Abstract
The identification of “asymptomatic” (i.e., protective) epitopes recognized by T cells from herpes simplex virus (HSV)-seropositive healthy individuals is a prerequisite for an effective vaccine. Using the PepScan epitope mapping strategy, a library of 179 potential peptide epitopes (15-mers overlapping by 10 amino acids) was identified from HSV type 1 (HSV-1) glycoprotein B (gB), an antigen that induces protective immunity in both animal models and humans. Eighteen groups (G1 to G18) of 10 adjacent peptides each were first screened for T-cell antigenicity in 38 HSV-1-seropositive but HSV-2-seronegative individuals. Individual peptides within the two immunodominant groups (i.e., G4 and G14) were further screened with T cells from HLA-DR-genotyped and clinically defined symptomatic (n = 10) and asymptomatic (n = 10) HSV-1-seropositive healthy individuals. Peptides gB161-175 and gB166-180 within G4 and gB661-675 within G14 recalled the strongest HLA-DR-dependent CD4+ T-cell proliferation and gamma interferon production. gB166-180, gB661-675, and gB666-680 elicited ex vivo CD4+ cytotoxic T cells (CTLs) that lysed autologous HSV-1- and vaccinia virus (expressing gB)-infected lymphoblastoid cell lines. Interestingly, gB166-180 and gB666-680 peptide epitopes were strongly recognized by CD4+ T cells from 10 of 10 asymptomatic patients but not by CD4+ T cells from 10 of 10 symptomatic patients (P < 0.0001; analysis of variance posttest). Inversely, CD4+ T cells from symptomatic patients preferentially recognized gB661-675 (P < 0.0001). Thus, we identified three previously unrecognized CD4+ CTL peptide epitopes in HSV-1 gB. Among these, gB166-180 and gB666-680 appear to be “asymptomatic” peptide epitopes and therefore should be considered in the design of future herpes vaccines.
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are ubiquitous viruses that infect a majority of people worldwide (3, 22, 68). Shedding of reactivated HSV is estimated to occur at rates of 3 to 28% in adults who harbor latent HSV in their sensory neurons (32, 66-68). However, the vast majority of these individuals do not experience recurrent herpetic disease and are designated “asymptomatic patients” (22, 40, 68). In contrast, in some individuals (symptomatic patients), reactivation of latent virus leads to induction of “pathogenic” HSV-specific CD4+ and CD8+ T cells (22, 68) and recurrent disease, ranging from rare episodes occurring every 5 to 10 years to outbreaks occurring monthly or even more frequently among a small proportion of subjects (22, 26, 60). Interestingly, for genital herpes, symptomatic and asymptomatic patients have similar virus shedding rates (68). It is likely that the same is true for ocular and oro-facial herpes, since shedding rates in tears and saliva of asymptomatic individuals have been reported to be as high as 33.5% (22, 29, 40, 42). Latently infected patients are at risk of developing severe immunopathology, such as blinding herpetic stromal keratitis (HSK) (primarily due to HSV-1), painful genital ulcerations (primarily due to HSV-2), and in rare cases, fatal HSV encephalitis (reviewed in reference 72).
Considering the wealth of data addressing the role of T cells in animal models, it is surprising how few reports explore the immunologic basis of symptomatic and asymptomatic HSV infections in humans. The HSV-1-specific CD4+ T-cell responses in the cornea are much more likely to cause pathology than those in the genital tract or anus/buttocks region. Indeed, the involvement of CD4+ T cells that produce Th1 cytokines (interleukin-2 [IL-2] and gamma interferon [IFN-γ]) in HSK has been established with the mouse model of ocular herpes, and corneal herpetic diseases can be abrogated by depletion of CD4+ T cells or neutralization of Th1 cytokines (24, 25, 50, 51, 62, 63). HSV-specific CD4+ T cells are key participants in the development of recurrent herpetic sores in symptomatic individuals (14, 21, 39, 46, 47, 65, 75, 79). Paradoxically, HSV-specific CD4+ T cells may also be important in controlling the severity of genital herpes infection (1, 47, 49). CD4+ T-cell infiltrates appear to correlate with HSV clearance from mucocutaneous genital lesions (38). Individuals with severe immunodeficiency involving a lack of CD4+ T cells can have severe recurrent herpes with a longer duration of symptomatic lesions (17, 43, 57), thus supporting the thought that CD4+ T cells are one of the main mediators of protective immunity against recurrent herpes (34, 47, 75, 76). Early in the development of recurrent lesions, HSV-1-specific IFN-γ-producing CD4+ T cells that display a cytotoxic activity predominate in the mononuclear infiltrate surrounding HSV-1-infected epidermal cells, which strongly express HLA-DR (47). For many years, the association of HLA-DR haplotypes with susceptibility to both labial and genital herpes has been appreciated (27, 41, 44, 55). Among the HLA-DR alleles, DRB1*04, DRB1*07, DRB1*11, and DRB1*13 are most frequent in the populations studied (26, 35, 41, 55). DRB1*13 is frequently seen in symptomatic HSV infection, while DRB1*04 is most frequent in individuals with asymptomatic HSV infection (41). Obvious and immediate consequences of variations in HLA-DR allele distribution are changes in functional epitopes presented to the host's CD4+ T cells (26, 27, 35, 41, 44, 47, 49, 55).
We hypothesize that the clinical spectrum of herpes, ranging from asymptomatic infection to frequently distressing outbreaks, may be reflected in T-cell recognition of different sets of epitopes of one or several HSV protein antigens (Ags). Thus, recognition by CD4+ T cells of a set of viral epitopes designated “symptomatic” might be associated with severe immunopathological diseases, while recognition of “asymptomatic” epitopes might, in turn, lead to immunoprotection. However, identification of HSV target Ags and the sets of epitopes recognized by “asymptomatic” versus “symptomatic” CD4+ T cells (i.e., “protective” versus “pathogenic” T cells) is far from being completed. Due to the lack of an animal model with a high incidence of recurrent eye disease, it is currently not feasible to demonstrate that “symptomatic” T-cell epitopes are pathogenic. However, if people with a history of severe recurrent disease (i.e., symptomatic people) tend to develop T cells that recognize a subset of epitopes (i.e., symptomatic epitopes) that differ from those recognized by T cells from asymptomatic people (i.e., asymptomatic epitopes), it would be logical to exclude such symptomatic epitopes from vaccines on the grounds that they may enhance rather than diminish recurrent disease.
Results from a number of studies indicate that HSV glycoprotein B (gB), one of the major HSV Ags that produce protective immunity in both animal models and humans (7, 10, 12, 14, 19, 45, 61), is recognized by CD4+ T cells from both symptomatic and asymptomatic HSV-seropositive humans (14, 39, 45, 75-77). In the late 1980s and early 1990s, Zarling and coworkers generated gB-specific CD4+ T-cell clones from HSV-2-seropositive severely symptomatic patients who had recurrent HSV-2 genital infections approximately every 8 weeks and from HSV-1-seropositive symptomatic patients who suffered frequent recurrent oral lesions (75-77). gB-specific CD4+ T cells have also been cloned from HSV-seropositive healthy asymptomatic individuals (76, 77). Five of these CD4+ cytotoxic T-lymphocyte (CTL) clones lysed autologous HSV-1- or both HSV-1- and -2-infected lymphoblastoid cell lines (LCLs), and their cytotoxicity was restricted to HLA-DR molecules (73, 76). In another study, HSV-specific CD4+ CTL clones were recovered from recurrent herpes lesions of five patients (33, 38). In the late 1990s and early 2000s, Cunningham and coworkers reported that gB is a major target for IFN-γ-producing CD4+ CTLs recovered from skin lesions (47, 49). Although the above studies suggest that gB is a target of both “symptomatic” and “asymptomatic” CD4+ T cells, no specific “symptomatic” or “asymptomatic” gB epitopes have ever been defined.
In this study, we hypothesize that different sets of HSV-1 gB epitopes might be recognized by CD4+ T cells from symptomatic versus asymptomatic individuals. We used the PepScan peptide library strategy and identified the following four previously unrecognized CD4+ T-cell peptide epitopes from HSV-1 gB: gB161-175, gB166-180, gB661-675, and gB666-680. Among these, gB166-180, gB661-675, and gB666-680 were targeted by CD4+ CTLs that lysed autologous HSV-1- and vaccinia virus (expressing gB [VVgB])-infected LCLs. Interestingly, gB166-180 and gB666-680 appeared to be recognized preferentially by CD4+ T cells from HSV-1-seropositive healthy “asymptomatic” individuals, while gB661-675 appeared to be recognized preferentially by CD4+ T cells from severely “symptomatic” individuals. We propose that an effective immunotherapeutic herpes vaccine should exclude the potential “symptomatic” gB661-675 epitope.
