Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia. The transforming ability of Tax, the viral oncoprotein, is believed to depend on interactions with cell cycle regulators and on transactivation of genes that control cellular proliferation, including proliferating cell nuclear antigen (PCNA), a cofactor associated with DNA replication and repair. Tax associates with cellular transcription factors to alter their affinity for cognate DNA elements, leading to increased or decreased transcription from that promoter. Although it has been demonstrated that Tax transactivates the PCNA promoter, the mechanism of transcriptional activation is unknown. Here we report a cellular complex that binds specifically to a novel site within the minimal Tax-responsive element of the TATAA-less PCNA promoter. Mutation at this binding site or Tax expression inhibited complex formation and increased promoter activity, suggesting that the complex is a transcriptional repressor. The activation of PCNA gene expression by Tax and consequential decrease in nucleotide excision repair mediated by PCNA overexpression could contribute to the reduced DNA repair capacity and genomic instability observed in HTLV-1-infected cells.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia (ATL), a disease characterized by malignant proliferation of CD4+ T lymphocytes. An estimated 10 to 20 million people worldwide are infected with HTLV-1 (6), but only a small percentage of HTLV-1-infected individuals develop ATL (11, 16, 22, 30). Disease onset occurs after a long period of clinical latency, consistent with a multistep process of T-lymphocyte immortalization and transformation. HTLV-1 is also associated with other clinical disorders, including tropical spastic paraparesis/HTLV-1-associated myelopathy, HTLV-1-associated arthropathy, HTLV-1-associated uveitis, infective dermatitis, and polymyositis (2, 8, 21, 26). The mechanism by which infected individuals develop ATL is unknown, but the accumulation of DNA damage in HTLV-transformed cells has been associated with the viral oncoprotein, Tax (20).
HTLV-1 Tax is essential for viral gene expression and also regulates the expression of cellular genes involved in proliferation and regulation of cellular processes, including pathways involved in cell cycle regulation, apoptosis, and DNA damage responses. Microarray analyses have identified over 300 genes that are upregulated in Tax-expressing cells (5, 23), including that encoding proliferating cell nuclear antigen (PCNA), a sliding clamp that affects DNA replication and repair. Since Tax does not bind DNA directly, its ability to transactivate promoters depends on interactions with cellular transcription factors and coactivators. These interactions can alter the DNA binding affinity and specificity of cellular DNA binding proteins (14). Tax has been shown to activate gene expression through cyclic AMP response element and activating transcription factor-1 (CRE/ATF-1), nuclear factor κB (NF-κB), and serum response element (SRE) pathways. We have previously shown that Tax is able to transactivate the PCNA promoter (18, 24) to increase endogenous PCNA protein expression (12, 13). Although a Tax-responsive element (TRE) of the PCNA promoter was identified, the mechanism of Tax transactivation of this promoter is currently unknown. Analysis of Tax mutant proteins demonstrated that transactivation of the PCNA promoter did not require the CRE or NF-κB pathways (18, 24). The PCNA promoter TRE contains no direct homology to any known cellular transcription factor binding sites or to any previously determined TREs. Identification of a cellular protein(s) that binds to the PCNA TRE and further characterization of TRE function would advance our understanding of how Tax regulates the expression of this important gene.
In this study, we examined Tax-mediated transactivation of the PCNA promoter. TATAA-binding protein (TBP) was shown to associate specifically with a novel transcription factor site within the minimal TRE of the TATAA-less PCNA promoter. The TBP-TRE complex was a moderate repressor of transcription, and Tax expression disrupted formation of this complex. Disruption of the repressive TBP complex at the PCNA promoter could contribute to the increased PCNA expression that is observed in Tax-expressing cells. PCNA overexpression has been shown to repress nucleotide excision repair (12, 13, 18), which may provide a mechanism by which Tax can suppress DNA repair and contribute to cellular transformation.
MATERIALS AND METHODS
Cell lines.
293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Jurkat, CEM, MS-9, and MT-2 cells were maintained in RPMI medium supplemented with 10% fetal bovine serum. MT-2 cells were also supplemented with interleukin-2 (100 U/ml).
Plasmids and oligonucleotides.
Oligonucleotides were synthesized by Sigma-Genosys. The PCNA promoter probes used for electrophoretic mobility shift assay (EMSA) analysis are listed in Table 1. The c-fos SRE oligonucleotide was described previously (29). The TBP consensus binding site oligonucleotide sense strand was 5′-GCAGAGCATATAAAATGAGGTAGGA-3′. The multimerized PCNA promoter elements used for promoter activation assays were as follows: 2X −21 to −1, 5′-TCGAGCGCGCTTGCGGACGCGGCGGCTAGACGCGCTTGCGGACGCGGCGGCATTAAACGGTTA-3′; 2X Δ PIR, 5′-TCGAGACATCTTGCGGACGCGGCGGCTAGAACATCTTGCGGACGCGGCGGCA TTAAACGGTTA-3′; and 2X Δ PDR, 5′-TCGAGCGCGCTTGCGATCTGGGCGGCTAGACGCGCTTGCGATCTGGGCGGCATTAAACGGTTA-3′ (underlined sequences identify the positions of mutations). The PCNA promoter luciferase reporter plasmids 2X −21 to −1, 2X Δ PIR, and 2X Δ PDR were made by ligating the multimerized PCNA promoter elements into the promoterless pGL3 basic luciferase reporter plasmid between the XbaI and HindIII cloning sites. The Tax and negative control expression plasmids were pCMV-Tax and pCMV-1 and were described previously (25). pGL3 basic and pRL-SV40, an internal control reporter, were purchased from Promega (Madison, WI).
