Abstract
Infection of mice with pneumonia virus of mice (PVM) is used as a natural host experimental model for studying the pathogenesis of infection with the closely related human respiratory syncytial virus. We analyzed the contribution of T cells to virus control and pathology after PVM infection. Control of a sublethal infection with PVM strain 15 in C57BL/6 mice was accompanied by a 100-fold increase in pulmonary cytotoxic T lymphocytes, 20% of which were specific for PVM. T-cell-deficient mice failed to eliminate PVM and became virus carriers in the absence of the clinical or histopathological signs of pneumonia that occurred after infection of control mice. Mice with limited T-cell numbers did not achieve virus control without weight loss, indicating that T-cell-mediated virus control was closely linked to immunopathology. Both CD4 and CD8 T cells independently contributed to virus elimination and disease. Virus control and disease were similar in the absence of perforin, gamma interferon, or tumor necrosis factor alpha. Interestingly, disease and mortality after lethal high-dose PVM infection were independent of T cells. These data illustrate a key role for T cells in control of PVM infection and demonstrate that both T-cell-dependent and -independent pathways contribute to disease in a viral dose-dependent fashion.
Human respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infection in infants and young children (20). The development of a safe vaccine, as well as of novel therapeutic approaches, hinges on a better understanding of the immune response to RSV. The role of T cells in virus control and disease pathogenesis is controversial. In RSV-infected infants, little correlation has been found between clinical disease parameters and the level of the virus-specific cytotoxic-T-lymphocyte (CTL) response in bronchoalveolar lavage (BAL) fluid or blood (22). Moreover, only low numbers of T cells were detected in postmortem lung tissues of children with lethal RSV infections (25, 36). On the other hand, infants with congenital T-cell deficiency in the context of SCID fail to eliminate RSV (15, 16, 21) and virus control during T-cell reconstitution can significantly aggravate the pulmonary disease (15; our unpublished observations). Experiments in the RSV mouse model have shown that T cells are necessary and sufficient for virus elimination and that T-cell-mediated immunopathology contributes significantly to the disease (3, 17, 29). However, RSV is not a natural pathogen of mice, and only a limited respiratory infection can be established after intranasal inoculation of virus at high titers (>105 PFU) (18). The significance of these findings for human RSV infection is therefore unclear.
Infection of mice with pneumonia virus of mice (PVM), a virus of the same genus as RSV, is increasingly used as a natural host experimental model for human RSV infection (13). PVM replicates to high titers in the mouse lung, causing a rapid onset of severe, and eventually fatal, granulocytic bronchiolitis at doses as low as 102 PFU (10, 11). Thus far, there are only limited data on the role of T cells in PVM infection. Early observations in spontaneously infected T-cell-deficient nude mice indicated a role for T cells in virus control (5, 32, 35). However, in other studies, very few lymphocytes were detected in the alveolar space after experimental PVM infection (2, 9). Virus-specific CD8+ T cells have been isolated at later time points after sublethal infection, but the frequency was low, and evidence of functional CTL inactivation was presented (7). These data suggested that T cells may play a limited role in the PVM model.
In the present study we performed detailed analyzes of the role of T cells in the murine PVM infection. Experiments were designed to address the following questions. (i) What is the kinetic, the magnitude, and the virus-specific functional activity of the T-cell response, and how does this correlate to disease parameters? (ii) What is the course of the infection in the absence of T cells? (iii) What are the relevant T-cell-dependent effector mechanisms during PVM infection? (iv) Are there differences concerning the role of T cells between sublethal low-dose and lethal high-dose PVM infections? Our data show that T cells are important for control of PVM replication but also significantly contribute to PVM disease in a viral dose-dependent fashion.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were obtained from Charles River (Sulzfeld, Germany). T-cell-receptor β (TCRβ)-chain- and TCRβδ-chain-deficient C57BL/6 mice were provided by F. von Loewenich (Institute for Medical Microbiology and Hygiene, Freiburg, Germany). CD4 (B6.129S2-Cd4)- and CD8 (B6.129S2-Cd8a)-deficient mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Gamma interferon (IFN-γ) receptor α-chain knockout mice (GKO) were originally generated by M. Aguet (Lausanne, Switzerland) (23) and backcrossed to C57BL/6 for six generations. C57BL/6 mice deficient in perforin (PKO) (26) were generously provided by Hans Hengartner (Zürich, Switzerland). Tumor necrosis factor receptor p55 (TNFRp55)-deficient mice were kindly provided by K. Pfeffer (Institute for Medical Microbiology and Clinical Hygiene, Düsseldorf, Germany) and TNF-α-deficient mice by M. Freudenberg (MPI for Immunobiology, Freiburg, Germany). All mice were bred and kept under specific-pathogen-free conditions in microisolator cages and used in age- and sex-matched experimental groups at 6 to 12 weeks of age. Mice were infected intranasally under ketamine and xylazine anesthesia with PVM15 in 80 μl of serum-free Eagle minimal essential medium. After infection, the inocula were retitrated. Inocula between 50 and 250 PFU are termed low-dose infections, and inocula between 2,000 and 5,000 PFU are termed high-dose infections throughout the present study. These doses had been established to cause sublethal, respective lethal infections in previous experiments. All animal experiments were performed in accordance with guidelines of the local animal care commission (accreditation no. 35/9185.81/G-06/81).