MATERIALS AND METHODS
Study population.
From August 2003 to October 2007, we screened 283 HSV-1- and/or HSV-2-seropositive individuals. Among these, a cohort of 79 immunocompetent individuals who were seropositive or seronegative for HSV-1 and/or HSV-2, with an age range of 18 to 63 years (median, 31 years), were enrolled in the present study. Figure 1 shows the characteristics of this study population with respect to sex, age, HSV serology, HSV disease, and HLA-DR frequency distribution. Forty-eight individuals were white, 31 were nonwhite (African, Asian, Hispanic, and others), 43 were females, and 36 were males. All patients were negative for human immunodeficiency virus and hepatitis B virus and had no history of immunodeficiency. Thirty-eight patients were HSV-1 seropositive and HSV-2 seronegative, among which 28 patients were healthy and asymptomatic (no history of recurrent HSV disease). The other 10 patients were defined as HSV-1-seropositive symptomatic patients who suffered from frequent and severe recurrent oral and/or oro-facial lesions, with 2 patients having had well-characterized HSK. Control individuals (n = 24) were seronegative for both HSV-1 and HSV-2 and had no history of ocular HSK, genital lesions, or oro-facial herpes disease. All subjects were enrolled at the University of California Irvine under an institutional review board-approved protocol. All subjects provided written informed consent.
FIG. 1.
Characteristics of the study population. A cohort of 79 immunocompetent individuals were enrolled in the present study and were characterized with respect to sex, race, age, HSV serology, and HSV disease (A). (B) The individuals were HLA-DR typed, and HLA-DRB1 allele frequency was calculated by the following formula: allele frequency = n/N, where n is the number of samples positive for the Ag, and N is the total number of samples.
HSV-1 and HSV-2 seropositivity screening.
The sera collected from 79 patients were tested for HSV-1 and HSV-2 status by use of HerpeSelect immunoglobulin G1 (IgG1) and IgG2 HSV enzyme-linked immunosorbent assay (ELISA) kits (Focus Diagnostics, Cypress, CA) as previously described (18). The sensitivities and specificities of these ELISAs were 91.2% (HSV-1) to 96.1% (HSV-2) and 92.3% (HSV-1) to 97% (HSV-2), respectively. Although these assays generally give clear-cut results, in some instances the stereotyping was also validated by Western blotting as previously described (53).
PepScan peptide library screening and synthesis.
gB from strain 17 (GenBank accession number P10211) was used in this study. One hundred seventy-nine synthetic peptides encompassing the entire sequence of HSV-1 gB (904 amino acids [aa]) were produced by using the multipin DKP system (Mimotopes). Figure 2A and B show examples of the 10 overlapping peptides forming group 4 (G4) and group 14 (G14). Peptides were synthesized as 15-mers overlapping by 10 aa with adjacent peptides. Synthesis was performed using classical 9-fluorenylmethoxy carbonyl/tert-butyl chemistry. After side chain deprotection with a mixture of trifluoroacetic acid, ethanedithiol, and anisole (38:1:1), the peptides were cleaved in ammonium bicarbonate buffer (0.1 M, pH 8.4) with 40% (vol/vol) acetonitrile via a cyclization mechanism, leaving a cyclo (lysylpropyl) moiety at the C terminus. Finally, the peptides were freeze-dried and stored as a dry powder at −80°C. Working stock solutions of peptides were made at 1 mg/ml in phosphate-buffered saline-10% dimethyl sulfoxide and were stored at −20°C.
FIG. 2.
The PepScan library was designed from the entire HSV-1 gB sequence (strain 17) (GenBank accession number P10211). (A) Sequences of the 10 overlapping 15-mer gB peptides in G4. (B) Sequences of the 10 overlapping 15-mer gB peptides in G14.
HLA-DR genotyping.
Peripheral blood mononuclear cells (PBMCs) were HLA typed genomically after isolation of their DNA by use of a Qiagen DNA kit. The amount of DNA obtained was quantified with a spectrophotometer. Olerup and Zetterquist (52) previously described the localization, sequences, lengths, and specificities of the sequence-specific primers used for typing the DRB1, DRB3, DRB4, and DRB5 alleles. HLA-DRB1 genotyping was performed using Dynal Reli SSO kits (Invitrogen). In brief, a target sequence of 272 bp in the second exon of the DRB1, DRB3, DRB4, and DRB5 genes was amplified by PCR. Genotyping of HLA-DRB1 alleles was carried out with a direct DNA probe test that, after PCR, uses a nucleic acid hybridization method for the differentiation of over 210 distinct alleles. This was performed using 54 specific probes immobilized on paper strips and was able to distinguish the polymorphic sequence motifs in the second exon of the DRB1, DRB3, DRB4, and DRB5 genes. HLA-DRB1 allele frequency was calculated by the following formula: allele frequency = n/N, where n is the number of samples positive for the Ag and N is the total number of samples.
Peptide binding assays specific for HLA-DR molecules.
HLA-DR molecules were immunopurified from homologous Epstein-Barr virus cell lines by affinity chromatography using the monomorphic monoclonal antibodies (MAbs) L243 and B7/21 (64). Binding to HLA-DR molecules was assessed by a competitive ELISA using biotinylated gB peptides as previously reported (64). Unlabeled forms of nonherpesvirus peptides were used as references to assess the validity of each experiment. The sequences of these reference peptides and their 50% inhibitory concentrations (IC50s) are as follows: HA 306-318 (PKYVKQNTLKLAT) for DRB1*0101 (1 nM), DRB1*0401 (5 nM), and DRB1*1101 (11 nM), YKL (AAYAAAKAAALAA) for DRB1*0701 (10 nM), A3 152-166 (EAEQLRAYLDGTGVE) for DRB1*1501 (35 nM), MT 2-16 (AKTIAYDEEARRGLE) for DRB1*0301 (129 nM), and B1 21-36 (TERVRLVTRHIYNREE) for DRB1*1301 (52 nM).
PBMC preparation and enzyme-linked immunospot (ELISPOT) assay.
PBMCs were isolated as we described previously (13). Approximately 175 ml of donor blood was drawn into a yellow-top Vacutainer tube (Becton Dickinson). The serum was isolated and centrifuged for 10 min at 800 × g. The PBMCs were isolated by gradient centrifugation using leukocyte separation medium (Cellgro). The cells were then washed in phosphate-buffered saline and resuspended in complete culture medium. Aliquots of freshly isolated PBMCs were also cryopreserved in liquid nitrogen for future testing.
T-cell stimulation was measured by IFN-γ production in peptide-stimulated PBMCs, using a BD-ELISpot IFN-γ kit (BD-Pharmingen) as we previously described (13). Briefly, 3 × 105 PBMCs were stimulated in ELISPOT plates (Millipore) for 2 days with 10 μg/ml of pooled peptides (10 peptides per pool), 10 μg/ml of an individual gB peptide, heat-inactivated HSV-1 (strain McKrae) (multiplicity of infection [MOI] of 5), or 1 μg/ml phytohemagglutinin (PHA) as a positive control. The plates were precoated with anti-human IFN-γ capture Ab in a humidified incubator at 37°C with 5% CO2. The spot-forming cells (SFCs) were developed as described by the manufacturer (BD-ELISpot IFN-γ kit; BD-Pharmingen) and were counted using a stereoscopic microscope.
T-cell activation and proliferation.
PBMCs were stained with carboxyfluorescein succinimidyl ester (CFSE) (2 mM) and then incubated with individual gB peptides (10 μg/ml) for 5 days. The cells were then washed and stained for CD4 molecule expression. For HLA restriction, CFSE-labeled cells were stimulated in the presence or absence of 10 μg/ml of anti-DR (L243; BD-Pharmingen, CA) or anti-DQ (SPV-L3; AbD Serotec, Oxford, United Kingdom) blocking Ab. Cycling cells were then analyzed by flow cytometry, and their absolute number was calculated using the following formula: (number of events in double-positive cells × number of events in gated lymphocytes)/total number of events acquired. To assess CD4+ T-cell activation, PBMCs were stimulated with individual gB peptides (10 μg/ml) for 24 h and then analyzed by flow cytometry for the expression of the T-cell early activated marker CD69 after double staining with fluorescent anti-human CD69 and anti-human CD4 Abs.
