Abstract
Kaposi's sarcoma (KS) is a vascular tumor of proliferative endothelial cells caused by KS-associated herpesvirus (KSHV) infection. Aberrant vascular permeability is a hallmark of KS manifested as multifocal edematous skin and visceral lesions with dysregulated angiogenesis and vast inflammatory infiltrations. In this study, we showed that KSHV infection increased the permeability of confluent endothelial monolayers to serum albumin, blood-derived cells, KSHV-infected cells, and KSHV virions. KSHV-induced permeability was associated with the disruption of adherens junctions and the degradation of vascular endothelial cadherin (VE-cadherin) protein. Both the inactivation of KSHV virions by UV irradiation and the blockage of de novo protein synthesis with cycloheximide failed to reverse the KSHV-induced disruption of adherens junctions. However, soluble heparin that blocked KSHV entry into cells completely inhibited KSHV-induced permeability. Furthermore, the KSHV-induced degradation of VE-cadherin was dose dependent on the internalized virus particles. Together, these results indicate that KSHV infection induces vascular permeability by inducing VE-cadherin degradation during virus entry into cells. KSHV-induced aberrant vascular permeability could facilitate virus spread, promote inflammation and angiogenesis, and contribute to the pathogenesis of KSHV-induced malignancies.
Endothelial cells form single-cell layers underlying the vascular or lymphatic circulation networks (71). Functional endothelial layers serve as physical barriers on the luminal surface of blood or lymph fluid to modulate the exchange of leukocytes and materials between the circulation systems and tissues. Selective permeability of the vasculatures to different macromolecules or cells ensures the dynamic needs of functional tissues under diverse physiological or pathological conditions (71).
Several plasma membrane junction structures, including gap junctions, adherens junctions, tight junctions, and desmosomal junctions, regulate the integrity and permeability of endothelial barriers (23). Adherens junctions consisting of vascular endothelial cadherins (VE-cadherins), and the associated intracellular partners α-catenin, β-catenin, plakoglobin (γ-catenin), and p120ctn, play a key role in this process. Both extracellular and intracellular factors, such as physical stress, infection by microorganisms, dysregulated cytokines, and abnormal intracellular signaling, alter the endothelial permeability by regulating adherens junctions (49). For example, abnormal permeability of the vasculatures is commonly seen in cancer and mediates the intravasation, extravasation, and dissemination of cancer cells as well as cancer-induced angiogenesis (15). A number of pathogenic viruses have also evolved to attain specific mechanisms, including the ability to disrupt adherens junctions, to compromise the integrity of the endothelium (62). The disruption of endothelial barriers allows the viruses to gain access to the entry receptors in the basolateral membranes and facilitates their trafficking to other tissues through the lymphatic and vascular networks. While some viruses, such as Ebola virus and human immunodeficiency virus type 1 (HIV-1), induce the disruption of endothelial barriers with specific cytotoxic viral products (42, 83), others such as respiratory syncytial virus and hantaviruses target the endothelium by inducing specific cellular cytokines (33, 47). The human cytomegalovirus disrupts adherens junctions by inducing the internalization and degradation of VE-cadherin to promote the extravasation of naive monocytes and the transfer of productive virus (7).
Infection by Kaposi's sarcoma-associated herpesvirus (KSHV) is associated with the development of Kaposi's sarcoma (KS), the most common malignancy in AIDS patients (22). KS is an angiogenic vascular tumor, primarily consisting of proliferative spindle endothelial cells with infiltration of red blood cells and inflammatory cells, such as mononuclear cells, plasma cells, and macrophages (38). KS is a highly disseminated tumor manifested as multifocal lesions and is often involved with visceral organs at the advanced stages of the disease. Furthermore, KS edema is commonly seen in patients with advanced disease, and a profound edematous KS lesion is associated with a poor prognosis (11, 25, 36). Together, these histological and clinical manifestations indicate that aberrant vascular permeability is one of the pathological hallmarks of KS (38).
A number of studies have shown that KSHV can efficiently infect human primary endothelial cells in vitro and induce an angiogenic and invasive phenotype (17, 26-28, 57, 84). However, how KSHV infection regulates endothelial permeability has not been examined. In this study, we have found that KSHV infection of confluent monolayers of primary human umbilical vein endothelial cells (HUVEC) increases the endothelial permeability to serum albumin, blood-derived cells, KSHV-infected cells, and KSHV virions. This leaky phenotype is associated with the virion-mediated disruption of adherens junctions and the degradation of VE-cadherin.
MATERIALS AND METHODS
Cell culture and virus infection.
HUVEC and the growth medium EGM2 were purchased from Lonza (Allendale, NJ). BCP-1 cells isolated from peripheral blood mononuclear cells of an HIV-negative primary effusion lymphoma (PEL) patient were used to prepare virus stocks (30). A KSHV-negative B-cell line, Bjab, was used as a control cell line for preparing control virus stocks. Virus induction and concentration were carried out as previously described (84). Briefly, log-phase BCP-1 cells cultured in RPMI 1640 supplemented with 10% fetal bovine serum and 50 μg/ml of gentamicin were induced with 20 ng/ml of 12-O-tetradecanoyl-phorbol-13-acetate and 0.3 mM of sodium butyrate for 2 days. The culture was then replaced with fresh medium without the inducing agents and cultured for another 3 days. The supernatant was then centrifuged twice at 5,000 × g for 10 min to eliminate cell debris and then at 100,000 × g for 1 h with 20% sucrose as a cushion. The final pellets were dissolved in endothelial cell culture medium overnight and used in the experiments. The titers of virus preparations were determined by infecting 293 cells and calculating the number of KSHV-infected cells (infectious units) generated by each ml of virus preparation at 48 h postinfection (hpi) (28). Positive cells were identified by staining for viral latent nuclear antigen (LANA). We used a multiplicity of infection of 5 for the experiments unless otherwise specified. Under our experimental conditions, we usually observed an 80 to 90% infection rate at 48 hpi in the confluent endothelial monolayers. In some experiments, cells were infected with UV light-irradiated KSHV (UV-KSHV), while other infection experiments were carried out in the presence of cycloheximide (CHX) at 75 μg/ml or soluble heparin at 10 μg/ml (Sigma, St. Louis, MO). UV irradiation was carried out under a 30 W UV lamp with a distance of 15 cm for 5 min. Under this condition, a typical infection rate of 80 to 90% of a virus preparation was reduced to <5%, while the internalization of viral particles per cell (vpc) was reduced by 40% (see Fig. 4E to G).
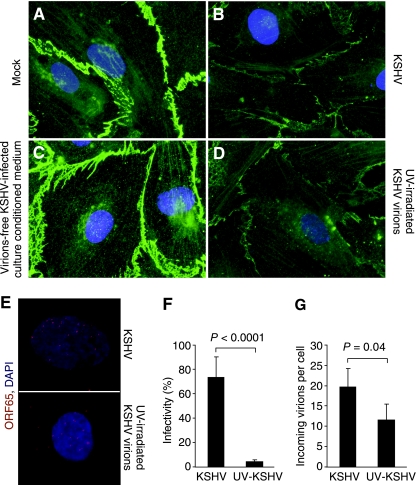
FIG. 4.