Virus and cells.
PVM strain 15 (ATCC no. VR-25) was propagated in BHK cells (ATCC, CCL-10) (27). Virus was purified via discontinuous sucrose gradient and stored at −80°C. Virus titers were determined by plaque assay on Vero cells (ATCC, CCL-81) under 0.8% methylcellulose as described previously (28). RAW309Cr1 (ATCC, TIB-69) is a murine macrophage cell line expressing both H-2d and H-2b MHCI molecules. These cells were found to be permissive for PVM and were used for the detection of PVM-specific CD8+ T cells as described below.
T-cell depletion and reconstitution experiments.
For T-cell depletion experiments, mice were injected intraperitoneally with a combination of 100 μg of anti-CD4 (clone YTS 191) and 200 μg of anti-CD8 (clone YTS 169) antibodies (8) or with phosphate-buffered saline as a control 1 day prior to infection. This treatment achieved a 500-fold reduction of circulating CD4+ T cells and a 20-fold reduction of CD8+ T cells as assessed by flow cytometry of peripheral blood cells at the end of the experiment. TCRβδ-deficient mice were reconstituted with defined numbers of naive spleen cells from sex-matched C57BL/6 donor mice injected intravenously 1 day before PVM15 infection.
Flow cytometry.
To obtain pulmonary inflammatory cells, mice were injected intraperitoneally with 10 mg of thiopental and exsanguinated via femoral vessels. BAL and isolation of pulmonary inflammatory cells was performed as described previously (31). Surface staining was performed for 30 min at 4°C using antibodies directed against the following molecules: CD3 (clone 145-2C11), CD4 (clone GK1.5), CD8 (clone 53-6.7), and streptavidin (all from BD Pharmingen, San Diego, CA). For intracellular IFN-γ staining, isolated BAL cells (1 × 105) were cultured with PVM15-infected or uninfected RAW309Cr1 cells (4 × 104) for 3 h in 96-well V-bottom plates in a volume of 200 μl of Iscove modified Dulbecco medium 10% fetal calf serum supplemented with 1 μl of monensin (Golgistop; BD Pharmingen)/ml. Cells were harvested, washed, surface stained (CD3 and CD8), and then subjected to intracellular cytokine staining using a Cytofix/Cytoperm kit according to the manufacturer's instructions (BD Pharmingen). Cells were either stained with antibody against IFN-γ (clone XMG1.2) or stained with an isotype control antibody (clone R3-43) (both from BD Pharmingen). Cells were analyzed on a FACSort cytometer using CellquestPro v4.02 software. Absolute cell numbers were calculated by multiplication of microscopically counted live cells (trypan blue exclusion) with the percentage of the respective population among live cells as determined by forward- and side-scatter analysis by flow cytometry.
Cytometric bead array.
BAL was performed with 0.8 ml of phosphate-buffered saline with a recovery of ∼0.6 ml. After centrifugation, the BAL supernatants were stored at −20°C. Then, 50-μl portions of the supernatants were analyzed for secreted cytokines—interleukin-6 (IL-6), IL-10, monocyte chemoattractant protein 1 (MCP-1), IFN-γ, TNF-α, and IL-12p70—using a CBA mouse inflammation kit (BD Pharmingen) according to the manufacturer's instructions. The data were analyzed by using CBA software (BD Pharmingen).
Tissue processing and histopathology.
Lung tissue of infected animals that had been instilled with 0.5 ml of 4% formalin intratracheally was dissected, immersed in 4% formalin overnight, and embedded in paraffin. Sections (4 μm thick) were stained with hematoxylin and eosin (H&E) and scored in a blinded fashion by a single experienced observer. The samples were examined for the extent and cellular composition of inflammatory infiltrates in bronchi, bronchioli, alveoli, and lung interstitium. In addition, injury to bronchoalveolar epithelium, including necrosis, apoptosis, and desquamation was evaluated.
Statistics.
Data were analyzed by using a Student's t test in the case of a normal distribution of raw data and equality of standard deviations. In cases where standard deviations differed significantly, the Welch t test was used. The nonparametric Mann-Whitney test was performed if the data did not follow a Gaussian distribution. All data were analyzed with GraphPad InStat software, version 3.06. Differences were considered significant at a P value of <0.05.
RESULTS
PVM15-induced weight loss is most pronounced during virus elimination, not at the peak of virus replication.
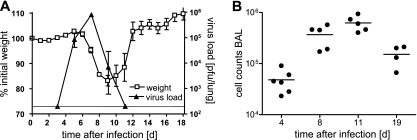
In previous experiments an optimal dose of 200 PFU or lower of PVM15 was established to cause a sublethal but symptomatic infection in C57BL/6 mice. Disease symptoms such as reduced activity and weight loss started at days 6 to 7 after infection and peaked with ca. 20% weight loss at days 8 to 9 (Fig. 1A). To assess the relationship between viral replication and weight loss, we determined PVM15 lung titers at different time points after infection. Peak virus titers of about 106 PFU per lung were observed on day 7 and PVM15 was eliminated below detection limit by day 11 after infection (Fig. 1A). Superimposition of the graphs for body weight and viral replication revealed a correlation between weight loss and the phase of virus elimination (days 7 to 11) rather than that of virus amplification (days 3 to 7). Weight loss correlated with the extent of alveolar cell infiltration, which achieved its maximum between days 8 and 11 after infection (Fig. 1B). This was interpreted as an indication for a contribution of the antiviral immune response to PVM15-induced disease.
