Abstract
The entry and dissemination of viruses in several families can be mediated by C-type lectins such as DC-SIGN. We showed that entry of the serotype II feline coronavirus strains feline infectious peritonitis virus (FIPV) WSU 79-1146 and DF2 into nonpermissive mouse 3T3 cells can be rescued by the expression of human DC-SIGN (hDC-SIGN) and that infection of a permissive feline cell line (Crandall-Reese feline kidney) was markedly enhanced by the overexpression of hDC-SIGN. Treatment with mannan considerably reduced infection of feline monocyte-derived cells expressing DC-SIGN, indicating a role for FIPV infection in vivo.
The entry of coronaviruses (CoVs) is mediated by a primary receptor, which in many cases has been well characterized (29). Aminopeptidase N (APN, or CD13) acts as a primary receptor for the entry of serotype II feline coronaviruses (FCoVs) in both feline infectious peritonitis virus (FIPV) and feline enteric coronavirus (FECV) biotypes (10, 29), as well as in transmissible gastroenteritis virus and human CoV strain 229E (HCoV-229E) (27). In addition to having a primary receptor, it is becoming increasingly clear that coronaviruses also make use of a variety of nonspecific receptors during entry, including sialic acid and C-type lectins (29). Notably, severe acute respiratory syndrome CoV was shown to utilize liver/lymph node-specific ICAM-3-grabbing nonintegrin (L-SIGN/CD209L) as a receptor in cis (11, 17), possibly in combination with a specific receptor, ACE2. Severe acute respiratory syndrome CoV also interacts with dendritic cell (DC)-specific ICAM-3-grabbing nonintegrin (DC-SIGN/CD209) in cis (11), as well as with DC-SIGN and/or L-SIGN in trans (19, 30). L-SIGN and DC-SIGN have also been shown to promote the entry of HCoV-229E and HCoV-NL63, respectively (12, 16).
The utilization of C-type lectins as entry factors was first demonstrated for human immunodeficiency virus type 1 (1, 9), and it is now known that viruses in various families use DC-SIGN and/or L-SIGN for entry and dissemination, acting either in cis or in trans (3, 18). C-type lectins act as entry factors by preferentially recognizing viral glycoproteins containing high-mannose carbohydrate residues (3, 7). Compared to that in the human system, the use of C-type lectins as entry factors in nonhuman animal viruses has received little attention. A notable exception is the demonstration that feline immunodeficiency virus can specifically interact with human DC-SIGN and allow virus transmission to target cells in cis and in trans (6). Based on this finding and the established role of L-SIGN/DC-SIGN for certain coronaviruses, we reasoned that FCoVs might be able to utilize C-type lectins as entry cofactors, with human DC-SIGN (hDC-SIGN) able to serve in this role.
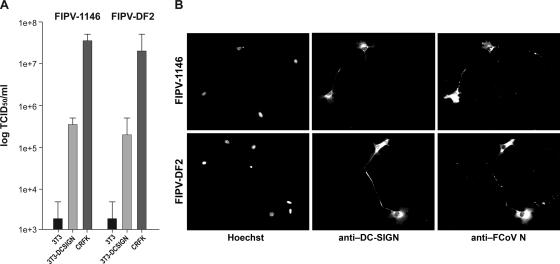
To examine the possible role of hDC-SIGN in FIPV infection, we first analyzed the infection of mouse 3T3 cell lines stably expressing hDC-SIGN (NIAID AIDS Research and Reference Reagent Program) and compared it to that of nonpermissive wild-type 3T3 cells and permissive Crandall-Reese feline kidney (CRFK) cells (ATCC). Cells were infected with either serotype II FIPV strain WSU 79-1146 (FIPV-1146) or FIPV-DF2 at a final concentration of 1 × 106 PFU/ml (multiplicity of infection [MOI] = 10 infectious units/cell), as determined by standard plaque assay of CRFK cells. The supernatants were collected, and the production of virus was determined at 24 h postinfection by 50% tissue culture infective dose assay of CRFK cells (Fig. 1A). As expected, 3T3 cells were not able to support FIPV infection; however, 3T3-hDCSIGN cells propagated the virus to a relatively high titer. The production of virus was approximately 2 log units lower than in permissive CRFK cells. To confirm that productive infection was mediated specifically by the expression of hDC-SIGN, we transfected wild-type 3T3 cells with plasmid pcDNA3-DCSIGN (NIAID AIDS Research and Reference Reagent Program) to transiently express hDC-SIGN. At twenty-four hours posttransfection, cells were infected with FIPV-1146 or FIPV-DF2 at a final concentration of 1 × 106 PFU/ml, and at 9 h postinfection, the cells were fixed. Cells were analyzed by immunofluorescence microscopy, using the anti-DC-SIGN monoclonal antibody (MAb) 120526 (an immunoglobulin G2a [IgG2a] isotype; NIAID AIDS Research and Reference Reagent Program) and the anti-FCoV nucleocapsid MAb 17B7.1 (an IgG2b isotype) (21) followed by isotype-specific secondary antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 569 (Invitrogen). Infection of 3T3 cells by either FIPV-1146 or FIPV-DF2 strictly correlated with hDC-SIGN expression (Fig. 1B).