Generation of gB peptide-specific CD4+ T-cell lines.
PBMC-derived CD4+ T cells were stimulated with mitomycin C-treated (50 μg/ml) autologous PBMCs (106/ml) incubated for 3 h with individual peptides (10 μg/ml). IL-2 (5 ng/ml; R&D Systems) was added to the culture every other day starting on day 3. On day 7, cells were restimulated with mitomycin C-treated autologous individual peptide-pulsed PBMCs (106/ml), and IL-7 (20 ng/ml) was added every other day. On day 14, cultures were expanded and restimulated as described above for another week and then used as effector cells for CD107 assay.
Target cells.
LCLs were generated as we recently described (13). Briefly, LCLs were derived by stimulating PBMCs with PHA (1 μg/ml) in RPMI 1640 plus 10% fetal calf serum (FCS) for 3 days. Half of the medium was then replaced with fresh medium containing IL-2 (10 ng/ml) and IL-7 (20 ng/ml). On day 6, the LCLs were infected overnight with VVgB, an empty vaccinia virus control (VVC), or HSV-1, each at an MOI of 10. The next day, infected LCLs were washed three times and treated with mitomycin C (50 μg/ml) for 30 min. The LCLs were then washed three more times before a 4-h incubation with effector CD4+ T cells at the indicated effector/target ratio. Cytotoxic activity was detected in a CD107a/b degranulation assay as we recently described (13). In order to demonstrate whether the strong T-cell responses detected against synthetic peptides gB161-175, gB166-180, gB661-675, and gB666-680 can be detected from naturally processed gB, PHA-stimulated blasts were infected with HSV-1, VVgB, or VVC and used as target cells. The response to VVC was subtracted from the response to VVgB (not shown).
T-cell cytotoxicity assay.
In order to detect cytolytic CD4+ T cells recognizing gB peptides in freshly isolated PBMCs, we used the CD107a/b cytotoxicity assay as we recently described (13). On the day of the assay, nonstimulated or gB peptide-stimulated PBMCs were incubated at 37°C for 5 to 6 h with BD-Golgi Stop, costimulatory Abs anti-CD28 and anti-CD49d (1 μg/ml), and 10 μl of CD107a-fluorescein isothiocyanate (CD107a-FITC) and CD107b-FITC. At the end of the incubation period, the cells were harvested into separate tubes, washed once with fluorescence-activated cell sorting (FACS) buffer, and then stained with Cy-phycoerythrin-conjugated anti-human CD4 for 30 min. The cells were then washed again and analyzed using a FACScan flow cytometer (Becton Dickinson, San Diego, CA).
Statistical analysis.
Data are expressed as means ± standard errors. Statistical significance was estimated by analysis of variance followed by Dunnett's test to identify differences between groups. Differences were considered significant when P values were <0.05. All P values were two-tailed unless stated otherwise.
RESULTS
Prevalence of gB epitope-specific IFN-γ-producing CD4+ T cells in healthy HSV-seropositive patients.
We first assessed the ability of HSV-specific CD4+ T cells from 38 HSV-1-seropositive, HSV-2-seronegative individuals to respond to a PepScan library of 179 gB peptides divided into 18 groups of 10 peptides each (G1 to G18). In an IFN-γ ELISPOT assay, we used fresh PBMC-derived CD4+ T cells isolated directly ex vivo to minimize artifacts that might arise from in vitro restimulation of CD4+ T cells. A preliminary analysis of IFN-γ-producing CD4+ T cells revealed that 20 or more spots/106 CD4+ T cells gave a 97% probability of defining a positive response. Of the 540 separate ELISPOT wells used to screen 20 HSV-seronegative individuals, only 16 wells (3%) exceeded 20 SFCs/106 CD4+ T cells. Of the 566 separate ELISPOT assays used to screen the 38 seropositive individuals, 96 (17%) had readings above 20 SFCs/106 CD4+ T cells. A level of 20 SFCs/106 CD4+ T cells was therefore used as the cutoff point for subsequent analyses.
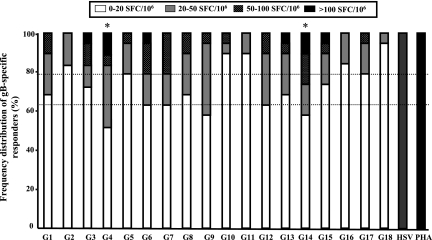
The number of IFN-γ-producing CD4+ T cells specific for each group of gB peptides was determined for each individual and ranked as follows: strong responses, >100 SFCs/106 PBMCs); medium responses, >50 and <100 SFCs/106 PBMCs; low responses, >20 and <50 SFCs/106 PBMCs; and no response, <20 SFCs/106 PBMCs. Positive IFN-γ-producing CD4+ T-cell responses were compared with responses in control wells without Ag. Figure 3 shows the frequency distribution of CD4+ T-cell responses induced by 18 groups of gB peptides and heat-inactivated HSV-1 (McKrae) or PHA as a positive control. Based on these criteria, significant IFN-γ-producing CD4+ T-cell responses were detected for all but five groups of gB peptides (G2, G10, G11, G16, and G18) (Fig. 3).
FIG. 3.
Prevalence of gB peptide-specific T-cell responses in HSV-1-seropositive responders. Eighteen groups (pools of 10 adjacent peptides per group) of peptides from the HSV-1 gB PepScan library were analyzed by ELISPOT assay for the capacity to elicit IFN-γ production by stimulated CD4+ T cells from 38 HSV-1-seropositive, HSV-2-seronegative individuals. The results represent the frequency distribution of gB peptide-specific responders in the presence of 10 μg/ml of each group of gB peptides or with heat-inactivated HSV-1 (strain McKrae) (MOI = 5) or PHA (1 μg/ml) as a positive control. The categories of immune response strength are as follows: no/low response, 0 to 20 SFCs; medium response, 20 to 50 SFCs; high response, 50 to 100 SFCs; and strong response, >100 SFCs.
The highest frequency of IFN-γ-producing CD4+ T-cell responses was detected against G4 (47.5%), while 42% of individuals responded to G14 and G9. In comparison, only 5 to 38% of individuals showed positive T-cell responses to the remaining 16 groups of peptides (G1, G2, G3, G5, G6, G7, G8, G10, G11, G12, G13, G15 G16, G17, and G18) (5%). The highest magnitude of response, against G4 and G14, was also recorded for 11% of HSV-1-seropositive individuals (Fig. 3). The lowest magnitude of response was detected against G18. All of the donors responded similarly to the PHA and heat-inactivated HSV-1 positive controls. The G6 group of peptides appeared to be recognized by a large percentage of individuals and to stimulate the highest magnitude of T-cell responses. However, G6 induced a significantly smaller proportion of responses in the >100 SFC range than did G4 (5% versus 11%). In addition, a smaller percentage of individuals responded to G6 than to G4 and G14 (38% versus 47.5% and 42%). Besides G6, the G9 group of peptides appeared to be next, after G4 and G14, to be recognized by a large percentage of individuals. However, G9 induced a smaller proportion of responses in the >100 SFC range than G4 and G14 did (0% versus 11%). In addition, although a majority of individuals responded to G9, their T-cell responses were at a low magnitude (i.e., 37% of individuals responded in the range of 20 to 50% SFCs) compared to the responses to G4 and G14 (31% and 15%). The borderline negative responses obtained with G6 and G9 were confirmed by the inability of individual peptides within G6 and G9 to stimulate significant CD4+ T-cell responses, based on both the magnitude and percentage of responders (not shown). Thus, both G6 and G9 do not contain an immunodominant epitope and were therefore excluded from further studies. Altogether, these results indicate that G4 and G14 might contain one or several immunodominant gB epitopes.
gB161-175, gB166-180, gB661-675, and gB666-680 are immunodominant peptide epitopes.
