by confocal microscopy after reaction with a mixture of anti-PML and anti-Daxx antibodies. In arsenite-treated cells, PML retained its punctate distribution, indicating that ND10 bodies remained essentially intact, but the distribution of Daxx was dramatically altered, with the signal dispersed throughout the nucleus and with little evidence of retention of the protein at ND10 (Fig. 8B). The punctate pattern of PML distribution was also changed, with an increase in the definition of the foci and a loss of the low background signal throughout the nucleus that was observed in untreated cells. Gramicidin D-treated cells retained both PML and Daxx in punctate structures, again with greater definition, but observation revealed that many nuclei had fewer foci than those in control cultures (Fig. 8C). To express this difference quantitatively, the numbers of ND10 (defined by the presence of PML) were counted in 300 cells taken from 10 random fields on each of two coverslips. The data (Fig. 8D) confirm that there was a significant change in the number of ND10 after gramicidin D treatment, with the average falling from 14.2 per cell (SD, 4.2) for control cultures to 8.7 per cell (SD, 4.2) for treated cultures (P < 0.001; two-tailed t test). Arsenite treatment did not significantly affect the number of ND10 (average, 14.6; SD, 4.6; P > 0.1).
FIG. 8.
Effects of arsenite and gramicidin D on ND10. HFFF2 monolayers were left untreated (A) or treated with 100 μM sodium arsenite (B) or 12 μg/ml gramicidin D (C) for 2 h at 37°C. Immunofluorescence staining was carried out using antibodies to Daxx and PML. Frequency distributions of ND10 in 300 nuclei of untreated or gramicidin D-treated cultures are presented in panel D.
DISCUSSION
Quiescent infection of human fibroblasts has, to date, appeared to be a very stable interaction, with the HSV-1 genome retained in a repressed state that can be reversed only by the provision of the viral transcription factor ICP0, ICP4, or pp71 (35, 54, 55, 63). The inability to provoke resumption of viral gene expression by treatments that alter cellular physiology has been a hurdle in understanding the nature of the quiescent state and has additionally aroused the suspicion that quiescence in fibroblasts represents a terminal interaction that cannot be reversed by cellular changes. However, the experiments reported here demonstrate that reactivation of viral gene expression and subsequent replication of HSV-1 are achievable and that the relevant treatments prevent repression of input IE-defective HSV-1 genomes and hence compensate for the absence of ICP0.
The observation that arsenite and gramicidin D are most effective if added as pretreatments to cultures rather than after infection indicates that their effects are due to changes in cell physiology rather than to a direct action of the compounds, or their metabolites, on the viral genome. In this respect, the results presented here confirm and extend the observation by Bringhurst and Schaffer that cellular stress induced by prior heat shock or UV irradiation of cultures enhances the plating efficiency of ICP0-null HSV-1 mutants in Vero cells (7). Although the two experimental systems differ in their details, their study and ours both demonstrate that efficient complementation of ICP0-null mutants can be achieved by defined pretreatments to cells. It should be noted that human fibroblasts are more stringent than Vero cells in their requirement for ICP0, and hence the magnitude of stimulation that we observe is greater than that reported by Bringhurst and Schaffer (16, 19, 68, 71).
The apparent lack of common elements in the responses to arsenite and gramicidin D suggest that there are at least two pathways leading to the complementation of ICP0-null mutants. Arsenite pretreatment, as expected, induced heat shock and oxidative stress. It is not clear whether the stress per se or the resultant new proteins are responsible for the effects on viral gene expression. The observation that the agent was more effective if it was added prior to infection suggests a role for newly synthesized proteins, but it is equally possible that other sequelae of the insult to the cell are responsible. Treatment of cultures with the antioxidant NAC abolished the effect of arsenite (results not shown), but we were unable to determine whether this signified a crucial role for oxidative stress or simply the unavailability of arsenite due to complexing with NAC. Gramicidin D did not induce heat shock or oxidative stress, demonstrating that neither of these responses is essential for activation of gene expression. Similarly, although heavy metals induced apoptosis and gramicidin D promoted necrosis, these pathologies were not obligatory for stimulation of viral gene expression.