Disruption of adherens junctions in UV-irradiated KSHV (UV-KSHV)-infected confluent endothelial monolayers but not in those treated with conditioned medium from KSHV-infected endothelial cells. (A and B) Adherens junctions revealed by VE-cadherin staining in mock- or KSHV-infected confluent endothelial monolayers at 6 hpi. (C) VE-cadherin staining in confluent endothelial monolayers treated with KSHV-conditioned medium for 6 h. Conditioned medium was obtained from confluent endothelial monolayers that displayed a clear disruption of adherens junctions after infection with KSHV for 6 hpi. The medium was subjected to high-speed centrifugation to eliminate any virions and cellular debris. A new confluent endothelial monolayer was then incubated with the medium for 6 h and stained for VE-cadherin. (D) VE-cadherin staining of a UV-KSHV-infected confluent endothelial monolayer at 6 hpi. (E) Representative pictures showing viral particles revealed by ORF65 staining in endothelial cells infected with KSHV or UV-KSHV at 4 hpi. (F) Infectivity of KSHV and UV-KSHV was examined by infecting cells for 2 days, staining for the expression of KSHV LANA, and calculating the percentages of positive cells. (G) Internalized viral particles in cells infected by KSHV or UV-KSHV for 4 h. Viral particles were detected by staining for the ORF65 protein.
Transwell endothelial permeability assay.
HUVEC were cultured on transwell inserts containing polycarbonate membranes with 8-μm pores (Nunc, Rochester, NY). Confluent endothelial monolayers were used for KSHV infection 2 to 3 days after they reached confluence. KSHV infection was carried out for 4 h as previously described (84). The inoculum was then washed away by rinsing the inserts several times with the culture medium. The KSHV-infected endothelial monolayers were evaluated for permeability to serum albumin, blood-derived cells, KSHV-infected cells, and virions. The cells were also evaluated for protein expression by immunofluorescence staining or Western-blotting analysis. To assess the permeability of endothelial monolayers to serum albumin, 100 μl of methylene blue-conjugated bovine serum albumin at 20% was added to the insert and incubated for 1 h. The amount of serum albumin that penetrated the endothelial monolayers and reached the lower chamber was determined by measuring the optical density at 405 nm with a Synergy HT microplate reader (BioTek Instruments, Inc., Winooski, VT). To assess the permeability of the endothelial monolayers to a blood-derived monocyte cell line U937, the cells were first prestained with VybrantDiO cell-labeling solution according to the instructions of the manufacturer (Invitrogen, Carlsbad, CA). A total of 105 labeled cells in 250 μl of culture medium were placed on endothelial monolayers and cultured for 6 h. The numbers of fluorescent cells that migrated through the endothelial monolayers and reached the lower chambers were then counted with a Zeiss Axiovert 200 M fluorescent microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY). BCBL-1 cells containing a recombinant KSHV BAC36 were also used in the permeability assay as described for U937 (86). Because of the presence of a green fluorescent protein (GFP) cassette in the viral genome, these cells express GFP and can be counted directly with the fluorescent microscope. To assess the permeability of endothelial monolayers to KSHV virions, virus preparations at 105 infectious units in 250 μl of culture medium were added to the monolayers and incubated for 1 h. Infectious virions that penetrated through the endothelial monolayers and reached the lower chambers were titrated as described above.
Immunofluorescence antibody assay.
The cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C for 20 min and then permeabilized with 0.5% Triton X-100 in PBS at 4°C for 10 min. The cells were blocked with 3% bovine serum albumin in PBS at 37°C for 1 h followed by incubation with a primary antibody at 37°C for 1 h. To detect VE-cadherin protein, the cells were incubated with a rabbit anti-VE-cadherin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and revealed with a goat anti-rabbit immunoglobulin G (IgG) Alexa Fluor 568 conjugate or a goat anti-rabbit IgG Alexa Fluor 488 conjugate (Invitrogen). To detect LANA, the cells were incubated with a rat anti-LANA monoclonal antibody (ABI, Columbia, MD) and revealed with a goat anti-rat IgG Alexa Fluor 568 conjugate (Invitrogen). KSHV particles were detected by staining KSHV small capsid protein encoded by open reading frame 65 (ORF65) with a mouse monoclonal antibody (28) and revealed with a goat anti-mouse IgG Alexa Fluor 568 conjugate (Invitrogen). To detect nuclei, the cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma). The stained cells were examined with a Zeiss Axiovert 200 M fluorescent microscope.
Western-blotting analysis.
Protein extracts were resolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes as previously described (29). To detect VE-cadherin protein, the membranes were first incubated with a mouse monoclonal antibody to VE-cadherin (Santa Cruz) and then with a goat anti-mouse IgG horseradish peroxidase conjugate (EMD Biosciences, Inc., San Diego, CA). A mouse antibody to β-tubulin (Sigma) was used to monitor sample loading. Specific signals were revealed with chemiluminescence substrates, and recorded on films or with a Kodak Image Station 2000 MM multimodal imager (Eastman Kodak Company, Rochester, NY).
RT-qPCR.
Reverse transcription real-time quantitative PCR (RT-qPCR) was carried out as previously described (85). Total RNA was extracted with an RNA isolation kit (Promega, Madison, WI). cDNA was obtained using Superscript II RNase H− reverse transcriptase with a poly(dT)18 primer (Invitrogen). A control without reverse transcriptase was conducted in parallel. qPCR was carried out in a DNA engine Opticon 2 continuous fluorescence detector (Bio-Rad Laboratories, Hercules, CA) as previously described (85). The primers for VE-cadherin were 5′-TCACCTGGTCGCCAATCC-3′ (forward) and 5′-AGGCCACATCTTGGGTTCCT-3′ (reverse), which amplified a product of 63 bp. The LANA primers were 5′-GCAGACACTGAAACGCTGAA-3′ (forward) and 5′-AGGTGAGCCACCAGGACTTA-3′ (reverse), which amplified a product of 101 bp. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization. The GAPDH primers were 5′-ACAGTCAGCCGCATCTTCTT-3′ (forward) and 5′-ACGACCAAATCCGTTGACTC-3′ (reverse).
RESULTS
KSHV infection increases the permeability of confluent endothelial monolayers to serum albumin, blood-derived cells, KSHV-infected PEL cells, and KSHV virions.
We employed a transwell permeability assay to examine the KSHV regulation of endothelial permeability. Confluent HUVEC monolayers cultured on transwell inserts were infected with KSHV at a multiplicity of infection of 5. Under this condition, we usually observed an 80 to 90% infection rate at 48 hpi. At different time points postinfection, we examined the permeability of the endothelial monolayers to serum albumin, the most abundant protein in the blood. As shown in Fig. 1A, KSHV infection increased the permeability of the endothelial monolayers to serum albumin as early as 6 hpi, and the permeability reached fourfold at 24 hpi. In these experiments, we used the complete endothelial cell culture medium as mock controls since the virus preparations were concentrated and resuspended in this medium. Furthermore, we did not observe any changes in the endothelial permeability when mock virus preparations from a KSHV-negative cell line Bjab were used in the experiments (data not shown), indicating that the observed effect was specific but not due to any cellular debris resulting from the cell culture.
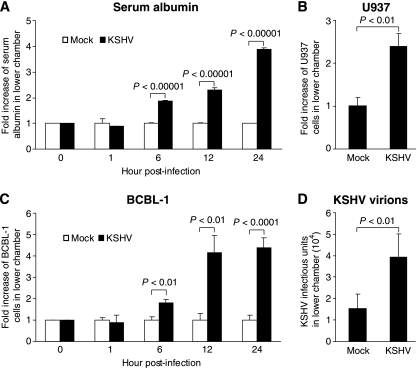
FIG. 1.