FIG. 1.
Weight loss, virus load, and alveolar inflammatory infiltrates in PVM-infected mice. (A and B) C57BL/6 mice (n = 20) were infected with low-dose PVM15 and weighed daily. On days 4, 8, 11, and 19 groups of four to six mice were sacrificed for cellular analysis (see also Fig. 2A and B). Open squares indicate the mean weight loss relative to the initial weight and standard deviation from 4 to 20 mice per day (left y axis). In a second, independent experiment (with equal kinetics of weight loss), groups of mice were sacrificed at days 3, 5, 7, 9, and 11 after infection for determination of pulmonary virus titers. The filled triangles (right y axis) indicate the mean virus titer and standard deviation from four mice per time point. (B) Absolute BAL cell counts as determined by microscopy. All experiments were performed twice with similar results.
The peak of the antiviral CTL response occurs at a time point when mice already start to recover.
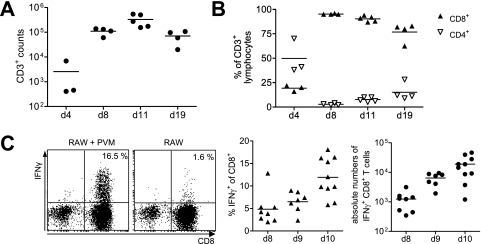
We then analyzed the cellular infiltrates in the lung by microscopy and flow cytometry of samples obtained by BAL. Although neutrophils and macrophages were the predominant cell populations (determined by cytospin analysis) up to day 6 after low-dose PVM15 infection (data not shown), lymphocytes were the dominating cell population thereafter. The absolute numbers of CD3+ T cells increased ∼100-fold between days 4 and 11 after infection (Fig. 2A). Whereas ca. 50% of the infiltrating CD3+ T cells were CD4+ T cells on day 4, almost all T lymphocytes were CD8+ CTLs by day 8, and this predominance of CTL persisted after the virus had been eliminated (Fig. 2B). In order to identify PVM-specific CTLs, we analyzed IFN-γ production of BAL CTLs in response to stimulation with PVM15-infected or uninfected RAW309Cr1 cells, a macrophage cell line expressing H-2b and H-2d that we identified to be permissive for PVM. The percentage of CTLs secreting IFN-γ in response to stimulation with infected RAW309Cr1 cells increased from <5% to 12 to 15% between days 8 and 10 after infection, and the absolute number of virus-specific CTLs increased by a factor of 20 (Fig. 2C). Thus, a high number of functional PVM-specific CTLs was generated after PVM15 infection.
FIG. 2.
Overall and virus-specific CD8+ T-cell response in the alveolar space during elimination of PVM15. (A and B) C57BL/6 mice (n = 20) were infected with low-dose PVM15. At the indicated time points groups of four to six mice were sacrificed, and BAL cells were analyzed by flow cytometry. (A) Absolute numbers of CD3+ T cells as determined by a combination of microscopic cell counts and flow cytometry. (B) Percentage of CD8+ and CD4+ T cells among CD3+ T cells. These experiments were performed twice with similar results. (C) Mice were infected with low-dose PVM15, and 8, 9, or 10 days later BAL lymphocytes were analyzed by flow cytometry. BAL cells were stimulated with PVM15-infected or uninfected RAW309Cr1 cells and stained for intracellular IFN-γ production (gate: CD3+ lymphocytes). The dot plots (left panel) show representative data obtained on day 10 after infection, the middle panel summarizes the data of two independent experiments. Absolute numbers of IFN-γ producing CTLs as determined by a combination of microscopic cell counts and flow cytometry are shown in the right panel (showing summarized data of two independent experiments). IFN-γ production of CD8 lung cells obtained from naive mice after stimulation with PVM15-infected RAW cells was <1% (data not shown).
Control of virus replication and weight loss after sublethal PVM infection are T cell dependent.
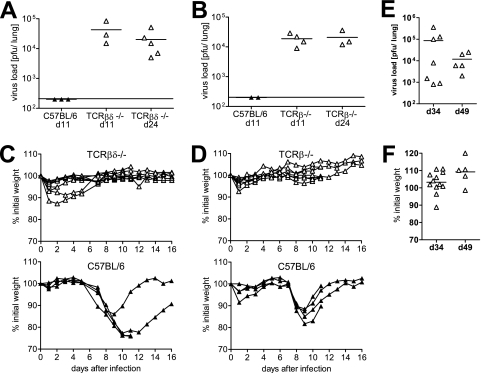
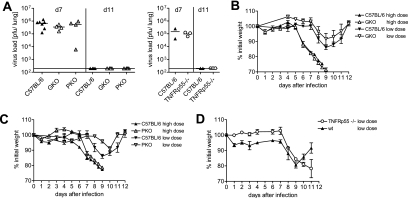
The contribution of T cells to virus control and disease pathogenesis after PVM15 infection was then analyzed using T-cell-deficient TCRβδ−/− mice. After inoculation with the low-dose of PVM15, C57BL/6 wild-type mice eliminated the virus below detection limit by day 11, as shown before (Fig. 1 and Fig. 3A and B). In contrast, virus remained detectable at significant titers in TCRβδ−/− mice up to day 24 after infection (Fig. 3A). Since γ/δ T cells may play an important role in microbial defense at mucosal surfaces (1), we addressed the question of whether γ/δ T cells could substitute for the loss of α/β T cells during PVM15 infection. However, PVM15 persisted at similar levels in TCRβ-deficient mice (Fig. 3B), arguing against a significant contribution of γ/δ T cells to virus elimination in this model. To analyze the further fate of these persistently infected mice, we performed an additional experiment where mice were observed until day 49 after infection. PVM persisted at significant, but variable titers in all of the mice analyzed (Fig. 3E). Interestingly, the T-cell-deficient mice did not loose any body weight, nor did they show any other clinical symptoms during the observation period (Fig. 3C, D, and F). This indicated that, in these experiments, PVM15-induced weight loss was also T cell dependent.