Alteration of ND10 is a likely basis for the effects of arsenite and gramicidin D on viral gene expression. These structures form on incoming HSV-1 genomes and are the sites where viral DNA is sequestered during quiescent infection (23, 24). Indeed, the ND10 components PML and Sp100 contribute to an intrinsic antiviral defense against HSV-1, since removal of the proteins by use of small interfering RNA increases the ability of ICP0-null mutants to initiate infection (26, 27). Daxx interacts with HSV-1 DNA even when PML and ND10 are absent and is known to be involved in repression of herpesvirus gene expression, although functional studies with this protein predominantly deal with early events of HCMV infection (9, 26, 57, 62, 70). Displacement of Daxx from ND10 by arsenite treatment would be expected to alleviate repression to some extent, since depletion of the protein increases expression from the HCMV MIEP after infection with in1382 (57), but additional effects must operate since ICP0-null mutants are only marginally complemented by removal of Daxx, similar to the findings upon depleting cells of PML or Sp100 (26; C. M. Preston, unpublished observations). The increased definition of PML foci in arsenite-treated cells may indicate a tighter association with ND10 and hence a decreased ability of the component proteins to recognize incoming genomes. Similarly, the reduced number and higher definition of PML foci in gramicidin D-treated cells may also underlie an inability to repress HSV-1 transcription.
In addition to the highly efficient complementation of ICP0-null HSV-1, arsenite and gramicidin D reactivated expression from quiescent genomes and provoked the resumption of virus replication. After optimal treatments, 5 to 10% of cells produced β-galactosidase at 38.5°C, compared with approximately 50% after infection with tsK. Thus, reactivation occurred in about 10% of cells containing a quiescent genome, a much higher level than those achieved with models of quiescence (excluding those that use viral gene products for reactivation) to date. The reasons for the acquisition of tolerance to the stressors during culture for 8 days, necessitating the use of harsher treatments to achieve effects, are not clear, but it is noteworthy that a similar response was observed when Vero cells containing quiescent HSV-1 were challenged with the deacetylase inhibitor trichostatin A (TSA) (71). It is likely that the extent of virus plaque formation at 32°C underestimated the extent of genome activation, since in1374 remains deficient for VP16 and ICP0 at this temperature and thus may not spread to form plaques efficiently in HFFF2 cells.
Systems of HSV-1 quiescence based on infection of cells of neuronal origin have been described. In general, these rely on the use of inhibitors to prevent viral replication and cell destruction but permit the earliest steps of the gene expression program to proceed. Reactivation of quiescence can be achieved in a number of ways, including heat shock, elevation of cAMP levels, removal of nerve growth factor, and addition of TSA, although only a small proportion of cells produce virus (2, 11, 12, 46, 66, 69, 75, 76). Treatment of cultured ganglia isolated from latently infected mice by heat shock or addition of dexamethasone, but not agents that increase cAMP levels, stimulated reactivation (32). In the HFFF2 system described here, severe heat shock or treatment with stressors was able to promote gene expression from a relatively large proportion of cells containing quiescent genomes, but raising cAMP levels or treatment with TSA was ineffective (55). At present, it is not possible to rationalize fully the apparent differences in requirements for reactivation in the various cell types. It may be that the methods used to generate neuronal cells harboring quiescent HSV-1, in which replication is blocked after initial viral gene expression, result in a less complete repression than that which occurs in fibroblasts, such that some genomes remain responsive to signaling pathways. Alternatively, the very silent state that invariably ensues after infection of fibroblasts with IE-defective HSV-1 mutants may prevent the genome from responding to stimuli that operate in neurons. A recent study demonstrated that repression of the HCMV MIEP was less stringent in cultured neurons than in fibroblasts (71), suggesting that silencing mechanisms may be relatively inefficient in neuronal cell types.