TABLE 1.
Sense sequence of oligonucleotides used for EMSA analysis
| Oligonucleotide | Position | Sequence (5′-3′)a |
|---|---|---|
| Minimal TRE | −59 to +1 | TCGAGCGTGGTGACGTCGCAACGCGGCGCAGGGTGAGAGCGCGCGCTTGCGGACGCGGCGGCAT |
| Distal | −48 to −30 | CTAGAGTCGCAACGCGGCGCAGGGA |
| Proximal | −21 to −1 | CTAGACGCGCTTGCGGACGCGGCGGCT |
| Δ PIR | −21 to −1 | CTAGAACATCTTGCGGACGCGGCGGCT |
| Δ PDR | −21 to −1 | CTAGACGCGCTTGCGATCTGGGCGGCT' |
Sequences in bold identify the positions of the inverted and direct repeats. Underlined sequences identify the positions of mutations.
Transfections.
293 cells at approximately 50% to 80% confluence were transfected using Lipofectamine reagent (Invitrogen, Carlsbad, CA) as directed by the manufacturer.
Whole-cell extract preparation.
Approximately 5 ×106 cells were washed in 0.5 volume of cold 0.01 M phosphate-buffered saline and pelleted. Cells were incubated in an equal volume of RIPA buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1% sodium deoxycholate, 1% Triton X) on ice for 20 min and centrifuged. The supernatant was collected, and protein concentrations were determined by Bradford assay.
EMSAs.
Double-stranded oligonucleotides were labeled with [α-32P]dCTP by use of Klenow enzyme (Invitrogen, Carlsbad, CA). EMSA reaction mixtures included 5× EMSA buffer (50 mM HEPES, pH 7.9, 250 mM KCl, 12.5 mM MgCl, 12.5% glycerol, 5 mM EDTA), 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 μg sheared salmon sperm DNA (Sigma), 10 μg whole-cell extract, and approximately 1 × 10−9 to 3 × 10−9 M labeled probe (30 kcpm) in a 20-μl total reaction volume. Reaction mixtures were incubated at room temperature for 20 min. Complexes were resolved in a 4% nondenaturing polyacrylamide gel in 0.6× Tris-borate-EDTA at 4°C at 155 V for 3.5 h. For competition assays, an approximately 150-fold molar excess of unlabeled oligonucleotide was added to the reaction mix, except where indicated. For antibody interference assays, 10 μg of whole-cell extract was preincubated with or without 0.5 μg antibody at 4°C for 1 h.
Antibodies.
Horseradish peroxidase-conjugated secondary antibodies and antibodies to actin (Sigma, St. Louis, MO), TFIID (TBP), and E2F-6 (Santa Cruz Biotechnology, Santa Cruz, CA) were purchased. A rabbit polyclonal antibody against Tax (568) was previously described (7).
Western blots.
One hundred micrograms of each sample was boiled with 4× sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%), transferred to a nitrocellulose membrane, and incubated with antibodies against Tax or actin. The membrane was then incubated with the appropriate horseradish peroxidase-conjugated secondary antibody. Immunoreactivity was detected with SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Promoter activation assays.
293 cells were distributed in 12-well plates and transfected with 1 ng of pRL-SV40 (to control for transfection efficiency) and 0.2 μg of pGL3 basic plasmid, 2X −21 to −1, 2X Δ PIR, or 2X Δ PDR luciferase reporter plasmid. Twenty-four hours after transfection, cells were lysed in passive lysis buffer (Promega, Madison, WI), and luciferase activity was measured using a dual-luciferase reporter assay system (Promega, Madison, WI). Luciferase activity was normalized to Renilla luciferase activity from the same well. Normalized luciferase activity from cells transfected with pGL3 basic was set to 100% activity, and luciferase values for each test plasmid were normalized to this value. For Tax transactivation assays, luciferase reporter plasmids were cotransfected with either 2 μg pCMV-Tax or sheared salmon sperm DNA, and cells were lysed and luciferase activity measured as described before, but 48 h after transfection. The degree of activation was determined by calculating the change in activity of each reporter in Tax-expressing cells compared to the activity in mock-transfected cells. Normalized luciferase activity from cells transfected with 2X −21 to −1 was set to 100% transactivation, and luciferase values for each test plasmid were normalized to this value.
RESULTS
A previously uncharacterized protein complex forms on the PCNA promoter.