KSHV infection increases the permeability of confluent endothelial monolayers to serum albumin, blood-derived U937 cells, KSHV-infected BCBL-1 cells, and KSHV virions. (A) Kinetics of endothelial permeability to serum albumin following KSHV infection. Confluent endothelial monolayers were mock infected or infected with KSHV and assayed for endothelial permeability to methylene blue-conjugated serum albumin at different hpi. The amount of serum albumin that migrated to the lower chamber was determined by measuring the optical density at 405 nm. (B) Endothelial permeability of KSHV-infected confluent endothelial monolayers to VybrantDiO cell-labeled U937 cells at 24 hpi. The number of U937 cells that migrated to the lower chamber was counted with a fluorescence microscope. (C) Kinetics of endothelial permeability to BCBL-1 cells following KSHV infection. BCBL-1 cells containing BAC36 and expressing GFP were used for the assay. The number of BCBL-1 cells that migrated to the lower chamber was counted with a fluorescence microscope. (D) Endothelial permeability of KSHV-infected confluent endothelial monolayers to KSHV virions at 24 hpi. Virus preparations containing 105 infectious units in 250 μl culture medium were added to the monolayers and incubated for 1 h. The amount of infectious virions that penetrated the endothelial monolayers and reached the lower chamber was titrated for infectious units.
In addition to soluble materials, there are abundant leukocytes in the bloodstream and lymph fluids that actively traffic between the circulation system and tissues. Conditions such as infections that trigger inflammatory responses promote the infiltration of leukocytes to tissues. Since inflammatory infiltrations are commonly seen in KS patients (38), we examined the permeability of endothelial monolayers to U937 cells, a blood-derived monocyte cell line. As shown in Fig. 1B, KSHV infection increased endothelial permeability to U937 by 2.3-fold at 12 hpi.
Next, we determined whether KSHV infection altered the permeability of endothelial monolayers to KSHV-infected cells and KSHV virions as these outcomes might be relevant to the dissemination of KSHV to adjacent and distal tissues in the infected subjects. As shown in Fig. 1C, KSHV infection altered the permeability of endothelial monolayers to BCBL-1 cells, a KSHV-infected cell line derived from PEL, with a pattern similar to that for serum albumin. The endothelial permeability to KSHV virions was also increased by 2.8-fold following KSHV infection at 12 hpi (Fig. 1D).
Together, these results indicated that KSHV infection increased the permeability of endothelial monolayers to blood soluble materials, blood-derived cells, KSHV-infected cells, and KSHV virions.
KSHV infection disrupts the adherens junctions of confluent endothelial monolayers.
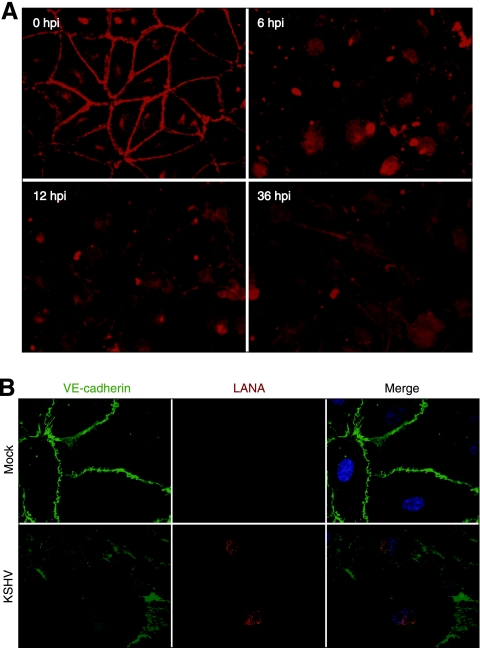
Since adherens junctions between endothelial cells regulate endothelial permeability, we examined the effect of KSHV infection on these membrane structures by staining VE-cadherin, the main component of these structures. As shown in Fig. 2A, confluent endothelial monolayers had strong and well-aligned VE-cadherin structures tightly distributed between the borders of the adjacent cells as well as some weak intracellular VE-cadherin staining. Following KSHV infection, the adherens junctions were disrupted. At 6 hpi, the intensity of VE-cadherin along the cell borders decreased significantly (Fig. 2A). After 12 hpi, the peripheral structures of VE-cadherin were disorganized and often became undetectable. To demonstrate that the endothelial cells were indeed infected by KSHV, we stained them for the expression of viral LANA. At 24 hpi, we observed the typical speckled nuclear LANA staining patterns in cells that manifested disrupted adherens junctions (Fig. 2B). Overall, as expected, we found that the kinetics of disruption of adherens junctions correlated with the increased permeability of the endothelial monolayers. Thus, KSHV infection induced the disruption of adherens junctions to promote endothelial permeability. Interestingly, while the disruption of adherens junctions was observed at the early stage of KSHV infection, it was sustained for at least up to 36 hpi, indicating that the disrupted membrane structures were not recovered. These results were consistent with the endothelial permeability results (Fig. 1) and suggested that KSHV infection continued to modulate vascular permeability at the later stages of infection.
FIG. 2.
KSHV infection disrupts the adherens junctions. (A) VE-cadherin staining of KSHV-infected confluent endothelial monolayers at different hpi. (B) Mock- and KSHV-infected confluent endothelial monolayers stained for VE-cadherin protein (green), KSHV LANA (red), and nuclei (blue) at 24 hpi.
KSHV infection of confluent endothelial monolayers downregulates the protein level of VE-cadherin.
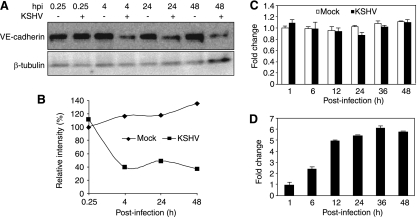
Since immunofluorescence staining had shown a reduced intensity of adherens junctions following KSHV infection (Fig. 2), we examined the alteration of the VE-cadherin protein level by Western-blotting analysis. In agreement with the immunofluorescence staining results, we found that KSHV infection reduced the VE-cadherin protein level by as much as 60% at 4 hpi (Fig. 3A and B). These results indicated that KSHV infection downregulated the protein level of VE-cadherin at the early stage of infection. Similar to the immunofluorescence staining results, we observed that the reduction of the VE-cadherin protein level was sustained for up to 48 hpi, the latest time point that we had examined (Fig. 3A and B). While RT-qPCR detected an expected increase in the expression of LANA transcripts following KSHV infection (Fig. 3D), we did not observe any significant alterations of the VE-cadherin transcripts (Fig. 3C), indicating that KSHV infection did not affect the expression of VE-cadherin at the transcription level. The fact that we did not observe a recovery of the VE-cadherin protein level at 48 hpi but detected a constant level of VE-cadherin transcripts suggested that, in addition to the downregulation of the protein level at the early stage of infection, another mechanism might be involved in regulating this protein at the posttranscriptional level at the later stages of KSHV infection. In the following experiments, we focused our efforts on the early stage of KSHV infection.
FIG. 3.
Expression kinetics of VE-cadherin protein and transcripts following KSHV infection. (A) Western-blotting analysis of VE-cadherin protein in mock- or KSHV-infected endothelial cells at different hpi. β-Tubulin was used to calibrate protein loading. (B) Quantification of VE-cadherin protein levels at the different hpi shown in panel A. The VE-cadherin protein amount of mock-infected endothelial cells at 0.25 hpi was set at 100%. (C to D) Expression of VE-cadherin (C) and LANA (ORF73) (D) transcripts in mock- or KSHV-infected endothelial cells at different hpi quantified by reverse transcription real-time quantitative PCR (RT-qPCR). RT-qPCR for GAPDH was used as a loading control. The level of VE-cadherin transcripts of mock-infected endothelial cells at 1 hpi was set at 1. The level of LANA transcripts of KSHV-infected endothelial cells at 1 hpi was set at 1. LANA transcripts were not detected in mock-infected cells at any time point.
The KSHV-induced disruption of adherens junctions and the downregulation of VE-cadherin at the early stage of infection are mediated by virus entry and do not require the expression of viral genes.