Relating the observations in cell culture systems of quiescence to latency in vivo is problematic but important given the difficulty and inefficiency of reactivating latent HSV-1. Explantation of ganglia is a widely used method of achieving reactivation, although even after this severe stress only a small number of latent genomes respond by resuming replication. It is difficult to provoke reactivation in vivo, but this has been achieved in the mouse by immunosuppression, hyperthermic treatment of animals, or injection of sodium butyrate and in rabbits by iontophoresis of epinephrine (4, 34, 50, 65). Nonetheless, recent studies have shown that the initiation of viral gene expression is not dependent on ICP0 after heat shock in vivo, suggesting that other changes to the intracellular environment are critical (72). In the systems studied, ranging from quiescence in fibroblasts and neuronal cells to latency in vivo, reactivation by heat shock has emerged as a common theme.
The low efficiency of reactivation is a hindrance to understanding in detail how the diverse reactivation stimuli influence neuronal physiology. Although the stimulation of quiescent viral gene expression and replication by arsenite and gramicidin D was most apparent when the stressors were used at high, almost lethal, concentrations, the effect was also observed when smaller amounts, consistent with cell survival, were used. This less efficient response may be more akin to reactivation in vivo. Since an obvious common feature of arsenite and gramicidin D is their toxicity, we suggest that the activation of death pathways, not necessarily to completion, may be linked to the events that trigger reactivation. There is increasing awareness that necrosis and apoptosis are related, rather than mutually exclusive, outcomes of insults to cells (59, 81), and reactivation of virus replication in response to the potential death of the host neuron would represent an important long-term survival mechanism for HSV-1.
Acknowledgments
We thank Duncan McGeoch for helpful comments on the manuscript.
This work was supported by the Medical Research Council.
Footnotes
Published ahead of print on 17 September 2008.
REFERENCES
- 1.Addison, C. L., M. Hitt, D. Kunsken, and F. L. Graham. 1997. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J. Gen. Virol. 781653-1661. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, J. L., C. G. Scarpini, V. Connor, R. H. Lachmann, A. M. Tolkovsky, and S. Efstathiou. 2001. Herpes simplex virus type 1 promoter activity during latency establishment, maintenance, and reactivation in primary dorsal root neurons in vitro. J. Virol. 753885-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block, G. J., C. H. Eskiw, G. Dellaire, and D. P. Bazett-Jones. 2006. Transcription regulation is affected by subnuclear targeting of reporter plasmids to PML nuclear bodies. Mol. Cell. Biol. 268814-8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blyth, W. A., D. A. Harbour, and T. J. Hill. 1980. Effect of immunosuppression on recurrent herpes simplex in mice. Infect. Immun. 29902-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourinbaiar, A. S., and C. F. Coleman. 1997. The effect of gramicidin, a topical contraceptive and antimicrobial agent with anti-HIV activity, against herpes simplex viruses type 1 and 2 in vitro. Arch. Virol. 1422225-2235. [DOI] [PubMed] [Google Scholar]
- 6.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bringhurst, R. A., and P. A. Schaffer. 2006. Cellular stress rather than stage of the cell cycle enhances the replication and plating efficiencies of herpes simplex virus type 1 ICP0− viruses. J. Virol. 804528-4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 654078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantrell, S. R., and W. A. Bresnahan. 2006. Human cytomegalovirus (HCMV) UL82 gene product (pp71) relieves hDaxx-mediated repression of HCMV replication. J. Virol. 806188-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrozza, M. J., and N. A. DeLuca. 1996. Interaction of the viral activator protein ICP4 with TFIID through TAF250. Mol. Cell. Biol. 163085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danaher, R. J., R. J. Jacob, and C. S. Miller. 2006. Reactivation from quiescence does not coincide with a global induction of herpes simplex virus type 1 transactivators. Virus Genes 33163-167. [DOI] [PubMed] [Google Scholar]
