Abstract
Kupffer cells (KCs) rapidly remove intravenously injected adenovirus (Ad) vectors from the circulation. A better understanding of the mechanisms involved could suggest strategies to improve Ad gene delivery by suppressing or evading KC uptake. We recently showed that clearance of Ad type 5 vectors by KCs does not involve the interaction of Ad with the well-established Ad receptors, namely, integrins or the coxsackievirus and Ad receptor (J. S. Smith, Z. Xu, J. Tian, S. C. Stevenson, and A. P. Byrnes, Hum. Gene Ther. 19:547-554, 2008). In the current study, we systematically quantified the contributions of various receptors and plasma proteins to the clearance of Ad by KCs. We found that scavenger receptors are a predominant mechanism for the clearance of Ad by KCs. In addition, we found that Ad is opsonized by natural immunoglobulin M antibodies and complement and that these opsonins play a contributory role in the clearance of Ad by KCs. We also examined additional mechanisms that have been postulated to be involved in the clearance of Ad, including the binding of Ad to platelets and vitamin K-dependent coagulation factors, but we found that neither of these were required for the clearance of Ad by KCs.
Adenovirus (Ad) vectors are promising tools for gene therapy because of their high transduction efficiency and wide tropism. However, when Ad is administered systemically by intravenous (i.v.) injection, the efficiency of gene delivery is greatly impaired due to rapid removal of virions by the reticuloendothelial system (RES), particularly by Kupffer cells (KCs) in the liver (2, 56, 63). Sequestration of Ad vector by the RES greatly reduces the ability of Ad to reach other tissues, provokes toxic responses, and triggers a rapid destruction of KCs (31, 32, 44, 56, 68). Understanding how KCs recognize Ad would be very helpful for designing methods to evade KCs, which might allow efficient systemic delivery of Ad vectors at doses lower than those currently possible.
The receptors used by Ad5 to enter cultured cells are well defined. First, the fiber knob binds to the coxsackievirus and Ad receptor (CAR). Subsequently, the penton base binds to integrins, which allows the virus to be internalized (67). These receptors can be important for transduction in vivo when Ad vectors are injected locally (36). Multiple studies have shown, however, that when vector is injected systemically by the i.v. route, an interaction of Ad with CAR and integrins is not required for hepatocyte transduction (1, 49). Likewise, we recently reported that the sequestration of Ad vector by KCs is completely unaffected when vectors are unable to bind to CAR and integrins (48). There is now extensive evidence that interactions between Ad and receptors in vivo are considerably more complicated than predicted by in vitro studies, including opsonization of Ad by plasma proteins and subsequent binding of these opsonins to receptors on cells (6). In particular, vitamin K-dependent coagulation factors opsonize Ad and greatly facilitate the transduction of hepatocytes (39).
Because in vivo studies are indispensable for obtaining a complete understanding of the mechanisms that KCs use to clear Ad, we recently developed a quantitative technique to measure the amount of Ad sequestered within KCs in the liver (47). Liver cryosections are stained with immunofluorescent antibodies against Ad and KCs, and confocal images are processed using computerized image analysis. In this manner, the amount of Ad within KCs can be specifically measured. We have shown that this technique is robust and reproducible and has a dynamic range that spans at least 2 orders of magnitude (47).
We undertook the current study to determine the mechanisms that are involved in the uptake of Ad by KCs. We and others recently showed that preinjecting mice with poly(I) can greatly reduce KC uptake and increase the circulating half-life of Ad in vivo (17, 47). Because poly(I) is a ligand for scavenger receptors (SRs), we wanted to further explore the involvement of SRs in vivo. In addition, given recent discoveries about the opsonization of Ad by coagulation factors, we decided to evaluate whether Ad is also opsonized by other mouse plasma proteins, including antibodies and complement, and whether this might contribute to the uptake of Ad by KCs. Finally, we reexamined earlier reports that opsonization by coagulation factors (45) or binding of Ad to platelets (55) might be important in the sequestration of Ad by KCs.
MATERIALS AND METHODS
Ad vectors.
Replication-defective E1/E3-deleted Ad5 vectors expressing nucleus-localized β-galactosidase (Av1nBg) or firefly luciferase (AdLuc) were grown and purified as described previously (47). Ad vector particle (vp) concentration was measured spectrophotometrically with no detectable aggregation, as described previously (47). Infectivity was measured as PFU on 293 cells. The vp-to-PFU ratios for Av1nBg and AdLuc were less than 15. Endotoxin levels in all Ad vectors were <0.15 EU/ml by the Limulus amoebocyte lysate method (Charles River Endosafe, Charleston, SC).
Animals.
Animal experiments were approved by the FDA CBER Animal Care and Use Committee. Male C57BL/6NCr mice were obtained from the National Cancer Institute (Frederick, MD). Rag-1 knockout (KO) and C3 KO mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in our specific-pathogen-free facilities. The background strain of these mice was C57BL/6J, and the corresponding control C57BL/6J mice were obtained directly from Jackson Labs. All mice were used at 7 to 10 weeks of age. Sera for enzyme-linked immunosorbent assays (ELISAs) were obtained from anesthetized mice by cardiac puncture and stored at −80°C until use. Tail vein injections were performed as previously described (47), and livers were removed 10 min after the injection of Ad (for KC uptake experiments) or 48 h after injection (for assays of liver expression of β-galactosidase).
Poly(I), poly(A), poly(C), poly(G), poly(U), dextran, dextran sulfate, and heparin (from porcine intestinal mucosa; 182 U/mg) were purchased from Sigma-Aldrich Inc. (St. Louis, MO). To evaluate the role of SRs, these substances were dissolved in water and dosed i.v. at 0.2 mg per mouse 5 min prior to Ad injection.
We used Rag-1 KO mice to examine the role of natural antibodies in the clearance of Ad by KCs. To examine whether transferring natural antibodies to Rag-1 KO mice would restore the uptake of Ad by KCs, a group of Rag-1 KO mice was injected i.v. with a total of 800 μl per mouse of normal C57BL/6 serum as follows: 400 μl 1 day in advance and 400 μl 2 h in advance.
To deplete complement, mice were injected intraperitoneally (i.p.) with cobra venom factor (CVF) (Quidel Corp., San Diego, CA) 1 day in advance. Mice were injected i.p. twice with 5 U of CVF 4 h apart. Control mice were injected i.p. with phosphate-buffered saline (PBS). The ability of CVF to deplete C3 was verified using a hemolytic activity assay (C3H50) (data not shown).
We used three different methods to deplete platelets. In one method, mice were injected i.v. 24 h in advance with a mixture of rat monoclonal antibodies against CD42b/GPIbα (Emfret Analytics, Wurzburg, Germany) at 2 μg/g of body weight. The same dose of nonimmune rat immunoglobulin G (IgG) (Emfret Analytics) was used as a negative control. In another method (54), platelets were depleted by injecting mice i.p. 24 h in advance with 0.1 U of Clostridium perfringens neuraminidase type VI (Sigma-Aldrich Inc., St. Louis, MO). In a third method, mice were injected i.v. 3 h in advance with 30 μg of anti-CD41/GPIIb (clone MWReg-30; BD Biosciences, San Jose, CA). Because MWReg-30 was available only in a formulation that contained sodium azide, the antibody was first dialyzed against PBS, and the protein concentration was determined by the Lowry method. Platelets in EDTA-blood were automatically counted using a veterinary hematology system (Hemavet 950; Drew Scientific, Dallas, TX).