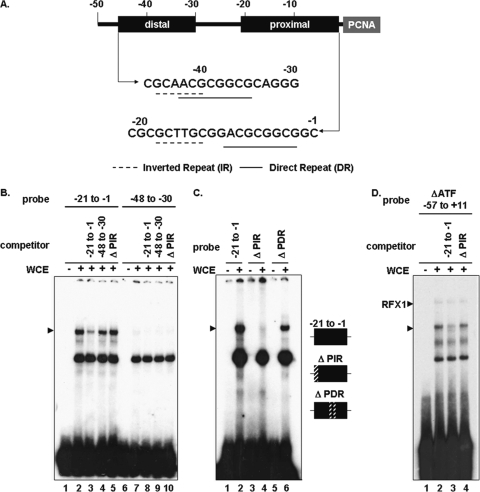
We have previously shown that the HTLV-1 Tax protein can transactivate the PCNA promoter (18, 24) to increase endogenous PCNA protein expression (12, 13). The minimum Tax-responsive region of the PCNA promoter was localized to a complex repeat element spanning nucleotides −45 to −7 (Fig. 1A) and is flanked by CRE/ATF-1 and RFX1 sites at the 5′ end and a YY1 site at the 3′ end (17, 24). This complex element contains an imperfect inverted repeat adjacent to a perfect direct repeat (ACGCGGCG). The GC-rich direct and indirect repeats have no obvious similarity to any characterized transcription factor binding site or to any previously determined TRE. The PCNA promoter does not contain a TATAA box to initiate RNA transcription. However, YY1 binding, together with histone H4 acetylation, can initiate transcriptional activation (27). We previously demonstrated that Tax transactivation of the PCNA promoter does not require CRE or NF-κB response elements (18, 24).
FIG. 1.
Formation of a specific protein complex on the PCNA promoter. (A) Schematic diagram of the PCNA promoter. Distal and proximal TREs are highlighted, and sequences are shown below (dashed lines, inverted repeats; solid lines, direct repeats). (B) EMSA using 32P-labeled proximal (lanes 1 to 5) or distal (lanes 6 to 10) probes incubated with 293 whole-cell extract (WCE) (lanes 2 to 5 and 7 to 10). Unlabeled competitor oligonucleotides (150-fold excess) were added as indicated above (lanes 3 to 5 and 8 to 10). The location of the specific complex is indicated on the left. (C) EMSA using 32P-labeled probes (lanes 1 and 2, proximal element; lanes 3 and 4, proximal element with a mutation in the inverted repeat; and lanes 5 and 6, proximal element with a mutation in the direct repeat) incubated with 293 whole-cell extract (lanes 2, 4, and 6). The position of the specific complex is indicated on the left. (D) EMSA using a 32P-labeled probe (ΔATF −57 to +11) (lanes 1 to 4) incubated with 293 whole-cell extract (lanes 2 to 4). Unlabeled competitor oligonucleotides (150-fold excess) were added as indicated above (lanes 3 and 4). The positions of the RFX1 and specific complexes are indicated on the left.
To determine whether a previously uncharacterized complex forms on the Tax-responsive region of the PCNA promoter, a proximal element probe, spanning nucleotides −21 to −1, or a distal element probe, spanning nucleotides −48 to −30, was incubated with whole-cell extracts and analyzed by EMSA. Two complexes formed on the proximal element (Fig. 1B, lane 2), while only a single complex formed on the distal element (Fig. 1B, lane 7). To determine the specificity of the complexes, unlabeled competitor oligonucleotides for each probe (Fig. 1B, lanes 3, 4, 8, and 9) or an oligonucleotide with a mutation in the proximal inverted repeat (Δ PIR) (Fig. 1B, lanes 5 and 10) was added to the indicated reactions. The proximal element (lane 3) competed for binding to the slowest-migrating complex (Fig. 1B, arrowhead). Since the distal element (lane 4) or proximal element (lane 5) mutant showed little or no competition for complex formation (Fig. 1B), the slower-migrating complex is considered to bind specifically to the proximal element.
Promoter mutations inhibit formation of the specific complex.
To further analyze this novel complex, mutations were introduced into the proximal element and their effect on binding activity was examined by EMSA. The wild-type proximal element, the proximal element containing a mutation of nucleotides −21 to −18 overlapping the inverted repeat (Δ PIR), or the proximal element containing mutations within nucleotides −11 to −7 in the direct repeat (Δ PDR) was used as a probe. Complex formation was observed on the wild-type proximal element and the proximal element with a mutation in the direct repeat (Fig. 1C, lanes 2 and 6). However, mutation of the inverted repeat disrupted complex formation (Fig. 1C, lane 4). This result is consistent with the inability of Δ PIR to compete for specific binding on the wild-type proximal element (Fig. 1B, lane 5). The Δ PIR mutant also did not compete for complex formation on the larger PCNA promoter TRE probe (Fig. 1D, lane 4). Taken together, these results suggest that a previously uncharacterized DNA-protein complex (PIR) binds specifically to the 5′ end of the inverted repeat within the proximal PCNA promoter element.
Tax interferes with PIR complex formation on the proximal TRE of the PCNA promoter.