The fact that we observed the disruption of adherens junctions and a reduced level of VE-cadherin protein at the early stage of KSHV infection suggested the involvement of virus entry in this process. Several studies have shown that KSHV infection of HUVEC induces the secretion of proinflammatory and proangiogenic cytokines at the early stage of KSHV infection (27, 57, 81, 84). While some proinflammatory and proangiogenic cytokines have been shown to regulate endothelial permeability in some pathological conditions (21, 33, 34, 47, 65), confluent endothelial cells are usually refractory to these cytokines partly because of the growth contact-inhibition effect exerted by the adherens junctions (13, 37, 46, 64). Indeed, we observed the contact inhibition and maintenance of intact adherens junctions in normal confluent endothelial monolayers cultured in medium containing growth and proangiogenic factors, such as vascular endothelial growth factor (VEGF), human fibroblast growth factor B, R3-IGF-1, human epidermal growth factor, and fetal bovine serum (Fig. 2 and 4A). To exclude any involvement of KSHV-induced cytokines and soluble factors in the disruption of adherens junctions at the early stage of viral infection, we infected confluent endothelial monolayers with KSHV for 6 h, at which time point we observed a clear disruption of adherens junctions (Fig. 4B). We then collected the culture supernatants and subjected them to high-speed centrifugation to eliminate virions and cellular debris. The remaining supernatants were added to the uninfected confluent endothelial monolayers. We followed the disruption of the adherens junctions of the endothelial monolayers. As shown in Fig. 4C, we did not observe any obvious signs of disruption of adherens junctions 6 h after the addition of the conditioned medium. These results indicated that the KSHV-induced disruption of adherens junctions at the early stage of infection was unlikely to be mediated by cytokines or other soluble factors induced by KSHV infection.
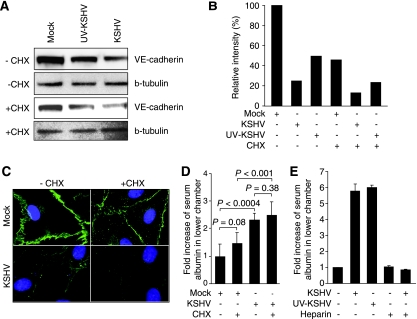
Next, we examined the direct effect of virus entry on adherens junctions. Our previous study has shown that there is minimal expression of viral genes at 6 hpi during KSHV primary infection (85). Since we observed the disruption of adherens junctions before 6 hpi, we postulated that the KSHV-induced disruption of adherens junctions did not require the expression of viral genes. To test this hypothesis, we inactivated the virus with UV. As shown in Fig. 4D, UV-KSHV remained almost as effective as the untreated KSHV in disrupting the adherens junctions. Under this condition, the virus infectivity was reduced by >95%, but the number of internalized vpc was only reduced 40% from an average of 20 to 12 per cell (Fig. 4E to G).
We then examined whether UV-KSHV also downregulated the protein level of VE-cadherin. As shown in Fig. 5A and B, the protein level of VE-cadherin was reduced by 50% in UV-KSHV-infected cells, while that of the KSHV-infected cells was reduced by 75% in Western-blotting analysis. These results confirmed that the KSHV-induced downregulation of VE-cadherin did not require the expression of viral genes. To further determine whether the expression of cellular genes was required for KSHV-induced VE-cadherin degradation, we infected the confluent endothelial monolayers with either untreated KSHV or UV-KSHV in the presence of CHX. Since CHX blocked de novo protein synthesis, the protein level of VE-cadherin in CHX-treated, mock-infected cells was reduced by 55%, while those of KSHV- or UV-KSHV-infected cells were further reduced, by a total of 88% and 77%, respectively (Fig. 5A and B). Thus, compared to mock-infected cells, in the presence of CHX, KSHV and UV-KSHV further reduced the protein level of VE-cadherin by 73% and 49%, respectively. Because of the lack of de novo protein synthesis in the presence of CHX, these results also indicated that the KSHV-induced downregulation of VE-cadherin protein at the early stage of infection was due to the KSHV-induced degradation of the protein.
FIG. 5.
The disruption of adherens junctions and the degradation of VE-cadherin protein during the KSHV primary infection of confluent endothelial monolayers are mediated by virus entry and do not require de novo synthesis of viral and cellular proteins. (A) Western-blotting analysis of VE-cadherin protein in mock-, KSHV-, and UV-KSHV-infected confluent endothelial monolayers with (+) or without (−) the presence of CHX. The infection was carried out for 4 h. β-Tubulin was used to calibrate protein loading. (B) Quantification of the VE-cadherin protein levels shown in panel A. The VE-cadherin protein level of mock-infected endothelial cells without the presence of CHX was set at 100%. (C) KSHV infection disrupted adherens junctions in the presence of CHX. Confluent endothelial monolayers were either mock-infected or infected with KSHV with or without the presence of CHX for 4 h and stained for VE-cadherin. (D) KSHV infection increased endothelial permeability to serum albumin in the presence of CHX. Confluent endothelial monolayers were either mock-infected or infected with KSHV with or without the presence of CHX. The permeability of the cells to serum albumin was examined as described in Fig. 1A. (E) UV-KSHV infection induced endothelial permeability to serum albumin, which was inhibited by soluble heparin. Confluent endothelial monolayers were either mock-infected or infected with KSHV or UV-KSHV with or without the presence of soluble heparin. The permeability of the cells to serum albumin was examined as described in the legend to Fig. 1A.
The results of the immunofluorescence staining were consistent with those of the Western-blotting analysis. While CHX-treated mock-infected cells had a reduced intensity of VE-cadherin staining, their adherens junctions remained largely intact (Fig. 5C). As expected, treatment with CHX further accelerated the disruption of the adherens junctions of the endothelial monolayers infected with KSHV (Fig. 5C). Consistent with these results, treatment with CHX did not significantly affect the endothelial permeability of both the mock- and KSHV-infected monolayers. However, the KSHV-infected monolayers had higher endothelial permeability than the mock-infected monolayers with or without the presence of CHX (Fig. 5D).
The above results indicated that KSHV-induced VE-cadherin degradation was mediated by KSHV entry. The first step in KSHV entry into cells is the attachment of virions to the cell surface, a step mediated by heparin sulfate. Previous studies have shown that the presence of soluble heparin in the culture medium can block KSHV entry into cells, and hence KSHV infection of cells (8, 75). To further confirm that KSHV entry into cells mediated the KSHV-induced degradation of VE-cadherin, we infected the endothelial monolayers with KSHV and UV-KSHV in the presence of soluble heparin. As expected, the addition of heparin totally blocked KSHV infection, as monitored by LANA staining of the infected cells at 48 hpi, and the entry of viral particles into the cells, as monitored by staining for the ORF65 viral small capsid protein (data not shown). In parallel experiments, we found that heparin totally inhibited KSHV and UV-KSHV induction of endothelial permeability (Fig. 5E).
Taken together, these results so far indicated that the KSHV-induced disruption of adherens junctions and endothelial permeability were mediated by virus entry and did not require the expression of viral and cellular genes.
Kinetics of KSHV-induced VE-cadherin degradation.
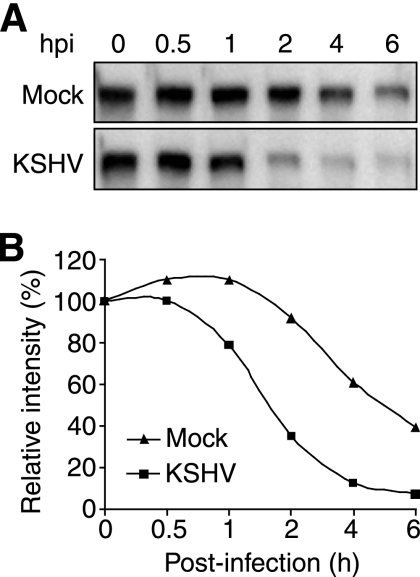
To further define KSHV-induced VE-cadherin degradation, we examined the kinetics of VE-cadherin degradation by blocking de novo protein synthesis with CHX. As shown in Fig. 6, the levels of VE-cadherin in mock-infected cells decreased following treatment with CHX; however, those of the KSHV-infected cells decreased at a much faster rate. While it took 5 h for the mock-infected cells to reduce the level of VE-cadherin by 50%, it only took 1.5 h for the KSHV-infected cells to reach the same level. At 5 hpi, less than 10% of the VE-cadherin protein pool remained in the KSHV-infected cells (Fig. 6). Thus, in the absence of de novo protein synthesis, KSHV infection increased the degradation rate of VE-cadherin by more than threefold.