FIG. 3.
Control of virus replication and weight loss after low-dose PVM infection are T cell dependent. TCRβδ−/− (A and C) and TCRβ−/− (B and D) mice (seven to eight mice per group) and C57BL/6 control mice were infected with low-dose PVM15 and weighed daily. (A and B) Mice were sacrificed for determination of lung virus titers at the indicated time points. (C and D) Weight curves of individual T-cell-deficient (upper panel) or wild-type control mice (lower panel). (E and F) Long-term observation of TCRβ−/− mice (n = 12) after low-dose infection with respect to pulmonary virus titers (E) and weight loss (F). The data are pooled from two independent experiments.
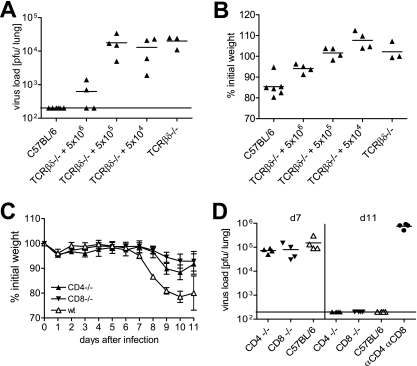
These findings were extended in reconstitution experiments. TCRβδ-deficient mice were transfused with increasing numbers of naive C57BL/6 spleen cells, followed by infection with PVM15. A transfusion of 5 × 106 spleen cells was required to achieve a significant reduction of virus titers by day 11 after PVM15 infection (Fig. 4A). At the same time this was accompanied by weight loss of ca. 10% on day 9 after infection (Fig. 4B). Mice that had received less than 5 × 106 spleen cells did not loose weight but also failed to significantly reduce PVM15 titers. This correlation between the efficacy of virus control and weight loss suggested a direct relationship between the beneficial and detrimental effects of T-cell responses during PVM15 infection.
FIG. 4.
T-cell-dependent virus control and weight loss are directly linked and CD4 and CD8 T cells both contribute. (A and B) Groups of four TCRβδ−/− mice were transfused with 5 × 104, 5 × 105, or 5 × 106 spleen cells from naive C57BL/6 mice. These partially reconstituted mice, as well as C57BL/6 (n = 6) and TCRβδ−/− (n = 3) control mice were infected with low-dose PVM15 and weighed daily. (A) Eleven days after infection, the pulmonary virus titers were determined. (B) Weight loss is shown as the percent initial weight at day 9 after infection. The experiment was repeated with TCRβ−/− mice with similar results. (C and D) CD4−/−, CD8−/−, and C57BL/6 control mice (eight mice per group) were infected with low-dose PVM15. (C) Weight curves. (D) Pulmonary virus titers of four mice per group at the indicated time points. The experiment was repeated in anti-CD4- or anti-CD8-depleted mice with similar results. The results from mice treated with both antibodies 1 day prior to infection are included in panel D.
To analyze the relative contribution of CD4 or CD8 T cells, we infected CD4- and CD8-deficient mice. Figure 4C shows that there was minimal weight loss in CD8-deficient mice and slightly more weight loss in CD4-deficient mice compared to wild-type mice. Although the virus titers were comparable in CD4-deficient, CD8-deficient, and control mice at day 7 after infection, both knockout strains had eliminated PVM15 below the detection limit by day 11 after infection (Fig. 4D). Depletion of both T-cell subsets from C57BL/6 mice led to a complete loss of virus control (Fig. 4D), illustrating that virus control in the knockout animals is mostly mediated by the still available T-cell subset and not by T-cell-independent mechanisms.
Reduced pulmonary inflammation despite viral persistence in T-cell-deficient mice.
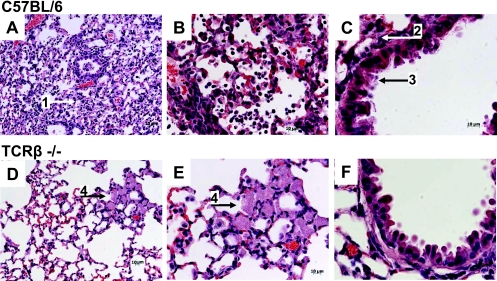
Histopathological analysis of lung tissue from TCRβ-deficient and wild-type mice on day 9 after sublethal PVM15 infection revealed significant differences between the two groups. The lungs of wild-type mice showed a severe pneumonia with widespread intra-alveolar and interstitial infiltrates consisting predominantly of neutrophils, macrophages, and scattered lymphocytes (Fig. 5A and B). Bronchi and bronchioli showed transmigration of neutrophils and focal epithelial necrosis and apoptosis (Fig. 5C). Sloughed epithelial cells, cellular debris, and inflammatory cells and fibrin partially filled the lumina (Fig. 5C). In contrast, inflammatory infiltrates in TCRβ-deficient mice were much less pronounced (Fig. 5D and E). These infiltrates consisted largely of focal aggregates of macrophage and foamy cell clusters (Fig. 5D and E), which sometimes contained multinucleated cells reminiscent of granulomas. The bronchial and bronchiolar epithelium was mostly intact (Fig. 5F).