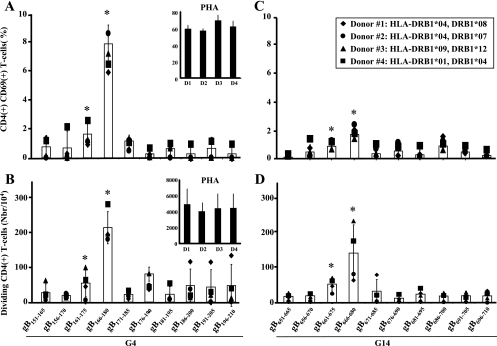
To identify the immunodominant CD4+ T-cell epitopes within G4 and G14, we analyzed the ability of the 10 individual peptides forming each group to induce HSV-specific CD4+ T-cell responses in four HSV-seropositive individuals with different HLA-DR haplotypes. Both activation (expression of the CD69 early activation marker) and proliferation (CFSE incorporation) were followed by FACS of gated CD4+ T cells, as described in Materials and Methods. Among the 10 peptides forming G4, peptides gB161-175 and gB166-180 induced the strongest CD4+ T-cell activation (Fig. 4A) and proliferation (Fig. 4B), with gB166-180 being the immunodominant peptide epitope within this group. In G14, peptides gB661-675 and gB666-680 induced the strongest CD4+ T-cell activation (Fig. 4C) and proliferation (Fig. 4D). Overall, T-cell responses induced by individual peptides within G4 were higher than those induced by peptides in G14. As a positive control, CD4+ T-cell responses to PHA were similar in all tested donors. Altogether, the results show that peptides gB161-175 and gB166-180 among G4 peptides and peptides gB661-675 and gB666-680 among G14 peptides are immunodominant peptide epitopes. In addition, the results indicate that regardless of HLA-DR haplotype, gB161-175, gB166-180, gB661-675, and gB666-680 induced significant magnitudes of CD4+ T-cell activation (expression of the CD69 early activation marker) and proliferation (CFSE incorporation), pointing to the promiscuity of these epitopes.
FIG. 4.
T-cell stimulation with individual gB peptides from the dominant groups (G4 and G14). (A and C) Induction of CD69 (early activation marker) expression in gB peptide-specific CD4+ T cells. (B and D) Absolute numbers of dividing CD4+ T cells after 5 days of stimulation with 10 μg/ml of individual peptides from groups G4 and G14.
In order to depict the magnitude and frequency of CD4+ T-cell responses induced by each HSV-1 gB peptide, we employed multiple immunological assays. Thus, to rank as an immunodominant peptide epitope, the peptide must induce strong responses (i.e., T-cell activation, proliferation, CD69 upregulation, and IFN-γ production) in at least four different donors. Looking at these immunological criteria, gB686-700 did induce T-cell stimulation but failed to induce cell division (Fig. 4B). Similarly, although gB176-190 induced a moderate proliferation of T cells, it also induced one of the lowest and least significant T-cell stimulations (Fig. 4C). Therefore, both gB176-190 and gB686-700 peptides were ranked as negative peptides, and gB166-180 andgB666-680 were ranked as immunodominant peptide epitopes.
In vitro binding to soluble HLA-DR molecules confirms gB161-175, gB166-180, gB661-675, and gB666-680 as strong peptide epitopes.
The four immunodominant gB responder peptides (i.e., gB161-175, gB166-180, gB661-675, and gB666-680), as well as four nonresponder control peptides (i.e., gB871-895, gB886-900, and gB890-904), were synthesized and tested in vitro for binding to seven soluble HLA-DR molecules (Table 1). This panel of available HLA-DR molecules consists of the most common HLA class II haplotypes, regardless of ethnicity (78). The relative binding capacity (IC50 [nanomolar]) for each peptide was calculated as the concentration of competitor peptide required to inhibit 50% of the binding of an allele-specific biotinylated peptide (indicator peptide), as described in Materials and Methods. Based on an upper threshold of 250 nM, which characterizes high-affinity peptide binders (11, 78), gB161-175, gB166-180, gB661-675, and gB666-680 bound to three or more different HLA-DR molecules, while the remaining peptide epitopes did not bind to any of the HLA-DR molecules tested. This suggests that the gB161-175, gB166-180, gB661-675, and gB666-680 peptide epitopes contain at least one universal T-cell epitope or several overlapping epitopes presented by multiple HLA-DR molecules.
TABLE 1.
In vitro binding capacities of HSV-1 gB-derived epitope peptides for soluble HLA-DR moleculesa
| Peptide | HLA-DR binding capacity (IC50 [nM])b
|
||||||
|---|---|---|---|---|---|---|---|
| DR1 (B1*0101) | DR3 (B1*0301) | DR4 (B1*0401) | DR7 (B1*0701) | DR11 (B1*1101) | DR13 (B1*1302) | DR15 (B1*1501) | |
| gB161-175 | 98 | 0.3 | 0.4 | 4 | 85 | >1,925 | 0.4 |
| gB166-180 | >9,759 | 18 | 224 | 289 | >1,690 | 233 | 139 |
| gB661-675 | 5,333 | 5 | 45 | 9 | 254 | >1,925 | 2 |
| gB666-680 | 436 | 3 | 15 | 612 | 131 | >1,925 | 7 |
| gB871-895 | >9,759 | >774 | >1,826 | >1,021 | >1,690 | >1,925 | >283 |
| gB886-900 | >9,759 | >774 | >1,826 | >1,021 | 1,265 | >1,925 | >283 |
| gB890-904 | >9,759 | >774 | >1,826 | >1,021 | >1,690 | >1,925 | >283 |
HSV-1 gB-derived peptides were subjected to ELISAs specific for HLA-DR molecules as described in Materials and Methods. Reference nonherpesvirus peptides were used to validate each assay. Data are expressed as relative activities (ratio of the IC50 of the peptides to the IC50 of the reference peptide) and are the means of two experiments. Peptide epitopes with high-affinity binding to HLA-DR molecules have IC50s below 250 and are shown in bold. “>” indicates peptide epitopes that failed to bind to tested HLA-DR molecules.
Population coverage was 8%, 6%, 21%, 15%, 12%, 14%, and 6% for DR1, DR3, DR4, DR7, DR11, DR13, and DR15, respectively.
T-cell responses to peptides gB161-175, gB166-180, and gB661-675 are HLA-DR dependent.
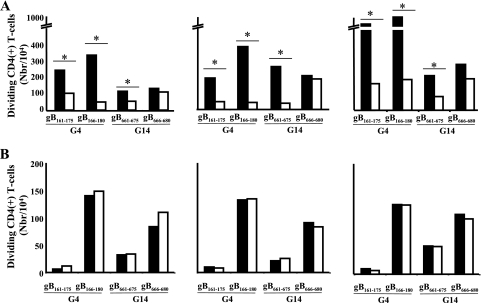
In order to ascertain that T-cell responses to the four immunodominant gB peptides are HLA-DR dependent, HLA blocking experiments were performed where antigen-presenting cell presentation of individual peptides to CD4+ T cells was assessed in the presence and absence of MAbs specific to the monomorphic region of HLA-DR or HLA-DQ molecules. Figure 5 shows that CD4+ T-cell proliferation induced by gB161-175, gB166-180, and gB661-675 was significantly (P < 0.005) abrogated in the presence of an anti-HLA-DR blocking MAb (4A) but not in the presence of an anti-HLA-DQ MAb (4B). CD4+ T-cell proliferation induced by gB666-680 was not affected by either anti-HLA-DR MAb or anti-HLA-DQ MAb. These results were confirmed for HSV-seropositive individuals with six different HLA-DRB1 haplotypes. Altogether, these results show that T-cell responses to peptides gB161-175, gB166-180, and gB661-675 are HLA-DR dependent.
FIG. 5.
CD4+ T-cell stimulation with individual gB peptides from the dominant groups (G4 and G14) in the presence or absence of anti-DR blocking MAb. (A) Absolute numbers of dividing CD4+ T cells stimulated for 5 days with 10 μg/ml of individual peptides gB161-175, gB166-180, gB661-675, and gB666-680 in the absence (solid bars) or presence (open bars) of anti-DR blocking Abs. (B) CD4+ T cells from three HLA-DRB1-genotyped donors were incubated with 10 μg/ml of gB peptide in the absence (solid bars) or presence (open bars) of anti-DQ blocking MAb. The graph shows the means plus standard deviations of the absolute numbers of dividing CD4+ T cells in the presence of the indicated gB peptides subtracted from the value without peptide. The results are representative of three experiments (n = 3 patients).
gB166-180-, gB661-675-, and gB666-680-specific CD4+ T cells display lytic activity.
In order to detect whether CD4+ T cells specific to the gB161-175, gB166-180, gB661-675, and gB666-680 peptides display cytotoxic activity, we used the CD107a/b degranulation assay on freshly isolated T cells. Figure 6 shows that peptides gB166-180, gB661-675, and gB666-680 were able to induce significant degranulation/mobilization of CD107 on the surfaces of specific CD4+ T cells. gB166-180 induced the highest cytotoxic activity, suggesting that this peptide contains a dominant CTL epitope. gB161-175 was unable to elicit CD4+ cytotoxic T cells, suggesting a helper function of this epitope.