FIG. 6.
Kinetics of VE-cadherin protein degradation during the KSHV primary infection of confluent endothelial monolayers. (A) Western-blotting analysis for VE-cadherin protein was carried out with confluent endothelial monolayers mock-infected or infected with KSHV in the presence of CHX at different hpi. (B) Quantification of the VE-cadherin protein levels at the different hpi shown in panel A. The VE-cadherin protein level of mock-infected cells at 0 hpi was set at 100%.
KSHV induction of VE-cadherin degradation correlates with the number of internalized viral particles.
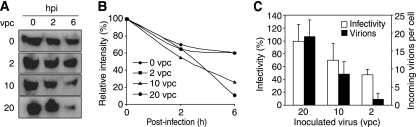
While UV-KSHV disrupted the adherens junctions as effectively as KSHV (Fig. 4D), compared to KSHV, they induced the degradation of VE-cadherin at a reduced rate (Fig. 5A and B). UV-KSHV induced about 33% less protein degradation of VE-cadherin than KSHV with or without the presence of CHX (50% versus 75% without CHX and 49% versus 73% with CHX in Fig. 5A and B, respectively). This reduced rate of VE-cadherin degradation appeared to correlate with the reduction of internalized viral particles (Fig. 4 and 5). To further provide evidence to support this relationship, we infected the endothelial monolayers with increased numbers of vpc in the presence of CHX. In these experiments, a 20-vpc inoculum gave an 80 to 90% infection rate, as examined by LANA staining at 48 hpi. As shown in Fig. 7A and B, we observed a clear vpc dose-dependent trend of VE-cadherin degradation. When the inoculum was reduced from 20 to 2 vpc, the number of actual internalized vpc was reduced from 18 to 1.8 as expected; however, the infectivity was only reduced 51% (Fig. 7C). These results demonstrated the dependence of VE-cadherin degradation on the number of internalized viral particles rather than virus infectivity.
FIG. 7.
The KSHV-induced degradation of VE-cadherin protein is dose dependent on the internalization of viral particles but not infectivity. (A) Western-blotting analysis for VE-cadherin protein was carried out with confluent endothelial monolayers mock-infected or infected with KSHV with different vpc in the presence of CHX at different hpi. (B) Quantification of the VE-cadherin protein levels shown in panel A. The VE-cadherin protein levels at 0 hpi were set at 100%. (C) Infectivity and internalized viral particles in cells infected with the same doses of vpc as described in panel A. The actual internalized viral particles were revealed by staining for the ORF65 protein at 4 hpi. KSHV infectivity was examined by staining for the expression of KSHV LANA and calculating the percentages of positive cells.
DISCUSSION
Both histological features and clinical manifestations indicate that KS is a disease with dysregulated endothelial permeability (38). In this study, we showed that KSHV infection enhanced the permeability of endothelial monolayers to plasma proteins, blood-derived cells, KSHV-infected cells and KSHV virions in an in vitro transwell-based permeability assay. In addition, we found that KSHV infection disrupted the adherens junctions at the early stage of infection, and this process was associated with the degradation of VE-cadherin. These results have illustrated how KSHV infection might directly contribute to the central pathology of KS and thus provide further support for a direct role for KSHV in the pathogenesis of this malignancy.
Extensive studies have shown that adherens junctions control the vascular permeability and growth of endothelial cells by regulating the vascular barriers and intracellular signaling (71, 72). Thus, adherens junctions play a crucial role in regulating inflammation and angiogenesis (15, 74). Typically, in response to extracellular insults or intracellular stress, an increase in vascular permeability could facilitate the infiltration of inflammatory cells to the affected tissues so that the damaged or infected tissues could be rapidly repaired. Dysregulation of this process could lead to extensive and prolonged inflammation (62, 74). In normal endothelia, adherens junctions also exert a contact-inhibition effect and block the responses of normal arrested endothelial cells to growth and proangiogenic factors (13, 37, 46, 52, 64). However, in cancer-induced angiogenesis, the formation and sprouting of new blood vessels require the growth of endothelial cells (15, 20, 74). The disruption of adherens junctions mitigates contact inhibition and facilitates the growth of endothelial cells by transmitting mitogenic and survival signals, such as Src, phosphatidylinositol 3-kinase (PI 3-kinase), proteinase kinase C, protein kinase B, and mitogen-activated protein kinases (MAPKs), activated by extracellular growth and proangiogenic factors and by releasing β-catenin to the nucleus to promote the transcription of oncogenes, such as c-myc and cyclin D1 (15, 71, 74). We and others have shown that KSHV infection of endothelial cells activates many of these pathways and promotes the growth, angiogenesis, and invasion of the infected cells while simultaneously inducing proinflammatory and proangiogenic cytokines (14, 27, 54, 57, 66, 67, 76, 81, 82, 84), which are consistent with the observed phenotypes of disruption of adherens junctions and increased endothelial permeability described in this study. Interestingly, these features are also characteristics of cellular transformation and the epithelial-to-mesenchymal transition of cancer cells (6, 39). Thus, it is tempting to speculate that KSHV infection might induce an endothelial-mesenchymal transition. Indeed, previous studies have not only found massive inflammation and dysregulated angiogenesis in KS lesions but also described a mesenchymal phenotype in KS tumor cells, all of which are consistent with the results of in vitro studies (38).
Several studies have observed an increase in endothelial permeability and the disruption of cellular barriers following viral infections (1, 7, 9, 16, 21, 34, 47, 65, 68). In some cases, the disruption of cell junctions facilitates the access of viruses to their receptors in the basolateral membranes. For example, group B coxsackieviruses disrupt tight junctions to facilitate virus entry into cells through the junctions (18, 19). Interestingly, alphaherpesviruses enter cells by interacting with receptors that normally reside in cell junctions (35). Several cellular receptors have been shown to mediate KSHV entry into target cells, including heparin sulfate, integrins α3β1 and α5β3, DC-SIGN, and transporter protein xCT (3, 4, 31, 41, 59, 60). Some of these membrane-associated molecules, such as integrins, have polarized basolateral distributions (40, 55). Thus, it would be interesting to determine whether the KSHV entry of endothelial cells could be enhanced in polarized cells and whether there were preferential KSHV entry sites. The disruption of adherens junctions could certainly facilitate KSHV entry through the cellular receptors located in the basolateral membranes.
The disruption of adherens junctions and increased endothelial permeability could also facilitate the intravasation, extravasation, and dissemination of KSHV through the vascular networks in the form of either virions or infected cells. Indeed, we observed enhanced permeability to KSHV-infected cells and KSHV virions following the KSHV infection of the endothelial monolayers. Clinically, an increase of KSHV blood viral loads has been associated with the progression of KS (12, 24, 29, 50, 58, 78). Thus, it could be speculated that multifocal and visceral involvements in KS could be facilitated by the leakiness of the endothelium induced by KSHV infection.
