Abstract
Herpesviruses use a cascade of interactions with different cell surface molecules to gain entry into cells. In many cases, this involves binding to abundant glycosaminoglycans or integrins followed by interactions with more limited cell surface proteins, leading to fusion with cellular membranes. Human cytomegalovirus (HCMV) has the ability to infect a wide variety of human cell types in vivo. However, very little is known about which HCMV glycoproteins mediate entry into various cell types, including relevant epithelial and endothelial cells. For other herpesviruses, studies of cell-cell fusion induced by viral proteins have provided substantial information about late stages of entry. In this report, we describe the fusion of epithelial, endothelial, microglial, and fibroblast cells in which HCMV gB and gH/gL were expressed from nonreplicating adenovirus vectors. Fusion frequently involved the majority of cells, and gB and gH/gL were both necessary and sufficient for fusion, whereas no fusion occurred when either glycoprotein was omitted. Coexpression of UL128, UL130, and UL131 did not enhance fusion. We concluded that the HCMV core fusion machinery consists of gB and gH/gL. Coimmunoprecipitation indicated that HCMV gB and gH/gL can interact. Importantly, expression of gB and gH/gL in trans (gB-expressing cells mixed with other gH/gL-expressing cells) resulted in substantial fusion. We believe that this is the first description of a multicomponent viral fusion machine that can be split between cells. If gB and gH/gL must interact for fusion, then these molecules must reach across the space between apposing cells. Expression of gB and gH/gL in trans with different cell types revealed surface molecules that are required for fusion on HCMV-permissive cells but not on nonpermissive cells.
Human cytomegalovirus (HCMV) is a betaherpesvirus that infects as many as 50 to 85% of humans, establishing a lifelong, persistent infection involving a cycle of latency and reactivation in some cell types and probably persistent, low-level replication in other cells. Infection of hosts with a functional immune system usually results in relatively minor symptoms, although it may involve fever, hepatitis, splenomegaly, and a mononucleosis-like disease. In contrast, hosts that are immunocompromised or immunodeficient often experience life-threatening diseases, including pneumonia, gastrointestinal disease, hepatitis, retinitis, and encephalitis. HCMV can be particularly devastating in neonates, causing defects in neurological development (2, 53). Moreover, HCMV is a major problem in transplantation, causing rejection of transplanted organs or cells (2, 69).
HCMV has the ability to infect a wide variety of cells in vivo, including endothelial cells, epithelial cells, fibroblasts, smooth muscle cells, monocytes, and macrophages (2, 44, 57). However, in the laboratory, HCMV is routinely propagated on normal human fibroblasts, nontransformed fibroblast lines, or fibroblasts immortalized after transfection with telomerase. Lab-adapted HCMV strains, e.g., AD169 and Towne, fail to infect endothelial and epithelial cells due to large deletions and point mutations found between open reading frames UL128 and UL150 of the HCMV genome (16, 21, 28, 49, 66). More specifically, the loss of the UL128, UL130, or UL131 gene was found to compromise virus infection of epithelial and endothelial cells, and rescue of wild-type UL128-131 genes restored the capacity to infect epithelial and endothelial cells (28, 74). These observations provided highly important insights into how HCMV infects endothelial and epithelial cells, two cell types that are critical for viral pathogenesis.
The UL128-131 genes encode relatively small proteins that possess signal sequences, but not membrane-spanning domains, and these proteins assemble onto the extracellular domain of HCMV glycoproteins gH and gL (gH/gL) (1, 61, 75). We showed that gH/gL/UL128-131 complexes were essential for HCMV entry into epithelial and endothelial cells, a process involving endocytosis and low-pH-dependent fusion with endosomal membranes (60). The UL128-131 proteins are not required for HCMV entry into human fibroblasts (28, 60, 74), and instead, a second gH/gL complex containing gO (34) may promote infection of these cells (22, 33). There is also evidence that deletion of gO from the genome compromises assembly and cell-to-cell spread of HCMV (38). Other herpesviruses, including Epstein-Barr virus (EBV) and human herpesvirus 6 (HHV-6), also possess distinct gH/gL complexes (46, 48, 78). There is substantial evidence that these different gH/gL complexes bind receptors that are specific to different cell types (7, 42, 48). Consistent with the notion that HCMV gH/gL/UL128-131 functions in receptor binding, we recently showed that expression of gH/gL/UL128-131, but not gB or gH/gL (without UL128-131), in ARPE-19 retinal epithelial cells interfered with infection of the cells (62). Interference had previously been used to provide evidence of herpes simplex virus (HSV) gD receptors that were subsequently identified as essential components of the entry pathways (15, 39). Together, these studies strongly support the hypothesis that HCMV gH/gL complexes, either gH/gL, gH/gL/gO, or gH/gL/UL128-131, function in virus entry, likely by binding receptors.
It has become clear that herpesviruses utilize different proteins, in some cases in a redundant fashion, to adsorb onto the surfaces of cells and then bind to more specific receptor proteins that activate fusion of the virion envelope with cellular membranes. The best characterized of the herpesvirus entry pathways involve HSV (reviewed in reference 68), with gB and gC acting redundantly to initiate adsorption of HSV onto heparan sulfate proteoglycans, followed by gD binding to herpesvirus entry mediator or nectins, to trigger fusion with the plasma membrane or endosomal membranes (17, 51). Both gB and gH/gL are required for this fusion (13, 25, 58). Recently, evidence was provided that expression of gH/gL along with gD in cellular membranes or the virion envelope can produce hemifusion, the mixing of the outer leaflets of membranes, whereas expression of gB, gD, and gH/gL produces full fusion, mixing of the contents of cells (70). This suggests that both gB and gH/gL have membrane fusogenic capacity, but both are clearly required for HSV fusion of the virion envelope with cell membranes. A different view of HSV gB and gH/gL came from studies of how these proteins function during virus egress from cells. When crossing the nuclear envelope, either gB or gH/gL can act to promote fusion between the virion envelope and the outer nuclear membrane (NM) (23). Thus, the studies of Subramanian and Geraghty (70) and our studies of nuclear egress are strong support for the notion that gB and gH/gL are both capable of causing membrane fusion under different circumstances.
Cell-cell fusion assays have been paramount in terms of understanding how HSV and other herpesvirus membrane proteins function in virus entry. Turner et al. (72) established that HSV gB, gD, and gH/gL are necessary and sufficient for cell-cell fusion of Chinese hamster ovary (CHO) cells (72). This fits well with observations that all of these glycoproteins are essential for entry as well as for fusion of cells infected with syncytial HSV mutants (13, 20, 25, 47, 58). Importantly, fusion was markedly diminished when any one of gB, gD, gH, or gL was omitted. Previous observations that HSV or HCMV gB (6, 12) or HSV gD alone (12, 14) could cause cell-cell fusion were difficult to reconcile with the results of Turner et al. (72) and with observations made using HSV gB, gD, and gH/gL mutants and may reflect the use of highly transformed cells, namely, CHO, bovine hamster kidney (BHK), and COS monkey cells, that have less extracellular matrix, which may preclude fusion. In addition, unlike nontransformed cells, highly transformed cells are also subject to spontaneous fusion even without expression of herpesvirus proteins. There was also evidence that cell-cell fusion differs from the fusion produced by syncytial HSV, which is diminished by deletion of gE/gI and gM (20). Thus, cell-cell fusion differs in some respects from HSV-induced fusion and the fusion that occurs during virus entry. However, these cell-cell fusion assays established HSV gB, gD, and gH/gL as the core viral fusion machinery. Recent studies involving split fluorescent proteins conjugated with HSV gB, gD and gH/gL support a model in which gD binding to its receptors triggers a core fusion complex, bringing together gB, gD, and gH/gL (3, 4). Cell fusion assays involving EBV (27) and HHV-8 (55) also found that gB and gH/gL (with gp42 in the case of EBV fusion of B cells) were sufficient for cell-cell fusion.
Our understanding of which HCMV glycoproteins are necessary for virus entry has lagged behind the knowledge of alpha- and gammaherpesvirus entry, largely because mutants lacking membrane proteins have not been characterized to define the steps in which virus replication is blocked. There is evidence that gB and gH/gL are essential for HCMV replication, based on an inability to rescue viruses containing mutations in these genes (22, 33). Previous evidence was presented to indicate that HCMV gH/gL expressed without gB promoted fusion of CHO cells and immortalized fibroblasts (41). These results probably reflect the fusogenic activity of gH/gL in the absence of gB.
We have been studying HCMV entry into epithelial and endothelial cells. It is important to understand the roles of gB and gH/gL in entry into these cells. Thus, we established conditions to efficiently express HCMV glycoproteins in retinal epithelial (ARPE-19) cells by using nonreplicating adenovirus (Ad) vectors. Here we show that expression of HCMV gB and gH/gL in ARPE-19 epithelial cells produced extensive cell-cell fusion. Expression of gB or gH/gL alone did not produce any detectable fusion, UL128-131 was not necessary for fusion, and certain mutant forms of gB were not active with gH/gL in fusion. Evidence is presented showing that gB and gH/gL can interact when they are coexpressed in ARPE-19 cells. Surprisingly, expression of gB in one set of cells mixed with other cells expressing gH/gL produced extensive fusion. Previously, the expression of HSV glycoproteins in trans did not lead to cell-cell fusion (10, 56).
MATERIALS AND METHODS
Cell lines.
Human epithelial cervical adenocarcinoma (HeLa), human laryngeal carcinoma (Hep-2), human kidney epithelial (HK2), human submaxillary salivary gland carcinoma (A253), human endometrium adenocarcinoma (HEC-1-A), human astroglioma (U-373-MG), baby hamster kidney (BHK-21), and rat pigmented retinal epithelial (RPE-J) cells were obtained from the American Type Culture Collection (ATCC [Manassas, VA]) and were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum (FBS). Human normal lung fibroblast (MRC-5) cells were provided by David Andrews from McMaster University (Hamilton, Ontario, Canada) and were maintained in DMEM plus 10% FBS. Primary human umbilical vein endothelial cells (HUVECs) and human neonatal dermal fibroblasts (NHDFs) were obtained from Cascade Biologics (Portland, OR) and were grown in medium 200 (Cascade Biologics) supplemented with the manufacturer's low-serum supplement kit and DMEM plus 10% FBS, respectively. Human retinal pigmented epithelial (ARPE-19) cells were obtained from ATCC and grown in DMEM-F-12 plus 10% FBS. 293 M cells were obtained from Microbix Biosystems (Toronto, Ontario, Canada) and were grown in minimal essential medium (Invitrogen) plus 10% FBS. All cells were maintained at 37°C, with the exception of RPE-J cells, which were maintained at 33°C.
Replication-defective Ad vectors.