To deplete vitamin K-dependent coagulation factors, mice were injected subcutaneously with 133 μg of warfarin (suspended in peanut oil) 3 days and 1 day before Ad dosing. Control mice were injected with peanut oil alone on the same schedule. The effectiveness of warfarin in depleting coagulation factors was verified by the finding of greatly prolonged prothrombin times (data not shown).
ELISAs.
Ninety-six-well plates were coated overnight with 3 × 109 vp of Ad5 vector in bicarbonate buffer, pH 9.6, and then blocked with 2% bovine serum albumin (BSA) in PBS. For IgM and IgG ELISAs, serum was diluted in PBS, 2% BSA, 0.05% Tween 20 and incubated on plates for 60 min at 4°C. Plates were washed and then incubated with horseradish peroxidase (HRP)-conjugated goat antisera that were specific for either the μ or γ chain of mouse Ig (Southern Biotech, Birmingham, AL). For all ELISAs, HRP was detected with tetramethylbenzidine. The background binding to BSA-coated wells was subtracted.
For C3 and C4 ELISAs, mouse serum was diluted 1:8 in 2% BSA, 0.05% Tween 20 in PBS containing 0.5 mM Mg2+ and 0.9 mM Ca2+. In some cases, specific complement pathways were inhibited by including 10 mM EDTA (Ca2+ and Mg2+ omitted) or 10 mM EGTA (with 2 mM Mg2+ but without Ca2+). Plates were incubated with mouse serum for 60 min at room temperature. Mouse C3 was detected with rat monoclonal anti-C3 (clone RmC11H9, 1:1,600 dilution; Cedarlane Laboratories Ltd., Burlington, Ontario, Canada). This antibody detects native C3 and fragments C3b, iC3b, and C3dg. Mouse C4 was detected using rabbit polyclonal anti-C4c (ab15982, 1:1,000 dilution; AbCam Inc., Cambridge, MA). This antibody detects native C4 and fragment C4c. Secondary antibodies were HRP-conjugated goat antisera against rat IgG (AbD Serotec, Oxford, United Kingdom) or against rabbit IgG (Southern Biotech) as appropriate.
Quantitation of Ad within KCs.
The amount of Ad within F4/80+ KCs at 10 min postinjection was measured exactly as previously described using computerized image analysis of immunofluorescently stained liver sections (47). A 10-min time point is appropriate because in mice the vast majority of Ad virions are cleared from the blood within 10 min of i.v. injection (1, 2, 14), and electron microscopy shows that Ad virions are taken up inside KCs at this time point (32). A standard curve of liver sections was evaluated concurrently with each assay, as previously described (47). The standard curves confirmed that there was a linear dose-response relationship and that changes in the amount of Ad associated with KCs could be quantitatively measured.
Statistical analysis.
In all figures, means ± standard deviations are shown. Experiments were analyzed by t test or analysis of variance, and post hoc comparisons were made using the Holm-Sidak test (SigmaStat 3.0; Systat Software, San Jose CA), with significance defined as a P value of ≤0.05. When necessary, data were log transformed prior to statistical analysis to equalize variances.
RESULTS
SRs play a major role in KC uptake of Ad.
SRs form a large family of receptors that recognize negatively charged materials with relatively broad specificity (34, 35). SRs have been shown to be responsible for the clearance of various ligands in vivo, including DNA, damaged erythrocytes, and endotoxin (18, 25, 27, 57, 62). Recently, the SR inhibitor poly(I) was shown by us and others to reduce the clearance of Ad in vivo (17, 47). In the current study, we systematically surveyed how KC clearance of Ad is affected by various additional polyanionic SR ligands. Furthermore, we demonstrated specificity by using control non-SR ligands with similar structure. This is a well-established strategy for defining the involvement of SRs in vivo (18, 25, 27, 57, 62).
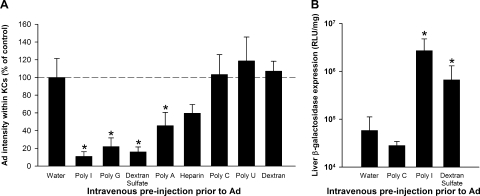
We found that the clearance of Ad by KCs could be greatly decreased by preinjecting mice 5 min beforehand with the broad-spectrum SR inhibitors poly(I), poly(G), and dextran sulfate (Fig. 1A). In contrast, there were no inhibitory effects with SR noninhibitors, namely, poly(C), poly(U), and dextran. We found that poly(A) and heparin were able to partially inhibit the clearance of Ad by KCs, although this was statistically significant only for poly(A) (Fig. 1A). Neither poly(A) nor heparin is a broad-spectrum SR inhibitor. Nonetheless, both poly(A) and heparin have previously been shown to inhibit the uptake of a select subset of SR ligands by macrophages both in vitro and in vivo (15, 53, 58, 65). Of note is that the experiment shown in Fig. 1A was performed with an Ad dose of 1.0 × 1011 vp/kg; in an additional experiment, we examined the effect of poly(I) on a higher dose of Ad (1.0 × 1012 vp/kg) and found that it caused a similar statistically significant decrease in the uptake of Ad by KCs (data not shown).
FIG. 1.
SR inhibitors reduce the uptake of Ad by KCs and enhance liver transduction. (A) C57BL/6 mice were injected i.v. with 0.2 mg of SR inhibitors or noninhibitors in water. Poly(I), poly(G), and dextran sulfate are broad-specificity SR inhibitors, while poly(A) and heparin inhibit a more limited subset of SRs. Five minutes later, mice were injected i.v. with 1.0 × 1011 vp/kg of Av1nBg. Mice were sacrificed 10 min after Ad injection, and the amount of Ad (intensity of immunofluorescent staining) was quantitated specifically within KCs. Three to five mice per group. *, P value of <0.05 versus the control water-predosed group (Holm-Sidak post hoc). (B) SR inhibitors enhanced liver transduction by Ad. C57BL/6 mice were injected i.v. with 0.2 mg of SR inhibitors [poly(I) and dextran sulfate] or an SR noninhibitor [poly(C)]. After 5 min, mice were injected i.v. with 5.0 × 1011 vp/kg of Av1nBg. After an additional 48 h, mice were sacrificed and the amount of β-galactosidase in the liver was quantitated. Four mice per group. *, P value of <0.05 versus control water-preinjected mice (Holm-Sidak post hoc). RLU, relative light units.
Because blockading KCs prior to Ad vector injection should make more Ad vector available for the transduction of other cells, we evaluated the effect of selected SR ligands on liver transduction. We found that liver transduction with 5.0 × 1011 vp/kg of Ad vector was significantly enhanced when the vector injection was preceded by poly(I) or dextran sulfate (Fig. 1B). In contrast, the SR noninhibitor poly(C) did not increase liver transduction.
In a pilot experiment evaluating liver transduction by a 10-fold-higher dose of Ad vector, we made the unexpected discovery that poly(I) could synergize with Ad to produce severe toxicity. When mice were injected with 0.2 mg of poly(I) followed by 5.0 × 1012 vp/kg of Ad vector, all mice showed dramatic behavioral symptoms, including reduced movement and rapid breathing, and four out of five mice died unexpectedly within 1 h. These behavioral symptoms and mortality were never seen when poly(I) was injected alone or when Ad vector was injected alone at this dose. Thus, although poly(I) is effective at blockading KCs and increasing liver transduction, it significantly increases the toxicity of Ad vectors in vivo.