The mechanism mediating the integrity of adherens junctions is complex. VE-cadherin is the main component of adherens junctions, which are complex plasma membrane structures (71). The extracellular domain of VE-cadherin interacts homophilically with other cadherins on the adjacent cells, while its cytoplasmic tail interacts with α-catenin, β-catenin, plakoglobin (γ-catenin) and p120ctn that couple the cadherin to the actin cytoskeleton. Signaling pathways that regulate components of the complex have been shown to alter adherens functions and vascular permeability (5, 43). A number of proinflammatory and proangiogenic factors such as tumor necrosis factor alpha, interleukin-6 and -8, and VEGF have been shown to mediate endothelial permeability during viral infections (21, 34, 47, 65). Some members of matrix metalloproteinases (MMPs) also mediate vascular permeability induced by viral proteins or during viral infections (48, 69). While KSHV infection of endothelial cells induces some of these proinflammatory and proangiogenic factors, as well as MMPs (14, 27, 54, 57, 66, 67, 76, 81, 82, 84), conditioned medium from acute KSHV-infected endothelial monolayers that manifested the clear disruption of adherens junctions failed to induce the disruption of adherens junctions when added to uninfected confluent endothelial monolayers (Fig. 4C). Furthermore, the inhibition of MMPs by a potent MMP inhibitor, GM6001, failed to rescue the KSHV-induced disruption of adherens junctions and permeability to serum albumin (data not shown), which are in contrast to those observed in dengue virus-induced permeability (48). Together, these results indicated that soluble factors were unlikely to be involved in KSHV-induced endothelial permeability at the early stage of infection. Nevertheless, it remained possible that some unidentified KSHV-induced soluble factors might mediate the observed increase in endothelial permeability, but they were associated with the cell surface or were labile. In this case, supernatants from the KSHV-infected cultures would not contain these factors and thus fail to induce endothelial permeability.
In contrast to soluble factors, our results showed that KSHV-induced vascular permeability was mediated by virus entry. This conclusion was supported by the fact that UV-KSHV effectively induced the disruption of adherens junctions, albeit their infectivity was abolished by the UV treatment (Fig. 4D to G). Consistent with these results, soluble heparin that blocked KSHV entry into the cells also prevented KSHV-induced vascular permeability (Fig. 5E). Furthermore, we found that the KSHV-induced disruption of adherens junctions did not require the expression of viral and cellular genes (Fig. 5A to C), and KSHV-induced VE-cadherin degradation was dose dependent on the internalization of viral particles (Fig. 7). In contrast, KSHV-induced VE-cadherin degradation was not robustly correlated with infectivity, as a cell could be infected without sufficient internalized viral particles to mediate the process (Fig. 7).
KSHV entry into cells is a multistep process, involving the attachment, binding, and fusion events, each mediated by distinct viral proteins and cellular receptors and triggering cascades of cellular signaling pathways (38). Some of these events, such as integrin-mediated signaling induced by KSHV binding to its integrin receptors, which have been implicated in regulating adherens junctions and vascular permeability (5, 10, 77), could mediate the KSHV-induced disruption of adherens junctions. For example, KSHV infection activates focal adhesion kinase, PI 3-kinase, protein kinase C-ζ, Src, Rho-GTPases, and the MEK/extracellular signal-regulated kinase, Jun N-terminal protein kinase, and p38 multiple MAPK pathways (4, 44, 51, 54, 63, 70, 81), and the activation of PI 3-kinase, focal adhesion kinase, MEK/extracellular signal-regulated kinase, Jun N-terminal protein kinase, and p38 MAPK is essential for KSHV entry into cells (44, 51, 54, 63). Clathrin endocytosis has been characterized as the pathway for KSHV entry into human fibroblasts (2). Since the internalization and degradation of VE-cadherin are mediated by endocytosis (79, 80), it is possible that some of these KSHV-activated pathways could regulate the KSHV-induced disruption of adherens junctions. Among the pathways activated during KSHV entry into cells, Rho-GTPases and Src are of particular interest since they have been shown to play pivotal roles in regulating adherens junctions. Rho-GTPases regulate the disassembly of endothelial adherens junctions in response to a variety of mediators. A recent study has shown that Rac activation is required for VEGF-induced VE-cadherin endocytosis through the p21-activated kinase and β-arrestin 2 phosphorylation of VE-cadherin (32). Src plays an important role in inducing the tyrosine phosphorylation of β-catenin, p120ctn, and VE-cadherin (53, 61, 73). The phosphorylation of VE-cadherin is linked to the dissociation of VE-cadherin/β-catenin/p120ctn complex (45, 56). Further studies are needed to determine whether the phosphorylation of VE-cadherin by Src or other tyrosine kinases during KSHV infection also serves as the initial signal to initiate VE-cadherin endocytosis and degradation.
While the KSHV-induced degradation of VE-cadherin and the disruption of adherens junctions occurred at the early stage of infection and did not require proinflammatory and proangiogenic cytokines, they were also observed at the later stages of infection (Fig. 2 and 3). Since the expression of VE-cadherin transcripts was not affected by KSHV infection (Fig. 3), it is likely that a distinct mechanism might be involved in the KSHV regulation of the VE-cadherin protein level at the later stages of KSHV infection. One possibility is that the disruption of adherens junctions could render the contact-inhibited endothelial cells susceptible to proinflammatory and proangiogenic cytokines induced by acute and prolonged KSHV infection. In this case, the disruption of adherens junctions at the early stage of KSHV infection could synergize with KSHV-induced proinflammatory and proangiogenic cytokines in sustaining the long-term disruption of adherens junctions and dysregulated endothelial permeability. Further studies are warranted to determine the existence of such a synergistic effect and the proinflammatory and proangiogenic cytokines involved.
Acknowledgments
This work was supported by an American Cancer Society research scholar grant (RSG-04-195) and grants from the National Institutes of Health (CA119889, CA096512, CA124332, and DE017333) to S.-J.G.
We thank Kenneth Izumi and Anthony Griffiths for their valuable suggestions for this work and appreciate the technical assistance from members of S.-J.G.'s laboratory.
Footnotes
Published ahead of print on 24 September 2008.