FIG. 6.
CD107a and -b expression ex vivo by activated gB peptide-specific CD4+ T cells. PBMCs from four different HLA-DR donors were stimulated with the dominant gB peptides in the presence of anti-CD28/49d, FITC-conjugated anti-CD107a and -b, and Golgi-Stop for 6 h. All peptides were used at a final concentration of 10 μg/ml. The graph shows the means ± standard deviations of the percentages of CD107a/b+ and CD4+ T cells in the presence of gB peptides or PHA for donors 1, 3, 7, and 8.
gB166-180, gB661-675, and gB666-680 peptide epitopes are naturally processed and presented from native gB.
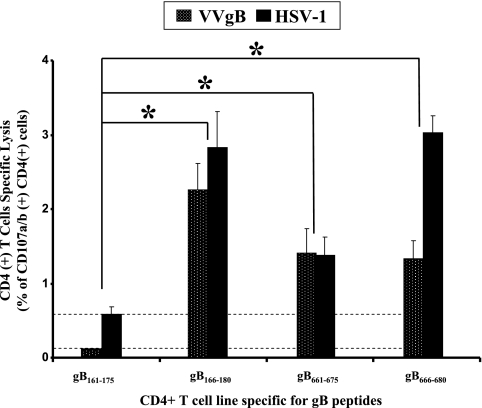
Because CD4+ CTLs were generated by in vitro stimulation with synthetic peptides, it was of interest to ascertain whether they recognized processed HSV gB-derived native epitopes in addition to the artificial synthetic peptide epitopes. Therefore, we analyzed the ability of CD4+ T-cell lines generated by gB161-175, gB166-180, gB661-675, and gB666-680 peptides to lyse HLA-DR-positive autologous HSV-1- and VVgB-infected LCLs. Figure 7 shows that CD4+ T-cell lines specific to gB166-180, gB661-675, and gB666-680 peptides undergo significant CD107a/b degranulation when incubated with HLA-DR-positive autologous target cells infected with VVgB or HSV-1 (strain McKrae) (P < 0.005). As a control, no cytolytic activity was detected when gB166-180-, gB661-675-, and gB666-680-specific CD4+ T-cell lines were incubated with target cells infected with empty vaccinia virus (VVC) not expressing gB (not shown). gB161-175 was unable to elicit CTL activity even after several rounds of T-cell-line expansion. Altogether, these results indicate that gB166-180, gB661-675, and gB666-680 are targets of CD4+ CTLs that are able to recognize naturally processed gB epitopes.
FIG. 7.
CD4+ T-cell lines generated by immunodominant CD4+ T-cell peptide epitopes recognized infected target cells expressing native gB. gB161-175, gB166-180, gB661-675, and gB666-680 peptide epitope-specific CD4+ T-cell lines were generated from HLA-DR-positive individuals, and their cytotoxic activity (CD107a/b degranulation) was tested in the presence or absence of target cells infected with HSV-1 or with VVgB. Target cells that were either uninfected or infected with VVC were used as negative controls. Dotted bars represent T-cell responses to VVgB-infected target cells after subtraction of the percent recognition of VVC-infected control cells. Black bars represent T-cell responses to HSV-1-infected target cells after subtracting the percent recognition of uninfected control cells.
T cells from asymptomatic and symptomatic patients recognize different sets of gB peptide epitopes.
Finally, we assessed whether CD4+ T cells from symptomatic and asymptomatic patients recognized different sets of gB epitopes. gB166-180 and gB666-680 T-cell peptide epitopes were strongly recognized by CD4+ T cells from 10 of 10 asymptomatic patients but not by CD4+ T cells from 10 of 10 symptomatic patients (P < 0.0001; analysis of variance posttest) (Fig. 8). Conversely, CD4+ T cells from symptomatic patients preferentially recognized gB661-675 (P < 0.0001) (Fig. 8). These findings greatly strengthen our hypothesis that T cells from symptomatic and asymptomatic patients recognize different sets of herpesvirus epitopes. Interestingly, the “asymptomatic” gB166-180 and gB666-680 peptide epitopes appeared to be highly conserved within and between HSV-1 and HSV-2 strains (not shown) and showed high binding affinities for a range of HLA-DR haplotypes (Table 1). Altogether, these results suggest that the “asymptomatic” gB166-180 and gB666-680 peptide epitopes are strong candidates to be included in the design of future herpes vaccines, while the “symptomatic” gB661-675 peptide epitope should be excluded.
FIG. 8.
IFN-γ-producing CD4+ T-cell responses to gB peptide epitopes in HSV-seropositive symptomatic and asymptomatic patients. CD4+ T cells isolated from HLA-DR-genotyped sex-matched symptomatic (n = 10) and asymptomatic (n = 10) patients were stimulated for 3 days with 10 μg/ml of the indicated gB peptide. IFN-γ-producing CD4+ T-cell responses specific to each epitope were determined as described in the legend to Fig. 2. Each circle represents one symptomatic (solid circle) or one asymptomatic (open circle) patient. “PHA,” T cells stimulated with the mitogen PHA (positive controls). The table shows the HLA-DR alleles of the symptomatic and asymptomatic individuals tested in this study.
DISCUSSION
This study identified four human CD4+ T-cell peptide epitopes from HSV-1 gB. Among these peptide epitopes, gB166-180 and gB666-680 appeared to be most highly recognized by CD4+ T cells from asymptomatic patients and were therefore designated “asymptomatic epitopes,” while gB661-675 appeared to be most highly recognized by CD4+ T cells from symptomatic patients and was therefore designated a “symptomatic epitope”.
CD4+ T-cell infiltrates appear to correlate with HSV clearance from mucocutaneous lesions (38). The target HSV protein Ags and derived epitopes remain to be identified. A number of studies indicate that gB is a major CD4+ T-cell target Ag in HSV-seropositive humans (14, 39, 45, 46, 75-77). Our studies suggest that gB epitopes are targets of both “symptomatic” and “asymptomatic” CD4+ T cells, thus supporting previous studies by Zarling et al., Koelle et al., and Cunningham et al. (33, 37, 38, 47-49, 73-77) showing that gB is targeted by T cells from both symptomatic and asymptomatic individuals. Recently, it was reported that CD4+ T-cell responses to HSV-2 gG differ in symptomatic and asymptomatic HSV-2-infected individuals (9, 16, 58). Similarly, HSV-1-infected symptomatic but not asymptomatic individuals respond to ICP8 and VP16 (59). Of the three identified CD4+ cytotoxic gB peptide epitopes described in the current report, gB166-180 and gB666-680 were recognized mostly by T cells from asymptomatic patients, while gB661-675 was recognized mostly by T cells from symptomatic patients. These findings strengthen our hypothesis that T cells from symptomatic and asymptomatic patients recognize different sets of herpesvirus peptide epitopes. To our knowledge, this is the first report to show a difference in the sets of epitopes recognized in a specific HSV protein by CD4+ cytotoxic T cells from HSV-1-infected asymptomatic compared to symptomatic individuals. Although the first goal of the present report was to investigate whether different and discrete sets of HSV-1 gB epitopes are recognize by CD4+ T cells from symptomatic versus asymptomatic individuals, an HLA association of CD4+ T-cell responses in symptomatic versus asymptomatic individuals is possible. Indeed, the set of viral epitopes recognized by CD4+ T cells from symptomatic individuals (i.e., “symptomatic epitopes”) might be associated with particular HLA haplotypes, while the different set of viral epitopes recognized by T cells from asymptomatic individuals (i.e., “asymptomatic epitopes”) might be associated with different HLA haplotypes. Investigating a possible association between HLA haplotypes and herpes disease would be useful to address in future studies, using a larger cohort of symptomatic and asymptomatic individuals.