- 12.Danaher, R. J., R. J. Jacob, M. R. Steiner, W. R. Allen, J. M. Hill, and C. S. Miller. 2005. Histone deacetylase inhibitors induce reactivation of herpes simplex virus type 1 in a latency-associated transcript-independent manner in neuronal cells. J. Neurovirol. 11306-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davison, M. J., V. G. Preston, and D. J. McGeoch. 1984. Determination of the sequence alteration in the DNA of the herpes simplex virus type 1 temperature-sensitive mutant ts K. J. Gen. Virol. 65859-863. [DOI] [PubMed] [Google Scholar]
- 14.Efstathiou, S., and C. M. Preston. 2005. Towards an understanding of the molecular basis of herpes simplex virus latency. Virus Res. 111108-119. [DOI] [PubMed] [Google Scholar]
- 15.Erl, W., C. Weber, and G. K. Hansson. 2000. Pyrollidone dithiocarbamate-induced apoptosis depends on cell type, density, and the presence of Cu(2+) and Zn(2+). Am. J. Physiol. Cell Physiol. 278C1116-C1125. [DOI] [PubMed] [Google Scholar]
- 16.Everett, R. D. 1989. Construction and characterisation of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 701185-1202. [DOI] [PubMed] [Google Scholar]
- 17.Everett, R. D. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays 22761-770. [DOI] [PubMed] [Google Scholar]
- 18.Everett, R. D. 1984. Transactivation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 33135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett, R. D., C. Boutell, and A. Orr. 2004. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J. Virol. 781763-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 181526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 726581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 135062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett, R. D., and J. Murray. 2005. ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J. Virol. 795078-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everett, R. D., J. Murray, A. Orr, and C. M. Preston. 2007. Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J. Virol. 8110991-11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 177161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everett, R. D., C. Parada, P. Gripon, H. Sirma, and A. Orr. 2008. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 822661-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everett, R. D., S. Rechter, P. Papior, N. Tavalai, T. Stamminger, and A. Orr. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 807995-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedberg, I., G. A. Weisman, and B. K. De. 1985. Permeability change in transformed mouse fibroblasts caused by ionophores, and its relationship to membrane permeabilization by exogenous ATP. J. Membr. Biol. 83251-259. [DOI] [PubMed] [Google Scholar]
- 29.Furuta, S., F. Ortiz, X. Z. Sun, H.-H. Wu, A. Mason, and J. Momand. 2002. Copper uptake is required for pyrrolidine dithiocarbamate-mediated oxidation and protein level increase of p53 in cells. Biochem. J. 365639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goeffroy, M. C., G. Chadeuf, A. Orr, A. Salvetti, and R. D. Everett. 2006. Impact of the interaction between herpes simplex virus type 1 regulatory protein ICP0 and ubiquitin-specific protease USP7 on activation of adeno-associated virus type 2 rep gene expression. J. Virol. 803650-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagglund, R., and B. Roizman. 2004. Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1. J. Virol. 782169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halford, W. P., B. M. Gebhardt, and D. J. Carr. 1996. Mechanisms of herpes simplex virus type 1 reactivation. J. Virol. 705051-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hancock, M. H., J. A. Corcoran, and J. R. Smiley. 2006. Herpes simplex virus regulatory proteins VP16 and ICP0 counteract an innate intranuclear barrier to viral gene expression. Virology 352237-252. [DOI] [PubMed] [Google Scholar]
- 34.Hill, J. M., J. B. Dudley, Y. Shimomura, and H. E. Kaufman. 1986. Quantitation and kinetics of induced HSV-1 ocular shedding. Curr. Eye Res. 5241-246. [DOI] [PubMed] [Google Scholar]