REFERENCES
- 1.Afonso, P. V., S. Ozden, M. C. Prevost, C. Schmitt, D. Seilhean, B. Weksler, P. O. Couraud, A. Gessain, I. A. Romero, and P. E. Ceccaldi. 2007. Human blood-brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganization. J. Immunol. 1792576-2583. [DOI] [PubMed] [Google Scholar]
- 2.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 777978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284235-249. [DOI] [PubMed] [Google Scholar]
- 4.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108407-419. [DOI] [PubMed] [Google Scholar]
- 5.Avizienyte, E., A. W. Wyke, R. J. Jones, G. W. McLean, M. A. Westhoff, V. G. Brunton, and M. C. Frame. 2002. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nat. Cell Biol. 4632-638. [DOI] [PubMed] [Google Scholar]
- 6.Baum, B., J. Settleman, and M. P. Quinlan. 2008. Transitions between epithelial and mesenchymal states in development and disease. Semin. Cell Dev. Biol. 19294-308. [DOI] [PubMed] [Google Scholar]
- 7.Bentz, G. L., M. Jarquin-Pardo, G. Chan, M. S. Smith, C. Sinzger, and A. D. Yurochko. 2006. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J. Virol. 8011539-11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkmann, A., K. Mahr, A. Ensser, S. Yaguboglu, F. Titgemeyer, B. Fleckenstein, and F. Neipel. 2001. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J. Virol. 7511583-11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bockeler, M., U. Stroher, J. Seebach, T. Afanasieva, N. Suttorp, H. Feldmann, and H. J. Schnittler. 2007. Breakdown of paraendothelial barrier function during Marburg virus infection is associated with early tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1. J. Infect. Dis. 196(Suppl. 2)S337-S346. [DOI] [PubMed] [Google Scholar]
- 10.Bos, J. L. 2005. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17123-128. [DOI] [PubMed] [Google Scholar]
- 11.Bower, M., M. Nelson, A. M. Young, C. Thirlwell, T. Newsom-Davis, S. Mandalia, T. Dhillon, P. Holmes, B. G. Gazzard, and J. Stebbing. 2005. Immune reconstitution inflammatory syndrome associated with Kaposi's sarcoma. J. Clin. Oncol. 235224-5228. [DOI] [PubMed] [Google Scholar]
- 12.Campbell, T. B., M. Borok, L. Gwanzura, S. MaWhinney, I. E. White, B. Ndemera, I. Gudza, L. Fitzpatrick, and R. T. Schooley. 2000. Relationship of human herpesvirus 8 peripheral blood virus load and Kaposi's sarcoma clinical stage. AIDS 142109-2116. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet, P., M. G. Lampugnani, L. Moons, F. Breviario, V. Compernolle, F. Bono, G. Balconi, R. Spagnuolo, B. Oostuyse, M. Dewerchin, A. Zanetti, A. Angellilo, V. Mattot, D. Nuyens, E. Lutgens, F. Clotman, M. C. de Ruiter, A. Gittenberger-de Groot, R. Poelmann, F. Lupu, J. M. Herbert, D. Collen, and E. Dejana. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98147-157. [DOI] [PubMed] [Google Scholar]
- 14.Carroll, P. A., E. Brazeau, and M. Lagunoff. 2004. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 3287-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavallaro, U., S. Liebner, and E. Dejana. 2006. Endothelial cadherins and tumor angiogenesis. Exp. Cell Res. 312659-667. [DOI] [PubMed] [Google Scholar]
- 16.Chiang, E. T., D. A. Persaud-Sawin, S. Kulkarni, J. G. Garcia, and F. Imani. 2006. Bluetongue virus and double-stranded RNA increase human vascular permeability: role of p38 MAPK. J. Clin. Immunol. 26406-416. [DOI] [PubMed] [Google Scholar]
- 17.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 755614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coyne, C. B., and J. M. Bergelson. 2006. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124119-131. [DOI] [PubMed] [Google Scholar]
- 19.Coyne, C. B., L. Shen, J. R. Turner, and J. M. Bergelson. 2007. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe 2181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dejana, E., R. Spagnuolo, and G. Bazzoni. 2001. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb. Haemost. 86308-315. [PubMed] [Google Scholar]
- 21.Dewi, B. E., T. Takasaki, and I. Kurane. 2004. In vitro assessment of human endothelial cell permeability: effects of inflammatory cytokines and dengue virus infection. J. Virol. Methods 121171-180. [DOI] [PubMed] [Google Scholar]
- 22.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebnet, K. 2008. Organization of multiprotein complexes at cell-cell junctions. Histochem. Cell Biol. 1301-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engels, E. A., R. J. Biggar, V. A. Marshall, M. A. Walters, C. J. Gamache, D. Whitby, and J. J. Goedert. 2003. Detection and quantification of Kaposi's sarcoma-associated herpesvirus to predict AIDS-associated Kaposi's sarcoma. AIDS 171847-1851. [DOI] [PubMed] [Google Scholar]
- 25.Feller, L., J. Masipa, N. Wood, E. Raubenheimer, and J. Lemmer. 2008. The prognostic significance of facial lymphoedema in HIV-seropositive subjects with Kaposi's sarcoma. AIDS Res. Ther. 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394588-592. [DOI] [PubMed] [Google Scholar]
- 27.Foglieni, C., S. Scabini, D. Belloni, F. Broccolo, P. Lusso, M. S. Malnati, and E. Ferrero. 2005. Productive infection of HUVEC by HHV-8 is associated with changes compatible with angiogenic transformations. Eur. J. Histochem. 49273-284. [DOI] [PubMed] [Google Scholar]
- 28.Gao, S. J., J. H. Deng, and F. C. Zhou. 2003. Productive lytic replication of a recombinant Kaposi's sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J. Virol. 779738-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao, S. J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335233-241. [DOI] [PubMed] [Google Scholar]
- 30.Gao, S. J., L. Kingsley, M. Li, W. Zheng, C. Parravicini, J. Ziegler, R. Newton, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. S. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2925-928. [DOI] [PubMed] [Google Scholar]
- 31.Garrigues, H. J., Y. E. Rubinchikova, C. M. Dipersio, and T. M. Rose. 2008. Integrin αvβ3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 821570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gavard, J., and J. S. Gutkind. 2006. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 81223-1234. [DOI] [PubMed] [Google Scholar]
- 33.Gavrilovskaya, I. N., E. E. Gorbunova, N. A. Mackow, and E. R. Mackow. 2008. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor, VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 825797-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geimonen, E., S. Neff, T. Raymond, S. S. Kocer, I. N. Gavrilovskaya, and E. R. Mackow. 2002. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. USA 9913837-13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 2801618-1620. [DOI] [PubMed] [Google Scholar]
- 36.Gill, P. S., A. Tulpule, B. M. Espina, S. Cabriales, J. Bresnahan, M. Ilaw, S. Louie, N. F. Gustafson, M. A. Brown, C. Orcutt, B. Winograd, and D. T. Scadden. 1999. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi's sarcoma. J. Clin. Oncol. 171876-1883. [DOI] [PubMed] [Google Scholar]
- 37.Grazia Lampugnani, M., A. Zanetti, M. Corada, T. Takahashi, G. Balconi, F. Breviario, F. Orsenigo, A. Cattelino, R. Kemler, T. O. Daniel, and E. Dejana. 2003. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J. Cell Biol. 161793-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greene, W., K. Kuhne, F. Ye, J. Chen, F. Zhou, X. Lei, and S. J. Gao. 2007. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat. Res. 13369-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guarino, M. 2007. Epithelial-mesenchymal transition and tumour invasion. Int. J. Biochem. Cell Biol. 392153-2160. [DOI] [PubMed] [Google Scholar]
- 40.Henning, W., W. Bohn, B. Nebe, A. Knopp, J. Rychly, and M. Strauss. 1994. Local increase of beta 1-integrin expression in cocultures of immortalized hepatocytes and sinusoidal endothelial cells. Eur. J. Cell Biol. 65189-199. [PubMed] [Google Scholar]
- 41.Kaleeba, J. A., and E. A. Berger. 2006. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 3111921-1924. [DOI] [PubMed] [Google Scholar]
- 42.Kim, T. A., H. K. Avraham, Y. H. Koh, S. Jiang, I. W. Park, and S. Avraham. 2003. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J. Immunol. 1702629-2637. [DOI] [PubMed] [Google Scholar]
- 43.Komarova, Y. A., D. Mehta, and A. B. Malik. 2007. Dual regulation of endothelial junctional permeability. Sci. STKE 2007re8. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan, H. H., N. Sharma-Walia, D. N. Streblow, P. P. Naranatt, and B. Chandran. 2006. Focal adhesion kinase is critical for entry of Kaposi's sarcoma-associated herpesvirus into target cells. J. Virol. 801167-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambeng, N., Y. Wallez, C. Rampon, F. Cand, G. Christe, D. Gulino-Debrac, I. Vilgrain, and P. Huber. 2005. Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circ. Res. 96384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lampugnani, M. G., F. Orsenigo, M. C. Gagliani, C. Tacchetti, and E. Dejana. 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174593-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee, C. G., H. J. Yoon, Z. Zhu, H. Link, Z. Wang, J. M. Gwaltney, M. Landry, and J. A. Elias. 2000. Respiratory syncytial virus stimulation of vascular endothelial cell growth factor/vascular permeability factor. Am. J. Respir. Cell Mol. Biol. 23662-669. [DOI] [PubMed] [Google Scholar]