It must also be remembered that humans are not immunologically naive and often have a large number of memory CD4+ T-cell populations that can cross-react with, and may disproportionately contribute to the responses to, other infectious pathogens. These cross-reactive T cells can become activated and modulate the immune response and outcome of subsequent heterologous infections, a phenomenon termed heterologous immunity (30, 69, 70). Therefore, we cannot exclude that some of the “symptomatic” and “asymptomatic” HSV-specific CD4+ T cells identified in this study are cross-reactive with self or other pathogen-derived epitopes. Such scenarios have been reported recently for murine heterologous infection and may be true for humans, as shown for CD4+ T-cell responses to cytomegalovirus and other pathogens (15, 54, 69, 70). Shedding of reactivated HSV is estimated to occur at rates of 3 to 33.5% in adults who harbor latent HSV-1 in their sensory neurons (32, 66-68). However, the vast majority of these individuals do not experience recurrent herpetic disease and are designated “asymptomatic patients” (22, 40, 68). In contrast, in some individuals (symptomatic patients) reactivation of latent virus leads to either an induction of ineffective or “symptomatic” HSV-specific T-cell immunity or a greater immunopathological response (20, 22, 68). Recurrent disease ranges from rare episodes occurring once every 5 to 10 years to outbreaks occurring monthly or even more frequently among a small proportion of subjects (22). Interestingly, for genital herpes, symptomatic and asymptomatic patients have similar virus shedding rates (68). It is likely to be the same for ocular herpes, since shedding rates in tears of asymptomatic individuals have been reported to be as high as 33.5% (22, 29, 40, 42).
Identification of the T-cell-mediated immune mechanism(s) by which asymptomatic patients control herpes disease and symptomatic patients do not, or at least the viral epitopes involved, is critical for the rational advance of herpes vaccine development. Among the multitude and complex mechanisms that might be in play are the following. (i) There may be differences in precursor frequencies, proliferative capacities, and functional properties of “symptomatic” versus “asymptomatic” epitope-specific T cells. Indeed, the T-cell repertoires of individuals with the same major histocompatibility complex restriction elements can vary significantly because of “heterologous immunity” and “private specificity” (69, 70). (ii) The differential level of infiltration/homing into sites of infection, i.e., corneas, genital areas, and/or sensory ganglia of T cells, specific to “symptomatic” versus “asymptomatic” epitopes may affect viral production and disease (23, 28, 38). (iii) “Asymptomatic epitopes” might trigger proliferation of “protective” T cells within the sites of infection, while “symptomatic” epitopes might trigger “pathogenic” T cells. (iv) The “symptomatic” epitopes may direct T-cell responses away from those that are best suited to clear the viral infection, with minimal pathogenic reaction. (v). An immunopathogenic T-cell response might occur through stimulating low-affinity oligoclonal responses that inhibit broad-based high-affinity T-cell responses to other well-presented epitopes, thus deviating protective responses to damaging responses. (vi) There may be differences in effector T cells lingering after recent shedding and/or disease versus memory T cells maintained in the absence of antigenic exposure. (vii) Finally, T-cell cross-reactivity with epitopes from other viruses, within or outside the herpesvirus family, can also play a role in protective heterologous immunity versus damaging heterologous immunopathology (31, 71). Regardless of the mechanism(s), if symptomatic individuals tend to generate T cells that recognize a discrete set of “symptomatic” epitopes that differs from the set of “asymptomatic” epitopes, it would be logical to exclude such “symptomatic” epitopes from future herpes vaccines on the grounds that they may enhance rather than diminish recurrent herpes diseases.
Rather than using only predictive computational algorithms to map CD4+ epitopes on HSV-1 gB, we instead employed a systematic strategy, including PepScan library scanning of the whole 904-aa gB sequence followed by multiple functional immunological assays, such as in vitro binding of gB peptides to a panel of HLA-DR molecules, ex vivo IFN-γ ELISPOT assay, CD107 expression, and CD4+ T-cell activation and proliferation analysis. Using these multiple screens, we identified four HSV-1 gB peptides, gB161-175, gB166-180, gB661-675, and gB666-680, that induced IFN-γ-producing CD4+ T cells. Among these peptides, gB166-180, gB661-675, and gB666-680 recalled CD4+ T cells that displayed cytotoxic activity. However, the results reported here do not imply that these are the only human CD4+ T-cell epitopes present on gB. Indeed, the 10-aa regions where these 15-mer peptides overlap might by themselves contain “junctional epitopes.” Shortening or lengthening a peptide by one or a few amino acids might sometimes result in missing junctional epitopes that might be present in the overlapping regions. Thus, to look for these “junctional epitopes,” we expect to synthesize and test a large number of peptide sequences, and the results from these experiments will be the subject of a future report.
A majority of the world's human population is infected with HSV-1 and/or HSV-2 and would most likely benefit from therapeutic vaccination designed to boost HSV-specific cellular immunity (36). Although a primary goal of this study was to identify human “asymptomatic” CD4+ CTL peptide epitopes on HSV-1 gB, the ultimate goal is to build the knowledge of HSV T-cell epitopes required for the development of a totally synthetic, self-adjuvanting lipopeptide human vaccine. We now plan to construct lipopeptide candidate vaccines incorporating the CD4+ T-cell epitopes (but excluding the “symptomatic” epitopes) identified in this study together with human CD8+ T-cell epitopes we recently described for gD (13) and other structural and regulatory herpesvirus proteins (unpublished data). Such a combination would produce CD4+-CD8+ chimeric lipopeptide candidate vaccines similar to those we recently described for mice against ocular and genital herpesvirus infection (79; X. Zhang, A. A. Chentoufi, M. Wu, Z. Zhu, D. Carpenter, A. B. Nesburn, S. L. Wechsler, and L. BenMohamed, submitted for publication). More importantly, each lipopeptide vaccine construct will contain promiscuous CD4+ epitopes identified in this study, thus covering a majority of the population rather than just those having specific HLA-DR alleles.
Although the high degree of HLA polymorphism is often pointed out as a major hindrance to the use of high-affinity epitope-based vaccines, this constraint can be dealt with through the inclusion of multiple supertype-restricted epitopes, recognized in the context of diverse related HLA alleles, and by designing vaccines with higher epitope densities (5, 8, 56, 80). Thus, an effective vaccine could include multiple “asymptomatic” T-cell epitopes present in diverse herpesvirus protein Ags, chosen to represent at least the HLA-DR supertypes, known to provide recognition in up to 95% of the global population, regardless of race and ethnicity (2, 4, 6, 56). Hence, bearing in mind the particular properties that would be required in a possible human vaccine, we have conceived studies to identify HLA class II “promiscuous” T-cell epitopes in HSV protein Ags targeted by CD4+ T cells from “asymptomatic” individuals. These epitopes, along with similarly identified HLA class I supertype-restricted “asymptomatic” CD8+ CTL epitopes, would provide the rationale needed to develop multiepitope CD4-CD8 vaccines that are broadly recognized in the majority of outbred racial and ethnic populations.
Acknowledgments
This work was supported by Public Health Service grants EY14900, EY15225, and EY16663 from the NIH, by The Discovery Eye Foundation, by The Henry L. Guenther Foundation, and by a Research to Prevent Blindness Challenge grant. L. BenMohamed is an RPB Special Award Investigator.
We thank Dennis Zaller (Merck & Co., Inc.) for providing the HLA-DR transgenic mice and David Koelle (University of Washington) for providing the vaccinia viruses used in this study.
Footnotes
Published ahead of print on 17 September 2008.