The construction of replication-defective (E1-negative) Ad vectors expressing HCMV TR glycoproteins gH, gL, UL128, UL130, and UL131 has been described previously (30, 61). Similar methods were used to generate a nonreplicating Ad vector expressing gB from HCMV strain TR. Briefly, the gB open reading frame was PCR amplified from the start to the stop codon from DNA derived from HCMV TR and subcloned into plasmid pAdTet-7 as previously described (71). The pAdTet-7 plasmid containing the gB open reading frame was verified by DNA sequencing and then cotransfected along with DNA derived from Ad psi-5, which has packaging sequences flanked by loxP sites, into 293 cells expressing the Cre-4 recombinase (30, 52). Viruses derived from these cotransfections were passaged at least four times on Cre-4 293 cells to remove psi-5 viruses. The construction of replication-defective (E1-negative) Ad vectors expressing full-length gB, a mutant gB (gBΔCT) lacking the cytoplasmic tail, or a mutant gB lacking both the cytoplasmic tail and the transmembrane domain (gBΔCTΔTM) from HCMV AD169 has been described previously (32, 36). The construction and characterization of an Ad vector expressing a soluble form of gH (sgH) from HCMV TR have been described previously (61). Ad vector stocks were generated by infecting 293 M cells at 0.1 PFU/cell. Cells were harvested 6 to 10 days after infection and centrifuged at 800 × g for 5 min. Cell pellets were suspended in DMEM plus 10% FBS and sonicated to release cell-associated virus, followed by centrifugation at 3,000 × g for 5 min to remove large cellular debris. Virus-containing cell lysates were stored at −80°C. Ad stock titers were determined by plaque assays on 293 M cells.
Antibodies and immunofluorescent staining.
The rabbit polyclonal antibody anti-IE-86 (R683) has been described previously (37). The monoclonal antibodies (MAbs) 27-156 and 27-78, specific for gB, and the MAb 14-4b, specific for gH, were kindly provided by W. Britt (University of Alabama, Birmingham, AL) (64, 65). MAbs to human epidermal growth factor receptor (EGFR) (clone LA1) and human integrin αVβ3 (clone 23C6) were obtained from Chemicon International (Billerica, MA). A rabbit polyclonal antibody to human β-catenin (C2206) was obtained from Sigma (St. Louis, MO). Secondary fluorescein isothiocyanate-conjugated donkey anti-mouse and donkey anti-rabbit antibodies were obtained from Molecular Probes (Eugene, Oregon). For fluorescence staining, cells were fixed with phosphate-buffered saline (PBS) containing 2% formaldehyde for 10 min at room temperature. The fixed cells were permeabilized with immunofluorescence buffer (IF buffer; 0.5% Triton X-100, 0.5% deoxycholate, 2% goat serum, and 0.05% sodium azide in PBS) for 30 min and then incubated with primary antibodies diluted in IF buffer for 1 h. Cells were washed several times with IF buffer and stained with secondary antibodies in IF buffer for 1 h. In some cases, after removal of the secondary antibody solution, the cells were incubated in PBS containing propidium iodide (Molecular Probes) at a concentration of 0.5 μg/ml to stain cell nuclei. Images were captured with a Nikon Eclipse TS100 microscope fitted with a Qiacam digital charge-coupled device camera.
Cell-cell fusion assays.
Cell-to-cell fusion assays were performed by transducing cells with Ad vectors expressing HCMV glycoproteins at 25 to 100 PFU per cell. Cells were also concomitantly transduced with an Ad vector supplying the Tet transactivator required to activate protein expression at one-fifth of the total PFU. Transductions were performed with cells seeded 24 h earlier on 60- or 100-mm dishes in 2 or 5 ml of DMEM supplemented with 2% FBS. After a 12-h incubation period with the Ad vectors, the cells were washed thoroughly with PBS and trypsinized with 0.025% trypsin-EDTA buffer. After the cells were released from the dish, they were counted, mixed with an equal number of uninfected target cells, replated onto culture dishes, and maintained in growth medium for 60 h. These conditions were also used for assays in which glycoproteins were expressed in trans.
For experiments in which Ad vectors were directly transduced onto cell monolayers, cells were incubated with Ad vectors for 12 h, washed thoroughly with PBS, and incubated in growth medium for 48 h. For quantitative analysis of cell-cell fusion, cells were fixed in 2% paraformaldehyde, rinsed in PBS containing 0.1% Triton X-100, and analyzed under a bright-field microscope. Cell-cell fusion was quantified by counting the total number of nuclei involved in syncytium formation compared to the total number of cells within the same field and was expressed as the percentage of cells fused. Only syncytia containing five nuclei or more were analyzed.
Radiolabeling of cells and immunoprecipitation of proteins.
To metabolically label cells, cell monolayers were washed extensively with medium lacking methionine and cysteine and then incubated in this medium for 1 h before the addition of medium supplemented with [35S]methionine-cysteine (50 to 200 μCi/ml; Amersham). Cell extracts were made with NP-40 lysis buffer (0.5% NP-40 in PBS supplemented with 1 mg/ml bovine serum albumin and 1 mM phenylmethylsulfonyl fluoride) and clarified by centrifugation at 1,500 × g for 10 min. Culture supernatants and cell extracts were clarified by centrifugation at 60 to 100,000 × g for 30 to 60 min. For cell surface iodination, ARPE-19 cells were transduced with glycoprotein-expressing Ad vectors as described above. At 48 h posttransduction, cells were transferred to ice for 10 min, followed by two washes with ice-cold PBS. Cells were then incubated with 3 ml of ice-cold DMEM containing 1 mCi of 125I and 15 μg/ml of lactoperoxidase (Sigma). After the labeling solution was added, 40 μl of 0.1% H2O2 was added every 2 min for 12 min. Finally, the cells were incubated on ice for an additional 8 min. Surface labeling was ceased by washing the cell monolayers extensively with ice-cold PBS, and cells were lysed in 1 ml of NP-40 lysis buffer and clarified as described above. For immunoprecipitation, lysates were precleared by incubation with irrelevant mouse sera and protein A-agarose beads for 1 to 2 h. Precleared lysates were then incubated with the appropriate antibody for 1 to 2 h, and the IgG-protein complexes were captured with protein A-agarose for 1 to 2 h. Samples were washed extensively in NP-40 lysis buffer, and proteins were eluted from the protein A-agarose by the addition of sodium dodecyl sulfate (SDS) sample buffer containing 2% β-mercaptoethanol and boiled for 5 min. Proteins were analyzed by electrophoresis through 10% SDS-polyacrylamide gels. For phosphorimaging, gels were fixed in 10% glacial acetic acid and 30% methanol, dried, and then analyzed using a BAS 2500 phosphorimager system (Molecular Dynamics, Sunnyvale, CA).
HCMV and virus entry assays.
HCMV TR is a wild-type clinical strain that was derived from the retina and was cloned into a bacterial artificial chromosome after limited passage on fibroblasts (49, 67). HCMV stocks were produced by infecting NHDF cells at 0.1 PFU/cell for 10 to 16 days. To enrich for HCMV particles, infected cells were sonicated, large cellular debris was removed by centrifugation at 6,000 × g for 15 min, and virus particles were centrifuged through a 20% sorbitol cushion at 50,000 × g for 1 h. Pellets were resuspended in DMEM plus 10% FBS and frozen at −70°C. Virus titers were determined by plaque assay on NHDF cells. For virus infection, cell monolayers were inoculated with 3 PFU/cell in DMEM plus 2% FBS. For all cell types except fibroblasts, culture dishes were centrifuged at 800 × g for 2 h at room temperature and then moved to 37°C for 1 h. Infection of fibroblast cells was performed by inoculating cell monolayers with 3 PFU/cell in DMEM plus 2% FBS, followed by incubation at 37°C for 3 h. After infection, all cells were washed twice with PBS and incubated in growth medium for 24 h. Virus entry was determined by fixing cells and staining for IE-86 by immunofluorescence.
Antibody inhibition of virus entry and cell-cell fusion.
For antibody inhibition of cell-cell fusion, ARPE-19 cells were transduced with Ad vectors expressing gB, gH, and gL as described above. After mixing of glycoprotein-expressing cells with non-glycoprotein-expressing cells, the culture medium was removed 6 h later and replaced with culture medium containing MAb 14-4b, 27-156, or 27-78, EGFR MAb, integrin αVβ3 MAb, or purified mouse IgG at concentrations of 10 to 50 μg/ml and incubated for 54 h. For antibody inhibition of virus entry, ARPE-19 cells seeded in 96-well plates were incubated in culture medium containing EGFR MAb, integrin αVβ3 MAb, or purified mouse IgG at a concentration of 50 μg/ml at 4°C for 1 h. Alternatively, virus inoculum diluted to 3 PFU/cell was incubated with MAb 14-4b, 27-156, or 27-78 at 50 μg/ml at 37°C for 1 h prior to infection. Infections were performed by adding the virus-antibody mixture to ARPE-19 cells or by adding HCMV TR diluted to 3 PFU/cell to antibody-treated cells. Infections were enhanced by centrifuging the culture plates at 800 × g for 2 h at room temperature and then moving them to 37°C for 1 h. The cells were then washed twice with PBS and incubated in growth medium for 24 h. Virus entry was analyzed by immunofluorescence to detect HCMV IE-86.
RESULTS
Cell-cell fusion of ARPE-19 human retinal epithelial cells by HCMV glycoproteins.