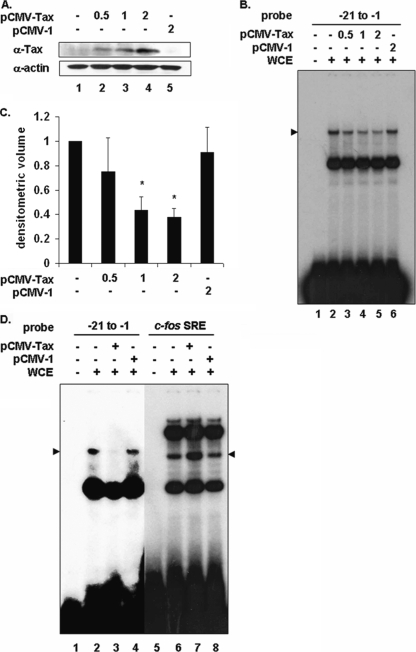
The association of Tax with cellular transcription factors has been shown to alter their affinity for cognate DNA elements, leading to increased or decreased transcription from that promoter (14). To determine the effect of Tax on the PIR complex, 293 cells were transfected with increasing amounts of a vector encoding Tax or the empty parental plasmid. Tax protein levels were measured by immunoblotting (Fig. 2A), and formation of the PIR complex on the proximal PCNA promoter was measured by EMSA. A dose-dependent decrease in complex formation was observed in Tax-expressing cells (Fig. 2B, lanes 3 to 5) but not in mock-transfected cells or cells transfected with empty plasmid (Fig. 2B, lanes 2 and 6). Densitometric analyses of three independent experiments (Fig. 2C) demonstrated that the PIR complex was reduced approximately 67% in cells transfected with the largest amount of Tax.
FIG. 2.
Transient Tax expression interferes with PIR complex formation on the proximal PCNA promoter TRE. (A) Western blot of 293 whole-cell extracts from mock-transfected cells (lane 1) or cells transfected with increasing amounts of pCMV-Tax (lanes 2 to 4) or pCMV-1 (lane 5), using antibodies to Tax or actin as indicated. (B) EMSA using 32P-labeled proximal probe (lanes 1 to 6) incubated with whole-cell extracts from mock-transfected cells (lane 2) or 293 cells transfected with increasing amounts of pCMV-Tax (lanes 3 to 5) or pCMV-1 (lane 6). The position of the specific complex is indicated on the left. (C) Densitometric volume analyses of samples from panel B quantitated with ImageQuant software. Each reaction signal from the specific complex was divided by the total signal and normalized to mock-transfected cells. Error bars represent the standard errors of the means for three independent experiments. *, significant difference in specific complex formation between Tax-negative and Tax-positive cells (Student t test; P < 0.05). (D) EMSA using 32P-labeled proximal (lanes 1 to 4) or c-fos SRE (lanes 5 and 6) probe incubated with whole-cell extracts from mock-transfected cells (lanes 2 and 6) or 293 cells transfected with 2 μg pCMV-Tax (lanes 4 and 7) or pCMV-1 (lanes 5 and 8). The position of the specific proximal complex is indicated on the left, and the position of the specific SRE complex is indicated on the right (arrowheads).
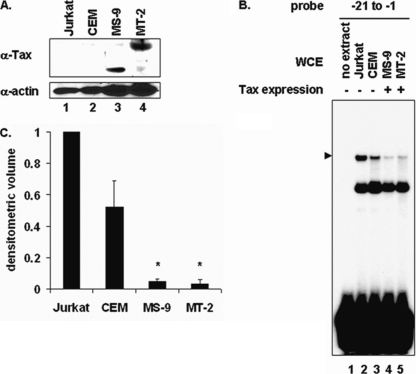
To determine whether the decrease in PIR complex formation induced by Tax was specific, 293 cells were transfected with a Tax expression plasmid or the empty parental plasmid, and whole-cell extracts were prepared and analyzed by EMSA (Fig. 2D). Since Tax is known to interact with SRF to enhance its binding to SREs located within the c-fos promoter (28, 29), the c-fos promoter SRE was used as a control. Tax expression disrupted formation of the PIR complex on the −21 to −1 proximal element probe (Fig. 2D, lane 3). In contrast, the binding of SRF to the c-fos SRE probe was enhanced in Tax-expressing cells (Fig. 2D, lane 7), demonstrating that the inhibitory effect of Tax on PIR complex formation was specific. In addition, extracts from human T cells that stably express Tax (Fig. 3B, lanes 4 and 5) also showed less PIR complex formation than did extracts from cells that do not express Tax (Fig. 3B, lanes 2 and 3). Quantitation of the EMSA bands is shown in Fig. 3C, and a Western blot showing Tax expression in these extracts is shown in Fig. 3A.
FIG. 3.
Stable Tax expression in T cells interferes with PIR complex formation on the proximal PCNA promoter TRE. (A) Western blot of whole-cell extracts from Jurkat (lane 1), CEM (lane 2), MS-9 (lane 3), and MT-2 (lane 4) T cells, using antibodies to Tax or actin as indicated. (B) EMSA using 32P-labeled proximal probe (lanes 1 to 5) incubated with whole-cell extract from Jurkat (lane 2), CEM (lane 3), MS-9 (lanes 4), or MT-2 (lane 5) T cells. The position of the specific complex is indicated on the left. (C) Densitometric volume analyses of the samples from panel B quantitated with ImageQuant software. Each reaction signal from the specific complex was divided by the total signal and normalized to that of Jurkat T cells. Error bars represent the standard errors of the means for two independent experiments. *, significant difference in specific complex formation between Tax-negative and Tax-positive cells (Student t test; P < 0.05).