FIG. 1.
Expression of hDC-SIGN rescues FCoV infection of nonpermissive cells. (A) 3T3, 3T3-DCSIGN, or CRFK cells were infected with FIPV-1146 or FIPV-DF2 at a MOI of 10. Cells were rinsed and placed in fresh media after 2 h. At 24 h, cells and the supernatants were collected. The production of virus was determined from at least three independent replicates of the experiment by 50% tissue culture infective dose (TCID50) assay. Error bars represent the standard deviations of the means. (B) 3T3 cells were transfected with pCDNA3-hDCSIGN. Twenty-four hours posttransfection, cells were infected with virus at a MOI of 10. Cells were fixed 9 h postinfection and stained for immunofluorescence microscopy with the IgG2a anti-hDC-SIGN MAb 120526 and the IgG2b anti-FCoV nucleocapsid (anti-FIPV N) MAb 17B7.1.
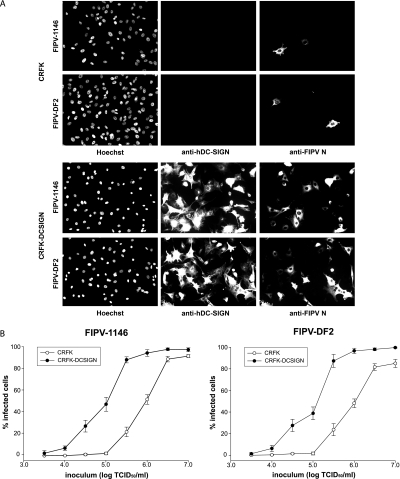
To examine how DC-SIGN might function in the context of FIPV infection of permissive cells, we created CRFK cells lines stably expressing hDC-SIGN. CRFK or CRFK-hDCSIGN cells were infected with FIPV-1146 and FIPV-DF2 at a low MOI of 0.01 infectious units/cell (1 × 103 PFU/ml). Cells were fixed at 6 h postinfection and analyzed by immunofluorescence microscopy after being stained with the IgG2a anti-hDC-SIGN MAb 120526 and the IgG2b anti-FCoV nucleocapsid MAb 17B7.1. Infection of CRFK cells was markedly increased in CRFK-hDCSIGN cells (Fig. 2A). To quantify the increase in infection following hDC-SIGN expression, the experiments were repeated with inocula of various amounts of FIPV (0.0005 to 1.0 infectious units/cell), and images from at least three independent experiments (>1,000 cells) were scored for infection. As shown in Fig. 2B, the expression of hDC-SIGN significantly enhanced infection of FIPV over a range of MOI values but with more-limited effects at the lowest and highest infectious doses.
FIG. 2.