Natural antibodies bind to Ad in vitro and are involved in KC clearance of Ad in vivo.
Natural antibodies are polyreactive germ line-encoded antibodies that are able to recognize antigens even in the absence of prior exposure to the antigen (8, 38, 69). Although these natural antibodies tend to have relatively low affinity for monomeric antigens (69), viral capsids have highly repetitive structures that should allow multivalent binding with much higher avidity.
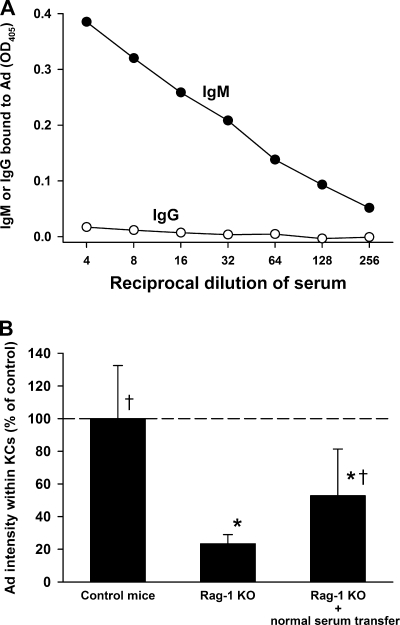
We first examined the ability of natural antibodies to bind to Ad in vitro. Using an ELISA, we found that naïve mouse serum contains IgM antibodies that opsonize Ad but that the binding of IgG to Ad was minimal (Fig. 2A). To examine the role of natural antibodies in vivo, we examined the KC uptake of Ad in antibody-null Rag-1 KO mice. Rag-1 KO mice showed a statistically significant decrease in the amount of Ad within KCs (Fig. 2B), demonstrating a contribution of natural antibodies to the clearance of Ad by KCs. To examine whether the defect in the uptake by KCs in Rag-1 KO mice could be reversed by natural antibody, a group of Rag-1 KO mice was preinjected with serum from naïve C57BL/6 mice. This resulted in a significant increase in KC uptake of Ad compared to that seen for Rag-1 KO mice (Fig. 2B). Supplementing Rag-1 KO mice with normal mouse serum did not completely restore the uptake of Ad by KCs, and we speculate that this may have been due to limitations on the amount of serum that we could transfer.
FIG. 2.
Natural antibodies bind to Ad and influence the KC uptake of Ad. (A) ELISA plates were coated with Ad and then incubated with naïve mouse serum. IgM was able to bind to Ad (filled circles), but the binding of IgG to Ad was minimal (open circles). An additional independent experiment yielded similar results. OD405, optical density at 405 nm. (B) Ad uptake by KCs was reduced in antibody-deficient Rag-1 KO mice, and uptake was partially restored by transferring normal mouse serum to Rag-1 KO mice. Prior to the injection of Ad, one group of Rag-1 KO mice was given i.v. injections of normal C57BL/6 serum as described in Materials and Methods. Mice were injected i.v. with 1.0 × 1011 vp/kg AdLuc vector and sacrificed 10 min later, and the amount of Ad within KCs was quantitated. Six to 11 mice per group. *, P value of <0.05 versus control C57BL/6 mice; †, P value of <0.05 versus Rag-1 KO mice (Holm-Sidak post hoc). Additional confirmatory experiments at Ad vector doses of 1.0 × 1011 and 1.0 × 1012 vp/kg also showed statistically significant reductions in the amount of Ad within KCs of Rag-1 KO mice.
Mouse complement opsonizes Ad via the classical pathway in vitro and is involved in KC uptake of Ad in vivo.
Several previous in vitro studies with human plasma or serum have shown that Ad5 can bind to and activate complement (4, 9, 23, 41). One issue with using human material is that individual humans have variable levels of specific anti-Ad antibodies that activate complement via the classical pathway (9). For mice, in vivo studies have shown that complement component C3 enhances liver transduction by Ad and also augments the cytokine response to Ad (26, 70). In vitro, the isolated Ad5 fiber knob can bind mouse complement proteins C3, C4, and C4-binding protein (45), but it is not clear whether this binding is direct or indirect; nor is it apparent which of the complement activation pathways may be involved. In particular, we were interested to learn whether natural antibodies play a role in the opsonization of Ad by complement. Therefore, we established ELISAs to characterize the opsonization of Ad by mouse C3 and C4.
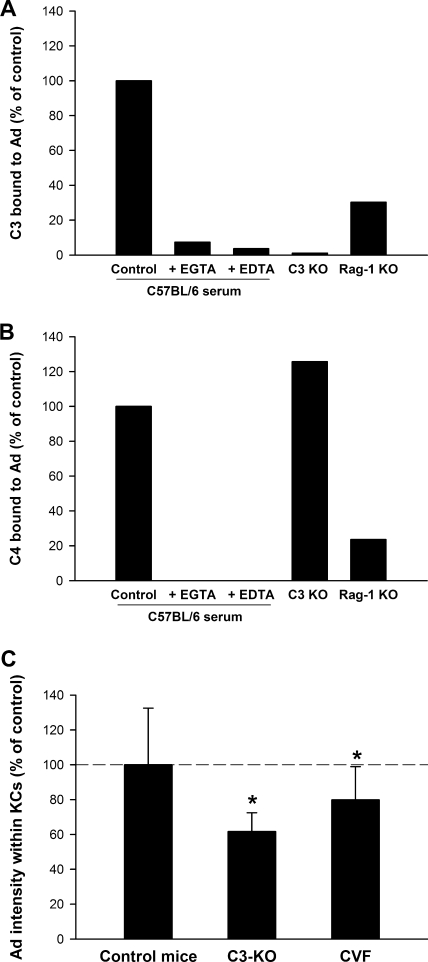
Complement activation can occur through the classical, alternative, or mannan-binding lectin pathways. C3 is a central member of the complement cascade in all three pathways, but C4 is involved only in the classical and lectin pathways. When naïve mouse serum was incubated on Ad-coated 96-well plates, we detected binding of both C3 and C4 (Fig. 3A and B). Complement activation requires divalent cations, and our finding that EDTA blocked binding of C3 and C4 to Ad indicates that the interaction of complement proteins with Ad was not merely through nonspecific association (Fig. 3A and B). In addition, we found that binding of C3 and C4 was blocked by EGTA, which selectively blocks the classical and lectin-mediated pathways without blocking the alterative pathway. These results indicate that the alternative pathway is not responsible for the opsonization of Ad by complement.
FIG. 3.