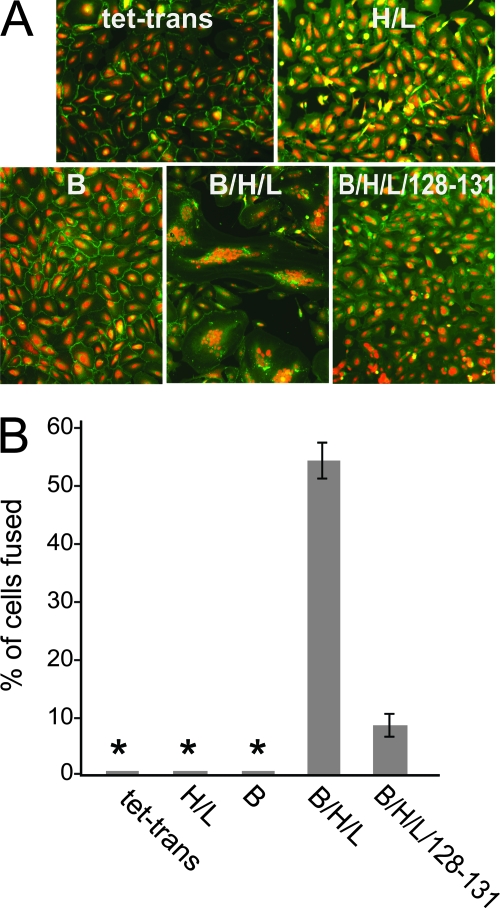
Fusion assays have been very important in helping us to understand how other herpesviruses enter cells by defining the proteins necessary for fusion of virion and cellular membranes. There have not been reports of HCMV proteins able to mediate fusion of biologically relevant epithelial or endothelial cells. We and others have used ARPE-19 retinal epithelial cells to characterize HCMV entry (60, 62, 74). We previously used nonreplicating Ad vectors (which do not express significant amounts of Ad proteins) to express HCMV glycoproteins in cells to characterize structure and function (61, 62). Here we used these vectors to test whether ARPE-19 cells expressing certain HCMV glycoproteins would fuse to form multinucleated syncytia. HCMV glycoproteins gB, gH, gL, UL128, UL130, and UL131 were expressed individually or in combination in ARPE-19 cells for 12 h. The cells were then trypsinized to remove them from dishes and plated at a 1:1 ratio with other ARPE-19 cells that had not been transduced with Ad vectors. To more readily distinguish fused cells, nuclei were fluorescently stained with propidium iodide, and plasma membranes were fluorescently stained with an antibody to β-catenin. With untransduced cells and cells transduced with a control Ad vector, Ad tet-trans, expressing the tetracycline transactivator protein, not a single syncytium was detected in any case in the entire monolayer (2 × 105 cells) in any experiment (Fig. 1A and B). When ARPE-19 cells were transduced with Ad vectors expressing either gB or gH and gL, again not a single syncytium was observed in any experiment (Fig. 1A and B). In contrast, with ARPE-19 cells transduced with Ad vectors expressing gB and gH/gL (using a dose of 25 PFU/cell), there was extensive cell-cell fusion, in many cases representing over 50% of the cells (Fig. 1A and B). In these samples, syncytia began to form by 36 h, and there was extensive fusion by 60 h after cell mixing (Fig. 1A and B). ARPE-19 cells transduced with six Ad vectors expressing gB, gH, gL, and UL128-131 (using a dose of 25 PFU/cell) exhibited lower levels of cell-cell fusion (Fig. 1A and B). This lower level of fusion observed with UL128-131 is investigated further below.
FIG. 1.
Cell-cell fusion of ARPE-19 human retinal epithelial cells by HCMV glycoproteins. (A) ARPE-19 cells were transduced with nonreplicating Ad vectors expressing the tet transactivator (tet-trans), gH and gL (H/L), gB (B), a combination of gB, gH, and gL (B/H/L), or a combination of gB, gH, and gL plus UL128, -130, and -131 (B/H/L/128-131) at 25 PFU/cell for each construct. Cell monolayers were transduced with the appropriate Ad vector for 12 h, trypsinized, mixed with nontransduced ARPE-19 cells, and incubated for 60 h to allow formation of syncytia. Cell monolayers were then fixed and fluorescently stained with propidium iodide and a rabbit polyclonal antibody to β-catenin, followed by a fluorescein isothiocyanate-conjugated donkey anti-rabbit antibody, to label cell nuclei and plasma membranes, respectively. (B) Quantification of cell-cell fusion from ARPE-19 cells expressing the tet transactivator (tet-trans), gH and gL (H/L), gB (B), a combination of gB, gH, and gL (B/H/L), or a combination of gB, gH, and gL plus UL128, -130, and -131 (B/H/L/128-131). Cell-cell fusion was quantified from micrographs by counting the number of nuclei involved in syncytium formation, dividing it by the total number of nuclei within the same field, and expressing it as the percentage of cells fused. Values represent the averages derived from three separate images, and the error bars indicate the standard deviations. Asterisks indicate that no fusion was observed.
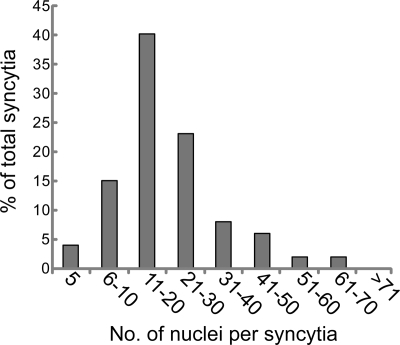
To quantify the cell-cell fusion produced by gB and gH/gL, we counted the number of individual nuclei involved in syncytia. The results from these experiments are shown in Fig. 2. Again, there was no background fusion observed in untransduced ARPE-19 monolayers or after cells were transduced with Ad vectors expressing Ad tet-trans, gB, or gH/gL (data not shown). The number of nuclei included in gB/gH/gL-induced syncytia ranged from 5 to 70 nuclei. Syncytia including either 5 nuclei/syncytium or >50 nuclei/syncytium accounted for less than 10% of the total syncytia. The largest group included syncytia that contained between 11 and 20 nuclei, representing 40% of the total number of syncytia. We concluded that HCMV gB and gH/gL are necessary and sufficient to mediate cell-cell fusion of ARPE-19 cells. There was no fusion in cells expressing gB or gH/gL alone.
FIG. 2.
Quantification of the extent of syncytium formation induced by HCMV glycoproteins gB, gH, and gL in ARPE-19 cells. ARPE-19 cells transduced with Ad vectors expressing gB, gH, and gL at 25 PFU/cell were mixed with nontransduced ARPE-19 cells and incubated for 60 h to induce syncytium formation. Images from fixed monolayers were analyzed to determine the number of independent nuclei within each syncytium. The x axis indicates the number of nuclei per syncytium, and the y axis represents the frequency of the total syncytia analyzed. Only syncytia containing at least five nuclei were included in the analysis. No syncytia were observed in non-glycoprotein-expressing ARPE-19 cells.
Analyses of dose responses and the effects of UL128-131 on expression of gB and gH/gL.
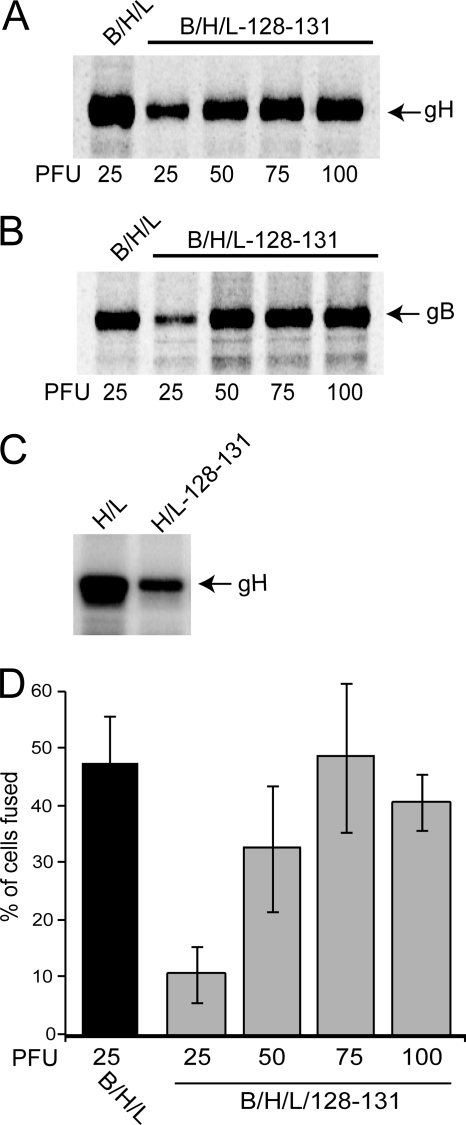
The observation that ARPE-19 cells transduced with Ad vectors expressing gB and gH/gL plus UL128-131 were less extensively fused than cells transduced with Ad vectors expressing gB and gH/gL alone was surprising. We previously showed that the expression of UL128-131 promoted cell surface transport of gH/gL in Ad vector-transduced U373 microglial cells (61). Moreover, we showed that expression of the entire gH/gL/UL128-131 complex, but not gH/gL alone, blocked HCMV entry into ARPE-19 epithelial cells (62). To address possible reasons for why there was less fusion with gB and gH/gL/UL128-131, we characterized the expression of gH and gB in cells transduced with different combinations of Ad vectors by using different doses. Multiple Ad vectors can compete for transcription factors and reduce the expression of other transgenes, especially when as many as six different Ad vectors are involved. ARPE-19 cells were transduced with Ad vectors expressing HCMV glycoproteins for 24 h, the cells were labeled for 3 h with [35S]methionine-cysteine, and then gB or gH was immunoprecipitated. Transduction of cells with Ad vectors expressing gB and gH/gL produced substantially higher levels of both gB and gH than those in cells transduced with gB, gH/gL, and UL128-131, using a dose of 25 PFU/cell for each Ad vector (Fig. 3A and B). Moreover, the cell surface expression of gH, measured by lactoperoxidase-catalyzed iodination, was substantially higher in cells transduced with Ad vectors expressing gH/gL alone (25 PFU/cell) than in cells transduced with Ad vectors expressing gH/gL/UL128-131 (25 PFU/cell) (Fig. 3C). Thus, to increase the levels of expression of gB, gH/gL, and UL128-131, we used higher doses of Ad vectors (50, 75, or 100 PFU/cell). Expression of both gB and gH increased substantially when cells were transduced with Ad vectors at a dose of 50 PFU/cell for each vector and appeared to level off at a dose of 75 PFU/cell (Fig. 3A and B). When we increased the doses of gB, gH/gL, and UL128-131 to 50 and 75 PFU/cell for each Ad vector, cell-cell fusion was increased to the levels observed with just gB and gH/gL at 25 PFU/cell (Fig. 3D). Higher levels of transduction (100 PFU/cell) with all six Ad vectors began causing cytopathic effects by 72 h (not shown), beginning to affect fusion (Fig. 3D). Thus, when the levels of the HCMV proteins were similar, comparing gB/gH/gL/UL128-131 to gB/gH/gL, the levels of fusion were very similar. Lower levels of each of the Ad vectors (10 PFU/cell) produced lower levels of fusion than those obtained with 25 PFU/cell of each Ad vector. It must be kept in mind that there is no replication of these Ad vectors and that the DNA is transcribed but not replicated. Expression of the HCMV glycoproteins was seen in all cells, and a majority of cells fused, usually producing large syncytia of 20 to 30 nuclei. Certainly, one could argue that Ad vectors are capable of high-level expression, but the concentration of HCMV glycoproteins in cellular membranes is unlikely to be higher than that in the virion envelope during entry. We concluded that gB and gH/gL form the minimal set of HCMV proteins required for fusion of ARPE-19 epithelial cells. However, when UL128-131 was added to gB and gH/gL, there was no inhibition or increase of fusion.
FIG. 3.