- 48.Luplertlop, N., D. Misse, D. Bray, V. Deleuze, J. P. Gonzalez, V. Leardkamolkarn, H. Yssel, and F. Veas. 2006. Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 71176-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta, D., and A. B. Malik. 2006. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 86279-367. [DOI] [PubMed] [Google Scholar]
- 50.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 3321181-1185. [DOI] [PubMed] [Google Scholar]
- 51.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-ζ-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 771524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson, C. M., and C. S. Chen. 2003. VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J. Cell Sci. 1163571-3581. [DOI] [PubMed] [Google Scholar]
- 53.Owens, D. W., G. W. McLean, A. W. Wyke, C. Paraskeva, E. K. Parkinson, M. C. Frame, and V. G. Brunton. 2000. The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell-cell contacts. Mol. Biol. Cell 1151-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan, H., J. Xie, F. Ye, and S. J. Gao. 2006. Modulation of Kaposi's sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J. Virol. 805371-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philp, N. J., and V. T. Nachmias. 1987. Polarized distribution of integrin and fibronectin in retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 281275-1280. [PubMed] [Google Scholar]
- 56.Potter, M. D., S. Barbero, and D. A. Cheresh. 2005. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J. Biol. Chem. 28031906-31912. [DOI] [PubMed] [Google Scholar]
- 57.Qian, L. W., J. Xie, F. Ye, and S. J. Gao. 2007. Kaposi's sarcoma-associated herpesvirus infection promotes invasion of primary human umbilical vein endothelial cells by inducing matrix metalloproteinases. J. Virol. 817001-7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quinlivan, E. B., C. Zhang, P. W. Stewart, C. Komoltri, M. G. Davis, and R. S. Wehbie. 2002. Elevated virus loads of Kaposi's sarcoma-associated human herpesvirus 8 predict Kaposi's sarcoma disease progression, but elevated levels of human immunodeficiency virus type 1 do not. J. Infect. Dis. 1851736-1744. [DOI] [PubMed] [Google Scholar]
- 59.Rappocciolo, G., H. R. Hensler, M. Jais, T. A. Reinhart, A. Pegu, F. J. Jenkins, and C. R. Rinaldo. 2008. Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC-SIGN. J. Virol. 824793-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rappocciolo, G., F. J. Jenkins, H. R. Hensler, P. Piazza, M. Jais, L. Borowski, S. C. Watkins, and C. R. Rinaldo, Jr. 2006. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J. Immunol. 1761741-1749. [DOI] [PubMed] [Google Scholar]
- 61.Roura, S., S. Miravet, J. Piedra, A. Garcia de Herreros, and M. Dunach. 1999. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 27436734-36740. [DOI] [PubMed] [Google Scholar]
- 62.Sahni, S. K. 2007. Endothelial cell infection and hemostasis. Thromb. Res. 119531-549. [DOI] [PubMed] [Google Scholar]
- 63.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 7910308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shay-Salit, A., M. Shushy, E. Wolfovitz, H. Yahav, F. Breviario, E. Dejana, and N. Resnick. 2002. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 999462-9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shresta, S., K. L. Sharar, D. M. Prigozhin, P. R. Beatty, and E. Harris. 2006. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 8010208-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sivakumar, R., N. Sharma-Walia, H. Raghu, M. V. Veettil, S. Sadagopan, V. Bottero, L. Varga, R. Levine, and B. Chandran. 2008. Kaposi's sarcoma-associated herpesvirus induces sustained levels of vascular endothelial growth factors A and C early during in vitro infection of human microvascular dermal endothelial cells: biological implications. J. Virol. 821759-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Speciale, L., R. Biffi, R. Mancuso, E. Borghi, R. Mazziotti, and P. Ferrante. 2006. Big endothelin-1 and interleukin-6 modulation in human microvascular endothelial cells after human herpesvirus 8 infection. Exp. Biol. Med. (Maywood) 2311171-1175. [PubMed] [Google Scholar]
- 68.Tabata, T., S. McDonagh, H. Kawakatsu, and L. Pereira. 2007. Cytotrophoblasts infected with a pathogenic human cytomegalovirus strain dysregulate cell-matrix and cell-cell adhesion molecules: a quantitative analysis. Placenta 28527-537. [DOI] [PubMed] [Google Scholar]
- 69.Toschi, E., G. Barillari, C. Sgadari, I. Bacigalupo, A. Cereseto, D. Carlei, C. Palladino, C. Zietz, P. Leone, M. Sturzl, S. Butto, A. Cafaro, P. Monini, and B. Ensoli. 2001. Activation of matrix-metalloproteinase-2 and membrane-type-1-matrix-metalloproteinase in endothelial cells and induction of vascular permeability in vivo by human immunodeficiency virus-1 Tat protein and basic fibroblast growth factor. Mol. Biol. Cell 122934-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veettil, M. V., N. Sharma-Walia, S. Sadagopan, H. Raghu, R. Sivakumar, P. P. Naranatt, and B. Chandran. 2006. RhoA-GTPase facilitates entry of Kaposi's sarcoma-associated herpesvirus into adherent target cells in a Src-dependent manner. J. Virol. 8011432-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vestweber, D. 2008. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 28223-232. [DOI] [PubMed] [Google Scholar]
- 72.Vincent, P. A., K. Xiao, K. M. Buckley, and A. P. Kowalczyk. 2004. VE-cadherin: adhesion at arm's length. Am. J. Physiol. Cell. Physiol. 286C987-C997. [DOI] [PubMed] [Google Scholar]
- 73.Wallez, Y., F. Cand, F. Cruzalegui, C. Wernstedt, S. Souchelnytskyi, I. Vilgrain, and P. Huber. 2007. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene 261067-1077. [DOI] [PubMed] [Google Scholar]
- 74.Wallez, Y., and P. Huber. 2008. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta 1778794-809. [DOI] [PubMed] [Google Scholar]
- 75.Wang, F. Z., S. M. Akula, N. Sharma-Walia, L. Zeng, and B. Chandran. 2003. Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence. J. Virol. 773131-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi's sarcoma. Nat. Genet. 36687-693. [DOI] [PubMed] [Google Scholar]
- 77.Wang, Y., G. Jin, H. Miao, J. Y. Li, S. Usami, and S. Chien. 2006. Integrins regulate VE-cadherin and catenins: dependence of this regulation on Src, but not on Ras. Proc. Natl. Acad. Sci. USA 1031774-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. E. Suggett, D. M. Aldam, A. S. Denton, et al. 1995. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346799-802. [DOI] [PubMed] [Google Scholar]
- 79.Xiao, K., D. F. Allison, M. D. Kottke, S. Summers, G. P. Sorescu, V. Faundez, and A. P. Kowalczyk. 2003. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J. Biol. Chem. 27819199-19208. [DOI] [PubMed] [Google Scholar]
- 80.Xiao, K., J. Garner, K. M. Buckley, P. A. Vincent, C. M. Chiasson, E. Dejana, V. Faundez, and A. P. Kowalczyk. 2005. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell 165141-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xie, J., H. Pan, S. Yoo, and S. J. Gao. 2005. Kaposi's sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J. Virol. 7915027-15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu, Y., and D. Ganem. 2007. Induction of chemokine production by latent Kaposi's sarcoma-associated herpesvirus infection of endothelial cells. J. Gen. Virol. 8846-50. [DOI] [PubMed] [Google Scholar]
- 83.Yang, Z. Y., H. J. Duckers, N. J. Sullivan, A. Sanchez, E. G. Nabel, and G. J. Nabel. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6886-889. [DOI] [PubMed] [Google Scholar]
- 84.Ye, F. C., D. J. Blackbourn, M. Mengel, J. P. Xie, L. W. Qian, W. Greene, I. T. Yeh, D. Graham, and S. J. Gao. 2007. Kaposi's sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J. Virol. 813980-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoo, S. M., F. C. Zhou, F. C. Ye, H. Y. Pan, and S. J. Gao. 2005. Early and sustained expression of latent and host modulating genes in coordinated transcriptional program of KSHV productive primary infection of human primary endothelial cells. Virology 34347-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 766185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]