REFERENCES
- 1.Aurelian, L. 2004. Herpes simplex virus type 2 vaccines: new ground for optimism? Clin. Diagn. Lab. Immunol. 11437-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BenMohamed, L., Y. Belkaid, E. Loing, K. Brahimi, H. Gras-Masse, and P. Druilhe. 2002. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur. J. Immunol. 322274-2281. [DOI] [PubMed] [Google Scholar]
- 3.BenMohamed, L., G. Bertrand, C. D. McNamara, H. Gras-Masse, J. Hammer, S. L. Wechsler, and A. B. Nesburn. 2003. Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J. Virol. 779463-9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BenMohamed, L., R. Krishnan, C. Auge, J. F. Primus, and D. J. Diamond. 2002. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology 106113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BenMohamed, L., A. Thomas, and P. Druilhe. 2004. Long-term multiepitopic cytotoxic-T-lymphocyte responses induced in chimpanzees by combinations of Plasmodium falciparum liver-stage peptides and lipopeptides. Infect. Immun. 724376-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BenMohamed, L., S. L. Wechsler, and A. B. Nesburn. 2002. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect. Dis. 2425-431. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein, D. I., C. J. Harrison, L. J. Jenski, M. G. Myers, and L. R. Stanberry. 1991. Cell-mediated immunologic responses and recurrent genital herpes in the guinea pig. Effects of glycoprotein immunotherapy. J. Immunol. 1463571-3577. [PubMed] [Google Scholar]
- 8.Bettahi, I., X. Zhang, R. E. Afifi, and L. BenMohamed. 2006. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol. 19220-236. [DOI] [PubMed] [Google Scholar]
- 9.Burchett, S. K., L. Corey, K. M. Mohan, J. Westall, R. Ashley, and C. B. Wilson. 1992. Diminished interferon-gamma and lymphocyte proliferation in neonatal and postpartum primary herpes simplex virus infection. J. Infect. Dis. 165813-818. [DOI] [PubMed] [Google Scholar]
- 10.Cantin, E. M., R. Eberle, J. L. Baldick, B. Moss, D. E. Willey, A. L. Notkins, and H. Openshaw. 1987. Expression of herpes simplex virus 1 glycoprotein B by a recombinant vaccinia virus and protection of mice against lethal herpes simplex virus 1 infection. Proc. Natl. Acad. Sci. USA 845908-5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellino, F., and R. N. Germain. 2007. Chemokine-guided CD4+ T cell help enhances generation of IL-6Ralphahigh IL-7Ralpha high prememory CD8+ T cells. J. Immunol. 178778-787. [DOI] [PubMed] [Google Scholar]
- 12.Chan, W. L., M. L. Lukig, and F. Y. Liew. 1985. Helper T cells induced by an immunopurified herpes simplex virus type I (HSV-I) 115 kilodalton glycoprotein (gB) protect mice against HSV-I infection. J. Exp. Med. 1621304-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chentoufi, A. A., X. Zhang, K. Lamberth, G. Dasgupta, I. Bettahi, A. Nguyen, M. Wu, X. Zhu, A. Mohebbi, S. Buus, S. L. Wechsler, A. B. Nesburn, and L. BenMohamed. 2008. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J. Immunol. 180426-437. [DOI] [PubMed] [Google Scholar]
- 14.Corey, L., A. G. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, and S. E. Straus. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282331-340. [DOI] [PubMed] [Google Scholar]
- 15.Cornberg, M., A. T. Chen, L. A. Wilkinson, M. A. Brehm, S. K. Kim, C. Calcagno, D. Ghersi, R. Puzone, F. Celada, R. M. Welsh, and L. K. Selin. 2006. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J. Clin. Investig. 1161443-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson, K., L. Bellner, S. Gorander, G. B. Lowhagen, P. Tunback, K. Rydberg, and J. A. Liljeqvist. 2004. CD4(+) T-cell responses to herpes simplex virus type 2 (HSV-2) glycoprotein G are type specific and differ in symptomatic and asymptomatic HSV-2-infected individuals. J. Gen. Virol. 852139-2147. [DOI] [PubMed] [Google Scholar]
- 17.Fauci, A. S. 1988. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science 239617-622. [DOI] [PubMed] [Google Scholar]
- 18.Field, P. R., D. W. Ho, W. L. Irving, D. Isaacs, and A. L. Cunningham. 1993. The reliability of serological tests for the diagnosis of genital herpes: a critique. Pathology 25175-179. [DOI] [PubMed] [Google Scholar]
- 19.Fleck, M., J. Podlech, K. Weise, and D. Falke. 1994. A vaccinia virus-herpes simplex virus (HSV) glycoprotein B1 recombinant or an HSV vaccine overcome the HSV type 2 induced humoral immunosuppression and protect against vaginal challenge in BALB/c mice. Med. Microbiol. Immunol. 18387-94. [DOI] [PubMed] [Google Scholar]
- 20.Freeman, M. L., B. S. Sheridan, R. H. Bonneau, and R. L. Hendricks. 2007. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J. Immunol. 179322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankins, G. D., F. G. Cunningham, J. P. Luby, S. L. Butler, J. Stroud, and M. Roark. 1984. Asymptomatic genital excretion of herpes simplex virus during early labor. Am. J. Obstet. Gynecol. 150100-101. [DOI] [PubMed] [Google Scholar]
- 22.HEDS. 1998. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N. Engl. J. Med. 339300-306. [DOI] [PubMed] [Google Scholar]
- 23.Hendricks, R. L. 1997. An immunologist's view of herpes simplex keratitis: Thygeson Lecture 1996, presented at the Ocular Microbiology and Immunology Group meeting, October 26, 1996. Cornea 16503-506. [PubMed] [Google Scholar]
- 24.Hendricks, R. L., M. Janowicz, and T. M. Tumpey. 1992. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J. Immunol. 1482522-2529. [PubMed] [Google Scholar]
- 25.Hendricks, R. L., T. M. Tumpey, and A. Finnegan. 1992. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J. Immunol. 1493023-3028. [PubMed] [Google Scholar]
- 26.Hobbs, M. R., B. B. Jones, B. E. Otterud, M. Leppert, and J. D. Kriesel. 2008. Identification of a herpes simplex labialis susceptibility region on human chromosome 21. J. Infect. Dis. 197340-346. [DOI] [PubMed] [Google Scholar]
- 27.Jabbar, A. A., A. M. al-Samarai, and N. S. al-Amar. 1991. HLA antigens associated with susceptibility to herpes simplex virus infection. Dis. Markers 9281-287. [PubMed] [Google Scholar]
- 28.Johnson, A. J., C. F. Chu, and G. N. Milligan. 2008. Effector CD4+ T-cell involvement in clearance of infectious herpes simplex virus type 1 from sensory ganglia and spinal cords. J. Virol. 829678-9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman, H. E., A. M. Azcuy, E. D. Varnell, G. D. Sloop, H. W. Thompson, and J. M. Hill. 2005. HSV-1 DNA in tears and saliva of normal adults. Investig. Ophthalmol. Vis. Sci. 46241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, H. S., M. H. Park, E. Y. Song, H. Park, S. Y. Kwon, S. K. Han, and Y. S. Shim. 2005. Association of HLA-DR and HLA-DQ genes with susceptibility to pulmonary tuberculosis in Koreans: preliminary evidence of associations with drug resistance, disease severity, and disease recurrence. Hum. Immunol. 661074-1081. [DOI] [PubMed] [Google Scholar]
- 31.Kim, S. K., M. Cornberg, X. Z. Wang, H. D. Chen, L. K. Selin, and R. M. Welsh. 2005. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J. Exp. Med. 201523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knaup, B., S. Schunemann, and M. H. Wolff. 2000. Subclinical reactivation of herpes simplex virus type 1 in the oral cavity. Oral Microbiol. Immunol. 15281-283. [DOI] [PubMed] [Google Scholar]
- 33.Koelle, D. M., H. Abbo, A. Peck, K. Ziegweid, and L. Corey. 1994. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J. Infect. Dis. 169956-961. [DOI] [PubMed] [Google Scholar]
- 34.Koelle, D. M., J. Benedetti, A. Langenberg, and L. Corey. 1992. Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital herpes. Ann. Intern. Med. 116433-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koelle, D. M., and T. L. Bergemann. 2008. Doctor, why is my herpes so bad? The search continues. J. Infect. Dis. 197331-334. [DOI] [PubMed] [Google Scholar]
- 36.Koelle, D. M., and L. Corey. 2008. Herpes simplex: insights on pathogenesis and possible vaccines. Annu. Rev. Med. 59381-395. [DOI] [PubMed] [Google Scholar]
- 37.Koelle, D. M., L. Corey, R. L. Burke, R. J. Eisenberg, G. H. Cohen, R. Pichyangkura, and S. J. Triezenberg. 1994. Antigenic specificities of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 682803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 1011500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langenberg, A. G., L. Corey, R. L. Ashley, W. P. Leong, S. E. Straus, et al. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. N. Engl. J. Med. 3411432-1438. [DOI] [PubMed] [Google Scholar]
- 40.Leigh, J. F., N. Acharya, V. Cevallos, and T. P. Margolis. 2008. Does asymptomatic shedding of herpes simplex virus on the ocular surface lead to false-positive diagnostic PCR results? Br. J. Ophthalmol. 92435-436. [DOI] [PubMed] [Google Scholar]