Analysis of dose responses and contribution of UL128-131 to cell-cell fusion of ARPE-19. (A and B) ARPE-19 cells were transduced with Ad vectors expressing HCMV glycoproteins at the indicated PFU/cell for 24 h and then radiolabeled with [35S]methionine-cysteine for 3 h. Proteins were immunoprecipitated with gB- or gH-specific MAbs 27-156 and 14-4b, respectively, and then analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). (C) ARPE-19 cells were transduced with Ad vectors expressing either gH and gL or gH, gL, and UL128-131, using 25 PFU/cell, for 24 h and then surface labeled by lactoperoxidase-catalyzed iodination. gH was immunoprecipitated using anti-gH MAb 14-4b. Proteins were analyzed by SDS-PAGE. (D) Cell-cell fusion of ARPE-19 cells transduced with Ad vectors expressing gB, gH, and gL or gB, gH, gL, and UL128-131 at the doses indicated and incubated for 60 h. Cell fusion was quantified as described in the legend to Fig. 1. Values represent the averages derived from three separate images, and the error bars indicate the standard deviations.
HCMV gB and gH/gL cause extensive fusion when transduced into monolayers of epithelial cells.
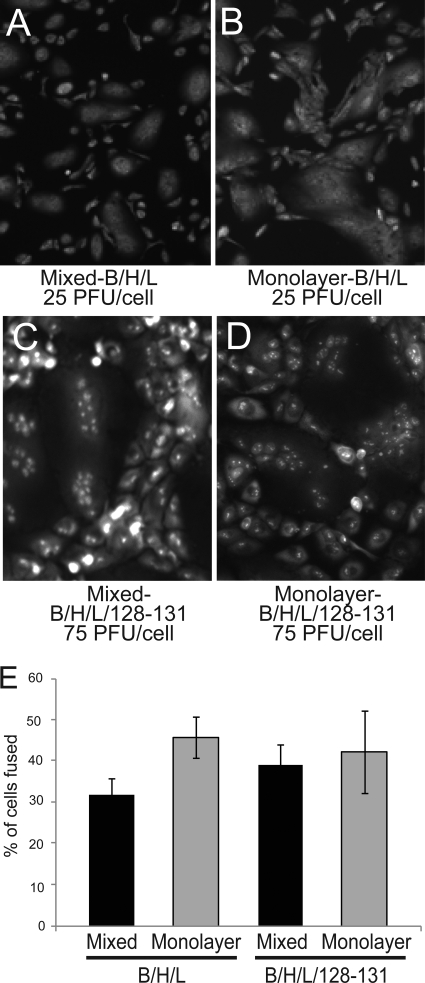
Previous fusion assays described for HSV glycoproteins involved mixing of glycoprotein-expressing cells with other cells not expressing viral glycoproteins (56, 72). This was important in order to avoid interference. If HSV gD was present, gD receptors were downregulated and fusion did not occur. However, HCMV may be different, and there was no interference of HCMV entry with gH/gL (without UL128-131) (62). In the experiments above, we used a mixing protocol. However, here we tested whether transduction of Ad vectors expressing gB and gH/gL or gH/gL/UL128-131 directly onto monolayers of ARPE-19 cells produced fusion. Indeed, there was extensive fusion of epithelial cells expressing gB and gH/gL throughout the monolayer (Fig. 4B and E). There were differences in the kinetics of fusion for cells directly transduced with Ad vectors (monolayer) versus those transduced and then mixed with other cells (mixed) (Fig. 4A, B, and E). Fusion was slower under the mixed conditions, likely related to delays in viral glycoprotein expression produced during trypsinization and mixing with other cells. Moreover, only half of the cells in the dish had been transduced to express HCMV proteins. As in previous experiments, there was no detectable fusion with gB alone or gH/gL alone (data not shown). We also observed extensive fusion with Ad vectors coexpressing gB and gH/gL/UL128-131 when cells were mixed or when vectors were transduced directly onto ARPE-19 cell monolayers (Fig. 4C, D, and E). This suggests that interference mechanisms involving gH/gL/UL128-131 that block HCMV entry into ARPE-19 cells (62) did not block cell-cell fusion. These results illustrate differences in what is learned by studying interference versus fusion and with alpha- versus betaherpesviruses.
FIG. 4.
ARPE-19 cells fuse when gB and gH/gL are transduced into all of the cells in a monolayer. Two conditions for transduction of ARPE-19 cells were compared. In some instances, ARPE-19 cells were transduced with Ad vectors expressing gB, gH, and gL (A) or gB, gH, gL, and UL128-131 (C) and then mixed with untransduced ARPE-19 cells (mixed). In other cases, Ad vectors expressing gB, gH, and gL (B) or gB, gH, gL, and UL128-131 (D) were used to directly transduce monolayers of ARPE-19 cells (monolayers). Transductions of gB, gH, and gL were performed at 25 PFU/cell. To attain similar levels of expression, transductions involving gB, gH, gL, and UL128-131 involved higher doses (75 PFU/cell). (E) Quantification of cell-cell fusion under the conditions in panels A to D by the methods described in the legend to Fig. 1. Values represent the averages derived from three separate images, and the error bars indicate the standard deviations.
Analysis of cell-cell fusion in different cell types.
To gain a broader understanding of cell-cell fusion mediated by HCMV gB and gH/gL, we tested a number of other human and nonhuman cell types from a variety of tissues for the ability to fuse when expressing HCMV gB and gH/gL. HCMV can enter many different human cells and can also enter, but not replicate in, certain nonhuman cells. For these experiments, fusion assays were performed as described for Fig. 1 by transducing one set of cells with gB and gH/gL, removing the cells from plastic dishes, and mixing them with untransduced cells of the same type. To ensure that Ad vectors were capable of transducing different cell types, an Ad vector expressing green fluorescent protein (GFP) (45) was used (not shown). Expression of HCMV gB and gH/gL induced substantial fusion in U-373-MG astroglioma cells (28%), MRC-5 fibroblasts (34%), and HUVECs (11%) (Table 1). In contrast, expression of gB and gH/gL in human cervical adenocarcinoma (HeLa), laryngeal carcinoma (Hep-2), kidney epithelial (HK2), endometrium adenocarcinoma (HEC-1-A), or salivary gland carcinoma (A253) cells did not produce detectable cell-cell fusion. In addition, we were unable to detect cell-cell fusion with NHDFs, consistent with a previous report (41). No cell-cell fusion was observed with either of the nonhuman cells, hamster kidney fibroblasts (BHK-21) or rat retinal epithelial cells (RPE-J), after transduction with gB and gH/gL (Table 1).
TABLE 1.
Analysis of HCMV entry and gB/gH/gL-mediated fusion in different cell types
| Species | Derivative tissue | Cell name | Fusion (%)a | IE expression (%)b |
|---|---|---|---|---|
| Human | Epithelial cervical adenocarcinoma | HeLa | 0 | 1 |
| Retinal pigment epithelium | ARPE | 49.2 | 17 | |
| Laryngeal carcinoma | Hep-2 | 0 | 1 | |
| Astroglioma | U-373-MG | 28 | 38 | |
| Kidney epithelium | HK2 | 0 | 0 | |
| Neonatal dermal fibroblasts | NHDF | 0 | 39 | |
| Normal lung fibroblasts | MRC-5 | 34.3 | 56 | |
| Endometrium adenocarcinoma | HEC-1-A | 0 | 0 | |
| Umbilical vein vascular endothelium | HUVECs | 11.5 | 5 | |
| Submaxillary salivary gland carcinoma | A253 | 0 | 0 | |
| Hamster | Kidney fibroblasts | BHK21 | 0 | 0 |
| Rat | Retinal pigment epithelium | RPE-J | 0 | 0 |
Cell-cell fusion was quantified by counting the total number of nuclei involved in syncytium formation compared to the total number of cells within the same field and expressed as the percentage of cells fused. Only syncytia containing five nuclei or more were scored as positive for fusion.
Virus entry was measured following infection of cell monolayers with 3 PFU/cell of HCMV TR for 24 h, followed by immunofluorescence to detect HCMV IE-86. For all cell types except NHDF and MRC-5 fibroblasts, infection was enhanced by centrifugation as described in Materials and Methods. Samples were scored based on the number of IE-86-positive cells compared to the total number of cells and expressed as a percentage.
To determine if there was a correlation between cell-cell fusion and the entry of HCMV into these different cells, we also tested the ability of these different cell types to support immediate-early (IE) expression after infection with HCMV clinical strain TR (60). HCMV was able to enter all of the cell types that also fused when transduced with Ad vectors expressing gB and gH/gL. These included ARPE-19, U-373-MG, and MRC-5 cells and HUVECs (Table 1). In contrast, HCMV did not enter or very poorly entered HeLa, Hep-2, HK2, HEC-1-A, A253, BHK-21, and RPE-J cells. In most instances, there were fewer than 1% IE-positive cells, consistent with the inability to fuse. However, normal human fibroblasts supported HCMV entry but did not exhibit cell-cell fusion. Thus, with the exception of normal fibroblasts, there was a strong correlation between the ability of HCMV to enter various cell types and the ability of gB and gH/gL to mediate cell-cell fusion.
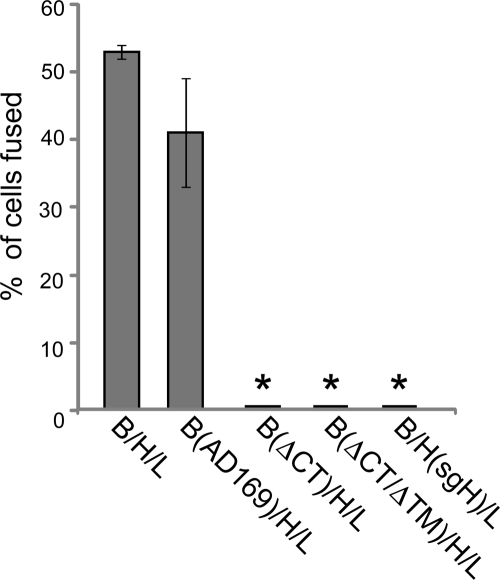
Analysis of cell-cell fusion with gB and gH mutant constructs.