- 35.Hobbs, W. E., D. E. Brough, I. Kovesdi, and N. A. DeLuca. 2001. Efficient activation of viral genomes by levels of herpes simplex virus ICP0 insufficient to affect cellular gene expression or cell survival. J. Virol. 753391-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homer, E. G., A. Rinaldi, M. J. Nicholl, and C. M. Preston. 1999. Activation of herpesvirus gene expression by the human cytomegalovirus protein pp71. J. Virol. 738512-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamieson, D. R. S., L. H. Robinson, J. I. Daksis, M. J. Nicholl, and C. M. Preston. 1995. Quiescent viral genomes in human fibroblasts after infection with herpes simplex virus Vmw65 mutants. J. Gen. Virol. 761417-1431. [DOI] [PubMed] [Google Scholar]
- 38.Jones, C. 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clin. Microbiol. Rev. 1679-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelkar, D. A., and A. Chattopadhyay. 2007. The gramicidin ion channel: a model membrane protein. Biochim. Biophys. Acta 17682011-2025. [DOI] [PubMed] [Google Scholar]
- 40.Khalil, S., J. Luciano, W. Chen, and A. Y.-C. Liu. 2006. Dynamic regulation and involvement of heat shock transcriptional response in arsenic carcinogenesis. J. Cell. Physiol. 207562-569. [DOI] [PubMed] [Google Scholar]
- 41.Kitchin, K. T. 2001. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 172249-261. [DOI] [PubMed] [Google Scholar]
- 42.Lau, A. T. Y., Q.-Y. He, and J.-F. Chiu. 2004. A proteome analysis of the arsenite response in cultured lung cells: evidence for in vitro oxidative stress-induced apoptosis. Biochem. J. 382641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall, K. R., K. V. Rowley, A. Rinaldi, I. P. Nicholson, A. M. Ishov, G. G. Maul, and C. M. Preston. 2002. Activity and intracellular localization of the human cytomegalovirus protein pp71. J. Gen. Virol. 831601-1612. [DOI] [PubMed] [Google Scholar]
- 44.Mastrocola, T., A. Flamigni, and M. Rugulo. 1991. Hypotonic shock activated Cl− and K+ pathways in human fibroblasts. Biochim. Biophys. Acta 1069201-208. [DOI] [PubMed] [Google Scholar]
- 45.McFarlane, M., J. I. Daksis, and C. M. Preston. 1992. Hexamethylene bisacetamide stimulates herpes simplex virus immediate early gene expression in the absence of trans-induction by Vmw65. J. Gen. Virol. 73285-292. [DOI] [PubMed] [Google Scholar]
- 46.Miller, C. S., R. J. Danaher, and R. J. Jacob. 2006. ICP0 is not required for efficient stress-induced reactivation of herpes simplex virus type 1 from cultured quiescently infected neuronal cells. J. Virol. 803360-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minaker, R. L., K. L. Mossman, and J. R. Smiley. 2005. Functional inaccessibility of quiescent herpes simplex virus genomes. Virol. J. 285-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosca, J. D., D. P. Bednarik, N. B. Raj, C. A. Rosen, J. G. Sodroski, W. A. Haseltine, G. S. Hayward, and P. M. Pitha. 1987. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeats that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 847408-7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mossman, K. L., P. F. MacGregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neumann, D. M., P. S. Bhattacharjee, and J. M. Hill. 2007. Sodium butyrate: a chemical inducer of in vivo reactivation of herpes simplex virus type 1 in the ocular mouse model. J. Virol. 816106-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Hare, P. 1993. The virion transactivator of herpes simplex virus. Semin. Virol. 4145-155. [Google Scholar]
- 52.O'Hare, P., and G. S. Hayward. 1985. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J. Virol. 53751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preston, C. M. 1979. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature sensitive mutant tsK. J. Virol. 29275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preston, C. M. 2007. Reactivation of expression from quiescent herpes simplex virus type 1 genomes in the absence of immediate-early protein ICP0. J. Virol. 8111781-11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Preston, C. M., and M. J. Nicholl. 2005. Human cytomegalovirus tegument protein pp71 directs long-term gene expression from quiescent herpes simplex virus genomes. J. Virol. 79525-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Preston, C. M., and M. J. Nicholl. 1997. Repression of gene expression upon infection of cells with herpes simplex virus type 1 mutants impaired for immediate-early protein synthesis. J. Virol. 717807-7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Preston, C. M., and M. J. Nicholl. 2006. Role of the cellular protein hDaxx in human cytomegalovirus immediate-early gene expression. J. Gen. Virol. 871113-1121. [DOI] [PubMed] [Google Scholar]