FIG. 5.
Pulmonary histopathology after low-dose PVM15 infection is T cell dependent. Histopathological analysis of lung sections (H&E staining) from C57BL/6 control (A to C) and TCRβ-deficient (D to F) mice that had been infected with low-dose PVM15 9 days previously. The slides show representative sections from histological analysis of four mice per group. Scale bars are indicated in the figures. Numbered arrows mark the following features: 1, intra-alveolar infiltrates; 2, peribronchiolar infiltrates; 3, necrotic airway epithelial cells; and 4, cluster of foamy macrophages.
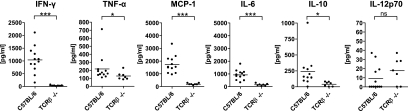
To further define the characteristics of T-cell-mediated disease, we determined several inflammatory cytokines in the BAL fluid 8 days after PVM15 infection. Except for IL-12p70, the level of all investigated cytokines was significantly reduced in T-cell-deficient mice compared to wild-type mice. This was particularly pronounced for IFN-γ, MCP-1, and IL-6 (Fig. 6). Thus, the pulmonary inflammatory response as determined by these marker cytokines correlated with the histological data and was significantly reduced in the absence of T cells.
FIG. 6.
Different cytokine patterns in BAL fluids of C57BL/6 and TCRβ−/− mice. C57BL/6 or TCRβ−/− mice were infected with low-dose PVM15, and the indicated cytokines were determined on day 8 after infection in BAL fluids by cytometric bead array. In the graphs the data of two independent experiments were combined. ***, P < 0.0001; **, P < 0.005; *, P < 0.05; ns, not significant.
Virus control and weight loss after PVM15 infection are independent of IFN-γ, perforin, and TNF-α.
To characterize the molecular effector mechanisms involved in CTL-mediated virus control and immunopathology, we performed experiments with mice deficient for perforin (PKO), the IFN-γ receptor (GKO), or for the TNFRp55 (TKO). All strains showed similar virus titers at day 7 after infection with a sublethal dose of PVM15 (Fig. 7A). Surprisingly, all of the mice had eliminated PVM15 by day 11, revealing that neither of these T-cell effector molecules is absolutely required for virus elimination (Fig. 7A). Weight loss after low-dose infection was similar in GKO and control mice (Fig. 7B) but appeared slightly reduced in PKO mice, indicating a minor role for perforin in PVM15-mediated weight loss (Fig. 7C). These minor differences were not observed in experiments using lethal doses of PVM15 where weight loss development of PKO and GKO mice was identical to those of wild-type control mice (Fig. 7B and C). TKO mice lost slightly more weight than control mice after low-dose PVM15 infection, but this difference was not statistically significant (Fig. 7D).
FIG. 7.
Virus control and weight loss after PVM15 infection are largely independent of IFN-γ, perforin, and TNF. C57BL/6, perforin−/−, IFN-γ receptor−/−, and TNFRp55−/− mice were infected with low-dose or high-dose PVM15. (A) At 7 and 11 days after infection with low-dose PVM15, three to four mice per group were sacrificed for determination of pulmonary virus titers. The left panel shows the virus titers of GKO, PKO, and C57BL/6 control mice, and the right panel shows TNFRp55−/− and control mice on days 7 and 11, respectively. (B to D) Weight curves indicating the mean and standard deviation from three to four mice per group. The experiments for GKO and PKO mice were performed twice with similar results. The experiment for TNFRp55−/− mice was repeated with TNF-α-deficient mice with similar results.
The role of T cells in PVM-induced disease is dose dependent.
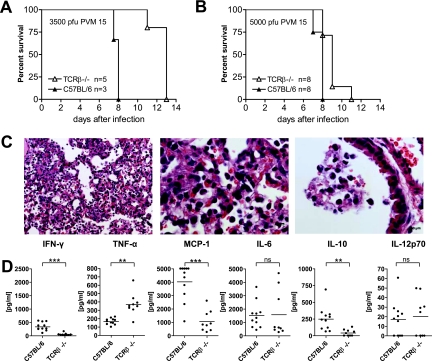
C57BL/6 mice infected with doses of PVM15 higher than 1,000 PFU show enhanced weight loss and usually succumb to severe pneumonia by day 9 after infection (data not shown). To study the contribution of T cells to disease and mortality at lethal doses of PVM, TCRβ−/− and wild-type mice were infected with 3,500 (Fig. 8A) or 5,000 (Fig. 8B) PFU of PVM15 and monitored for weight loss and survival. Unexpectedly, these lethal doses induced similar weight loss in both groups (data not shown), and survival was only slightly prolonged in T-cell-deficient mice (Fig. 8A and B). These observations were confirmed by histopathological analysis. Nine days after infection with 5,000 PFU, TCRβ-deficient mice still showed the characteristic macrophage clusters observed in Fig. 5B, but they also developed the extensive intra-alveolar granulocytic infiltrates and disruption of the bronchial epithelium that were observed in wild-type mice (Fig. 8C, compare to Fig. 5A). The inflammatory response pattern for MCP-1, IL-10, IFN-γ, and IL-12p70 was similar to that observed after low-dose infection. However, in contrast to infection with sublethal doses, lethally infected T-cell-deficient mice and wild-type mice showed similar levels of IL-6. Most strikingly, T-cell-deficient mice had a significantly higher TNF-α response (Fig. 8D). Overall, these findings showed that disease and mortality were determined by T-cell-independent pathways after PVM infection with lethal doses.