Little is known about how HCMV glycoproteins participate in either entry or cell-cell fusion. However, with other herpesvirus gB and gH homologues, deletion of the cytoplasmic (CT) domains abolishes the capacity to mediate entry and cell-cell fusion (11, 26, 27, 31, 59). We tested whether a form of HCMV gB lacking the large C-terminal CT domain or lacking both the CT and transmembrane (TM) domains of gB (secreted) would promote cell-cell fusion. These mutant forms of gB were constructed previously using the gB gene of the laboratory strain AD169 (32). Full-length AD169 gB coexpressed with TR gH/gL in ARPE-19 cells produced similar levels of fusion to those with TR-derived gB and gH/gL (Fig. 5). Cells transduced with an Ad vector expressing a form of AD169 gB lacking the CT domain (gBΔCT) and gH/gL failed to fuse. Previous studies showed that gBΔCT was found much more extensively on the surfaces of cells (32). Furthermore, a soluble form of gB lacking both the CT and TM domains (gBΔCTΔTM) coexpressed with gH/gL did not promote fusion (Fig. 5). We also tested whether a truncated form of TR gH (sgH) lacking the CT and TM domains (61) could function in cell-cell fusion. We observed no fusion when this protein was coexpressed with gB and gL (Fig. 5). The results with soluble forms of gB and gH demonstrated that gB and gH/gL must be anchored in the plasma membrane in order to function in a cell-cell fusion assay. Moreover, HCMV gB absolutely requires the CT domain for membrane fusion.
FIG. 5.
Analysis of cell-cell fusion of ARPE-19 cells transduced with mutant forms of gB and gH. Cell-cell fusion of ARPE-19 cells transduced with Ad vectors expressing gB, gH, and gL derived from HCMV strain TR was analyzed. Cell-cell fusion assays were also performed with Ad vectors expressing gB from AD169, AD169 gB lacking the CT domain (ΔCT), or AD169 gB lacking both the CT and TM domains (ΔCTΔTM). Fusion assays were also performed with an Ad vector expressing a mutant gH (sgH) that lacked both the CT and TM domains. Cell-cell fusion was quantified from images as described in the legend to Fig. 1. Values represent the averages derived from three separate images, and the error bars indicate the standard deviations. Asterisks indicate that no fusion was observed.
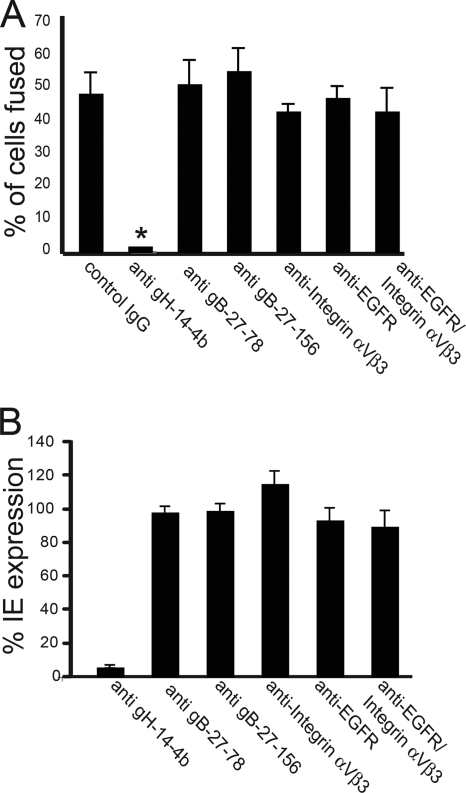
Inhibition of cell-cell fusion by anti-gH, -gB, -EGFR, or -integrin antibody.
To investigate whether anti-gB and anti-gH antibodies could block cell-cell fusion, ARPE-19 cells were transduced with gB and gH/gL, and then these cells were mixed with untransduced cells. MAbs were applied 6 h after cell mixing. The anti-gH MAb 14-4b, which neutralizes HCMV AD169 (65), was also able to effectively block cell-cell fusion (Fig. 6A) and to neutralize HCMV TR entry into cells, as measured by IE expression (Fig. 6B). In contrast, two anti-gB MAbs, 27-156 and 27-78, which neutralize HCMV AD169 (64), did not block cell-cell fusion (Fig. 6A) and also did not neutralize HCMV TR entry (Fig. 6B). It was reported that EGFR and integrins (such as αVβ3) can facilitate entry of HCMV AD169 into fibroblasts or other cells (24, 76, 77), although the role of EGFR in entry has been challenged (35). Incubation of ARPE-19 cells with an anti-EGFR MAb that was reported to reduce HCMV AD169 entry into fibroblasts at 1 μg/ml did not reduce gB/gH/gL-induced fusion of ARPE-19 cells (Fig. 6A) and did not inhibit HCMV TR entry at 50 μg/ml (Fig. 6B). Similarly, an anti-integrin αVβ3 MAb that blocked entry into fibroblast cells at 20 μg/ml (76) did not inhibit gB/gH/gL-induced cell fusion and did not block HCMV TR entry into ARPE-19 cells at 50 μg/ml (Fig. 6A and B). We also incubated epithelial cells with a combination of EGFR- and αVβ3 integrin-specific antibodies and observed no inhibition of either cell-cell fusion or virus entry (Fig. 6A and B).
FIG. 6.
Effects of gH-, gB-, EGFR-, and integrin-specific antibodies on infectivity of HCMV and cell-cell fusion. (A) ARPE-19 cells were transduced with Ad vectors expressing gB, gH, and gL (25 PFU/cell) for 12 h and then mixed with nontransduced cells. Six hours after cell mixing, the medium was replaced with culture medium containing anti-gH MAb 14-4b at 10 μg/ml and anti-gB MAbs 27-156 and 27-78, anti-EGFR MAb LA1, anti-integrin αVβ3 MAb 23C6, or purified control mouse IgG at 50 μg/ml, and the cells were then incubated for 54 h. Cell-cell fusion was observed in triplicate wells and quantified as described in the legend to Fig. 1. Asterisks indicate that no fusion was observed. (B) For antibody inhibition of virus entry, ARPE-19 cells were incubated in culture medium containing EGFR MAb, integrin αVβ3 MAb, or purified mouse IgG at 50 μg/ml prior to infection with HCMV. Alternatively, HCMV virus inoculum diluted to 3 PFU/cell was incubated with MAb 14-4b, 27-156, or 27-78 or with purified mouse IgG at 50 μg/ml prior to being added to ARPE-19 cells. After 24 h, the cells were fixed and stained for HCMV IE protein IE-86. The number of cells expressing HCMV IE protein was normalized to that observed with the control mouse IgG.
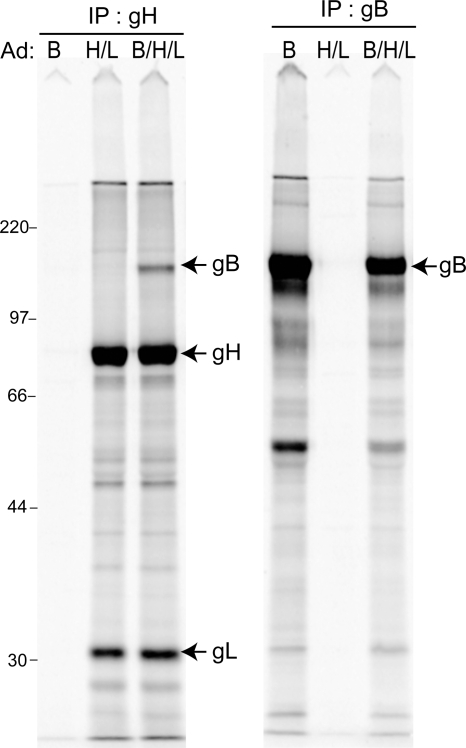
Coimmunoprecipitation of gB with gH/gL.
HSV gB and gH/gL interact during membrane fusion (3, 4). To characterize potential interactions between HCMV gB and gH/gL, we attempted to coimmunoprecipitate these glycoproteins. ARPE-19 cells were transduced with Ad vectors expressing gB and gH/gL, cells were labeled with [35S]methionine-cysteine, and extracts were made using 0.5% NP-40. gH-specific MAb 14-4b precipitated both gH and gL, as expected, but also precipitated a small amount of the total gB expressed in these cells (Fig. 7, left panel). This gH-specific antibody did not precipitate any detectable gB from extracts of cells expressing gB alone. gB-specific MAb 27-156 precipitated gB, and we did not detect gH or gL (Fig. 7, right panel). Note that there might have been smaller amounts of gH present, as the gH band was obscured by other bands. A viral or cellular protein similar to gL was detected in MAb 27-156 precipitates, but closer examination shows that this protein has a slightly slower electrophoretic mobility than that of gL. These results may be related to the observation that HSV gB and gH/gL only interact when gD and gD receptors are present, consistent with the notion that this is a functional interaction (3, 4). Only a small amount of HCMV gB was coprecipitated with gH/gL. Note that there is no quantification of how much HSV gB interacts with gH/gL, and it is not known whether gB must interact with gH/gL for fusion to occur. More studies are required to establish the functional consequences, if any, of HCMV gB interactions with gH/gL.
FIG. 7.
Coimmunoprecipitation of HCMV gB and gH. ARPE-19 cells were transduced with Ad vectors expressing gB alone (B), gH and gL (H/L), or a combination of gB, gH, and gL (B/H/L), using 25 PFU/cell, for 24 h and then labeled with [35S]methionine-cysteine for 3 h. Cell extracts were made with 0.5% NP-40 lysis buffer and subjected to immunoprecipitation with anti-gH (14-4b) or anti-gB (27-156) MAb, and proteins were analyzed by SDS-PAGE under reducing conditions. The molecular mass marker, in kilodaltons, is indicated on the left.
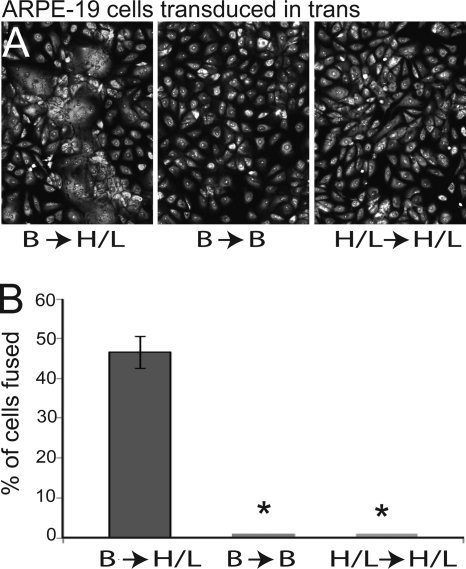
Cell-cell fusion of epithelial cells in which gB and gH/gL are expressed in trans.
Cell-cell fusion assays normally involve expressing the core fusion or entry proteins in one set of cells followed by mixing of these cells with untransduced cells (72). When a subset of HSV proteins, e.g., gB and gD, were expressed in one set of cells and gH/gL was expressed in the other set of cells, there was no fusion (10, 56). Other combinations did not promote cell-cell fusion. We tested whether expression of HCMV gB in one set of cells and HCMV gH/gL in the other set of cells might produce fusion between the cells. We were surprised to find that expressing HCMV gB and gH/gL in trans produced cell-cell fusion in ARPE-19 cells similar to that observed when these glycoproteins were expressed in cis (Fig. 8A and B). In contrast, expressing gB or gH/gL in both sets of cells did not produce any fusion (Fig. 8A and B). These observations, coupled with the immunoprecipitation of gB with gH/gL, are consistent with the possibility that gB and gH/gL might interact across the spaces separating apposing epithelial cell junctions (Fig. 9). It is also possible that interactions between gB and gH/gL are not necessary for fusion.