Ad interacts with complement proteins in vitro and in vivo. (A) The binding of C3 could be detected when mouse serum was incubated on Ad-coated plates. The binding of C3 required Ca2+ and was partially dependent on antibody. Where indicated, EDTA was added at 10 mM to chelate Ca2+ and Mg2+. To chelate Ca2+ selectively, we used 10 mM EGTA and 2 mM MgCl2. One per group, measured in duplicate. Results are representative of three independent experiments. (B) Similarly, the binding of C4 to Ad-coated plates could also be detected. The binding of C4 was dependent on Ca2+, partially dependent on antibody, and independent of C3. One per group, measured in duplicate. Results are representative of three independent experiments. (C) The amount of Ad within KCs was significantly reduced in C3 KO mice and in C57BL/6 mice whose complement had been depleted with CVF. Mice were injected i.v. with 1.0 × 1011 vp/kg of Av1nBg vector and sacrificed after 10 min, and the amount of Ad within KCs was quantitated. Six to 17 mice per group. *, P value of <0.05 versus control C57BL/6 mice (Holm-Sidak post hoc). An additional independent experiment with C3 KO mice at an Ad dose of 1.0 × 1012 vp/kg also found a statistically significant decrease in uptake of Ad by KCs.
The activation of the classical pathway is typically initiated by the binding of antibody followed by the sequential binding and activation of C1, C4, C2, and C3. In line with this sequence, we found that a normal C4 opsonization of Ad was seen with serum from C3 KO mice (Fig. 3B). We also found that the opsonization of Ad by C3 and C4 was reduced in antibody-deficient Rag-1 KO mice, but this reduction was only partial (Fig. 3A and B). This demonstrates that natural antibodies play a role in the opsonization of Ad by complement but also that there exists another antibody-independent opsonization pathway.
To examine whether complement is involved in the uptake of Ad by KCs in vivo, we used both C3 KO mice and wild-type mice that had been depleted of complement by use of CVF. CVF acts as a C3 convertase and depletes C3 and other complement proteins that are downstream of C3 (10). The amount of Ad taken up by KCs was significantly decreased both in C3 KO mice and in complement-depleted wild-type mice (Fig. 3C). However, the decreases were small and were statistically significant only when experiments contained large numbers of mice. In sum, we found that complement opsonizes Ad in vitro and that complement proteins play a modest role in the clearance of Ad by KCs in vivo.
Platelets are not necessary for KC uptake of Ad.
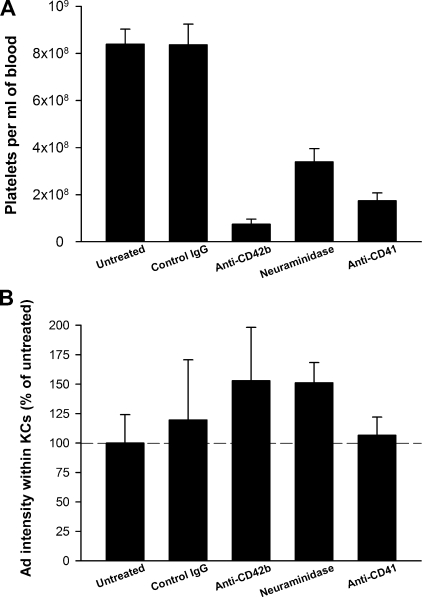
A recent study by Stone et al. (55) reported a role for platelets in the clearance of Ad5 vectors by the liver. It was proposed that when Ad is injected i.v., it binds first to platelets in the blood, and subsequently Ad-laden platelets are taken up by KCs in the liver. However, the amount of Ad within KCs was not quantitated in this study. To examine whether platelets are required for the KC uptake of Ad, we depleted mouse platelets by use of three different methods. We found that antibodies against the platelet antigen CD42b were the most effective method of depleting platelets (Fig. 4A). A second method—i.p. neuraminidase injection—also resulted in platelet depletion, although the platelets were reduced by only 60% (Fig. 4A). Neuraminidase treatment causes a decrease in the amount of sialic acid on the surfaces of platelets and the subsequent removal of platelets from the circulation (54). A third method, using an anti-CD41 antibody according to the method of Stone et al. (55), depleted the majority of platelets, and the amount of depletion was similar to that previously reported (79% depletion of platelets in the current study versus 66% depletion in the work of Stone et al.).
FIG. 4.
Depletion of platelets has no effect on the amount of Ad within KCs. (A) Platelets were depleted by injecting C57BL/6 mice with anti-CD42b, neuraminidase, or anti-CD41. Control mice were either left untreated or injected with control IgG. Mice were injected i.v. with 1.0 × 1012 vp/kg AdLuc vector and sacrificed 10 min later, and platelets were counted in EDTA-blood. (B) Liver sections were collected and the amount of Ad within KCs was quantitated. No significant difference in terms of the amount of Ad within KCs was detected when the untreated group was compared to the treatment groups (P > 0.05; Holm-Sidak post hoc). Four to six mice per group. We repeated the experiment using the most effective method of platelet depletion (anti-CD42b) and again found a lack of effect on the KC uptake of Ad.
None of the three methods of platelet depletion resulted in any significant difference in Ad uptake by KCs (Fig. 4B). Thus, we conclude that KC uptake of Ad does not require platelets.
Vitamin K-dependent coagulation factors are not required for KC uptake of Ad.
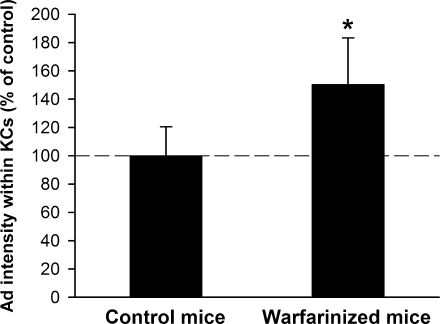
Vitamin K-dependent coagulation factors in the plasma have been shown to facilitate the transduction of hepatocytes by Ad both in vitro and in vivo (24, 39, 45, 59). To examine whether depleting vitamin-K-dependent coagulation factors would affect the uptake of Ad by KCs, mice were treated with warfarin according to the protocol of Parker et al. (39). In a pilot experiment, we found that depleting coagulation factors in this manner caused an almost 1,000-fold decrease in the ability of a luciferase-expressing Ad vector to transduce the liver and express luciferase (data not shown), in agreement with previous findings (39). However, warfarin treatment did not decrease the amount of Ad within KCs. In fact, warfarin treatment unexpectedly caused a statistically significant increase in the amount of Ad found in KCs (Fig. 5), a finding that we reproduced in a second independent experiment (data not shown).
FIG. 5.
Depletion of vitamin K-dependent coagulation factors results in increased accumulation of Ad within KCs. Warfarin-treated or control mice were injected i.v. with 1.0 × 1012 vp/kg of AdLuc vector and sacrificed 10 min later, and the amount of Ad within KCs was quantitated. Seven or nine mice per group. *, P value of <0.05 versus control mice (t test). An additional independent experiment produced a similar statistically significant increase in KC uptake of Ad.
DISCUSSION
One of the most important physiological roles of KCs is to scavenge invading pathogens such as viruses and bacteria and remove them from the blood (7). Many studies have confirmed that KCs play a major role in the rapid clearance of Ad vectors from the circulation and that this reduces the amount of Ad that is available for the transduction of hepatocytes and other target tissues (2, 32, 56, 63). Understanding the mechanisms through which KCs recognize Ad should suggest new strategies to circumvent KC uptake.