TBP is a component of the proximal TRE binding complex.
Labrie et al. resolved multiple EMSA complexes with a probe spanning nucleotides −87 to +62 of the PCNA promoter and coding region (17). Each complex bound the probe specifically, and their supershift analyses identified ATF-1, RFX1, and YY1 as components of these complexes. Although the PCNA promoter does not contain a consensus TATAA box, competitor assays using an unlabeled oligonucleotide containing the adenovirus major late promoter TATAA box reduced the formation of an uncharacterized complex, suggesting that general transcription factors such as TBP can associate with the PCNA promoter and play a role in regulating PCNA transcription. However, several mutations within the PCNA promoter, including one at positions −27 to −20 to disrupt any potential TATAA element, did not affect complex formation, so the association of TBP with the PCNA promoter is likely to be indirect.
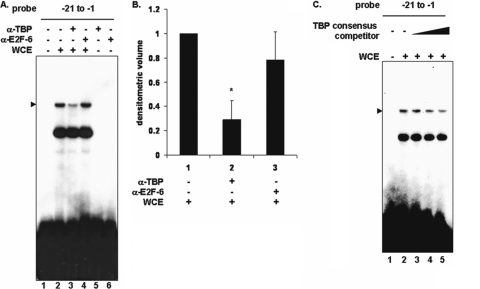
Tax has been shown to directly interact with TBP (3), and specific Tax mutants that fail to bind TBP also fail to suppress nucleotide excision repair (18). The position of a putative TATAA box (TGAGA) at nucleotides −29 to −25 is very close to the PCNA promoter PIR, which is required for formation of the PIR complex identified above. Therefore, we investigated whether TBP contributes to formation of this complex. To address this question, we asked whether a TBP-specific antibody could affect formation of the PIR complex. An antibody to TBP disrupted the complex (Fig. 4A, lane 3), while a control antibody to E2F-6 did not affect the complex (Fig. 4A, lane 4). An unlabeled consensus TBP binding site oligonucleotide competed for specific complex formation on the PCNA promoter, in a dose-dependent manner (Fig. 4C, lanes 3 to 5). However, since TBP is relatively abundant in cells, 100- to 400-fold excess competitor was required to compete for complex formation. These results suggest that TBP is part of the PIR complex that forms on the PCNA promoter-proximal TRE.
FIG. 4.
TBP forms a complex on the PCNA promoter-proximal TRE. (A) EMSA using a 32P-labeled proximal probe (lanes 1 to 6) was performed with 293 cell whole-cell extract (lanes 2 to 4). Antibodies to TBP or E2F-6 were added as indicated (lanes 3 to 6). The position of the specific complex is indicated on the left. (B) Densitometric volume analyses of the samples from panel A quantitated with ImageQuant software. Each reaction signal from the specific complex was divided by the total signal and normalized to reactions in the absence of antibody. Error bars represent the standard errors of the means for three independent experiments. *, significant difference in specific complex formation between reactions with and without antibody to TBP (Student t test; P < 0.05). (C) EMSA using a 32P-labeled proximal probe (lanes 1 to 5) incubated with 293 whole-cell extract (lanes 2 to 5). Increasing amounts of unlabeled TBP consensus competitor oligonucleotide (100-, 200-, and 400-fold excess) were added (lanes 3 to 5). The position of the specific complex is indicated on the left.
TBP negatively regulates the PCNA initiator element.
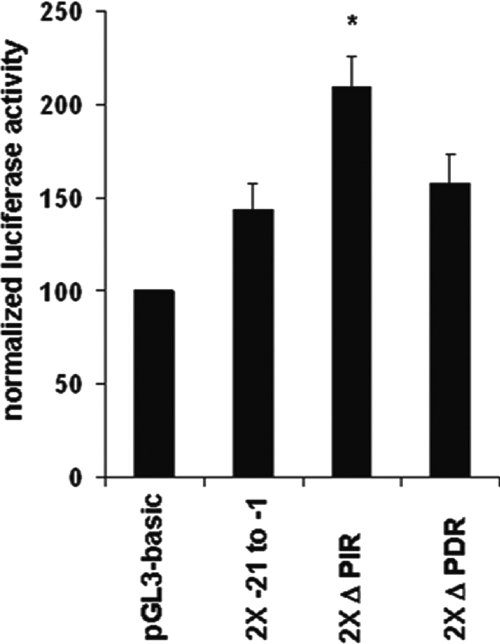
The ability of Tax to diminish formation of the TBP-containing complex raised the possibilities that the PIR complex may negatively regulate the PCNA promoter and that this negative regulation may be relieved by Tax. To characterize the effect of the PIR binding site on basal activity of the PCNA initiator element, two copies of either the wild-type proximal PCNA promoter TRE (2X −21 to −1) or the proximal elements containing mutations in the inverted (2X Δ PIR) or direct (2X Δ PDR) repeats were cloned upstream of the PCNA initiator. The wild-type proximal element and 2X Δ PDR showed similarly low PCNA promoter activities (Fig. 5). However, mutation of the inverted repeat (2X Δ PIR), which disrupts PIR complex formation, resulted in 45% more PCNA promoter activity than that with the wild-type proximal element. This modest increase in transcription is similar to levels of Tax transactivation of the PCNA promoter reported in previous studies (18, 24).