Expression of hDC-SIGN enhances FCoV infection of permissive cells. (A) CRFK or CRFK-hDCSIGN cells were infected with FIPV-1146 or FIPV-DF2 at a MOI of 0.01. Six hours postinfection, cells were fixed and stained for immunofluorescence microscopy with the IgG2a anti-hDC-SIGN MAb 120526 and the IgG2b anti-FCoV nucleocapsid (anti-FIPV N) MAb 17B7.1. (B) CRFK or CRFK-hDCSIGN cells were infected with FIPV-1146 or FIPV-DF2 at the indicated concentrations. Six hours postinfection, cells were fixed and stained for immunofluorescence microscopy with the anti-FCoV nucleocapsid MAb 17B7.1. Images from at least three independent experiments were captured and quantified. For quantification, >1,000 cells from three independent replicates of each experimental condition were scored. Error bars represent the standard deviations of the means. TCID50, 50% tissue culture infective dose.
To confirm that the enhancement of FIPV infection following DC-SIGN expression was specific, we infected CRFK or CRFK-DCSIGN cells with FIPV-1146 and FIPV-DF2 at an MOI of 0.01 infectious unit/cell (1 × 103 PFU/ml) in the presence of mannan (50 mg/ml) as a competitor of DC-SIGN binding or in the presence of the anti-DC-SIGN MAbs 9E9A8 and 120526 (20 mg/ml; NIAID AIDS Research and Reference Reagent Program) (Fig. 3). Both mannan and the two MAbs tested reduced FIPV infection to the control levels found in the absence of hDC-SIGN expression.
FIG. 3.
Mannan and anti-hDC-SIGN MAbs block the enhancement of infection by FCoVs. CRFK, CRFK-hDCSIGN, or CRFK-hDCSIGN cells pretreated with either mannan (50 μg/ml), anti-hDC-SIGN MAb 9E9A8 (20 μg/ml), or anti-hDC-SIGN MAb 120526 (20 μg/ml) were infected with FIPV-1146 or FIPV-DF2 at a MOI of 0.01. Cells were fixed at 6 h postinfection and stained for immunofluorescence microscopy with the anti-FCoV nucleocapsid MAb 17B7.1. Images from at least three independent experiments were captured and quantified. For quantification, >1,000 cells from three independent replicates of each experimental condition were scored. Error bars represent the standard deviations of the means.
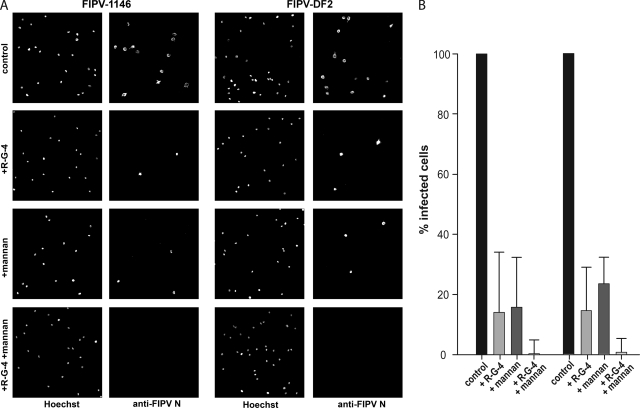
Dissemination of FIPV in the cat relies on the ability of the virus to infect monocytes and macrophages (10). To examine the role of DC-SIGN in such in vivo situations, we isolated primary feline monocytes (23) and cultured them for 4 days in the presence of 20% fetal bovine serum. These cells were specifically recognized by the hDC-SIGN MAb DC6 (NIAID AIDS Research and Reference Reagent Program) (data not shown), indicating that they had undergone differentiation and were expressing DC-SIGN. We then treated these cells with either the MAb R-G-4 (200 mg/ml) to inhibit infection via the feline aminopeptidase N (fAPN) receptor (14) or with mannan (50 mg/ml). Monocytes were then infected with FIPV strains 1146 and DF2 at 1 × 106 PFU/ml (MOI = 10 infectious units/cell). Cells were fixed at 6 h postinfection and analyzed by immunofluorescence microscopy as described above. Compared to the untreated controls, both cells treated with R-G-4 and those treated with mannan showed a significant inhibition of FIPV infection, in both cases to around 15% of the level for the untreated cells (Fig. 4). The addition of R-G-4 and mannan combined resulted in an almost complete blockage of FIPV infection (Fig. 4). These data confirm previous reports that the blockage of fAPN with R-G-4 alone could not completely inhibit the infection of feline macrophages with serotype II FIPV (24) and suggest that both APN and DC-SIGN are important for infection in vivo.