As noted in the introduction, in vitro and in vivo investigations into the roles of various Ad receptors often yield strikingly different results; therefore, in vivo studies are vital to ensure that findings are actually relevant to gene therapy. Although it is straightforward to evaluate Ad uptake by KCs in a qualitative fashion, quantitative measurements are much more difficult. Traditional methods of isolating KCs from the liver may be problematic after i.v. injection of Ad, because we have shown that Ad vectors rapidly disrupt and kill KCs in vivo (32). Our recently developed immunofluorescent method has proven to be an effective method for quantitating the uptake of Ad by KCs in situ (47). Because we previously found that neither of the main Ad receptors are required for uptake by KCs (48), we initiated a wider survey to identify receptors and opsonins that influence the uptake of Ad by KCs.
SRs.
SRs are a diverse array of receptors that are defined more by their function than by their structure. There are at least eight different classes of SRs that share little sequence homology, but they all possess the ability to recognize negatively charged materials, without any need for opsonization by plasma proteins (34, 35). Studies with SR KO mice have so far not reported any major impact on the clearance of SR ligands in vivo (19), most likely due to the presence of multiple additional functionally redundant SRs from different classes. Thus, the most definitive method available to establish the role of SRs in vivo is to compete clearance by preinjecting known SR ligands (18, 25, 27, 57, 62).
Interestingly, the Ad capsid bears an overall negative charge, with the amounts of negative charge differing among serotypes due primarily to differences in the hypervariable hexon loops (28). The serotypes most commonly used for gene therapy—Ad5 and the closely related Ad2—are two of the most negatively charged serotypes (28, 29). Alemany et al. (2) were the first to speculate that the negative charge of the Ad capsid might lead to an interaction with SRs. They deleted a 13-residue stretch of acidic residues from the Ad5 hexon to reduce the negative charge of the capsid, but this had no effect on the rate of Ad clearance from the circulation, possibly because the overall charge of the capsid was still negative (2). Recently we found that a polyribonucleotide, poly(I), decreases the KC uptake of Ad in vivo (47), and Haisma et al. showed that poly(I) reduces the transduction of macrophages by Ad in vitro, slows the clearance of Ad from the circulation, and enhances the ability of Ad to transduce hepatocytes in vivo (17). In addition, Haisma et al. recently reported that the expression of a class A SR enhances the susceptibility of CHO cells to transduction by Ad vector (16).
In the current study, we extended these investigations beyond poly(I) and surveyed a wide variety of SR ligands for their ability to reduce the KC clearance of Ad. We found that the broad-specificity SR ligands poly(I), poly(G), and dextran sulfate all had similar inhibitory effects on the KC uptake of Ad. Importantly, the non-SR ligands poly(U), poly(C), and dextran had no effect, demonstrating the specificity of this phenomenon. Although all of the polyribonucleotides used in this study bear an overall negative charge, they differ in their abilities to bind SRs due to structural factors. For example, it has been shown that poly(I) and poly(G) can adopt a four-stranded helical conformation that greatly enhances their recognition by a bovine class A SR, but poly(A), poly(C), and poly(U) cannot adopt this conformation and are not recognized (40). We found that poly(A) and heparin caused a partial reduction of Ad uptake by KCs, although this was statistically significant only for poly(A). Both poly(A) and heparin are known to inhibit the clearance of distinct subsets of SR ligands, apparently by binding to only a subset of SRs (15, 53, 58, 65). Since a reduction in the amount of Ad taken up by KCs should increase the availability of Ad to other tissues, we also tested selected SR ligands for their ability to enhance Ad transduction of hepatocytes in vivo. We found that the SR ligands that strongly inhibited the KC uptake of Ad, such as poly(I) and dextran sulfate, were able to substantially enhance liver transduction, but the non-SR ligand poly(C) had no effect.
Because SRs recognize negatively charged materials, it may be interesting to investigate whether Ad serotypes that bear less negative charge on their capsids might be more successful than Ad5 at evading KCs. Altering the hexon sequences to engineer a neutrally charged capsid might be another strategy to limit SR binding, although there could be practical barriers to producing such Ad vectors, given the vital role that surface charge plays in preventing the aggregation of nanoparticles (30). An alternative strategy may be to modify Ad with nonreactive polymers, which is proving to be an effective method for slowing the clearance of Ad from the circulation (14, 33), likely due to a decreased removal of Ad by KCs (reference 33 and our unpublished observations). It is also worth noting that SRs have been implicated in the uptake of certain types of liposomes (43), and predosing mice with liposomes can reduce the KC uptake of Ad and enhance hepatocyte transduction in vivo (50).
Although poly(I) and Ad were well tolerated in mice when administered individually, we noted considerable mortality when poly(I) was injected prior to higher doses of Ad vector. The mechanism for this is unknown and we are currently investigating whether this enhanced toxicity is specific to poly(I) or is a general feature of SR inhibitors. Much further work will be required to evaluate whether pretreatment with SR ligands might have the potential to be a safe or practical clinical strategy for enhancing the efficiency of Ad gene therapy.
Natural antibodies and complement.
Although natural antibodies tend to have low affinity and low abundance, they can have profound biological effects in inhibiting both viral infection and viral gene therapy. Polyreactive natural IgM antibodies play an important role in the control of viral infections in vivo, where they are a vital innate defense during the early stages of an infection, before the adaptive immune system has mounted an antigen-specific response (8, 37, 38, 69). In addition, natural antibodies are a major obstacle to in vivo gene therapy with retroviruses, bacteriophage, herpes simplex virus type 1, and baculovirus (21, 42, 51, 52, 61).
In the current study, we discovered that Ad is opsonized by natural IgM antibodies from naïve mouse serum, while opsonization by IgG was not detected. Similar findings have previously been made with a number of other viruses (21, 22, 37, 61). Although the monomeric affinity of natural IgM tends to be much lower than that of affinity-matured antigen-specific IgG antibodies, the avidity of IgM can be very high when bound to repetitive antigens (such as viral capsids), because IgM is a pentamer or a hexamer that contains 10 or 12 Fabs (69). Interestingly, IgM changes shape when bound to multivalent antigens and becomes an extremely effective initiator of the classical complement pathway (5, 13). Only a single IgM molecule is needed to activate the hemolytic activity of complement, a task that requires roughly 1,000 IgG molecules (11). Natural antibodies and subsequent complement activation cause the lysis or neutralization of many viruses (21, 22, 38, 42, 51, 52, 61). However, we were unable to detect any neutralizing effect of 90% mouse serum on the infectious titer of Ad vectors (our unpublished observations), in agreement with a previous report (2).
We found that mouse complement components C3 and C4 opsonize Ad in vitro. Further, we observed that the mechanism for this complement opsonization was partially dependent on natural antibodies and independent of the alternative pathway. Complement activation involves a complex enzymatic cascade, and caution is warranted when evaluating these mechanisms and extrapolating them to the in vivo situation, where many of the conditions are significantly different. Complement activation can be influenced by many factors, including how blood is collected and processed, the format in which the virus is presented (fixed or soluble), the extent of the dilution of serum or plasma, the time and temperature of incubation, the particular assays that are used to measure complement activation, and the level of preexisting antiviral antibodies. These technical factors may help to explain why there is considerable disagreement among previous studies on the mechanisms by which Ad5 activates human complement. Although all studies agree that the lectin pathway plays no role, some studies found that only the classical pathway is involved (9, 66), other studies found that the alternative but not the classical pathway is involved (4, 23), and an additional study found that both the classical and alternative pathways are involved depending on the concentration of Ad (41).