FIG. 5.
TBP binding site negatively regulates the PCNA initiator element. Two copies of either wild-type proximal TRE (2X −21 to −1), Δ PIR, or Δ PDR sequences in a luciferase reporter plasmid were cotransfected with the pRL-SV40 Renilla reporter plasmid into 293 cells. Luciferase activity was normalized to Renilla luciferase activity from the same well. Error bars represent the standard errors of the means for three independent experiments performed in duplicate. *, significant difference in normalized luciferase activity between cells transfected with 2X Δ PIR and cells transfected with empty plasmid (Student t test; P < 0.05).
Promoter mutations diminish Tax transactivation.
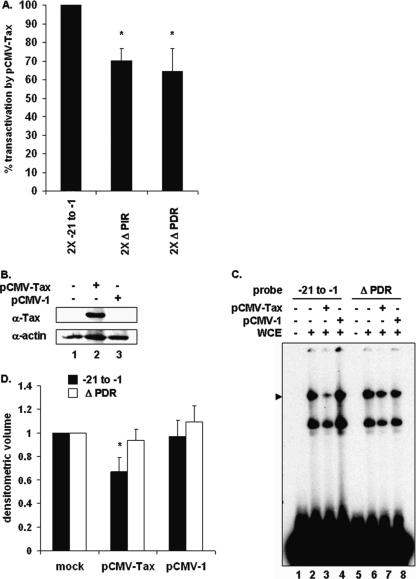
To determine whether the PIR binding site was Tax responsive, a reporter plasmid containing the multimerized wild-type proximal PCNA promoter element or constructs containing mutations in the inverted (2X Δ PIR) or direct (2X Δ PDR) repeats were cotransfected with or without Tax. Mutation of the inverted repeat (2X Δ PIR) diminished Tax transactivation (Fig. 6A). Since this mutation disrupted formation of the PIR complex, this result was anticipated. Interestingly, mutation of the adjacent GC-rich direct repeat (2X Δ PDR) also diminished Tax transactivation even though the Δ PDR mutant did not compete for formation of the PIR complex (Fig. 1C). To examine whether the direct repeat affected the ability of Tax to disrupt PIR complex formation, cells were transfected with Tax or an empty parental plasmid. Tax protein levels were measured by immunoblotting (Fig. 6B), and formation of the PIR complex on the wild-type proximal or Δ PDR PCNA promoter probe was measured by EMSA. Decreased PIR complex formation on the wild-type probe was again observed in Tax-expressing cells (Fig. 6C, lane 3) but not in mock-transfected cells or cells transfected with empty plasmid (Fig. 6C, lanes 2 and 4). Interestingly, mutation of the direct repeat abrogated the ability of Tax to disrupt PIR complex formation (Fig. 6C, lane 7). These results suggest that the PIR binding site, together with downstream direct repeat sequences, is required for Tax to interfere with PIR complex formation and to transactivate the PCNA promoter.
FIG. 6.
TBP binding site negatively regulates the PCNA initiator element. (A) Two copies of either wild-type proximal TRE (−21 to −1), Δ PIR, or Δ PDR sequences in a luciferase reporter plasmid were cotransfected into 293 cells together with the pRL-SV40 Renilla luciferase reporter plasmid and either pCMV-Tax or sheared salmon sperm DNA. Luciferase activity was normalized to Renilla luciferase activity from the same well. Error bars represent the standard errors of the means for two independent experiments performed in duplicate. *, significant difference in normalized luciferase activity between cells transfected with 2X Δ PIR or 2X Δ PDR and cells transfected with wild-type 2X −21 to −1 plasmid (Student t test; P < 0.05). (B) Western blot of 293 whole-cell extracts from mock-transfected cells (lane 1) or cells transfected with pCMV-Tax (lane 2) or pCMV-1 (lane 3), using antibodies to Tax or actin as indicated. (C) EMSA using a 32P-labeled proximal probe (lanes 1 to 6) incubated with whole-cell extracts from mock-transfected cells (lane 2) or 293 cells transfected with pCMV-Tax (lane 3) or pCMV-1 (lane 4). The position of the specific complex is indicated on the left. (D) Densitometric volume analyses of the samples from panel C quantitated with ImageQuant software. Each reaction signal from the specific complex was divided by the total signal and normalized to that of mock-transfected cells. Error bars represent the standard errors of the means for three independent experiments. *, significant difference in specific complex formation between Tax-negative and Tax-positive cells (Student t test; P < 0.05).