FIG. 8.
Mortality and histopathology are largely determined by T-cell-independent factors after infection with high doses of PVM15. (A and B) C57BL/6 and TCRβ−/− mice were infected with 3,500 (A) or 5,000 (B) PFU of PVM15 and monitored for weight loss and survival twice daily. Mice that were in extremis or showed more than 30% weight loss were euthanized, as a lethal outcome of the infection was assumed. The graphs show the percentage of surviving mice. (C) Histopathological analysis of lung sections from TCRβ-deficient mice that had been infected with 5,000 PFU were sacrificed on day 9 after infection. Representative lung sections (H&E staining) of five mice per group are shown. (D) Cytokine profile in BAL fluids of C57BL/6 or TCRβ−/− mice after high-dose infection with PVM15. The indicated cytokines were measured 8 days after infection in BAL supernatants by cytometric bead array (combined data of two independent experiments). ***, P < 0.0001; **, P < 0.005; ns, not significant.
DISCUSSION
This study demonstrates an important role for T cells in virus control and immunopathology after infection of mice with PVM15. T-cell-deficient mice became chronic virus carriers in the absence of acute disease, illustrating the dual role of T cells for virus control and immunopathology. Controlled variation of host and viral parameters showed a tight balance between beneficial and detrimental effects of T cells but also revealed pathways of disease after high-dose PVM infection that appeared to be T cell independent.
The contribution of T cells to virus control and pathology after murine PVM infection has not been investigated in detail previously. In studies analyzing the early, innate immune response to PVM in C57BL/6 mice (13), very poor pulmonary lymphocyte responses were observed (12). Analysis of BALB/c mice at later time points after infection showed significant CD8+ T-cell infiltrates, and up to 11% of these were specific for a single PVM-derived CTL epitope (7). However, epitope-specific IFN-γ production was not detected in more than 2 to 3% of BAL CTLs to this or any of the other tested epitopes. In analogy to similar findings in other respiratory virus infections (6, 7, 19), these data were interpreted as indication for a PVM-mediated functional inactivation of T cells.
In our study, we observed a strong T-cell infiltrate in the BAL of PVM15-infected C57BL/6 mice. We analyzed the function of PVM-specific T cells after restimulation with PVM-infected antigen-presenting cells. This leads to presentation of the full spectrum of PVM-derived peptides in more physiological concentrations and allowed quantification of the overall T-cell response to the virus, including the response to yet-undefined epitopes. Using this approach we found PVM-specific IFN-γ production in up to 20% of BAL CTLs, demonstrating a strong virus-specific pulmonary T-cell response to PVM that is comparable to other murine respiratory virus infections, including RSV (31). Our observations also suggest that during the time of virus elimination (days 8 to 11) the functional CTL response to PVM is not significantly impaired. This is compatible with our previous finding that functional silencing of T cells in the alveolar space does not impair virus control and probably represents a physiological mechanism to limit pulmonary inflammation rather than a viral escape strategy (30, 34).
Extending early observations in nude mice (5, 32, 35), we show that genetically T-cell-deficient or T-cell-depleted mice cannot eliminate PVM15 during an observation period of 49 days. Persisting virus titers in these chronically infected mice were ∼10-fold lower than peak titers, suggesting that T-cell-independent factors, e.g., components of the innate immune response, can provide some level of virus control but fail to eliminate the pathogen. These results mirror previous findings in human and murine RSV infection: infants with congenital T-cell deficiencies fail to eliminate RSV (15, 16, 21), and depletion of T cells leads to persistent infection in BALB/c mice (17). Adoptive transfer experiments have shown that T cells are necessary and sufficient to clear RSV from infected mice (3, 4, 29). Similar to the RSV model (17), both CD4+ and CD8+ T cells contributed to the clearance of PVM from the lung.
The extent of PVM-induced airway disease, however, was only partly determined by the kinetics of virus elimination and viral cytopathogenicity but significantly enhanced by the immune response to the viral infection. After infection with sublethal doses of PVM, weight loss as a rough measure of clinical disease was clearly dependent on T cells. T-cell-deficient mice also showed a significantly attenuated pulmonary inflammatory response after low-dose PVM infection. This concerned not only T-cell-dependent cytokines such as IFN-γ but also cytokines mostly secreted by macrophages and epithelial cells such as MCP-1 and IL-6. Pulmonary inflammatory infiltrates were also significantly attenuated in T-cell-deficient mice. Also, during prolonged observation T-cell-deficient mice did not develop signs of disease despite failure to control the virus. This is in contrast to previous reports that nude mice spontaneously infected with PVM develop chronic pneumonia that is eventually lethal (32, 35). The limited observation period may explain this discrepancy, but secondary infections in non-SPF mouse facilities may also contribute to this effect. Significantly, despite the T-cell dependence of the pulmonary pathology in low-dose PVM-infected mice, the inflammatory cells were predominantly granulocytes. This may be important for the interpretation of recent histopathological studies of lung tissue from infants with RSV infection (25, 36).