FIG. 8.
HCMV gB and gH/gL induce cell-cell fusion when expressed in ARPE-19 cells in trans. (A) Cell-cell fusion after ARPE-19 cells expressing gB were mixed with ARPE-19 cells expressing gH and gL (B→H/L). Control assays were performed after mixing ARPE-19 cells expressing either gB or gH and gL alone (B→B and H/L→H/L, respectively). Cell monolayers were fixed and fluorescently stained with propidium iodide to label cell nuclei. (B) Quantification of cell-cell fusion mediated by gB and gH/gL in trans under the conditions described in the legend to Fig. 1. Values represent the averages derived from three separate images, and the error bars indicate the standard deviations. Asterisks indicate that no fusion was observed.
FIG. 9.
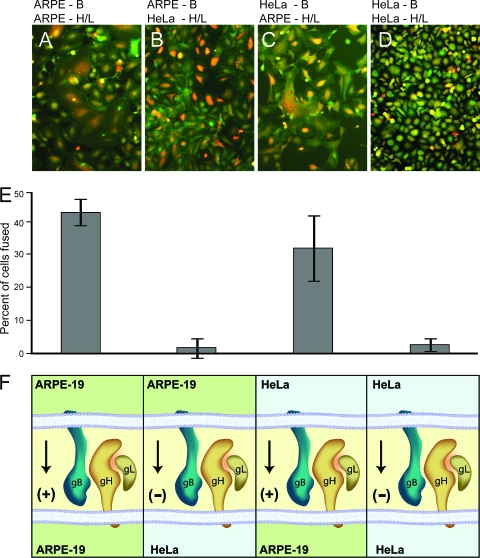
Analysis of cell-cell fusion mediated by gB and gH/gL in trans, using different cell types. (A) ARPE-19 cells expressing gB were mixed with ARPE-19 cells expressing gH and gL. (B) ARPE-19 cells expressing gB were mixed with HeLa cells expressing gH and gL. (C) HeLa cells expressing gB were mixed with ARPE-19 cells expressing gH and gL. (D) HeLa cells expressing gB were mixed with HeLa cells expressing gH and gL. The cells were fixed and stained with propidium iodide and β-catenin MAb (a cell surface marker) after 60 h. (E) Quantification of cell-cell fusion in the samples described above. Values represent the averages derived from three separate images, and the error bars indicate the standard deviations. (F) Cartoon depicting the orientation of gB and gH/gL in ARPE-19 and HeLa cells. Arrows and + or − signs indicate whether or not fusion was observed.
HeLa and A253 salivary gland tumor cells did not fuse when gB and gH/gL were expressed in these cells in cis (Table 1). Moreover, HCMV enters these cells very poorly (HeLa) or not at all (A253). One interpretation is that these cells are missing a molecule or molecules that are required both for fusion and for entry. Based on the fusion of ARPE-19 cells in trans, it was of substantial interest to determine whether HeLa or A253 cells expressing gB might fuse with ARPE-19 cells expressing gH/gL or vice versa. As expected, HeLa cells expressing gB did not fuse with HeLa cells expressing gH/gL (Fig. 9D and E). Similarly, HeLa cells expressing gH/gL did not fuse with APRE-19 cells expressing gB (Fig. 9B and E). In contrast, there was extensive fusion (32%) when HeLa cells expressing gB were mixed with ARPE-19 cells expressing gH/gL (Fig. 9C and E). In other experiments, when A253 cells expressing gB were mixed with ARPE-19 cells expressing gH/gL, we also observed fusion (31%), but again, no fusion was observed when A253 cells expressing gH/gL were mixed with ARPE-19 cells expressing gB (data not shown). No fusion was observed when A253 cells expressing gH/gL were mixed with A253 cells expressing gB.
One potential concern in interpreting these experiments in which gB and gH/gL were expressed in trans relates to the possibility that Ad vectors used to express gB might remain associated with cells after cell mixing and then deliver gB into other cells expressing gH/gL, such that expression actually occurs in cis. There are two solid arguments for why this cannot be the case. First, we did not see substantial amounts of fusion at 72 h when 5 PFU/cell of the Ad vectors was applied to ARPE-19 cells in cis (not shown). The cells were washed extensively after the addition of Ad vectors and incubated for 12 h before cell mixing. Thus, it is unlikely that over 20% of the Ad vector inoculum remained. Second, it was possible that there were functionally significant quantities of Ad vectors that remained on the surfaces of cells and were then transferred to other cells after cell mixing. This might deliver gB and gH/gL into some cells in cis. However, arguing against this, ARPE-19 cells transduced with gB did not fuse with HeLa cells transduced with gH/gL. Other experiments involving an Ad vector that expresses GFP (AdGFP) also demonstrated that ARPE-19 cells transduced with AdGFP and mixed with other ARPE-19 cells (labeled with tracker dye) did not produce GFP expression in the second set of cells (not shown). In addition, ARPE-19 cells transduced with AdGFP for 12 h and then subsequently overlaid with Ad-permissive 293 cells displayed very few infectious Ad particles (<100 in an entire monolayer). The total numbers of Ad particles remaining on the surfaces of ARPE-19 cells would correspond to 0.001 PFU/cell. We do not observe much or any fusion of ARPE-19 cells below 5 PFU/cell (data not shown). These results rule out the transfer of Ad vector from one cell to the next. We concluded that HCMV glycoproteins gB and gH/gL can mediate fusion of ARPE-19 cells when the glycoproteins are expressed in trans.
DISCUSSION
Much of what has been learned about the entry of alpha- and gammaherpesviruses comes from studies of cell-cell fusion. Especially prominent are recent studies showing that HSV gB and gH/gL can mediate different steps of the fusion process, namely, hemifusion followed by full fusion (70), and that HSV gB interacts with gH/gL once gD interacts with receptors (3, 4). Far less is known about how betaherpesviruses enter cells and even whether HCMV gB and gH/gL are necessary for entry. Antibody studies have indicated a critical role for gB and gH/gL in entry (9, 19, 65, 73). The inability to obtain gB and gH null mutants of HCMV is also suggestive but is not direct evidence that these proteins mediate entry. Here we demonstrated that expression of HCMV gB and gH/gL caused ARPE-19 epithelial cells to fuse. Fusion was extensive, such that about half of the cells in a monolayer became part of syncytia involving 10 to 30 cells. Omitting either gB, gH, or gL from the assay completely abolished fusion. We stress that these epithelial cells differ markedly from certain highly transformed tumor cell lines (CHO and BHK cells) that have been used in previous fusion assays, where there are often significant numbers of fused cells without herpesvirus glycoproteins. ARPE-19 cells never showed syncytia without HCMV proteins. Moreover, a majority of cells were transduced, and in most cases, half of the cells fused.
Expression of HCMV gB and gH/gL in several other human cell lines, i.e. U-373-MG microglial cells, MRC-5 fibroblasts, and HUVECs, caused fusion of a substantial fraction of the cells (Table 1). Again, there was no detectable fusion with just gB or gH/gL alone. With each of these cells, there was a good correlation between the ability of gB and gH/gL to cause cell-cell fusion and the ability of HCMV to enter the cells. This is consistent with the presence of HCMV receptors being required for entry as well as for gB and gH/gL to cause cell-cell fusion on certain cells. One exception to the correlation was human fibroblasts, cells used to propagate HCMV, which did not display any cell fusion, as reported previously (41). We concluded that gB and gH/gL make up the HCMV core fusion complex, i.e., these glycoproteins are necessary and sufficient for fusion, for several cell types. These results are the first to define betaherpesvirus proteins that promote fusion of biologically relevant epithelial and endothelial cells. Moreover, our results fit well with the results for the gammaherpesviruses EBV and HHV-8, where gB and gH/gL complexes (including gp42 for EBV) are sufficient for fusion (27, 55). For HSV, the receptor-binding protein gD is required in addition to gB and gH/gL both for entry (47) and for cell-cell fusion (72). Putting these observations together might suggest that HCMV gB or gH/gL (or both) might also be sufficient to bind cellular ligands or receptors that promote fusion in some instances.
Our observations that both HCMV gB and gH/gL are required for all cells tested differ from previous observations that gH/gL was sufficient to cause fusion of CHO cells or immortalized fibroblasts (41). Previous results differ substantially from ours in that we used Ad vectors to obtain high levels of expression of HCMV glycoproteins. We argue that these high concentrations of HCMV glycoproteins are not unlike those in the virion envelope. Moreover, we did not test fusion of CHO cells or immortalized fibroblasts. The previous observations fit well with the growing belief that herpesvirus gB and gH/gL each have inherent fusogenic properties either expressed alone or in NM fusion (23, 70). To explain gH/gL-mediated fusion of CHO cells, it is important to note that CHO cells are highly transformed cells (with an altered extracellular matrix) and are known to spontaneously fuse, even without HCMV or HSV proteins (41, 56). Similar arguments might be made to explain the more limited fusion observed with BHK cells expressing HSV gD alone (14) and with COS or U-373-MG cells expressing HSV or HCMV gB alone (6, 12). In all of these cases, the level of fusion (a small percentage of the cells) was much lower than the level of fusion we observed (50%). Our studies also differed from previous studies in that we used glycoproteins of the wild-type HCMV strain TR rather than the lab strain AD169. Exemplifying this, two anti-gB MAbs known to block AD169 entry did not block entry of TR and did not block cell-cell fusion, although a gH-specific MAb was able to block both TR entry and cell-cell fusion. We also found no evidence that anti-EGFR or anti-integrin αVβ3 antibodies could inhibit either entry or cell-cell fusion with TR and epithelial cells.
We also investigated mutant forms of gB and gH. Deletions that produced soluble gB or gH did not function in fusion, probably because both gB and gH/gL must be anchored in membranes in order to promote fusion. This is also the case with other herpesviruses (31, 40, 42). Furthermore, the HCMV gB CT domain was absolutely required for cell-cell fusion, like the case with HSV and EBV gB molecules (27, 59). It is likely that HCMV gB must traffic properly within cells, reach the plasma membrane, form dimers or trimers (29), interact with gH/gL (3, 4), and undergo conformational changes (29) in order to cause fusion. It is important that our previous studies of the gB CT domain mutant (32) demonstrated that this molecule was present on cell surfaces at higher, not lower, levels. Therefore, the inability of the gB CT mutant to fuse is not related to reduced cell surface levels, but this does not rule out the need for oligomerization or conformational changes.