- 58.Preston, C. M., A. Rinaldi, and M. J. Nicholl. 1998. Herpes simplex virus type 1 immediate early gene expression is stimulated by inhibition of protein synthesis. J. Gen. Virol. 79117-124. [DOI] [PubMed] [Google Scholar]
- 59.Proskuryakov, S. Y., A. G. Knonplyannikov, and V. L. Gabai. 2003. Necrosis: a specific form of programmed cell death? Exp. Cell Res. 2831-16. [DOI] [PubMed] [Google Scholar]
- 60.Rea, M. A., J. P. Gregg, Q. Qin, M. A. Phillips, and R. H. Rice. 2003. Global alteration of gene expression in human keratinocytes by inorganic arsenite. Carcinogenesis 24747-756. [DOI] [PubMed] [Google Scholar]
- 61.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in culture. J. Virol. 61829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saffert, R. T., and R. F. Kalejta. 2006. Inactivating a cellular intrinsic defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 803863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samaniego, L. A., L. Neiderhiser, and N. A. DeLuca. 1998. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J. Virol. 723307-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sampath, P., and N. A. DeLuca. 2008. Binding of ICP4, TATA-binding protein, and RNA polymerase II to herpes simplex virus type 1 immediate-early, early and late promoters in virus-infected cells. J. Virol. 822339-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sawtell, N. M., and R. L. Thompson. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 662150-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith, R. L., J. M. Escudero, and C. L. Wilcox. 1992. Activation of second messenger pathways activates latent herpes simplex virus in neuronal cultures. Virology 188311-318. [DOI] [PubMed] [Google Scholar]
- 67.Stow, E. C., and N. D. Stow. 1989. Complementation of a herpes simplex virus type 1 Vmw110 deletion mutant by human cytomegalovirus. J. Gen. Virol. 70695-704. [DOI] [PubMed] [Google Scholar]
- 68.Stow, N. D., and E. C. Stow. 1986. Isolation and characterisation of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 672571-2585. [DOI] [PubMed] [Google Scholar]
- 69.Su, Y.-H., R. L. Meegalla, R. Chowhan, C. Cubitt, J. E. Oakes, R. N. Lausch, N. W. Fraser, and T. M. Block. 1999. Human corneal cells and other fibroblasts can stimulate the appearance of herpes simplex virus from quiescently infected PC12 cells. J. Virol. 734171-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tavalai, N., P. Papior, S. Rechter, and T. Stamminger. 2008. Nuclear domain 10 components promyelocytic leukemia protein and hDaxx independently contribute to an intrinsic antiviral defense against human cytomegalovirus infection. J. Virol. 82126-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Terry-Allison, T., C. A. Smith, and N. A. DeLuca. 2007. Relaxed repression of herpes simplex virus type 1 genomes in murine trigeminal neurons. J. Virol. 8112394-12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson, R. L., and N. M. Sawtell. 2006. Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J. Virol. 8010919-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallace, B. A. 2000. Common structural features in gramicidin and other ion channels. Bioessays 22227-234. [DOI] [PubMed] [Google Scholar]
- 75.Wigdahl, B. L., R. J. Ziegler, M. Sneve, and F. Rapp. 1983. Herpes simplex virus latency in isolated rat sensory neurons. Virology 127159-167. [DOI] [PubMed] [Google Scholar]
- 76.Wilcox, C. L., R. L. Smith, C. R. Freed, and E. M. Johnson. 1990. Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J. Neurosci. 1041268-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wysocka, J., and W. Herr. 2003. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem. Sci. 28294-304. [DOI] [PubMed] [Google Scholar]
- 78.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 696249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yih, L.-H., K. Peck, and T.-C. Lee. 2002. Changes in gene expression profiles of human fibroblasts in response to sodium arsenite treatment. Carcinogenesis 23867-876. [DOI] [PubMed] [Google Scholar]
- 80.Zabierowski, S., and N. A. DeLuca. 2004. Differential cellular requirements for activation of herpes simplex virus type 1 early (tk) and late (gC) promoters by ICP4. J. Virol. 786162-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zong, W. X., and C. B. Thompson. 2006. Necrotic death as a cell fate. Genes Dev. 201-15. [DOI] [PubMed] [Google Scholar]