The molecular mechanisms of T-cell-mediated virus clearance and immunopathology in PVM infection remain elusive. Similar to the RSV mouse model, both processes did not require perforin, TNF, and—as also shown previously for PVM (14)—IFN-γ. This is in contrast to previous reports in the RSV mouse-model, where TNF has been postulated to be the main mediator of virus-induced illness (24, 33). We have preliminary evidence indicating that a combination of these effector mechanisms is required for the control of PVM, but this needs to be defined in more detail. It will be important for the development of targeted immunotherapeutic interventions.
Given the clear role of T cells after low-dose infection, it was an unexpected finding that infection with a lethal dose induced weight loss and mortality to a similar extent in both T-cell-deficient and control mice. One may speculate that viral cytopathogenicity is more important under these conditions. Although virus persistence at moderate levels (∼104 PFU/lung) was tolerated long term without significant signs of disease, the high virus load achieved after lethal infection (106 to 107 PFU/lung; data not shown) may have a more drastic influence on pulmonary homeostasis. Another possibility is damage by inflammatory cytokines. In particular, the levels of IL-6 and MCP-1 were increased in high-dose compared to low-dose infections of TCRβ−/− mice. This may indicate that innate immune cells and molecules significantly contribute to immunopathology in the absence of T cells.
In summary, analysis of the role of T cells in the PVM mouse model revealed an important contribution to virus control and a dose-dependent contribution to virus induced immune pathology. Our observations in the natural host PVM model show some parallels to human RSV infection. Further study of T-cell-dependent and T-cell-independent pathways of disease may therefore have implications for the development of immunotherapeutic approaches to human RSV infection.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (Eh145/4-1 to S.E. and C.D.K.).
We thank Friederike von Loewenich for kindly providing TCRβ- and TCRβδ-deficient mice and Klaus Pfeffer and Marina Freudenberg for TNFRp55- and TNF-deficient mice.
Footnotes
Published ahead of print on 24 September 2008.
REFERENCES
- 1.Aljurf, M., A. Ezzat, and O. M. M. 2002. Emerging role of γδ T cells in health and disease. Blood Rev. 16203-206. [DOI] [PubMed] [Google Scholar]
- 2.Bonville, C. A., A. J. Easton, H. F. Rosenberg, and J. B. Domachowske. 2003. Altered pathogenesis of severe pneumovirus infection in response to combined antiviral and specific immunomodulatory agents. J. Virol. 771237-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon, M. J., P. J. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 1681163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon, M. J., E. J. Stott, G. Taylor, and B. A. Askonas. 1987. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology 62133-138. [PMC free article] [PubMed] [Google Scholar]
- 5.Carthew, P., and S. Sparrow. 1980. Persistence of pneumonia virus of mice and Sendai virus in germ-free (nu/nu) mice. Br. J. Exp. Pathol. 61172-175. [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, J., and T. J. Braciale. 2002. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 854-60. [DOI] [PubMed] [Google Scholar]
- 7.Claassen, E. A., P. A. van der Kant, Z. S. Rychnavska, G. M. van Bleek, A. J. Easton, and R. G. van der Most. 2005. Activation and inactivation of antiviral CD8 T-cell responses during murine pneumovirus infection. J. Immunol. 1756597-6604. [DOI] [PubMed] [Google Scholar]
- 8.Cobbold, S. P., A. Jayasuriya, A. Nash, T. D. Prospero, and H. Waldmann. 1984. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312548-551. [DOI] [PubMed] [Google Scholar]
- 9.Domachowske, J. B., C. A. Bonville, D. Ali-Ahmad, K. D. Dyer, A. J. Easton, and H. F. Rosenberg. 2001. Glucocorticoid administration accelerates mortality of pneumovirus-infected mice. J. Infect. Dis. 1841518-1523. [DOI] [PubMed] [Google Scholar]
- 10.Domachowske, J. B., C. A. Bonville, K. D. Dyer, A. J. Easton, and H. F. Rosenberg. 2000. Pulmonary eosinophilia and production of MIP-1α are prominent responses to infection with pneumonia virus of mice. Cell. Immunol. 20098-104. [DOI] [PubMed] [Google Scholar]
- 11.Domachowske, J. B., C. A. Bonville, A. J. Easton, and H. F. Rosenberg. 2002. Differential expression of proinflammatory cytokine genes in vivo in response to pathogenic and nonpathogenic pneumovirus infections. J. Infect. Dis. 1868-14. [DOI] [PubMed] [Google Scholar]
- 12.Domachowske, J. B., C. A. Bonville, J. L. Gao, P. M. Murphy, A. J. Easton, and H. F. Rosenberg. 2000. The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J. Immunol. 1652677-2682. [DOI] [PubMed] [Google Scholar]