Our observations that HCMV UL128-131 proteins were not necessary for cell-cell fusion of ARPE-19 epithelial cells or endothelial cells were surprising. These results might, at first glance, appear to be at odds with observations that all five gH/gL/UL128-131 proteins were required for entry into epithelial and endothelial cells (58), as well as with our interference studies demonstrating that gH/gL/UL128-131 expression in epithelial cells blocked HCMV entry (62). The interference observations led us to propose gH/gL/UL128-131-specific ligands that are a necessary part of the entry pathway into epithelial cells. However, there are two important and related points that help to explain why UL128-131 proteins are not required for cell-cell fusion. First, cell-cell fusion assays define the minimal set of herpesvirus proteins required for membrane fusion, i.e., membrane proteins that act during the last stages of entry, namely, lipid mixing. HCMV gB and gH/gL are sufficient for this fusion with the cells tested. Importantly, UL128-131 did not inhibit cell-cell fusion or increase it. Second, it must be recognized that there can be substantial differences between the requirements for cell-cell fusion and virus entry into cells. It is important to recognize that entry of HCMV into ARPE-19 cells involves endocytosis and low-pH-dependent fusion with endosomes (60). Interference (expression of gH/gL/UL128-131 in cells) blocks entry processes that involve endosomes. In contrast, cell-cell fusion involves the plasma membrane of one cell (expressing gB and gH/gL) fusing with another cell at neutral pH. Thus, the requirement for UL128-131 in the virion envelope for entry and in cell membranes for entry correlates with the low-pH pathway. It should also be noted that HCMV can enter fibroblasts without UL128-131 (60) and that this entry occurs at neutral pH and with fusion with the plasma membrane (18). Our studies involving interference with fibroblasts showed significant interference with gH/gL alone (58). Again, the involvement of UL128-131 correlates with the endocytic, low-pH pathway.
One way of explaining the requirement for UL128-131 in early stages of HCMV entry is that gH/gL/UL128-131 interacts with cell surface proteins that are required to trigger endocytosis or with endosomal proteins required for triggering of fusion. Cell-cell fusion may not require these interactions because it occurs with juxtaposed plasma membranes. Alternatively, UL128-131 might prevent premature triggering of the gH/gL fusion activity so that this occurs only after HCMV enters endosomes. Again, there might not be a requirement for UL128-131 to block triggering in order for cell-cell fusion to occur. Together, all of these results suggest that the UL128-131 proteins are specific to the low-pH entry pathway defined for epithelial and endothelial cells and not for the neutral-pH pathway defined for fibroblasts. We also believe that the interference and cell-cell fusion assays depict different stages in the HCMV entry process. Interference represents early stages of the entry process into epithelial cells (requires UL128-131 and saturable receptor ligands), while cell-cell fusion depicts the latter stages of membrane fusion, defining proteins that disrupt and mix lipid bilayers.
Related to these differences, we characterized whether cell-cell fusion might be enhanced after a short pulse with low-pH buffer (to simulate conditions in endosomes), with and without UL128-131, and found no enhancement (A. Vanarsdall, unpublished results). Similar results have been observed for HSV, as the virus enters certain cells in a process requiring low pH, yet the same cells fuse when transduced with gB, gD, and gH/gL at neutral pH (50, 56). For pseudorabies virus, there are also differences between the related processes of virus entry and cell-to-cell spread; the latter does not require gD, but entry absolutely requires gD (54).
We made the very surprising observation that when gB-expressing ARPE-19 cells were mixed with gH/gL-expressing ARPE-19 cells (in trans), there was extensive fusion (42% of all cells fused). As far as we are aware, this is the first example of viral fusion proteins acting in trans. Other less complex viruses, e.g., paramyxoviruses, have a single fusion protein that is triggered by a separate receptor binding protein (reviewed in reference 43), and all of the evidence suggests that both viral proteins must be present in the same membrane for fusion. The herpesviruses all express two fusion proteins, gB and gH/gL, that, at least for HSV, can promote lipid mixing under different conditions (70). There is also evidence that either gB or gH/gL is largely sufficient for fusion at the nuclear envelope (23). But when HSV gB, gD, and gH/gL were expressed in trans, in any combination, there was no fusion (10, 56). This may relate to the fact that there are three proteins (gB, gD, and gH/gL) required for fusion with HSV, where gD is required for receptor binding, and only two (gB and gH/gL) required with HCMV. However, fusion in trans is very different from fusion involving these two putative fusion proteins in the same membrane. There are also observations that EBV gH/gL/gp42 can enhance fusion between cells expressing gB and gH/gL and other cells (42), but again the membrane-anchored proteins are present in the same membrane.
One might argue that HCMV glycoproteins are all expressed in cis in the virion envelope during virus entry and, thus, that the biologically important orientation of these glycoproteins is in cis. However, observations that HCMV gB and gH/gL can function in trans have broad and important implications for how we understand herpesvirus membrane fusion and how gB and gH/gL function in this process. For example, HSV virions can fuse with the outer NM during virus egress. In this case, it is likely that gB and gH/gL are in both the virion envelope and the outer NM. At the NM, membrane fusion appears to be rapid and might not require receptor binding related to this in trans expression of glycoproteins.
It could be argued that these experiments should be reproduced by using transfection or retroviruses, as was the case with previous cell-cell fusion assays. Two comments are warranted here. First, Ad vectors do not express significant amounts of Ad proteins, and we used many controls, e.g., cells transduced with gB alone do not fuse at all. Second, Ad vectors allow relatively high levels of expression in cells that are very difficult to transfect. Previous studies may have missed much of this because there was both low-level expression and a high background. Higher levels of HCMV glycoproteins more likely reflect the levels in infected cells and the virion envelope. It is very important to perform these experiments with biologically relevant cells. These cells do not spontaneously fuse, but when transduced with Ad vectors, a large fraction of cells fused. Thus, we believe that it is highly unlikely that this high level of fusion can be obtained by using transfection or retroviruses. Even if there were some fusion, it would not add substantially to our observations.
There is evidence that HSV gB and gH/gL bind one another (triggered by gD) just before or during membrane fusion (3, 4), although it is not yet proven whether this interaction is required for fusion. We observed that anti-gH antibody could precipitate a fraction of the total gB expressed in cells. It is not clear whether more gB might interact with gH/gL when detergents are not present. These observations of gB and gH/gL interactions should be regarded as preliminary, but it was important to include these results in terms of understanding the results of cell-cell fusion in trans. If HCMV gB and gH/gL interactions are necessary for fusion, then these glycoproteins expressed in trans must reach across the space separating apposing cells in order to interact (Fig. 9). Our studies involving mutant gB molecules lacking the CT or CT and TM domains support the notion that both gB and gH/gL must be anchored in the membrane for fusion to occur. However, it is also very possible that HCMV gB and gH/gL expressed either in cis or in trans do not require interactions between glycoproteins to mediate fusion. For HSV, it is also not clear whether gB-gH/gL interactions are functionally important. Perhaps HCMV gB and gH/gL carry out different steps in the fusion process, acting independently (70). However, what is clear is that both HCMV gB and gH/gL are absolutely required for fusion in trans, because expression of gH/gL or gB alone in both populations gave no fusion.
Additional information about HCMV entry was obtained by expressing HCMV glycoproteins in trans in nonpermissive cells, i.e., HeLa and A253 cells. HCMV does not enter either of these cells well, and <1% of the cells displayed IE expression. Moreover, neither of these cells fused when gB and gH/gL were expressed in cis. This is consistent with the hypothesis that HeLa and A253 cells are missing ligands or receptors for gB, gH/gL, or both. HeLa cells are clearly capable of cell-cell fusion, as HSV glycoproteins cause fusion (56). There was no fusion when HeLa or A253 cells expressing gH/gL were mixed with ARPE-19 cells expressing gB. In contrast, when HeLa or A253 cells expressing gB were mixed with ARPE-19 cells expressing gH/gL, there was extensive fusion (Fig. 9). These results suggest that cell surface proteins or ligands recognized by gB might be missing (or limiting in concentration) on the surfaces of HeLa and A253 cells, such that fusion does not occur. There may also be gH/gL ligands involved in this process and found on ARPE-19 cells, as indicated by our interference studies (62), but such ligands must also be coexpressed by HeLa and A253 cells. Previous studies of HSV (5, 63) and HCMV (8) have provided evidence for gB ligands that can function in entry.
Our observations that gB and gH/gL are necessary and sufficient for cell-cell fusion define these two glycoproteins as the core fusion machinery for HCMV. That UL128-131 was not required suggests that gH/gL/UL128-131 acts in early stages of HCMV entry, not in the latter stages defined by gB/gH/gL-induced cell-cell fusion. Observations of HCMV glycoproteins expressed in trans have important mechanistic implications for our understanding of how herpesvirus gB and gH/gL function.
Acknowledgments
This work was supported by grants AI055051 and EY11245 from the National Institutes of Health (to D.C.J.). Additional support was received from an individual Ruth L. Kirschstein National Research Service Award from the NEI (F32-EY015965) (to B.J.R.) and an institutional training grant (T32-AI07472) (to A.L.V.).
We are very grateful to William Britt for supplying important MAbs. We also thank Tiffani Howard for computer graphics and Chris Langford for help with online manuscript submission. Finally, we thank Todd Wisner for his advice and technical assistance and also thank the members of the Johnson laboratory for their support.
Footnotes
Published ahead of print on 24 September 2008.
REFERENCES
- 1.Adler, B., L. Scrivano, Z. Ruzcics, B. Rupp, C. Sinzger, and U. Koszinowski. 2006. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 872451-2460. [DOI] [PubMed] [Google Scholar]
- 2.Alford, C. A., and W. J. Britt. 1990. Cytomegalovirus, p. 1981-2010. In B. N. Fields and D. M. Knipe (ed.), Fields virology. Raven Press Ltd., New York, NY.