FIG. 4.
Mannan inhibits FCoV infection of primary feline monocyte-derived cells expressing DC-SIGN. (A) Primary feline cells expressing DC-SIGN were pretreated with mannan (50 μg/ml), anti-fAPN MAb R-G-4 (200 μg/ml), or both in combination for 60 min before being infected with FIPV-1146 or FIPV-DF2 at a MOI of 10. Cells were fixed 12 h postinfection and stained for immunofluorescence microscopy with the anti-FCoV nucleocapsid (anti-FCoV N) MAb 17B7.1. (B) Images from at least three independent experiments were captured and quantified. For quantification, >250 cells from three independent replicates of each experimental condition were scored. Error bars represent the standard deviations of the means.
Our studies show that the C-type lectin hDC-SIGN can rescue FIPV infection of nonpermissive cells and enhance infection of permissive cells, acting in cis. To date, we have not examined the use of hDC-SIGN in trans for FIPV infection in vivo. As the feline homolog of hDC-SIGN has not yet been cloned (6), the role of FCoV-DC-SIGN interactions in cats remains to be demonstrated, and we have not examined serotype I FCoVs due to the difficulty in propagating these viruses in the laboratory. In our studies, we also examined human L-SIGN, which was able to rescue infection with FIPV-1146 (data not shown). However, as L-SIGN is as yet unreported except in humans, this was not examined further.
It is interesting to note that both APN and DC-SIGN are localized to cell surface microdomains, or lipid rafts (2, 20), and that this localization is important for the function of DC-SIGN during virus entry (2). This suggests that the colocalization of the two cell surface molecules may be a factor in successful FCoV entry into host cells. At present, however, little is known regarding the internalization pathway of FCoVs, although HCoV-299E (which uses a combination of APN and L-SIGN for entry) is believed to enter cells via cell surface microdomains that contain caveolin (20). Other likely factors in the successful use of DC-SIGN as an entry factor for coronaviruses are that viral spike proteins are heavily glycosylated and that the virus buds from the endoplasmic reticulum-Golgi intermediate compartment (13); the coronavirus spike protein is therefore likely to contain high-mannose sugars that would be a recognition site for C-type lectins (3, 4, 7).
The majority of natural infections with FCoVs are classified as being in the FECV biotype and cause, at most, mild enteritis in the intestinal epithelial tract. It is believed that mutations within FECV viruses allow the dissemination and systemic spread of the newly generated FIPV biotype viruses, and monocytes and macrophages are well established as cell types that act as a conduit for such systemic spread (10). DC-SIGN is considered to be widely expressed in monocyte-derived macrophages which are thought to be the targets of FIPV infection in vivo (10), so in the course of our studies, we examined whether there were any differences in the utilization of DC-SIGN by FIPV-1146 and the FECV biotype WSU 79-1638. However, we found no significant difference in the effects of hDC-SIGN expression between these two viruses (data not shown), which suggests that any change in cell tropism between these viruses appears to occur at a postreceptor step of infection. At present, there is only limited information on the possible role of DCs in the dissemination of FIPV. With increased characterization of feline DCs (8, 25), it will be very interesting to explore these cells in the context of FIPV infection in cats.
Although murine APN is not considered to support FIPV infection by itself (28), it remains possible that the entry of serotype II FCoV is mediated through a combination of DC-SIGN and mouse APN. In this scenario, DC-SIGN would act as an entry cofactor, allowing for the use of mouse APN as a viral receptor. Alternatively, DC-SIGN may act an alternate receptor for FCoV rather than acting as an accessory factor. Such APN-independent entry of FIPV has been known for some time, based on Fc-mediated uptake of the virus into mouse macrophages in the presence of anti-S antibodies (5, 15, 22, 26). To address these possible models, hDC-SIGN was overexpressed in primary fibroblast cells from an APN knockout mouse and from a parental wild-type mouse (kindly provided by Renata Pasqualini and Wadih Arap, Anderson Cancer Center, Houston, TX). While overexpression of hDC-SIGN in wild-type murine fibroblasts rescued infection by FIPV, it did not allow infection in APN knockout cells (data not shown), suggesting that DC-SIGN does not act as an alternate receptor for FIPV but rather acts as an entry cofactor. However, it cannot be ruled out that DC-SIGN may act as an independent FCoV receptor under certain conditions.