- 13.Easton, A. J., J. B. Domachowske, and H. F. Rosenberg. 2004. Animal pneumoviruses: molecular genetics and pathogenesis. Clin. Microbiol. Rev. 17390-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis, J. A., B. V. Martin, C. Waldner, K. D. Dyer, J. B. Domachowske, and H. F. Rosenberg. 2007. Mucosal inoculation with an attenuated mouse pneumovirus strain protects against virulent challenge in wild type and interferon-gamma receptor deficient mice. Vaccine 251085-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Saleeby, C. M., J. Suzich, M. E. Conley, and J. P. DeVincenzo. 2004. Quantitative effects of palivizumab and donor-derived T cells on chronic respiratory syncytial virus infection, lung disease, and fusion glycoprotein amino acid sequences in a patient before and after bone marrow transplantation. Clin. Infect. Dis. 39e17-e20. [DOI] [PubMed] [Google Scholar]
- 16.Fishaut, M., D. Tubergen, and K. McIntosh. 1980. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J. Pediatr. 96179-186. [DOI] [PubMed] [Google Scholar]
- 17.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 881026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26153-162. [DOI] [PubMed] [Google Scholar]
- 19.Gray, P. M., S. Arimilli, E. M. Palmer, G. D. Parks, and M. A. Alexander-Miller. 2005. Altered function in CD8+ T cells following paramyxovirus infection of the respiratory tract. J. Virol. 793339-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall, C. B. 1999. Respiratory syncytial virus: a continuing culprit and conundrum. J. Pediatr. 1352-7. [PubMed] [Google Scholar]
- 21.Hall, C. B., K. R. Powell, N. E. MacDonald, C. L. Gala, M. E. Menegus, S. C. Suffin, and H. J. Cohen. 1986. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 31577-81. [DOI] [PubMed] [Google Scholar]
- 22.Heidema, J., M. V. Lukens, W. W. van Maren, M. E. van Dijk, H. G. Otten, A. J. van Vught, D. B. van der Werff, S. J. van Gestel, M. G. Semple, R. L. Smyth, J. L. Kimpen, and G. M. van Bleek. 2007. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J. Immunol. 1798410-8417. [DOI] [PubMed] [Google Scholar]
- 23.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 2591742-1745. [DOI] [PubMed] [Google Scholar]
- 24.Hussell, T., A. Pennycook, and P. J. Openshaw. 2001. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 312566-2573. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, J. E., R. A. Gonzales, S. J. Olson, P. F. Wright, and B. S. Graham. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 20108-119. [DOI] [PubMed] [Google Scholar]
- 26.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zinkernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 36931-37. [DOI] [PubMed] [Google Scholar]
- 27.Krempl, C. D., E. W. Lamirande, and P. L. Collins. 2005. Complete sequence of the RNA genome of pneumonia virus of mice (PVM). Virus Genes 30237-249. [DOI] [PubMed] [Google Scholar]
- 28.Krempl, C. D., A. Wnekowicz, E. W. Lamirande, G. Nayebagha, P. L. Collins, and U. J. Buchholz. 2007. Identification of a novel virulence factor in recombinant pneumonia virus of mice. J. Virol. 819490-9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostler, T., W. Davidson, and S. Ehl. 2002. Virus clearance and immunopathology by CD8+ T cells during infection with respiratory syncytial virus are mediated by IFN-γ. Eur. J. Immunol. 322117-2123. [DOI] [PubMed] [Google Scholar]
- 30.Ostler, T., and S. Ehl. 2002. Pulmonary T cells induced by respiratory syncytial virus are functional and can make an important contribution to long-lived protective immunity. Eur. J. Immunol. 322562-2569. [DOI] [PubMed] [Google Scholar]
- 31.Ostler, T., T. Hussell, C. D. Surh, P. Openshaw, and S. Ehl. 2001. Long-term persistence and reactivation of T-cell memory in the lung of mice infected with respiratory syncytial virus. Eur. J. Immunol. 312574-2582. [DOI] [PubMed] [Google Scholar]
- 32.Richter, C. B., J. E. Thigpen, C. S. Richter, and J. M. Mackenzie, Jr. 1988. Fatal pneumonia with terminal emaciation in nude mice caused by pneumonia virus of mice. Lab. Anim. Sci. 38255-261. [PubMed] [Google Scholar]
- 33.Rutigliano, J. A., and B. S. Graham. 2004. Prolonged production of TNF-alpha exacerbates illness during respiratory syncytial virus infection. J. Immunol. 1733408-3417. [DOI] [PubMed] [Google Scholar]
- 34.Vallbracht, S., H. Unsold, and S. Ehl. 2006. Functional impairment of cytotoxic T cells in the lung airways following respiratory virus infections. Eur. J. Immunol. 361434-1442. [DOI] [PubMed] [Google Scholar]
- 35.Weir, E. C., D. G. Brownstein, A. L. Smith, and E. A. Johnson. 1988. Respiratory disease and wasting in athymic mice infected with pneumonia virus of mice. Lab. Anim. Sci. 38133-137. [PubMed] [Google Scholar]
- 36.Welliver, T. P., R. P. Garofalo, Y. Hosakote, K. H. Hintz, L. Avendano, K. Sanchez, L. Velozo, H. Jafri, S. Chavez-Bueno, P. L. Ogra, L. McKinney, J. L. Reed, and R. C. Welliver, Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 1951126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]