DISCUSSION
The experimental results presented herein extend our understanding of Tax-mediated transactivation of the TATAA-less PCNA promoter. The PIR complex was shown to contain TBP and to be a moderate repressor of transcription via an element located at the 5′ end of the PCNA promoter-proximal TRE inverted repeat. We further demonstrated that Tax expression disrupts the PIR complex and that disruption of this complex leads to increased transcription from the PCNA initiator. These results suggest that full Tax responsiveness of the PCNA promoter requires the entire proximal element, inclusive of the direct and inverted repeats. The TRE includes the novel PIR binding site that begins at the GC-rich region upstream of the imperfect PIR and extends into the inverted repeat (base pairs −21 to −13). The downstream, GC-rich proximal direct repeat (base pairs −10 to −3) also contributes to Tax transactivation of the PCNA promoter.
The mechanism of transactivation of the PCNA promoter by HTLV-1 Tax is unique from those of other viral proteins. Adenovirus E1A 243R protein relieves the repressive effects of the RFX1-p107 complex by interacting with the retinoblastoma-like tumor suppressor protein p107 to disrupt complex formation on the PCNA E1A-responsive element at base pairs −59 to −45 (19). Hepatitis B virus X protein has been shown to physically occupy the CREB-binding element of the PCNA promoter to recruit p300 and synergistically enhance CREB activity and CREB phosphorylation by protein kinase A (4).
One mechanism by which Tax could target and transactivate the PCNA promoter at the TRE is by interacting with TBP. Binding of Tax to TBP may result in conformational changes that reduce the stability of the PIR complex on the PCNA promoter, thereby relieving negative regulation. The repressive effect of TBP on PCNA transcription is likely to involve factors such as negative coregulator 2 (NC2) (15) as a component of the TBP complex. NC2 represses RNA polymerase II transcription by binding to TBP and inhibiting TFIIA and TFIIB (10). NC2 also affects the sequence specificity of TBP for TATAA-box binding (9) and has been found to be associated with a large number of human promoters, including the PCNA promoter (1). It is also possible that Tax may recruit an undetected complex to the GC-rich PCNA promoter direct repeat which may hinder complex formation on the indirect repeat.
Tax-mediated disruption of the repressive PIR complex on the PCNA promoter opens the possibility that Tax can affect other TATAA-less promoters. Genome-scale computational analyses have indicated that approximately 76% of human core promoters lack TATAA-like elements and have a high GC content (27) similar to that of the PCNA promoter TRE. The release of TBP from the PCNA promoter may also contribute to PCNA activation in other forms of cancer.
Although we hypothesize that suppression of DNA repair may play a critical role in the transformation of Tax-expressing cells, factors other than PCNA are certainly involved in the transformation process. HTLV-1 Tax has been shown to upregulate over 300 genes (5, 23), several of which have been implicated as having roles in cellular transformation. Continued study and characterization of the effects of Tax on PCNA expression and function will increase our understanding of Tax-mediated cellular immortalization and transformation. Analysis of Tax-mediated transactivation of the PCNA promoter has demonstrated that the TBP-TRE complex is a moderate repressor of transcription and that Tax expression can disrupt this complex.
Acknowledgments
We thank members of the Marriott lab for helpful discussions and support.
These studies were supported by Public Health Service grants CA-55684 and CA-77371, awarded to S.J.M., from the National Cancer Institute. D.C.E. was supported in part by NIH training grant T32 AI-07471.
Footnotes
Published ahead of print on 17 September 2008.
REFERENCES
- 1.Albert, T. K., K. Grote, S. Boeing, G. Stelzer, A. Schepers, and M. Meisterernst. 2007. Global distribution of negative cofactor 2 subunit-alpha on human promoters. Proc. Natl. Acad. Sci. USA 10410000-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buggage, R. R. 2003. Ocular manifestations of human T-cell lymphotropic virus type 1 infection. Curr. Opin. Ophthalmol. 14420-425. [DOI] [PubMed] [Google Scholar]
- 3.Caron, C., R. Rousset, C. Beraud, V. Moncollin, J. M. Egly, and P. Jalinot. 1993. Functional and biochemical interaction of the HTLV-I Tax1 transactivator with TBP. EMBO J. 124269-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cougot, D., Y. Wu, S. Cairo, J. Caramel, C. A. Renard, L. Levy, M. A. Buendia, and C. Neuveut. 2007. The hepatitis B virus X protein functionally interacts with CREB-binding protein/p300 in the regulation of CREB-mediated transcription. J. Biol. Chem. 2824277-4287. [DOI] [PubMed] [Google Scholar]
- 5.de La Fuente, C., L. Deng, F. Santiago, L. Arce, L. Wang, and F. Kashanchi. 2000. Gene expression array of HTLV type 1-infected T cells: up-regulation of transcription factors and cell cycle genes. AIDS Res. Hum. Retrovir. 161695-1700. [DOI] [PubMed] [Google Scholar]