Acknowledgments
We thank Fred Scott, Joel Baines, and Sandrine Belouzard for helpful advice and discussions during the course of this work and Ed Dubovi for kind provision of reagents. We also thank A. Damon Ferguson for technical assistance and Renata Pasqualini and Wadih Arap for provision of cells from APN-null mice.
This work was supported in part by a research grant (to G.R.W.) from the Winn Feline Foundation. A.D.R. was supported by grant T32AI007618 (Training in Molecular Virology and Pathogenesis) from the National Institutes of Health.
Footnotes
Published ahead of print on 17 September 2008.
REFERENCES
- 1.Baribaud, F., S. Pohlmann, and R. W. Doms. 2001. The role of DC-SIGN and DC-SIGNR in HIV and SIV attachment, infection, and transmission. Virology 2861-6. [DOI] [PubMed] [Google Scholar]
- 2.Cambi, A., F. de Lange, N. M. van Maarseveen, M. Nijhuis, B. Joosten, E. M. van Dijk, B. I. de Bakker, J. A. Fransen, P. H. Bovee-Geurts, F. N. van Leeuwen, N. F. Van Hulst, and C. G. Figdor. 2004. Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells. J. Cell Biol. 164145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cambi, A., M. Koopman, and C. G. Figdor. 2005. How C-type lectins detect pathogens. Cell Microbiol. 7481-488. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh, D. 1983. Coronavirus IBV glycopolypeptides: size of their polypeptide moieties and nature of their oligosaccharides. J. Gen. Virol. 641187-1191. [DOI] [PubMed] [Google Scholar]
- 5.Corapi, W. V., R. J. Darteil, J. C. Audonnet, and G. E. Chappuis. 1995. Localization of antigenic sites of the S glycoprotein of feline infectious peritonitis virus involved in neutralization and antibody-dependent enhancement. J. Virol. 692858-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Parseval, A., S. V. Su, J. H. Elder, and B. Lee. 2004. Specific interaction of feline immunodeficiency virus surface glycoprotein with human DC-SIGN. J. Virol. 782597-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinberg, H., D. A. Mitchell, K. Drickamer, and W. I. Weis. 2001. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 2942163-2166. [DOI] [PubMed] [Google Scholar]
- 8.Freer, G., D. Matteucci, P. Mazzetti, L. Bozzacco, and M. Bendinelli. 2005. Generation of feline dendritic cells derived from peripheral blood monocytes for in vivo use. Clin. Diagn. Lab. Immunol. 121202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100587-597. [DOI] [PubMed] [Google Scholar]
- 10.Haijema, B. J., P. J. Rottier, and R. J. de Groot. 2007. Feline coronaviruses: a tale of two-faced types, p. 183-203. In V. Thiel (ed.), Coronaviruses. Molecular and cellular biology. Caister Academic Press, Norfolk, United Kingdom.
- 11.Han, D. P., M. Lohani, and M. W. Cho. 2007. Specific asparagine-linked glycosylation sites are critical for DC-SIGN- and L-SIGN-mediated severe acute respiratory syndrome coronavirus entry. J. Virol. 8112029-12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann, H., G. Simmons, A. J. Rennekamp, C. Chaipan, T. Gramberg, E. Heck, M. Geier, A. Wegele, A. Marzi, P. Bates, and S. Pohlmann. 2006. Highly conserved regions within the spike proteins of human coronaviruses 229E and NL63 determine recognition of their respective cellular receptors. J. Virol. 808639-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogue, B. G., and C. E. Machamer. 2008. Coronavirus structural proteins and virus assembly, p. 179-200. In S. Perlman, T. Gallagher, and E. J. Snijder (ed.), Nidoviruses. ASM Press, Washington, DC.