The ultimate test of the relevance of complement is an in vivo study, and therefore we measured whether the absence of complement component C3 changed the uptake of Ad by KCs. We found that the uptake of Ad by KCs was significantly decreased in two different models: C3 KO mice and complement-depleted mice. However, the reduction in uptake was modest, indicating the presence of alternative clearance mechanisms.
Macrophages express multiple receptors for Igs and complement, and future investigation will be needed to delineate the mechanisms by which KCs recognize Ad that has been opsonized with IgM and complement. Macrophages express at least one Fc receptor that can recognize IgM, the Fcα/μ receptor (46). However IgM also powerfully activates complement, and in vivo studies actually show that the KC uptake of IgM-opsonized erythrocytes is mediated in large part by the complement receptor CR3, as demonstrated with CR3 KO mice (64). In addition, it has recently been discovered that KCs express CRIg, a C3b receptor that is important for the clearance of opsonized bacteria from the circulation (20). It should be pointed out that in our experiments we targeted C3, but it is also possible that opsonization by other complement proteins might be relevant to clearance from C3-deficient mice. For example, C1-opsonized material can bind to C1q receptors on macrophages (3, 12).
Platelets and coagulation factors are not required for uptake of Ad by KCs.
Platelets and coagulation factors have previously been proposed to be involved in the clearance of Ad by KCs (45, 55), so we examined their roles quantitatively in our system. We depleted platelets from the circulation by use of multiple methods but found no impact on the clearance of Ad by KCs, demonstrating that there is no requirement for platelets to deliver Ad to KCs. This is in contrast to the study of Stone et al. (55), who reported that depleting platelets reduces the amount of Ad5 sequestered in the liver. However, Stone et al. did not specifically quantitate the amount of Ad within KCs. In addition to this methodological difference, there were a number of other differences from our experiments that may have contributed to the divergent results, including a dose of Ad fourfold higher than that in our study and an earlier time point of 5 min. Notably as well, our study was performed using normal C57BL/6 mice, while Stone et al. used mice that were transgenic for CD46, a complement control protein.
We and others have previously found using electron microscopy that platelets associate with KCs in the liver soon after the i.v. injection of Ad (32, 44). Because KCs begin to degenerate within minutes after the i.v. injection of Ad (32), we interpret this platelet association with KCs as evidence that platelets are activated by disrupted KCs and associated intravascular coagulation rather than as evidence that platelets bind to Ad and then ferry virions to KCs. We have extensively searched for Ad virions in mouse liver by electron microscopy; although we have observed enormous numbers of virions inside KCs 10 min after an i.v. injection of Ad at 1013 vp/kg (32), the association of virions with platelets is extraordinarily uncommon (our unpublished observations).
Regarding the role of coagulation factors, it has been shown that the Ad transduction of hepatocytes in vitro can be enhanced by FVII, FIX, FX, and protein C (6, 39, 45). In vivo, warfarin can deplete these coagulation factors, resulting in a substantial reduction in the ability of Ad to transduce hepatocytes (39). In particular, FX opsonizes Ad via high-affinity direct binding to the Ad hexon protein (24, 59), and injecting warfarin-treated mice with FX is sufficient to restore the ability of Ad to transduce hepatocytes in vivo (24, 39, 60). Regarding the influence of coagulation factors on KCs, FIX has been reported to enhance the uptake of Ad by KCs (45), but these findings were obtained using a liver perfusion system and therefore their in vivo relevance may be limited. Because of the profound effect of coagulation factors on hepatocyte transduction, we examined whether depleting coagulation factors with warfarin could also affect the KC uptake of Ad in vivo.
We found not only that coagulation factors were not necessary for Ad uptake by KCs but also that KCs of warfarin-treated mice contained significantly more Ad than did those of control mice. This might be due to the fact that warfarin-treated mice sequester much less Ad in hepatocytes, and we speculate that this might lead to a corresponding increase in the amount of Ad available to KCs. This scenario is consistent with recent studies showing that the total amount of Ad sequestered in the liver is unchanged in warfarin-treated mice, as measured by the amount of Ad DNA in the liver at 1 h after Ad injection (24, 60). An important implication of our results is that although ablating the ability of Ad vectors to bind coagulation factors is a very effective method to detarget Ad from hepatocytes (24, 59), this may not necessarily detarget KCs.
In conclusion, our results show that SRs play a major role in the sequestration of Ad by KCs in vivo. Natural antibodies and complement also contribute to an extent. However, we found no evidence of any requirement for platelets or coagulation factors in the uptake of Ad by KCs. Many different strategies are currently being developed to redirect the tropism of Ad vectors to various receptors on specific tissues, but it is clear that efficient systemic gene delivery will also require techniques to detarget Ad from the RES. Although we found that multiple mechanisms were involved in the uptake of Ad by KCs, it appears from our results that reducing the interaction of Ad with SRs might have the best potential to improve systemic Ad gene delivery in vivo.
Acknowledgments
This research was supported by the FDA, including support from the Critical Path Program.
We thank Mike Havert and Takele Argaw for helpful reviews of the manuscript.
Footnotes
Published ahead of print on 24 September 2008.
REFERENCES
- 1.Alemany, R., and D. T. Curiel. 2001. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 81347-1353. [DOI] [PubMed] [Google Scholar]
- 2.Alemany, R., K. Suzuki, and D. T. Curiel. 2000. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 812605-2609. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Dominguez, C., E. Carrasco-Marin, and F. Leyva-Cobian. 1993. Role of complement component C1q in phagocytosis of Listeria monocytogenes by murine macrophage-like cell lines. Infect. Immun. 613664-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appledorn, D. M., A. Kiang, A. McBride, H. Jiang, S. Seregin, J. M. Scott, R. Stringer, Y. Kousa, M. Hoban, M. M. Frank, and A. Amalfitano. 2008. Wild-type adenoviruses from groups A-F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Ther. 15885-901. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong, S. J., M. C. Outlaw, and N. J. Dimmock. 1990. Morphological studies of the neutralization of influenza virus by IgM. J. Gen. Virol. 712313-2319. [DOI] [PubMed] [Google Scholar]