- 3.Atanasiu, D., J. C. Whitbeck, T. M. Cairns, B. Reilly, G. H. Cohen, and R. J. Eisenberg. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. USA 10418718-18723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avitabile, E., C. Forghieri, and G. Campadelli-Fiume. 2007. Complexes between herpes simplex virus glycoproteins gD, gB, and gH detected in cells by complementation of split enhanced green fluorescent protein. J. Virol. 8111532-11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 7911588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bold, S., M. Ohlin, W. Garten, and K. Radsak. 1996. Structural domains involved in human cytomegalovirus glycoprotein B-mediated cell-cell fusion. J. Gen. Virol. 772297-2302. [DOI] [PubMed] [Google Scholar]
- 7.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8594-599. [DOI] [PubMed] [Google Scholar]
- 8.Boyle, K. A., and T. Compton. 1998. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J. Virol. 721826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britt, W. J. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135369-378. [DOI] [PubMed] [Google Scholar]
- 10.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 821419-1422. [DOI] [PubMed] [Google Scholar]
- 11.Browne, H. M., B. C. Bruun, and A. C. Minson. 1996. Characterization of herpes simplex virus type 1 recombinants with mutations in the cytoplasmic tail of glycoprotein H. J. Gen. Virol. 772569-2573. [DOI] [PubMed] [Google Scholar]
- 12.Butcher, M., K. Raviprakash, and H. P. Ghosh. 1990. Acid pH-induced fusion of cells by herpes simplex virus glycoproteins gB and gD. J. Biol. Chem. 2655862-5868. [PubMed] [Google Scholar]
- 13.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 622596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campadelli-Fiume, G., E. Avitabile, S. Fini, D. Stirpe, M. Arsenakis, and B. Roizman. 1988. Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology 166598-602. [DOI] [PubMed] [Google Scholar]
- 15.Campadelli-Fiume, G., M. Arsenakis, F. Farabegoli, and B. Roizman. 1988. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 62159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 7078-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocchi, F., D. Fusco, L. Menotti, T. Gianni, R. J. Eisenberg, G. H. Cohen, and G. Campadelli-Fiume. 2004. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 1017445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191387-395. [DOI] [PubMed] [Google Scholar]
- 19.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, et al. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 53057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis-Poynter, N., S. Bell, T. Minson, and H. Browne. 1994. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J. Virol. 687586-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolan, A., C. Cunningham, R. D. Hector, A. F. Hassan-Walker, L. Lee, C. Addison, D. J. Dargan, D. J. McGeoch, D. Gatherer, V. C. Emery, P. D. Griffiths, C. Sinzger, B. P. McSharry, G. W. G. Wilkinson, and A. J. Davison. 2004. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 851301-1312. [DOI] [PubMed] [Google Scholar]
- 22.Dunn, W., C. Chou, L. Hong, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 10014223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farnsworth, A., T. W. Wisner, M. Webb, R. Roller, G. Cohen, R. Eisenberg, and D. C. Johnson. 2007. Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc. Natl. Acad. Sci. USA 10410187-10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feire, A. L., H. Koss, and T. Compton. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 10115470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galdiero, M., A. Whiteley, B. Bruun, S. Bell, T. Minson, and H. Browne. 1997. Site-directed and linker insertion mutagenesis of herpes simplex virus type 1 glycoprotein H. J. Virol. 712163-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haan, K. M., S. K. Lee, and R. Longnecker. 2001. Different functional domains in the cytoplasmic tail of glycoprotein B are involved in Epstein-Barr virus-induced membrane fusion. Virology 290106-114. [DOI] [PubMed] [Google Scholar]
- 28.Hahn, G., M. G. Revello, M. Patrone, E. Percivalle, G. Campanini, A. Sarasini, M. Wagner, A. Gallina, G. Milanesi, U. Koszinowski, F. Baldanti, and G. Gerna. 2004. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 7810023-10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hannah, B. P., E. E. Heldwein, F. C. Bender, G. H. Cohen, and R. J. Eisenberg. 2007. Mutational evidence of internal fusion loops in herpes simplex virus glycoprotein B. J. Virol. 814858-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hardy, S., M. Kitamura, T. Harris-Stansil, Y. Dai, and M. L. Phipps. 1997. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 711842-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harman, A., H. Browne, and T. Minson. 2002. The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion. J. Virol. 7610708-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegde, N. R., C. Dunn, D. M. Lewinsohn, M. A. Jarvis, J. A. Nelson, and D. C. Johnson. 2005. Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL. J. Exp. Med. 2021109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 747720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber, M. T., and T. Compton. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 728191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaacson, M. K., A. L. Feire, and T. Compton. 2007. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J. Virol. 816241-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarvis, M. A., T. R. Jones, D. D. Drummond, P. P. Smith, W. J. Britt, J. A. Nelson, and C. J. Baldick. 2004. Phosphorylation of human cytomegalovirus glycoprotein B (gB) at the acidic cluster casein kinase 2 site (Ser900) is required for localization of gB to the trans-Golgi network and efficient virus replication. J. Virol. 78285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarvis, M. A., C. E. Wang, H. L. Meyers, P. P. Smith, C. L. Corless, G. J. Henderson, J. Vieira, W. J. Britt, and J. A. Nelson. 1999. Human cytomegalovirus infection of Caco-2 cells occurs at the basolateral membrane and is differentiation state dependent. J. Virol. 734552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang, X. J., B. Adler, K. L. Sampaio, M. Digel, G. Jahn, N. Ettischer, Y. D. Stierhof, L. Scrivano, U. Koszinowski, M. Mach, and C. Sinzger. 2008. UL74 of human cytomegalovirus contributes to virus release by promoting secondary envelopment of virions. J. Virol. 822802-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson, R. M., and P. G. Spear. 1989. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J. Virol. 63819-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones, N. A., and R. J. Geraghty. 2004. Fusion activity of lipid-anchored envelope glycoproteins of herpes simplex virus type 1. Virology 324213-228. [DOI] [PubMed] [Google Scholar]
- 41.Kinzler, E. R., and T. Compton. 2005. Characterization of human cytomegalovirus glycoprotein-induced cell-cell fusion. J. Virol. 797827-7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirschner, A. N., J. Omerovic, B. Popov, R. Longnecker, and T. S. Jardetzky. 2006. Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion. J. Virol. 809444-9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamb, R. A. 1993. Paramyxovirus fusion: a hypothesis for changes. Virology 1971-11. [DOI] [PubMed] [Google Scholar]
- 44.Landolfo, S., M. Gariglio, G. Gribaudo, and D. Lembo. 2003. The human cytomegalovirus. Pharmacol. Ther. 98269-297. [DOI] [PubMed] [Google Scholar]
- 45.Lewinsohn, D. A., R. A. Lines, and D. M. Lewinsohn. 2002. Human dendritic cells presenting adenovirally expressed antigen elicit Mycobacterium tuberculosis-specific CD8+ T cells. Am. J. Respir. Crit. Care Med. 166843-848. [DOI] [PubMed] [Google Scholar]
- 46.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 693987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 621486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mori, Y., P. Akkapaiboon, S. Yonemoto, M. Koike, M. Takemoto, T. Sadaoka, Y. Sasamoto, S. Konishi, Y. Uchiyama, and K. Yamanishi. 2004. Discovery of a second form of tripartite complex containing gH-gL of human herpesvirus 6 and observations on CD46. J. Virol. 784609-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy, E., D. Yu, J. Grimwood, J. Schmutz, M. Dickson, M. A. Jarvis, G. Hahn, J. A. Nelson, R. M. Myers, and T. E. Shenk. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 10014976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 775324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicola, A. V., J. Hou, E. O. Major, and S. E. Straus. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 797609-7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 9313565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2706. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Willliams & Wilkins, Philadelphia, PA.
- 54.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pertel, P. E. 2002. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J. Virol. 764390-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279313-324. [DOI] [PubMed] [Google Scholar]
- 57.Plachter, B., C. Sinzger, and G. Jahn. 1996. Cell types involved in replication and distribution of human cytomegalovirus. Adv. Virus Res. 46195-261. [DOI] [PubMed] [Google Scholar]
- 58.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 672285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruel, N., A. Zago, and P. G. Spear. 2006. Alanine substitution of conserved residues in the cytoplasmic tail of herpes simplex virus gB can enhance or abolish cell fusion activity and viral entry. Virology 346229-237. [DOI] [PubMed] [Google Scholar]
- 60.Ryckman, B. J., M. A. Jarvis, D. D. Drummond, J. A. Nelson, and D. C. Johnson. 2006. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 80710-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryckman, B. J., B. L. Rainish, M. C. Chase, J. A. Borton, J. A. Nelson, M. A. Jarvis, and D. C. Johnson. 2008. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 8260-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryckman, B. J., M. C. Chase, and D. C. Johnson. 2008. HCMV gH/gL/UL128-131 interferes with virus entry into epithelial cells: evidence for cell type-specific receptors. Proc. Natl. Acad. Sci. USA 10514118-14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Satoh, T., J. Arii, T. Suenaga, J. Wang, A. Kogure, J. Uehori, N. Arase, I. Shiratori, S. Tanaka, Y. Kawaguchi, P. G. Spear, L. L. Lanier, and H. Arase. 2008. PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 132935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoppel, K., E. Hassfurther, W. Britt, M. Ohlin, C. A. Borrebaeck, and M. Mach. 1996. Antibodies specific for the antigenic domain 1 of glycoprotein B (gpUL55) of human cytomegalovirus bind to different substructures. Virology 216133-145. [DOI] [PubMed] [Google Scholar]
- 65.Simpson, J. A., J. C. Chow, J. Baker, N. Avdalovic, S. Yuan, D. Au, M. S. Co, M. Vasquez, W. J. Britt, and K. L. Coelingh. 1993. Neutralizing monoclonal antibodies that distinguish three antigenic sites on human cytomegalovirus glycoprotein H have conformationally distinct binding sites. J. Virol. 67489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sinzger, C., K. Schmidt, J. Knapp, M. Kahl, R. Beck, J. Waldman, H. Hebart, H. Einsele, and G. Jahn. 1999. Modification of human cytomegalovirus tropism through propagation in vitro is associated with changes in the viral genome. J. Gen. Virol. 802867-2877. [DOI] [PubMed] [Google Scholar]
- 67.Smith, I. L., I. Taskintuna, F. M. Rahhal, H. C. Powell, E. Ai, A. J. Mueller, S. A. Spector, and W. R. Freeman. 1998. Clinical failure of CMV retinitis with intravitreal cidofovir is associated with antiviral resistance. Arch. Ophthalmol. 116178-185. [DOI] [PubMed] [Google Scholar]
- 68.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Streblow, D. N., S. L. Orloff, and J. A. Nelson. 2007. Acceleration of allograft failure by cytomegalovirus. Curr. Opin. Immunol. 19577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subramanian, R. P., and R. J. Geraghty. 2007. Herpes simplex virus type 1 mediates fusion through a hemifusion intermediate by sequential activity of glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 1042903-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 51039-1043. [DOI] [PubMed] [Google Scholar]
- 72.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urban, M., M. Klein, W. J. Britt, E. Hassfurther, and M. Mach. 1996. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J. Gen. Virol. 771537-1547. [DOI] [PubMed] [Google Scholar]
- 74.Wang, D., and T. Shenk. 2005. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 7910330-10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, D., and T. Shenk. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. USA 10218153-18158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang, X., D. Y. Huang, S. M. Huong, and E. S. Huang. 2005. Integrin alphavbeta3 is a coreceptor for human cytomegalovirus. Nat. Med. 11515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424456-461. [DOI] [PubMed] [Google Scholar]
- 78.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 725552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]