- 41.Lekstrom-Himes, J. A., P. Hohman, T. Warren, A. Wald, J. M. Nam, T. Simonis, L. Corey, and S. E. Straus. 1999. Association of major histocompatibility complex determinants with the development of symptomatic and asymptomatic genital herpes simplex virus type 2 infections. J. Infect. Dis. 1791077-1085. [DOI] [PubMed] [Google Scholar]
- 42.Liesegang, T. J. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 201-13. [DOI] [PubMed] [Google Scholar]
- 43.Lopez, C., and R. J. O'Reilly. 1977. Cell-mediated immune responses in recurrent herpesvirus infections. I. Lymphocyte proliferation assay. J. Immunol. 118895-902. [PubMed] [Google Scholar]
- 44.Malo, A., E. Kampgen, and R. Wank. 1998. Recurrent herpes simplex virus-induced erythema multiforme: different HLA-DQB1 alleles associate with severe mucous membrane versus skin attacks. Scand. J. Immunol. 47408-411. [DOI] [PubMed] [Google Scholar]
- 45.Mertz, G. J. 1990. Genital herpes simplex virus infections. Med. Clin. N. Am. 741433-1454. [DOI] [PubMed] [Google Scholar]
- 46.Mertz, G. J., R. Ashley, R. L. Burke, J. Benedetti, C. Critchlow, C. C. Jones, and L. Corey. 1990. Double-blind, placebo-controlled trial of a herpes simplex virus type 2 glycoprotein vaccine in persons at high risk for genital herpes infection. J. Infect. Dis. 161653-660. [DOI] [PubMed] [Google Scholar]
- 47.Mikloska, Z., and A. L. Cunningham. 1998. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J. Gen. Virol. 79353-361. [DOI] [PubMed] [Google Scholar]
- 48.Mikloska, Z., V. A. Danis, S. Adams, A. R. Lloyd, D. L. Adrian, and A. L. Cunningham. 1998. In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions—do herpes simplex virus-infected keratinocytes contribute to their production? J. Infect. Dis. 177827-838. [DOI] [PubMed] [Google Scholar]
- 49.Mikloska, Z., A. M. Kesson, M. E. Penfold, and A. L. Cunningham. 1996. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J. Infect. Dis. 1737-17. [DOI] [PubMed] [Google Scholar]
- 50.Niemialtowski, M. G., and B. T. Rouse. 1992. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J. Immunol. 1481864-1870. [PubMed] [Google Scholar]
- 51.Niemialtowski, M. G., and B. T. Rouse. 1992. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J. Immunol. 1493035-3039. [PubMed] [Google Scholar]
- 52.Olerup, O., and H. Zetterquist. 1992. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens 39225-235. [DOI] [PubMed] [Google Scholar]
- 53.Schmid, D. S., D. R. Brown, R. Nisenbaum, R. L. Burke, D. Alexander, R. Ashley, P. E. Pellett, and W. C. Reeves. 1999. Limits in reliability of glycoprotein G-based type-specific serologic assays for herpes simplex virus types 1 and 2. J. Clin. Microbiol. 37376-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selin, L. K., M. A. Brehm, Y. N. Naumov, M. Cornberg, S. K. Kim, S. C. Clute, and R. M. Welsh. 2006. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol. Rev. 211164-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seppanen, M., M. L. Lokki, T. Timonen, M. Lappalainen, H. Jarva, A. Jarvinen, S. Sarna, V. Valtonen, and S. Meri. 2001. Complement C4 deficiency and HLA homozygosity in patients with frequent intraoral herpes simplex virus type 1 infections. Clin. Infect. Dis. 331604-1607. [DOI] [PubMed] [Google Scholar]
- 56.Sette, A., and J. Sidney. 1999. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50201-212. [DOI] [PubMed] [Google Scholar]
- 57.Siegal, F. P., C. Lopez, G. S. Hammer, A. E. Brown, S. J. Kornfeld, J. Gold, J. Hassett, S. Z. Hirschman, C. Cunningham-Rundles, B. R. Adelsberg, et al. 1981. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N. Engl. J. Med. 3051439-1444. [DOI] [PubMed] [Google Scholar]
- 58.Singh, R., A. Kumar, W. D. Creery, M. Ruben, A. Giulivi, and F. Diaz-Mitoma. 2003. Dysregulated expression of IFN-gamma and IL-10 and impaired IFN-gamma-mediated responses at different disease stages in patients with genital herpes simplex virus-2 infection. Clin. Exp. Immunol. 13397-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spatz, M., H. M. Wolf, V. Thon, J. M. Gampfer, and M. M. Eibl. 2000. Immune response to the herpes simplex type 1 regulatory proteins ICP8 and VP16 in infected persons. J. Med. Virol. 6229-36. [DOI] [PubMed] [Google Scholar]
- 60.Stamm, W. E., H. H. Handsfield, A. M. Rompalo, R. L. Ashley, P. L. Roberts, and L. Corey. 1988. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA 2601429-1433. [PubMed] [Google Scholar]
- 61.Stanberry, L. R., C. J. Harrison, D. I. Bernstein, R. L. Burke, R. Shukla, G. Ott, and M. G. Myers. 1989. Herpes simplex virus glycoprotein immunotherapy of recurrent genital herpes: factors influencing efficacy. Antivir. Res. 11203-214. [DOI] [PubMed] [Google Scholar]
- 62.Tang, Q., W. Chen, and R. L. Hendricks. 1997. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J. Immunol. 1581275-1283. [PubMed] [Google Scholar]
- 63.Tang, Q., and R. L. Hendricks. 1996. Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J. Exp. Med. 1841435-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Texier, C., S. Pouvelle, M. Busson, M. Herve, D. Charron, A. Menez, and B. Maillere. 2000. HLA-DR restricted peptide candidates for bee venom immunotherapy. J. Immunol. 1643177-3184. [DOI] [PubMed] [Google Scholar]
- 65.Torseth, J. W., and T. C. Merigan. 1986. Significance of local gamma interferon in recurrent herpes simplex infection. J. Infect. Dis. 153979-984. [DOI] [PubMed] [Google Scholar]
- 66.Wald, A., L. Corey, R. Cone, A. Hobson, G. Davis, and J. Zeh. 1997. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J. Clin. Investig. 991092-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wald, A., J. Zeh, S. Selke, T. Warren, R. Ashley, and L. Corey. 2002. Genital shedding of herpes simplex virus among men. J. Infect. Dis. 186(Suppl. 1)S34-S39. [DOI] [PubMed] [Google Scholar]
- 68.Wald, A., J. Zeh, S. Selke, T. Warren, A. J. Ryncarz, R. Ashley, J. N. Krieger, and L. Corey. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342844-850. [DOI] [PubMed] [Google Scholar]
- 69.Welsh, R. M. 2006. Private specificities of heterologous immunity. Curr. Opin. Immunol. 18331-337. [DOI] [PubMed] [Google Scholar]
- 70.Welsh, R. M., S. K. Kim, M. Cornberg, S. C. Clute, L. K. Selin, and Y. N. Naumov. 2006. The privacy of T cell memory to viruses. Curr. Top. Microbiol. Immunol. 311117-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welsh, R. M., and L. K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2417-426. [DOI] [PubMed] [Google Scholar]
- 72.Whitley, R. J., and J. W. Gnann. 2002. Viral encephalitis: familiar infections and emerging pathogens. Lancet 359507-513. [DOI] [PubMed] [Google Scholar]
- 73.Yasukawa, M., and Y. Kobayashi. 1985. Inhibition of herpes simplex virus replication in vitro by human cytotoxic T cell clones and natural killer cell clones. J. Gen. Virol. 662225-2229. [DOI] [PubMed] [Google Scholar]
- 74.Yasukawa, M., and J. M. Zarling. 1985. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. III. Analysis of viral glycoproteins recognized by CTL clones by using recombinant herpes simplex viruses. J. Immunol. 1342679-2682. [PubMed] [Google Scholar]
- 75.Zarling, J. M., P. A. Moran, L. Brewer, R. Ashley, and L. Corey. 1988. Herpes simplex virus (HSV)-specific proliferative and cytotoxic T-cell responses in humans immunized with an HSV type 2 glycoprotein subunit vaccine. J. Virol. 624481-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zarling, J. M., P. A. Moran, R. L. Burke, C. Pachl, P. W. Berman, and L. A. Lasky. 1986. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J. Immunol. 1364669-4673. [PubMed] [Google Scholar]
- 77.Zarling, J. M., P. A. Moran, L. A. Lasky, and B. Moss. 1986. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J. Virol. 59506-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang, X., F. A. Castelli, X. Zhu, M. Wu, B. Maillère, and L. BenMohamed. 2008. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin. Vaccine Immunol. 151436-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, X., A. Issagholian, E. A. Berg, J. B. Fishman, A. B. Nesburn, and L. BenMohamed. 2005. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nɛ-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J. Virol. 7915289-15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu, X., T. V. Ramos, H. Gras-Masse, B. E. Kaplan, and L. BenMohamed. 2004. Lipopeptide epitopes extended by Ne-palmitoyl lysine moiety increases uptake and maturation of dendritic cell through a Toll-like receptor 2 pathway and triggers a Th1-dependent protective immunity. Eur. J. Immunol. 341142-1149. [DOI] [PubMed] [Google Scholar]