- 6.Edlich, R. F., L. G. Hill, and F. M. Williams. 2003. Global epidemic of human T-cell lymphotrophic virus type-I (HTLV-I): an update. J. Long Term Eff. Med. Implants 13127-140. [DOI] [PubMed] [Google Scholar]
- 7.Gatza, M. L., and S. J. Marriott. 2006. Genotoxic stress and cellular stress alter the subcellular distribution of human T-cell leukemia virus type 1 Tax through a CRM1-dependent mechanism. J. Virol. 806657-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calander, and G. DeThe. 1985. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet ii407-409. [DOI] [PubMed] [Google Scholar]
- 9.Gilfillan, S., G. Stelzer, E. Piaia, M. G. Hofmann, and M. Meisterernst. 2005. Efficient binding of NC2. TATA-binding protein to DNA in the absence of TATA. J. Biol. Chem. 2806222-6230. [DOI] [PubMed] [Google Scholar]
- 10.Goppelt, A., G. Stelzer, F. Lottspeich, and M. Meisterernst. 1996. A mechanism for repression of class II gene transcription through specific binding of NC2 to TBP-promoter complexes via heterodimeric histone fold domains. EMBO J. 153105-3116. [PMC free article] [PubMed] [Google Scholar]
- 11.Hinuma, Y., K. Nagata, M. Misoka, T. Nakai, T. Matsumoto, K. Kiroshita, S. Shirakwa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 786476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao, S. Y., and S. J. Marriott. 1999. Disruption of nucleotide excision repair by the human T-cell leukemia virus type 1 Tax protein. J. Virol. 734299-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao, S. Y., and S. J. Marriott. 2000. Suppression of DNA repair by HTLV-I Tax is rescued by a functional p53 signaling pathway. J. Biol. Chem. 27535926-35931. [DOI] [PubMed] [Google Scholar]
- 14.Kashanchi, F., and J. N. Brady. 2005. Transcriptional and post-transcriptional gene regulation of HTLV-1. Oncogene 245938-5951. [DOI] [PubMed] [Google Scholar]
- 15.Kim, T. K., Y. Zhao, H. Ge, R. Bernstein, and R. G. Roeder. 1995. TATA-binding protein residues implicated in a functional interplay between negative cofactor NC2 (Dr1) and general factors TFIIA and TFIIB. J. Biol. Chem. 27010976-10981. [DOI] [PubMed] [Google Scholar]
- 16.Kondo, T., H. Kono, H. Nonaka, N. Miyamoto, R. Yoshida, F. Bando, H. Inoue, I. Miyoshi, Y. Hinuma, and M. Hanaoka. 1987. Risk of adult T-cell leukaemia/lymphoma in HTLV-I carriers. Lancet ii159. [DOI] [PubMed] [Google Scholar]
- 17.Labrie, C., B. H. Lee, and M. B. Mathews. 1995. Transcription factors RFX1/EF-C and ATF-1 associate with the adenovirus E1A-responsive element of the human proliferating cell nuclear antigen promoter. Nucleic Acids Res. 233732-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemoine, F. J., S. Y. Kao, and S. J. Marriott. 2000. Suppression of DNA repair by HTLV-I Tax correlates with Tax transactivation of PCNA gene expression. AIDS Res. Hum. Retrovir. 161623-1627. [DOI] [PubMed] [Google Scholar]
- 19.Liu, M., B. H. Lee, and M. B. Mathews. 1999. Involvement of RFX1 protein in the regulation of the human proliferating cell nuclear antigen promoter. J. Biol. Chem. 27415433-15439. [DOI] [PubMed] [Google Scholar]
- 20.Marriott, S. J., and O. J. Semmes. 2005. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene 245986-5995. [DOI] [PubMed] [Google Scholar]
- 21.Nicot, C., R. L. Harrod, V. Ciminale, and G. Franchini. 2005. Human T-cell leukemia/lymphoma virus type 1 nonstructural genes and their functions. Oncogene 246026-6034. [DOI] [PubMed] [Google Scholar]
- 22.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i1031-1032. [DOI] [PubMed] [Google Scholar]
- 23.Pise-Masison, C. A., M. Radonovich, R. Mahieux, P. Chatterjee, C. Whiteford, J. Duvall, C. Guillerm, A. Gessain, and J. N. Brady. 2002. Transcription profile of cells infected with human T-cell leukemia virus type I compared with activated lymphocytes. Cancer Res. 623562-3571. [PubMed] [Google Scholar]
- 24.Ressler, S., G. F. Morris, and S. J. Marriott. 1997. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J. Virol. 711181-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith, M. R., and W. C. Greene. 1990. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 41875-1885. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe, T. 1997. HTLV-1-associated diseases. Int. J. Hematol. 66257-278. [DOI] [PubMed] [Google Scholar]
- 27.Weng, J. J., and B. Y. Yung. 2005. Nucleophosmin/B23 regulates PCNA promoter through YY1. Biochem. Biophys. Res. Commun. 335826-831. [DOI] [PubMed] [Google Scholar]
- 28.Winter, H. Y., T. Dayaram, and S. J. Marriott. 2007. Activation of the human T-cell leukemia virus type 1 long terminal repeat by the ternary complex factor Elk-1. J. Virol. 8113075-13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winter, H. Y., and S. J. Marriott. 2007. Human T-cell leukemia virus type 1 Tax enhances serum response factor DNA binding and alters site selection. J. Virol. 816089-6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its importance in the disease. Proc. Natl. Acad. Sci. USA 792031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]