- 14.Hohdatsu, T., Y. Izumiya, Y. Yokoyama, K. Kida, and H. Koyama. 1998. Differences in virus receptor for type I and type II feline infectious peritonitis virus. Arch. Virol. 143839-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohdatsu, T., J. Tokunaga, and H. Koyama. 1994. The role of IgG subclass of mouse monoclonal antibodies in antibody-dependent enhancement of feline infectious peritonitis virus infection of feline macrophages. Arch. Virol. 139273-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffers, S. A., E. M. Hemmila, and K. V. Holmes. 2006. Human coronavirus 229E can use CD209L (L-SIGN) to enter cells. Adv. Exp. Med. Biol. 581265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffers, S. A., S. M. Tusell, L. Gillim-Ross, E. M. Hemmila, J. E. Achenbach, G. J. Babcock, W. D. Thomas, Jr., L. B. Thackray, M. D. Young, R. J. Mason, D. M. Ambrosino, D. E. Wentworth, J. C. Demartini, and K. V. Holmes. 2004. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 10115748-15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lozach, P. Y., L. Burleigh, I. Staropoli, and A. Amara. 2007. The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins. Methods Mol. Biol. 37951-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzi, A., T. Gramberg, G. Simmons, P. Moller, A. J. Rennekamp, M. Krumbiegel, M. Geier, J. Eisemann, N. Turza, B. Saunier, A. Steinkasserer, S. Becker, P. Bates, H. Hofmann, and S. Pohlmann. 2004. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J. Virol. 7812090-12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nomura, R., A. Kiyota, E. Suzaki, K. Kataoka, Y. Ohe, K. Miyamoto, T. Senda, and T. Fujimoto. 2004. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 788701-8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen, C. W., W. V. Corapi, C. K. Ngichabe, J. D. Baines, and F. W. Scott. 1992. Monoclonal antibodies to the spike protein of feline infectious peritonitis virus mediate antibody-dependent enhancement of infection of feline macrophages. J. Virol. 66956-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlman, S., and A. A. Dandekar. 2005. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 5917-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regan, A. D., R. Shraybman, R. D. Cohen, and G. R. Whittaker. 29 May 2008, posting date. Differential role for low pH and cathepsin-mediated cleavage of the viral spike protein during entry of serotype II feline coronaviruses. Vet. Microbiol. [Epub ahead of print.] doi: 10.1016/j.vetmic.2008.05.019. [DOI] [PMC free article] [PubMed]
- 24.Rottier, P. J., K. Nakamura, P. Schellen, H. Volders, and B. J. Haijema. 2005. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J. Virol. 7914122-14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sprague, W. S., M. Pope, and E. A. Hoover. 2005. Culture and comparison of feline myeloid dendritic cells vs macrophages. J. Comp. Pathol. 133136-145. [DOI] [PubMed] [Google Scholar]
- 26.Takano, T., Y. Katada, S. Moritoh, M. Ogasawara, K. Satoh, R. Satoh, M. Tanabe, and T. Hohdatsu. 2008. Analysis of the mechanism of antibody-dependent enhancement of feline infectious peritonitis virus infection: aminopeptidase N is not important and a process of acidification of the endosome is necessary. J. Gen. Virol. 891025-1029. [DOI] [PubMed] [Google Scholar]
- 27.Tresnan, D. B., R. Levis, and K. V. Holmes. 1996. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 708669-8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tusell, S. M., S. A. Schittone, and K. V. Holmes. 2007. Mutational analysis of aminopeptidase N, a receptor for several group 1 coronaviruses, identifies key determinants of viral host range. J. Virol. 811261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wentworth, D. E., and K. V. Holmes. 2007. Coronavirus binding and entry, p. 3-31. In V. Thiel (ed.), Coronaviruses. Molecular and cellular biology. Caister Academic Press, Norfolk, United Kingdom.
- 30.Yang, Z. Y., Y. Huang, L. Ganesh, K. Leung, W. P. Kong, O. Schwartz, K. Subbarao, and G. J. Nabel. 2004. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 785642-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]