- 6.Baker, A. H., J. H. McVey, S. N. Waddington, N. C. Di Paolo, and D. M. Shayakhmetov. 2007. The influence of blood on in vivo adenovirus bio-distribution and transduction. Mol. Ther. 151410-1416. [DOI] [PubMed] [Google Scholar]
- 7.Bilzer, M., F. Roggel, and A. L. Gerbes. 2006. Role of Kupffer cells in host defense and liver disease. Liver Int. 261175-1186. [DOI] [PubMed] [Google Scholar]
- 8.Boes, M. 2000. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 371141-1149. [DOI] [PubMed] [Google Scholar]
- 9.Cichon, G., S. Boeckh-Herwig, H. H. Schmidt, E. Wehnes, T. Muller, P. Pring-Akerblom, and R. Burger. 2001. Complement activation by recombinant adenoviruses. Gene Ther. 81794-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cochrane, C. G., H. J. Muller-Eberhard, and B. S. Aikin. 1970. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J. Immunol. 10555-69. [PubMed] [Google Scholar]
- 11.Cooper, N. R. 1985. The classical complement pathway: activation and regulation of the first complement component. Adv. Immunol. 37151-216. [DOI] [PubMed] [Google Scholar]
- 12.Coremans, I. E., W. M. Bogers, R. K. Stad, E. A. van der Voort, F. A. Prins, N. van Rooijen, F. C. Breedveld, and M. R. Daha. 1993. Role of liver endothelial and Kupffer cells in clearance of human C1q in rats. Eur. J. Immunol. 231942-1947. [DOI] [PubMed] [Google Scholar]
- 13.Feinstein, A., N. Richardson, and M. J. Taussig. 1986. Immunoglobulin flexibility in complement activation. Immunol. Today 7169-174. [DOI] [PubMed] [Google Scholar]
- 14.Green, N. K., C. W. Herbert, S. J. Hale, A. B. Hale, V. Mautner, R. Harkins, T. Hermiston, K. Ulbrich, K. D. Fisher, and L. W. Seymour. 2004. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 111256-1263. [DOI] [PubMed] [Google Scholar]
- 15.Haba, M., K. Urano, H. Yuasa, and J. Watanabe. 1996. Molecular weight dependency in the uptake of fractionated [3H]heparin in isolated rat Kupffer cells. Biol. Pharm. Bull. 19864-868. [DOI] [PubMed] [Google Scholar]
- 16.Haisma, H. J., M. Boesjes, and A. R. Bellu. 2008. Scavenger receptor A: a route for adenovirus internalization into macrophages. Mol. Ther. 16(Suppl. 1)S302. [Google Scholar]
- 17.Haisma, H. J., J. A. Kamps, G. K. Kamps, J. A. Plantinga, M. G. Rots, and A. R. Bellu. 2008. Polyinosinic acid enhances delivery of adenovirus vectors in vivo by preventing sequestration in liver macrophages. J. Gen. Virol. 891097-1105. [DOI] [PubMed] [Google Scholar]
- 18.Hampton, R. Y., D. T. Golenbock, M. Penman, M. Krieger, and C. R. Raetz. 1991. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature 352342-344. [DOI] [PubMed] [Google Scholar]
- 19.Hansen, B., B. Arteta, and B. Smedsrod. 2002. The physiological scavenger receptor function of hepatic sinusoidal endothelial and Kupffer cells is independent of scavenger receptor class A type I and II. Mol. Cell. Biochem. 2401-8. [DOI] [PubMed] [Google Scholar]
- 20.Helmy, K. Y., K. J. Katschke, Jr., N. N. Gorgani, N. M. Kljavin, J. M. Elliott, L. Diehl, S. J. Scales, N. Ghilardi, and C. M. van Lookeren. 2006. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 124915-927. [DOI] [PubMed] [Google Scholar]
- 21.Hoare, J., S. Waddington, H. C. Thomas, C. Coutelle, and M. J. McGarvey. 2005. Complement inhibition rescued mice allowing observation of transgene expression following intraportal delivery of baculovirus in mice. J. Gene Med. 7325-333. [DOI] [PubMed] [Google Scholar]
- 22.Jayasekera, J. P., E. A. Moseman, and M. C. Carroll. 2007. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 813487-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang, H., Z. Wang, D. Serra, M. M. Frank, and A. Amalfitano. 2004. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-Ad antibodies. Mol. Ther. 101140-1142. [DOI] [PubMed] [Google Scholar]
- 24.Kalyuzhniy, O., N. C. Di Paolo, M. Silvestry, S. E. Hofherr, M. A. Barry, P. L. Stewart, and D. M. Shayakhmetov. 2008. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 1055483-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawabata, K., Y. Takakura, and M. Hashida. 1995. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm. Res. 12825-830. [DOI] [PubMed] [Google Scholar]
- 26.Kiang, A., Z. C. Hartman, R. S. Everett, D. Serra, H. Jiang, M. M. Frank, and A. Amalfitano. 2006. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 14588-598. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi, N., T. Kuramoto, K. Yamaoka, M. Hashida, and Y. Takakura. 2001. Hepatic uptake and gene expression mechanisms following intravenous administration of plasmid DNA by conventional and hydrodynamics-based procedures. J. Pharmacol. Exp. Ther. 297853-860. [PubMed] [Google Scholar]
- 28.Konz, J. O., R. C. Livingood, A. J. Bett, A. R. Goerke, M. E. Laska, and S. L. Sagar. 2005. Serotype specificity of adenovirus purification using anion-exchange chromatography. Hum. Gene Ther. 161346-1353. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn, I., B. Larsen, C. Gross, and T. Hermiston. 2007. High-performance liquid chromatography method for rapid assessment of viral particle number in crude adenoviral lysates of mixed serotype. Gene Ther. 14180-184. [DOI] [PubMed] [Google Scholar]
- 30.Liang, Y., N. Hilal, P. Langston, and V. Starov. 2007. Interaction forces between colloidal particles in liquid: theory and experiment. Adv. Colloid Interface Sci. 134-135:151-166. [DOI] [PubMed] [Google Scholar]
- 31.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 718798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manickan, E., J. S. Smith, J. Tian, T. L. Eggerman, J. N. Lozier, J. Muller, and A. P. Byrnes. 2006. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 13108-117. [DOI] [PubMed] [Google Scholar]
- 33.Mok, H., D. J. Palmer, P. Ng, and M. A. Barry. 2005. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 1166-79. [DOI] [PubMed] [Google Scholar]
- 34.Mukhopadhyay, S., and S. Gordon. 2004. The role of scavenger receptors in pathogen recognition and innate immunity. Immunobiology 20939-49. [DOI] [PubMed] [Google Scholar]
- 35.Murphy, J. E., P. R. Tedbury, S. Homer-Vanniasinkam, J. H. Walker, and S. Ponnambalam. 2005. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 1821-15. [DOI] [PubMed] [Google Scholar]
- 36.Nalbantoglu, J., G. Pari, G. Karpati, and P. C. Holland. 1999. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum. Gene Ther. 101009-1019. [DOI] [PubMed] [Google Scholar]
- 37.Ochsenbein, A. F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R. M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science 2862156-2159. [DOI] [PubMed] [Google Scholar]
- 38.Ochsenbein, A. F., and R. M. Zinkernagel. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21624-630. [DOI] [PubMed] [Google Scholar]
- 39.Parker, A. L., S. N. Waddington, C. Nicol, D. M. Shayakhmetov, S. M. Buckley, L. Denby, G. Kemball-Cook, S. Ni, A. Lieber, J. H. McVey, S. A. Nicklin, and A. H. Baker. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes in vitro and in vivo. Blood 1082554-2561. [DOI] [PubMed] [Google Scholar]
- 40.Pearson, A. M., A. Rich, and M. Krieger. 1993. Polynucleotide binding to macrophage scavenger receptors depends on the formation of base-quartet-stabilized four-stranded helices. J. Biol. Chem. 2683546-3554. [PubMed] [Google Scholar]
- 41.Perreau, M., M. C. Guerin, C. Drouet, and E. J. Kremer. 2007. Interactions between human plasma components and a xenogenic adenovirus vector: reduced immunogenicity during gene transfer. Mol. Ther. 151998-2007. [DOI] [PubMed] [Google Scholar]
- 42.Rother, R. P., W. L. Fodor, J. P. Springhorn, C. W. Birks, E. Setter, M. S. Sandrin, S. P. Squinto, and S. A. Rollins. 1995. A novel mechanism of retrovirus inactivation in human serum mediated by anti-alpha-galactosyl natural antibody. J. Exp. Med. 1821345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothkopf, C., A. Fahr, G. Fricker, G. L. Scherphof, and J. A. Kamps. 2005. Uptake of phosphatidylserine-containing liposomes by liver sinusoidal endothelial cells in the serum-free perfused rat liver. Biochim. Biophys. Acta 166810-16. [DOI] [PubMed] [Google Scholar]
- 44.Schiedner, G., W. Bloch, S. Hertel, M. Johnston, A. Molojavyi, V. Dries, G. Varga, N. van Rooijen, and S. Kochanek. 2003. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum. Gene Ther. 141631-1641. [DOI] [PubMed] [Google Scholar]
- 45.Shayakhmetov, D. M., A. Gaggar, S. Ni, Z. Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 797478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibuya, A., N. Sakamoto, Y. Shimizu, K. Shibuya, M. Osawa, T. Hiroyama, H. J. Eyre, G. R. Sutherland, Y. Endo, T. Fujita, T. Miyabayashi, S. Sakano, T. Tsuji, E. Nakayama, J. H. Phillips, L. L. Lanier, and H. Nakauchi. 2000. Fc alpha/mu receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 1441-446. [DOI] [PubMed] [Google Scholar]
- 47.Smith, J. S., Z. Xu, and A. P. Byrnes. 2008. A quantitative assay for measuring clearance of adenovirus vectors by Kupffer cells. J. Virol. Methods 14754-60. [DOI] [PubMed] [Google Scholar]
- 48.Smith, J. S., Z. Xu, J. Tian, S. C. Stevenson, and A. P. Byrnes. 2008. Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum. Gene Ther. 19547-554. [DOI] [PubMed] [Google Scholar]
- 49.Smith, T. A., N. Idamakanti, M. L. Rollence, J. Marshall-Neff, J. Kim, K. Mulgrew, G. R. Nemerow, M. Kaleko, and S. C. Stevenson. 2003. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 14777-787. [DOI] [PubMed] [Google Scholar]
- 50.Snoeys, J., G. Mertens, J. Lievens, T. van Berkel, D. Collen, E. A. Biessen, and B. De Geest. 2006. Lipid emulsions potently increase transgene expression in hepatocytes after adenoviral transfer. Mol. Ther. 1398-107. [DOI] [PubMed] [Google Scholar]
- 51.Sokoloff, A. V., I. Bock, G. Zhang, S. Hoffman, J. Dama, J. J. Ludtke, A. M. Cooke, and J. A. Wolff. 2001. Specific recognition of protein carboxy-terminal sequences by natural IgM antibodies in normal serum. Mol. Ther. 3821-830. [DOI] [PubMed] [Google Scholar]
- 52.Srivastava, A. S., T. Kaido, and E. Carrier. 2004. Immunological factors that affect the in vivo fate of T7 phage in the mouse. J. Virol. Methods 11599-104. [DOI] [PubMed] [Google Scholar]
- 53.Stehle, G., E. A. Friedrich, H. Sinn, A. Wunder, J. Harenberg, C. E. Dempfle, W. Maier-Borst, and D. L. Heene. 1992. Hepatic uptake of a modified low molecular weight heparin in rats. J. Clin. Investig. 902110-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenberg, P. E., J. Levin, G. Baker, Y. Mok, and L. Corash. 1991. Neuraminidase-induced thrombocytopenia in mice: effects on thrombopoiesis. J. Cell. Physiol. 1477-16. [DOI] [PubMed] [Google Scholar]
- 55.Stone, D., Y. Liu, D. Shayakhmetov, Z. Y. Li, S. Ni, and A. Lieber. 2007. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J. Virol. 814866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tao, N., G. P. Gao, M. Parr, J. Johnston, T. Baradet, J. M. Wilson, J. Barsoum, and S. E. Fawell. 2001. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 328-35. [DOI] [PubMed] [Google Scholar]
- 57.Terpstra, V., and T. J. van Berkel. 2000. Scavenger receptors on liver Kupffer cells mediate the in vivo uptake of oxidatively damaged red blood cells in mice. Blood 952157-2163. [PubMed] [Google Scholar]
- 58.Vlassara, H., M. Brownlee, and A. Cerami. 1986. Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J. Exp. Med. 1641301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waddington, S. N., J. H. McVey, D. Bhella, A. L. Parker, K. Barker, H. Atoda, R. Pink, S. M. Buckley, J. A. Greig, L. Denby, J. Custers, T. Morita, I. M. Francischetti, R. Q. Monteiro, D. H. Barouch, N. van Rooijen, C. Napoli, M. J. Havenga, S. A. Nicklin, and A. H. Baker. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132397-409. [DOI] [PubMed] [Google Scholar]
- 60.Waddington, S. N., A. L. Parker, M. Havenga, S. A. Nicklin, S. M. Buckley, J. H. McVey, and A. H. Baker. 2007. Targeting of adenovirus serotype 5 (Ad5) and 5/47 pseudotyped vectors in vivo: a fundamental involvement of coagulation factors and redundancy of CAR binding by Ad5. J. Virol. 819568-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wakimoto, H., K. Ikeda, T. Abe, T. Ichikawa, F. H. Hochberg, R. A. Ezekowitz, M. S. Pasternack, and E. A. Chiocca. 2002. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol. Ther. 5275-282. [DOI] [PubMed] [Google Scholar]
- 62.Willekens, F. L., J. M. Werre, J. K. Kruijt, B. Roerdinkholder-Stoelwinder, Y. A. Groenen-Dopp, A. G. van den Bos, G. J. Bosman, and T. J. van Berkel. 2005. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 1052141-2145. [DOI] [PubMed] [Google Scholar]
- 63.Wolff, G., S. Worgall, N. van Rooijen, W. R. Song, B. G. Harvey, and R. G. Crystal. 1997. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J. Virol. 71624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan, J., V. Vetvicka, Y. Xia, M. Hanikyrova, T. N. Mayadas, and G. D. Ross. 2000. Critical role of Kupffer cell CR3 (CD11b/CD18) in the clearance of IgM-opsonized erythrocytes or soluble beta-glucan. Immunopharmacology 4639-54. [DOI] [PubMed] [Google Scholar]
- 65.Yuasa, H., and J. Watanabe. 2003. Are novel scavenger-like receptors involved in the hepatic uptake of heparin? Drug Metab. Pharmacokinet. 18273-286. [DOI] [PubMed] [Google Scholar]
- 66.Zaiss, A. K., M. J. Cotter, L. R. White, S. A. Clark, N. C. Wong, V. M. Holers, J. S. Bartlett, and D. A. Muruve. 2008. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 822727-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, Y., and J. M. Bergelson. 2005. Adenovirus receptors. J. Virol. 7912125-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3697-707. [DOI] [PubMed] [Google Scholar]
- 69.Zhou, Z. H., A. G. Tzioufas, and A. L. Notkins. 2007. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J. Autoimmun. 29219-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zinn, K. R., A. J. Szalai, A. Stargel, V. Krasnykh, and T. R. Chaudhuri. 2004. Bioluminescence imaging reveals a significant role for complement in liver transduction following intravenous delivery of adenovirus. Gene Ther. 111482-1486. [DOI] [PubMed] [Google Scholar]